
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 10 February 2025
Sec. Veterinary Epidemiology and Economics
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1504640
This article is part of the Research TopicAntimicrobial Resistance in Veterinary Medicine: Epidemiology, Economic Impact, and Mitigation StrategiesView all 4 articles
 Essam M. Abdelfattah1,2
Essam M. Abdelfattah1,2 Pius S. Ekong1
Pius S. Ekong1 Emmanuel Okello1,3
Emmanuel Okello1,3 Tapakorn Chamchoy1
Tapakorn Chamchoy1 Betsy M. Karle4
Betsy M. Karle4 Randi A. Black5
Randi A. Black5 Wagdy ElAshmawy1,6
Wagdy ElAshmawy1,6 David Sheedy1
David Sheedy1 Deniece R. Williams1
Deniece R. Williams1 Terry W. Lehenbauer1,3
Terry W. Lehenbauer1,3 Barbara A. Byrne7
Barbara A. Byrne7 Sharif S. Aly1,3*
Sharif S. Aly1,3*The current study objective was to investigate the risk factors associated with the isolation of antimicrobial-resistant Escherichia coli, Enterococcus spp., and Streptococcus spp. (ES) from the feces of dairy cows in California (CA). A longitudinal study was conducted on ten dairies, and a random sample of cattle (late pregnant heifers and dry cows) stratified by each herd’s parity distribution were followed monthly from close-up to 120 days in milk during fall to winter 2018 (winter season) and spring to summer 2019 (summer season). Gastrointestinal commensals were isolated from fecal samples and tested for antimicrobial susceptibility using the broth microdilution method against a selected panel of antimicrobial drugs (AMD). Eight dairies used blanket AMD therapy at dry-off for all lactating cows, while the remaining two dairies did not use any AMD treatment at dry-off. Clinical mastitis was identified as the most common indication for AMD use across the study dairies. Intramuscular administration of ceftiofur hydrochloride to treat lameness and unknown disease during lactation was significantly associated with the isolation of tetracycline-resistant fecal E. coli. Resistance to ceftiofur, tetracycline, or trimethoprim-sulfamethoxazole in fecal E. coli was significantly higher in the winter than in the summer season. In contrast, resistance to tetracycline, florfenicol, tilmicosin, tildipirosin, or tiamulin in fecal gram-positive commensals was significantly higher in the summer than in the winter. In conclusion, AMD usage practices and seasonal variations significantly influenced the AMR of E. coli and ES in the feces of dairy cattle.
Antimicrobial resistance (AMR) is a significant global health issue with considerable financial and health implications for both humans and animals (1). The use of antimicrobial drugs (AMD) and the subsequent development of AMR involves a complex, multifactorial process (1). In response to societal concerns about AMR in livestock, the US Food and Drug Administration (FDA) implemented the final rule of the Veterinary Feed Directive (VFD) in 2017, along with additional guidance. These regulations aim to ensure the judicious use of all medically important antimicrobial drugs (MIADs) administered through feed or water to food-producing animals (2). In 2018, California (CA) implemented Senate Bill 27 (SB 27), requiring veterinary prescriptions under a valid veterinarian-client-patient relationship (VCPR) for all other dosage forms of MIADs used for livestock and administered by routes other than in feed or water, which includes injectable, intramammary (IMM), and other oral AMD dosage forms (3). The CA state law has resulted in moving the AMDs that were available in livestock supply and feed stores as over-the-counter (OTC) products to prescription only under a valid VCPR. Similarly, the FDA issued Guidance for Industry #263 in 2019, moving OTC AMD nationwide to prescription-only status for all animal uses, and in 2021, initiated a two-year timeline for full implementation beginning on 11th June 11, 2023 (4).
Fecal commensals, including Escherichia coli and Enterococcus spp., have been used as indicator organisms in various studies on AMR because they can acquire resistance genes and act as a reservoir for the spread of resistance genes (5). Commensal bacteria in livestock can serve as a reservoir for resistance genes that could be transferred to other bacteria that may cause cattle disease (6). To control the spread and prevent AMR in commensal bacteria, it is important to identify the risk factors that are associated with such resistance. Understanding the association between the use of AMD and AMR in animal production systems will help develop effective control measures (7). Differences in exposure to AMD, management practices, and exposure to other risk factors, such as the age of the animal, herd size, sampling season, and region, were found to be associated with the development of AMR (7, 8). Previous studies have focused on AMR in calves (9, 10), AMR bacteria isolated directly from clinical mastitis cases (11, 12), or focused on AMR dynamics related to a specific drug (8). However, there is little information regarding the effect of locally applied IMM AMDs or systemically administered AMDs on Gram-negative or Gram-positive enteric commensals in dairy cows.
To the best of our knowledge, this is the first study conducted on California dairies after the implementation of SB 27 to study the effect of AMD use on AMR of fecal commensals. The current study uniquely followed cow cohorts across various regions and seasons, providing comprehensive insights into the regional and seasonal dynamics of AMR. The primary objective of this longitudinal study was to explore the associations between herd demographics, management practices, and AMD use as predictors of AMR in fecal commensal bacteria isolated from cows on CA dairies followed from the dry period to 120 days in milk (DIM) post-calving. We hypothesized that various factors contribute to the development of AMR in fecal commensal bacteria isolated from dairy cows. These factors include herd demographics such as herd size, breed, region, and season; management practices including dry-off protocols and disease condition; and the use of antimicrobial drugs, specifically the timing of AMD administration and the type of AMDs used, whether systemic or IMM.
The study was approved by the University of California Davis’ Institutional Animal Care and Use Committee (protocol number 19871). The current study was part of a prospective longitudinal study conducted to describe the epidemiology and patterns of AMR phenotypes among fecal commensal bacteria from adult dairy cows in CA dairies across the state’s regions and seasons. Details of the study herds, management practices, sampling, and laboratory procedures were described in (13). The current study was conducted on 10 dairies, with each dairy enrolling two cohorts of cows over two distinct seasons. The first cohort was sampled during the fall and winter of 2018 and is referred to as the winter cohort. The second cohort was sampled during the spring and summer of 2019 and is referred to as the summer cohort. Each dairy was visited five times per cohort at intervals of 4–5 weeks. A random sample of 12 cows per dairy per cohort was enrolled before calving (close-up stage), and up to 120, with a total of 240 cows, were enrolled in this study. During the 12-month study period, a total of 240 cows were identified and enrolled using a parity-stratified random sample of the 10 study herds (12 cows per dairy over two seasons). The study dairies were distributed throughout CA’s three dairy regions: three in Northern California (NCA), two in Northern San Joaquin Valley (NSJV), and five in Greater Southern California (GSCA) based on Love et al. (14).
In each season, fecal samples were collected during monthly sampling points from a stratified random sample of 12 late-pregnancy heifers and cows, identified proportional to each herd’s parity distribution, from close-up (approximately 2 weeks prior to calving) to 120 DIM. Therefore, the number of pregnant heifers enrolled corresponded to the frequency of the first lactation cows in each study herd. Upon calving, these heifers were sampled as cows up to 120 DIM (DIM). Fecal samples were collected from enrolled cows monthly from 2 weeks before calving up to 120 DIM with a total of five sampling points (closeup, 30, 60, 90, and 120 DIM). Fecal samples were collected in 50 mL polypropylene tubes and transported to the laboratory on wet ice for culture within 24 h. Data on the study cows’ body condition and fecal scores were also collected during sampling. Body condition score (BCS) was assessed on a 5-point scale [1 = thin, 3 = average, and 5 = obese, according to (15)]. Each cow’s fecal consistency was assessed on a 3-point scale (1 = normal, 2 = loose, 3 = watery), which had been used previously in cattle (16).
The collected fresh fecal samples were directly plated onto E. coli ChromoSelect agar and Enterococci ChromoSelect agar (Sigma–Aldrich, St. Louis, MO, USA) for isolation of E. coli and Enterococcus spp., respectively, within 24 h of fecal sample collection. Due to a recent taxonomical update that distinguishes the genus Enterococcus faecalis and other members of the genus from Streptococcus spp., Enterococci ChromoSelect agar was only able to identify Enterococcus spp. and Streptococcus spp. by colony appearance (17). Therefore, colonies isolated from the Enterococci ChromoSelect agar from here onward were referred to as Enterococcus spp./Streptococcus spp. (ES). From each fecal sample, two isolated E. coli and two ES colonies were selected from ChromoSelect agars for antimicrobial susceptibility testing using the broth microdilution method (18) using the Sensititre™ Bovine BOPO7F Plate (Thermo Scientific, Remel Inc., Lenexa, KS, USA). Antimicrobial susceptibility testing was performed against the panel of AMD, including ampicillin, penicillin, ceftiofur, danofloxacin, enrofloxacin, florfenicol, gamithromycin, gentamicin, neomycin, sulfadimethoxine, spectinomycin, trimethoprimsulfamethoxazole, tetracycline, tiamulin, tilmicosin, tildipirosin, tulathromycin, and tylosin tartrate. Subsequently, isolates were classified as susceptible or resistant (intermediate isolates were classified as resistant) based on MIC breakpoints set by the Clinical and Laboratory Standards Institute if available (19); otherwise, MIC breakpoints were suggested by other publications as detailed in (13). Susceptibility testing quality control measures were conducted weekly using five control strains, including E. coli ATCC 35218, E. coli ATCC 25922, Enterococcus faecalis ATCC 29212, Strep. pneumoniae ATCC 49619, and Histophilus somni 700025.
Data on additional predictors were collected at the start and end of each sampling season. Specifically, each dairy’s enrollment questionnaire was completed to characterize its herd management practices and AMD use for dry cow therapy (DCT) and other therapeutic uses. The questionnaire was also completed for each dairy after the sampling periods, focusing on documenting changes in AMD use or management practices that the dairy management adopted during the study period. The questionnaire was reimplemented from a previously published antimicrobial stewardship survey about herd demographics, health management and antibiotic use, and antimicrobial stewardship practices and perspectives (20).
Data regarding the AMD exposure of the 12 individual study cows at each dairy during each season were systematically recorded in eight of the 10 study herds using computer management systems—Dairy Comp 305 for five herds and DHI Plus for three herds. Backup copies of these records were collected at each sampling visit across the two study seasons, totaling 10 backups per dairy. The remaining two study dairies maintained their records on paper, which were accessed monthly during each sampling visit. The individual cow data included the animal identification number, the AMD used, dose, route of administration, and date of AMD administration in addition to DCT. In addition, treatment protocols for each dairy were accessed through their respective record-keeping software. Treatment data and antimicrobial resistance data were time-matched in a relational database (Microsoft Access, Microsoft Corp., Redmond, WA, USA), preserving the temporality of the study cows’ AMD exposure with respect to their fecal sample isolates’ AMR phenotype.
Adapted from (21, 22), the treatment records were stratified by use categories (UC) into a dry cow, clinical mastitis, lameness, metritis, gastrointestinal, and unknown disease condition or syndrome. The UC dry cow represented IMM administration of long-acting AMD treatments administered at the end of lactation, while the UC clinical mastitis represented IMM AMD treatments to treat clinical mastitis during the lactation. The UC lameness represented the systemic administration of AMD for treatment of lameness or foot rot as detected by lameness signs and/or hoof trimmer examination. The UC gastrointestinal represented the systemic AMD for the treatment of diarrhea and other disease conditions related to the gastrointestinal system. The UC unknown disease represented the AMD administered to sick animals without identifying the specific cause (cow record information did not specify the underlying disease condition). Multiple measures were calculated to standardize the AMD use across the study dairies based on (22). These measures included the concentration of active substance (AS) in each AMD product. The active substance concentration was identified from the product label and, where needed, converted to milligrams (mg/mL).
Products containing active substances (AS) indicated in International Units (IU) instead of milligrams (mg), such as procaine penicillin, were converted to mg using a conversion factor of 1,000 IU per mg (23). For each AS in our study, the following measures were calculated based on the formulas used in (22): gram per administration (g/admin = grams of AS per administration); gram per regimen (g/reg = grams of AS per regimen), where a regimen refers to the prescribed course of treatments; and administration per regimen (Admin = number of administrations per regimen) (21).
In addition, the Defined Daily Dose for the study (DDDstudy) was calculated for cows randomly selected from their respective herds. This estimation aimed to characterize the typical dose that a standard dairy cow (680 kg) would receive if treated according to the FDA-approved label dosage. The dosage was calculated in units of mg/kg/day. A single administration was a drug product administered at a single cow treatment administration event. Multiple administrations were considered a single regimen when product administrations were consecutive, with no time gap between administrations greater than an interval of 2 days. Treatment intervals were explored, and a new case of the same disease was identified if a gap of 5 or more days was observed between treatments for the same cow.
The number of herds included in the study was determined using a convenience sample of 10 dairies. These dairies were selected to represent different milk sheds across California, ensuring geographic diversity and variability in management practices. Additionally, this number was chosen to facilitate the practical management of data collection across the study period.
The study sample size was determined a priori using the formula for comparing a dichotomous outcome between two groups with repeated measures, as described in Equation 1 by Brown and Prescot (24). The number of dairy cows to enroll in a given herd was based on the difference (∆) in the proportion of cows with resistant fecal commensals in AMD-treated cows (Group 1, P1 = 33%) compared to untreated cows (Group 2, P2 = 3%), where ∆ is measured on the logit scale such that ∆ = logit(P1) – logit(P2) for m repeated measures (5 sampling events/cow) assuming a compound symmetry covariance structure with a correlation ρ (where ρ = 0.25), and the quantity ν, the inverse of the P(1-P), where P is the mean of group proportions P1 and P2.
A two-sided hypothesis test framework tested a null hypothesis that the OR = 1 and an alternative hypothesis that OR ≠ 1 with an alpha of 0.05 and a power of 80%.
Given these inputs, a sample size of 12 cows (treated and untreated) per herd was deemed necessary to detect a significant difference in the proportions of resistant fecal commensals in AMD-treated and untreated cows. Given that 10 herds were enrolled and followed up over 2 seasons, a total of 240 cows were enrolled (12 cows/herd x 2 seasons x 10 herds = 240).
Descriptive statistics were used to calculate the proportion of AMR at the isolate and cow levels. Resistance status at the cow level was defined as one or more isolates resistant to one or more AMD at one or more time points during the collection time from calving up to 120 DIM. Cow-level AMR incidence was estimated for each specific drug and for E. coli and ES separately, as the total number of cows with at least 1 AMR isolate was divided by the total number of cows sampled. The mean, median, standard deviation, minimum, and maximum were calculated for AMD use measures, including the g/admin, g/reg, Admin, and DDD.
Collinearity of all potential explanatory variables was checked using Spearman’s rank correlation statistic. Mixed effects logistic regression models were specified for AMR outcomes at the animal level. The random effect structure included the cow as a random intercept and the sampling point as a random slope to account for the lack of independence of observations over time within the same cow. Robust standard error estimates were calculated with a clustered sandwich variance estimator that allowed for intragroup (farm-level) correlations. All independent variables, including AMD use, herd demographics (sampling region, sampling season, sampling points, herd average milk production, breed, milking herd average size, milking herd average somatic cell count (SCC), dry-off protocol), and animal-level factors (BCS, fecal score, disease condition, parity), were explored using univariate models for cow-level resistance to each AMD. Predictors associated with an outcome of interest at p ≤ 0.20 were considered for further modeling. A manual forward model-building approach was used, and variables were retained in the model if significant at p ≤ 0.05 or an improvement in model goodness of fit was observed using the Akaike Information Criterion (AIC), where a lower AIC estimate was considered a better fit. Confounding was assessed using the method of change in estimates, where a change of 30% or more in model estimates was considered evidence of confounding. All biologically meaningful interaction terms were explored using significance testing. Finally, previously excluded variables were offered once again to the resulting model and retained at p ≤ 0.05. The coefficients, odds ratios (OR), and their associated 95% confidence intervals were estimated in the final model for significant factors (p ≤ 0.05). All statistical analyses were performed using Stata 15 and 16 (Stata Corp, College Station, TX, USA).
The study herds included five herds in GSCA, two in NSJV, and three in NCA. The average number of milking cows in the study herds was 1,605 cows/herd, with a minimum of 130 cows/herd and a maximum of 5,000 cows/herd. The annual rolling herd average milk production was 11,390 ± 530.57 kg/cow/year. Regarding breed distribution, six herds were 100% Holstein, one herd was 100% Jersey, one was 100% crossbred, and two were mixed breeds. The herd average SCC was 140,000 ± 17,950 cells per mL.
The body condition score (BCS) of the enrolled cows, expressed as mean ± standard error (SE), was recorded on different days in milk (DIM). The body condition score of enrolled cows (mean ± SE) was 3.4 ± 0.01, 3.2 ± 0.02, 3.1 ± 0.02, 3.1 ± 0.02, 3.1 ± 0.02 during close-up, 30, 60, 90, 120 DIM respectively.
Culture of some fecal samples did not yield E. coli (68 samples) or ES (75 samples) after three culture attempts. Hence, a total of 2,169 E. coli and 2,157 ES isolates were available for antimicrobial susceptibility testing. Table 1 summarizes the proportion of E. coli and ES isolates with AMR against drugs that these species are otherwise known to be susceptible to. At the isolate level, the highest proportion of resistant E. coli was against florfenicol (83.31%), followed by sulfadimethoxine (32.45%) and tetracycline (16.82%). For ES, the highest proportion of resistant isolates was to tildipirosin (50.2%), followed by tilmicosin (47.90%), florfenicol (46.50%), and tiamulin (42.4%) (Table 1). Overall, cow-level incidence of AMR in E. coli ranged from 2.50 to 99.58%, while in ES, AMR ranged from 1.68 to 95.38% (Table 1).
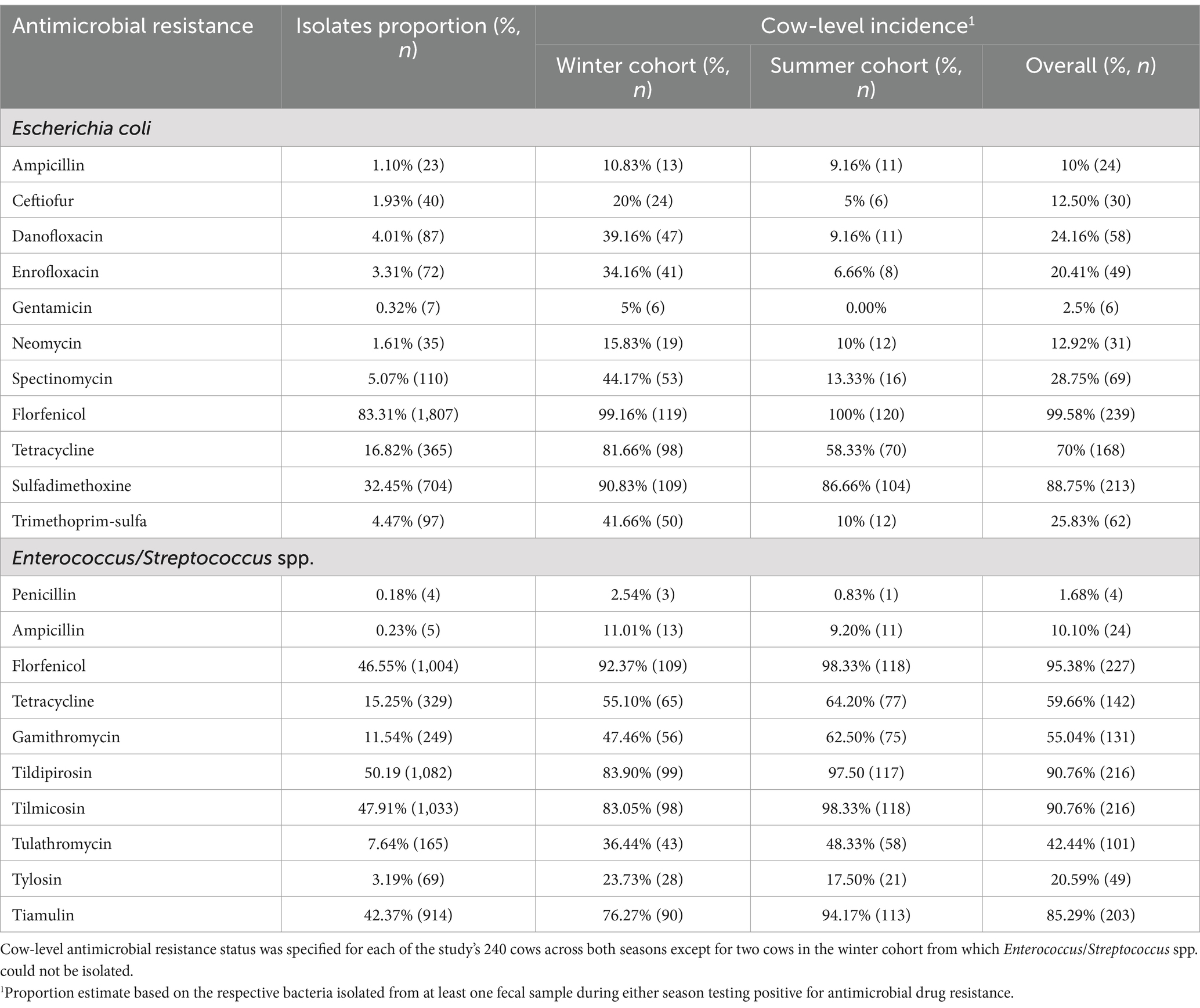
Table 1. The proportion of E. coli and Enterococcus/Streptococcus spp. resistant to antimicrobial drugs and cow-level incidence of antimicrobial resistance based on fecal commensal resistance.
Eight of our study herds reported the use of blanket dry-cow therapy (BDCT) at dry-off, employing either IMM AMD alone or in combination with an internal teat sealant. The remaining two herds did not administer IMM AMD or teat sealant at dry-off.
For BDCT, four herds reported the use of ceftiofur hydrochloride at a dose of 2 g/regimen, three herds used cephapirin benzathine at a dose of 1.2 g/regimen, and one herd reported using a combination product containing procaine penicillin G (4 g/regimen) and dihydrostreptomycin (4 g/regimen). However, among 4 of the 12 cows enrolled during the summer season, each had only two functional quarters, resulting in a mean dose of 3.38 g/regimen for the combined AMD. In Supplementary materials, descriptive statistics of AMD administered to the study cows and drug mass per regimen are summarized in Supplementary Tables 1, 2. Of the 240 study cows, only 37 (15.4% ± 2.33) in the study dairies received AMD for treating clinical disease conditions. Specifically, during both study seasons, 41 cases among the 37 treated cows consisted of either clinical mastitis (n = 21), metritis (n = 3), gastrointestinal (n = 2), lameness (n = 1), or an unknown disease condition (n = 14) (Figure 1). The number of grams per administration for the active drug substances administered in our study is summarized in Supplementary Table 3.
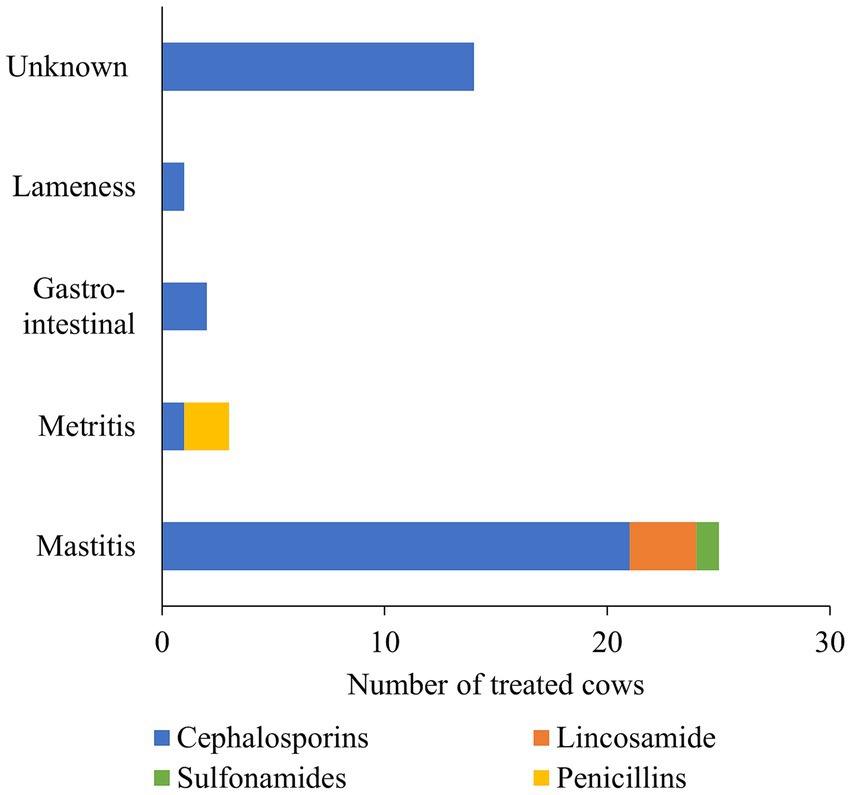
Figure 1. A stacked bar chart depicts the breakdown of therapeutic antimicrobial drugs (AMD) administered by drug class and the number of cows treated for different disease conditions. The AMD use information was collected for a random sample of 12 cows stratified by parity distribution on each of 10 California dairies and followed up from close up (approximately 2 weeks prior to calving) to 120 days in milk over two seasons (Winter and Summer).
Ceftiofur hydrochloride was administered as follows: an average rate of 2 g/administration intramammary for DCT, 1 g/admin intramuscular for the treatment of lameness, 0.75 g/admin subcutaneously for the treatment of clinical metritis, and 0.125 g/administration intramammary for the treatment of clinical mastitis.
Clinical mastitis cases were treated intramammarily with 0.125 g of ceftiofur hydrochloride per quarter. The overall ceftiofur hydrochloride regimen ranged from 0.25 to 1g/regimen, with a mean of 0.57 g/regimen across 2 to 8 administrations (Supplementary Tables 2–4).
One clinical mastitis case was treated with 0.125 g of ceftiofur hydrochloride per quarter administered intramammarily at 12-h intervals (Supplementary Table 1). Each administration, defined as treatment following a single restraint, counted as a separate event for a total of 2 administrations in a 24 hour period. Hence, despite such a cow being treated with a daily dose of 0.250 g ceftiofur hydrochloride, it had 0.125 g/administration. Cephapirin sodium was used for the treatment of clinical mastitis at a dose range from 0.2 to 0.4 g/reg, with a mean dose of 0.20 g/administration (adjusting for treatment of multiple simultaneous quarters with clinical mastitis), with a range of 1 to 2 administrations per regimen. Pirlimycin hydrochloride was used for the treatment of bovine clinical mastitis at a dose range from 0.15 to 0.2 g/reg, a mean dose of 0.05 g/administration, with a range of 3 to 4 administrations per regimen, and one dairy reporting a case with all 4 quarters treated for clinical mastitis for 4 days using pirlimycin. Records for one enrolled herd showed the use of sulfadimethoxine for the treatment of clinical mastitis at a dose of 100 g/reg, with a mean dose of 20 g/administration for a total of five administrations. Ceftiofur hydrochloride was used to treat lameness at 1 g per administration, with five administrations per regimen reported by study dairies. Cows diagnosed with clinical metritis were treated with ampicillin at a dose of 18.75 g/reg administered intramuscularly or ceftiofur hydrochloride at a dose of 2.25 g/reg administered subcutaneously. The DDD study for each active substance for treated cows is reported in Table 2. The mean calculated DDD study for ceftiofur hydrochloride for DCT was 2.94 mg/kg/day based on all four quarters receiving intramammary infusions at dry-off, while the average calculated DDDstudy for ceftiofur hydrochloride for treatment of clinical mastitis was 0.183 mg/kg/day after adjusting for clinical mastitis in multiple quarters in the same cow.
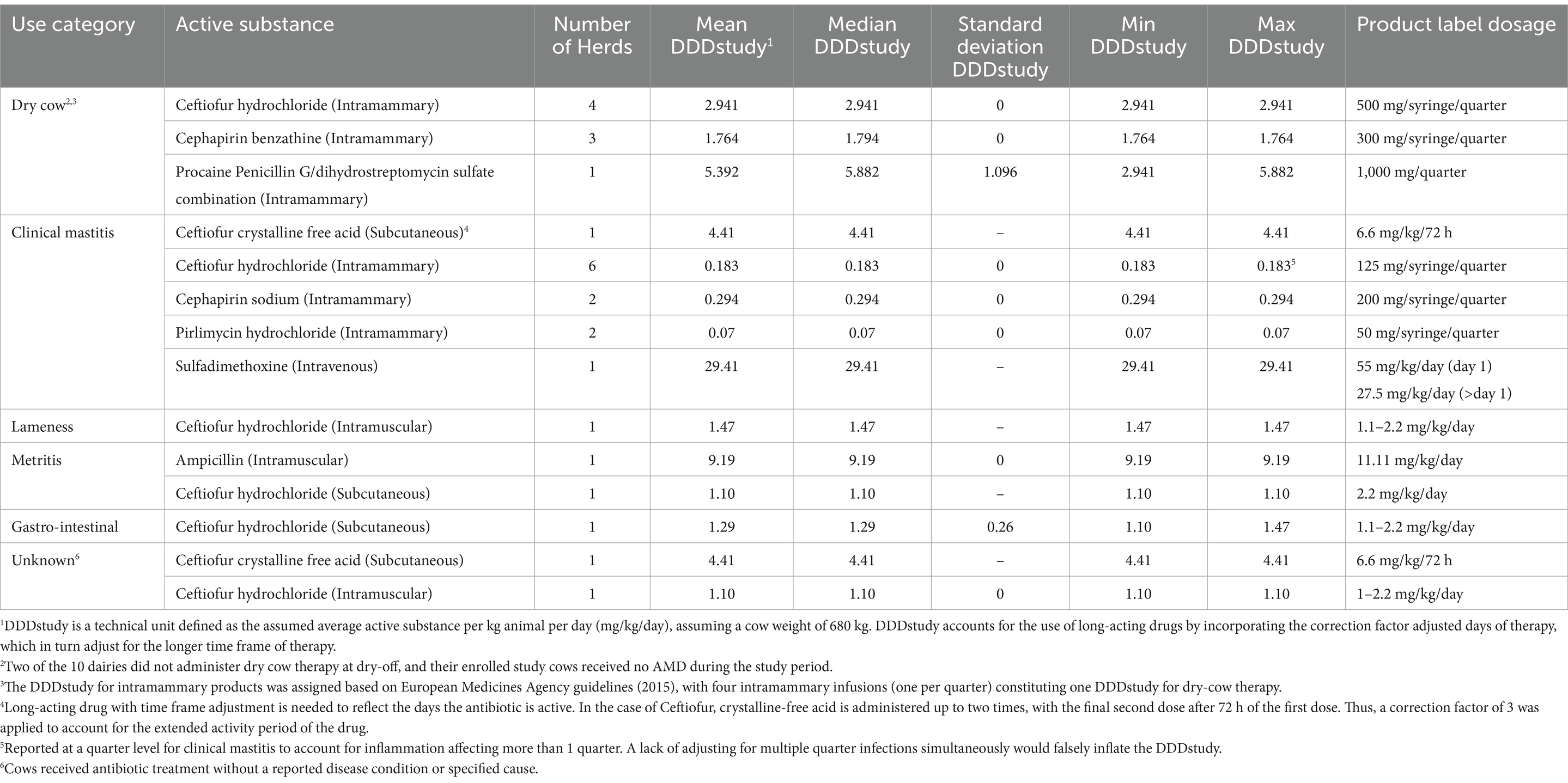
Table 2. Descriptive statistics of defined daily doses (DDDstudy) for each active substance administered on 8 California dairies to a random sample of 12 cows stratified by parity distribution on each of 10 premises followed up from close-up (approximately 2 weeks prior to calving) to 120 days in milk over two seasons (Winter and Summer) for a total of 240 cows.
Generalized linear mixed models with a logit link were specified for the following E. coli AMR outcomes: ceftiofur, tetracyclines, florfenicol, sulfadimethoxine, and trimethoprim-sulfamethoxazole. In addition, we could not specify E. coli models for resistance against gentamicin, spectinomycin, ampicillin, or neomycin due to the low frequency of resistant isolates. Similar models were specified for the following ES AMR outcomes: tetracycline, florfenicol, gamithromycin, tilmicosin, tildipirosin, tulathromycin, tylosin tartrate, and tiamulin.
Similar to the abovementioned limitations relevant to the rare outcomes (resistance in isolates), exposure to treatments may have been either non-identifiable with other factors or of low frequency, resulting in the inability to model such treatment effect. Due to the non-identifiability between DCT and herd effect, we were not able to estimate the distinct effect of DCT on AMR in fecal commensal bacteria. Specifically, only one herd reported the use of the procaine penicillin G and dihydrostreptomycin sulfate combination for DCT. Therefore, a comparison of the effect of DCT may have been confounded by the herd. In contrast, cephapirin sodium was used in our study herds (3 cows) for IMM treatment of clinical mastitis. However, we were not able to model the effect of its administration on AMR due to the low frequency of administration.
Table 3 summarizes the final models for resistance in E. coli isolated from fecal samples of the study cows. The odds of resistance against ceftiofur, tetracycline, or trimethoprim-sulfamethoxazole in fecal E. coli were significantly higher in the winter than in the summer (p < 0.05). In addition, E. coli isolated from fecal samples from NCA study cows had lower odds of resistance against ceftiofur, tetracycline, sulfadimethoxine, or trimethoprim-sulfamethoxazole (p ≤ 0.05) in comparison to those from GSCA. Systemic administration of ceftiofur hydrochloride IM for treatment of lameness and unknown disease conditions during lactation was significantly associated with higher odds of tetracycline-resistant E. coli (p = 0.02). Cows with fecal scores of 2 had higher odds of shedding florfenicol-resistant E. coli (OR = 2.03) in comparison to cows with fecal scores of 1 (p = 0.02). In contrast, cows with fecal scores of 2 had lower odds of shedding trimethoprim-sulfamethoxazole-resistant E. coli (OR = 0.42) in comparison to cows with fecal score 1 (p < 0.01, Table 3).
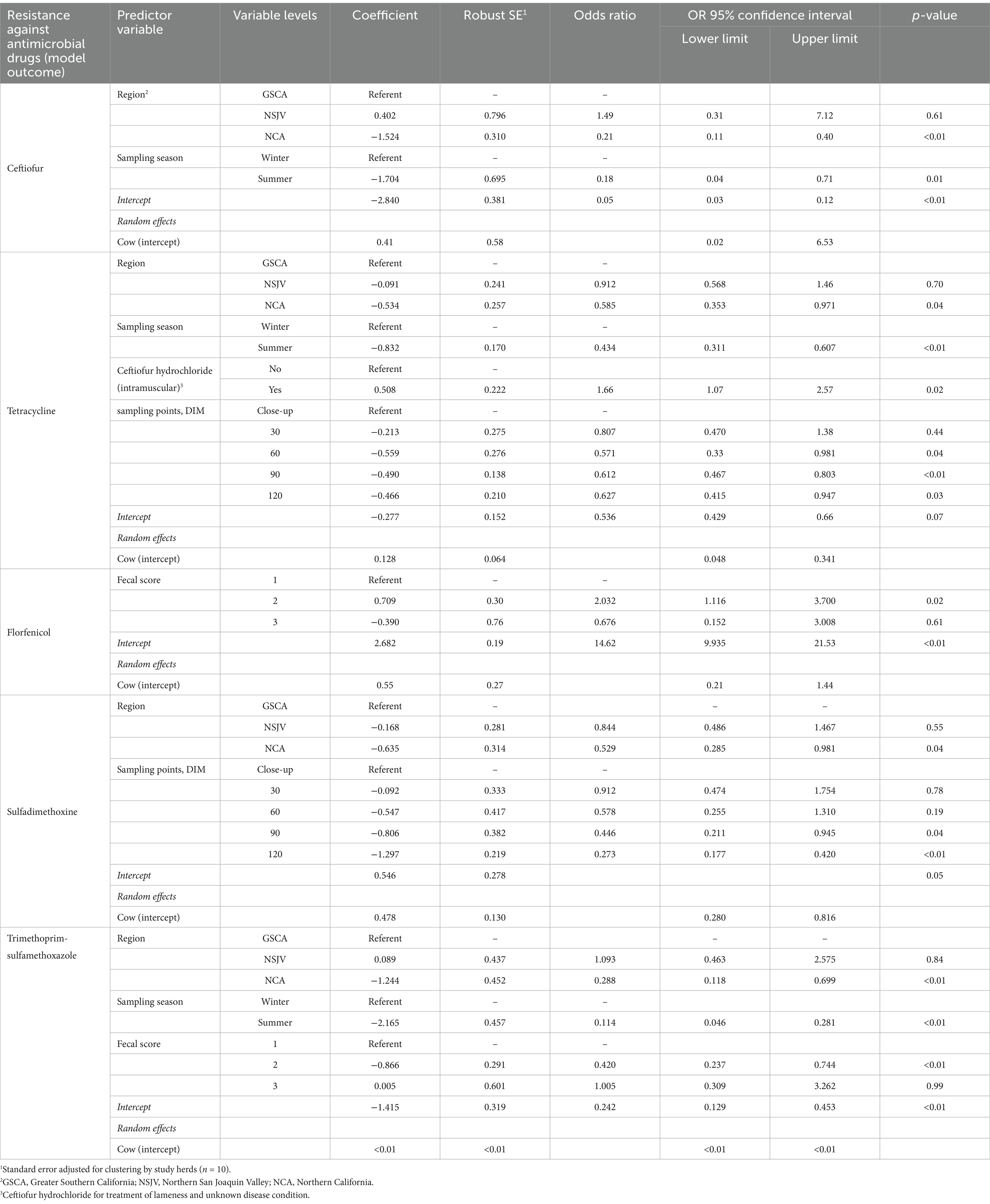
Table 3. Final mixed-effects logistic regression model for risk factors associated with the prevalence of antimicrobial-resistant E. coli isolated from fecal samples collected on 10 California dairies from a random sample of 12 cows stratified by parity distribution on each of 10 California dairies and followed up from close up (approximately 2 weeks prior to calving) to 120 days in milk over two seasons (Winter and Summer).
Table 4 summarizes the final models for resistance in ES isolated from fecal samples of the study cows. The odds of resistance against tetracycline, florfenicol, tilmicosin, tildipirosin, or tiamulin in fecal ES were significantly higher in the summer than in the winter (p < 0.01). Fecal ES isolates from study cows in NCA had significantly lower odds of resistance against florfenicol, tilmicosin, tulathromycin, tildipirosin, or tiamulin than those from GSCA (p < 0.01). In addition, cows with fecal scores of 2 or 3 had higher odds of shedding florfenicol, tilmicosin, or tildipirosin-resistant ES in comparison to cows with fecal scores of 1 (p < 0.05), while only cows with fecal scores of 2 had higher odds of shedding tiamulin-resistant E. coli in comparison to cows with fecal scores of 1 (p = 0.04).

Table 4. Final mixed-effects logistic regression models for risk factors associated with the prevalence of antimicrobial-resistant Enterococcus/Streptococcus spp. isolated from fecal samples collected on 10 California dairies from a random sample of 12 cows stratified by parity distribution on each of 10 California dairies and followed up from close up (approximately 2 weeks prior to calving) to 120 days in milk over two seasons (Winter and Summer).
Our study demonstrated that AMD treatments and the location of the dairy and sampling season were significant risk factors associated with AMR in fecal commensal species in dairy cattle. However, AMR isolates were detected even in adult cows, even in the absence of exposure to specific drugs. BDCT was the most common indication for AMD use in the study’s dairy herds. Ceftiofur hydrochloride and cephapirin benzathine were the most frequent active substances used for BDCT, a finding that agrees with a prior survey of CA dairies (20). Similarly, a survey study conducted on Pennsylvania dairies (25) found that cephapirin benzathine was the most common DCT, followed by penicillins (procaine penicillin, cloxacillin) and third-generation cephalosporin (ceftiofur).
Cephalosporins and penicillin were the most common parenterally administered AMDs in the current study, in agreement with the Dairy 2014 study from the National Animal Health Monitoring System (NAHMS) (26), which reported that 73% of dairies nationwide used cephalosporins as the primary AMD, followed by penicillins, lincosamides, and tetracyclines. In our study, mastitis was the most commonly diagnosed indication for therapeutic use of AMD, followed by metritis and gastrointestinal disease conditions. Respondents to a previous survey of CA dairies also reported that clinical mastitis was the most common cause of AMD treatment (20). Our records showed that sulfadimethoxine was used for the treatment of clinical mastitis on one study dairy at a dose of 29 mg/kg/day for 5 days. However, there is no indication for using sulfadimethoxine to treat clinical mastitis (Sulfadimethoxine Injection 40%; Aspen Veterinary Resources, ® Ltd.). Furthermore, sulfadimethoxine dosage per label calls for a loading dose on the first day of treatment (55 mg/kg/day), followed by half that dose for the remaining treatment days. In 2005, AVMA published a reminder on the prohibited extra-label drug use (ELDU) of sulfonamides in lactating dairy cattle and mentioned that unapproved use of sulfonamides is one of the most frequent causes of violative residues in food-producing animals (27). In addition, our records showed the use of ampicillin for the treatment of metritis and retained placenta. While ampicillin is an effective therapeutic AMD for the treatment of metritis, its use for the treatment of metritis in cattle is considered ELDU (28). Ceftiofur crystalline free acid (subcutaneous) was administered for treatment of clinical mastitis on a study dairy at the dose of 15 mL IM using a 2-dose regimen, which is considered ELDU because there is no label indication for clinical mastitis (EXCEDE®; Zoetis USA). Extra-label drug use must be approved by the prescribing veterinarian and used under the Animal Medicine Use Clarification Act (AMDUCA). Study herds’ treatment records indicated that 5.8% of cows treated with an AMD had no disease indication reported in the computer software. An unknown disease indication is possible if management recorded a treatment but not the indication. However, other possible reasons could be if the cows were treated for undiagnosed illness (e.g., fever of unknown origin) or other health findings that were not associated with a specific disease condition. Further outreach and extension education to veterinarians and dairy staff on following treatment protocols developed and updated regularly by the herd veterinarian may reduce the number of cows receiving AMD therapy designated under unknown illness.
Our study reports on differences in resistance between the two seasons within the study period. Specifically, E. coli isolated from fecal samples in dairy farms during the study’s winter season were more resistant to ceftiofur, tetracycline, or trimethoprim-sulfamethoxazole than E. coli recovered during the summer. Possible reasons for the increased occurrence of AMR in E. coli during the winter season are the influence of temperature differences and AMD use. The winter season in CA can be associated with rain, which could provide suitable conditions for environmental bacteria to proliferate and increase the risk of clinical mastitis, metritis, and lameness, thus leading to increased antimicrobial usage. Seasonal differences in AMR were reported in similar studies. Massé et al. (29) found that extended-spectrum β-lactamase (ESBL)/AmpC-producing E. coli were recovered from fecal samples in dairy farms in Canada more frequently in the autumn season than in spring.
Although our study included 10 dairies across the three California milk sheds, it included 3 or 4 dairies in any one of the study regions, limiting the interpretation of differences in resistance between the study regions. The locations of the study dairies were a significant risk factor in the odds of AMR E. coli isolated from fecal samples of the study dairy cattle. Specifically, E. coli isolated from fecal samples of study cows in NCA had significantly lower odds of resistance against ceftiofur, tetracycline, sulfadimethoxine, or trimethoprim-sulfamethoxazole compared to those from GSCA and NSJV. The location differences reported in our study could be attributed to differences in management practices, environmental conditions, or herd size in the study herds. Amongst the management differences, two out of three study dairies in NCA did not report the use of any antimicrobials for the prevention and treatment of clinical mastitis in the study cows enrolled. On the remaining 8 study dairies, ceftiofur hydrochloride was the most common IMM AMD for treating clinical mastitis in lactating cows on six dairies, followed by cephapirin sodium on two dairies. Our finding is in agreement with Berge et al. (30), where the location of dairy farms was a significant factor in the odds of AMR E. coli isolated from cattle feces, attributing this difference to management-related factors. Regional differences in management practices within CA dairies were previously identified by (14), with dairies in NCA being significantly smaller in herd size, having fewer Holsteins compared to other milking cow breeds, and raising their own calves onsite compared to dairies in the GSCA and NSJV regions. The regional differences in AMR could also be attributed to the regional differences in climate and its associated management practices, such as exposure to pasture. Compared to southern regions, the NCA climate is milder and has more precipitation,1 allowing cows on one of the NCA dairies to be exclusively on pasture, on a second dairy with some pasture access, while the third had no access to pasture. In contrast, the higher temperatures and lower rainfall in NSJV and GSCA limited their dairy cow housing to dry lots or free stall pens. Regional differences in AMR prevalence were observed in previous studies (11). A Canadian study found that Staphylococcus aureus from cows in Ontario had significantly lower odds of tetracycline resistance compared to those from Québec (11). Another study conducted in Sweden (9) found that a higher prevalence of chloramphenicol resistance in E. coli was found in calves raised on farms in southern Sweden than in calves raised in northern Sweden.
In our study, systemic administration of ceftiofur hydrochloride for treatment of lameness and unknown disease was significantly associated with tetracycline resistance in E. coli isolates from fecal samples of the study dairy. Mann et al. (31) found that systemic antimicrobial treatment with ceftiofur hydrochloride decreased tetracycline susceptibility in fecal E. coli isolates in the treated group on day 2 in comparison to untreated cows and that isolates resistant to ceftiofur were more likely to show tetracycline resistance. At the gene level, Kanwar et al. (32) found an increase in the proportion of fecal E. coli isolates harboring tet(A) and tet(B) genes following ceftiofur treatment in feedlot cattle. Several studies on fecal E. coli have recorded that all ceftiofur-resistant isolates were found to be co-resistant to tetracycline because ceftiofur and tetracycline determinants are usually found together on the same plasmid (33, 34). Studies conducted on Salmonella isolates derived from cattle have indicated that the blaCMY-2 gene is usually located on a large IncA/C plasmid that harbors several other resistance genes, including tetracycline resistance genes (6, 34). Co-resistance to multiple classes of AMD, such as fluoroquinolones, tetracyclines, chloramphenicol, aminoglycosides, sulfonamides, and/or trimethoprim, has been reported in E. coli isolated from dairy cattle in the US (35).
Although ceftiofur was the most administered AMD in our study, E. coli isolates recovered from cows treated with ceftiofur were all susceptible to ceftiofur. Daniels et al. (36) reported that ceftiofur administration frequency did not impact levels of commensal E. coli containing the blaCMY-2 gene at the herd level. The absence of detection of the association between ceftiofur treatment and resistance in our study could be attributed to two possible factors. First, our fecal samples were collected monthly, which could have missed the resistance against ceftiofur in enteric E. coli, given that a recent study that compared resistance post-treatment at different ceftiofur concentrations showed loss of ceftiofur resistance at the 8 μg/mL level within 3–4 days post-treatment (8). Taylor et al. (37) found that on day 16 after cephalosporin treatment of cows with metritis, the population of cephalosporin-resistant bacteria in the treatment group was reduced by 0.5 log10 CFU. By day 28, the bacterial population returned to pre-treatment resistance levels. Second, the majority of ceftiofur used in our study dairies was IMM. Such non-parenteral administration may be less likely to confer resistance among fecal E. coli (37). In summary, changes in the AMD susceptibility of fecal E. coli were associated with the choice of AMD, geographical location, and sampling season, which suggests that AMR is a multifactorial phenomenon.
Cow-level ES AMR incidence ranged from 1.7% for penicillin to >90% for florfenicol and macrolides (tildipirosin or tilmicosin), which showed that enteric ES had a higher rate of AMR for drugs not approved for use in lactating dairy cows despite the study herds’ treatment records showing no administrations of macrolides or florfenicol in adult cows. Other studies similarly reported AMR to florfenicol and macrolides in Enterococcus spp. isolated from dairy cows (38, 39). Another study reported that the predominant AMR genes in Enterococcus spp. from dairy cattle in CA targeted macrolide drugs (40). The high levels of resistance for florfenicol and macrolides in enteric ES detected in our study could be attributed to the mechanism of co-selection, similar to that related to systemic administration of ceftiofur hydrochloride and its associated increase in tetracycline resistance in E. coli. Florfenicol resistance in E. coli is associated with the floR gene, which is co-located on plasmids with genes that confer resistance to other antimicrobial drugs, which may explain florfenicol resistance spread with exposure to drugs other than florfenicol (40). In our study herds, the common practice of flushing free-stall pens with recycled lagoon water may have played a role in exposing adult cattle to resistant bacteria or AMD residues in excreta or secretions of cows and/or calves treated with AMD. Florfenicol and macrolides are commonly used to treat respiratory diseases in calves. Hence, recycled lagoon water may have exposed adult cows to the florfenicol and macrolide residues from treated calves.
Regional differences in cows harboring resistant ES were also observed in our study, with lower odds of having cows harboring ES resistance to florfenicol, tilmicosin, tulathromycin, tildipirosin, or tiamulin-resistant ES in NCA in comparison to cows in GSCA and NSJV regions. Previous studies reported that different bacteria with varying AMR exist in different regions (13, 41, 42). Farms have unique ecosystems composed of each farm’s environment, geography, weather, and management factors that may serve as suitable media for amplifying and disseminating AMR in bacteria (43). Another study reported regional differences in AMR prevalence in Canada. It attributed those findings to differences in management, environment, geography, weather, and resource availability that might influence AMR. However, differences were only observed for bovine mastitis Staphylococcus aureus but not gram-negative pathogens (11, 12).
The presence of AMR in adult cows, despite the absence of specific drug exposure, may be linked to environmental contamination with resistant bacteria, direct colonization, or horizontal gene transfer. In addition, drug residues from treated calves may expose adult cows through the recycled flush system. Further research is required to confirm such hypotheses. The current study identified a high proportion of florfenicol resistance in E. coli and ES on the study dairies with no record of florfenicol use in the adult herd. This drug is not currently labeled for use in dairy cattle 20 months or older. Therefore, the link between AMD treatment of youngstock and AMR of fecal commensal bacteria in adult cows in the same herds needs further investigation.
In addition to the residual confounding characteristic of our observational study design, the current study herds were a convenience sample of CA dairies and not a random sample of the state’s dairies. Experimental study designs, such as double-blind, complete block, randomized trials, would be more suitable than observational studies to investigate the causal association between exposure to a single drug and antimicrobial resistance against it without accounting for sources of variability in the real world. The use of selective media for the isolation of Enterococcus spp. selected both Enterococcus spp. and Streptococcus spp., only recently distinguished, could have introduced a bias (13). While the spatial distribution of the herds captured the state’s three milk sheds identified in earlier studies, it is possible that the study herds were not representative. Our study was conducted on dairy cows from close-up (2 weeks pre-calving) to 120 DIM only. It could not provide information on the epidemiology of AMD use and resistance later in the lactation.
The current estimates of the DDDstudy may not accurately reflect the actual amount of drug administered per kg per day on a study dairy if the body weight of a treated cow differs from the assumed weight of 680 kg used for calculating the DDDstudy. Actual administered DDDstudy estimates would have required knowledge of each cow’s body weight, data that could have been but was not collected.
The observed associations were based on AMD use reported by dairies using computer management systems or paper records and not based on empty vial verification. However, dairy owners allowed us full access to the study herds and their dairy records during the study period. Further, the study associations were modeled using the phenotypic data with no information on determinants of resistance.
Several drugs and their resistance patterns were not modeled due to their infrequent use or observation. Several drugs and their resistance patterns could not be modeled due to their low frequency of use or observation, respectively.
Specifically, each of the eight dairies that implemented dry cow AMD therapy administered only a single AMD, confounding any comparison by herd-specific factors. In addition, only one dairy used a combination of procaine penicillin G and dihydrostreptomycin sulfate, leading to perfect non-identifiability due to the lack of variability in treatment across the study sample. A more robust experimental approach, such as comparing dry cow IMM AMD treatment with untreated controls and more frequent sampling of milk post-calving, would provide a clearer assessment of this association. A recent study of a random sample of non-aureus Staphylococcus spp. isolated from milk samples collected at dry-off and post-calving found increased resistance to cephalothin, ceftiofur, and oxacillin in cows treated at dry-off with AMD of the same class or a class with a shared resistance mechanism (44).
Our study showed that florfenicol, tetracycline, or sulfadimethoxine-resistant E. coli and florfenicol, tildipirosin, tilmicosin, or tiamulin-resistant ES were commonly isolated from feces of the study dairy cows in CA. Extra-label use of AMD was observed in sporadic cases in our study dairies. The finding of AMD-resistant E. coli and ES isolates from the feces of dairy cows was associated with multiple factors. Systemic administration of ceftiofur hydrochloride for treatment of lameness and unknown disease during lactation was significantly associated with higher odds of isolating fecal E. coli resistant to tetracycline. Regional and seasonal differences were reported in our study, which could be attributed to differences in management practices, environmental conditions, or herd size in California dairies. Resistance to calf hood AMD in adult dairy cattle was observed. Therefore, follow-up research is needed to understand the association between the use of AMD on a dairy, including for calf treatments, and AMR.
The datasets presented in this article are not readily available because the study data were collected under Food and Agriculture Law which requires maintaining confidentiality of the participating farms including publishing only aggregate analyzed data. This study was sponsored by the California Department of Food and Agriculture and is subject to California Food and Agriculture Code (FAC) Sections 14400 to 14408. FAC section 14407 requires that data collected be held confidential to prevent individual identification of a farm or business. Reasonable requests for additional data in aggregate form can be provided by contacting Dr. Sharif Aly, email: U0FseUB1Y2RhdmlzLmVkdQ==.
The animal studies were approved by the University of California Davis. The studies were conducted in accordance with the local legislation and institutional requirements. Oral informed consent was obtained from the owners for the participation of their animals in this study.
EA: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. PE: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. EO: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. TC: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. BK: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing. RB: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing. WE: Data curation, Investigation, Methodology, Validation, Writing – review & editing. DS: Data curation, Investigation, Methodology, Validation, Writing – review & editing. DW: Investigation, Methodology, Resources, Validation, Writing – review & editing. TL: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. BB: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. SA: Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. California Department of Food and Agriculture funded the research conducted.
The authors are grateful to the owners of the study dairies, their staff, and herd veterinarians for their contribution to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1504640/full#supplementary-material
1. WHO. Antimicrobial resistance. Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (Accessed September 30, 2024).
2. FDA C for V. FACT SHEET: veterinary feed directive final rule and next steps. FDA. (2024). Available at: https://www.fda.gov/animal-veterinary/development-approval-process/fact-sheet-veterinary-feed-directive-final-rule-and-next-steps (Accessed September 30, 2024)
3. California Code, FAC 14400-14408. Available at: https://leginfo.legislature.ca.gov/faces/codes_displaySection.xhtml?sectionNum=14401.&lawCode=FAC (Accessed September 30, 2024).
4. FDA C for V. FDA CVM GFI #263 recommendations for sponsors of medically important antimicrobial drugs approved for use in animals to voluntarily bring under veterinary oversight all products that continue to be available over-the-counter. (2021). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-263-recommendations-sponsors-medically-important-antimicrobial-drugs-approved-use-animals (Accessed September 30, 2024)
5. Witte, W. Ecological impact of antibiotic use in animals on different complex microflora: environment. Int J Antimicrob Agents. (2000) 14:321–5. doi: 10.1016/s0924-8579(00)00144-8
6. Winokur, PL, Vonstein, DL, Hoffman, LJ, Uhlenhopp, EK, and Doern, GV. Evidence for transfer of CMY-2 AmpC -lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob Agents Chemother. (2001) 45:2716–22. doi: 10.1128/AAC.45.10.2716-2722.2001
7. Benedict, KM, Gow, SP, McAllister, TA, Booker, CW, Hannon, SJ, Checkley, SL, et al. Antimicrobial resistance in Escherichia coli recovered from feedlot cattle and associations with antimicrobial use. PLoS One. (2015) 10:e0143995. doi: 10.1371/journal.pone.0143995
8. Sheedy, DB, Okello, E, Williams, DR, Precht, K, Cella, E, Lehenbauer, TW, et al. Effect of antimicrobial treatment on the dynamics of Ceftiofur resistance in Enterobacteriaceae from adult California dairy cows. Microorganisms. (2021) 9:828. doi: 10.3390/microorganisms9040828
9. Duse, A, Waller, KP, Emanuelson, U, Unnerstad, HE, Persson, Y, and Bengtsson, B. Risk factors for antimicrobial resistance in fecal Escherichia coli from preweaned dairy calves. J Dairy Sci. (2015) 98:500–16. doi: 10.3168/jds.2014-8432
10. Pereira, RV, Siler, JD, Ng, JC, Davis, MA, Grohn, YT, and Warnick, LD. Effect of on-farm use of antimicrobial drugs on resistance in fecal Escherichia coli of preweaned dairy calves. J Dairy Sci. (2014) 97:7644–54. doi: 10.3168/jds.2014-8521
11. Saini, V, McClure, JT, Scholl, DT, DeVries, TJ, and Barkema, HW. Herd-level association between antimicrobial use and antimicrobial resistance in bovine mastitis Staphylococcus aureus isolates on Canadian dairy farms. J Dairy Sci. (2012) 95:1921–9. doi: 10.3168/jds.2011-5065
12. Saini, V, McClure, JT, Scholl, DT, DeVries, TJ, and Barkema, HW. Herd-level relationship between antimicrobial use and presence or absence of antimicrobial resistance in gram-negative bovine mastitis pathogens on Canadian dairy farms. J Dairy Sci. (2013) 96:4965–76. doi: 10.3168/jds.2012-5713
13. Abdelfattah, EM, Ekong, PS, Okello, E, Chamchoy, T, Karle, BM, Black, RA, et al. Epidemiology of antimicrobial resistance (AMR) on California dairies: descriptive and cluster analyses of AMR phenotype of fecal commensal bacteria isolated from adult cows. PeerJ. (2021) 9:e11108. doi: 10.7717/peerj.11108
14. Love, WJ, Lehenbauer, TW, Karle, BM, Hulbert, LE, Anderson, RJ, Van Eenennaam, AL, et al. Survey of management practices related to bovine respiratory disease in preweaned calves on California dairies. J Dairy Sci. (2016) 99:1483–94. doi: 10.3168/jds.2015-9394
15. Wildman, EE, Jones, GM, Wagner, PE, Boman, RL, Troutt, HF, and Lesch, TN. A dairy cow body condition scoring system and its relationship to selected production characteristics. J Dairy Sci. (1982) 65:495–501. doi: 10.3168/jds.S0022-0302(82)82223-6
16. Feldmann, HR, Williams, DR, Champagne, JD, Lehenbauer, TW, and Aly, SS. Effectiveness of zinc supplementation on diarrhea and average daily gain in pre-weaned dairy calves: a double-blind, block-randomized, placebo-controlled clinical trial. PLoS One. (2019) 14:e0219321. doi: 10.1371/journal.pone.0219321
17. Pinto, B, Pierotti, R, Canale, G, and Reali, D. Characterization of “faecal streptococci” as indicators of faecal pollution and distribution in the environment. Lett Appl Microbiol. (1999) 29:258–63. doi: 10.1046/j.1472-765X.1999.00633.x
18. CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: M07-A11. 1st ed. Wayne, PA: Committee for Clinical Laboratory Standards (2018). 92 p.
19. CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Clinical and Laboratory Standards Institute (CLSI). vol. VET08. 4th ed (2018). 170 p.
20. Abdelfattah, EM, Ekong, PS, Okello, E, Williams, DR, Karle, BM, Rowe, JD, et al. 2019 survey of antimicrobial drug use and stewardship practices in adult cows on California dairies: post senate bill 27. Microorganisms. (2021) 9:1507. doi: 10.3390/microorganisms9071507
21. Schrag, NFD, Apley, MD, Godden, SM, Singer, RS, and Lubbers, BV. Antimicrobial use quantification in adult dairy cows – part 2 – developing a foundation for pharmacoepidemiology by comparing measurement methods. Zoonoses Public Health. (2020) 67:69–81. doi: 10.1111/zph.12772
22. Schrag, NFD, Apley, MD, Godden, SM, Lubbers, BV, and Singer, RS. Antimicrobial use quantification in adult dairy cows - part 1 - standardized regimens as a method for describing antimicrobial use. Zoonoses Public Health. (2020) 67:51–68. doi: 10.1111/zph.12766
23. Plumb, DC. Plumb’s veterinary drug handbook 9th edition. 9th ed. Stockholm, Wisconsin: Pharma Vet Inc. Blackwell Pub.
25. Redding, LE, Bender, J, and Baker, L. Quantification of antibiotic use on dairy farms in Pennsylvania. J Dairy Sci. (2019) 102:1494–507. doi: 10.3168/jds.2018-15224
26. USDA. Milk quality, milking procedures, and mastitis on U.S. dairies. (2016).Available at: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_Mastitis.pdf (Accessed May 29, 2022).
27. Reminder: extralabel drug use of sulfonamides in lactating dairy cattle prohibited | American Veterinary Medical Association. Available at: https://www.avma.org/javma-news/2005-10-01/reminder-extralabel-drug-use-sulfonamides-lactating-dairy-cattle-prohibited (Accessed September 30, 2024).
28. Credille, BC, and Giguere, S. Acute puerperal metritis: antimicrobial therapy and the relationship between antimicrobial pharmacodynamics and therapeutic success. Bov pract. (2015) 49:18–24. doi: 10.21423/bovine-vol49no1p18-24
29. Massé, J, Lardé, H, Fairbrother, JM, Roy, J-P, Francoz, D, Dufour, S, et al. Prevalence of antimicrobial resistance and characteristics of Escherichia coli isolates from fecal and manure pit samples on dairy farms in the province of Québec, Canada. Front Vet Sci. (2021) 8:654125. doi: 10.3389/fvets.2021.654125
30. Berge, AC, Hancock, DD, Sischo, WM, and Besser, TE. Geographic, farm, and animal factors associated with multiple antimicrobial resistance in fecal Escherichia coli isolates from cattle in the western United States. J Am Vet Med Assoc. (2010) 236:1338–44. doi: 10.2460/javma.236.12.1338
31. Mann, S, Siler, JD, Jordan, D, and Warnick, LD. Antimicrobial susceptibility of fecal Escherichia coli isolates in dairy cows following systemic treatment with Ceftiofur or penicillin. Foodborne Pathog Dis. (2011) 8:861–7. doi: 10.1089/fpd.2010.0751
32. Kanwar, N, Scott, HM, Norby, B, Loneragan, GH, Vinasco, J, McGowan, M, et al. Effects of Ceftiofur and chlortetracycline treatment strategies on antimicrobial susceptibility and on tet(A), tet(B), and blaCMY-2 resistance genes among E. coli isolated from the feces of feedlot cattle. PLoS One. (2013) 8:e80575. doi: 10.1371/journal.pone.0080575
33. Tragesser, LA, Wittum, TE, Funk, JA, Winokur, PL, and Rajala-Schultz, PJ. Association between ceftiofur use and isolation of Escherichia coli with reduced susceptibility to ceftriaxone from fecal samples of dairy cows. Am J Vet Res. (2006) 67:1696–700. doi: 10.2460/ajvr.67.10.1696
34. Lowrance, TC, Loneragan, GH, Kunze, DJ, Platt, TM, Ives, SE, Scott, HM, et al. Changes in antimicrobial susceptibility in a population of Escherichia coli isolated from feedlot cattle administered ceftiofur crystalline-free acid. Am J Vet Res. (2007) 68:501–7. doi: 10.2460/ajvr.68.5.501
35. Gelalcha, BD, Ensermu, DB, Agga, GE, Vancuren, M, Gillespie, BE, D’Souza, DH, et al. Prevalence of antimicrobial resistant and extended-Spectrum Beta-lactamase-producing Escherichia coli in dairy cattle farms in East Tennessee. Foodborne Pathog Dis. (2022) 19:408–16. doi: 10.1089/fpd.2021.0101
36. Daniels, JB, Call, DR, Hancock, D, Sischo, WM, Baker, K, and Besser, TE. Role of Ceftiofur in selection and dissemination of Bla CMY-2 -mediated cephalosporin resistance in Salmonella enterica and commensal Escherichia coli isolates from cattle. Appl Environ Microbiol. (2009) 75:3648–55. doi: 10.1128/AEM.02435-08
37. Taylor, EA, Jordan, ER, Garcia, JA, Hagevoort, GR, Norman, KN, Lawhon, SD, et al. Effects of two-dose ceftiofur treatment for metritis on the temporal dynamics of antimicrobial resistance among fecal Escherichia coli in Holstein-Friesian dairy cows. PLoS One. (2019) 14:e0220068. doi: 10.1371/journal.pone.0220068
38. Li, X, Aly, SS, Su, Z, Pereira, RV, Williams, DR, Rossitto, P, et al. Phenotypic antimicrobial resistance profiles of E. coli and Enterococcus from dairy cattle in different management units on a Central California dairy. Clin Microbiol. (2018) 7. doi: 10.4172/2327-5073.1000311
39. Liu, Y, Wang, Y, Wu, C, Shen, Z, Schwarz, S, Du, X-D, et al. First report of the multidrug resistance gene cfr in Enterococcus faecalis of animal origin. Antimicrob Agents Chemother. (2012) 56:1650–4. doi: 10.1128/AAC.06091-11
40. Jeamsripong, S, Li, X, Aly, SS, Su, Z, Pereira, RV, and Atwill, ER. Antibiotic resistance genes and associated phenotypes in Escherichia coli and Enterococcus from cattle at different production stages on a dairy farm in Central California. Antibiotics. (2021) 10:1042. doi: 10.3390/antibiotics10091042
41. Kirk, JH, McCowan, B, Atwill, ER, Glenn, KS, Higginbotham, GE, Collar, CA, et al. Association of minimum inhibitory concentration cluster patterns with dairy management practices for environmental bacteria isolated from bulk tank milk. J Dairy Sci. (2005) 88:3710–20. doi: 10.3168/jds.S0022-0302(05)73057-5
42. Rossitto, PV, Ruiz, L, Kikuchi, Y, Glenn, K, Luiz, K, Watts, JL, et al. Antibiotic susceptibility patterns for environmental streptococci isolated from bovine mastitis in Central California dairies. J Dairy Sci. (2002) 85:132–8. doi: 10.3168/jds.S0022-0302(02)74061-7
43. Acar, JF, and Moulin, G. Antimicrobial resistance at farm level. Rev Sci Tech. (2006) 25:775–92. doi: 10.20506/rst.25.2.1695
Keywords: antimicrobial resistance, antimicrobial drug use, antibiotics, dairy cows, California, season, region, dose
Citation: Abdelfattah EM, Ekong PS, Okello E, Chamchoy T, Karle BM, Black RA, ElAshmawy W, Sheedy D, Williams DR, Lehenbauer TW, Byrne BA and Aly SS (2025) Antimicrobial drug use and its association with antimicrobial resistance in fecal commensals from cows on California dairies. Front. Vet. Sci. 11:1504640. doi: 10.3389/fvets.2024.1504640
Received: 01 October 2024; Accepted: 30 December 2024;
Published: 10 February 2025.
Edited by:
Benti Deresa Gelalcha, The University of Tennessee, Knoxville, United StatesReviewed by:
Jianzhong Wang, Shanxi Agricultural University, ChinaCopyright © 2025 Abdelfattah, Ekong, Okello, Chamchoy, Karle, Black, ElAshmawy, Sheedy, Williams, Lehenbauer, Byrne and Aly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharif S. Aly, c2FseUB1Y2RhdmlzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.