- 1Unit of Veterinary Public Health and Epidemiology, Institute of Food Safety, Food Technology and Veterinary Public Health, University of Veterinary Medicine, Vienna, Austria
- 2Department of Population Health Sciences, Faculty of Veterinary Medicine, Utrecht University, Utrecht, Netherlands
- 3Division for Animal Health, Austrian Agency for Health and Food Safety (AGES), Vienna, Austria
- 4Department of Epidemiological Sciences, Animal Plant and Health Agency, Weybridge, United Kingdom
- 5National Food Safety Centre, National Diagnostic and Research Veterinary Medical Institute, Sofia, Bulgaria
- 6Department of Food Hygiene, Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise “G. Caporale”, Teramo, Italy
- 7Department of Biological Safety, German Federal Institute for Risk Assessment (BfR), Berlin, Germany
- 8Department of Rural Development and Agriculture, Ministry of Agriculture, Environment and Climate Protection of the State of Brandenburg, Potsdam, Germany
Salmonella spp. and hepatitis E virus (HEV) are significant foodborne zoonotic pathogens that impact the health of livestock, farmers, and the general public. This study aimed to identify biosecurity measures (BSMs) against these pathogens on swine farms in Europe, the United States, and Canada. Overall, 1,529 articles from three scientific databases were screened manually and with the artificial intelligence (AI) tool ASReview. We identified 54 BSMs from 32 articles, primarily focused on Salmonella spp. control. Amongst the extracted BSMs, only five measures for Salmonella spp. control, namely, ‘acidification of feed’, ‘acidification of drinking water’, ‘rodent control’, ‘all-in and all-out production’, and ‘disinfection’ had sufficient observations to conduct a meta-analysis. Of these five, acidification and rodent control were found to be protective measures, that is, their summary odds ratios in the corresponding meta-analyses were lower than 1, indicating lower odds of Salmonella spp. presence on farms which implemented these BSM compared to farms which did not implement them (odds ratio [OR] around 0.25). All-in and all-out production showed a non-significant protective effect (OR = 0.71), while disinfection showed a statistically non-significant lack of association between disinfection and the presence of Salmonella spp. on the farm (OR = 1.03). For HEV, no meta-analysis could be performed. According to multiple articles, two BSMs were significantly associated with a lower risk of HEV presence, namely, disinfecting vehicles (OR = 0.30) and quarantining pigs before introducing them on the farm (OR = 0.48). A risk of bias assessment for each included article revealed a high risk in the majority of the articles, mainly due to selection and performance bias. This emphasises the lack of standardised, high-quality study designs and robust empirical evidence linking BSM implementation to pathogen reduction. The limited data available for meta-analysis, coupled with the high risk of bias (RoB) in the literature, highlights the urgent need for more substantial evidence on the effectiveness of BSMs in mitigating the transmission and spread of zoonotic pathogens, such as Salmonella spp. and HEV on pig farms.
1 Introduction
1.1 Rationale
Livestock-associated zoonotic pathogens are a serious public health threat and an occupational hazard for farmers and personnel who are continuously in contact with farm animals and their products (1). Amongst other pathogens, Salmonella spp. and hepatitis E virus (HEV) are zoonotic pathogens that can cause foodborne disease in humans and are difficult to control in pig farms.
Salmonellosis is the second most reported gastrointestinal infection in humans after campylobacteriosis. In 2021, there were 60,050 human cases of salmonellosis in the European Union (EU), of which 6,755 were cases associated with foodborne outbreaks. In particular, the most common vehicle for Salmonella spp. included ‘pig meat and products thereof” (European Food Safety Authority [EFSA] and European Centre for Disease Prevention and Control [ECDC]) (2). In 2018, the United States Department of Agriculture (USDA) estimated the annual economic cost of human illness due to foodborne salmonellosis alone was $4.1 billion (USDA) (3). There is, therefore, a strong incentive to control Salmonella spp. in the pig food chain both from a healthcare and an economic perspective.
Similar to Salmonella spp. infections, HEV infections are subclinical in pigs. Pigs may be infected with HEV genotype 3 or 4 and shed the virus in faeces. Seroprevalences of up to 93% (4) have been reported in individual pigs and on the farm level, with up to 100% of farms being affected by HEV in European countries (5, 6). Humans can become infected by consuming raw or undercooked pork, particularly pork liver. In industrialised countries, pigs are presumed to be a significant source of human HEV infection (EFSA) (7). Contrary to pigs, HEV infection in humans may cause acute hepatitis, and in immunocompromised patients, can result in acute or acute-to-chronic liver failure and even become fatal (8), with 44,000 fatalities recorded worldwide in 2015 [WHO (9)].
Current policies emphasize prevention-based food production systems to address pathogens, such as Salmonella spp., and HEV and ensure food safety. Vora et al. (10) identified on-farm biosecurity measures (BSMs) as crucial to preventing zoonotic transmission from livestock to humans. A BSM is defined as “the implementation of a segregation, hygiene, or management procedure (excluding medically effective feed additives and preventive/curative treatment of animals) that specifically aims at reducing the probability of the introduction, establishment, survival, or spread of any potential pathogen to, within, or from a farm, operation or geographical area” (11). Implementing BSMs in swine production can reduce pathogen levels from farm to fork, leading to less contaminated pork and lower foodborne outbreak risks (12). Thus, the knowledge of effective BSMs on pig farms is vital for animal health, public health, and economic reasons.
Numerous types of BSMs in pig farming have been described in the scientific literature either in general or specifically against Salmonella spp. or HEV infections (13–15). One recent systematic review studied animal health/veterinary interventions’ effectiveness against pathogens relevant to pig herds and found feed additives and vaccination were appropriate for Salmonella spp. control, but not for HEV (16). Nevertheless, the effectiveness of such measures in reducing the risk of pathogen introduction or spread on or between farms has not yet been systematically reviewed and quantified comprehensively.
To our knowledge, this systematic review represents the first attempt to collate the published literature on BSMs against Salmonella spp. and HEV infections in domestic pigs from different exploratory study designs, analyse their effectiveness in reducing the prevalence of these pathogens at the farm level, and assess the eligible scientific literature regarding a potential risk of bias.
1.2 Aims and objectives
The primary aims of this review were to (i) provide an overview of the existing literature on BSMs for the control of Salmonella spp. and HEV infections at different production stages of domestic pigs on farms and (ii) outline the quantitative effectiveness of these BSMs. The secondary aim was to identify areas where future research is needed.
1.3 Research question
The research question was framed according to the Population, Intervention, Comparison, and Outcome (PICO) format.
P (Population): Swine farms in the EU, UK, USA, and Canada.
I (Intervention): Biosecurity measures (e.g., acidification, rodent control).
C (Comparison): Farms that did not implement these biosecurity measures.
O (Outcome): Reduction in prevalence of Salmonella spp. or HEV.
2 Methods
2.1 Systematic literature search
The outcome investigated in this study was the quantitative effectiveness of BSMs in reducing the occurrence of Salmonella spp. and HEV in pig farms. The geographical regions of interest were the United States, Canada and Europe and its associated political territories (e.g., French territories overseas which—although having a different climate than the US/Canada/Europe—are still subject to EU legislation on pathogen control). The decision to restrict the study to the EU, USA, Canada, and the UK was based on the socioeconomic similarities and established biosecurity frameworks that facilitated a more comparable analysis. The inclusion and exclusion criteria used to select studies for this systematic review are displayed in Table 1.
Initially the purpose of our search included pathogenic E. coli (PEC) as well. Since the literature search resulted in only two eligible publications, we excluded PEC from this analysis. However, PEC was still included in the search terms and is documented in the search process, as shown in Figure 1 and Supplementary Table S1.
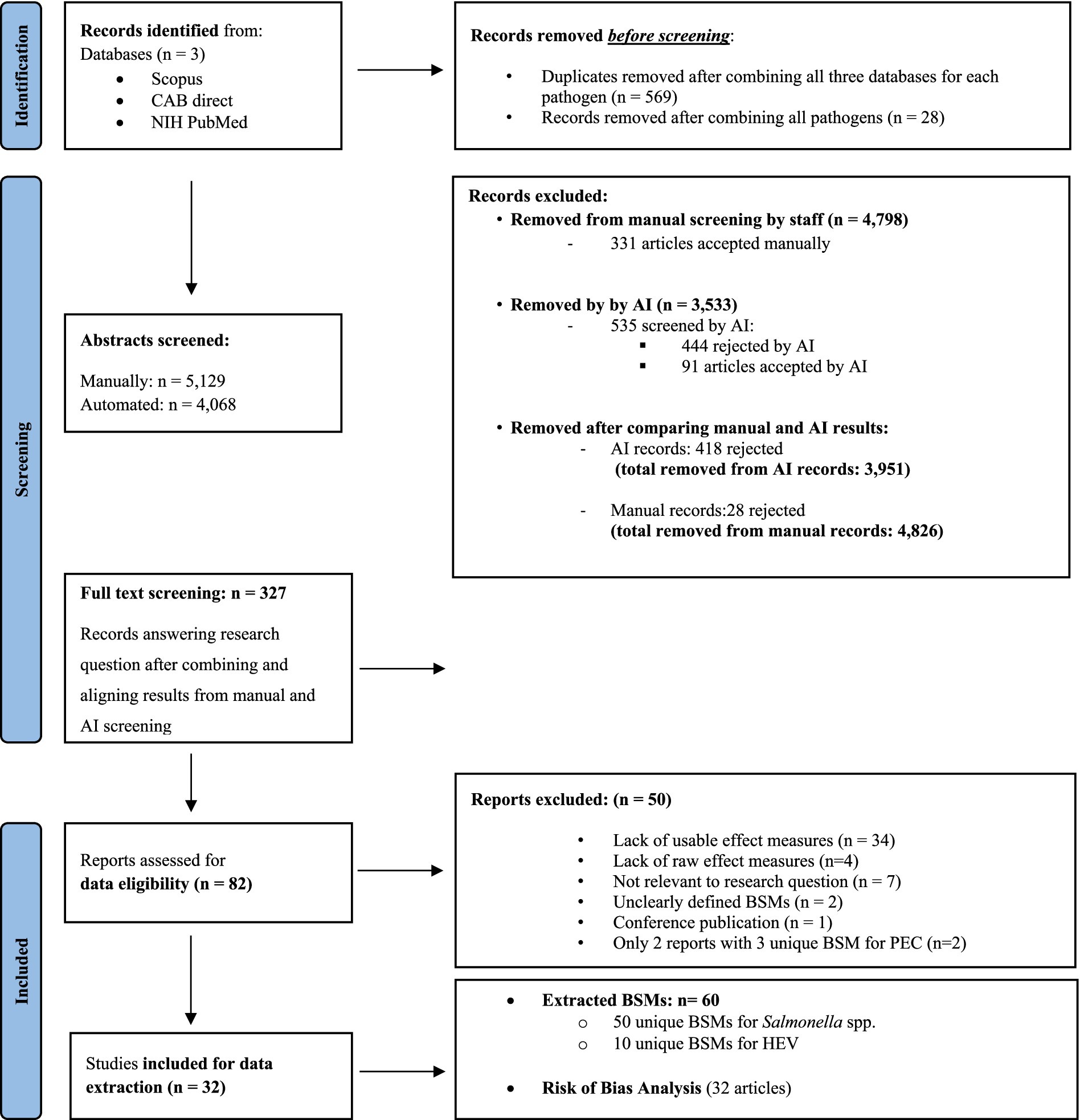
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the workflow of the systematic review. Source: Adapted from reference (71). 35 articles made it to the final list, but BSMs were only extracted and analysed from 32 articles as one article reported confidence intervals for pathogen being not transformable into confidence intervals (CIs) for an OR and only 2 articles with 3 unique BSMs were identified for PEC and were, therefore, excluded from further analysis.
Three scientific databases were used based on the author’s institutional access to the following databases: Scopus, CAB Direct, and National Institutes of Health (NIH) PubMed. The queries (Supplementary Table S1) were run separately for each of the pathogens but on all the databases, leading to nine searches conducted in April 2021.
A list of three example articles for both HEV and Salmonella spp. was created based on expert knowledge about available literature on these pathogens to validate that the search query was picking up appropriate articles. We deemed the query as effective if at least two of the three selected articles were identified.
2.1.1 Data collection process
From each database, a list of search results per pathogen was exported from the Web interface, and the results were merged per pathogen into a Microsoft Excel file, from which duplicates were manually removed. Each article was provided with a unique identifier. Any articles that did not have author metadata were removed from the final list. In total, 2,314 titles were identified through the database search for Salmonella spp. (n = 2,003) and for HEV (n = 311; and 5,129 when including PEC; Figure 1). Titles and abstracts were screened in duplicate: once by one of the 12 researchers during a manual screening round and once in parallel using the AI reviewing software ASReview version 0.18.rc0 1 (17).
2.1.2 Screening process
Articles were initially screened by one of the primary authors according to the criteria in Table 1 and excluded if one of these criteria was not met. After that, they were equally divided amongst the 12 authors for three rounds of selection: (i) title and abstract screening stage, (ii) full-text screening, and (iii) data extraction. During these stages, reviewers were provided with an Excel file containing the details of the articles alongside a list of questions which referred to the review’s eligibility criteria (Table 1), with the article either accepted or rejected at the end of the review. Further details on this process are shown in Figure 1.
2.1.3 ASReview
Screening was also performed using ASReview. This software uses an active learning technique. At first, some articles classified by a researcher as ‘positive’ (i.e., relevant/match selection criteria) and ‘negative’ (i.e., irrelevant/not matching selection criteria) are selected; thus, creating a starting point to classify publications as relevant or not. Then, ASReview starts screening the remaining publications and proposes articles that may be most relevant. The researcher checks each proposed publication and classifies them as relevant or not. ASReview picks up from the relevant and irrelevant articles defined by the researcher and continuously updates its knowledge, proposing further publications most likely to be relevant as well. This process is repeated until ASReview is proposing no more relevant articles, so one may assume that all relevant articles have been selected and the articles left to screen are irrelevant. Therefore, screening can be stopped before all articles are reviewed, thereby improving the efficiency of the reviewing process. Because of its efficiency, a single researcher could act as the “duplicate” reviewer through the software. Articles were re-extracted from the search databases following the same aforementioned query, yet in a format useable for ASReview, and after removing duplicates, 4,068 articles were uploaded to the ASReview software. The positive articles were uploaded separately and consisted of one article per pathogen (18–20). Additionally, three randomly proposed articles by the software that did not meet the selection criteria were selected as negative articles. Subsequently, 536 articles (13% of the total number of articles) were screened by the reviewer, and screening was stopped after 100 abstracts in a row were marked as irrelevant, which is considered a conservative heuristic for when to stop screening using this method (21). In total, 91 articles were accepted using ASRreview, and the reviewer rejected 444 articles. Results from both the authors that screened manually and from screening via ASreview were then manually merged into the final list of selected articles, with any final duplicates removed (Figure 1). Where there was a divergence between the manually reviewed and the software-reviewed articles, one author came in as a third reviewer to decide to reject or accept the article for the next round.
At the end of the abstract screening stage, and after aligning with ASReview results, 327 articles out of 5,129 were moved to full-text analysis, the majority of which were related to Salmonella spp. (195 titles), then E. coli (111 titles), and finally HEV (21) (Figure 1). Eighty-two articles (25%) were found to be entirely relevant to our research question after full-text analysis. They were selected for data extraction and the risk of bias analysis.
2.2 Extracted data
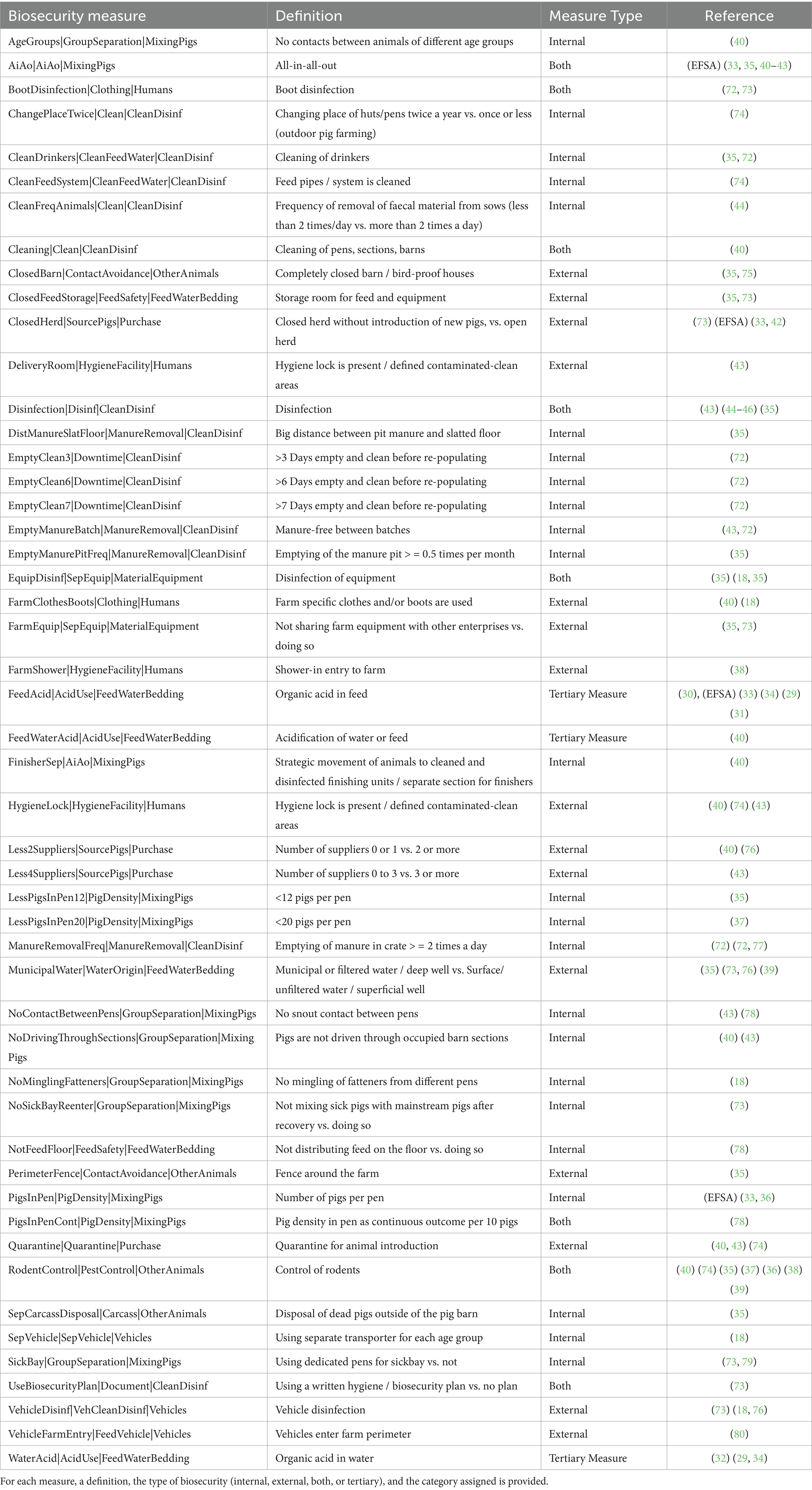
The following data were extracted from the articles by two reviewers independently to ensure harmonisation: effect measures (e.g., odds ratios or risk ratios), type of BSM, study location, the language used, title and author, year of publication, type of study, and production stage where the BSM was applied. From each article, one or more point estimates of the effect measures and their associated confidence intervals (CI) were extracted and sometimes recalculated from raw data when possible (see next section). Each extracted point estimate with its CI represents an “observation.” A publication may contain several observations, as it may study several BSMs. All observations were subsequently grouped based on the definition (where reported) or description of the BSM they best fell into, which was reported in the present study as a BSM. Therefore, a BSM in this article was defined as the combination of the main category, subcategory, and specific action taken on the farm to prevent pathogen presence (Tables 2, 3) Extracted effect measures from the articles were then cleaned by six authors, resulting in the removal of 50 articles at this stage. The reasons for exclusion at this point were (i) a lack of usable effect measures (e.g., unclear at which stage BSMs were administrated, or the data was inconsistent (in some tables, the numbers did not add up to info from other tables; n = 34), (ii) a lack of raw effect measures (n = 4), (iii) not relevant to our research question (n = 7), (iv) unclearly defined BSMs (n = 2); conference publication (n = 1), and (v) only two reports with three unique BSM for PEC (n = 2). In total, 50 articles were excluded at this stage, leading to a final dataset of 32 articles for analysis (for details on reasons for exclusion, see Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] flow diagram in Figure 1).
2.3 Data analysis
The extracted data was analysed using the statistical software R version 4.0.3 (22). For the evidence synthesis of the data analysis, the R package metafor version 3.0-2 (23) was used.
Some studies provided raw data from which the effect measures could be calculated, whereas others reported an effect measure derived from a univariate and/or multivariate regression analysis. Whenever a study provided raw data, this was used to calculate the OR and its variance using the escalc() function from the metafor package. Otherwise, the reported effect size and the corresponding confidence interval (CI) were used.
As the aim was to compare the effectiveness of BSMs in different stages of pig production, we pooled all observations for a given combination of production stage, pathogen, and BSM. Since the considered publications studied pig production in different countries and periods, we expected some heterogeneity between the studies. Additionally, many publications assessed BSM effectiveness at stages different from their implementation stage, further contributing to heterogeneity. We employed a random-effects model to account for expected heterogeneity. We established that a minimum of five observations were needed for performing a meta-analysis, in line with the established methodology, as suggested, for example, refer to references (24, 25). Thus, whenever for a given combination of the production stage, pathogen, and BSM, five or more observations could be found, we performed a meta-analysis using the metafor function rma.uni() based on a random-effects model using restricted maximum likelihood estimator for estimating the amount of statistical heterogeneity. Statistical heterogeneity was assessed using the I2 statistic, and our interpretation of whether the corresponding I2 values may represent “moderate” or “substantial” heterogeneity followed (26). There were only two combinations of production stage, pathogen, and BSM, where five or more observations were available: acidification of feed and acidification of drinking water for Salmonella during the fattening stage. To extract more insights, we aggregated publications on the same BSM regardless of production stage, performing a meta-analysis for combinations with at least five observations. For five combinations of pathogen and BSM, there were five observations: acidification of feed, acidification of drinking water, all-in and all-out production, disinfection, and rodent control.
Therefore, evidence synthesis was carried out in two different ways. The first approach described above considered a combination of BSM, pathogen and production stage. This approach is referred to as “considering the production stage.” The second approach considered combinations of BSM and pathogen, irrespective of the production stage. This approach is referred to as “ignoring the production stage.”
To provide a formal assessment of publication bias concerning the observations included in the meta-analyses, we created contour-enhanced funnel plots using the function funnel() from the metafor package. We performed Egger’s test using the regtest() function, also from the metafor package (which performed a weighted regression with multiplicative dispersion).
Finally, we did a sensitivity analysis for the meta-analyses using the influence() function from the metafor package.
We only provided bias analyses and sensitivity analyses for the meta-analyses, which showed statistically significant summary OR estimates.
2.4 Risk of bias analysis
All studies included in the data extraction stage were evaluated for risk of bias (RoB) by using protocols adapted from the study mentioned in Kim et al. (27) for observational studies and from the study in Higgins et al. (28) for experimental studies. Both protocols provided a framework for assessing the likelihood or risk that bias concerning the study’s outcome could have been introduced. All RoB analyses were conducted in duplicate independently by two reviewers. In total, either seven or eight domains of bias, depending on the study type, were assessed: selection bias, allocation concealment (for experimental studies only), confounding variables, performance bias, detection bias, attrition bias, reporting bias, and an “other bias” domain including questions on funding sources and potential conflicts of interest. To assess the RoB in the context of BSMs and the respective article, specific signalling questions for each domain were formulated to guide the authors in classifying RoB as either low, medium, or high. The masks applied during the RoB assessment, including the signalling questions, are presented in Supplementary Tables S2 and S3. The overall assessment of each article was based on the combined assessments of all RoB domains. In cases where the majority of the signalling questions were at a medium level, or one was rated as a high level, the domain was classified as having a high RoB. If one of the domains was rated as having a high RoB, the overall assessment was also rated as having a high RoB.
3 Results
This analysis included 157 observations of 54 BSMs extracted from 32 articles. Amongst the 32 studies reviewed, eight main types of BSMs were identified, which were as follows: (a) humans, that is, interventions that involved humans, such as hand washing, showering, etc.; (b) mixing of pigs; (c) cleaning and disinfection; (d) vehicles; (e) feed, water, and bedding; (f) purchase of animals; (g) equipment, and (h) other animals. The number of observations for each type of BSM by the two pathogens studied in this review can be found in Supplementary Figure S2.
3.1 Study characteristics
3.2 Salmonella spp.
3.2.1 Number of BSMs associated with Salmonella spp. in this study
Overall, 2,003 articles about the BSMs against Salmonella spp. were identified in the literature, with 28 of those (1.4%) eligible for final analysis (Figure 1). These articles were published between 1997 and 2019 (median: 2009, interquartile range [IQR] = 15). In total, 50 BSMs were identified for Salmonella spp. (Table 2).
3.2.2 Internal and external biosecurity measures for Salmonella spp.
Overall, the majority of the extracted BSMs belonged to internal biosecurity (n = 24, Table 2, note that the number 24 refers to BSMs which are classified as strictly “internal” in Table 2), that is, addressing measures that would prevent the spread of a pathogen within the farm. However, BSMs that showed a high protective effect were mostly those covering external biosecurity, aimed at preventing transmission of pathogens between farms and represented 15 of the extracted BSMs for Salmonella spp. in Table 2. Of note, eight BSMs were attributed to both internal and external biosecurity measures for this pathogen and a small proportion of three BSMs were identified as relating to tertiary biosecurity, which is usually described as measures aiming to control the ability of animals to deal with the pathogen.
3.2.3 Meta-analysis for Salmonella spp.
a. Considering the production stage
When considering the production stage, two BSMs met the inclusion criteria for meta-analysis, being the acidification of feed (FeedAcid|AcidUse|FeedWaterBedding; Figure 2) and the acidification of water (WaterAcid|AcidUse|FeedWaterBedding; Figure 3). The observations and the corresponding articles are designated with unique identifiers throughout the forest plots presented in this analysis. A list of the identifiers and information on the corresponding publication (title, author, country of study, etc.) can be found in Supplementary Table S6.
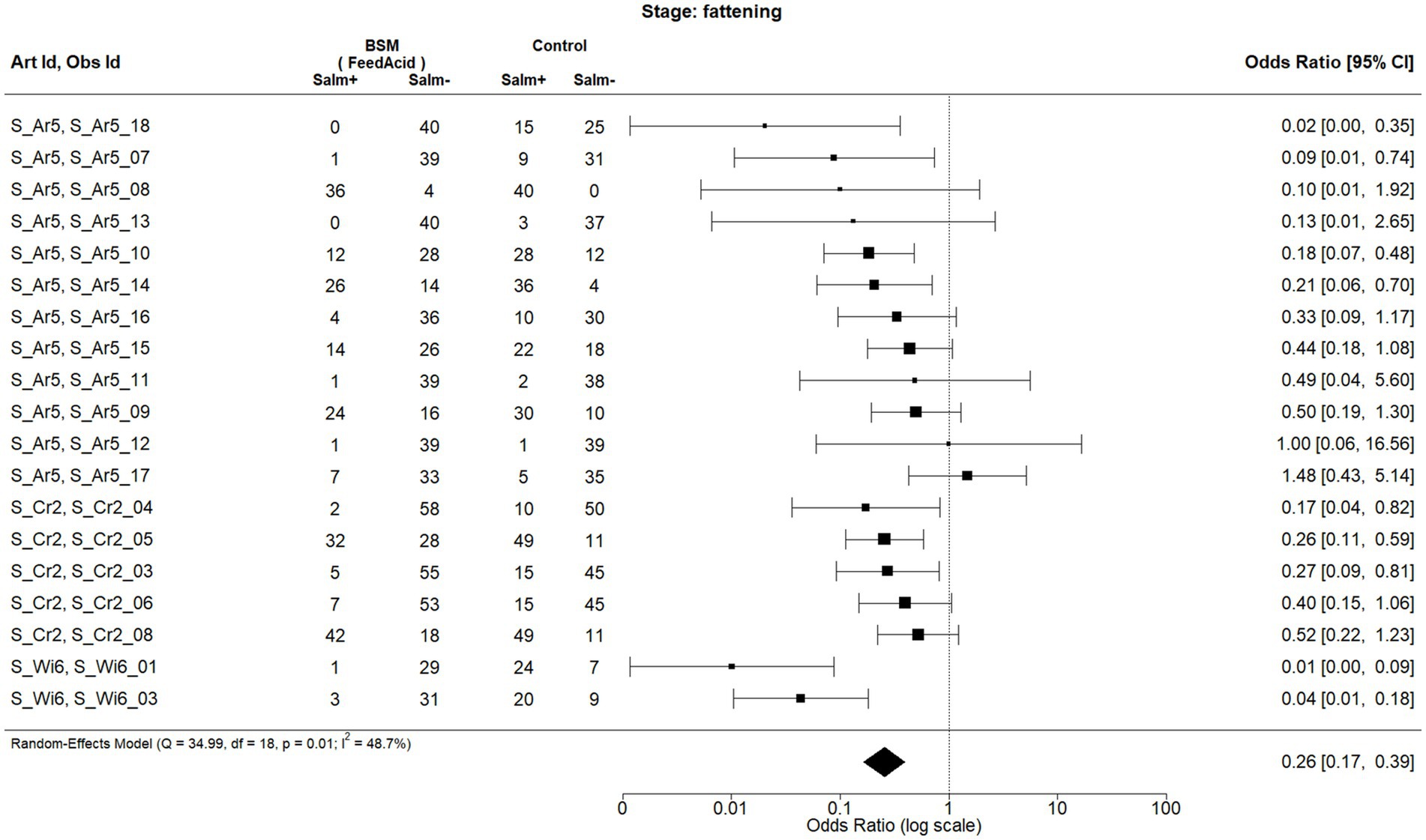
Figure 2. Forest plot of random-effects meta-analysis (with restricted maximum likelihood estimator of the amount of heterogeneity) for the biosecurity measure (BSM) acidification of feed for Salmonella spp. at the fattening stage. Each observation is encoded with internal BIOPIGEE designations for the article ID (Art Id) and the observation ID (Obs Id). Where available, the number of Salmonella spp. positive (‘Salm+’) and negative (‘Salm−’) samples for the BSM and the control condition, as stated in the corresponding publication, are provided. Each square indicates the odds ratio (OR) for one observation, with the size of the square indicating the weight with which that observation contributed to the summary value of the meta-analysis. The whiskers on the squares indicate the 95% confidence intervals (CIs) for each observation. The diamond at the bottom showed the summary value of the meta-analysis, indicating a summary OR of 0.26 with a 95% CI of 0.17–0.39.
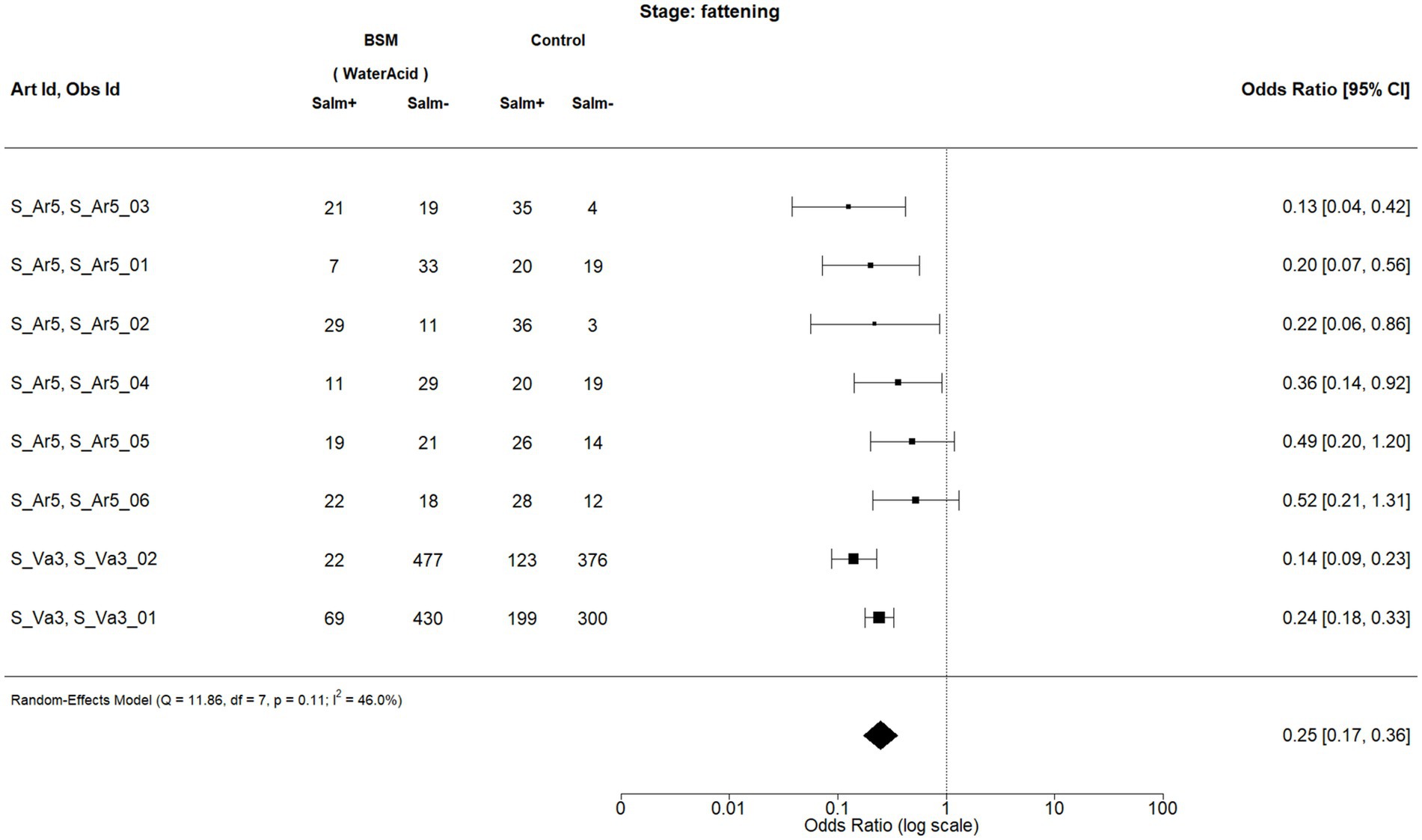
Figure 3. Forest plot of random-effects meta-analysis (with restricted maximum likelihood estimator of the amount of heterogeneity) for the biosecurity measure (BSM) water acidification for Salmonella spp. at the fattening stage. Each observation is encoded with internal BIOPIGEE designations for the article ID (Art Id) and the observation ID (Obs Id). Where available, the number of Salmonella spp. positive (‘Salm+’) and negative (‘Salm−’) samples for the BSM and the control condition, as stated in the corresponding publication, are provided. Each square indicates the odds ratio (OR) for one observation, with the size of the square indicating the weight with which that observation contributed to the summary value of the meta-analysis. The whiskers on the squares indicate the 95% confidence intervals (CIs) for each observation. The diamond at the bottom indicated the summary value of the meta-analysis, indicating a summary OR of 0.25 with a 95% CI of 0.17–0.36.
For feed acidification, there were 19 observations from three publications, that is, S_Ar5, which corresponds to (29), S_Cr2 (30) and S_Wi6 (31) (Figure 2). All three publications dealt with pigs in Spain and were published between 2007 and 2013. The summary estimate for the OR due to feed acidification is 0.26, 95% CI: 0.17–0.39. The heterogeneity statistic I2 has a point estimate of 48.7%, indicating a moderate heterogeneity between the observations.
For water acidification, there were eight observations from two publications: S_Ar5 and S_Va3 (32) (Figure 3). While the first publication was from Spain in 2013, the latter was from The Netherlands in 2001. The summary estimate for the OR due to water acidification is 0.25, 95% CI: 0.17–0.36. The I2 is 46.0%, indicating a moderate heterogeneity between the observations.
a. Ignoring the production stage
When pooling all observations for a BSM, regardless of the production stage, five BSMs had sufficient data for meta-analysis: acidification of feed and water, all-in and all-out production (AiAo|AiAo|MixingPigs), disinfection (Disinfection|Disinf|CleanDisinf), and rodent control (RodentControl|PestControl|OtherAnimals). Acidification of feed and water, along with rodent control, demonstrated statistically significant protective effects. Note that in this section, we only show the forest plot (Figure 4), the funnel plot (Figure 5), and the sensitivity analysis (Figure 6) for rodent control. The forest plots for the remaining four BSMs can be found in the Supplementary material (Supplementary Figure S3). As noted above, funnel plots (Supplementary Figure S4) and sensitivity analysis (Supplementary Figure S5) were only provided for the BSMs which demonstrated statistically significant protective effects, in this case of ignoring the production stage which means acidification of feed and acidification of water.
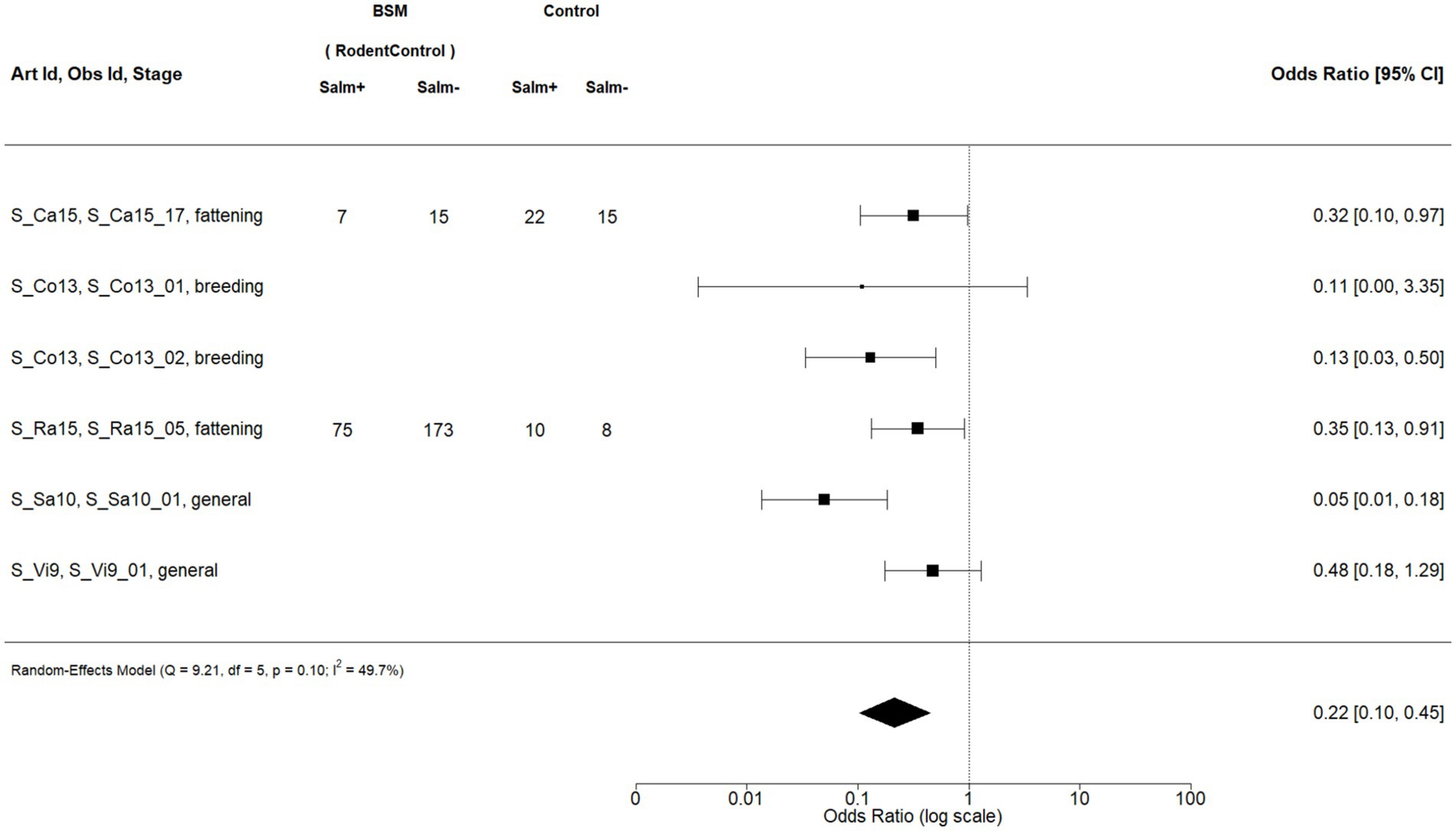
Figure 4. Forest plot of random-effects meta-analysis (with restricted maximum likelihood estimator of the amount of heterogeneity) for the biosecurity measure (BSM) rodent control for Salmonella spp. ignoring the production stage. Each observation is encoded with internal BIOPIGEE designations for the article ID (Art Id), the observation ID (Obs Id), and the production stage. Where available, the number of Salmonella spp. positive (‘Salm+’) and negative (‘Salm−’) samples for the BSM and the control condition, as stated in the corresponding publication, are provided. Each square indicates the odds ratio (OR) for one observation, with the size of the square indicating the weight with which that observation contributed to the summary value of the meta-analysis. The whiskers on the squares indicate the 95% confidence intervals (CI) for each observation. The diamond at the bottom indicated the summary value of the meta-analysis, indicating a summary OR of 0.22 with a 95% CI of 0.10–0.45.
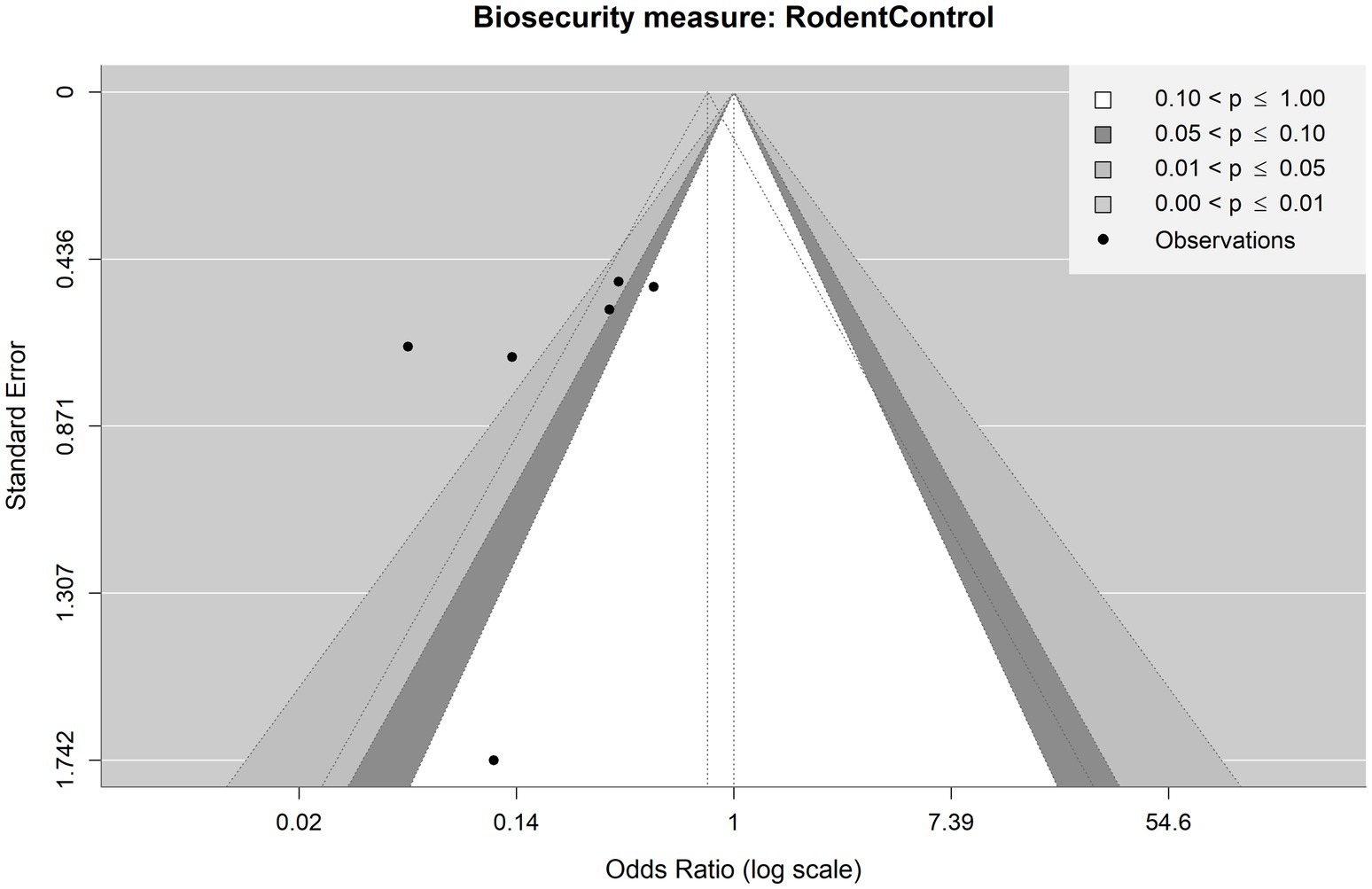
Figure 5. Contour-enhanced funnel plot for observations included in the meta-analysis on the mitigating effect of rodent control on Salmonella spp. infection in pig production, ignoring the stage. Each point is an observation from the literature. The shaded areas help identify whether observations lie above or under statistical significance. The funnel with dotted lines indicates how the observations are scattered around the summary OR of 0.22.
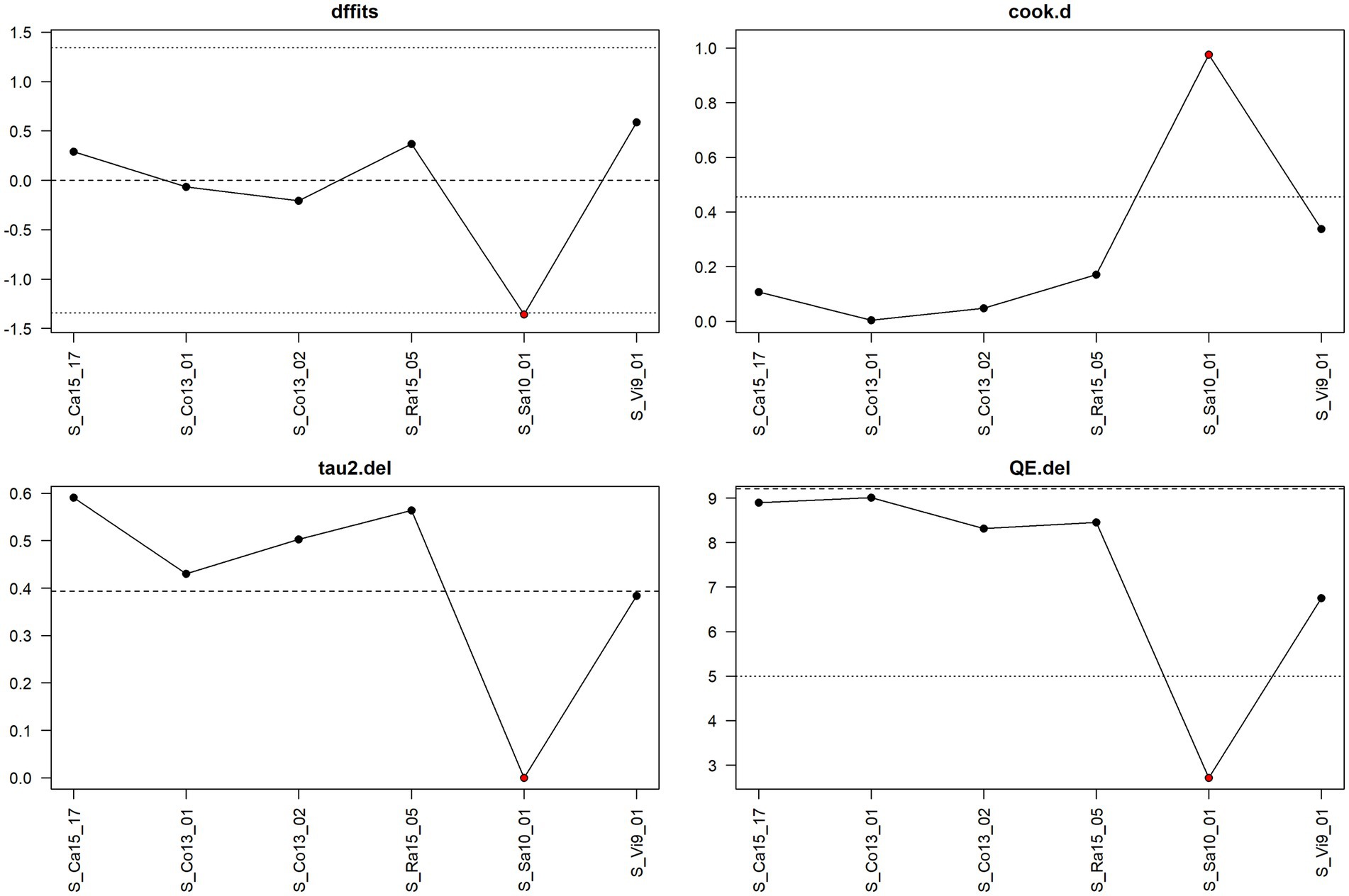
Figure 6. Diagnostic plot for sensitivity analysis for the meta-analysis on the mitigating effect of rodent control on Salmonella spp. infection ignoring the stage in pig production.
For feed acidification, 21 observations from five publications were finally included. That is, in addition to the aforementioned observation for the fattening stage from S_Ar5, S_Cr2 and S_Wi6, there were now observations from S_Ef1, which is (33) and considers acidification generally throughout the EU and from S_Ru5 which is (34) and considers feed acidification at the weaning stage in Italy. The summary estimate for the OR was 0.27, 95% CI 0.18–0.41. The heterogeneity statistic I2 was 62.2%, indicating a substantial heterogeneity between the observations.
One more observation was included for water acidification compared to the analysis considering the production stage, which came from S_Ru5 considering water acidification at the weaning stage in Italy, published in 2018. The summary estimate for the OR is 0.23, 95% CI: 0.16–0.34. The heterogeneity statistic I2 was 44.1%, indicating a moderate heterogeneity between the observations.
For rodent control, there were six observations from five publications (Figure 4): S_Ca15 (35) considers fattening pigs on Reunion Island, an overseas department of France in the Indian Ocean; S_Co13 (36) considers breeding pigs in Portugal, S_Ra15 (37) considers finishing pigs in Canada, whereas S_Sa10 (38) and S_Vi9 (39) consider pigs in Spain. The summary estimate for the OR was 0.22, 95% CI: 0.10–0.45. The heterogeneity statistic I2 was 49.7%, indicating a moderate statistical heterogeneity between the observations.
For all-in and all-out production, there were six observations from six publications (see Supplementary Figure S3 A1): S_Al19 (40) for pigs in Germany in 2000, S_Ca15 (35) for pigs on Reunion Island in 2010, S_Da15 (41) for pigs in the US in 1997, S_Do12 (42) for pigs in Poland in 2015, S_Ef1 (33) for pigs in the EU in 2011, and S_St6 (43) for pigs in Denmark in 2001. The summary estimate for the OR was 0.79 (95% CI: 0.52–1.18). The heterogeneity statistic (I2) was 19.6%, suggesting low statistical heterogeneity. However, some heterogeneity exists, given the varied geographical locations and study periods. One observation (S_Da15_01) had a wide confidence interval due to a zero value in its 2 × 2 table. Removing this observation increased I2 to 29.3%, indicating borderline to moderate heterogeneity. With a small number of observations and a p-value of 0.39 for the heterogeneity test, the precision of the heterogeneity estimate was low, suggesting the true I2 might be even higher than 29.3%.
For disinfection, there were eight observations from six publications (see Supplementary Figure S3B): S_Al19 (40) for pigs in Germany in 2000, S_Ca15 (35) on Reunion Island in 2010, S_Fa8 (44) in France in 2003, S_Ma12 (45) in the UK in 2017, S_St6 (43) for pigs in Denmark in 2001, and S_Va8 (46) in The Netherlands in 2001. The summary estimate for OR was 1.03, 95% CI: 0.46–2.28. The heterogeneity statistic I2 of 94.3% indicated considerable heterogeneity.
a. Bias analysis
First, we consider the meta-analyses, which considered the production stage. Figure 7 shows the funnel plot for the observations concerning feed acidification at the fattening stage. Visual inspection of the funnel with the dotted lines (i.e., the funnel plot centred at the summary OR of 0.26) suggests a possible small-study bias: there are three observations at the bottom (i.e., observations with high standard errors and, therefore, smaller sample sizes) left-hand side from the vertical line (i.e., showing an effectivity against Salmonella spp. with lower than summary OR, meaning a relatively high effectivity relative to the summary OR). However, there is only one corresponding small study observation at the bottom right-hand side with higher than summary OR. This visually suggestive asymmetry between more publications of small studies reporting high effectivity and fewer with low effectivity is in contrast to the result of Egger’s test, which does not provide evidence of asymmetry in the funnel plot and hence possible publications bias (p = 0.0623) at the 0.05 significance level. Still, as the p-value is close to the cut-off of 0.05, it may be interpreted as a tendency that there might be some bias present.
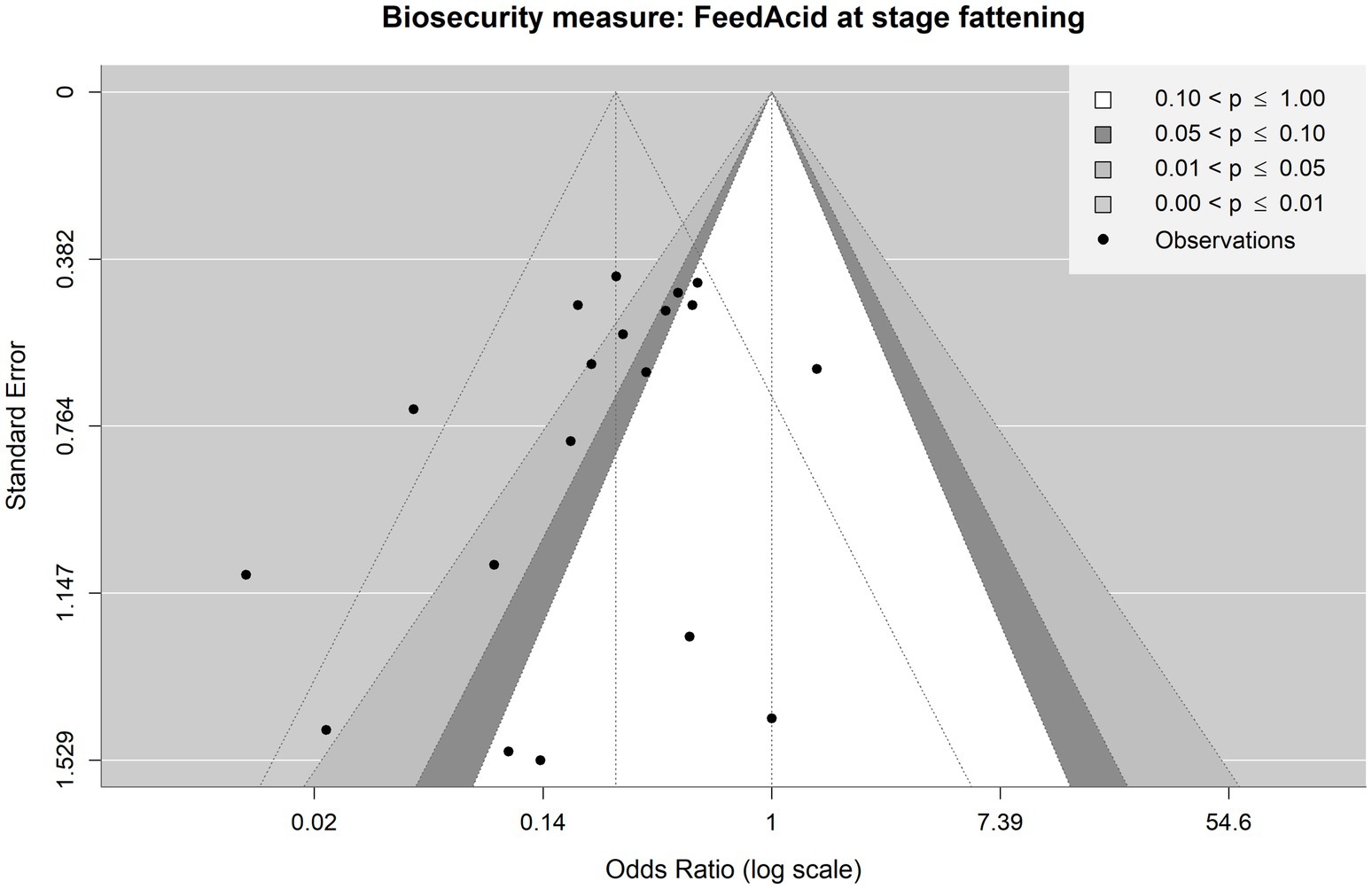
Figure 7. Contour-enhanced funnel plot for observations included in the meta-analysis on the mitigating effect of feed acidification on Salmonella spp. Infection at the fattening stage in pig production. Each point is an observation from the literature. The shaded areas help identify whether observations lie above or under statistical significance. The funnel with dotted lines indicates how the observations are scattered around the summary OR of 0.26.
However, taking the statistical significance of the individual observations into account (the contoured plot with the shaded funnel centred at OR = 1), no particular asymmetry in terms of statistical significance is evident upon visual inspection: 9 observations are lying in the areas of statistical significance while 10 lies in the area of no statistical significance. From this perspective, there appears to be no publication bias based on statistical significance.
Figure 8 shows the funnel plot for water acidification at the fattening stage. Visual inspection of the dotted funnel plot suggests a possible small-study bias, with the two observations on the bottom left-hand side having no counterparts on the right-hand side. However, Egger’s test again provided no evidence for funnel plot asymmetry and, hence, publication bias (p = 0.5779). It should be noted that in this case, there are less than 10 observations in the meta-analysis. This situation is where Egger’s test can only detect a substantial small-study bias (47). Visual inspection of the contoured funnel plot suggests an asymmetry concerning the significance of the observations (six observations show statistical significance, whereas two observations do not show it), which suggests that there might be a publication bias due to underreported non-significant observations.
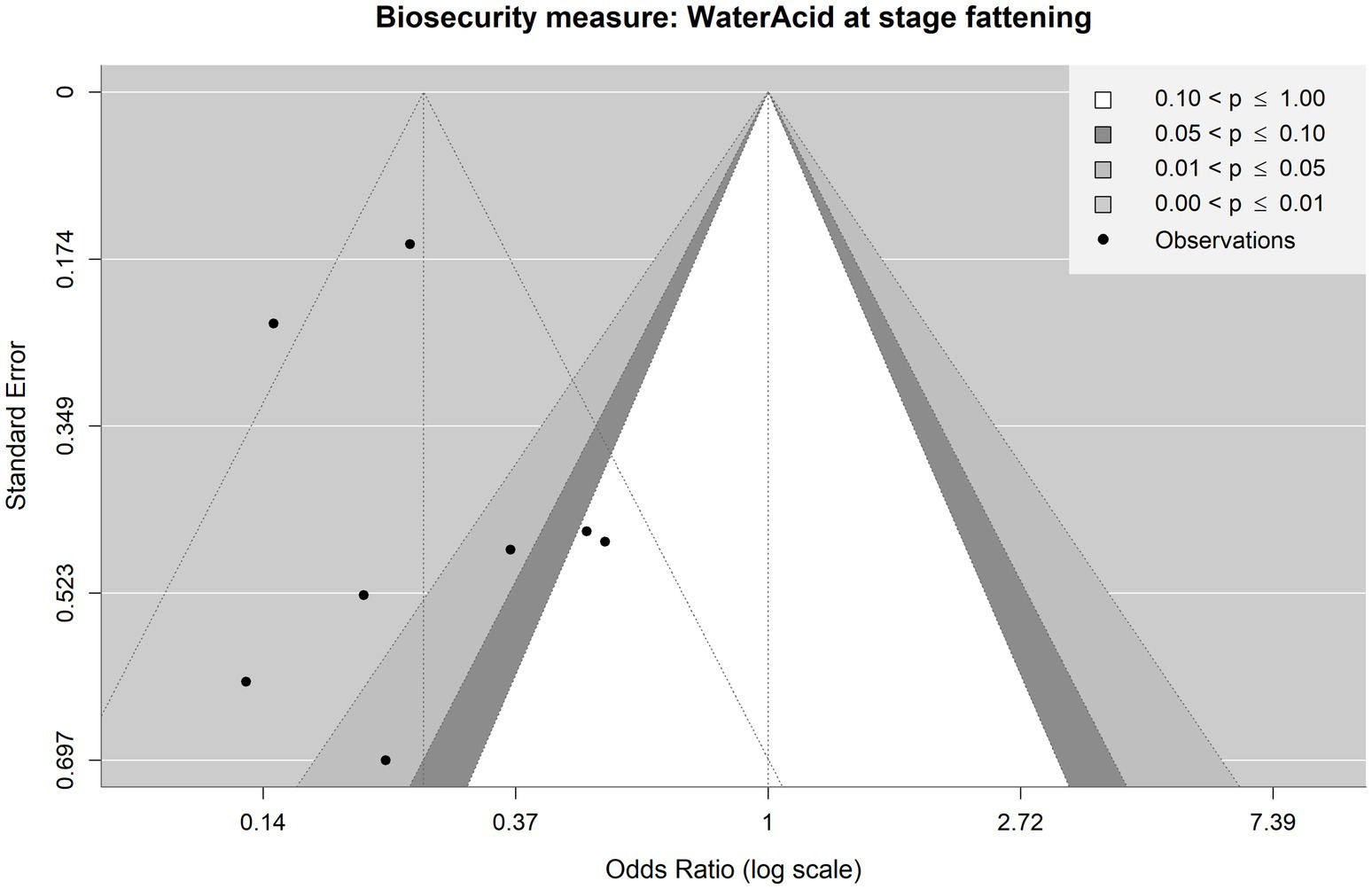
Figure 8. Contour-enhanced funnel plot for observations included in the meta-analysis on the mitigating effect of water acidification on Salmonella spp. infection at the fattening stage in pig production. Each point is an observation from the literature. The shaded areas help identify whether observations lie above or under statistical significance. The funnel with dotted lines indicates how the observations are scattered around the summary OR of 0.25.
Then, we considered the meta-analyses which ignored the production stage. Figure 5 shows the funnel plot for the meta-analysis on rodent control when ignoring the production stage. Visual inspection shows an apparent asymmetry. There are only publications left to the vertical lines of the dotted funnel. A small-study bias is suggested by a single observation with a relatively high standard error left from the perpendicular line with no corresponding observations on the right. However, Egger’s test did not provide evidence for asymmetry (p = 0.3320). As noted above, Egger’s test for less than 10 observations is somewhat limited in its informative value. In terms of sensitivity, two observations were non-significant, whereas four observations were significant. Given the small number of observations, there is no apparent bias in terms of significance.
Visual inspection of the funnel plot for acidification of feed ignoring the production stage (Supplementary Figure S4A) indicates a considerable small-study bias, corroborated by Egger’s test (p < 0.0001). The contoured funnel plot detects no large asymmetry in terms of statistically significant (11 observations) vs. non-significant (11 observation) data points.
The funnel plot for water acidification ignoring the production stage (Supplementary Figure S4B) shows only one observation in the “small-study” area on the bottom left-hand side and none on the bottom right-hand side. However, these low numbers in observations do not allow us to draw any conclusions. In line with this result Egger’s test did not provide evidence for funnel asymmetry and hence publication bias (p = 0.9508).
A large bias with respect to the statistical significance of the individual observations is present as only two observations show non-significant results, while seven observations show significant results, hinting at a possible publication bias for non-significant observations.
a. Sensitivity analysis
First, we looked at the meta-analyses which considered the production stage.
Figure 9 shows the result of sensitivity analysis for feed acidification at the fattening stage based on a leave-one-out approach. That is, one observation at a time is left out, and the meta-analysis is re-run to see how the results change. In particular, the values of the following four measures are considered under leaving out one observation: dffits (the studentised difference in fits), cook.d (Cook’s distances), tau2.del (estimate of τ2—a measure of between-study variance—the variance of the effect when leaving one particular study out), and QE.del (test statistic for (residual) heterogeneity when leaving one particular study out). The author of the metafor package chose cut-off values for dffits and cook.d values to select whether a corresponding observation might be considered an “influential” one, that is, an observation to which the meta-analytical result is, to a certain extent, sensitive. The cut-off for dffits is defined by values which are larger than , where is the number of model coefficients (in our case with the random effects model, we always have = 1), and is the number of observations. In the case of Figure 9 and feed acidification at the fattening stage, = 19; hence, the cut-offs are about 0.71. The cut-off for cook.d is reached if the lower tail area of a chi-square distribution with p degrees of freedom cut-off by the Cook’s distance is larger than 50%.
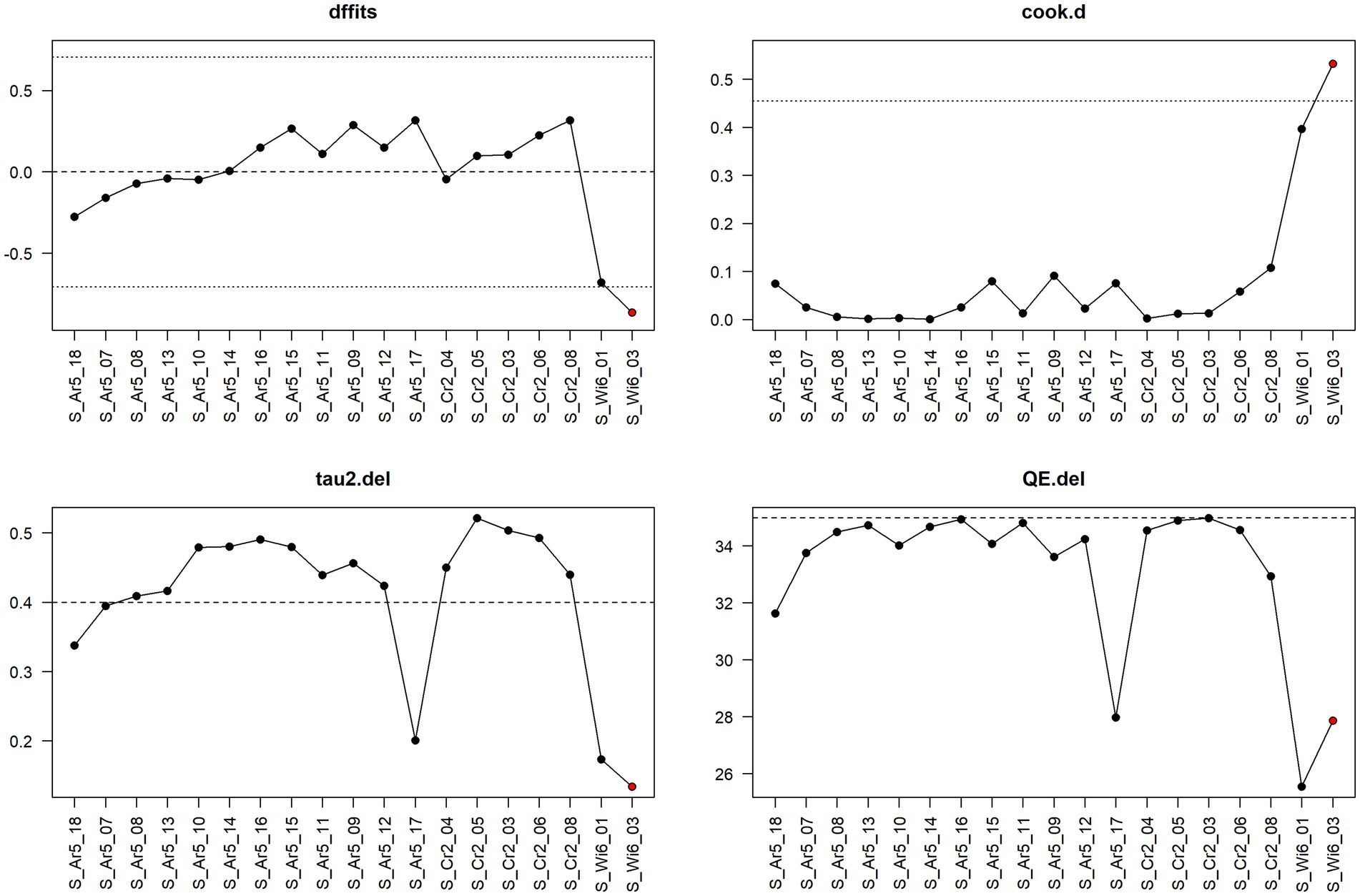
Figure 9. Diagnostic plot for sensitivity analysis for the meta-analysis on the mitigating effect of feed acidification on Salmonella spp. infection at the fattening stage in pig production.
The cut-offs for dffits and cook.d are indicated in the sensitivity analysis plots as dotted lines. If leaving out one observation leads to meta-analytical results which exceed the cut-offs for dffits and/or cook.d, the corresponding point in the sensitivity plot is indicated in red. Hence, red points indicate that the corresponding observations could be interpreted as being considerably influential.
Thus, Figure 9 shows that the observation S_Wi6_03 is the only one that has a considerable influence, and one also sees that leaving it out decreases considerably the heterogeneity as measured by tau2.del as well as QE.del.
Figure 10 shows the sensitivity analysis for water acidification at the fattening stage. Here no single observation is considered influential according to the considered measures. Note that the dotted line in the panel for the measure QE.del is not a cut-off for potentially influential observations but one of two horizontal reference lines drawn at the test statistic based on the case that all observations are considered (i.e., without leaving one observation out) and at k–p, i.e., the degrees of freedom of the test statistic. In this case, the test statistic in question is the Q-statistic, which is also shown in Figure 3 and has the value 11.86 (dashed line in Figure 10), and the degrees of freedom are 7 (dotted line in Figure 10).
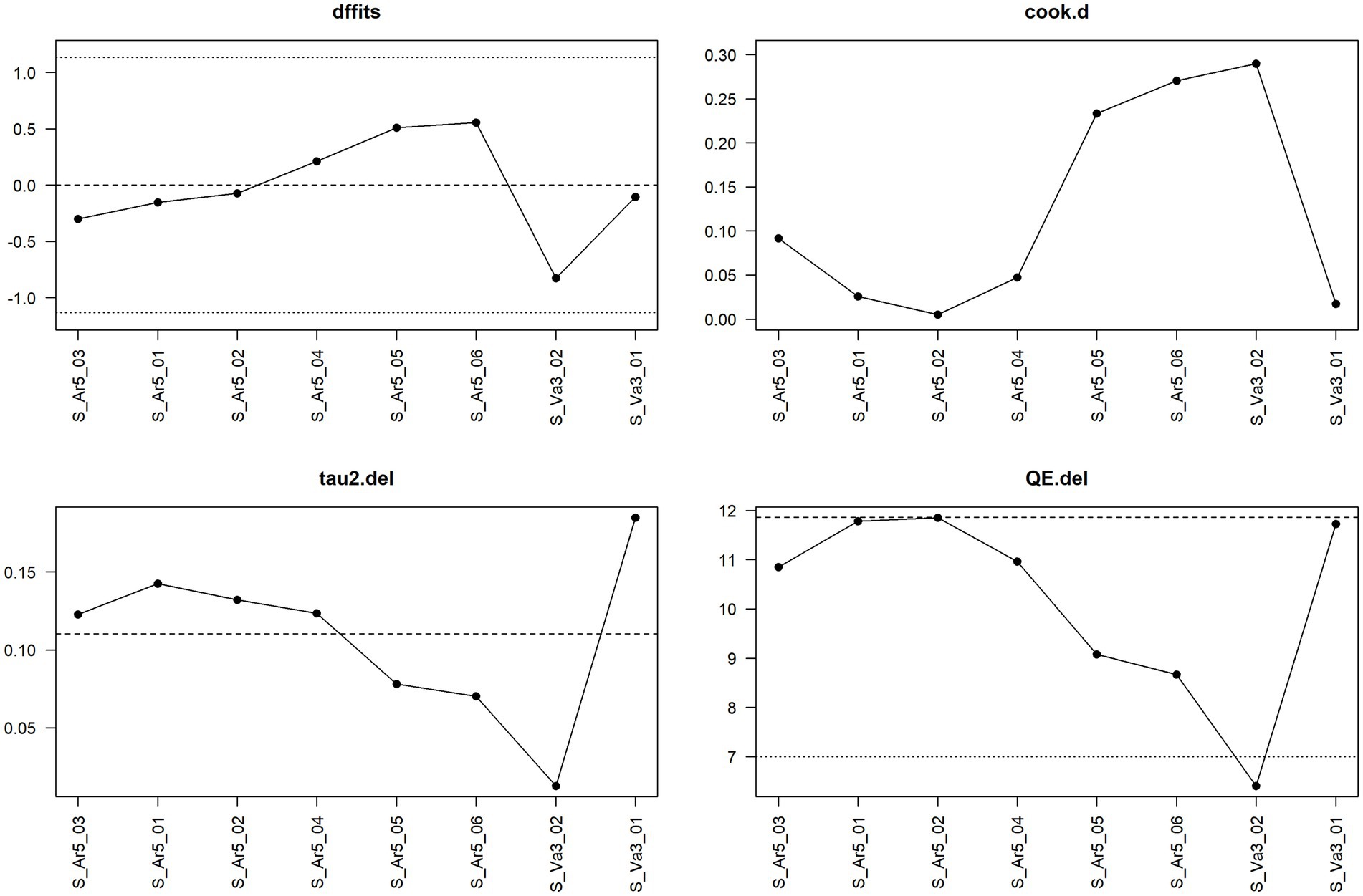
Figure 10. Diagnostic plot for sensitivity analysis for the meta-analysis on the mitigating effect of water acidification on Salmonella spp. infection at the fattening stage in pig production.
Then, we looked at the meta-analyses which ignored the production stage.
The sensitivity plot in Figure 6 indicated that the observation S_Sa10_01 considerably exceeds the cook.d cut-off, and leaving it out considerably lowers the heterogeneity of the meta-analysis.
3.3 Hepatitis E virus
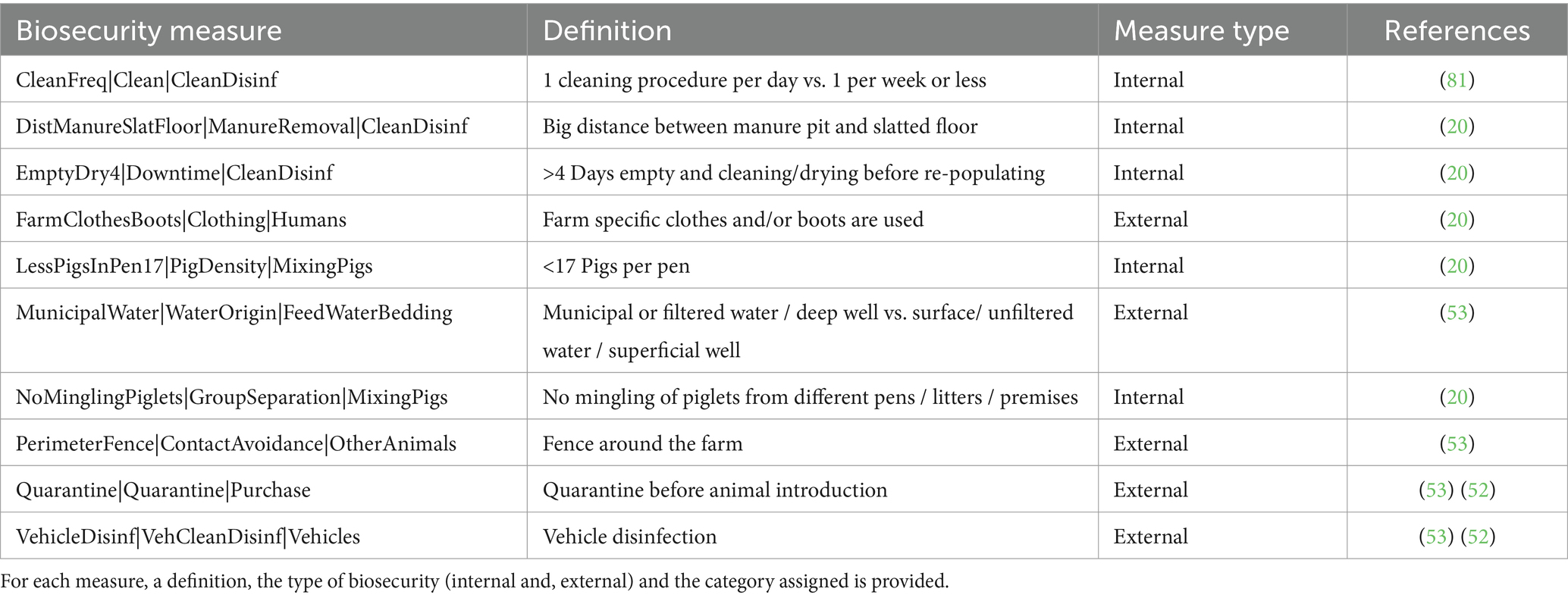
The database search retrieved 311 hits for HEV, of which 21 articles were screened by full text. Finally, data was extracted from four articles published between 2014 and 2021 (median = 2017.5, IQR = 4.75), from which 10 protective BSMs were found (Table 3). Two BSMs, quarantining pigs and disinfecting vehicles, were reported in two articles, while the others were reported in only one article. All BSMs reduced the odds of an observational unit being HEV-positive. They were attributed 50/50 to external and internal BSMs. There was insufficient information to run a meta-analysis on the BSMs for HEV.
3.4 Risk of bias analysis
The outcome of the RoB analysis classified all studies in the final selection stage (observational studies n = 27, experimental n = 5) to be at a high overall RoB (Figure 11). The high overall rating of bias risk was mainly driven by the high scores in the selection bias (87.5%), performance bias- (75%), and “other bias” (59.4%) domains (Supplementary Table S4). The high rating in the selection bias domain, especially in observational (i.e., mainly cross-sectional) study designs, was based on the lack of or unclear random selection of animals or animal groups and even more so by missing consideration of potential confounders in the respective studies. In the context of performance bias, the collection of samples in the respective studies was either unclear, not described, or performed non-blinded. The main signalling question in ‘other bias’ concerning conflict of interest led to the high rating in this domain. The detailed ratings of each bias domain for all studies in the final selection stage are presented in Supplementary Table S5.
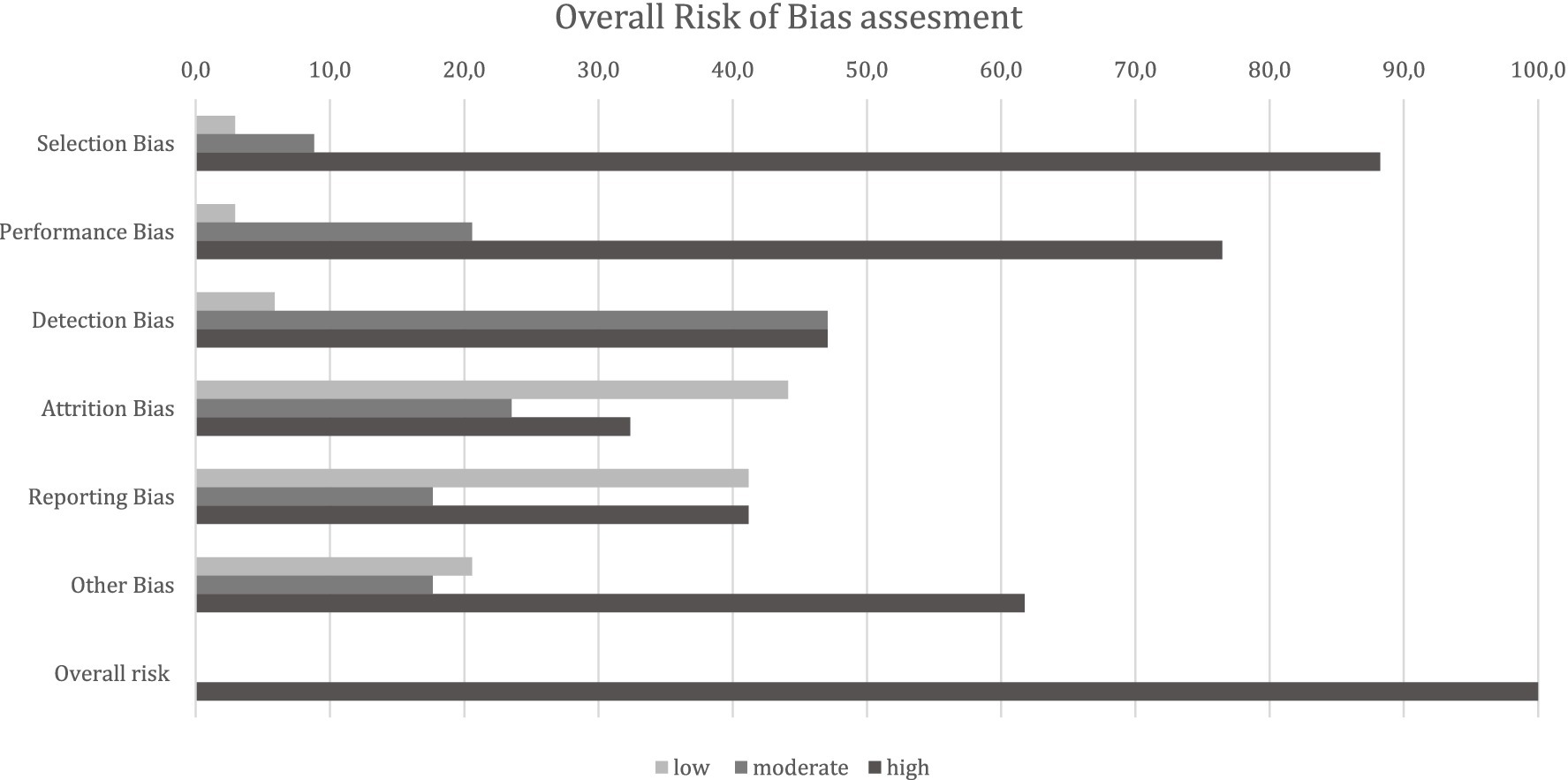
Figure 11. Risk of bias ratings per bias domain for all studies in the final selection stage (n = 32). The numbers shown are percentages of articles for each rating by domain.
4 Discussion
4.1 Identified BSMs in the context of specific pathogen control
4.1.1 Identified BSMs in the context of Salmonella control
Only two BSMs had sufficient data to perform a meta-analysis with respect to Salmonella spp. control and considering the production stage: acidification of water and feed. The meta-analysis revealed that farms that did not apply acidification of feed or water had about 4 times the odds of being Salmonella spp. favourable than farms which used acidification. This outcome was primarily based on observations made during the fattening stage and was, therefore, only “evidence-based” for this production stage. However, if one considers that some evidence from feed acidification at other stages of production found in the literature points in the same direction (33, 34), and if one considers the general hypothesis that acidification of the intestinal tract should prevent the colonisation of Salmonella spp. in the gastrointestinal tract of pigs (32), then acidification could be an effective BSM against Salmonella spp. at all stages throughout the pig production line. However, the studies in this analysis often lacked details on acid concentrations and application methods (to water or feed) and typically measured pathogen burden by bacteria count in pig tissues. As such, further research on the effects of organic acids on Salmonella spp. in feed and water would strengthen the evidence supporting their use as an effective BSM for controlling Salmonella spp. in pigs.
When ignoring the production stage, the meta-analysis showed one additional BSM to be effective against Salmonella spp., namely rodent control. The analysis showed that the reduction of the odds for Salmonella spp. in pigs for this BSM is similar to the one found for acidification. Rodents can acquire and carry Salmonella spp. infections for several months. They are considered highly efficient vectors and amplifiers of Salmonella spp. on farms. Due to their high carriage rates and rapid reproduction (48, 49). Mouse droppings can contain up to 104 CFU/g of Salmonella spp., and a single mouse can shed 100 faecal pellets per day (50). The importance of rodent control, specifically of rodent bait, on pig farms in limiting Salmonella spp. was recently confirmed in a cross-sectional study performed within the biosecurity practices for pig farming across Europe (BIOPIGEE) project (51).
4.1.2 Identified BSMs in the context of HEV control
There was not enough data in the included publications to perform a meta-analysis on BSMs for HEV. Therefore, the literature on this pathogen is discussed here qualitatively. The included publications report several external BSMs to be effective in preventing HEV from entering farms. The external BSM quarantining pigs may prevent direct and indirect transmission between pigs of different origins (52, 53). A European study of biosecurity measures to control HEV that was published after the current reviewing process was finalised, also found a quarantine area as a key biosecurity measure related to a lower HEV risk (54). Quarantine is a common practice before the introduction of gilts to the herd (55), and gilts are often purchased at an age category in which HEV is most prevalent (fattening period (6),). Therefore, quarantining may prevent transmission to other gilts and sows on the farm.
Indirect transmission of HEV between farms may be facilitated by vehicles, rodents, or people that enter multiple farms (15). Studies have found a significant reduction in the odds of a positive HEV farm when disinfecting vehicles, having a fence around the farm (53), and wearing farm-specific clothes or boots (20), which may all prevent the indirect transmission of HEV. Municipal water is more protective for HEVs than private wells. Possibly, private wells can be HEV contaminated, for instance, via run-off of water or manure (56). HEV is known to be prevalent (and probably survive) in water (57), and pig infection occurs orally (58). Still, more research is needed on how wells can become contaminated with HEV and whether water from the nozzle inside the pig barns is HEV contaminated.
Five BSMs are related to internal farm biosecurity, with the associated main categories being cleaning and disinfection, and avoiding mixing of pigs. Distances exceeding 0.8 meters between the manure pit and slatted floor, extended downtime periods, and frequent cleaning significantly increase the likelihood of farms testing negative for HEV. These measures align with strategies aimed at interrupting HEV transmission within farms by minimizing direct contact and faecal exposure. The effectiveness of implementing specific biosecurity measures to control HEV in pig farms may vary based on infection status, age group affected, farm type, and other farm-specific epidemiological factors (15). For instance, on farms with sporadic HEV outbreaks of the implementation of external measures may prevent new outbreaks from coming from outside. However, internal biosecurity measures may be more effective because the within-farm and farm-level prevalence of HEV are both high in majority of the European countries (6).
4.1.3 Risk of bias
The result of an overall high RoB in all studies in the final article selection requires caution regarding the use and interpretation of the results presented in our study. Here, it must be noted that the applied approach for assessing RoB was strict and comprehensive and that this outcome does not necessarily invalidate the results and conclusions of these studies. However, the result does highlight recurring issues that need to be addressed to aid future research in the context of BSM application and their effectiveness. Particularly in the selection of farm operations or animal groups, the consideration of potential confounders in connection with the outcome of the efficacy of BSMs, the sampling design, and the reporting of negative results ought to receive closer attention. Two other factors that contributed strongly to the rating of a high bias were the randomisation process in the selection bias domain and blinding in the detection and performance bias domain, especially in observational studies. Another aspect was that a statement about conflicts of interest was often missing in primary research articles, which also contributed to the outcome of a high risk of bias in several publications. However, in many cases, this was not a requirement by scientific journals at the time of publication.
The overall outcome of a high RoB for all studies emphasises that studies conducted on the effectiveness of BSMs often fail to reach the required quality, and reported results are problematic when it comes to a true meta-analytical approach. This may stem from the need for mandatory preregistration and protocol preparation in observational studies, leading to publication bias and selective outcome reporting. Moreover, the high RoB also reflects the absence of harmonised study designs and insufficient empirical evidence to support causal relationships between BSMs and pathogen reduction.
4.2 Generalisations and cross-protective effect of some BSMs across pathogens
The leading and potentially most effective BSMs for Salmonella spp. and HEV were all related to preventing indirect contact between pig herds. Indirect contact between herds is possible via persons, and the BSMs related to such indirect contact were either related to showering, wearing farm-specific clothes and/or boots, or a combination of these two BSMs. According to a review on biosecurity in pig production (13), hand-washing and outwear changes are probably effective for many pathogens to prevent transmission into a new herd. A higher level of biosecurity would include a compulsory shower (13). Experimental studies showed that showering and changing outwear could prevent Foot-and-Mouth disease virus transmission between infected and susceptible pigs (59). Nevertheless, a study in 2008 in Belgian pig herds reported that only 2% of pig farmers used showering before entering. This low percentage may apply to other countries as well (60). Since BSMs related to humans entering the farm have shown to be effective for multiple pathogens (38, 59), complete showering and changing outwear could be advised to farmers aiming to improve their level of biosecurity.
Acidification of feed and water was found as a protective measure in relation to Salmonella spp. presence on farms. However, these measures are only relevant if Salmonella is already present, yet they cannot prevent the introduction of Salmonella on farms. Therefore, when veterinarians use the current meta-analysis to advise their clients about Salmonella control, they must first assess whether the focus must be on prevention of introduction or reduction in prevalence. If the primary focus is on preventing Salmonella introduction, a farm-specific assessment of potential breaches in external biosecurity for all production stages is advised. The effectiveness of acidification to prevent HEV infection is irrelevant, as viruses cannot colonise tissues, and HEV primarily infects hepatocytes of the liver, instead of enterocytes of the gastrointestinal tract like Salmonella spp. (61).
4.2.1 Counterintuitive associations
While cleaning and disinfection are established, parts of Salmonella spp. control, when aggregating the data we found, there was no evidence of a positive effect on Salmonella spp. prevalence in pens/farms. The forest plot (Supplementary Figure S3B) revealed a split amongst the observations: four indicated a statistically non-significant protective effect, while four suggested disinfections as a risk factor. The two observations with the largest sample size belong to the four observations identifying disinfection as a risk factor. However, these two observations came from the same publication (S_MA12 (45)) and are therefore not independent. The following issues contributed to the fact that the aggregated data on disinfection only provided an inconclusive result. Of the six articles investigating the role of disinfection on Salmonella spp. prevalence on farms, only one actively detailed the procedure for disinfecting pens in their study. As many of the articles also relied on farmers’ self-reporting their protocols, the introduction of information bias may explain the results of these studies.
4.3 Limitations and strengths
4.3.1 Limitations
This systematic review identified biosecurity measures (BSMs) likely to reduce Salmonella spp. or HEV occurrence on farms. However, many BSMs were supported by only a single observation in one article, preventing an accurate assessment of their true effectiveness due to potential bias in included studies. The scarcity of observations per BSM in the literature may be due to publication bias, favouring the publication of novel findings over studies that confirm or refute existing evidence (11). Also, BSMs incorporated in multiple studies may have been assessed differently per study (i.e., the question or answer options were different), leading to some variation of the results between studies. Finally, it is essential to acknowledge that some information on BSMs against our pathogens of interest may have been overlooked for several reasons. For instance, this study considered only peer-reviewed publications and only articles available in the native language of at least one of the study authors, which may cause an information bias for “non-usual” European languages. Additionally, case reports describing the effect of depopulation in eradicating a pathogen from a herd (62) were not included in the meta-analysis as the effect of this measure could not be quantified despite depopulation being potentially a highly effective measure (63). Moreover, publication bias may have resulted in missing data on the ineffectiveness of certain BSMs. This bias can occur in meta-analyses and literature reviews, leading to gaps in knowledge. By restraining our literature search to only published and peer-reviewed articles, we aimed to target high-quality research. However, this approach may have excluded unpublished data or grey literature, potentially leading to an overrepresentation of positive findings, as negative results are less likely to be published in peer-reviewed journals.
4.3.2 Strengths
In systematic quantitative reviews and meta-analyses, RoB assessment is crucial but often absent in traditional narrative or scoping reviews, representing the vast majority of publications on farm biosecurity. This study is likely the first to include a RoB assessment of primary studies on specific BSMs. It adds a layer of scrutiny to their effectiveness in reducing on-farm pathogen occurrence. Additionally, this review introduced an innovative use of AI software for screening titles and abstracts, alongside manual reviewing, to reduce the time to screen articles without losing screening sensitivity. ASReview has been used in more than 10 systematic reviews since its launch in 2021 (64). The decision about when to stop screening abstracts using ASReview was recently investigated and was proposed to be carried out after at least 10% of the abstracts is screened and 50 consecutive abstracts are marked as irrelevant by the reviewer (21). Our screening of 13% of abstracts and stopping after 100 irrelevant abstracts can therefore be seen as a safe and conservative approach, minimizing the risk of missing relevant articles. Although the sensitivity of the method depends on the clarity of the research question, the choice of relevant articles at the start of the reviewing process, and the reliability of the researcher that does the screening, we also traditionally screened all articles, so the articles that may have been missed using the AI software could be included using the other method. Additionally, research has shown that medical scientific researchers also falsely in- and exclude almost 11% of studies, showing that traditional reviewing is not better per se (65). Our combination of the two methods may be ideal.
Majority of the final publications in our study were observational studies, which were inherently prone to bias and confounding. This impaired assessing a causal relationship between intervention measures and pathogen reduction (66, 67).
However, through the rigorous application of the RoB methodology (see Methodology section for comprehensive details), the conclusions drawn from our study maintained their robustness and validity.
4.4 Recommendations and future outlook
In general, the literature examined here provided limited evidence-based knowledge about the control of Salmonella spp., its introduction, and its spread on farms. There remains a lack of scientific evidence on areas such as the method of animal purchase or feed storage. These cannot be supplemented with findings on protection from HEV, for which evidence was even more scarce. The categorisation of the effectiveness of BSMs in this review helped to highlight which measures should be investigated further as a matter of top priority. In addition, accurate reporting of the methodology and results of experimental and observational studies examining the efficacy of BSMs against pathogens on farms would further improve the evidence base and allow for further prioritisation of specific BSMs.
This review, highlights the following:
• There was a lack of knowledge of BSMs, particularly against HEV.
• Further research should examine two of the BSMs found to be non-significant in the meta-analysis of the “ignoring production stage” approach, that is, all-in and all-out production and disinfection for Salmonella spp. This may settle the question of whether these BSMs are truly effective, while requiring less effort compared to other BSMs with less data available for evidence synthesis.
• The cost-effectiveness of implementing food and water acidification, based on systematic and standardised application of acids, as well as rodent control measures on farms, should also be assessed urgently to provide farmers with the knowledge and tools for implementation.
• Available literature often lacks quality and carries a high bias risk. Future research on BSMs in swine production and peer-review processes should prioritise standardised reporting. To further improve the scientific evidence regarding (farm) animal and zoonotic diseases epidemiology and the effectiveness of respective BSMs, standardized/harmonized study designs based on guidelines (including checklists such as COHERE (68) may foster the quality of studies in this field, reduce risk of bias and facilitate comparability of BSMs between studies). Additionally, using guidelines such as STROBE-vet (69) will help reduce bias when reporting the study design and participant selection.
In summary, further research is essential to assess BSMs’ efficacy in reducing pathogen burden on pig farms, enhancing food safety, and minimizing public health risks. Implementing studies within national control programs, enhancing sample sizes, and incorporating power-based estimates can improve evidence quality. This review also highlights the need for more studies on preventive measures as well as field and experimental studies that can confirm the protectiveness of the BSMs that have not been covered in this review. For instance, comparing various cleaning and disinfection methods and addressing water contamination within farms.
Moreover, it is worth mentioning that the results of this literature review were used to generate two self-assessment checklists that aim to support pig farmers in identifying possible gaps in their on-farm implemented biosecurity towards Salmonella spp. and HEV. These checklists are open-access and available in eight European languages (70).
In conclusion, our systematic review and meta-analysis emphasise the importance of dedicating more effort and conducting further research to truly understand the effectiveness of BSMs against zoonotic pathogens in swine farms. This study provides a starting point by identifying BSMs that should be given priority for additional investigation, as well as those that could offer benefits for use on farms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
NH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Visualization. MM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. ES: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing, Visualization, Conceptualization, Funding acquisition. EW: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Formal analysis, Supervision. GK-V: Data curation, Writing – original draft, Writing – review & editing, Investigation. GA: Conceptualization, Data curation, Funding acquisition, Writing – original draft, Writing – review & editing, Investigation, Methodology. DD΄A: Data curation, Writing – original draft, Writing – review & editing, Investigation. VZ-G: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition. SS: Data curation, Writing – original draft, Writing – review & editing. EM: Data curation, Writing – original draft, Writing – review & editing. RS: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing, Methodology. EB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. GC: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Data curation, Visualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was part of the European One Health EJP Consortium, which is funded by the European Union’s Horizon 2020 research and innovation programme under Grant Agreement Number 773830. In the frame of this study, Nikolaus Huber was funded by VET-Austria, a cooperation between the Austrian Federal Ministry of Health, the Austrian Agency for Health and Food Safety, and the University of Veterinary Medicine, Vienna, Austria.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1494870/full#supplementary-material
References
1. Libera, K, Konieczny, K, Grabska, J, Szopka, W, Augustyniak, A, and Pomorska-Mól, M. Selected livestock-associated Zoonoses as a growing challenge for public health. Infect Dis Rep. (2022) 14:63–81. doi: 10.3390/idr14010008
2. EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control). The European Union one health 2020 Zoonoses report. EFSA J. (2021) 19:e06971. doi: 10.2903/j.efsa.2021.6971
3. USDA (United States Department of Agriculture) (2018). Cost estimates of foodborne illness. Available at: https://www.ers.usda.gov/data-products/cost-estimates-of-foodborne-illnesses/
4. Grierson, S, Heaney, J, Cheney, T, Morgan, D, Wyllie, S, Powell, L, et al. Prevalence of hepatitis E virus infection in pigs at the time of slaughter, United Kingdom, 2013. Emerg Infect Dis. (2015) 21:1396–401. doi: 10.3201/eid2108.141995
5. Meester, M, Bouwknegt, M, Hakze-van der Honing, R, Vernooij, H, Houben, M, van Oort, S, et al. Repeated cross-sectional sampling of pigs at slaughter indicates varying age of hepatitis E virus infection within and between pig farms. Vet Res. (2022) 53:50. doi: 10.1186/s13567-022-01068-3
6. Salines, M, Andraud, M, and Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: a comprehensive review. Vet Res. (2017) 48:31. doi: 10.1186/s13567-017-0436-3
7. EFSA (European Food Safety Authority), Panel on Biological HazardsRicci, A, Allende, A, Bolton, D, Chemaly, M, et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. (2017) 15:e04886. doi: 10.2903/j.efsa.2017.4886
8. Kamar, N, and Pischke, S. Acute and persistent hepatitis E virus genotype 3 and 4 infection: clinical features, pathogenesis, and treatment. Cold Spring Harb Perspect Med. (2019) 9:1872. doi: 10.1101/cshperspect.a031872
9. WHO (World Health Organisation) (2022). Hepatitis E [Webpage]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (Accessed November 15, 2022).
10. Vora, NM, Hannah, L, Lieberman, S, Vale, MM, Plowright, RK, and Bernstein, AS. Want to prevent pandemics? Stop spillovers. Nature. (2022) 605:419–22. doi: 10.1038/d41586-022-01312-y
11. Huber, N, Andraud, M, Sassu, EL, Prigge, C, Zoche-Golob, V, Käsbohrer, A, et al. What is a biosecurity measure? A definition proposal for animal production and linked processing operations. One Health. (2022) 15:100433. doi: 10.1016/j.onehlt.2022.100433
12. Aiyar, A, and Pingali, P. Pandemics and food systems - towards a proactive food safety approach to disease prevention & management. Food Secur. (2020) 12:749–56. doi: 10.1007/s12571-020-01074-3
13. Alarcón, LV, Allepuz, A, and Mateu, E. Biosecurity in pig farms: a review. Porcine Health Manag. (2021) 7:5. doi: 10.1186/s40813-020-00181-z
14. Andres, VM, and Davies, RH. Biosecurity measures to control Salmonella and other infectious agents in pig farms: a review. Compr Rev Food Sci F. (2015) 14:317–35. doi: 10.1111/1541-4337.12137
15. Meester, M, Tobias, TJ, Bouwknegt, M, Kusters, NE, Stegeman, JA, and van der Poel, WHM. Infection dynamics and persistence of hepatitis E virus on pig farms – a review. Porcine Health Manag. (2021) 7:16. doi: 10.1186/s40813-021-00189-z
16. Rodrigues da Costa, M, Pessoa, J, Meemken, D, and Nesbakken, T. A systematic review on the effectiveness of pre-harvest meat safety interventions in pig herds to control Salmonella and other foodborne pathogens. Microorganisms. (2021) 9:1825. doi: 10.3390/microorganisms9091825
17. van de Schoot, R, de Bruin, J, Schram, R, Zahedi, P, de Boer, J, Weijdema, F, et al. An open source machine learning framework for efficient and transparent systematic reviews. Nat Mach Intell. (2021) 3:125–33. doi: 10.1038/s42256-020-00287-7
18. Gotter, V, Klein, G, Koesters, S, Kreienbrock, L, Blaha, T, and Campe, A. Main risk factors for Salmonella-infections in pigs in North-Western Germany. Prev Vet Med. (2012) 106:301–7. doi: 10.1016/j.prevetmed.2012.03.016
19. Amass, S, Halbur, P, Byrne, B, Schneider, J, Koons, C, Cornick, N, et al. Mechanical transmission of enterotoxigenic Escherichia coli to weaned pigs by people, and biosecurity procedures that prevented such transmission. J Swine Health Prod. (2003) 11:61–8. doi: 10.54846/jshap/365
20. Walachowski, S, Dorenlor, V, Lefevre, J, Lunazzi, A, Eono, F, Merbah, T, et al. Risk factors associated with the presence of hepatitis E virus in livers and seroprevalence in slaughter-age pigs: a retrospective study of 90 swine farms in France. Epidemiol Infect. (2013) 142:1934–44. doi: 10.1017/S0950268813003063
21. Boetje, J, and van de Schoot, R. The SAFE procedure: a practical stopping heuristic for active learning-based screening in systematic reviews and meta-analyses. Syst Rev. (2024) 13:81. doi: 10.1186/s13643-024-02502-7
22. R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
23. Viechtbauer, W. Conducting Meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
24. Tufanaru, C, Munn, Z, Stephenson, M, and Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. (2015) 13:196–207. doi: 10.1097/XEB.0000000000000065
25. Jackson, D, and Turner, R. Power analysis for random-effects meta-analysis. Res Synth Methods. (2017) 8:290–302. doi: 10.1002/jrsm.1240
26. Deeks, J, Higgins, J, and Altman, D. (2023). Chapter 10: Analysing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of interventions version 64 (updated august 2023) [internet]. Available at: www.training.cochrane.org/handbook (Accessed April 17, 2024)/
27. Kim, SY, Park, JE, Lee, YJ, Seo, HJ, Sheen, SS, Hahn, S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. (2013) 66:408–14. doi: 10.1016/j.jclinepi.2012.09.016
28. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ Clin Res Ed. (2011) 343:d5928. doi: 10.1136/bmj.d5928
29. Argüello, H, Carvajal, A, Costillas, S, and Rubio, P. Effect of the addition of organic acids in drinking water or feed during part of the finishing period on the prevalence of Salmonella in finishing pigs. Foodborne Pathog Dis. (2013) 10:842–9. doi: 10.1089/fpd.2013.1497
30. Creus, E, Pérez, JF, Peralta, B, Baucells, F, and Mateu, E. Effect of acidified feed on the prevalence of Salmonella in market-age pigs. Zoonoses Public Health. (2007) 54:314–9. doi: 10.1111/j.1863-2378.2007.01069.x
31. Willamil, J, Creus, E, Pérez, J, Mateu, E, and Martín-Orúe, S. Effect of a microencapsulated feed additive of lactic and formic acid on the prevalence of Salmonella in pigs arriving at the abattoir. Arch Anim Nutr. (2011) 65:431–44. doi: 10.1080/1745039X.2011.623047
32. van der Wolf, PJ, van Schie, FW, Elbers, ARW, Engel, B, van der Heijden, HMJF, Hunneman, WA, et al. Administration of acidified drinking water to finishing pigs in order to prevent salmonella infections. Vet Q. (2001) 23:121–5. doi: 10.1080/01652176.2001.9695097
33. EFSA (European Food Safety Authority). Analysis of the baseline survey on the prevalence of Salmonella in holdings with breeding pigs, in the EU, 2008 - part B: factors associated with Salmonella pen positivity. EFSA J. (2011) 9:2329. doi: 10.2903/j.efsa.2011.2329
34. Ruggeri, J, Foresti, F, Pavesi, R, Terrini, A, Giudici, F, Padoan, D, et al. The synergistic effect of organic acids, phytochemicals and a permeabilizing complex reduces Salmonella Typhimurium 1,4,[5],12:i-shedding in pigs. Vet Res Commun. (2018) 42:209–17. doi: 10.1007/s11259-018-9723-3
35. Cardinale, E, Maeder, S, Porphyre, V, and Debin, M. Salmonella in fattening pigs in Reunion Island: herd prevalence and risk factors for infection. Prev Vet Med. (2010) 96:281–5. doi: 10.1016/j.prevetmed.2010.06.006
36. Correia-Gomes, C, Economou, T, Mendonça, D, Vieira-Pinto, M, and Niza-Ribeiro, J. Assessing risk profiles for Salmonella serotypes in breeding pig operations in Portugal using a Bayesian hierarchical model. BMC Vet Res. (2012) 8:226. doi: 10.1186/1746-6148-8-226
37. Rajić, A, O'Connor, BP, Deckert, AE, Keenliside, J, McFall, ME, Reid-Smith, RJ, et al. Farm-level risk factors for the presence of Salmonella in 89 Alberta swine-finishing barns. Can J Vet Res. (2007) 71:264–70.
38. San Román, B, Garrido, V, Sánchez, S, Martínez-Ballesteros, I, Garaizar, J, Mainar-Jaime, RC, et al. Relationship between Salmonella infection, shedding and serology in fattening pigs in low–moderate prevalence areas. Zoonoses Public Health. (2018) 65:481–9. doi: 10.1111/zph.12453
39. Vico, JP, Rol, I, Garrido, V, Román, BS, Grilló, MJ, and Mainar-Jaime, RC. Salmonellosis in finishing pigs in Spain: prevalence, antimicrobial agent susceptibilities, and risk factor analysis. J Food Prot. (2011) 74:1070–8. doi: 10.4315/0362-028X.JFP-10-515
40. von Altrock, A, Schütte, A, and Hildebrandt, G. Results of the German investigation on the EU project "Salmonella in pork (Salinpork)"--1. Investigations in the farms. Berl Munch Tierarztl Wochenschr. (2000) 113:191–201.
41. Davies, PR, Morrow, WEM, Jones, FT, Deen, J, Fedorka-Cray, PJ, and Harris, IT. Prevalence of salmonella in finishing swine raised in different production systems in North Carolina, USA. Epidemiol Infect. (1997) 119:237–44. doi: 10.1017/S095026889700784X
42. Dors, A, Pomorska-Mól, M, Czyżewska, E, Wasyl, D, and Pejsak, Z. Prevalence and risk factors for Lawsonia intracellularis, Brachyspira hyodysenteriae and Salmonella spp. in finishing pigs in polish farrow-to-finish swine herds. Pol J Vet Sci. (2015) 18:825–31. doi: 10.1515/pjvs-2015-0107
43. Stege, H, Christensen, J, Nielsen, JP, and Willeberg, P. Data-quality issues and alternative variable-screening methods in a questionnaire-based study on subclinical Salmonella enterica infection in Danish pig herds. Prev Vet Med. (2001) 48:35–54. doi: 10.1016/S0167-5877(00)00182-3
44. Fablet, C, Fravalo, P, Jolly, J-P, Eveno, E, Madec, F, and Beloeil, P-A. Recherche des facteurs de risque de l’excrétion de Salmonella enterica par les porcs en croissance. Enquête épidémiologique analytique en élevage naisseur-engraisseur. Epidemiol Santé Animale. (2003):43.
45. Martelli, F, Lambert, M, Butt, P, Cheney, T, Tatone, FA, Callaby, R, et al. Evaluation of an enhanced cleaning and disinfection protocol in Salmonella contaminated pig holdings in the United Kingdom. PLoS One. (2017) 12:e0178897. doi: 10.1371/journal.pone.0178897
46. van der Wolf, PJ, Wolbers, WB, Elbers, ARW, van der Heijden, HMJF, Koppen, JMCC, Hunneman, WA, et al. Herd level husbandry factors associated with the serological Salmonella prevalence in finishing pig herds in the Netherlands. Vet Microbiol. (2001) 78:205–19. doi: 10.1016/S0378-1135(00)00294-7
47. Sterne, JA, Gavaghan, D, and Egger, M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. (2000) 53:1119–29. doi: 10.1016/S0895-4356(00)00242-0
48. Umali, DV, Lapuz, RR, Suzuki, T, Shirota, K, and Katoh, H. Transmission and shedding patterns of Salmonella in naturally infected captive wild roof rats (Rattus rattus) from a Salmonella-contaminated layer farm. Avian Dis. (2012) 56:288–94. doi: 10.1637/9911-090411-Reg.1
49. Rebeca, Z-S, and Andrea, MA. Preharvest Salmonella risk contamination and the control strategies In: M Mihai, editor. Current topics in Salmonella and salmonellosis. Rijeka: IntechOpen (2017). p. Ch. 11.
50. Henzler, DJ, and Opitz, HM. The role of mice in the epizootiology of Salmonella enteritidis infection on chicken layer farms. Avian Dis. (1992) 36:625–31. doi: 10.2307/1591757
51. Smith, RP, May, HE, Burow, E, Meester, M, Tobias, TJ, Sassu, EL, et al. Assessing pig farm biosecurity measures for the control of Salmonella on European farms. Epidemiol Infect. (2023) 151:e130. doi: 10.1017/S0950268823001115
52. Lopez-Lopez, P, Risalde, MLA, Frias, M, García-Bocanegra, I, Brieva, T, Caballero-Gomez, J, et al. Risk factors associated with hepatitis E virus in pigs from different production systems. Vet Microbiol. (2018) 224:88–92. doi: 10.1016/j.vetmic.2018.08.020
53. Caruso, C, Peletto, S, Rosamilia, A, Modesto, P, Chiavacci, L, Sona, B, et al. Hepatitis E virus: a cross-sectional serological and virological study in pigs and humans at zoonotic risk within a high-density pig farming area. Transbound Emerg Dis. (2017) 64:1443–53. doi: 10.1111/tbed.12533
54. Dubbert, T, Meester, M, Smith, RP, Tobias, TJ, Di Bartolo, I, Johne, R, et al. Biosecurity measures to control hepatitis E virus on European pig farms. Front Vet Sci. (2024) 11:11. doi: 10.3389/fvets.2024.1441544
55. Bernaerdt, E, Dewulf, J, Verhulst, R, Bonckaert, C, and Maes, D. Purchasing policy, quarantine and acclimation practices of breeding gilts in Belgian pig farms. Porc Health Manag. (2021) 7:25. doi: 10.1186/s40813-021-00205-2
56. Steyer, A, Naglič, T, Močilnik, T, Poljšak-Prijatelj, M, and Poljak, M. Hepatitis E virus in domestic pigs and surface waters in Slovenia: prevalence and molecular characterization of a novel genotype 3 lineage. Infect Genet Evol. (2011) 11:1732–7. doi: 10.1016/j.meegid.2011.07.007
57. Takuissu, GR, Kenmoe, S, Ndip, L, Ebogo-Belobo, JT, Kengne-Ndé, C, Mbaga, DS, et al. Hepatitis E virus in water environments: a systematic review and Meta-analysis. Food Env Virol. (2022) 14:223–35. doi: 10.1007/s12560-022-09530-3
58. Kasorndorkbua, C, Guenette, DK, Huang, FF, Thomas, PJ, Meng, XJ, and Halbur, PG. Routes of transmission of swine hepatitis E virus in pigs. J Clin Microbiol. (2004) 42:5047–52. doi: 10.1128/JCM.42.11.5047-5052.2004
59. Amass, SF, Pacheco, JM, Mason, PW, Schneider, JL, Alvarez, RM, Clark, LK, et al. Procedures for preventing the transmission of foot-and-mouth disease virus to pigs and sheep by personnel in contact with infected pigs. Vet Rec. (2003) 153:137–40. doi: 10.1136/vr.153.5.137
60. Ribbens, S, Dewulf, J, Koenen, F, Mintiens, K, De Sadeleer, L, de Kruif, A, et al. A survey on biosecurity and management practices in Belgian pig herds. Prev Vet Med. (2008) 83:228–41. doi: 10.1016/j.prevetmed.2007.07.009
61. Todt, D, Friesland, M, Moeller, N, Praditya, D, Kinast, V, Brüggemann, Y, et al. Robust hepatitis E virus infection and transcriptional response in human hepatocytes. PNAS. (2020) 117:1731–41. doi: 10.1073/pnas.1912307117
62. Erdman, MM, Harris, IT, Torremorell, M, Wilt, VM, and Harris, DLH. Occurrence of Salmonella serotype typhimurium DT104 on a commercial swine farm before, during, and after depopulation and repopulation. J Am Vet Med Assoc. (2005) 227:460–6. doi: 10.2460/javma.2005.227.460
63. Dee, SA, Joo, HS, Polson, DD, Park, BK, Pijoan, C, Molitor, TW, et al. Evaluation of the effects of nursery depopulation on the persistence of porcine reproductive and respiratory syndrome virus and the productivity of 34 farms. Vet Rec. (1997) 140:247–8. doi: 10.1136/vr.140.10.247
64. Zotero (2024). ASReview publications [internet]. Available at: https://www.zotero.org/groups/4597652/asreview_public/library
65. Wang, Z, Nayfeh, T, Tetzlaff, J, O'Blenis, P, and Murad, MH. Error rates of human reviewers during abstract screening in systematic reviews. PLoS One. (2020) 15:e0227742. doi: 10.1371/journal.pone.0227742
66. Metelli, S, and Chaimani, A. Challenges in meta-analyses with observational studies. Evid-Based Ment Health. (2020) 23:83–7. doi: 10.1136/ebmental-2019-300129
67. Mueller, M, D’Addario, M, Egger, M, Cevallos, M, Dekkers, O, Mugglin, C, et al. Methods to systematically review and meta-analyse observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol. (2018) 18:44. doi: 10.1186/s12874-018-0495-9
68. Davis, MF, Rankin, SC, Schurer, JM, Cole, S, Conti, L, and Rabinowitz, P. Checklist for one health epidemiological reporting of evidence (COHERE). One Health. (2017) 4:14–21. doi: 10.1016/j.onehlt.2017.07.001
69. Sargeant, JM, O'Connor, AM, Dohoo, IR, Erb, HN, Cevallos, M, Egger, M, et al. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology - veterinary (STROBE-vet) statement. Prev Vet Med. (2016) 134:188–96. doi: 10.1016/j.prevetmed.2016.09.005
70. Burow, E, Smith, RP, Tobias, TJ, Meester, M, Sassu, E, and Prigge, C. (2022). OHEJP BIOPIGEE checklists for Salmonella and HEV. Zenodo.
71. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ Clin Res Ed. (2021) 372:n71. doi: 10.1136/bmj.n71
72. Belœil, PA, Fravalo, P, Fablet, C, Jolly, JP, Eveno, E, Hascoet, Y, et al. Risk factors for Salmonella enterica subsp. enterica shedding by market-age pigs in French farrow-to-finish herds. Prev Vet Med. (2004) 63:103–20. doi: 10.1016/j.prevetmed.2004.01.010
73. Twomey, DF, Miller, AJ, Snow, LC, Armstrong, JD, Davies, RH, Williamson, SM, et al. Association between biosecurity and Salmonella species prevalence on English pig farms. Vet Rec. (2010) 166:722–4. doi: 10.1136/vr.b4841
74. Meyer, C, Beilage, E, and Krieter, J. Salmonella seroprevalence in different pig production systems. Tierarztl Prax Ausg G: Grosstiere/Nutztiere. (2005) 33:104–12. doi: 10.1055/s-0038-1624096
75. Poljak, Z, Dewey, CE, Friendship, RM, Martin, SW, and Christensen, J. Multilevel analysis of risk factors for Salmonella shedding in Ontario finishing pigs. Epidemiol Infect. (2008) 136:1388–400. doi: 10.1017/S0950268807009855
76. García-Feliz, C, Carvajal, A, Collazos, JÁ, and Rubio, P. Herd-level risk factors for faecal shedding of Salmonella enterica in Spanish fattening pigs. Prev Vet Med. (2009) 91:130–6. doi: 10.1016/j.prevetmed.2009.05.011
77. Davies, PR, Morrow, WE, Jones, FT, Deen, J, Fedorka-Cray, PJ, and Gray, JT. Risk of shedding Salmonella organisms by market-age hogs in a barn with open-flush gutters. J Am Vet Med Assoc. (1997) 210:386–9. doi: 10.2460/javma.1997.210.03.386
78. Wilkins, W, Rajić, A, Waldner, C, McFall, M, Chow, E, Muckle, A, et al. Distribution of Salmonella serovars in breeding, nursery, and grow-to-finish pigs, and risk factors for shedding in ten farrow-to-finish swine farms in Alberta and Saskatchewan. Can J Vet Res. (2010) 74:81–90.
79. Cevallos-Almeida, M, Fablet, C, Houdayer, C, Dorenlor, V, Eono, F, Denis, M, et al. Longitudinal study describing time to Salmonella seroconversion in piglets on three farrow-to-finish farms. Vet Rec Open. (2019) 6:e000287. doi: 10.1136/vetreco-2018-000287
80. Smith, RP, Andres, V, Cheney, TE, Martelli, F, Gosling, R, Marier, E, et al. How do pig farms maintain low Salmonella prevalence: a case-control study. Epidemiol Infect. (2018) 146:1909–15. doi: 10.1017/S0950268818002248
Keywords: biosecurity, swine herds, interventions, zoonoses, meta-analysis, risk reduction, HEV, Salmonella spp.
Citation: Huber N, Meester M, Sassu EL, Waller ESL, Krumova-Valcheva G, Aprea G, D’Angelantonio D, Zoche-Golob V, Scattolini S, Marriott E, Smith RP, Burow E and Carreira GC (2024) Biosecurity measures reducing Salmonella spp. and hepatitis E virus prevalence in pig farms—a systematic review and meta-analysis. Front. Vet. Sci. 11:1494870. doi: 10.3389/fvets.2024.1494870
Edited by:
Christopher Hamilton-West, University of Chile, ChileReviewed by:
Folorunso Oludayo Fasina, University of Pretoria, South AfricaRita Albernaz-Gonçalves, Instituto Federal Catarinense, Brazil
Copyright © 2024 Huber, Meester, Sassu, Waller, Krumova-Valcheva, Aprea, D’Angelantonio, Zoche-Golob, Scattolini, Marriott, Smith, Burow and Carreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina Meester, bS5tZWVzdGVyQHV1Lm5s
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Nikolaus Huber
Nikolaus Huber Marina Meester
Marina Meester Elena L. Sassu3†
Elena L. Sassu3† Elisabeth S. L. Waller
Elisabeth S. L. Waller Gergana Krumova-Valcheva
Gergana Krumova-Valcheva Giuseppe Aprea
Giuseppe Aprea Daniela D’Angelantonio
Daniela D’Angelantonio Silvia Scattolini
Silvia Scattolini Richard P. Smith
Richard P. Smith Guido Correia Carreira
Guido Correia Carreira