- 1Primate Resources Center, Korea Research Institute of Bioscience and Biotechnology, Jeongeup, Republic of Korea
- 2Department of Laboratory Animal Medicine, College of Veterinary Medicine, Jeonbuk National University, Iksan, Republic of Korea
Introduction: Assisted reproductive technologies (ARTs), such as intracytoplasmic sperm injection and embryo transfer, are essential for generating genetically edited monkeys. Despite their importance, ARTs face challenges in recipient selection in terms of time and the number of animals required. The potential of superovulated monkeys, commonly used as oocyte donors, to serve as surrogate mothers, remains underexplored. The study aimed to compare the efficacy of superovulated and uterine-embryo synchronized recipients of embryo transfer in cynomolgus monkeys (Macaca fascicularis).
Methods: This study involved 23 cynomolgus monkeys divided into two groups–12 superovulated recipients and 11 synchronized recipients. The evaluation criteria included measuring endometrial thickness on the day of embryo transfer and calculating pregnancy and implantation rates to compare outcomes between groups.
Results: The study found no statistically significant differences in endometrial thickness (superovulated: 4.48 ± 1.36 mm, synchronized: 5.15 ± 1.58 mm), pregnancy rates (superovulated: 30.8%, synchronized: 41.7%), and implantation rates (superovulated: 14.3%, synchronized: 21.9%) between the groups (p > 0.05).
Conclusion: The observations indicate that superovulated recipients are as effective as synchronized recipients for embryo transfer in cynomolgus monkeys. This suggests that superovulated recipients can serve as viable options, offering an efficient and practical approach to facilitate the generation of gene-edited models in this species.
1 Introduction
Advanced gene-editing technologies such as CRISPR/Cas9 play a pivotal role in generating gene-edited animal models (1), enabling precise modifications in animal embryos. Traditionally, these models have been developed using mice, favoring genetic tractability and cost-effectiveness. However, nonhuman primates (NHPs), which closely mirror humans, offer a more accurate representation of human diseases (2). This feature has been underscored in several studies that have successfully developed gene-edited NHP models (3–6).
Assisted reproductive technologies (ARTs), initially devised to treat infertility in humans, have significantly broadened their applications to include the development of gene-edited animals. These technologies, including in vitro fertilization, intracytoplasmic sperm injection (ICSI), and embryo transfer, have been established in NHPs (7–10). However, the development of ARTs has been slow due to financial constraints, limited resources, and the complexity of the procedures.
Cynomolgus monkeys (Macaca fascicularis) are preferred for gene-edited NHP models due to their continuous breeding capability, suitable size, and similarities to humans in terms of their reproductive cycles and uterine structure (11). The successful generation of gene-edited cynomolgus monkeys conventionally requires superovulated females for oocyte donation and uterine-embryo synchronized recipients for embryo transfer. This approach can, however, pose challenges because synchronizing the embryo stage with the cycle phase of the recipient candidate is not always straightforward and often necessitates a larger number of female monkeys. Selecting female recipients using a more direct and efficient method for embryo transfer is, therefore, crucial. Very few reports have described the selection of recipients for embryo transfer during the generation of cynomolgus monkeys. Moreover, no previous studies have used donors as recipients for embryo transfer in this species. Research has only reported similar practices in other laboratory animals such as dogs and marmosets (12, 13).
This study, therefore, aimed to compare the efficacy of superovulated and uterine-embryo-synchronized recipients in cynomolgus monkeys for embryo transfer, an essential step in ARTs. By investigating the effects of superovulation on the condition of recipients and pregnancy outcomes, this study sought to enhance the efficiency of developing genetically edited animals using cynomolgus monkeys and contribute to advancing ARTs in this species.
2 Materials and methods
2.1 Animals
Sexually mature cynomolgus monkeys (88–116 months old) were imported from China by Biomedical Research and housed at the Primate Resources Center (Jeongeup, South Korea). They were individually caged in a room maintained at a temperature of 23 ± 3°C and a humidity of 55 ± 15%. The lighting was regulated on a 12-h light/12-h dark cycle. The monkeys had ad libitum access to water and were fed a primate-specific diet supplemented with multivitamins twice daily, with fruits or vegetables provided once daily. Qualified animal caretakers closely monitored all the animals at least twice daily for injuries and illnesses. Additionally, any abnormalities, including signs of pain and unusual behavior, were promptly reported to the veterinarians. Health and medical records were obtained for each animal. All the necessary steps were taken to ensure their well-being and minimize any potential stress or discomfort. All animal procedures performed in this research were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology (approval numbers: KRIBB-AEC-21306, KRIBB-AEC-24098).
2.2 Ovarian stimulation and oocyte recovery
The ovarian stimulation protocol was adapted from previously published studies (14), as illustrated in Figures 1A,B. The regimen included the administration of a gonadotropin-releasing hormone (GnRH) antagonist, ganirelix (Orgalutran Inj, ORGANON, Seoul, Korea), at a dosage of 0.125 mg once daily, and recombinant human follicle-stimulating hormone (hFSH) (Gonal-F Pen, Merck, Serono, Italy) at 37.5 IU twice daily intramuscularly on days 1–6. Human menopausal gonadotropin (IVF-M HP, LG Chem, Cheongju, Korea) was administered at 37.5 IU twice daily intramuscularly on days 7–9. Human chorionic gonadotropin (hCG) (chorionic gonadotropin human, Sigma) was administered intramuscularly at a dose of 1,000 IU 36–38 h before oocyte recovery on day 9. Immediately before oocyte recovery, the developmental status of the follicles was confirmed via ultrasonography (USG), and females with a poor response to stimulation were excluded. During oocyte recovery, the monkeys were anesthetized with an intramuscular dose of 5 mg/kg Zoletil® 50 (Virbac, Carros, France). The ovaries were exposed through an incision in the middle of the lower abdomen, and cumulus-oocyte complexes (COCs) were aspirated using an 18-gage needle attached to a 10.0 mL syringe. The syringe was filled with Tyrode’s albumin lactate pyruvate-4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (TALP-HEPES) medium, according to a method described by another study (15), supplemented with 4 mg/mL bovine serum albumin (A3311, Sigma, United States) and 5 IU/mL heparin (H3149-25KU, Sigma, USA).
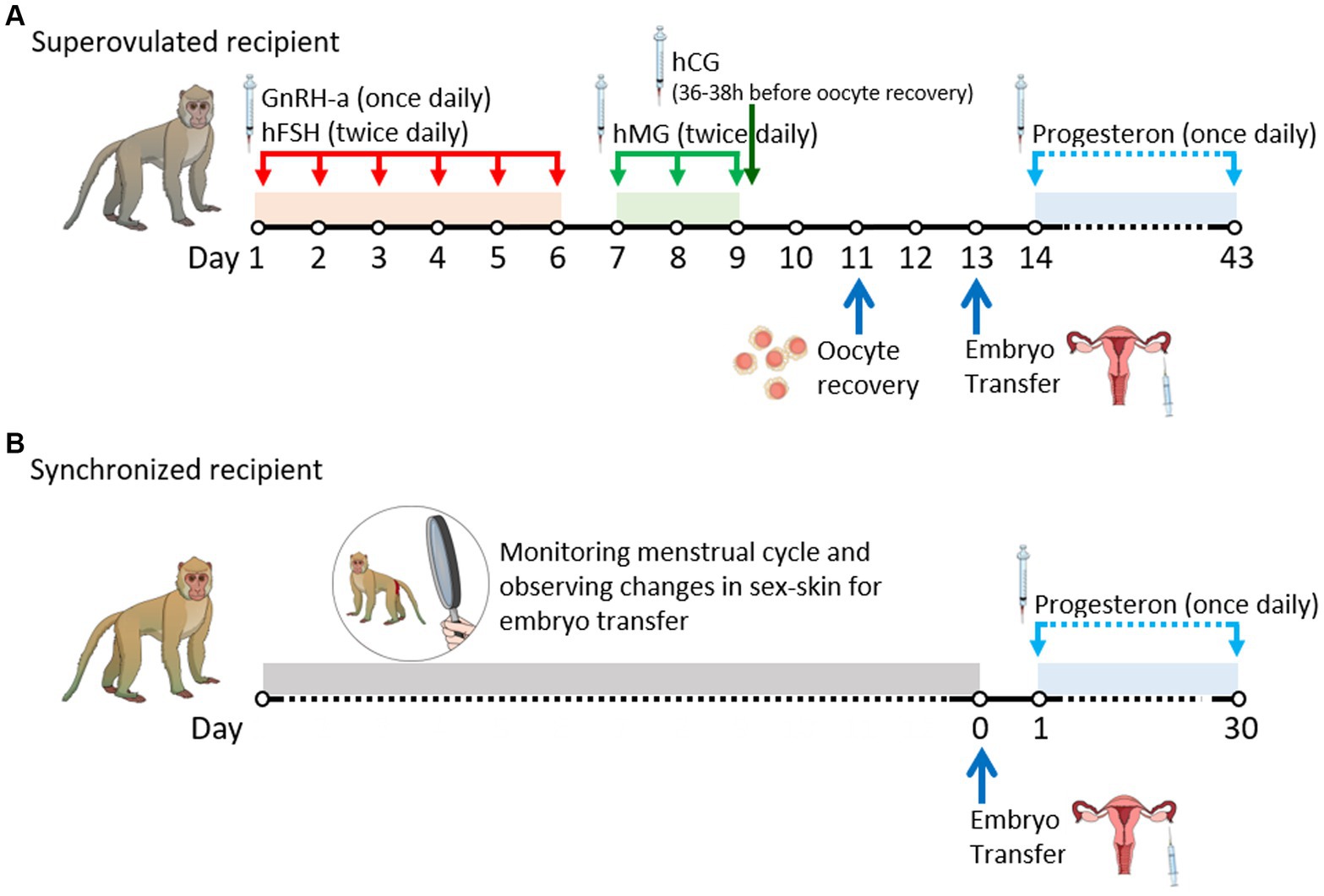
Figure 1. Timeline of experimental procedures by date. (A) Schedule of superovulated recipients. (B) Schedule of synchronized recipients. GnRH-a, gonadotrophin-releasing hormone antagonist; hCG, human chorionic gonadotrophin; hFSH, human follicle-stimulating hormone; hMG, human menopausal gonadotrophin.
2.3 Semen collection
Semen was obtained from male cynomolgus monkeys (71–110 months old) with proven fertility (16) via electrical stimulation and diluted in TALP-HEPES medium supplemented with 5 mg/mL bovine serum albumin. The sperm were then centrifuged at 2,000 rpm for 20 min using a PureSperm 90 gradient (PS90-100, Nidacon, Sweden) to separate the active sperm from the seminal plasma. The supernatant was discarded, and the sperm pellet was further washed by centrifugation at 2,000 rpm for 10 min in PureSperm Wash (PSW-100, Nidacon, Sweden). The top layer of the sperm was collected for use in ICSI.
2.4 Intracytoplasmic sperm injection and embryo culture
The COCs were initially rinsed with TALP-HEPES supplemented with 4 mg/mL bovine serum albumin, 5 IU/mL heparin, and 0.2% hyaluronidase (H4272, Sigma-Aldrich) to remove cumulus cells. Oocyte maturation was assessed under an inverted microscope (Leica DMI8; Leica Microsystems, Germany) at magnifications of ×100 or ×200 to identify the germinal vesicle (GV), metaphase I (MI), and metaphase II (MII) stages for analysis. Immature oocytes at the GV and MI stages were then cultured for up to 24 h until they reached the MII stage, in 50 μL drops of mCMRL-1066 medium (11,530,037, GIBCO, United States), supplemented with 10 mM sodium DL-lactate (L7900, Sigma, United States), 25 μg/mL 20% fetal bovine serum (16,000,044, Gibco, United States) and 5 μg/mL PMSG (Pregnant Mare Serum Gonadotropin, Prospec, Israel), 10 ug/ml hCG (CG10, Sigma, United States). The cultures were kept at 37°C in a 5% CO2 and 6% O2 atmosphere (Heracell 150i, Thermo Fisher Scientific, United States), under embryo-tested mineral oil (M3516, Sigma, United States). MII stage oocytes, either identified or derived from immature oocytes, were injected with monkey spermatozoa for genome editing according to a previously described method (17, 18). For the intracytoplasmic sperm injection (ICSI), spermatozoa were prepared in PureSperm Wash 10 min before the microinjection. A part of the suspended sperm was mixed with 10% polyvinylpyrrolidone. The zona pellucida of oocytes in injection media was covered with mineral oil and penetrated by several piezo pulses. The oolemma was punctured by the application of 1–2 piezo pulses, with the pipette tips reaching the opposite side of the oocyte cortex and the oolemma stretched without being broken. The sperm head was injected into the oocyte cytoplasm with a minimum amount of medium. Injected oocytes were incubated for at least 10 min in micromanipulation medium (TALP-HEPES) for stabilization. The oocytes were then transferred into mCMRL medium containing 0.4% BSA (A3311, Sigma, United States) and further cultured under embryo-tested mineral oil at 37°C in an atmosphere of 5% CO2 and 6% O2 for 48 h prior to embryo transfer.
2.5 Recipient selection
Oocyte donors also served as recipients in the superovulated group. In the synchronized group, recipient selection was based on monitoring the regular menstrual cycle and observing changes in sex-skin color and swelling (Supplementary Figure S1), which typically occur during ovulation between days 13 and 19 after menstruation. These observations established a 6–9 day window period for embryo transfer (19). Additionally, only those with a uterus presenting normal echo, as verified by USG before embryo transfer, were selected as embryo recipients in both groups.
2.6 Embryo transfer
Embryo transfer was performed 2 days after oocyte recovery, with the monkeys under anesthesia which was administered via an intramuscular dose of 5 mg/kg Zoletil® 50 (Virbac, Carros, France). Only the embryos that reached the four-cell stage were selected. Using a microglass capillary, 1 to 2 μL of BSA-free mCMRL medium containing the selected embryos were carefully picked up from the culture dishes. The embryos were surgically transferred to the oviduct via the infundibulum. In the standard procedure, two to three embryos were deposited in the oviduct. Luteal phase support was provided through daily intramuscular injections of progesterone (Taiyu Progesterone; Taiyu Chemical & Pharm, Taiwan) at a dosage of 3.5 mg, commencing the day after embryo transfer and continuing until ultrasonographic confirmation of pregnancy at 30 days.
2.7 Abdominal ultrasonography
USG was employed to assess ovarian conditions in oocyte donor monkeys before oocyte recovery and to evaluate uterine conditions in recipient monkeys before embryo transfer. Specifically, endometrial thickness in recipient monkeys was measured in the transverse plane at the point of the greatest uterine diameter. Pregnancy was diagnosed on day 30 following the transfer, confirming the presence of a yolk sac and embryonic cardiac motion using USG (20) (Supplementary Figure S2; Supplementary Video S1). The procedure was conducted under anesthesia induced by 10 mg/kg ketamine (Yuhan Ketamin 50 Inj., Yuhan Corporation, South Korea) by an experienced veterinarian using a high-resolution ultrasound device (LOGIQ e, GE Healthcare Technologies, Inc., Chicago, IL, United States) equipped with a 12.0 MHz probe.
2.8 Statistical analysis
All the data analyses were performed using the GraphPad Prism 8 software (GraphPad Software, LLC). Comparisons between groups for continuous variables were performed using the Student’s t-test. Pregnancy and implantation rates were compared between groups using Fisher’s exact test. The data were presented as mean ± standard deviation (SD). Differences were considered statistically significant at a p value of less than 0.05.
3 Results
3.1 Comparative characteristics of the superovulated vs. synchronized group
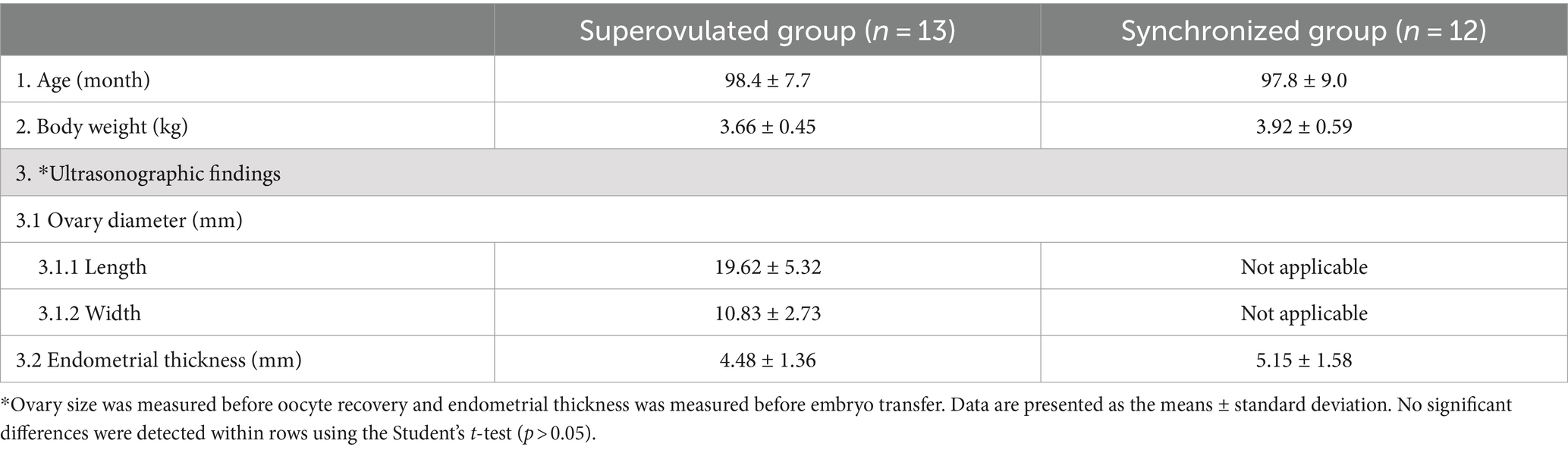
Table 1 provides a comparative overview of the characteristics of the superovulated and synchronized recipients in this study. The superovulated group had an average age of 98.4 ± 7.7 months, closely aligning with the average age of the synchronized group of 97.8 ± 9.0 months. The body weight for the superovulated group averaged 3.66 ± 0.45 kg, slightly less than the synchronized group at 3.92 ± 0.59 kg. There was no statistically significant difference in age and body weight between the two groups, as confirmed by the Student’s t-test (p > 0.05).
The ovary size was measured before oocyte recovery and endometrial thickness was measured before embryo transfer using USG (Figures 2A,B). The ovary size in the superovulated group was measured before oocyte recovery, yielding dimensions of 19.62 ± 5.32 mm in length and 10.83 ± 2.73 mm in width. The ovary size in the synchronized group was not assessed. In terms of the endometrial thickness before embryo transfer, the superovulated group presented a slightly thinner endometrium at 4.48 ± 1.36 mm compared to 5.15 ± 1.58 mm in the synchronized group. However, these differences were not statistically significant, as determined by the Student’s t-test (p > 0.05).
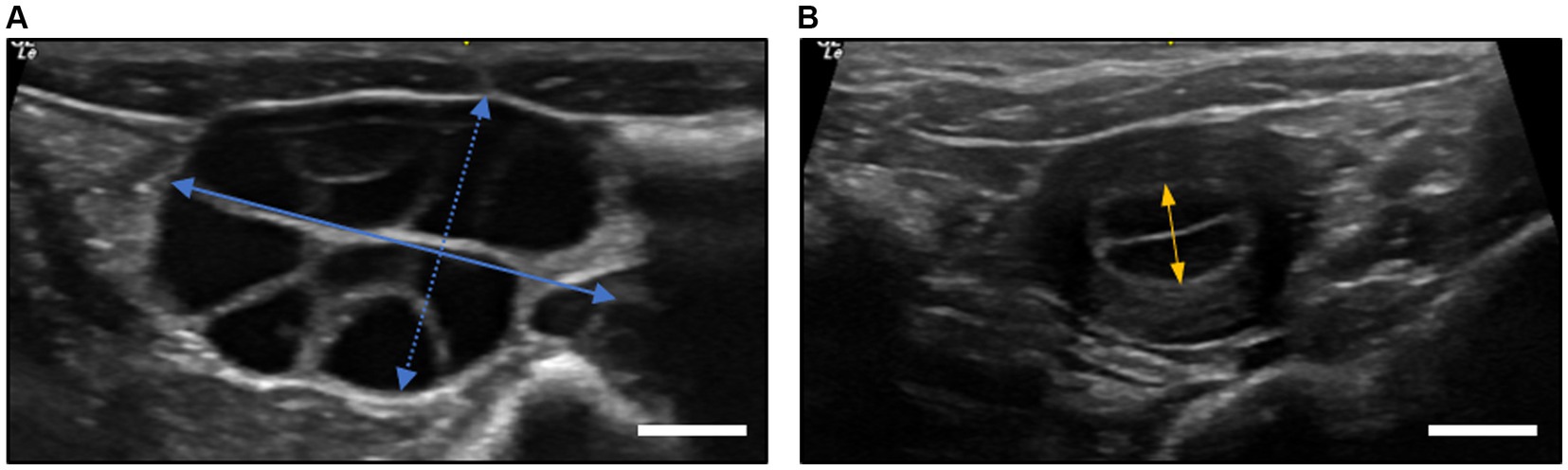
Figure 2. Ultrasonographic measurements of ovary diameter and endometrial thickness in cynomolgus monkeys. (A) Ovary diameter after superovulation procedures at oocyte recovery. The length of the ovary was defined as the longest axis (straight blue line), while the width was measured perpendicular to the length at its widest point (dotted blue line). (B) The endometrial thickness of recipients at embryo transfer. The measurement was taken at the thickest part of the endometrium, typically in the transverse plane of the uterus (straight orange line). All measurements were taken using a digital caliper in ultrasound device software with an accuracy of ±0.01 mm. The scale bar is 5.0 mm.
3.2 Pregnancy outcomes following embryo transfer in two groups
The data on pregnancy and implantation rates are summarized in Table 2. In the superovulated group, 35 embryos were transferred to 13 recipients, resulting in four pregnancies (30.8%), including one twin pregnancy. The remaining participants had singletons. In the synchronized group, 32 embryos were transferred to 12 recipients, leading to five pregnancies (41.7%), one of which was a triplet pregnancy. The implantation rates were 14.3% (five of 35 transferred embryos) in the superovulated group and 24.1% (seven of 32 transferred embryos) in the synchronized group. Although the pregnancy and implantation rates were higher in the synchronized group, these differences were not statistically significant (p > 0.05). Additionally, direct observations after embryo transfer revealed skin suture dehiscence in two cases, one in each group, which was attributed to the actions of the monkeys; however, no major infections, incision abnormalities, or other significant complications were observed.
4 Discussion
4.1 Principal findings of the study
The observations suggest that in embryo transfer, superovulated recipients are as effective as synchronized recipients and could be considered a preferable option because of their comparable pregnancy outcomes in cynomolgus monkeys.
4.2 Limitations of previous studies
The superovulated oocyte donor is commonly the recipient in human ARTs; however, its application is limited in NHPs. In NHPs, surrogate recipients have primarily been used to increase the number of offspring produced for research purposes and avoid complications associated with transferring embryos back into the oocyte donor during the stimulation cycle. To date, numerous cynomolgus monkey offspring have been produced using ARTs with and without gene editing methods. To the best of our knowledge, no reports have documented the use of superovulated recipients; instead, most studies have employed embryo-uterus-synchronized recipients (Table 3). Research has predominantly focused on selecting the most suitable synchronized recipients because of the widely recognized importance of aligning the developmental stage of embryos with the uterine conditions of the recipients, which is essential for successful embryo transfer (8, 21).

Table 3. Previous studies on recipient selection and pregnancy outcomes in cynomolgus monkeys through ARTs.
4.3 Challenges in identifying uterine-embryo synchronized recipients
Identifying uterine embryo-synchronized recipients in cynomolgus monkeys can be challenging. In humans, oocyte donors often serve as recipients and synchronous embryo transfer has been associated with high pregnancy rates (22). Surrogates other than oocyte donors are usually employed in cynomolgus monkeys. The process of identifying a synchronized recipient typically involves confirming synchronization through various methods such as assessing estradiol levels, monitoring menses and sex-skin changes, and observing new stigma or new corpus luteum in the ovaries via USG or laparoscopy, either separately or in combination.
Hormone assays that detect serum estradiol levels are commonly used, with embryo transfer typically occurring 1–3 days after a peak in estradiol levels (8). However, this process requires daily blood collection over a long period to monitor estradiol levels, as the peak can occur anywhere from seven to 20 days after menstruation (19). This can cause stress in monkeys and complicate the synchronization of embryonic development with the recipient’s uterine condition.
Predicting the ovulation date by monitoring the menstrual cycle and sex-skin changes may be straightforward. The menstrual cycle of cynomolgus monkeys is approximately 29 days, with ovulation occurring approximately 11–14 days after the onset of menstruation (23, 24). Sexual swelling and reddening are highly accurate indicators of ovulation timing (25). Nevertheless, monitoring a regular menstrual cycle is time-consuming, and not all female’s exhibit changes in sex-skin (26).
The technique of detecting ovulation through the observation of a new stigma or corpus luteum using USG or laparoscopy is highly accurate for confirming synchronization. This method, however, requires specialized equipment and skilled personnel and may not always detect ovulation points during a normal menstrual cycle (19).
Selecting a synchronized recipient, therefore, requires significant time and resources as well as several female monkeys. This increases the complexity and ethical challenges involved in effective embryo transfer in NHP studies.
4.4 Analysis of endometrial thickness in superovulated and synchronized recipients
The endometrium of cynomolgus monkeys undergoes significant changes during the menstrual cycle, which are primarily driven by ovarian hormones and their receptors (26, 27).
The endometrium typically expands during the follicular phase and reaches its peak immediately after ovulation (28), and is considered a critical aspect of uterine receptivity. Uterine receptivity is crucial for embryo transfer, not only in humans but also in NHPs (29, 30).
In humans, studies on the effect of endometrial thickness have shown varied outcomes, with some studies suggesting a more favorable outcome for pregnancies with an endometrial thickness of at least 10 mm and negative outcomes with thicknesses below 6 mm (31, 32) while others have reported successful pregnancies with thicknesses as low as 4 mm (33). Interestingly, additional research has indicated that endometrial thickness may not be directly related to pregnancy outcomes (34).
The comparison of endometrial thicknesses measured using ultrasound revealed no statistically significant differences between the superovulated and synchronized groups in this study. Furthermore, the observations presented no significant differences compared to those of a previous study, which reported an endometrial thickness of 5.7 mm (35). These findings suggest that the endometrial changes induced by superovulation are comparable to those occurring during the natural menstrual cycle and do not adversely affect the endometrial thickness, implying that superovulated recipients have a uterine receptivity similar to that of synchronized recipients.
For additional analysis, the results of this study were categorized into pregnant and non-pregnant recipients, with endometrial thicknesses measured at 4.89 ± 0.71 mm and 4.75 ± 1.79 mm, respectively. No significant differences were observed between the two groups (p > 0.05). The results suggest that endometrial thickness does not significantly affect pregnancy outcomes in cynomolgus monkeys, because endometrial thickness is often considered a factor in successful implantation and pregnancy.
4.5 The effect of superovulation on the pregnancy outcomes
In human ARTs, the impact of controlled ovarian hyperstimulation—the use of hormonal medications to stimulate the ovaries to produce multiple follicles, similar to superovulation in this study—on pregnancy and implantation rates has been extensively studied. Several studies have indicated that hyperstimulation does not negatively affect endometrial receptivity or pregnancy outcomes (36–39); however, others have highlighted the potential detrimental effects on the outcomes of assisted reproduction (40–43). Laboratory animal studies involving rats and mice have demonstrated mixed results in terms of the effects of ovarian hyperstimulation. Research on rats suggests that hyperstimulation can maintain normal uterine receptivity (44), while findings from mouse studies indicate potential negative impacts on implantation due to endometrial alterations (45). Despite this controversy, ovarian hyperstimulation is a critical component of fertility treatments and the generation of mutant animals, enhancing both the number of oocytes and the quality of embryos available for fertilization and subsequent development.
In the current study, no significant differences were observed in the pregnancy and implantation rates between the superovulated and synchronized recipient groups. It is believed that a superovulation protocol can effectively optimize the conditions for both follicular development and endometrial preparation by forcefully controlling the menstrual cycle. Furthermore, the administration of progesterone after embryo transfer, which is commonly used in human fertility treatments to aid embryo implantation and maintain pregnancy (46), is considered to have similar beneficial effects.
This study additionally achieved moderate success rates for pregnancy and implantation, comparable to those reported in other studies (Table 3). These findings support the effective use of superovulated recipients as synchronized recipients for embryo transfer in cynomolgus monkeys.
4.6 Strengths and weaknesses of the study
This innovative study confirmed that superovulated monkeys, traditionally used only as oocyte donors, can also serve as surrogate mothers. This finding is beneficial in terms of the time, cost, and reduction in the number of animals needed, as well as alleviating the cumbersome process associated with selecting surrogate recipients. The meticulous division and control of the two groups enhanced the reliability of the findings, suggesting that both methods were equally effective. Moreover, the study included measurements of endometrial thickness, which not only enhanced the understanding of the effects of superovulation but also enabled comparisons of endometrial thickness between pregnant and non-pregnant monkeys. This detail is particularly relevant as it may influence clinical approaches to reproductive technologies. This study, therefore, makes a practical contribution by identifying superovulated recipients as viable and efficient alternatives for generating genetically edited models and broadening the knowledge base of ARTs in cynomolgus monkeys.
This study was, however, limited by its small sample size, which may have restricted the generalizability of the results. The slightly higher pregnancy and implantation rates observed in the synchronized group suggest a trend that may become more apparent with larger sample sizes. Focusing predominantly on short-term outcomes additionally limits a comprehensive understanding of the long-term implications of these ART methods, such as complications of repeated surgery, pregnancy maintenance, and birth rates.
4.7 Unanswered questions and proposals for future studies
In future studies, it will be necessary to confirm these findings in superovulated recipients with larger sample sizes, slightly different superovulation protocols, and long-term outcomes to ensure the reliability and applicability of the results. Additionally, exploring biological mechanisms such as hormonal profiles, endometrial gene expression, and the uterine microenvironment in superovulated recipients will provide deeper insights into the underlying processes. Employing refined surgical methods such as laparoscopy can enhance animal welfare by reducing stress and increasing the safety of procedures. Ultimately, these studies will improve the overall efficacy and safety of ARTs for cynomolgus monkeys.
5 Conclusion
ARTs are vital for producing mutant monkeys; they, however, often encounter challenges in recipient selection. To the best of our knowledge, this is the first study to compare superovulated and uterine-embryo synchronized recipients in cynomolgus monkeys. The observations from this study highlighted that superovulated recipients, who are also oocyte donors, effectively serve as surrogates (Figure 3). This approach not only simplifies recipient selection and reduces the number of animals needed but also enhances the practical application of ARTs, facilitating the creation of gene-edited models in this species.
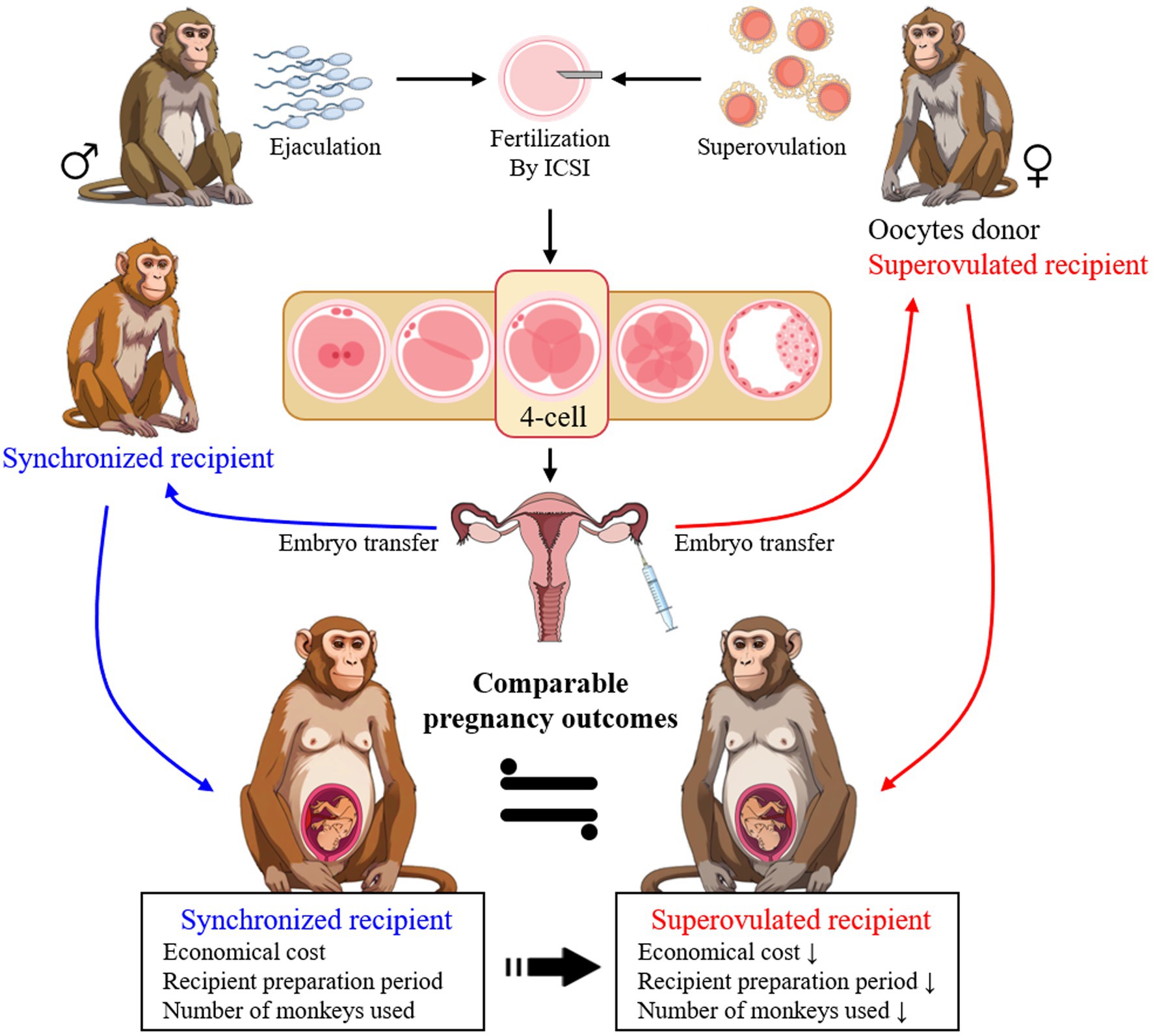
Figure 3. The highlight of superovulated recipients as effective surrogates compared to synchronized recipients in embryo transfer for generating gene-edited monkeys. The selection of superovulated recipients enhances efficiency and the practical application of ARTs in this species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
D-HL: Data curation, Formal analysis, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. S-BY: Data curation, Investigation, Resources, Visualization, Writing – review & editing. Y-JJ: Data curation, Investigation, Resources, Writing – review & editing. JM: Data curation, Investigation, Resources, Writing – review & editing. JeK: Data curation, Investigation, Resources, Writing – review & editing. SL: Data curation, Investigation, Resources, Writing – review & editing. JuK: Conceptualization, Project administration, Supervision, Writing – review & editing. J-SK: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant from the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM5162423).
Acknowledgments
We express our heartfelt thanks to the dedicated animal care staff at the Primate Resources Center for their exceptional care and support in ensuring the well-being of our research subjects.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1452631/full#supplementary-material
References
1. Faddy, MJ, Gosden, MD, and Gosden, RG. A demographic projection of the contribution of assisted reproductive technologies to world population growth. Reprod Biomed Online. (2018) 36:455–8. doi: 10.1016/j.rbmo.2018.01.006
2. Smith, DG. Taxonomy of nonhuman primates used in biomedical research. Amsterdam, Netherlands: Nonhuman Primates in Biomedical Research, Elsevier, pp. 57–85 (2012).
3. Niu, Y, Shen, B, Cui, Y, Chen, Y, Wang, J, Wang, L, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. (2014) 156:836–43. doi: 10.1016/j.cell.2014.01.027
4. Wan, H, Feng, C, Teng, F, Yang, S, Hu, B, Niu, Y, et al. One-step generation of p53 gene biallelic mutant Cynomolgus monkey via the CRISPR/Cas system. Cell Res. (2015) 25:258–61. doi: 10.1038/cr.2014.158
5. Liu, H, Chen, Y, Niu, Y, Zhang, K, Kang, Y, Ge, W, et al. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell. (2014) 14:323–8. doi: 10.1016/j.stem.2014.01.018
6. Liu, Z, Li, X, Zhang, J-T, Cai, Y-J, Cheng, T-L, Cheng, C, et al. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature. (2016) 530:98–102. doi: 10.1038/nature16533
7. Bavister, BD, Boatman, DE, Collins, K, Dierschke, DJ, and Eisele, SG. Birth of rhesus monkey infant after in vitro fertilization and nonsurgical embryo transfer. Proc Natl Acad Sci. (1984) 81:2218–22.
8. Sun, Q, Dong, J, Yang, W, Jin, Y, Yang, M, Wang, Y, et al. Efficient reproduction of cynomolgus monkey using pronuclear embryo transfer technique. Proc Natl Acad Sci. (2008) 105:12956–60. doi: 10.1073/pnas.0805639105
9. Sasaki, E, Suemizu, H, Shimada, A, Hanazawa, K, Oiwa, R, Kamioka, M, et al. Generation of transgenic non-human primates with germline transmission. Nature. (2009) 459:523–7. doi: 10.1038/nature08090
10. Shimozawa, N, Nakamura, S, Takahashi, I, Hatori, M, and Sankai, T. Characterization of a novel embryonic stem cell line from an ICSI-derived blastocyst in the African green monkey. Reproduction. (2010) 139:565.
11. Yang, S. Assisted reproductive technologies in nonhuman primates. Reprod Technol Anim. (2020) 1:181–91. doi: 10.1016/B978-0-12-817107-3.00012-6
12. Zou, Q, Wang, X, Liu, Y, Ouyang, Z, Long, H, Wei, S, et al. Generation of gene-target dogs using CRISPR/Cas9 system. J Mol Cell Biol. (2015) 7:580–3. doi: 10.1093/jmcb/mjv061
13. Abe, Y, Nakao, H, Goto, M, Tamano, M, Koebis, M, Nakao, K, et al. Efficient marmoset genome engineering by autologous embryo transfer and CRISPR/Cas9 technology. Sci Rep. (2021) 11:20234.
14. Kim, J-S, Yoon, S-B, Jeong, K-J, Sim, B-W, Choi, S-A, Lee, S-I, et al. Superovulatory responses in cynomolgus monkeys (Macaca fascicularis) depend on the interaction between donor status and superovulation method used. J Reprod Dev. (2017) 63:149–55. doi: 10.1262/jrd.2016-074
15. Ramsey, C, and Hanna, C. In vitro culture of rhesus macaque (Macaca mulatta) embryos. Comp Embryo Cult Methods Protoc. (2019) 1:341–53. doi: 10.1007/978-1-4939-9566-0_23
16. American Association for Laboratory Animal Science. Association of Primate Veterinarians Guideline for semen collection in nonhuman Primates in biomedical research. J Am Assoc Lab Anim Sci. (2022) 61:223–5.
17. Meng, Q, Li, X, Wu, T, Dinnyes, A, and Zhu, S. Piezo-actuated zona-drilling improves the fertilisation of OPS vitrified mouse oocytes. Acta Vet Hung. (2007) 55:369–78.
18. Jo, Y-J, Lee, I-W, Jung, S-M, Kwon, J, Kim, N-H, and Namgoong, S. Spire localization via zinc finger—containing domain is crucial for the asymmetric division of mouse oocyte. FASEB J. (2019) 33:4432–47. doi: 10.1096/fj.201801905R
19. Huang, Z, Li, Y, Jiang, Q, Wang, Y, Ma, K, and Li, Q. Generation of cynomolgus monkey fetuses with intracytoplasmic sperm injection based on the MII-stage oocytes acquired by personalized superovulation protocol. J Vet Sci. (2020) 21:e48. doi: 10.4142/jvs.2020.21.e48
20. Chen, Y, Niu, Y, Yang, S, He, X, Ji, S, Si, W, et al. The available time window for embryo transfer in the Rhesus monkey (M acaca mulatta). Am J Primatol. (2012) 74:165–73. doi: 10.1002/ajp.21017
21. Santos, MA, Kuijk, EW, and Macklon, NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. (2010) 139:23–34.
22. Mandelbaum, J, Junca, AM, Plachot, M, Alnot, MO, Alvarez, S, Debache, C, et al. Human embryo cryopreservation, extrinsic and intrinsic parameters of success. Hum Reprod. (1987) 2:709–15. doi: 10.1093/oxfordjournals.humrep.a136619
23. Yoshida, T, Nakajima, M, Hiyaoka, A, Suzuki, MT, Cho, F, and Honjo, S. Menstrual cycle lengths and the estimated time of ovulation in the cynomolgus monkey (Macaca fascicularis). Jikken Dobutsu. (1982) 31:165–74.
24. van Diepen, HA, Pansier, J, Oude Wesselink, P, van Drie, A, van Duin, M, and Mulders, S. Non-invasive translational Cynomolgus model for studying folliculogenesis and ovulation using color Doppler ultrasonography. J Med Primatol. (2012) 41:18–23. doi: 10.1111/j.1600-0684.2011.00514.x
25. Tardif, S, Carville, A, Elmore, D, Williams, LE, and Rice, K. Reproduction and breeding of nonhuman primates. San Diego: Academic press, pp. 197–249. (2012).
26. Weinbauer, GF, Niehoff, M, Niehaus, M, Srivastav, S, Fuchs, A, Van Esch, E, et al. Physiology and endocrinology of the ovarian cycle in macaques. Toxicol Pathol. (2008) 36:7S–23S.
27. Brenner, RM, Carlisle, KS, Hess, DL, Sandow, BA, and West, NB. Morphology of the oviducts and endometria of cynomolgus macaques during the menstrual cycle. Biol Reprod. (1983) 29:1289–302.
28. Van Esch, E, Cline, JM, Buse, E, and Weinbauer, GF. The macaque endometrium, with special reference to the cynomolgus monkey (Macaca fascicularis). Toxicol Pathol. (2008) 36:67S–100S. doi: 10.1177/0192623308326149
29. Revel, A. Defective endometrial receptivity. Fertil Steril. (2012) 97:1028–32. doi: 10.1016/j.fertnstert.2012.03.039
30. Yoshinaga, K. Uterine receptivity for blastocyst implantation. Ann N Y Acad Sci. (1988) 541:424–31.
31. Mahutte, N, Hartman, M, Meng, L, Lanes, A, Luo, Z-C, and Liu, KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. (2022) 117:792–800. doi: 10.1016/j.fertnstert.2021.12.025
32. Israel, R, Isaacs, JD Jr, Wells, CS, Williams, DB, Odem, RR, Gast, MJ, et al. Endometrial thickness is a valid monitoring parameter in cycles of ovulation induction with menotropins alone. Fertil Steril. (1996) 65:262–6. doi: 10.1016/S0015-0282(16)58082-0
33. Sundström, P. Establishment of a successful pregnancy following in-vitro fertilization with an endometrial thickness of no more than 4 mm. Hum Reprod. (1998) 13:1550–2.
34. Ng, EHY, Chan, CCW, Tang, OS, Yeung, WSB, and Ho, PC. Factors affecting endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound during IVF treatment. Hum Reprod. (2006) 21:1062–9. doi: 10.1093/humrep/dei442
35. Maaskant, A, Scarsi, KK, Meijer, L, Roubos, S, Louwerse, AL, Remarque, EJ, et al. Long-acting reversible contraception with etonogestrel implants in female macaques (Macaca mulatta and Macaca fascicularis). Front Vet Sci. (2024) 10:1319862. doi: 10.3389/fvets.2023.1319862
36. Chemerinski, A, Shen, M, Valero-Pacheco, N, Zhao, Q, Murphy, T, George, L, et al. The impact of ovarian stimulation on the human endometrial microenvironment. Hum Reprod. (2024) 39:1023–41. doi: 10.1093/humrep/deae048
37. Levi, AJ, Drews, MR, Bergh, PA, Miller, BT, and Scott, RT Jr. Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril. (2001) 76:670–4.
38. Sharara, FI, and McClamrock, HD. High estradiol levels and high oocyte yield are not detrimental to in vitro fertilization outcome. Fertil Steril. (1999) 72:401–5. doi: 10.1016/S0015-0282(99)00293-9
39. Meldrum, DR. Female reproductive aging—ovarian and uterine factors. Fertil Steril. (1993) 59:1–5. doi: 10.1016/S0015-0282(16)55608-8
40. Simon, C, Cano, F, Valbuena, D, Remohi, J, and Pellicer, A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. (1995) 10:2432–7. doi: 10.1093/oxfordjournals.humrep.a136313
41. Yu Ng, EH, Yeung, WSB, Yee Lan Lau, E, So, WWK, and Ho, PC. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen–thawed embryo transfer cycles. Hum Reprod. (2000) 15:250–5. doi: 10.1093/humrep/15.2.250
42. Shapiro, BS, Daneshmand, ST, Garner, FC, Aguirre, M, Hudson, C, and Thomas, S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen–thawed embryo transfer in normal responders. Fertil Steril. (2011) 96:344–8. doi: 10.1016/j.fertnstert.2011.05.050
43. Montoya-Botero, P, and Polyzos, NP. The endometrium during and after ovarian hyperstimulation and the role of segmentation of infertility treatment. Best Pract Res Clin Endocrinol Metab. (2019) 33:61–75. doi: 10.1016/j.beem.2018.09.003
44. Xu, C, and Tang, S. Alteration of endometrial receptivity in rats with ovarian hyperstimulation syndrome. J Obstet Gynaecol. (2014) 34:146–52. doi: 10.3109/01443615.2013.832735
45. Ertzeid, G, and Storeng, R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod. (2001) 16:221–5.
46. Labarta, E, and Rodríguez, C. Progesterone use in assisted reproductive technology. Best Pract Res Clin Obstet Gynaecol. (2020) 69:74–84.
47. Seita, Y, Tsukiyama, T, Iwatani, C, Tsuchiya, H, Matsushita, J, Azami, T, et al. Generation of transgenic cynomolgus monkeys that express green fluorescent protein throughout the whole body. Sci Rep. (2016) 6:24868. doi: 10.1038/srep24868
48. Ke, Q, Li, W, Lai, X, Chen, H, Huang, L, Kang, Z, et al. TALEN-based generation of a cynomolgus monkey disease model for human microcephaly. Cell Res. (2016) 26:1048–61. doi: 10.1038/cr.2016.93
49. Zhao, H, Tu, Z, Xu, H, Yan, S, Yan, H, Zheng, Y, et al. Altered neurogenesis and disrupted expression of synaptic proteins in prefrontal cortex of SHANK3-deficient non-human primate. Cell Res. (2017) 27:1293–7. doi: 10.1038/cr.2017.95
50. Chen, Y, Yu, J, Niu, Y, Qin, D, Liu, H, Li, G, et al. Modeling Rett syndrome using TALEN-edited MECP2 mutant cynomolgus monkeys. Cell. (2017) 169:e10:945–55. doi: 10.1016/j.cell.2017.04.035
51. Zhang, W, Wan, H, Feng, G, Qu, J, Wang, J, Jing, Y, et al. SIRT6 deficiency results in developmental retardation in cynomolgus monkeys. Nature. (2018) 560:661–5. doi: 10.1038/s41586-018-0437-z
52. Cui, Y, Niu, Y, Zhou, J, Chen, Y, Cheng, Y, Li, S, et al. Generation of a precise Oct4-hrGFP knockin cynomolgus monkey model via CRISPR/Cas9-assisted homologous recombination. Cell Res. (2018) 28:383–6. doi: 10.1038/cr.2018.10
53. Zhou, Y, Sharma, J, Ke, Q, Landman, R, Yuan, J, Chen, H, et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature. (2019) 570:326–31. doi: 10.1038/s41586-019-1278-0
54. Qiu, P, Jiang, J, Liu, Z, Cai, Y, Huang, T, Wang, Y, et al. BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. Natl Sci Rev. (2019) 6:87–100. doi: 10.1093/nsr/nwz002
55. Tsukiyama, T, Kobayashi, K, Nakaya, M, Iwatani, C, Seita, Y, Tsuchiya, H, et al. Monkeys mutant for PKD1 recapitulate human autosomal dominant polycystic kidney disease. Nat Commun. (2019) 10:5517. doi: 10.1038/s41467-019-13398-6
56. Schmidt, JK, Strelchenko, N, Park, MA, Kim, YH, Mean, KD, Schotzko, ML, et al. Genome editing of CCR5 by CRISPR-Cas9 in Mauritian cynomolgus macaque embryos. Sci Rep. (2020) 10:18457. doi: 10.1038/s41598-020-75295-z
57. Wang, F, Zhang, W, Yang, Q, Kang, Y, Fan, Y, Wei, J, et al. Generation of a Hutchinson–Gilford progeria syndrome monkey model by base editing. Protein Cell. (2020) 11:809–24. doi: 10.1007/s13238-020-00740-8
58. Chen, Z-Z, Wang, J-Y, Kang, Y, Yang, Q-Y, Gu, X-Y, Zhi, D-L, et al. PINK1 gene mutation by pair truncated sgRNA/Cas9-D10A in cynomolgus monkeys. Zool Res. (2021) 42:469–77. doi: 10.24272/j.issn.2095-8137.2021.023
Keywords: assisted reproductive technologies, cynomolgus monkey, embryo transfer, pregnancy outcome, recipient
Citation: Lee D-H, Yoon S-B, Jo Y-J, Mo JW, Kwon J, Lee SIl, Kwon J and Kim J-S (2024) Comparative analysis of superovulated versus uterine-embryo synchronized recipients for embryo transfer in cynomolgus monkeys (Macaca fascicularis). Front. Vet. Sci. 11:1452631. doi: 10.3389/fvets.2024.1452631
Edited by:
Stefan Gregore Ciornei, University of Life Science, RomaniaReviewed by:
Emoke Pall, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaHiroyuki Imai, Yamaguchi University, Japan
Copyright © 2024 Lee, Yoon, Jo, Mo, Kwon, Lee, Kwon and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Su Kim, a2ltanNAa3JpYmIucmUua3I=; Jungkee Kwon, amt3b25AamJudS5hYy5rcg==
†These authors have contributed equally to this work and share first authorship
 Dong-Ho Lee
Dong-Ho Lee Seung-Bin Yoon
Seung-Bin Yoon Yu-Jin Jo
Yu-Jin Jo Jun Won Mo1
Jun Won Mo1 Jeongwoo Kwon
Jeongwoo Kwon