- 1Department of Veterinary Surgery, College of Veterinary Medicine, Chungnam National University, Daejeon, Republic of Korea
- 2Department of Veterinary Surgery, College of Veterinary Medicine, Gyeongsang National University, Jinju, Republic of Korea
An 11-year-old spayed female Maltese dog presented with a 2-month history of gait alterations, wide-based stance, and chronic vomiting. Neurological examination revealed cerebellovestibular signs, including head tilt, nystagmus, strabismus, intentional tremor, and hypermetric gait. MRI showed a mass with iso- to hypointensity on T1-weighted (T1W) images and heterogeneous hyperintensity on T2-weighted (T2W) images, with marked non-uniform contrast enhancement. The tumor was removed via a telovelar approach without intraoperative complications. Postoperatively, the dog developed non-ambulatory paraparesis with the rigidity of the pelvic limbs but recovered ambulation within 6 days. Preoperative neurological signs progressively improved, and the patient was discharged without complications 10 days after surgery. Histological examination revealed dense spindle cells with an abundant collagen matrix and oval-shaped nucleated cells with small whorls, leading to a diagnosis of transitional meningioma of the fourth ventricle. MRI follow-up at 8 months postoperatively showed no definitive evidence of recurrence. At the final follow-up, 15.4 months postoperatively, mild neurological signs, including a slight head tilt and subtle strabismus, remained, but the rest of the neurological examination was normal. This is the first reported case of a meningioma in the fourth ventricle of a dog successfully removed using the telovelar approach.
1 Introduction
Primary ventricular tumors are relatively uncommon in veterinary medicine. The most common tumor located in the ventricular system is the choroid plexus tumor (CPT), which accounts for up to 7% of primary brain tumors and is the third most common type of primary intracranial tumor in dogs (1, 2). Other primary tumors, such as ependymoma, astrocytoma, and meningioma, can also occur within the ventricles, although reported cases are limited (3–9). The prevalence of primary fourth ventricular tumors is presumed to be less than 5% of all primary brain tumors. This estimation is based on the finding that 49% of CPT are located in the fourth ventricle, while other primary fourth ventricular tumors have been reported only rarely as single-case reports (7, 10). The clinical signs associated with fourth ventricle tumors are predominantly cerebellovestibular signs, including strabismus, head tilt, obtunded mental status, and delayed postural reactions (7, 11). Additionally, an absent menace response has also been reported (11).
Meningiomas typically occur in the rostrotentorial region, accounting for 62.2 to 84.4% of all cases (12, 13). In contrast, intraventricular meningiomas are extremely rare; only two lateral ventricular meningiomas and one-fourth ventricular meningioma have been described (6, 7, 9). The single case of a fourth ventricular meningioma was diagnosed postmortem through histopathologic examination and classified as a microcystic subtype, which is a rare form of meningioma. In human medicine, intraventricular meningioma is similarly rare, constituting 0.7–3% of intracranial meningiomas, with only 6.6% of these occurring in the fourth ventricle (14, 15).
While a standard treatment protocol for fourth ventricular tumors has not been established in veterinary medicine, surgical removal is considered the first line of treatment in human medicine (16). The surgical approach to the fourth ventricle is particularly challenging due to its narrow anatomical location between the brain stem, specifically the medulla oblongata and the cerebellum (17). In the human literature, the transvermian and telovelar approach are well-described methods for accessing the fourth ventricle. The telovelar approach has been used to surgically resect a choroid plexus carcinoma (CPC) within the fourth ventricle in a dog (11). This technique offers several advantages over the transvermian approach, such as sparing the vermis and providing better exposure in craniocaudal and lateral directions (11, 18, 19).
The author found no existing literature describing the surgical removal of a fourth ventricular meningioma and its outcome in veterinary medicine. This case report aims to describe the neuroradiological findings, detailed surgical technique, and long-term outcome of a primary fourth ventricular meningioma, classified as the transitional subtype.
2 Case description
A client-owned, 11-year-old spayed female Maltese dog weighing 3.8 kg was referred with a 2-month history of falling episodes with gait alterations, wide-based stance, nausea, and vomiting. Nausea and vomiting could not be controlled by maropitant (1 mg/kg, orally, q24hr) at the primary veterinary clinic. The physical examination revealed a systolic murmur of grade 4/6 at the left heart apex, which was later evaluated as grade B1 myxomatous mitral valve disease; other findings were normal. Neurological examination revealed bright and alert mentation, an obvious left head tilt, positional, horizontal nystagmus with fast phase to the right, ventrolateral positional strabismus of left eye, intentional head tremor, and hypermetric gait. Concurrent vestibular and cerebellar signs indicated a lesion on the left central vestibular system and cerebellum. The lesion was thought to be a space-occupying mass, and differential diagnoses included neoplasia (CPT, glioma, meningioma, and lymphoma), cyst, or cyst-like lesion. Inflammatory diseases, anomalies, and degenerative diseases were thought to be less likely. Blood tests were within normal reference range, except for a mildly elevated liver panel. No abnormal findings were identified on the thoracic radiographs. MRI revealed a 10.4 × 11 × 16.2 mm sized, oval-shaped, and slightly left-sided intraventricular mass. The mass showed iso- to hypointensity on T1W images, heterogeneous hyperintensity on T2W images, hyperintensity on fluid-attenuated inversion recovery sequences (FLAIR), and marked non-uniform contrast enhancement (Figures 1A–D). The mass compressed and displaced the medulla oblongata ventrally and the cerebellum dorsally. Obstructive ventriculomegaly was observed rostral to the lesion. The neuroradiological findings corresponded with the symptoms. A presumptive diagnosis of an intraventricular tumor was made, with CPT considered the most likely differential diagnosis.
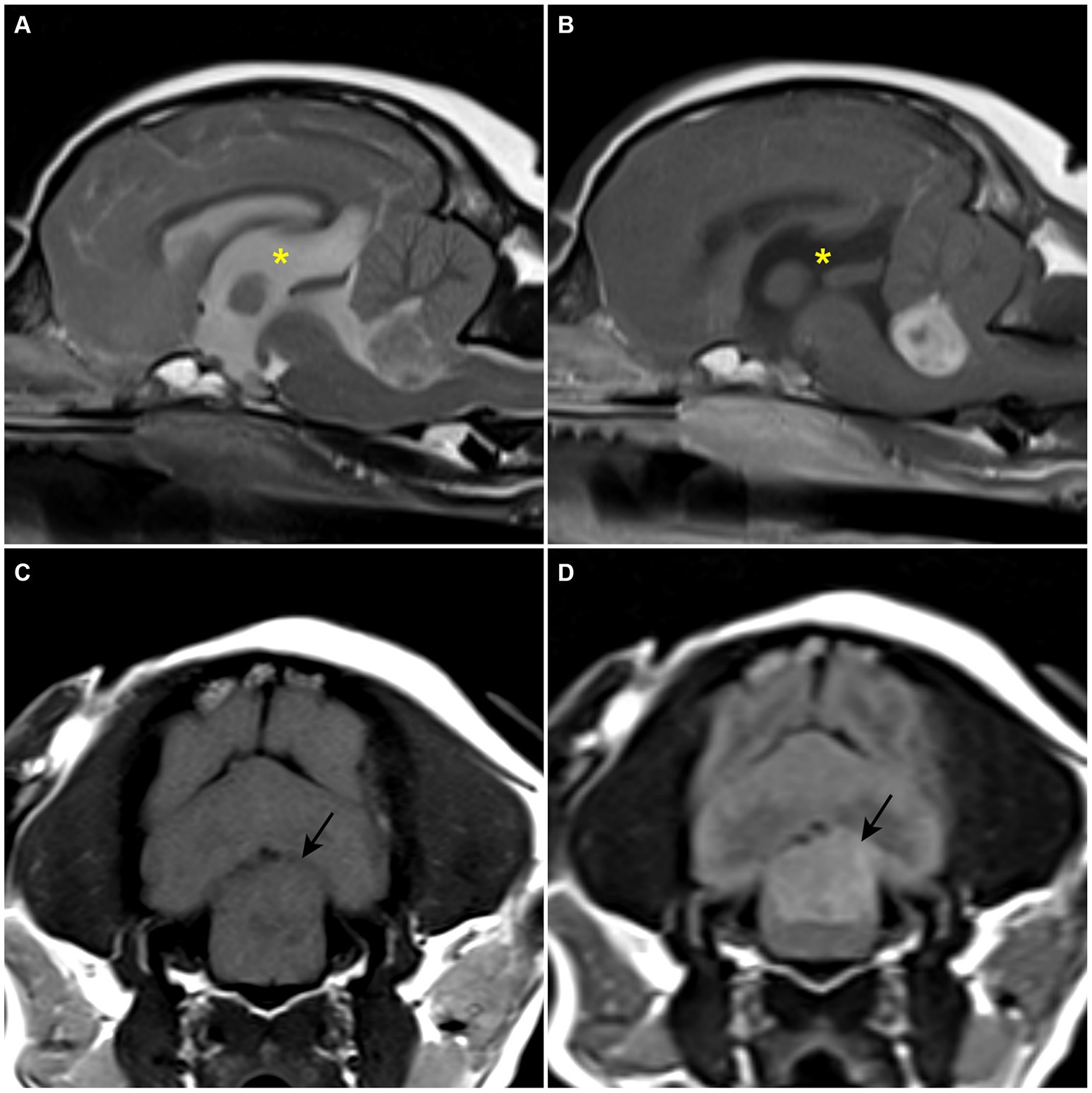
Figure 1. Preoperative MRI. (A) T2-weighted sagittal plane. A heterogenous hyperintense space-occupying the lesion is confirmed within the fourth ventricle. (B) T1-weighted post-contrast sagittal plane. A strong enhanced mass-like lesion is confirmed within the fourth ventricle. An enlarged third ventricle (asterisk) is revealed. (C) T1-weighted transverse plane. Hypo- to isointense lesion is confirmed. (D) T2 fluid-attenuated inversion recovery transverse plane. The lesion is revealed as a hyperintensive signal.
Surgical removal of the tumor using the telovelar approach was planned as described in a previous study (11). Prior to the anesthetic induction, maropitant (1 mg/kg, IV), dexamethasone (0.2 mg/kg, IV), and cefazoline (22 mg/kg, IV) were administered. The patient was premedicated with midazolam (0.2 mg/kg, IV) and administered propofol (4 mg/kg, IV) slowly, followed by intubation with a reinforced endotracheal tube to avoid kinking due to surgical position. The dog was maintained with 1–1.5% (vaporizer setting) isoflurane with a constant 100% O2 flow of 2 L/min. The patient was positioned with the neck flexed as much as possible, slightly over 90 degrees, to achieve the maximum opening of the foramen magnum, taking care not to compress the jugular vein. After the patient preparation, mannitol (0.25 g/kg, IV) was infused slowly over 15 min to achieve better brain relaxation and prevent reperfusion injury (20–22). During anesthesia, the monitoring parameters, including electrocardiogram, heart rate (HR), respiratory rate (RR), oxygen blood saturation (SpO2), rectal temperature, non-invasive blood pressure (NIBP), end-tidal CO2, tidal volume, airway pressure, and compliance, were within the normal range. Remifentanil was administered IV at a flow rate of 5–6ug/kg/h for analgesia.
The surgical approach to suboccipital craniectomy was performed routinely (11, 23). Suboccipital craniectomy was performed and additional partial dorsal laminectomy of the atlas was performed to expose the widest opening of the caudal fossa. Both procedures were conducted meticulously using a Kerrison rongeur. When the caudal fossa was sufficiently exposed, a neurosurgical microscope was used to magnify the surgical site and capture the image (Figure 2). Micro-bleeding from the dura mater was coagulated by bipolar electrocautery to maintain a clear surgical site. The dura mater was picked with a double-pronged tissue pick instrument and incised with a von Graefe knife without excessive tension. The incision was made from the level of the cerebellar uvula to the level of the atlas, longitudinally. The dura mater was retracted laterally without suturing. The tela choroidea was identified as the thin, avascular, membranous layer located between the uvula and the medulla oblongata. The tela choroidea was easily incised with a blunt-ended, 90°-angled Sisson nerve hook, and the tumor was identified through the incision. Neurosurgical lint-free sponges were packed around the tumor to visualize the tumor and retract the brain parenchyma. Visible blood vessels on the tumor were coagulated with a bipolar electrocautery. The hook probe, a micro-ring curette, and tumor forceps were used alternately to perform blunt dissection around the tumor with minimal traction. Meticulous maneuvering of the cerebellum dorsally with a semi-blunt Sachs nerve elevator through the sponge provided a wider surgical site. During the dissection, minor bleeding was controlled with sponges and ophthalmic triangular swabs. After the dissection, the mass was detached from the ventricle by grasping it with tumor forceps and removed as a single solitary piece. Minor bleeding after removal was controlled by attaching absorbable oxidized regenerated celluloses to the ventral surface of the ventricle and filling the ventricle with Hartmann’s solution. Sponges were removed and generous irrigation was performed to confirm the absence of residual bleeding and to spare the ventricle space. A thin sheath, thought to be a residual tumor capsule, was confirmed on the caudodorsal surface of the medulla oblongata and partially removed with Castroviejo scissors. The tela choroidea remained open, and the dura mater was sutured with a synthetic dura substitute (ReDura, Medprin Biotech, Frankfurt, Germany) to maximize the space over the foramen magnum using 6/0 absorbable suture material (6/0 PDS II, Ethicon, Raritan, NJ) in a single interrupted pattern. Closure of the muscle layer and skin was performed routinely. The entire removed mass was submitted for histopathological examination.
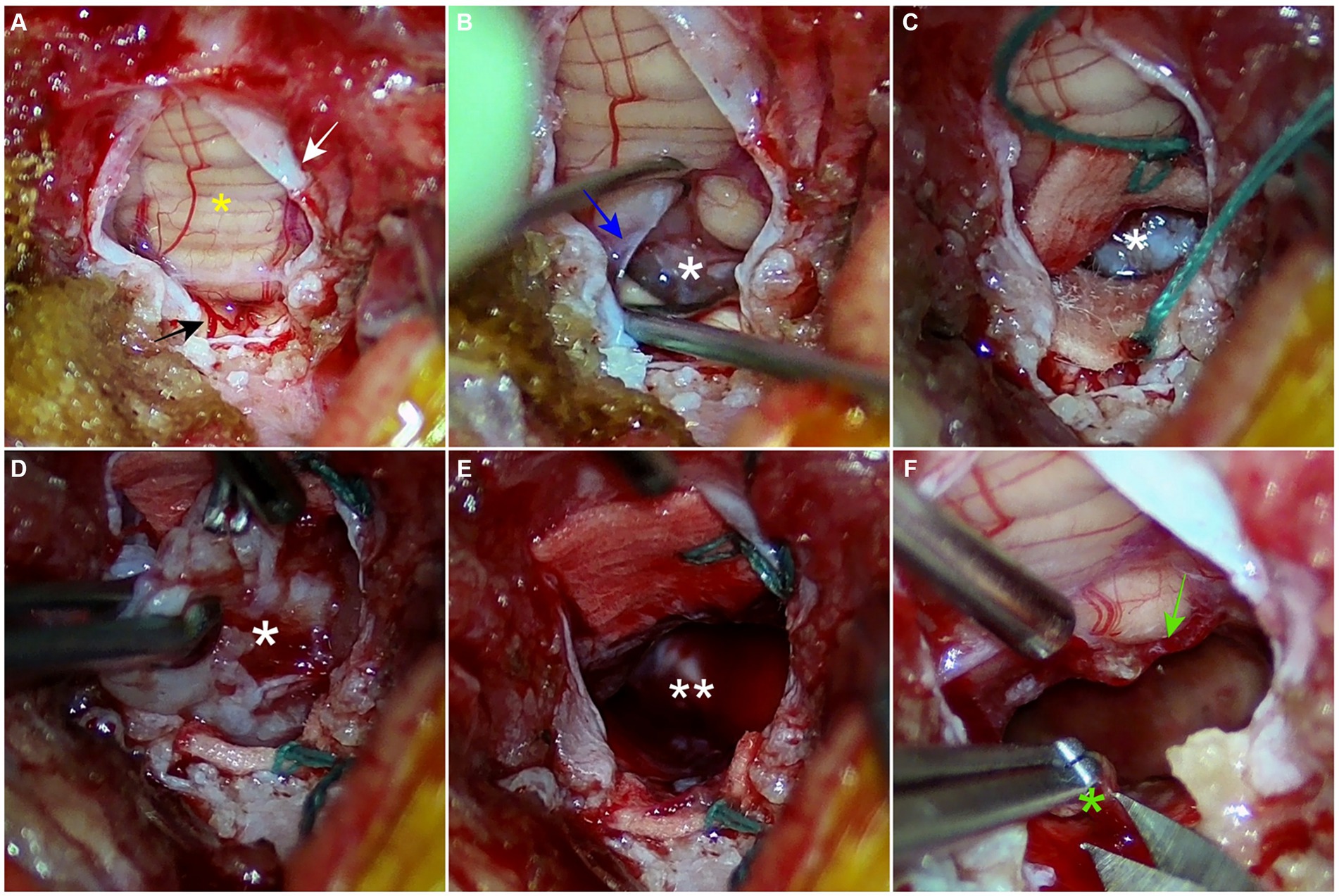
Figure 2. Intraoperative images from neurosurgical microscope. (A) A dural incision is made longitudinally from the level of the cerebellar uvula to the level of the atlas. The vermis and dura mater are indicated as a yellow asterisk and a white arrow, respectively. A branch of the caudal cerebral artery is highlighted with a black arrow. (B) An avascular tela choroidea (blue arrow) is incised with a nerve hook probe. The tumor (white asterisk) is confirmed through the incision. (C) Two green-stringed neurosurgical sponges are packed around the tumor to retract the brain parenchyma and secure the surgical field. (D) Through repeated meticulous maneuvers using tumor forceps, a hook probe, and a micro-ring curette, the tumor is bluntly dissected from the fourth ventricle (E) The tumor is removed, and the space (double white asterisks) of the fourth ventricle is confirmed. (F) The remaining suspected tumor capsule (green asterisk) is partially resected. The remaining choroid plexus is highlighted with a green arrow.
The patient was moved for a postoperative MRI to confirm the surgical removal of the tumor. Immediate postoperative MRI revealed the removal of the tumor, but a strongly enhancing structure was confirmed ventral to the caudal cerebellar lobe on T1W with gadolinium images (Figures 3A,B). The tumor was white to gray, oval-shaped, 15.8 × 10.5 × 10 mm, and firm (Figure 4A). Histological examination revealed dense spindle cells with an abundant collagen matrix, the typical finding of the fibrous subtype, and oval-shaped nucleated cells with small whorls, the common finding of the meningothelial subtype (Figures 4B,C). The combination of the findings, including medical imaging, gross appearance, and histopathologic findings, led to a definitive diagnosis of primary fourth ventricular transitional meningioma.
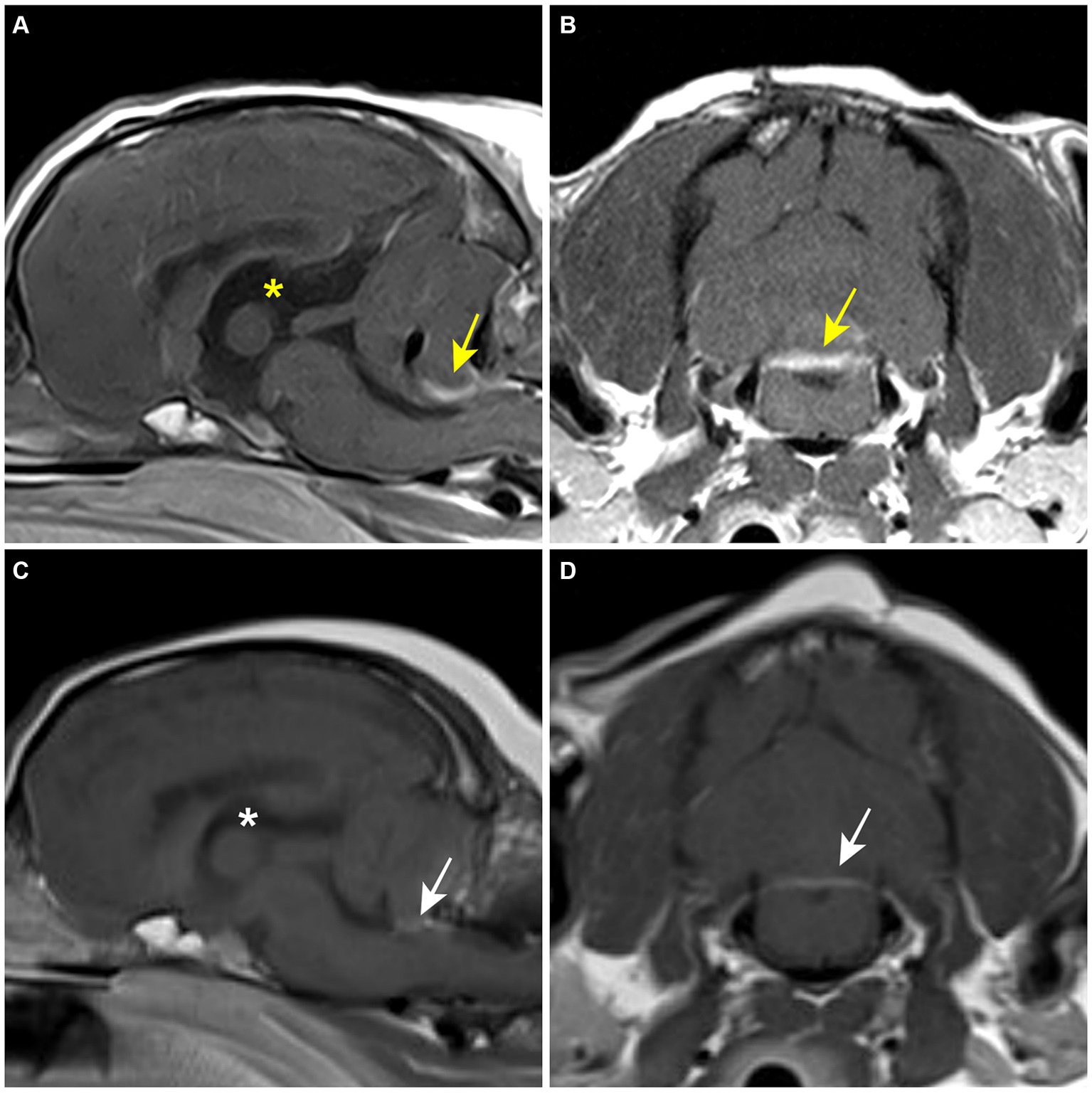
Figure 3. Postoperative MRI. (A,B) Immediate postoperative T1-weighted post-contrast MRI. A contrast-enhanced lesion (yellow arrow) is revealed on the caudoventral aspect of the cerebellum. The third ventricle (yellow asterisk) is still enlarged. (C,D) 8-month postoperative T1-weighted post-contrast MRI. Contrast-enhanced lesion (white arrow) and the third ventricle (white asterisk) are reduced compared to the former MRI findings.
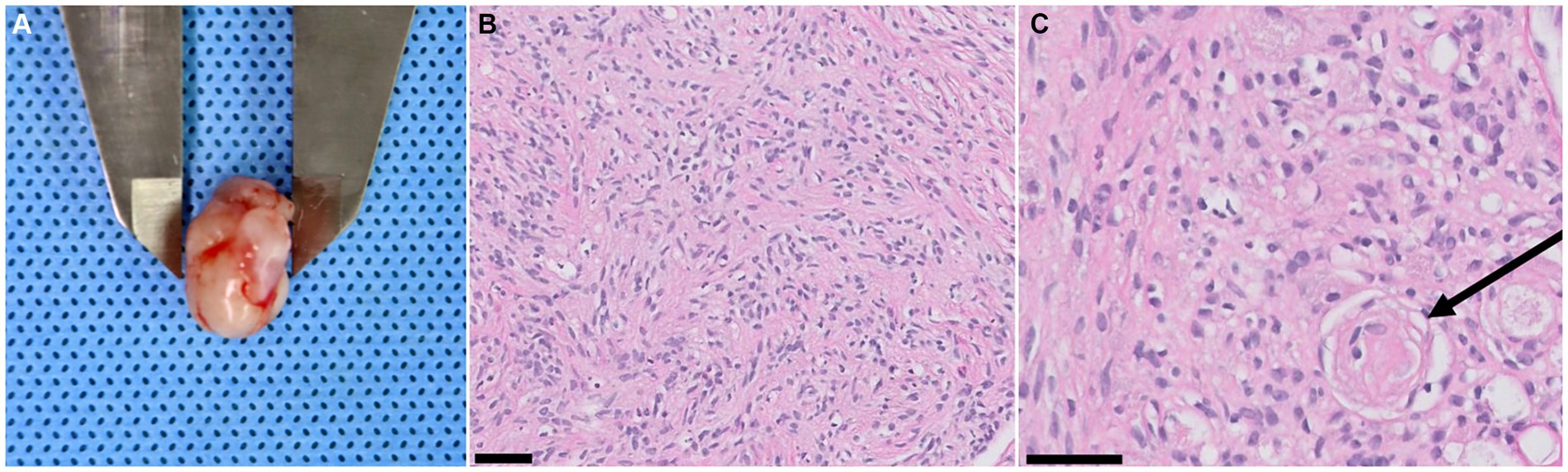
Figure 4. Tumor gross appearance and biopsy results. (A) A resected fourth ventricular tumor. The gross appearance is white to gray (relatively low vascularization), oval-shaped, 15.8 × 10.5 × 10 mm in size, and has a firm texture. (B) Dense spindle cells with an abundant collagen matrix are confirmed. These findings are typical findings of the fibrous subtype of meningioma (magnification ×20, black 50 μm scale bar, hematoxylin and eosin stain). (C) Oval-shaped nucleated cells with small whorls (black arrow) are revealed, indicating the meningothelial subtype meningioma. (magnification ×40, black 50 μm scale bar, hematoxylin and eosin stain).
The patient recovered smoothly from anesthesia. Vital signs were monitored and neurological examinations were performed at regular intervals throughout the convalescent period. There was no significant alteration of vital parameters, such as HR, PR, NIBP, and body temperature, during monitoring. On the day of the surgery, non-ambulatory paraparesis with hindlimb rigidity was observed but improved after a single infusion of mannitol (0.5 g/kg, IV, slowly over 15 min). A single event of mental dullness was observed 3 days after the surgery, but it soon recovered after a single administration of mannitol (0.5 g/kg, IV, slowly over 15 min). Preoperative signs, such as vomiting and vestibular signs, improved progressively, and ambulation recovered 6 days after the surgery. During hospitalization, amoxicillin-clavulanic acid (20 mg/kg, IV, q12hr), prednisolone (0.5 mg/kg, PO, q12hr), levetiracetam (20 mg/kg, IV, q8hr), gabapentin (10 mg/kg, PO, q12hr), esomeprazole (1 mg/kg, IV, q12hr), and maropitant (1 mg/kg, IV, q24hr) were administered. Acetazolamide (10 mg/kg, PO, q8hr) was added after the event of mental dullness. Remifentanil was infused (6ug/kg/h, IV) for analgesia, gradually tapered according to the monitored pain response, and withdrawn at 3 days post-surgery. The patient was discharged 10 days after surgery with remaining neurological signs, including positional nystagmus, slight head tilt, and hypermetria. The antibiotic was discontinued and hydroxyurea (50 mg/kg, orally, every other day) was added to the medication upon discharge.
At the first follow-up, 43 days after surgery, the patient showed head tilt, positional nystagmus, and hypermetria, but other clinical signs had resolved. Medication was modified to exclude acetazolamide and reduce the dosage of prednisolone to 0.3 mg/kg, q12hr. At 6-month postoperative follow-up, neurologic signs recover to normal, except for a slight head tilt. Levetiracetam was discontinued, and prednisolone was reduced to 0.5 mg/kg, q24hr. Eight months after the surgery, a follow-up MRI was requested by the client to verify no recurrence of the tumor. The former contrast-enhanced structure had reduced significantly and appeared to be a membranous structure (Figures 3C,D). The size of the lateral ventricle returned to within the normal reference; preoperative ventricle/brain index: 0.61, 8-month postoperative ventricle/brain- index: 0.56 (24). There was no recurrence of the mass-like lesion in the fourth ventricle. At the final follow-up at 15.4 months postoperatively, the patient showed a slight head tilt and subtle positional strabismus. The client was satisfied with the outcome. Throughout the follow-up, regular blood work, including complete blood count, total protein, globulin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase and gamma-glutamyl transferase, was performed every 2 months to monitor the effect of the medication, especially prednisolone and hydroxyurea. Mild anemia and elevated ALT and ALP were observed throughout the medication but without further deterioration. Based on the MRI findings and the absence of further deterioration in neurological signs, it was presumed that there was no recurrence of the tumor.
3 Discussion
This case report describes the first successful surgical removal of a primary fourth ventricular transitional meningioma in a dog, providing a detailed account of MRI findings, surgical technique, postoperative care, and outcome. Despite the uncommon occurrence of intraventricular meningiomas in dogs, it is essential to consider meningioma in the differential diagnosis for fourth ventricular masses due to their differing characteristics from CPTs.
The recurrence of the brain tumor is closely related to the completeness of the resection. Although there are limited cases of surgical treatment of fourth ventricle tumors, meningiomas are thought to be more feasible to resect totally in a gross manner than CPT. In our case, the tumor was well-encapsulated and relatively less vascularized. Therefore, it was able to be removed en bloc without piecemeal maneuvering and with minimal bleeding. A previous study described that a CPT was removed in three pieces and the figures showed that the sponge was notably red, indicating more bleeding from the tumor resection compared to our case (11). Another report, describing the removal of lateral ventricular CPT in a dog, reported bleeding as the main intraoperative complication (25). Additionally, human literature indicates that uncontrollable hemorrhage during CPT resection is associated with perioperative mortality, which can range up to 30% (26). Although it is difficult to draw definitive conclusions from a single case, these characteristics can impact surgical outcomes. Therefore, further research through a larger number of case studies on a fourth ventricular meningioma is necessary to better understand the prognosis related to surgery.
Preoperatively, CPT was the primary consideration due to its neuroanatomical prevalence and histological origin. The choroid plexus consists of pia, ependyma, and proliferated capillaries and is found within the ventricular system (17). CPTs, including choroid plexus papilloma (CPP) and carcinoma (CPC), originate from the choroid plexus epithelium (10, 27). In contrast, meningiomas typically arise from arachnoid cap cells (1). To date, only four cases of intraventricular meningiomas have been reported in the veterinary literature, including one case of primary fourth ventricular microcystic meningioma, two cases of lateral ventricular meningioma, and the current case (6, 7, 9). Although studies on the origin of intraventricular meningiomas in veterinary medicine are limited, human literature suggests they may arise from the stroma of the choroid plexus or tela choroidea (28). The diagnosis of meningioma in our case adds further evidence that meningiomas can indeed occur in this location within the ventricular system of dogs.
MRI is considered the gold standard for diagnosing brain diseases. However, differentiating between CPC and intraventricular meningioma using MRI is challenging. Some pathological differences may be reflected in MRI findings (29). CPCs are highly vascular, with abundant microvasculature, making them more likely to appear hypointense on T1W, hyperintense on T2W, and exhibit signal voids on FLAIR (30). Additionally, their gross appearance can be indicated by an irregular margin on MRI images (31). According to previous veterinary studies, these MRI features of CPT align with the pathological hypotheses (1, 10, 13, 32–34). In our case, the tumor showed iso- to hypointensity on T1W, heterogeneous hyperintensity on T2W, hyperintensity on FLAIR, and strong enhancement after gadolinium infusion. However, the few reported intraventricular meningioma cases, including our case, showed significant variation in MRI findings (6, 7, 9). Therefore, MRI alone is not reliable for differentiating between CPTs and intraventricular meningiomas. Further studies are needed to correlate MRI findings with histological characteristics to improve diagnostic accuracy.
In our case, postoperative MRI sequences confirmed that the tumor was removed and showed no signs of recurrence at 8 months postoperatively. An initial contrast-enhanced lesion observed on the immediate postoperative MRI was found to have reduced in size on the 8-month postoperative MRI. Differential diagnoses for this lesion included residual choroid plexus, inflammation, and remaining tumor tissue. The choroid plexus was considered the most likely due to the surgical findings, the lesion’s size reduction, and prolonged use of prednisolone. The choroid plexus, located beneath the caudal lobe of the cerebellum and extending into the lumen of the fourth ventricle, can enhance with contrast due to its rich blood supply and lack of a blood–brain barrier (17, 35). Furthermore, a residual sheath, presumed to be the tumor capsule, was identified on the caudodorsal surface of the brainstem, which did not correspond to the lesion’s location. However, there remains a possibility of residual neoplastic cells in the choroid plexus since intraventricular meningiomas can originate from it. Thus, regular follow-ups to monitor neurological status and two postoperative MRI scans have been implemented.
The temporary deterioration of neurological signs observed post-surgery, including non-ambulatory paraparesis and mental dullness, may be attributed to retraction and maneuvering during the procedure, as well as to reperfusion damage and subsequent edema following decompression Additionally, it could be a result of acute postoperative hydrocephalus. According to human literature, residual hemostatic material is known to be a risk factor for acute postoperative hydrocephalus since it can cause occlusion of cerebrospinal fluid flow (36, 37). The interaction between cerebrospinal fluid, blood, and foreign bodies tends to promote coagulation (38). Predictive factors for acute hydrocephalus following lateral ventricular tumor resection include preoperative hydrocephalus and the presence of intraventricular hemostatic agents, both of which were relevant in our case (39). Therefore, meticulous handling and protection of the parenchyma during surgery, along with generous irrigation with warm fluid, are crucial for preventing complications after the fourth ventricular tumor resection (36). Additionally, careful monitoring and immediate treatment are essential for managing postoperative complications (40).
This case report has several limitations. First, it is based on a single case, thus further studies are needed to verify MRI findings for diagnosing intraventricular tumors and to evaluate the prognosis of surgically resected intraventricular meningiomas. Additionally, the pathomechanism of mental dullness observed 3 days after the surgery could not be explained, as MRI or CT images were not taken at the time. Finally, although there was no evidence of recurrence according to neurological examination and MRI at the final follow-up, the risk of recurrence remains. Therefore, further investigation of the long-term outcomes is necessary.
In conclusion, this case report presents the first instance of a surgically treated primary fourth ventricular meningioma in a dog, providing a detailed account of MRI findings, surgical technique, postoperative care, and its satisfactory outcome. This case highlights the importance of including meningioma in the differential diagnoses for fourth ventricular masses, despite the rarity of intraventricular meningiomas in dogs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethic statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because we report a case study of a veterinary hospital. We have the consent of both the owner and veterinarian that this dog (underwent the listed examinations and surgical intervention) was treated not for experimental but for medical reasons. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JJ: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YR: Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. YJ: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a basic science research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00247989).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miller, AD, Miller, CR, and Rossmeisl, JH. Canine primary intracranial Cancer: a Clinicopathologic and comparative review of glioma, meningioma, and choroid plexus tumors. Front Oncol. (2019) 9:1151. doi: 10.3389/fonc.2019.01151
2. Rossmeisl, JH, and Pancotto, TE. Tumors of the nervous system In: DM Vail, DH Thamm, and JM Liptak, editors. Withrow & Macewen's small animal clinical oncology. 6th ed. St. Louis, MI: Elsevier Health Sciences (2019). 657–74.
3. Miller, AD, Koehler, JW, Donovan, TA, Stewart, JE, Porter, BF, Rissi, DR, et al. Canine Ependymoma: diagnostic criteria and common pitfalls. Vet Pathol. (2019) 56:860–7. doi: 10.1177/0300985819859872
4. Lehner, L, Czeibert, K, Csébi, P, Diószegi, K, and Nagy, G. Case report: intraventricular tumor removal using Transcallosal approach and follow-up in three dogs. Front Vet Sci. (2023) 10:1240934. doi: 10.3389/fvets.2023.1240934
5. Rissi, DR, Levine, JM, Eden, KB, Watson, VE, Griffin, JF IV, Edwards, JF, et al. Cerebral Oligodendroglioma mimicking intraventricular neoplasia in three dogs. J Vet Diagn Invest. (2015) 27:396–400. doi: 10.1177/1040638715584619
6. Ortiz-Nisa, S, de la Fuente, C, Sant’Ana, F, Pumarola, M, and Añor, S. Clinical, imaging and histopathological characteristics of a malignant intracranial meningioma with pulmonary metastasis in a dog. Vet Rec Case Rep. (2022) 10:e365. doi: 10.1002/vrc2.365
7. Salvadori, C, Pintore, MD, Ricci, E, Konar, M, Tartarelli, CL, Gasparinetti, N, et al. Microcystic meningioma of the fourth ventricle in a dog. J Vet Med Sci. (2011) 73:367–70. doi: 10.1292/jvms.10-0337
8. Hansen, KS, Li, CF, Théon, AP, and Kent, MS. Stereotactic radiotherapy outcomes for intraventricular brain Tumours in 11 dogs. Vet Comp Oncol. (2023) 21:665–72. doi: 10.1111/vco.12929
9. Ijiri, A, Yoshiki, K, Tsuboi, S, Shimazaki, H, Akiyoshi, H, and Nakade, T. Surgical resection of twenty-three cases of brain meningioma. J Vet Med Sci. (2014) 76:331–8. doi: 10.1292/jvms.12-0373
10. Westworth, D, Dickinson, P, Vernau, W, Johnson, E, Bollen, A, Kass, P, et al. Choroid plexus tumors in 56 dogs (1985–2007). J Vet Intern Med. (2008) 22:1157–65. doi: 10.1111/j.1939-1676.2008.0170.x
11. Antonakakis, MG, Carletti, BE, Anselmi, C, McGrath, S, and Minguez, JJ. Use of a Telovelar approach for complete resection of a choroid plexus tumor in a dog. Vet Surg. (2022) 51:1273–9. doi: 10.1111/vsu.13859
12. Minato, S, Cherubini, GB, Della Santa, D, Salvadori, S, and Baroni, M. Incidence and type of brain herniation associated with intracranial meningioma in dogs and cats. J Vet Med Sci. (2021) 83:267–73. doi: 10.1292/jvms.20-0111
13. Snyder, JM, Shofer, FS, Van Winkle, TJ, and Massicotte, C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med. (2006) 20:669–75. doi: 10.1111/j.1939-1676.2006.tb02913.x
14. Nakamura, M, Roser, F, Bundschuh, O, Vorkapic, P, and Samii, M. Intraventricular Meningiomas: a review of 16 cases with reference to the literature. Surg Neurol. (2003) 59:490–503. doi: 10.1016/S0090-3019(03)00082-X
15. Lyngdoh, BT, Giri, PJ, Behari, S, Banerji, D, Chhabra, DK, and Jain, VK. Intraventricular Meningiomas: a surgical challenge. J Clin Neurosci. (2007) 14:442–8. doi: 10.1016/j.jocn.2006.01.005
16. Sufianov, R, Pitskhelauri, D, and Bykanov, A. Fourth ventricle tumors: a review of series treated with microsurgical technique. Front Surg. (2022) 9:915253. doi: 10.3389/fsurg.2022.915253
17. de Lahunta, A, Glass, E, and Kent, M. Neuroanatomy gross description and atlas of transverse sections and magnetic resonance images In: De Lahunta's veterinary neuroanatomy and clinical neurology. Philadelphia, PA: Elsevier (2021). 6–44.
18. Deshmukh, VR, Figueiredo, EG, Deshmukh, P, Crawford, NR, Preul, MC, and Spetzler, RF. Quantification and comparison of Telovelar and Transvermian approaches to the fourth ventricle. Oper Neurosurg. (2006) 58:ONS-202–7. doi: 10.1227/01.NEU.0000207373.26614.BF
19. Ebrahim, KS, and Toubar, AF. Telovelar approach versus Transvermian approach in management of fourth ventricular tumors. Egypt J Neurosurg. (2019) 34:1–10. doi: 10.1186/s41984-019-0036-9
20. Zhang, W, Neal, J, Lin, L, Dai, F, Hersey, DP, McDonagh, DL, et al. Mannitol in critical care and surgery over 50+ years: a systematic review of randomized controlled trials and complications with Meta-analysis. J Neurosurg Anesthesiol. (2019) 31:273–84. doi: 10.1097/ANA.0000000000000520
21. Shan, R, Zhou, H, Liu, X, Su, G, Liu, G, Zhang, X, et al. Neuroprotective Effects of Four Different Fluids on Cerebral Ischaemia/Reperfusion Injury in Rats through Stabilization of the Blood–Brain Barrier. Eur J Neurosci. (2021) 54:5586–600. doi: 10.1111/ejn.15385
22. Grana, IL, Mariné, AF, MPI, B, and Feliu-Pascual, AL. Successful surgical resection of an ependymal cyst in the fourth ventricle of a dog. J Am Anim Hosp Assoc. (2024) 60:25–30. doi: 10.5326/JAAHA-MS-7373
23. Akin, EY, and Shores, A. Suboccipital Craniectomy/foramen magnum decompression In: A Shores and BA Brisson, editors. Current techniques in canine and feline neurosurgery. Hoboken, NJ: John Wiley and Sons (2017). 115–20.
24. Laubner, S, Ondreka, N, Failing, K, Kramer, M, and Schmidt, MJ. Magnetic resonance imaging signs of high intraventricular pressure-comparison of findings in dogs with clinically relevant internal hydrocephalus and asymptomatic dogs with Ventriculomegaly. BMC Vet Res. (2015) 11:1–11. doi: 10.1186/s12917-015-0479-5
25. Lehner, L, Czeibert, K, Benczik, J, Jakab, C, and Nagy, G. Transcallosal removal of a choroid plexus tumor from the lateral ventricle in a dog Case Report. Front Vet Sci. (2020) 7:536. doi: 10.3389/fvets.2020.00536
26. Asmaro, K, Pawloski, J, and Skoch, J. Giant choroid plexus papilloma resection utilizing a Transcollation system. Oper Neurosurg. (2020) 18:47–51. doi: 10.1093/ons/opz096
27. Higgins, RJ, Bollen, AW, Dickinson, PJ, and Sisó-Llonch, S. Tumors of the nervous system In: DJ Meuten, editor. Tumors in domestic animals. 5th ed. Ames, Iowa: John Wiley and Sons (2017). 834–91.
28. Osborn, A. Meningiomas and other nonglial neoplasms In: A Patterson, editor. Diagn Neuroradiol. St. Louis, MI: Mosby-Year Book (1994). 620–2.
29. Takeuchi, S, Sugawara, T, Masaoka, H, and Takasato, Y. Fourth ventricular meningioma: a case report and literature review. Acta Neurol Belg. (2012) 112:97–100. doi: 10.1007/s13760-012-0040-2
30. Nakano, S, Uehara, H, Wakisaka, S, and Kinoshita, K. Meningioma of the fourth ventricle—case report—. Neurol Med-Chir. (1989) 29:52–4. doi: 10.2176/nmc.29.52
31. Alver, I, Abuzayed, B, Kafadar, AM, Muhammedrezai, S, Sanus, GZ, and Ziya, A. Primary fourth ventricular meningioma: case report and review of the literature. Turk Neurosurg. (2011) 21:249–53. doi: 10.5137/1019-5149.jtn.2869-09.0
32. Ródenas, S, Pumarola, M, Gaitero, L, Zamora, À, and Añor, S. Magnetic resonance imaging findings in 40 dogs with histologically confirmed intracranial Tumours. Vet J. (2011) 187:85–91. doi: 10.1016/j.tvjl.2009.10.011
33. Wisner, ER, Dickinson, PJ, and Higgins, RJ. Magnetic resonance imaging features of canine intracranial neoplasia. Vet Radiol Ultrasound. (2011) 52:S52–61. doi: 10.1111/j.1740-8261.2010.01785.x
34. Bentley, RT. Magnetic resonance imaging diagnosis of brain tumors in dogs. Vet J. (2015) 205:204–16. doi: 10.1016/j.tvjl.2015.01.025
35. McKinney, AM. Choroid plexus: Normal locations and appearances In: Atlas of Normal imaging variations of the brain, skull, and Craniocervical vasculature. Cham, Switzerland: Springer (2017). 177–237.
36. Hendricks, BK. Principles of intraventricular surgery: neurosurgical atlas. (2016). Available at: https://www.neurosurgicalatlas.com/volumes/brain-tumors/intraventricular-tumors/principles-of-intraventricular-surgery.
37. Ktari, O, Frassanito, P, Gessi, M, Bianchi, F, Tamburrini, G, and Massimi, L. Gelfoam migration: a potential cause of recurrent hydrocephalus. World Neurosurg. (2020) 142:212–7. doi: 10.1016/j.wneu.2020.06.214
38. Vandersteene, J, Baert, E, Planckaert, GM, Van Den Berghe, T, Van Roost, D, Dewaele, F, et al. The influence of cerebrospinal fluid on blood coagulation and the implications for Ventriculovenous shunting. J Neurosurg. (2018) 130:1244–51. doi: 10.3171/2017.11.jns171510
39. Zhang, C, Ge, L, Li, Z, Zhang, T, and Chen, J. Single-center retrospective analysis of risk factors for hydrocephalus after lateral ventricular tumor resection. Front Surg. (2022) 9:886472. doi: 10.3389/fsurg.2022.886472
Keywords: meningioma, fourth ventricle, fourth ventricular meningioma, telovelar approach, dog
Citation: Jeong J, Lee H, Rho Y and Jeon Y (2024) Case report: Gross total resection of a primary fourth ventricular meningioma using the telovelar approach in a dog. Front. Vet. Sci. 11:1450332. doi: 10.3389/fvets.2024.1450332
Edited by:
Theresa Elizabeth Pancotto, Virginia Tech, United StatesReviewed by:
Sam Long, Veterinary Referral Hospital, AustraliaAnita Shea, Massey University, New Zealand
Copyright © 2024 Jeong, Lee, Rho and Jeon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: YoungJin Jeon, b3JhbmdlZTAxMTVAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Jaemin Jeong
Jaemin Jeong Haebeom Lee
Haebeom Lee Yoonho Rho
Yoonho Rho YoungJin Jeon
YoungJin Jeon