- 1National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido, Japan
- 2Department of Parasitology, Faculty of Veterinary Medicine, Vietnam National University of Agriculture, Hanoi, Vietnam
- 3Department of Parasitology, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand
- 4Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
Dog owners are greatly concerned about tick infestations in their pets. The prevalence and dispersion of ticks and their disease-causing microorganisms have been limited from the viewpoint of dog owners in Vietnam. This study investigated the presence of tick infestation and the pathogens associated with it in canines that were brought to veterinary hospitals in Vietnam. In the survey, 1,423 dogs participated from February to October 2022. Molecular and morphological methods were utilized to identify ticks and the associated pathogens. In addition,risk variables linked to tick infestation were documented and analyzed using statistical methods. The total exposure to the brown dog tick (Rhipicephalus sanguineus sensu lato) was 29.01%. Nam Dinh has the highest tick prevalence among the research areas. Tick infestation reached its highest point between June and September in the northern region of the country, with distinct seasons showing a strong correlation with tick infestation in dogs. Out of 177 tick pools examined, 146(82.49%) tested positive for at least one infection. Mycoplasma spp. (78.53%) was the most common, followed by Anaplasma spp. (37.29%), Rickettsia felis (5.08%), Babesia vogeli, and Hepatozoon canis (2.82%). In the current study, there was a statistically significant link between tick infestation and characteristics such as age, breed, body size, lifestyle, and bathing frequency. Understanding the seasonal behavior of vector ticks is crucial for identifying individuals or animals susceptible to tick-borne diseases. Studying the distribution of ticks and their ability to carry and disseminate zoonotic germs in specific places could assist veterinarians and policymakers in implementing effective strategies to manage zoonotic infections.
1 Introduction
Ticks are significant blood-feeding carriers of disease-causing agents that impact both pets and humans worldwide. Ticks can transfer many zoonotic pathogens such as viruses (tick-borne encephalitis virus), bacteria (Anaplasma, Ehrlichia, Rickettsia), and protozoa (Babesia and Hepatozoon) to vertebrate hosts during blood feeding, which might damage their health (1). More than seven tick species have been recently found in dogs in Asia, such as Rhipicephalus haemaphysaloides, Rhipicephalus sanguineus, Haemaphysalis longicornis, Haemaphysalis campanulata, Haemaphysalis wellingtoni, Haemaphysalis hystricis, and species within the genus Ixodes (2). Among these, R. sanguineus, also known as the brown dog tick and taxonomically designated as R. sanguineus sensu lato (s.l.) of tropical lineage, was the predominant tick species infesting dogs in numerous nations (2). Rhipicephalus sanguineus is believed to transmit many infections that cause babesiosis, hepatozoonosis, ehrlichiosis, mycoplasmosis, and rickettsiosis in dogs throughout Southeast Asia (3, 4). Apicomplexan protozoa parasites often found in dogs worldwide include species of Babesia (Babesia canis, Babesia gibsoni, and Babesia vogeli) and species of Hepatozoon (Hepatozoon canis and Hepatozoon americanum) (5, 6). Anaplasmataceae bacteria, including Ehrlichia canis and Anaplasma platys, are the most common bacterial tick-borne diseases found in dogs in Asia. All these infections are transferred through the vector’s bite during blood feeding, except for Hepatozoon, which is transmitted through the ingestion of R. sanguineus (7).
Vietnam is one of the rapidly developing economies in Asia, leading to an increase in the number of companion animals. Vietnam is geographically organized into three primary regions: Northern, Central, and Southern. It features both tropical and temperate temperature zones. In contrast to the South, which has two different seasons—dry and rainy—the North experiences four distinct seasons: spring, summer, autumn, and winter. The northern regions experience summertime averages of 22°C–27.5°C and wintertime averages of 15°C–20°C. Southern regions see consistently high temperatures ranging from 26°C to 29°C year-round.1 So far, the only type of tick found on dogs during studies on ticks in dogs in Vietnam was the brown dog tick (2, 8, 9). Protozoa and bacterial tick-borne organisms were recently found in dogs in Vietnam, with a detection rate ranging from 0.8% to 25.8% (2). The presence of a large dog population, favorable weather conditions, and close contact between humans and pet dogs facilitate the spread of zoonotic tick-borne diseases in the area. There have been few surveillance efforts to quantify tick abundance in dogs and to comprehend their frequency and spatial distribution in Vietnam. This study aimed to survey ticks infesting privately owned dogs at veterinary hospitals in Vietnam and detection of selective tick-borne pathogens that are reported commonly in dogs and ticks in Asia. It involved morphological and genetic species characterization of the collected ticks. Factors contributing to tick infestation were documented and examined.
2 Materials and methods
2.1 Ethical consent
The Ethics Committee of Obihiro University of Agriculture and Veterinary Medicine accepted the protocol for using animal samples in this work (Permit for animal experiment: 21-25; DNA experiment: 1725-5).
2.2 Area of study, collection of samples, and tick identification
A survey on tick infestation in dogs was conducted from February to October 2022 with voluntary participation from veterinary clinics in Hanoi (21°01′N, 105°51′E), Ho Chi Minh City (10°46’N; 106°42′E), Nam Dinh (20°25′N; 106°10′E), and Dak-Lak (12°4′N, 108°3′E) in Vietnam (Figure 1). Veterinarians conducted a comprehensive inspection of the animals to detect ticks by inspecting the entire body surface for more than 5 min. The examinations involved assessing the animals’ physical condition and noting any abnormalities in the skin/haircoat and mucous membranes such as jaundice, paleness, or hemorrhaging. A questionnaire was created to collect data about the sampled dogs’ location, breed, sex, age, and body size for analyzing several categories related to tick infestation. Further inquiries were made regarding the living environment (indoor, outdoor, or semi-outdoor) and bathing frequency (weekly, monthly, every 2 months, or annually) if applicable.
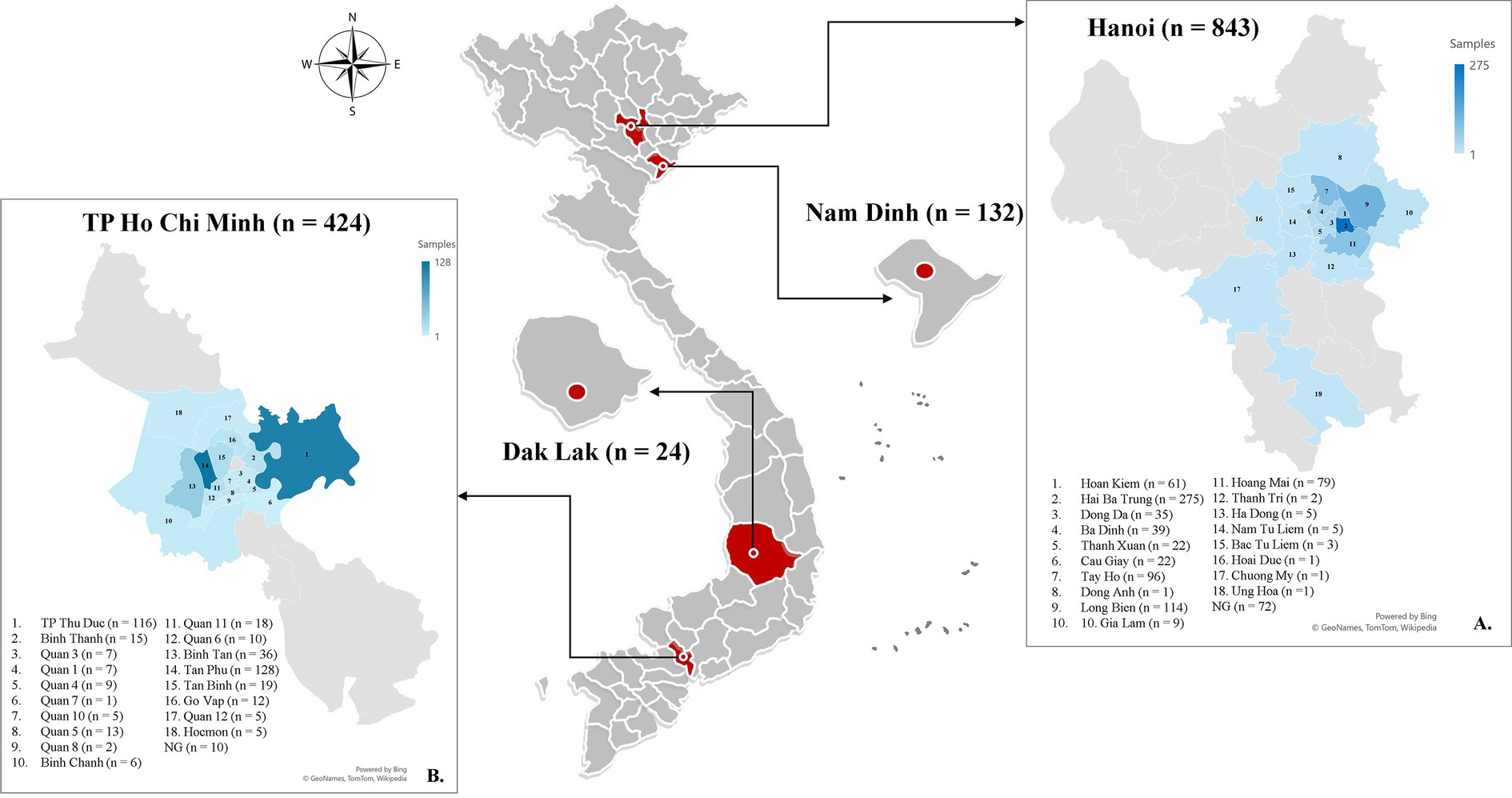
Figure 1. Map showing the locations where tick infestation on dogs is being studied in Vietnam, with red circles indicating the sampling sites in each province. The blue highlighted districts indicate the specific geographic areas where samples were gathered in Hanoi (A) and Ho Chi Minh City (B).
From one to five ticks attached to the dog were collected and stored in 1.5 mL tubes containing 70% ethanol. The specimens were subsequently transferred to the National Research Center for Protozoan Diseases in Obihiro, Hokkaido, Japan for identification using morphological and molecular analysis. A total of 555 ticks were submitted for species identification and detection of microorganisms. Ticks were examined using a stereomicroscope (Olympus SZX16) to determine their species and life stage (larva, nymph, or adult) by utilizing specific physical characteristics (10). Following the initial morphological identification, the ticks were rinsed extensively with phosphate-buffered saline. Afterwards, ticks in the identical life stage and obtained from the identical dog were pooled for the DNA extraction using an DNeasy Blood and Tissue Kits (Quiagen, Germany) as per the manufacturer’s guidelines. Following the pooling process, a total of 177 pools was obtained, with each pool containing between one and five individual ticks for DNA extraction. Following DNA extraction, the sequences aimed at the species-specific gene were amplified to molecularly confirm the tick species and their associated microorganism (Table 1).
2.3 Molecular detection and phylogenetic analysis of microorganism
The DNA samples underwent screening for apicomplexan protozoa (such as Babesia spp., Hepatozoon spp.), bacteria of Anaplasmataceae family (such as A. platys, E. canis), Mycoplasma spp., Rickettsia spp., and Bartonella spp. using primer sets by conventional PCR (cPCR). The cPCR amplified targeted sequences of Ehrlichia/Anaplasma spp. (16S rRNA gene), Babesia/Hepatozoon spp. (18S rRNA gene), and Rickettsia spp. from the spotted fever group (OmpA). Positive samples for the genus of the stated species were then analyzed using primers that target species-specific sequences by cPCR or nested PCR. All primers and the target genes utilized for pathogen detection were outlined in Table 1. Pathogen-positive samples and distilled deionized water were utilized as the positive and negative controls, respectively, for all experiments. The PCR amplicons were examined using electrophoresis in a 1.5% agarose gel (LE agarose, Thermo Fisher Scientific, Waltham, MA, United States) and seen under a UV transilluminator (ATTO, Tokyo, Japan). DNA was extracted and purified from the positive amplicon by excising it from a gel using a Gel Extraction Kit from QIAGEN, Germany. The result, purified and having the desired sequences, underwent sequencing using the BigDye v3.1 Terminator Cycle Sequencing Kit in an ABI PRISM 3100 Genetic Analyzer by Applied Biosystem, United States.
The sequences’ chromatograms were evaluated with BioEdit software (version 7.5.2) and compared to sequences in the Genbank database using the Basic Local Alignment Search Tool (BLAST) on the U.S. National Center for Biotechnology Information (NCBI) website. This work evaluated the genetic variation of Mycoplasma spp. and Rickettsia felis by examining 16S rRNA gene-based Mycoplasma spp. sequences (618 bp) and gltA gene-based R. felis sequences (654 bp) using phylogenetic analysis with the MEGA X tool. Phylogenetic trees were created using the maximum likelihood method and the most suitable substitution model. A bootstrap analysis with 1,000 replications was performed to evaluate the reliability of the branching patterns in the trees. Every sequence is provided with Genbank accession numbers, isolation sources, and countries of origin.
2.4 Statistical analysis
The OpenEpi application was utilized to calculate the detection rate of tick infestation, determine the percentage of discovered diseases, and estimate 95% confidence intervals (95% CI).2 A chi-square test was used to investigate the statistical relationship between the prevalence of tick infestation and the host independent factors. The odds ratio (OR) was calculated to assess the strength of the relationship between each category and tick infestation, determining if there was a significant association between them. The level of statistical significance was established at p ≤ 0.05. The study utilized a confidence level of 95%. The Statulator tool was utilized for data analysis.3
3 Results
3.1 Sample data and tick morphological identification
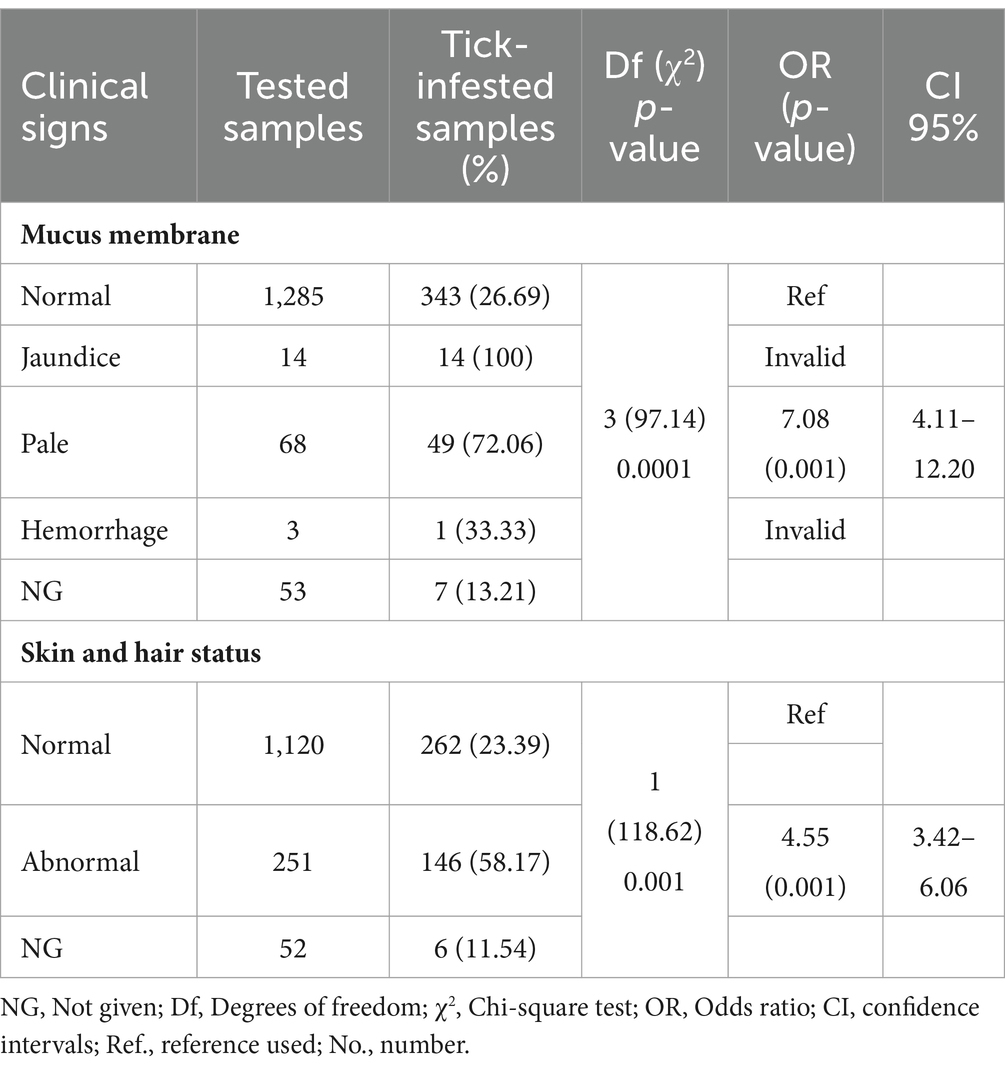
A total of 1,423 dogs participated in the survey, with 843 from Hanoi (59.24%), 424 from Ho Chi Minh City (29.8%), 132 from Nam Dinh (9.28%), and 24 from Dak-lak (1.68%; Figure 1). The enrolled dogs included: 630 females (44.27%), 729 males (51.23%), and 64 with unreported data (4.5%). The animals’ ages varied from 2 months to 20 years, with around 43.5% (619) of the population falling between one and 5 years old. The dogs were of more than 20 breeds, which were divided into three groups. Details of different breeds of dogs observed in the current study were presented in Figure 2; of which, 1,145 (80.46%) were of the exotic breed, 161 (11.31%) were of the domestic breed. The majority were indoor lifestyle (662, 46.52%), small size (≤3.5 kg; 462, 32.46%), and bathed at least once a week (707, 49.68%). Clinical symptoms such as skin and mucous membrane abnormalities were found to be statistically linked to the presence of tick infestation in dogs (Table A1). A total of 555 ticks were submitted for species identification and detection of microorganisms. In particular, based on the morphological typical traits, 33 larvae, 75 nymphs, and 447 adults (195 males and 252 females) are identified as belonging to the species complex R. sanguineus s.l. (Figure 3).
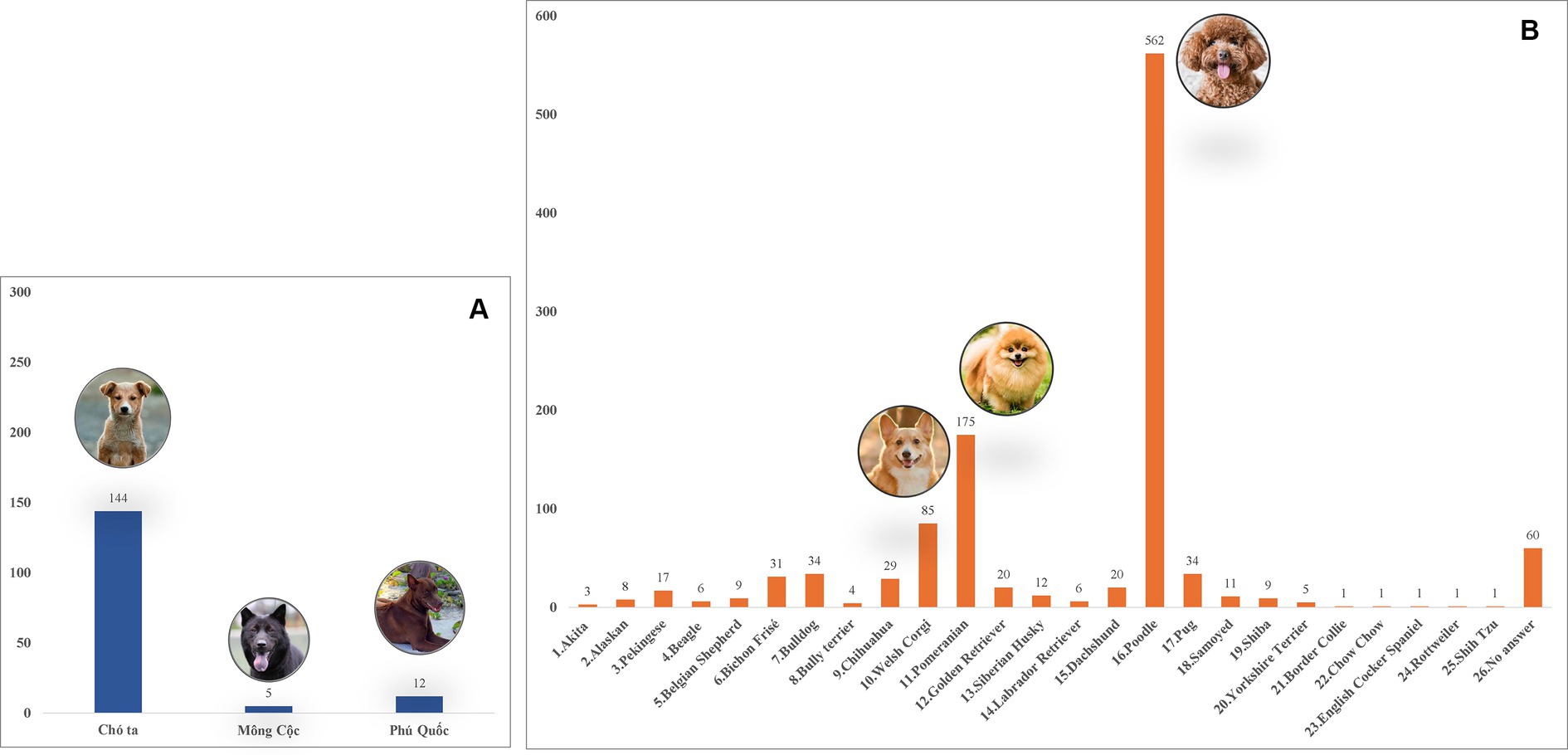
Figure 2. Different dog breeds surveyed in Vietnam during the study. (A) Domestic breeds. (B) Exotic breeds.
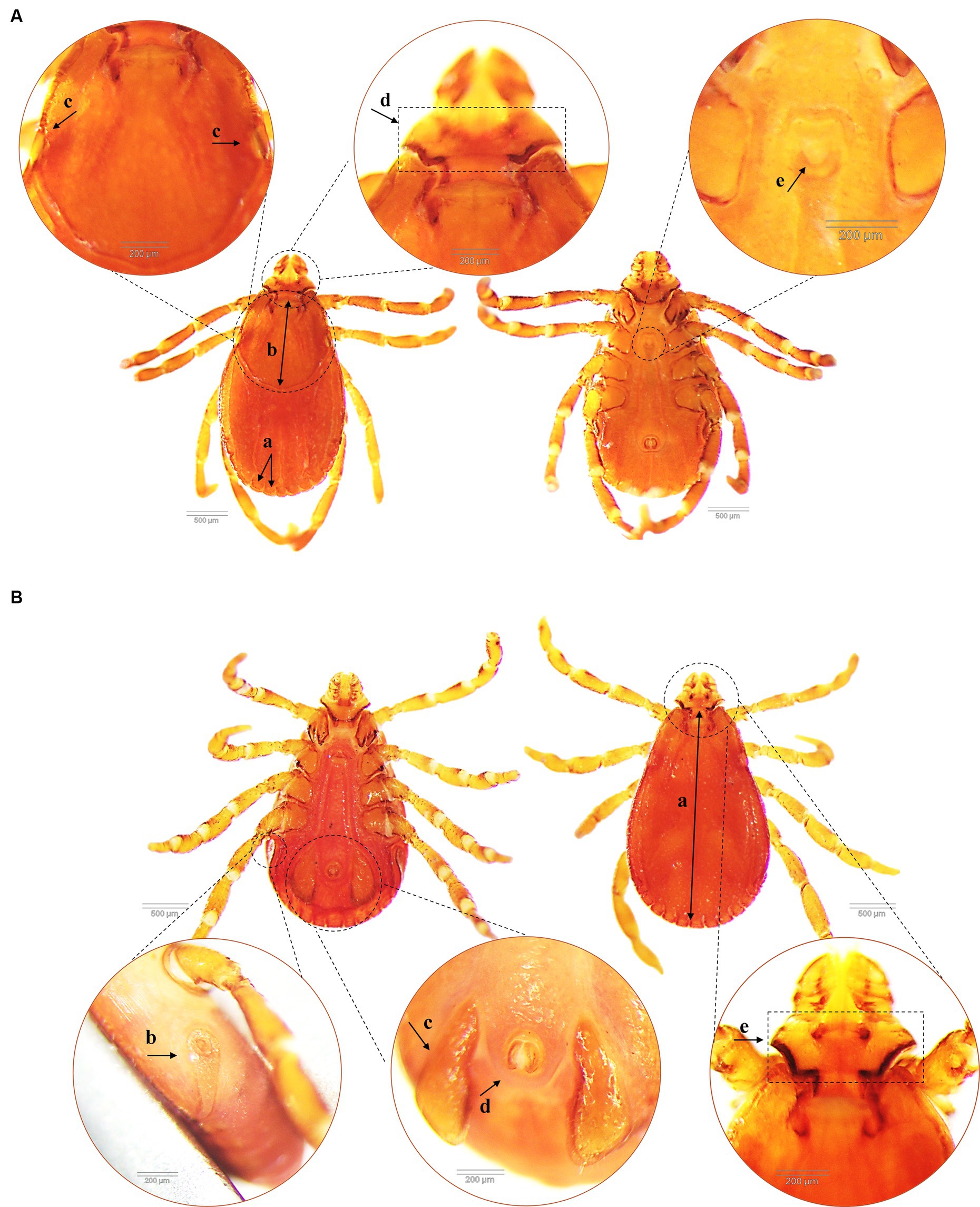
Figure 3. Morphological characteristics of Rhipicephalus sanguineus tick collected from dogs (dorsal and ventral side). (A) Female R. sanguineus with the festoon (similar for male) (a), the scutum covers approximately 1/3 of the dorsal surface (b), flat eyes (c), hexagonal angulate basis capituli (d) and U-shaped genital aperture (e). (B) Male R. sanguineus with the complete scutum covering most of the dosal surface (a), coma-shaped spiracular plates (similar for female) (b), subtriangular adanal plates (c), anus groove posteriorly (d) and hexagonal angulate basis capituli (e).
3.2 Geographical and tick seasonal distribution
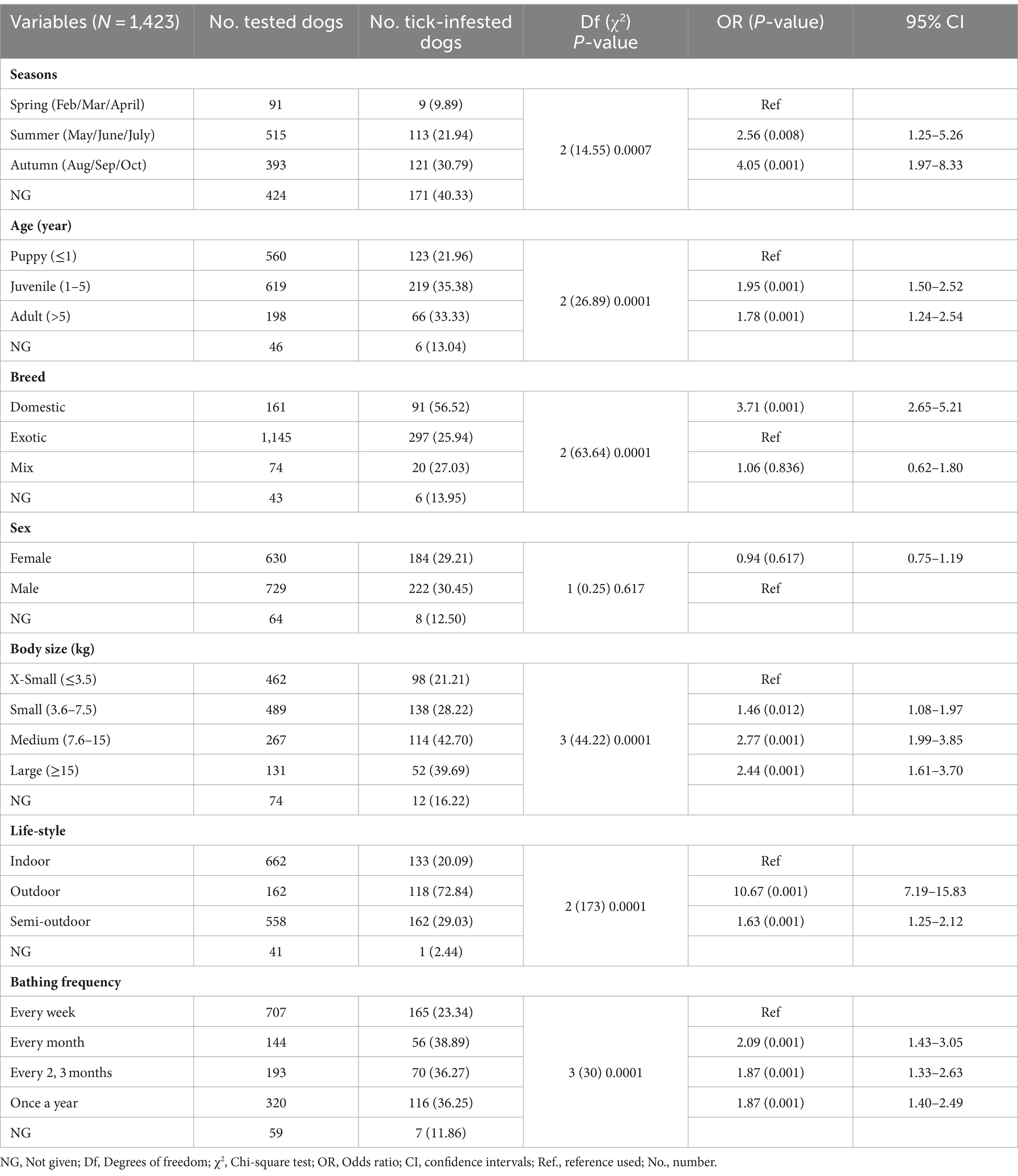
Of 1,423 dogs examined, 414 (29.01, 95% CI: 26.79–31.51%) were carrying at least one tick at the time of sampling. According to statistical analysis, there was a significant difference determined between geographical areas and tick infestation rate in dogs. Tick infestation rate was highest in dogs sampled in Nam Dinh at 55.3% (73/132, 95% CI: 46.79–63.52) followed by Ho Chi Minh City at 39.86% (169/424, 95% CI: 35.31–44.59) and Hanoi 20.7% (170/843, 95% CI: 17.6–23.01). In Dak-Lak, tick presence was found in two dogs (8.33, 95% CI, 2.3–25.85). Besides, dogs residing in Nam Dinh and Ho Chi Minh City were 4.9 times (p < 0.001, 95% CI: 3.34–7.18%) and 2.62 times (p < 0.001, 95% CI: 2.03–3.39%) more likely to get tick infestation compared to those in Hanoi. Samples collected from the north of Vietnam were subjected to tick seasonal distribution analysis. During different seasons, more dogs were tick-infested from May to September and the number of tick-infested dogs declined between February and April (Figure 4). Additionally, the detection rate of tick infestation in dogs was statistically associated with the different seasons in the north. Specifically, the majority of tick-infested dogs in the study were detected in the warmer months of summer (21.94%, 113/515) and autumn (30.79%, 121/393) compared to spring (9.89%, 9/91). In summer and autumn, dogs had 2.56 (p = 0.008) and 4.05 times (p < 0.001) the odds of getting tick infestation compared to those in spring, respectively (Table 2).
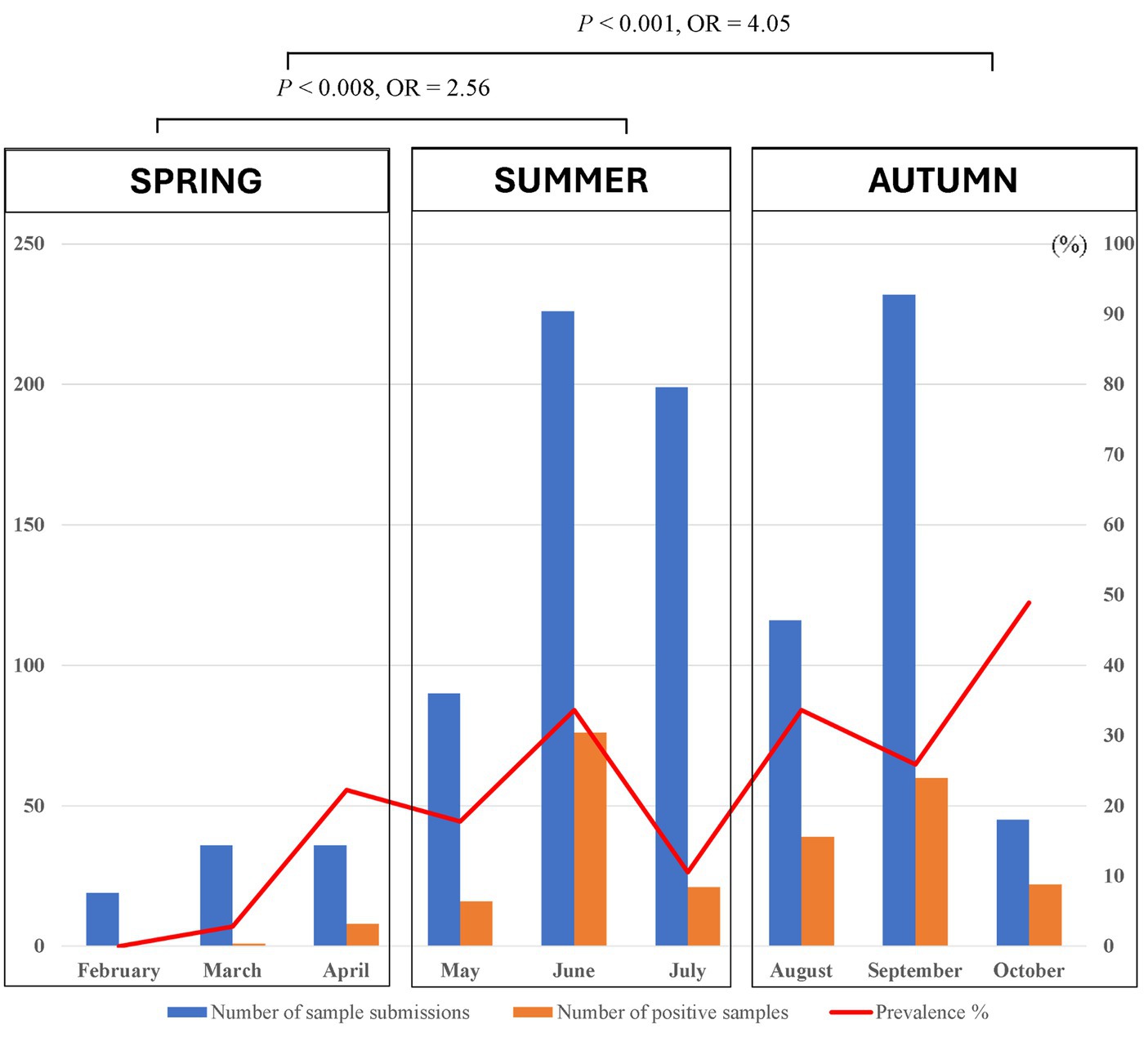
Figure 4. Monthly seasonal dynamics of the Rhipicephalus sanguineus tick found on owned dogs in northern Vietnam (2022).
3.3 Risk factors associated with tick infestation in dogs in Vietnam
The relationship of tick infestation with different host attributes was statistically assessed in the present study and reported in Table 2 along with the number and percentage of tick infestation. Samples with missing information were excluded from the analysis. Majority of tick-infested dogs detected were juveniles (35.38%, 219/619), domestic breed (56.52%, 91/161), medium size (42.7%, 114/267), outdoor lifestyle (72.84%, 118/162) and bathed less than once a month (36.83%, 242/657), compared to the other groups of accordingly category. There was no statistically significant association between tick positivity and sex. The juveniles and adults had 1.95 times (p < 0.001) and 1.78 times (p < 0.001) the odds of getting tick infestation than the puppies, respectively. Domestic dogs were 3.71 times more likely to get tick infestation than exotic dogs (p = 0.001, 95% CI: 2.65–5.21). Dogs in small sizes were 2 times less likely to get tick infestation than those in medium (p = 0.001, 95% CI: 1.99–3.85) or large size (p = 0.001, 95% CI: 1.61–3.7). Dogs with outdoor lifestyle were significantly correlated with tick detection and had 10 times the risk of getting tick infestation compared to those living in-house in this study (p < 0.001, 95% CI: 7.19–15.83).
3.4 Microorganism detection in tick samples collected from dogs in Vietnam
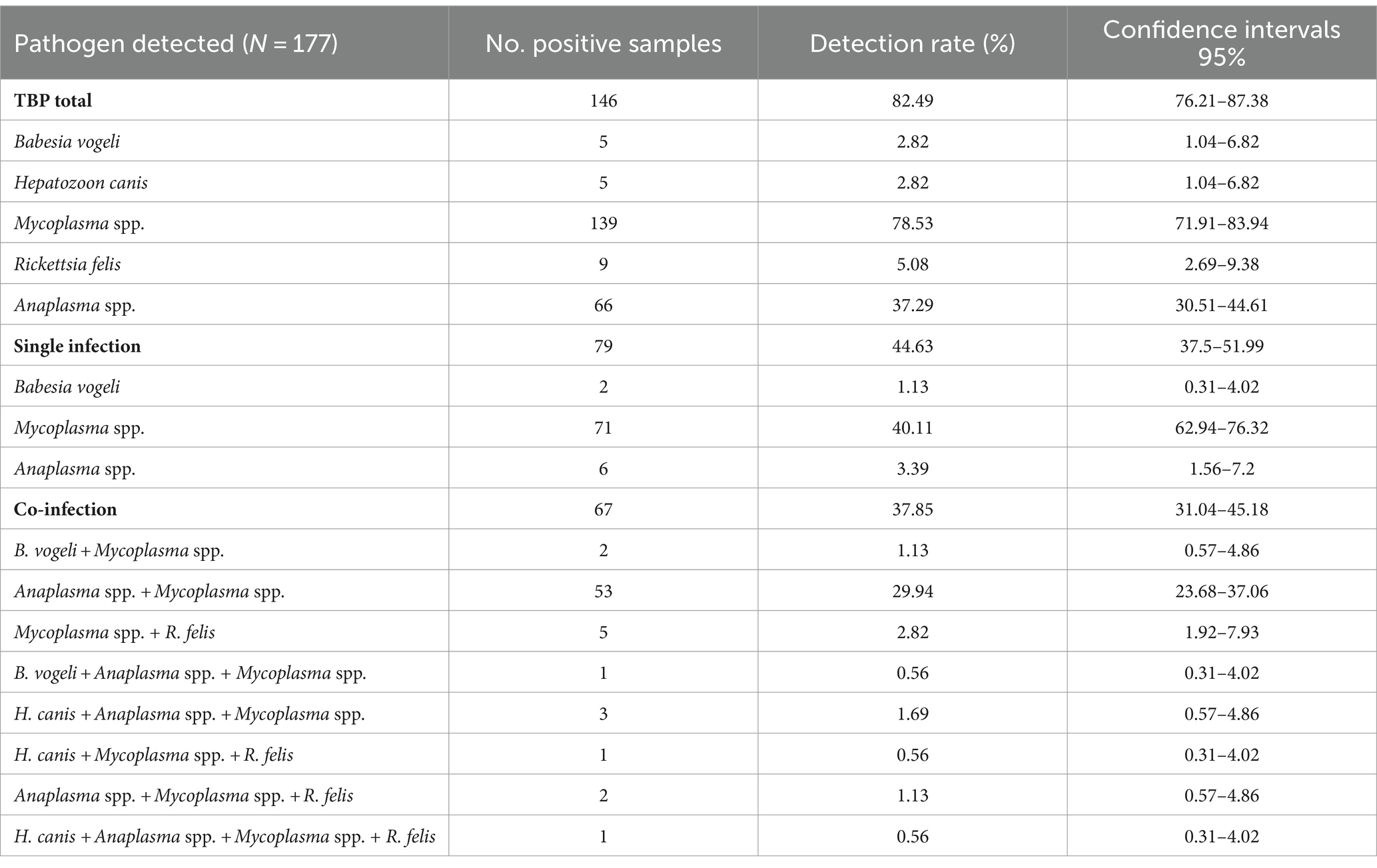
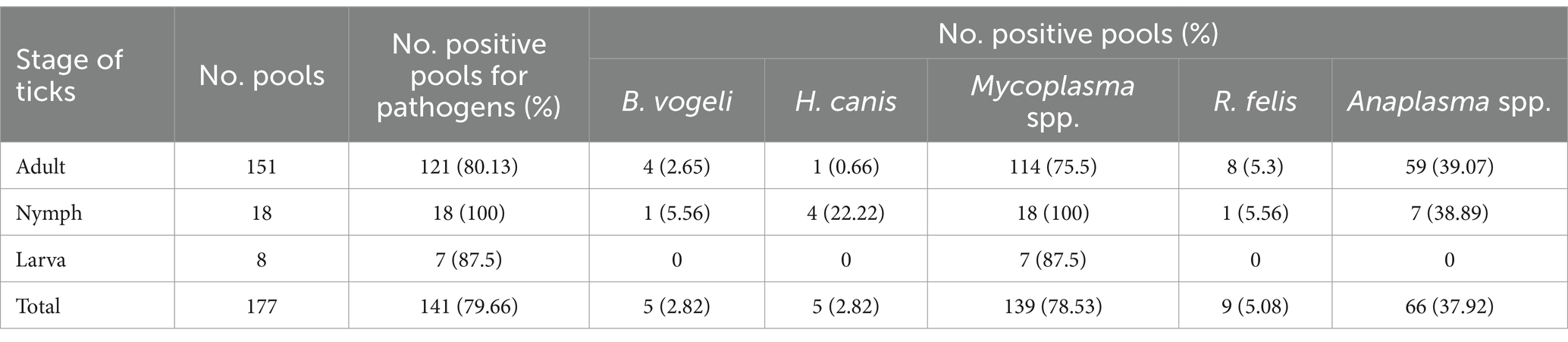
Among the 177 tick pools that were examined, 146 (82.49%) had at least one pathogen found, with Mycoplasma spp. being the most common (78.53%, CI: 71.91–83.94), followed by Anaplasma spp. (37.29%, CI: 30.51–44.61), R. felis (5.08%, CI: 2.69–9.38), B. vogeli, and H. canis (2.82%, CI: 1.04–6.82). In 79 pools, a single pathogen was discovered (44.63%, CI: 37.5–51.99). Multiple pathogens were detected in 67 pools (37.85%, CI: 31.04–45.18; Table 3). In terms of tick stages, out of 151 adult-stage tick pools, 121 (80.13%) pools had Mycoplasma spp. infections. These were followed by pools infected with Anaplasma spp. (39.07%, 59/151), R. felis (5.3%, 8/151), B. vogeli (2.65%, 4/151), and H. canis (0.66%, 1/151). In this investigation, nymphs and adults were found to have the same infections, while larvae primarily had Mycoplasma spp. (87.5%; Table 4).
3.5 Sequencing identities and phylogenetic analysis
The nucleotide sequences for tick and each identified pathogen showed 99–100% similarity with sequences in the Genbank database. Specimens of R. sanguineus s.l. were genetically identified as part of the tropical lineage with 100% nucleotide identity (GenBank accession numbers MF425992–MF425994). The 18S rRNA gene sequences of B. vogeli matched MT386936 with 100% identity, while H. canis showed 99.85% identity with MG050161. The gltA gene sequences of R. felis showed a high level of similarity, ranging from 99.84% to 100%, with sequences OM936910–MT019627. The 16S rRNA gene sequences of Mycoplasma spp. demonstrated a nucleotide identity of 99.51% to 99.68% with Mycoplasma wenyonii sequences MF377464–MG948627. Whereas, 16S rRNA gene sequences of Anaplasma spp. showed a high level of similarity, ranging from 99.03% to 99.67%, with sequences of the different species within the genus Anaplasma and Ehrlichia (EU090184–MN481611). However, none of the Anaplasma/Ehrlichia positive samples tested positive to E. canis or A. platys assays.
The molecular identification of particular sequences for Mycoplasma spp. and R. felis was confirmed by the clear separation of species-specific groups determined through phylogenetic analysis. The phylogenetic tree of the 16S rRNA gene-based Mycoplasma spp. indicated that all M. wenyonii sequences (618 bp in length) grouped together in a distinct clade with strong support (99% bootstrap value). This clade included sequences from the same species found in various geographic locations, while excluding other Mycoplasma spp. species (Figure 5). Phylogenetic analysis of incomplete gltA gene sequences (654 bp in length) showed that all R. felis from dogs grouped together in a clade with reference sequences of the same species, supported by a bootstrap value of 70% (Figure 6).
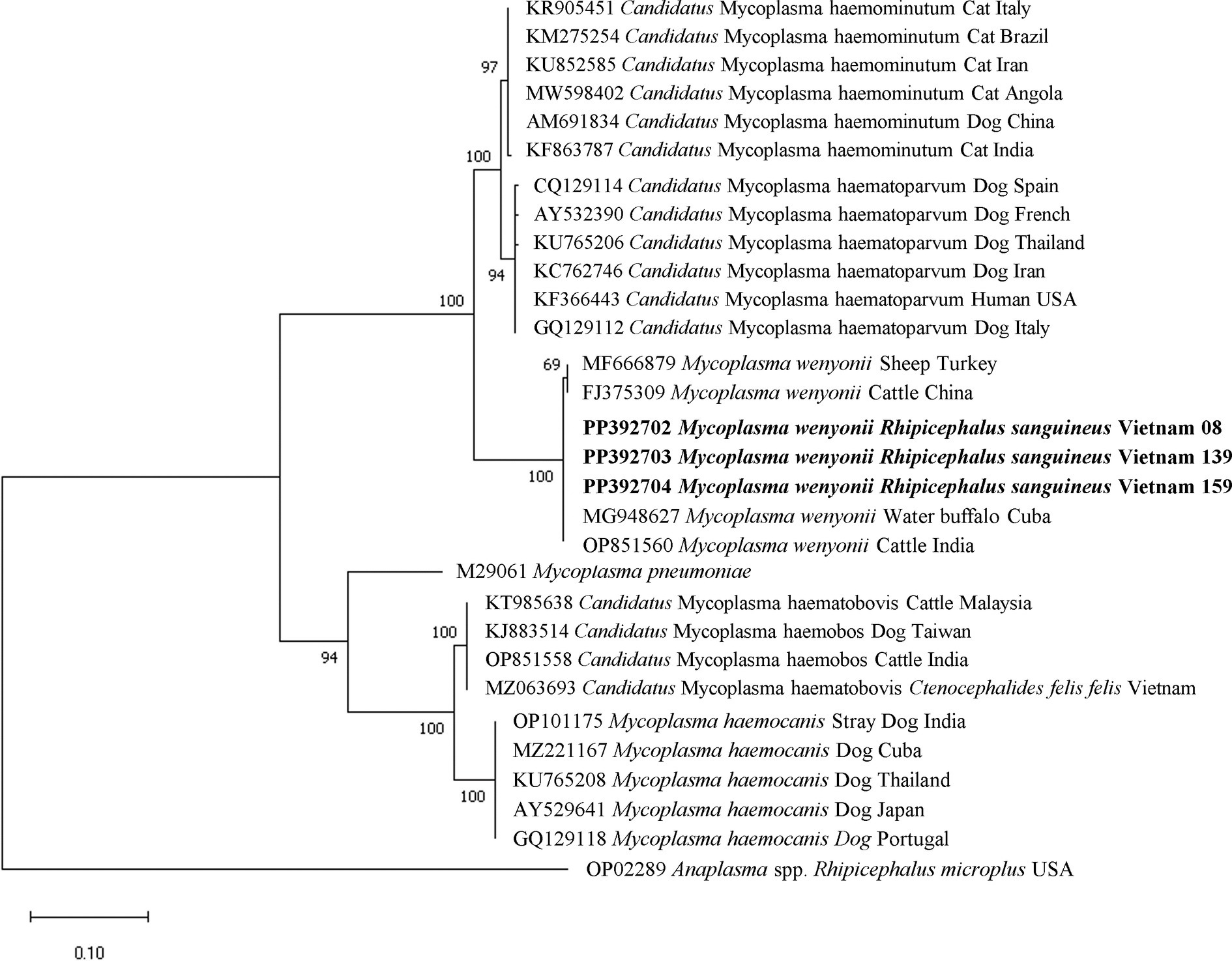
Figure 5. Phylogenetic analysis of Mycoplasma wenyonii based on the nucleotide sequences of a 618 bp fragment of 16S rRNA gene using Tamura 2 parameter model. Isolates obtained from this study are presented in bold text.

Figure 6. Phylogenetic analysis of Rickettsia felis based on the nucleotide sequences of a 654 bp fragment of gltA gene using Kimura 3 parameter model. Isolates obtained from this study are presented in bold text.
The representative sequences produced in this research have been deposited in Genbank database with the following accession numbers: PP389595 and PP389596 for R. sanguineus, PP377903–PP377905 for B. vogeli, PP373785 for H. canis, PP392702–PP392704 for M. wenyonii, and PP393500–PP393501 for R. felis.
4 Discussion
The survey assessed the occurrence of ticks and the microorganisms they harbor on 1,423 dogs owned by clients, mainly in the rapidly expanding Vietnamese cities of Hanoi and Ho Chi Minh City, which house the majority of the country’s human and animal population, along with two other provinces (Nam Dinh and Dak Lak). The overall exposure to brown dog ticks was 29.01%. This study represents the initial systematic examination of tick distribution and frequency on privately owned dogs in Vietnam, utilizing voluntary enrollment in veterinarian care. The study analyzed seasonal tick dynamics and risk factors associated with tick infestation in the canines studied for the first time. The findings and data will provide valuable information to epidemiologists, veterinarians, and legislators to develop treatment and prevention measures for vectors and their related infections.
The tick species found in dogs in this study was R. sanguineus s.l. from the tropical lineage. Previous research in various regions of Vietnam has also confirmed that dogs are commonly infested by R. sanguineus s.l. (2, 8, 9). Rhipicephalus sanguineus is the predominant tick species that targets domestic animals in Southeast Asia (2). The study found that most dogs with ticks were taken to hospitals throughout the summer and autumn months in the Northern region, characterized by hot, humid weather and high precipitation (22). During these periods, ectoparasites such as ticks are more active due to climate conditions that support the survival and reproduction of blood-feeding arthropods. Previous research has indicated that R. sanguineus ticks prefer to lay their eggs at temperatures ranging from 20 to 30°C (23). Optimal parameters for nymph survival include a temperature of 20°C and a relative humidity of 85%. Engorged larvae and nymphs of brown dog ticks successfully underwent molting under various combinations of constant temperature ranging from 10 to 35°C and relative humidity from 15% to 95% (24). The higher incidence of tick infestation in dogs in Ho Chi Minh City compared to Hanoi may be due to the ticks being consistently active throughout the year because of the favorable climate in the region, with an average temperature ranging between 26°C and 29°C annually, resulting in a greater risk of dogs being exposed to ticks.
This study identified variations in the likelihood of tick infection in dogs based on age, breed, body size, lifestyle, and bathing frequency (Table 2). The lifestyle group was the primary variable related with tick infestations. The study found that dogs allowed to roam freely were more prone to tick infestation compared to those kept indoors. Dogs that roam outdoors freely are more likely to encounter ticks due to greater exposure to the environment and contact with tick-infested wild animals nearby. Studies undertaken in ASEAN nations have revealed tick incidence exceeding 80% in stray animals (25, 26). If the outdoor activity of dogs is the mechanism by which host-seeking tick activity is increasingly successful, then the pattern of association between rural areas and the risk of tick infestation is understandable in Vietnam; since dogs in rural regions reported often semi-domiciled and have free access to outside environment, predisposing them to ticks (27). Our research supported the claim that the rate of tick infestation in dogs in Nam Dinh, a rural location, was notably greater than in Hanoi and Ho Chi Minh City, metropolitan areas. Tick infestation is notably higher in domestic dogs than in exotic breeds. The various reasons for owning a dog may have been the cause. Most domestic breed dogs in Vietnam are typically raised outdoors for guarding purposes and are kept in kennels, making them more susceptible to tick infestations compared to exotic breeds that are usually kept as pets for companionship and may even share a bed with their owners. Older dogs, including juveniles and adults, are more susceptible to tick infestation in this study, maybe because they spend more time outdoors than pups. Small dogs who are bathed frequently may have a reduced chance of tick attachment, according to the study. Small dogs may be easier to bathe than larger dogs due to their size, making them more approachable and manageable. Previous research on the relationship between tick infestation and gender found that male dogs had a much higher risk of infestation (28); however, this relationship was not seen in the current study.
The molecular testing revealed that the majority of tick samples were infected with Mycoplasma spp. (78.53%) rather than Anaplasma spp., Ehrlichia spp., H. canis, B. vogeli, or R. felis. The transmission pathways of hemoplasmas in dogs are under discussion, with blood-feeding arthropods including fleas and ticks proposed as potential vectors (29). Mycoplasmosis is not considered to be a tick-borne pathogen, however some hemotrophic mycoplasms that are significant in veterinary and public health can sometimes be found in ticks and co-infect hosts with established tick-borne pathogens. Mycoplasma spp. were found alongside other microorganisms, including Anaplasma spp., B. vogeli, H. canis, and R. felis in tick samples collected from the same host. The findings suggest that vertebrate hosts can be infected with several tick-borne diseases either by a single tick carrying multiple pathogens or by distinct ticks carrying individual pathogens. Hemotropic mycoplasma species, specifically Mycoplasma haemocanis and Candidatus Mycoplasma haematoparvum, have been recently identified in ticks and dogs in Vietnam and various regions of Southeast Asia, causing canine mycoplasmosis (3, 30, 31). A significant detection rate of M. wenyonii was unexpectedly detected in brown dog ticks and all three different stages of ticks (adults, nymph, larva) in this study, despite it being regularly reported to infect cattle and sheep after phylogenetic analysis. The role of ticks in the epidemiology of this pathogen remains uncertain.
The second most prevalent bacterium detected in tick samples in this study was Ehrlichia/Anaplasma spp. (37.29%). Anaplasma platys was the species found in dogs in Vietnam (30) and E. canis commonly detected in ticks and canines in different countries in Southeast Asia such as Thailand (3), Cambodia (32), or Philippines (33). However, all tick pools in the current study tested negative for A. platys and E. canis, when further analyzed for A. platys and E. canis identification using species-specific primers. These findings indicate other species belonging to the genus Ehrlichia and Anaplasma might be circulating in ticks in the studied areas. A further investigation on this bacteria in ticks with an increased number of samples is suggested to identify and investigate the phylogeny of these bacteria in Vietnam. Rickettsia felis is an emerging insect-borne rickettsial pathogen causing flea-borne spotted fever, which can be found in mammalian hosts and arthropods worldwide (34), and Ctenocephalides felis fleas—known as the main vector and reservoir for this pathogen (35). This pathogen has been recently detected in C. felis from dogs and cats in several parts of Vietnam (36–38). Our study found R. felis for the first time in both adult and nymph stages of R. sanguineus from owned dogs in Vietnam. The discovery of R. felis in both adult and nymph stages of R. sanguineus, as well as the high frequency of this tick species parasitizing dogs in the study, raises concerns about the potential transmission of R. felis to residents in the areas under investigation. Human cases of febrile sickness caused by R. felis have been reported in Thailand (39), Indonesia (40), and Laos (41). In Vietnam, R. felis was recently discovered and detected in humans by a molecular assay (42). The agent was reported to cause a severe undifferentiated fever in patients who displayed an eschar, a common clinical indicator that sometimes appeared at the location of the tick or mite bite (43, 44). Rhipicephalus sanguineus can transmit other species of the spotted fever group Rickettsiae, including Rickettsia rickettsii, which causes Rocky Mountain spotted fever, a severe and potentially fatal tick-borne disease; however, R. rickettsii has not been reported yet in Southeast Asia (43). The presence of R. felis and other pathogens found in R. sanguineus in this study suggests that these pathogens may also be present in the canines under investigation, posing a risk of infection to the host. This emphasizes the significance of performing epidemiological investigations on tick-borne infections in canines in the future. Moreover, H. canis and B. vogeli were discovered in a substantially lower percentage, which showed similar to the previous studies (9, 38). In summary, the identification of the above mentioned pathogens in ticks highlights the potential of this ectoparasite to transmit harmful organisms, posing a threat to both human and animal health (1, 4).
5 Conclusion
The study found that the brown dog tick (R. sanguineus s.l.) is common in dogs in the studied areas of Vietnam. This study validated multiple parameters that contribute to the probability of tick infestation in dogs, essential for successful veterinary therapies. The data indicate the presence of R. felis, a causative agent of spotted fever, in R. sanguineus ticks in Vietnam for the first time, which poses substantial health concerns to animals.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal studies were approved by the Ethics Committee of Obihiro University of Agriculture and Veterinary Medicine accepted the protocol for using animal samples in this work (Permit for animal experiment: 21-25; DNA experiment: 1725-5). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
TD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. LKB: Funding acquisition, Resources, Writing – original draft, Writing – review & editing. RU-S: Methodology, Writing – original draft, Writing – review & editing. TI: Methodology, Writing – original draft, Writing – review & editing. TH: Methodology, Writing – original draft, Writing – review & editing. IZ: Methodology, Writing – original draft, Writing – review & editing. ZM: Methodology, Writing – original draft, Writing – review & editing. LH: Methodology, Writing – original draft, Writing – review & editing. UKM: Methodology, Writing – original draft, Writing – review & editing. MA: Methodology, Writing – original draft, Writing – review & editing. SAE-S: Methodology, Writing – original draft, Writing – review & editing. XX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. KK: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by a Grant-in-Aid for Scientific Research (23KJ0074) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan and partially supported by Kasetsart University Research and Development Institute [KURDI, FF (KU) 32.67]. Boehringer Ingelheim Animal Health (Vietnam) provided partial support for the study. TD was supported by a research fellowship from the Japan Society for the Promotion of Science (JSPS) for young scientists, Japan (202313465).
Acknowledgments
The authors express gratitude to all partners at the collection sites for their involvement in the study and to the animal owners who supplied tick samples and responded to the questionnaires. We extend our appreciation to Ms. La Tra My (Key Account Manager) and Ms. Bui My Thuy Khanh (Associate Technical & Sale Manager) from Companion Animal Team, Boehringer Ingelheim Animal Health Vietnam LLC for their assistance in distributing and collecting questionnaires and specimens from veterinary clinics throughout Vietnam.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://climateknowledgeportal.worldbank.org/country/vietnam/climate-data-historical (Accessed 22 February 2024).
References
1. Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. (2010) 3:26. doi: 10.1186/1756-3305-3-26
2. Colella, V, Nguyen, VL, Tan, DY, Lu, N, Fang, F, Zhijuan, Y, et al. Zoonotic Vectorborne pathogens and Ectoparasites of dogs and cats in eastern and Southeast Asia. Emerg Infect Dis. (2020) 26:1221–33. doi: 10.3201/eid2606.191832
3. Do, T, Phoosangwalthong, P, Kamyingkird, K, Kengradomkij, C, Chimnoi, W, and Inpankaew, T. Molecular detection of tick-borne pathogens in stray dogs and Rhipicephalus sanguineus sensu lato ticks from Bangkok, Thailand. Pathogens. (2021b) 10:561. doi: 10.3390/pathogens10050561
4. Irwin, PJ, and Jefferies, R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. (2004) 20:27–34. doi: 10.1016/j.pt.2003.11.004
5. Ivanov, A, and Tsachev, I. Hepatozoon canis and hepatozoonosis in the dog. Trakia J Sci. (2008) 6:27–35.
6. Petra, B, Josipa, K, Renata, BR, and Vladimir, M. Canine Babesiosis: where Do we stand? Acta Vet. (2018) 68:127–60. doi: 10.2478/acve-2018-0011
7. Baneth, G, Samish, M, Alekseev, E, Aroch, I, and Shkap, V. Transmission of Hepatozoon canis to dogs by naturally-fed or percutaneously-injected Rhipicephalus sanguineus ticks. J Parasitol. (2001) 87:606–11. doi: 10.1645/0022-3395(2001)087[0606:TOHCTD]2.0.CO;2
8. Huynh, LN, Diarra, AZ, Pham, QL, Le-Viet, N, Berenger, J-M, Ho, VH, et al. Morphological, molecular and MALDI-TOF MS identification of ticks and tick-associated pathogens in Vietnam. PLoS Negl Trop Dis. (2021) 15:e0009813. doi: 10.1371/journal.pntd.0009813
9. Nguyen, VL, Colella, V, Iatta, R, Bui, KL, Dantas-Torres, F, and Otranto, D. Ticks and associated pathogens from dogs in northern Vietnam. Parasitol Res. (2019) 118:139–42. doi: 10.1007/s00436-018-6138-6
10. Walker, A. The genus Rhipicephalus (Acari, Ixodidae): a guide to the Brown ticks of the world. Trop Anim Health Prod. (2000) 32:417–8. doi: 10.1023/A:1005237804239
11. Hornok, S, Grima, A, Takács, N, and Kontschán, J. Infestation of Rhipicephalus sanguineus sensu lato on cats in Malta. Ticks Tick-Borne Dis. (2018) 9:1120–4. doi: 10.1016/j.ttbdis.2018.04.007
12. Kidd, L, Maggi, R, Diniz, PPVP, Hegarty, B, Tucker, M, and Breitschwerdt, E. Evaluation of conventional and real-time PCR assays for detection and differentiation of spotted fever group Rickettsia in dog blood. Vet Microbiol. (2008) 129:294–303. doi: 10.1016/j.vetmic.2007.11.035
13. Hii, S-F, Kopp, SR, Thompson, MF, O’Leary, CA, Rees, RL, and Traub, RJ. Molecular evidence of Rickettsia felis infection in dogs from northern territory, Australia. Parasit Vectors. (2011) 4:1–3. doi: 10.1186/1756-3305-4-198
14. Criado-Fornelio, A, Martinez-Marcos, A, Buling-Saraña, A, and Barba-Carretero, JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol. (2003) 93:307–17. doi: 10.1016/S0378-1135(03)00044-0
15. Inokuma, H, Raoult, D, and Brouqui, P. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J Clin Microbiol. (2000) 38:4219–21. doi: 10.1128/JCM.38.11.4219-4221.2000
16. Inokuma, H, Fujii, K, Matsumoto, K, Okuda, M, Nakagome, K, Kosugi, R, et al. Demonstration of Anaplasma (Ehrlichia) platys inclusions in peripheral blood platelets of a dog in Japan. Vet Parasitol. (2002a) 110:145–52. doi: 10.1016/S0304-4017(02)00289-3
17. Inokuma, H, Brouqui, P, Drancourt, M, and Raoult, D. Citrate synthase gene sequence: a new tool for phylogenetic analysis and identification of Ehrlichia. J Clin Microbiol. (2001) 39:3031–9. doi: 10.1128/JCM.39.9.3031-3039.2001
18. Jensen, WA, Fall, MZ, Rooney, J, Kordick, DL, and Breitschwerdt, EB. Rapid identification and differentiation of Bartonella species using a single-step PCR assay. J Clin Microbiol. (2000) 38:1717–22. doi: 10.1128/JCM.38.5.1717-1722.2000
19. Tabar, M-D, Altet, L, Francino, O, Sánchez, A, Ferrer, L, and Roura, X. Vector-borne infections in cats: molecular study in Barcelona area (Spain). Vet Parasitol. (2008) 151:332–6. doi: 10.1016/j.vetpar.2007.10.019
20. Kordick, SK, Breitschwerdt, EB, Hegarty, BC, Southwick, KL, Colitz, CM, Hancock, SI, et al. Coinfection with multiple tick-borne pathogens in a Walker hound kennel in North Carolina. J Clin Microbiol. (1999) 37:2631–8. doi: 10.1128/JCM.37.8.2631-2638.1999
21. Inokuma, H, Okuda, M, Ohno, K, Shimoda, K, and Onishi, T. Analysis of the 18S rRNA gene sequence of a Hepatozoon detected in two Japanese dogs. Vet Parasitol. (2002b) 106:265–71. doi: 10.1016/S0304-4017(02)00065-1
22. Acharya, N, and Bennett, E. Characteristic of the regional rainy season onset over Vietnam: tailoring to agricultural application. Atmosphere. (2021) 12:198. doi: 10.3390/atmos12020198
23. Sweatman, GK. Physical and biological factors affecting the longevity and oviposition of engorged Rhipicephalus sanguineus female ticks. J Parasitol. (1967) 53:432–45. doi: 10.2307/3276606
24. Koch, HG, and Tuck, MD. Molting and survival of the Brown dog tick (Acari: Ixodidae) under different temperatures and Humidities1. Ann Entomol Soc Am. (1986) 79:11–4. doi: 10.1093/aesa/79.1.11
25. Khovand, H, Fard, SRN, Khalili, M, and Jajarmi, M. A survey of Ixodid ticks in stray dogs and molecular detection of Ehrlichia canis from ticks in Central Iran. Turk J Vet Anim Sci. (2022) 46:209–17. doi: 10.55730/1300-0128.4168
26. Yan, LY, Peng, TL, and Goni, MD. Survey on tick infestation in stray dogs in localities of Malaysia. Vet Parasitol Reg Stud Rep. (2024) 47:100952. doi: 10.1016/j.vprsr.2023.100952
27. Nguyen, V-L, Dantas-Torres, F, and Otranto, D. Canine and feline vector-borne diseases of zoonotic concern in Southeast Asia. Curr Res Parasitol Vector-Borne Dis. (2021) 1:100001. doi: 10.1016/j.crpvbd.2020.100001
28. Raghavan, M, Glickman, N, Moore, G, Caldanaro, R, Lewis, H, and Glickman, L. Prevalence of and risk factors for canine tick infestation in the United States, 2002–2004. Vector-Borne Zoonotic Dis. (2007) 7:65–75. doi: 10.1089/vbz.2006.0570
29. Barker, EN, Tasker, S, Day, MJ, Warman, SM, Woolley, K, Birtles, R, et al. Development and use of real-time PCR to detect and quantify Mycoplasma haemocanis and “Candidatus Mycoplasma haematoparvum” in dogs. Vet Microbiol. (2010) 140:167–70. doi: 10.1016/j.vetmic.2009.07.006
30. Do, T, Bui, KL, Zafar, I, Inpankaew, T, Galon, ME, Ta, PA, et al. Molecular detection, risk factors, and phylogenetic analysis of tick-borne pathogens in dogs from northern Vietnam. Trob Biomed. (2024) 41:52–63. doi: 10.47665/tb.41.1.007
31. Liu, M, Ruttayaporn, N, Saechan, V, Jirapattharasate, C, Vudriko, P, Moumouni, PFA, et al. Molecular survey of canine vector-borne diseases in stray dogs in Thailand. Parasitol Int. (2016) 65:357–61. doi: 10.1016/j.parint.2016.04.011
32. Inpankaew, T, Hii, SF, Chimnoi, W, and Traub, R. Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia. Parasit Vectors. (2016) 9:253. doi: 10.1186/s13071-016-1552-z
33. Galay, RL, Manalo, AAL, Dolores, SLD, Aguilar, IPM, Sandalo, KAC, Cruz, KB, et al. Molecular detection of tick-borne pathogens in canine population and Rhipicephalus sanguineus (sensu lato) ticks from southern metro Manila and Laguna, Philippines. Parasit Vectors. (2018) 11:1–8. doi: 10.1186/s13071-018-3192-y
34. Parola, P. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect. (2011) 17:996–1000. doi: 10.1111/j.1469-0691.2011.03516.x
35. Dieme, C, Bechah, Y, Socolovschi, C, Audoly, G, Berenger, J-M, Faye, O, et al. Transmission potential of Rickettsia felis infection by Anopheles gambiae mosquitoes. Proc Natl Acad Sci. (2015) 112:8088–93. doi: 10.1073/pnas.1413835112
36. Do, T, Inpankaew, T, Duong, DH, and Bui, KL. First molecular evidence of pathogens in fleas collected from dogs in northern Vietnam. Pathogens. (2021a) 10:1185. doi: 10.3390/pathogens10091185
37. Nguyen, VT, Nguyen, HQ, Nguyen, VT, and Ng-Nguyen, D. Rickettsia felis and species of fleas parasitizing on household dogs in the central highlands of Vietnam. Comp Immunol Microbiol Infect Dis. (2023) 92:101926. doi: 10.1016/j.cimid.2022.101926
38. Nguyen, V-L, Colella, V, Greco, G, Fang, F, Nurcahyo, W, Hadi, UK, et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in east and Southeast Asia. Parasit Vectors. (2020) 13:420. doi: 10.1186/s13071-020-04288-8
39. Edouard, S, Bhengsri, S, Dowell, SF, Watt, G, Parola, P, and Raoult, D. Two human cases of Rickettsia felis infection, Thailand. Emerg Infect Dis. (2014) 20:1780–1. doi: 10.3201/eid2010.140905
40. Mawuntu, AHP, Johar, E, Anggraeni, R, Feliana, F, Bernadus, JBB, Safari, D, et al. Rickettsia felis identified in two fatal cases of acute meningoencephalitis. PLoS Negl Trop Dis. (2020) 14:e0007893. doi: 10.1371/journal.pntd.0007893
41. Dittrich, S, Phommasone, K, Anantatat, T, Panyanivong, P, Slesak, G, Blacksell, SD, et al. Rickettsia felis infections and comorbid conditions, Laos, 2003–2011. Emerg Infect Dis. (2014) 20:1402–4. doi: 10.3201/eid2008.131308
42. Hoang, MTT, Ngo, VP, Stenos, J, and Ng-Nguyen, D. The presence of Rickettsia felis in communities in the central highlands of Vietnam. Acta Trop. (2023) 248:107034. doi: 10.1016/j.actatropica.2023.107034
43. Álvarez-López, DI, Ochoa-Mora, E, Nichols Heitman, K, Binder, AM, Álvarez-Hernández, G, and Armstrong, PA. Epidemiology and clinical features of Rocky Mountain spotted fever from enhanced surveillance, Sonora, Mexico: 2015–2018. Am J Trop Med Hyg. (2021) 104:190–7. doi: 10.4269/ajtmh.20-0854
44. Le-Viet, N, Le, V-N, Chung, H, Phan, D-T, Phan, Q-D, Cao, T-V, et al. Prospective case-control analysis of the aetiologies of acute undifferentiated fever in Vietnam. Emerg Microbes Infect. (2019) 8:339–52. doi: 10.1080/22221751.2019.1580539
Appendix
Keywords: dogs, ticks, tick-borne pathogens, risk factors, Vietnam
Citation: Do T, Bui LK, Umemiya-Shirafuji R, Inpankaew T, Hasan T, Zafar I, Ma Z, Hang L, Mohanta UK, Amer M, El-Sayed SAE-S, Xuan X and Kamyingkird K (2024) The detection of zoonotic microorganisms in Rhipicephalus sanguineus (brown dog ticks) from Vietnam and the frequency of tick infestations in owned dogs. Front. Vet. Sci. 11:1435441. doi: 10.3389/fvets.2024.1435441
Edited by:
Mihaela Kavran, University of Novi Sad, SerbiaReviewed by:
Benjamin Cull, University of Minnesota Twin Cities, United StatesSara Savic, Scientific Veterinary Institute Novi Sad, Serbia
Copyright © 2024 Do, Bui, Umemiya-Shirafuji, Inpankaew, Hasan, Zafar, Ma, Hang, Mohanta, Amer, El-Sayed, Xuan and Kamyingkird. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuenan Xuan, Z2VuQG9iaWhpcm8uanAuYWM=; Ketsarin Kamyingkird, a2V0c2FyaW4ua2FAa3UudGg=; a2V0c2FyaW5rYW15QGhvdG1haWwuY29t
 Thom Do
Thom Do Linh Khanh Bui2
Linh Khanh Bui2 Rika Umemiya-Shirafuji
Rika Umemiya-Shirafuji Tawin Inpankaew
Tawin Inpankaew Tanjila Hasan
Tanjila Hasan Iqra Zafar
Iqra Zafar Li Hang
Li Hang Uday Kumar Mohanta
Uday Kumar Mohanta Moaz Amer
Moaz Amer Shimaa Abd El-Salam El-Sayed
Shimaa Abd El-Salam El-Sayed Xuenan Xuan
Xuenan Xuan Ketsarin Kamyingkird
Ketsarin Kamyingkird