
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 10 July 2024
Sec. Veterinary Epidemiology and Economics
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1432741
This article is part of the Research TopicWildlife-related zoonotic infections: Major threat to public healthView all 7 articles
Introduction: Blastocystis is one of the most critical intestinal protozoans in various hosts, including humans and mice. To determine the status of Blastocystis infection in wild rodents in China.
Methods: A total of 344 faecal samples were collected from seven wild rodent species from three provinces, and the small subunit ribosomal RNA (SSU rRNA) genes of Blastocystis were amplified to determine their prevalence and subtypes.
Results: Of the 344 samples, 54 (15.70%) were detected as Blastocystis-positive. The prevalence of Blastocystis was 26.14% (40/153), 7.95% (7/88), and 6.80% (7/103) in wild rodents from Hunan Province, Yunnan Province, and Guangxi Province, respectively. The prevalence of Blastocystis in different wild rodent species varied from 0.00% (0/13) in Mus musculus to 40.00% (2/5) in Rattus rattus sladeni. The prevalence of Blastocystis in samples from the lake beach area (27.40%, 40/146) was significantly higher than in those from the mountain (6.80%, 7/103) and field regions (7.37%, 7/95). The prevalence in different seasons was 26.14% in summer (40/153), 7.95% in autumn (7/88), and 6.80% in winter (7/103). Moreover, a total of two Blastocystis subtypes were identified in the investigated wild rodents, including ST4 and ST5.
Discussion: The present study discovered the existence of Blastocystis infection in Rattus favipectus, Microtus fortis, Apodemus agrarius, Bandicota indica, Rattus rattus sladeni, and Rattus losea, expanding the host range of this parasite. The findings also demonstrate that wild rodents may be an important potential infection source for Blastocystis infection in humans and other animals.
Wild rodents are widely distributed worldwide. They are one of the most pivotal reservoir hosts for many pathogens, including bacteria (1), viruses (2), and parasites (3), and can transmit these pathogens between animals and humans. Thus, an increasing amount of research is being conducted regarding the epidemiology of zoonotic pathogens in wild rodents. Blastocystis is one of the most critical zoonotic pathogens; it can infect a variety of hosts, including humans (4) and wild mice (5). Blastocystis infection mainly occurs through the faecal-oral route, such as the ingestion of Blastocystis cysts-contaminated food or water (6), and is often associated with irritable bowel syndrome (IBS) (7), cutaneous allergic disorders (8), nausea, and diarrhoea (9). However, recently, some research has also suggested that some subtypes of Blastocystis may also be beneficial for health (10, 11). Hence, investigating the distribution of subtypes of Blastocystis is of particular importance for prevention and control of Blastocystis infection.
To date, a total of 42 Blastocystis subtypes (STs) have been identified based on the small subunit ribosomal RNA (SSU rRNA) gene (12, 13). Of these, more than 40 Blastocystis subtypes have previously been found in humans and animals (14). ST1-ST10, ST12, ST14, ST16 ST23, ST35, and ST41 have been found in humans with a predominance of ST1-ST4 (15), and all other subtypes have only been documented in animals (14). ST1-ST5, ST8, ST13, ST14, and ST17 have been identified in wild rodents, which suggests a potential risk of the zoonotic transmission of Blastocystis from wild rodents to humans (16). More importantly, ST1-3 and ST8 have not only been found in humans and wild rodents, but also in water (16), suggesting a higher risk of transmission of Blastocystis between humans and rodents through water sources.
In view of the above, the investigation of the prevalence and distribution of Blastocystis in wild rodents is important to prevent and control Blastocystis infection in different hosts. While a few studies have been published on Blastocystis infection in some rodents, these studies did not specifically examine the prevalence of subtypes or conduct subtype distribution analysis of Blastocystis in Rattus favipectus, Microtus fortis, Apodemus agrarius, Bandicota indica, Rattus rattus sladeni, and Rattus losea. Considering the lack of current data related to the occurrence of Blastocystis and the prevalence of subtypes in the above rodent species, in the present study, a total of 344 wild rodents were collected from Hunan Province, Yunnan Province, and Guangxi Province, China, and the SSU rRNA genes were amplified to investigate the prevalence of Blastocystis.
A total of 344 wild rodents (n = 139, Microtus fortis; n = 31, Rattus norvegicus; n = 14, Apodemus agrarius; n = 39, Bandicota indica; n = 5, Rattus rattus sladeni; n = 39, Rattus flavipectus; n = 41, Rattus losea; n = 23, Niviventer lotipes; n = 13, Mus musculus) were randomly collected from Yunnan Province (n = 88, 21°8′ ~ 29°15′ N, 97°31′ ~ 106°11′ E), Hunan Province (n = 153, 24°38′ ~ 30°08′ N, 108°47′ ~ 114°15′ E), and Guangxi Province (n = 103, 20°54′ ~ 26°24′ N, 104°28′ ~ 112°04′ E), China, between September 2023 and February 2024. Information including gender, sampling times, seasons, regions, and species were recorded. All wild rodents were captured using mouse traps, and faecal samples were collected from the rectum of each rodent. Then, faecal samples were placed into a box with dry ice and sent to the laboratory. The DNA of each faecal sample was extracted using a Stool DNA kit (OMEGA, United States), according to the manufacturer’s instructions, and kept at −20°C until analysis via polymerase chain reaction (PCR).
The SSU rRNA genes of Blastocystis were amplified to determine their subtypes (6). Using the primers RD5 (5′- ATCTGGTTGATCCTGCCAGT-3′) and BhRDr (5′-GAGCTTTTTAACTGCAACAACG-3′), the SSU rRNA gene was amplified using PCR at a region of around 600 bp. Both the positive control (sequenced isolates of Blastocystis) and negative control (sterile distilled water) were also amplified in each test. The electrophoresis of 6 μL PCR products was performed on 1.0% agarose gel in TBE. All of the target products were detected under UV light and sequenced based on bidirectional sequencing at the General Biol. Company in Anhui, China. Then, the sequences were blasted with known reference sequences available in GenBank. The neighbour-joining (NJ) method (Kimura 2-parameter model and 1,000 replicates) was used to analyse the phylogenetic relationships of these Blastocystis based on Mega 5.0.1
The chi-square test in SAS (Statistical Analysis System, Version 9.0) was used to calculate the difference between the prevalence of Blastocystis (y) and different factors, including seasons (x1), species (x2), gender (x3), regions (x4), and environment (x5). The difference was considered as statistically significant if p < 0.05. Moreover, the chi-square test in SPSS (IBM Corp., Armonk, NY, United States) was used to calculate the odds ratios (ORs) and their 95% confidence intervals (95% CIs).
A total of 344 wild rodents were collected in the present study. Of these, 61 were identified as Blastocystis-positive through the PCR of SSU rRNA genes of Blastocystis. The total Blastocystis prevalence was 15.70% (54/344), with 26.14% (40/153) in Hunan, 7.95% (7/88) in Yunnan, and 6.80% (7/103) in Guangxi (Table 1). The prevalence of Blastocystis from the lake beach area (27.40%, 40/146) was significantly higher than that from the mountain (6.80%, 7/103) and field regions (7.37%, 7/95). The prevalence varied from 6.80% in winter (7/103) to 26.14% in summer (40/153). The prevalence in the wild rodents of according to gender was 16.24% for males and 14.97% for females.
The effect of regions, species, seasons, environment and gender regarding Blastocystis infection was analysed using forward stepwise logistic regression analysis based on Fisher’s scoring technique. Only region was included as a variable in final model, which has a close association with Blastocystis infection. The equation is described as follows: y = 0.9097×4 + 1.0973. Region had strongly effects on the Blastocystis infection in the wild rodents, for which the OR was 2.48 (95% CI 1.61–3.82). Wild rodents from Yunnan (OR 1.19, 95% CI 0.40–3.52) and Hunan (OR 4.85, 95% CI 2.08–11.33) were seen to be more susceptible than those from Guangxi (Table 1).
A total of two subtypes were identified in the present study, namely, ST4 and ST5. Of these, ST4 was the predominant Blastocystis subtype, being found in seven rodent species (n = 39, Microtus fortis; n = 2, Rattus norvegicus; n = 1, Apodemus agrarius; n = 1, Bandicota indica; n = 2, Rattus rattus sladeni; n = 2, Rattus flavipectus; n = 6, Rattus losea) in all three provinces (n = 40, Hunan Province; n = 6, Yunnan Province; n = 7, Guangxi Province), followed by ST5, which was only found in Rattus flavipectus (n = 1) collected in winter in Yunnan Province. However, ST4 and ST5 did not appear in the same rodent. ST4 was found in the lake beach, mountain, and field areas, whereas ST5 was only found in the mountain area (Table 2).
A total of six representative sequences were obtained from the 54 Blastocystis isolates in the present study. The ST4 sequences (PP622334, PP622335, PP622332, and PP622333) showed 100% homology with isolates from humans (MN836841, MH784408) reported in GenBank. The ST4 sequence (PP622336) in this study was also identical to sequences from other rodents such as rodents in Mexico (MK251246) and Rattus exulans in Indonesia (MH127488). On the other hand, the ST5 sequence (PP622331) was 100% homologous to isolates from pigs (MN526819, MK801414, and KY610202) reported in GenBank (Figure 1).
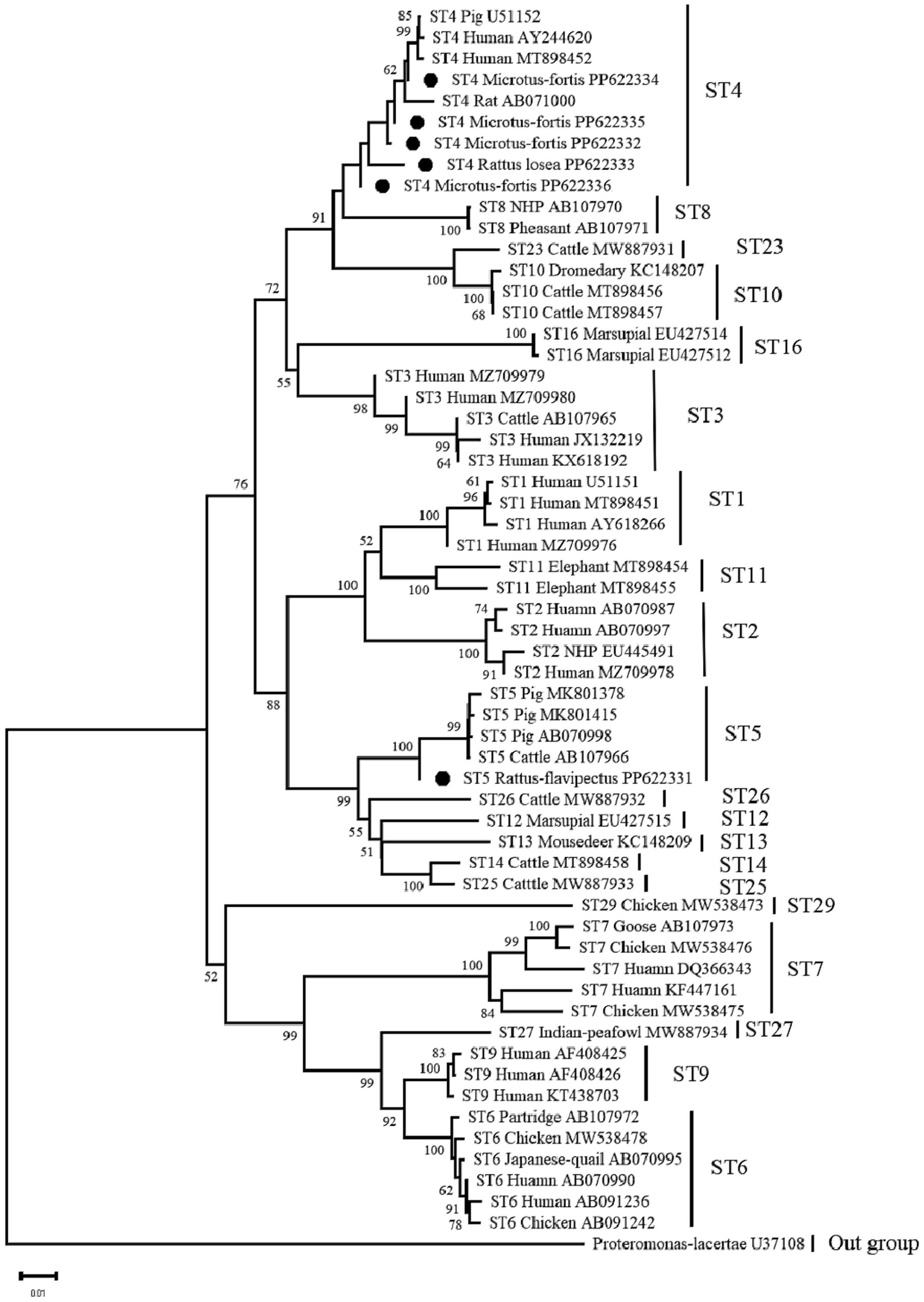
Figure 1. Neighbour-joining (NJ) phylogenetic analyses of Blastocystis based on the SSU rRNA genes. Bootstrap values more than 50% are shown. The Blastocystis isolates detected in present study are indicated by black circles.
In the present study, 54 out of 344 wild rodents were Blastocystis-positive based on a PCR of SSU rRNA genes. The overall prevalence was 15.70%, which was lower than that observed previously in rodents in Spain (83.5%) (17), Malaysia (45.9%) (18), Japan (44.4%) (19), England (43.5%) (20), and Ecuador (35.4%) (21); close to that in Indonesia (16.4%) (22), Iran (15.7%) (5); and higher than that previously reported in Brazil (8.7%) (23) and Mexico (13.3%) (24). Blastocystis infection in rodents has also been surveyed in other cities in China, with infection rates ranging from 0.0 to 27.3%, such as Sichuan, Hunan, Heilongjiang, Hainan, and Hubei, where the infection rates were 8.4, 4.6, 3.7, 2.2, and 30.4%, respectively (25–31). The different prevalence of Blastocystis in different studies may be due to the different sampling times, different susceptibility in different animals, and different pollution levels of the environment. Importantly, Blastocystis is widely found in different regions, suggesting that these regions are commonly contaminated by Blastocystis cysts, which should be paid more attention to during the prevention and control of the disease.
In the present study, the difference in the prevalence of Blastocystis in the summer, autumn, and winter was significant, indicating that the extent of parasitic infection varied significantly between seasons. Similar to a previous Japanese study, the rodents had lower rates of Blastocystis infection in winter (19). Several studies have shown that temperature and precipitation are positively correlated with parasites infection intensity, possibly because the higher temperatures and precipitation in summer and wet seasons are conducive to the growth and transmission of parasites. Moreover, the activity of wild rodents in winter was less than that in summer, which reduced the contact between wild rodents and other animals and water sources, thereby reducing the transmission of Blastocystis. Although the infection rate of Blastocystis was higher in summer than in winter, it was detected in summer, winter, and autumn, indicating that Blastocystis could be transmitted all year round and is a zoonotic parasite that requires significant attention. Blastocystis was reportedly found in water sources in 15 countries around the world, and the infection rate of some water sources, including fountain water, rainwater, rivers, stored water, and irrigation water, was as high as 100% (32). Studies also showed that drinking unboiled water was found to correlate with a high prevalence of Blastocystis infections in China (33, 34). Blastocystis is also one of the water-related pathogens in the WHO’s drinking water quality publications, which indicates the public health significance of this parasite (35, 36). Therefore, hosts can acquire Blastocystis infection through the contaminated water, which is also the most common route of infection. Noteworthy is that Blastocystis cysts can survive for 19–30 days in water at a temperature of 25°C and for 2 months at 4°C (37). This tendency is consistent with the results of this study, as more Blastocystis were detected near water sources. The prevalence of Blastocystis from lake beach areas (27.40%, 40/146) was significantly higher (p < 0.0001) than those from the mountain (6.80%, 7/103) and field regions (7.37%, 7/95); this suggests that access to safe drinking water to avoid Blastocystis infection is critical to public health.
In this study, the positive rate was 14.97% (22/147) in females, and 16.24% (32/197) in males. There was no significant difference in the positive rate between different gender groups (p > 0.05), which suggests that the infection rates are not affected by gender; similar results were also observed in a Japanese survey (19), where Blastocystis was found in seven species of wild rodents, including Bandicota indica, Microtus fortis, Rattus norvegicus, Rattus rattus sladeni, Rattus flavipectus, Rattus losea, and Apodemus agrarius, indicating that this zoonotic parasite is widely distributed in wild rodents. It is worth noting that Blastocystis infection was discovered for the first time in Bandicota indica, Microtus fortis, Rattus flavipectus, Rattus rattus sladeni, and Apodemus agrarius.
To date, a total of 42 Blastocystis subtypes (STs) have been identified through the utilisation of DNA-based techniques and sequence analysis of the small subunit ribosomal RNA (SSU rRNA) gene (12, 13). A large number of studies have reported that ST4 is the dominant subtype of wild rodents and is distributed in different species of rodents and different countries, such as China (25), Indonesia (22), Japan (19), Malaysia (18), Iran (38), and the United Kingdom (20). ST4 is also one of the most common subtypes in humans (10). In addition, ST4 has a peculiar geographical distribution and is the most influenced by geography and lifestyle (39). ST4 was originally isolated from a Wistar rat (40). Subsequently, ST4 was detected in wild rodents, cows, goats, pigs, and other wild mammals worldwide, and especially in the water sources that humans and animals are exposed to Liu et al. and Shams et al. (27, 41). These findings indicated that ST4 has a wide host range, infected wild animals can contaminate drinking water, and the consumption of contaminated water or contact with contaminated surface water may expose humans to Blastocystis. In the present study, ST4 was found in seven wild rodent species in all regions. The results demonstrated that ST4 is one of the most critical zoonotic subtypes of Blastocystis and requires more attention in future research.
Hoofed animals are the natural hosts of ST5, including cattle (42), pigs (43), sheep (44), and camels (45). Some studies have shown that ST5 is the predominant subtype in pigs, and has also been identified in humans working in commercial intensive pig farms (43), suggesting that close contact or exposure to infected animals may be an important route of infection for ST5 (46). Zoonotic ST5 has only been detected sporadically in rodents; for instance, Blastocystis ST5 was identified in Hydrochoerus hydrochaeris in France (47), in Clethrionomys glareolus in the United Kingdom (48), in Rattus norvegicus in Malaysia (18), and in Myocastor coypus, Rhizomys sinensis, and Callosciurus erythraeus in China (27, 28, 49). ST5 has also been found in rivers and lakes. In the present study, ST5 was only identified in Rattus flavipectus from the mountain regions in Yunnan. These findings suggest that although ST5 is rare in rodents, it can still be transmitted between humans and animals, posing a potential zoonotic risk.
Although this study provided valuable evidence of Blastocystis infections in rodents, there are several limitations that need to be acknowledged. The rodent samples obtained in this study encompassed a limited geographical area, which is insufficient for a comprehensive understanding of the prevalence of Blastocystis infection in rodents in China. Furthermore, the number of positive samples in this study was limited, which may have resulted in an inadequate representation of Blastocystis genetic diversity. To gain a more comprehensive understanding of the prevalence of Blastocystis, it is necessary to expand the sample size and collection area in follow-up studies.
The findings of this study demonstrated that Blastocystis infection in wild rodents is a frequently occurrence in China. The present study also discovered the existence of Blastocystis infection in Rattus favipectus, Microtus fortis, Apodemus agrarius, Bandicota indica, Rattus rattus sladeni, and Rattus losea, thereby broadening the host range of this parasite. Region, species, season, and environment had strong effects on Blastocystis infection in the investigated wild rodents. Crucially, ST4 and ST5, previously found in humans, were also found in this study, which suggests that wild rodents may be an important potential sources of human infections. Our study provided reliable data for future studies on Blastocystis subtype distribution in rodents and Blastocystis infection control in wild animals in China.
The representative gene sequence was submitted to GenBank (Accession nos. PP622331–PP622336).
The animal study was approved by the Animal Ethics Committee of Yancheng Teachers University. The study was conducted in accordance with the local legislation and institutional requirements.
Z-QG: Methodology, Software, Writing – original draft. H-TW: Methodology, Software, Writing – original draft. Q-YH: Data curation, Writing – review & editing. YQ: Data curation, Resources, Visualization, Writing – review & editing. XY: Data curation, Visualization, Writing – review & editing. QZ: Conceptualization, Resources, Supervision, Writing – review & editing. HM: Conceptualization, Resources, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Selmi, R, Belkahia, H, Dhibi, M, Abdelaali, H, Lahmar, S, Ben Said, M, et al. Zoonotic vector-borne bacteria in wild rodents and associated ectoparasites from Tunisia. Infect Genet Evol. (2021) 95:105039. doi: 10.1016/j.meegid.2021.105039
2. Bakker, JW, Pascoe, EL, van de Water, S, van Keulen, L, de Vries, A, Woudstra, LC, et al. Infection of wild-caught wood mice (Apodemus sylvaticus) and yellow-necked mice (A. flavicollis) with tick-borne encephalitis virus. Sci Rep. (2023) 13:21627. doi: 10.1038/s41598-023-47697-2
3. Lv, QB, Zeng, A, Xie, LH, Qiu, HY, Wang, CR, and Zhang, XX. Prevalence and riskfactors of toxoplasma Gondii infection among five wild rodent species from five provinces of China. Vector Borne Zoonotic Dis. (2021) 21:105–9. doi: 10.1089/vbz.2020.2658
4. Kwon, JY, Choi, JH, Lee, HI, Ju, JW, and Lee, MR. Molecular prevalence of Blastocystis sp. from patients with diarrhea in the republic of Korea. Microorganisms. (2024) 12:523. doi: 10.3390/microorganisms12030523
5. Mohammadpour, I, Bozorg-Ghalati, F, Gazzonis, AL, Manfredi, MT, Motazedian, MH, and Mohammadpour, N. First molecular subtyping and phylogeny of Blastocystis sp. isolated from domestic and synanthropic animals (dogs, cats and brown rats) in southern Iran. Parasit Vectors. (2020) 13:365. doi: 10.1186/s13071-020-04225-9
6. Yan, WL, Li, XM, Qin, SY, Xue, NY, Zou, Y, Li, JH, et al. Subtypes of Blastocystis in tibetan antelope (Pantholops hodgsonii). Res Vet Sci. (2024) 171:105233. doi: 10.1016/j.rvsc.2024.105233
7. Rostami, A, Riahi, SM, Haghighi, A, Saber, V, Armon, B, and Seyyedtabaei, SJ. The role of Blastocystis sp. and Dientamoeba fragilis in irritable bowel syndrome: a systematic review and meta-analysis. Parasitol Res. (2017) 116:2361–71. doi: 10.1007/s00436-017-5535-6
8. Aykur, M, Camyar, A, Türk, BG, Sin, AZ, and Dagci, H. Evaluation of association with subtypes and alleles of Blastocystis with chronic spontaneous urticaria. Acta Trop. (2022) 231:106455. doi: 10.1016/j.actatropica.2022.106455
9. Tan, KS, Mirza, H, Teo, JD, Wu, B, and Macary, PA. Current views on the clinical relevance of Blastocystis spp. Curr Infect Dis Rep. (2010) 12:28–35. doi: 10.1007/s11908-009-0073-8
10. Deng, L, Wojciech, L, Png, CW, Koh, EY, Aung, TT, Kioh, DYQ, et al. Experimental colonization with Blastocystis ST4 is associated with protective immune responses and modulation of gut microbiome in a DSS-induced colitis mouse model. Cell Mol Life Sci. (2022) 79:245. doi: 10.1007/s00018-022-04271-9
11. Wu, Z, Mirza, H, and Tan, KS. Intra-subtype variation in enteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7. PLoS Negl Trop Dis. (2014) 8:e2885. doi: 10.1371/journal.pntd.0002885
12. Koehler, AV, Herath, H, Hall, RS, Wilcox, S, and Gasser, RB. Marked genetic diversity within Blastocystis in Australian wildlife revealed using a next generation sequencing-phylogenetic approach. Int J Parasitol Parasites Wildl. (2024) 23:100902. doi: 10.1016/j.ijppaw.2023.100902
13. Santin, M, Figueiredo, A, Molokin, A, George, NS, Köster, PC, Dashti, A, et al. Division of Blastocystis ST10 into three new subtypes: ST42-ST44. J Eukaryot Microbiol. (2024) 71:e12998. doi: 10.1111/jeu.12998
14. Yang, F, Gou, JM, Yang, BK, Du, JY, Yao, HZ, Ren, M, et al. Prevalence and subtype distribution of Blastocystis in tibetan sheep in Qinghai province, northwestern China. Protist. (2023) 174:125948. doi: 10.1016/j.protis.2023.125948
15. Maloney, JG, Molokin, A, Seguí, R, Maravilla, P, Martínez-Hernández, F, Villalobos, G, et al. Identification and molecular characterization of four new Blastocystis subtypes designated ST35-ST38. Microorganisms. (2022) 11:46. doi: 10.3390/microorganisms11010046
16. Barati, M, KarimiPourSaryazdi, A, Rahmanian, V, Bahadory, S, Abdoli, A, Rezanezhad, H, et al. Global prevalence and subtype distribution of Blastocystis sp. in rodents, birds, and water supplies: a systematic review and meta-analysis. Prev Vet Med. (2022) 208:105770. doi: 10.1016/j.prevetmed.2022.105770
17. Galán-Puchades, MT, Trelis, M, Sáez-Durán, S, Cifre, S, Gosálvez, C, Sanxis-Furió, J, et al. One health approach to zoonotic parasites: molecular detection of intestinal protozoans in an urban population of Norway rats, Rattus norvegicus, in Barcelona, Spain. Pathogens. (2021) 10:311. doi: 10.3390/pathogens10030311
18. Farah Haziqah, MT, Mohd Zain, SN, Chandrawathani, P, Premaalatha, B, Mohd Khairul Nizam, MK, Arutchelvan, R, et al. Genetic diversity of rodent Blastocystis sp. from peninsular Malaysia. Trop Biomed. (2018) 35:586–92.
19. Masuda, A, Wada, M, Saho, H, Tokunaga, K, Kikuchi, Y, Yamasaki, F, et al. Prevalence and molecular characterization of the zoonotic enteric protozoans Cryptosporidium spp., Enterocytozoon bieneusi, and Blastocystis from Pallas's squirrels (Callosciurus erythraeus) in Kanagawa prefecture, Japan. Microbiol Spectr. (2021) 9:e0099021. doi: 10.1128/Spectrum.00990-21
20. Betts, EL, Gentekaki, E, Thomasz, A, Breakell, V, Carpenter, AI, and Tsaousis, AD. Genetic diversity of Blastocystis in non-primate animals. Parasitology. (2018) 145:1228–34. doi: 10.1017/s0031182017002347
21. González-Ramírez, LC, Vázquez, CJ, Chimbaina, MB, Djabayan-Djibeyan, P, Prato-Moreno, JG, Trelis, M, et al. Ocurrence of enteroparasites with zoonotic potential in animals of the rural area of San Andres, Chimborazo. Ecuador Vet Parasitol Reg Stud Reports. (2021) 26:100630. doi: 10.1016/j.vprsr.2021.100630
22. Katsumata, M, Yoshikawa, H, Tokoro, M, Mizuno, T, Nagamoto, T, Hendarto, J, et al. Molecular phylogeny of Blastocystis isolates from wild rodents captured in Indonesia and Japan. Parasitol Res. (2018) 117:2841–6. doi: 10.1007/s00436-018-5973-9
23. Oliveira-Arbex, AP, David, ÉB, Tenório, MDS, Cicchi, PJP, Patti, M, Coradi, ST, et al. Diversity of Blastocystis subtypes in wild mammals from a zoo and two conservation units in southeastern Brazil. Infect Genet Evol. (2020) 78:104053. doi: 10.1016/j.meegid.2019.104053
24. Martinez-Hernandez, F, Martinez-Ibarra, JA, Lopez-Escamilla, E, Villanueva-Garcia, C, Muñoz-Garcia, CI, Rendon-Franco, E, et al. Molecular genotyping of Blastocystis spp. in wild mammals from Mexico. Parasitol Res. (2020) 119:97–104. doi: 10.1007/s00436-019-06530-4
25. Chai, Y, Deng, L, Liu, H, Yao, J, Zhong, Z, Fu, H, et al. First subtyping of Blastocystis sp. from pet rodents in southwestern China. Int J Parasitol Parasites Wildl. (2020) 11:143–8. doi: 10.1016/j.ijppaw.2020.01.012
26. Deng, L, Yao, J, Chen, S, He, T, Chai, Y, Zhou, Z, et al. First identification and molecular subtyping of Blastocystis sp. in zoo animals in southwestern China. Parasit Vectors. (2021) 14:11. doi: 10.1186/s13071-020-04515-2
27. Liu, X, Ni, F, Wang, R, Li, J, Ge, Y, Yang, X, et al. Occurrence and subtyping of Blastocystis in coypus (Myocastor coypus) in China. Parasit Vectors. (2022) 15:14. doi: 10.1186/s13071-021-05126-1
28. Song, J, Yang, X, Ma, X, Wu, X, Wang, Y, Li, Z, et al. Molecular characterization of Blastocystis sp. in Chinese bamboo rats (Rhizomys sinensis). Parasite. (2021) 28:81. doi: 10.1051/parasite/2021081
29. Wang, J, Gong, B, Liu, X, Zhao, W, Bu, T, Zhang, W, et al. Distribution and genetic diversity of Blastocystis subtypes in various mammal and bird species in northeastern China. Parasit Vectors. (2018) 11:522. doi: 10.1186/s13071-018-3106-z
30. Xiao, X, Zhou, SH, Jiang, N, Tian, DZ, Zhou, ZM, Zhang, M, et al. First record of Leptospira and Blastocystis infections in captive flying squirrels (Trogopterus xanthipes) from Enshi County. China Acta Trop. (2019) 197:105065. doi: 10.1016/j.actatropica.2019.105065
31. Zhao, W, Zhang, Y, Li, J, Ren, G, Qiang, Y, Wang, Y, et al. Prevalence and distribution of subtypes of Blastocystis in Asiatic brush-tailed porcupines (Atherurus macrourus), bamboo rats (Rhizomys pruinosus), and masked palm civets (Paguma larvata) farmed in Hainan, China. Parasite. (2023) 30:45. doi: 10.1051/parasite/2023048
32. Attah, AO, Sanggari, A, Li, LI, Nik Him, N, Ismail, AH, and Meor Termizi, FH. Blastocystis occurrence in water sources worldwide from 2005 to 2022: a review. Parasitol Res. (2023) 122:1–10. doi: 10.1007/s00436-022-07731-0
33. Deng, Y, Zhang, S, Ning, C, Zhou, Y, Teng, X, Wu, X, et al. Molecular epidemiology and risk factors of Blastocystis sp. infections among general populations in Yunnan Province, Southwestern China. Risk Manag Healthc Policy. (2020) 13:1791–801. doi: 10.2147/RMHP.S269664
34. Zhang, SX, Kang, FY, Chen, JX, Tian, LG, and Geng, LL. Risk factors for Blastocystis infection in HIV/AIDS patients with highly active antiretroviral therapy in Southwest China. Infect Dis Poverty. (2019) 8:89. doi: 10.1186/s40249-019-0596-7
35. Organization, W. H . Guidelines for drinking-water quality. Geneva: World Health Organization (2021).
36. Organization, W. H . Guidelines for drinking-water quality: incorporating the first and second addenda. Geneva: World Health Organization (2022).
37. Yoshikawa, H, Yoshida, K, Nakajima, A, Yamanari, K, Iwatani, S, and Kimata, I. Fecal-oral transmission of the cyst form of Blastocystis hominis in rats. Parasitol Res. (2004) 94:391–6. doi: 10.1007/s00436-004-1230-5
38. Seifollahi, Z, Sarkari, B, Motazedian, MH, Asgari, Q, Ranjbar, MJ, and Abdolahi Khabisi, S. Protozoan parasites of rodents and their zoonotic significance in Boyer-Ahmad district, Southwestern Iran. Vet Med Int. (2016) 2016:3263868. doi: 10.1155/2016/3263868
39. Beghini, F, Pasolli, E, Truong, TD, Putignani, L, Cacciò, SM, and Segata, N. Large-scale comparative metagenomics of Blastocystis, a common member of the human gut microbiome. ISME J. (2017) 11:2848–63. doi: 10.1038/ismej.2017.139
40. Chen, XQ, Singh, M, Ho, LC, Moe, KT, Tan, SW, and Yap, EH. A survey of Blastocystis sp. in rodents. Lab Anim Sci. (1997) 47:91–4.
41. Shams, M, Shamsi, L, Yousefi, A, Sadrebazzaz, A, Asghari, A, Mohammadi-Ghalehbin, B, et al. Current global status, subtype distribution and zoonotic significance of Blastocystis in dogs and cats: a systematic review and meta-analysis. Parasit Vectors. (2022) 15:225. doi: 10.1186/s13071-022-05351-2
42. Badparva, E, Sadraee, J, and Kheirandish, F. Genetic diversity of Blastocystis isolated from cattle in Khorramabad, Iran. Jundishapur J Microbiol. (2015) 8:e14810. doi: 10.5812/jjm.14810
43. Pintong, AR, Sunyanusin, S, Prasertbun, R, Mahittikorn, A, Mori, H, Changbunjong, T, et al. Blastocystis subtype 5: predominant subtype on pig farms, Thailand. Parasitol Int. (2018) 67:824–8. doi: 10.1016/j.parint.2018.08.009
44. Wei, CN, Qin, RL, Zhang, ZH, Zheng, WB, Liu, Q, Gao, WW, et al. Prevalence and genetic characterization of Blastocystis in sheep and pigs in Shanxi province, North China: from a public health perspective. Animals (Basel). (2023) 13:2843. doi: 10.3390/ani13182843
45. Alfellani, MA, Stensvold, CR, Vidal-Lapiedra, A, Onuoha, ES, Fagbenro-Beyioku, AF, and Clark, CG. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. (2013) 126:11–8. doi: 10.1016/j.actatropica.2012.12.011
46. Wang, W, Owen, H, Traub, RJ, Cuttell, L, Inpankaew, T, and Bielefeldt-Ohmann, H. Molecular epidemiology of Blastocystis in pigs and their in-contact humans in Southeast Queensland, Australia, and Cambodia. Vet Parasitol. (2014) 203:264–9. doi: 10.1016/j.vetpar.2014.04.006
47. Cian, A, El Safadi, D, Osman, M, Moriniere, R, Gantois, N, Benamrouz-Vanneste, S, et al. Molecular epidemiology of Blastocystis sp. in various animal groups from two french zoos and evaluation of potential zoonotic risk. PLoS One. (2017) 12:e0169659. doi: 10.1371/journal.pone.0169659
48. Alfellani, MA, Taner-Mulla, D, Jacob, AS, Imeede, CA, Yoshikawa, H, Stensvold, CR, et al. Genetic diversity of Blastocystis in livestock and zoo animals. Protist. (2013) 164:497–509. doi: 10.1016/j.protis.2013.05.003
Keywords: Blastocystis , wild rodents, prevalence, subtype, China
Citation: Gao Z-Q, Wang H-T, Hou Q-Y, Qin Y, Yang X, Zhao Q and Ma H (2024) Prevalence and subtypes of Blastocystis in wild rodents from three provinces in China. Front. Vet. Sci. 11:1432741. doi: 10.3389/fvets.2024.1432741
Received: 14 May 2024; Accepted: 01 July 2024;
Published: 10 July 2024.
Edited by:
Fábio A. Abade Dos Santos, Lusofona University, PortugalReviewed by:
Manuel E. Cortés, Universidad Bernardo O’Higgins, ChileCopyright © 2024 Gao, Wang, Hou, Qin, Yang, Zhao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Zhao, emhhb3F1YW4wODI1QDE2My5jb20=; He Ma, bWFoZUBxYXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.