- 1Department of Pathophysiology, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 2Beijing Key Laboratory for Prevention and Control of Infectious Diseases in Livestock and Poultry, Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences, Beijing, China
The contagious respiratory pathogen, Avibacterium paragallinarum, contributes to infectious coryza in poultry. However, commercial vaccines have not shown perfect protection against infectious coryza. To search for an alternative approach, this research aimed to investigate whether the quorum-sensing system of pathogens plays a crucial role in their survival and pathogenicity. The LuxS/AI-2 quorum-sensing system in many Gram-negative and Gram-positive bacteria senses environmental changes to regulate physiological traits and virulent properties, and the role of the luxS gene in Av. paragallinarum remains unclear. To investigate the effect of the luxS gene in the quorum-sensing system of Av. paragallinarum, we constructed a luxS mutant. Bioluminescence analysis indicated that the luxS gene plays a vital role in the LuxS/AI-2 quorum-sensing system. The analysis of the LuxS/AI-2 system-related genes showed the level of pfs mRNA to be significantly increased in the mutant strain; however, lsrR, lsrK, and lsrB mRNA levels were not significantly different compared with the wild type. The ability of the luxS mutant strain to invade HD11 and DF-1 cells was significantly decreased compared with the wild-type strain. In addition, all chickens challenged with various doses of the luxS mutant strain developed infections and symptoms, and those challenged with the lowest dose exhibited only minor differences compared to chickens challenged with the wild-type strain. Thus, the deletion of the luxS gene reduces the invasion, but the luxS gene does not play an essential role in the pathogenesis of A. paragallinarum.
1 Introduction
Avibacterium paragallinarum, a member of the bacterial Pasteurellaceae family, is the etiological agent of infectious coryza, a highly contagious respiratory disease in laying hens (1, 2). Facial swelling, conjunctivitis, nasal discharge, diarrhea, and anorexia are the main clinical symptoms (3). Infectious coryza causes serious decreases in egg production and enormous economic losses for the poultry industry worldwide (4). Many of the microorganisms that reside in the respiratory tract are beneficial to the host’s normal development and physiology (5), and the bacteria with programmed therapeutic functions are now a tangible reality (6). The interactions between microbes therefore present possibilities for new strategies to treat and prevent infectious disease.
Quorum sensing is a cell-to-cell communication system by which bacteria sense their population density and other bacterial species through autoinducers (AIs) to regulate functions (7). Many Gram-negative bacteria produce N-acyl homoserine lactone signaling molecules, and the most Gram-positive bacteria use modified peptides as signal molecules. However, the enzyme, LuxS, which synthesizes the signaling molecule, AI-2, is the only quorum-sensing pathway in both Gram-positive and Gram-negative bacteria (8). Many pathogens use LuxS to exert AI-2 control over important virulence factors and essential metabolic pathways in pathogens, such as methionine synthesis and furanone production (7).
LuxS is an important enzyme in the activated methyl cycle, which is a means of methylating cellular components and recycling certain sulfur-containing amino acids in bacteria, archaea, and eukaryotes. A component of the activated methyl cycle, S-adenosyl-l-homocysteine, is detoxified to S-ribosyl-homocysteine by the Pfs enzyme, 5′-methylthioadenosine/s-adenosylhomocysteine nucleosidase. S-ribosyl-homocysteine, a substrate of LuxS, is catabolized to homocysteine, and 4,5-dihydroxy-2,3-pentanedione (the precursor of AI-2) (9, 10). In S. typhimurium and E. coli, AI-2 is recognized and bound by the LsrB protein, and then the AI-2 is phosphorylated by LsrK (11, 12). The phosphorylated form of AI-2 induces lsr transcription, and the process is acted upon by binding to LsrR, the repressor of the lsr operon (13). The LuxS/AI-2 quorum-sensing system is present in many Pasteurellaceae strains, and the system is associated with bacterial biofilms, cell adhesion, motility, and virulence (14–17). However, the role of the LuxS in Av. paragallinarum is unknown.
The involvement of LuxS in both the quorum-sensing system and the activated methyl cycle implies that it is important to study the function of LuxS in the in vivo pathogenicity of Av. paragallinarum. Here, we constructed a luxS deletion mutant of Av. paragallinarum to investigate the LuxS/AI-2 activity. We also determined differences in the expression of quorum sensing-related genes between the wild-type and the mutant strain. Furthermore, bacterial adherence and invasion were evaluated in the luxS knockout mutant. Finally, a pathogenicity analysis of the luxS mutant strain was performed.
2 Materials and methods
2.1 Bacterial strains, primers, and culture conditions
A clinical isolate of Av. paragallinarum strain 3005 (serogroup C) was isolated in China in 2018 (18). Wild-type, mutant, and complemented strains were grown in tryptic soy broth or tryptic soy agar medium supplemented with 10% chicken serum and 0.0025% nicotinamide adenine dinucleotide (NAD) at 37°C, and the agar plates were cultured under 5% carbon dioxide. Vibrio harveyi strains BB170 and BB152 were supplied by Professor Han (The Chinese Academy of Agricultural Sciences, Shanghai, China) and cultivated in a modified autoinducer bioassay (AB) medium at 30°C (19). Escherichia coli DH5α and BL21 (DE3) (Vazyme, Nanjing, China) were grown routinely in Lennox broth or on a solid medium containing 1.5% agar at 37°C. All primers used are listed in Table 1.
2.2 The expression of LuxS and production of LuxS antiserum
Primers pET32a-luxS-F/R and luxS-pET32a-F/R were used to amplify the luxS fragment from Av. paragallinarum strain 3005 and the expression vector from pET-32a, respectively. The PCR products were used to construct the pET32a-luxS expression plasmid using the Hieff Clone® Plus One Step Cloning Kit (Yeasen, Shanghai, China). Escherichia coli BL21 (DE3) transformed with the pET32a-luxS plasmid was used to express recombinant LuxS protein (His-LuxS). His-LuxS was purified using a His-tagged protein purification kit (CWbio, Jiangsu, China) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (22).
The His-LuxS protein was mixed with ‘QuickAntibody,’ a water-soluble immune adjuvant (Biodragon, Beijing, China), to prepare mouse antiserum. The His-LuxS protein was diluted to 1 mg/mL, and then 50 μL of protein was quickly mixed with the immune adjuvant by 1:1. Six male BALB/c mice were subcutaneously immunized with the mixture at a dose of 50 μg/mouse two times, with an interval of 14 days. Blood was collected from the tail vein, and immune serum titers were assessed by enzyme-linked immunosorbent assays (ELISAs). Mice with high antibody titers were euthanized in 30% CO2 cages, and their sera were collected.
2.3 Construction of luxS mutant and complemented strains
A fragment consisting of the luxS gene and luxS upstream and luxS downstream was amplified from the Av. paragallinarum strain 3005 using primers luxS-UF/luxS-UR and cloned into T Vector pMD19 (Takara, Dalian, China). The fragment was then inserted into the pGEM®-T Easy vector using HindIII and EcoRI restriction enzyme sites. The luxS gene of the obtained pGEM®-T Easy vector was replaced with a chloramphenicol cassette to generate the recombinant plasmid. The recombinant plasmid was introduced into Av. paragallinarum by electroporation (23). Briefly, wild-type Av. paragallinarum was washed three times with 9.3% (wt/vol) of cold sucrose, and 65 μL aliquots were mixed with recombinant plasmid (0.5 μg) and transferred to precooled electroporation cuvettes (gap size, 1 mm). After electrotransformation, the positive strain was selected on tryptic soy agar containing 10 μg/mL of chloramphenicol.
The complemented strain was constructed using the pBBR1MCS-2 vector as described, with a simple modification for the construction of the luxS mutant. The luxS gene was amplified using luxS-F/luxS-R primers and then cloned into T Vector pMD19. SalI and XbaI restriction enzymes were then used on the T-Vector pMD19 and pBBR1MCS-2 to establish the complemented plasmid. The plasmid was introduced into the luxS mutant strain by electrotransformation, and 50 μg/mL of kanamycin was used to select the complemented strain.
Complementation was verified by agarose gel electrophoresis analysis of the luxS gene using luxS-F/R primers, the chloramphenicol cassette using CM-F/R primers (20), and the total fragment using luxS-UF/luxS-UR primers. The deletion of the LuxS protein was confirmed by Western blot analysis.
2.4 AI-2 bioluminescence assay
The assay was performed essentially as previously described, with modification (24). Briefly, the test bacteria of Av. paragallinarum (wild-type, mutant strain, and complemented strain) was grown on tryptic soy agar plates and then washed with the AB medium. The washed medium and Vibrio harveyi strains BB152 in the logarithmic phase of growth were centrifuged at 12,000 g for 5 min, and the supernatants were collected and filtered through a 0.22 μm filter (Millipore, Billerica, USA). The V. harveyi BB170 reporter strain was grown in an orbital shaker for 16 h at 30°C. The cultured suspension was then diluted 1:5,000 in fresh AB medium. In total, 20 μL of filtered fluid were mixed with 180 μL of diluted BB170 in a flat-bottomed 96-well microtiter plate (Corning, New York, USA), followed by shaking at 30°C, 180 rpm for 6 h. Notably, 10% (v/v) filtered V. harveyi BB152 fluid was used as a positive control, and 10% (v/v) sterile PBS (0.01 M, pH = 7.4) was used as a negative control. Luminescence was measured every hour using a Synergy H1 microplate reader (BioTeK SYNERGY H1, Vermont, USA).
2.5 Detection of mRNAs of related quorum sensing genes
Total RNA was extracted from bacteria using a GenJET RNA Purification Kit (Thermo, Waltham, USA) following the manufacturer’s protocol and confirmed using a NanoDrop1000 spectrophotometer (NanoDrop Technologies Inc., Waltham, USA). RNA samples with a 260/280 nm ratio between 1.9 and 2.1 were used to synthesize cDNA using an EasyScript First-Strand cDNA Synthesis SuperMix kit (Trans, Beijing, China) according to the manufacturer’s instructions. The cDNA samples were stored at −20°C for later use.
Real-time quantitative PCR (RT-qPCR) was performed using SYBR Green SuperReal premix plus (Tiangen, Beijing, China). Total sample volumes of 25 μL contained 12.5 μL of PCR Master mix, 1 μL of DNA (or water as a negative control), 0.5 μL of forward/reverse primer, and 10.5 μL of water. Each reaction was performed in triplicate. To measure related quorum sensing genes, including pfs, lsrR, lsrB, lsrK, and rpoB (as a housekeeping control) (21), RT-qPCRs were performed with an initial denaturation step of 95°C for 15 min, followed by 40 cycles at 95°C for 10 s and 55°C for 30 s on a Bio-Rad IQ5 (Bio-Rad, Hercules, USA) (21, 25).
2.6 Bacterial adherence and invasion assays
The effects of the Av. paragallinarum luxS gene on cell adhesion and invasion were evaluated in assays using chicken macrophage (HD11, this laboratory) and chicken embryo fibroblast (DF-1, this laboratory) cells following a previously described method (26). Briefly, cells were seeded onto 24-well cell culture plates at 90% confluence. The wells were washed three times with PBS and infected with Av. paragallinarum (1 × 107 CFU/well) or its luxS mutant (1 × 107 CFU/well) in DMEM/F12 medium containing 10% (v/v) fetal bovine serum for HD11 cultures and DMEM medium containing 10% (v/v) fetal bovine serum for DF-1 cultures. After incubation at 37°C in 5% CO2 for 1 h, cells were rigorously washed with PBS three times to remove non-adherent bacteria and then incubated with 100 μL of 0.25% trypsin/EDTA containing 0.5% Triton X-100 for 10 min. The resulting cell suspension was serially diluted 10-fold with PBS and dispersed onto tryptic soy agar plates containing NAD and chicken serum. Finally, the number of adherent bacteria was counted.
To assess the effect of the Av. paragallinarum luxS gene on cell invasion, cell culture and infection were performed as described for the bacterial adherence assay. The infected cells were incubated for 3 h and 6 h. Following washing with PBS three times, 100 μg/mL of cefalexin and 50 μg/mL of kanamycin were added to each well and incubated at 37°C in 5% CO2 for 3 h to kill the extracellular bacteria. Wells were then treated with 100 μL of 0.25% trypsin/EDTA containing 0.5% Triton X-100 for 10 min, and the invaded bacteria were counted on tryptic soy agar plates containing NAD and chicken serum. Assays were performed for all treatment groups in duplicate three times.
2.7 Transmission electron microscopy (TEM)
TEM was used to visualize the in vitro invasion of cells by wild-type and luxS mutant Av. paragallinarum. DF-1 and HD11 cells were cultivated in culture dishes (10 cm diameter). The cell culture and infection were performed as described for the bacterial invasion assay. After pathogen infection for 6 h and antibiotic treatment for 3 h, the culture dishes were washed with PBS three times. The cells were then scraped from the bottom of the dishes with a cell scraper and collected in 1.5 mL sterile centrifuge tubes. Collected cells were fixed with 1 mL of fixative solution (2.5% glutaraldehyde) for 48 h. Ultrathin sections were then made and stained with 0.1% uranyl acetate and 0.1% lead citrate for transmission electron microscopy (Hitachi H-7500, Chiyoda, Japan).
2.8 Animal challenges
To assess potential changes in virulence associated with disrupting the luxS gene, wild-type and mutant Av. paragallinarum were used in chicken models (27). We carried out the animal experiments in strict accordance with the requirements of the Laboratory Animal Requirements of Environment and Housing Facilities (GB14925-2010, the National Laboratory Animal Standardization Technical Committee) and the Chinese Regulations of Laboratory Animals Guidelines (Ministry of Science and Technology of People’s Republic of China). All experimental procedures were approved and audited by the Beijing Academy of Agriculture and Forestry Sciences Animal Care and Use Committee guidelines [ID: SYXK (Jing) 2023–0005], which were approved by the Animal Welfare Committee of the Beijing Academy of Agriculture and Forestry Sciences (10 January 2023). Five-week-old male specific pathogen free White Leghorn chickens were randomly divided into seven experimental groups (n = 6 per group). The luxS mutant strain and wild strain infected three groups of chickens with doses of 1.5 × 105 CFU, 1.5 × 104 CFU, and 1.5 × 103 CFU, respectively. Each bird was challenged by infraorbital sinus inoculation with 0.2 mL of diluted bacteria. The control group was treated with PBS only. The clinical signs of nasal discharge and facial swelling in the challenged chickens were used to assess the morbidity of the chickens. According to a previously reported scoring system (28), 0 represents no clinical signs; 1 represents mild clinical signs (slight facial swelling); 2 represents moderate clinical signs (moderate facial swelling and nasal discharge); and 3 represents severe clinical signs (severe facial swelling, abundant nasal swelling, lacrimation, and partially or completely closed eye).
3 Results
3.1 Preparation of the mouse anti-LuxS antiserum
We cloned the luxS gene into pET32a and successfully expressed a recombinant His-LuxS fusion protein in E. coli BL21. The purified protein was a distinct band on SDS-PAGE analysis (Figure 1A). A His antibody was used to confirm the His-tag on the purified protein by Western blot analysis (Figure 1B). A mouse anti-LuxS antiserum diluted 10,000 times was assessed by ELISA and the antibody titer OD450 > 1.0.
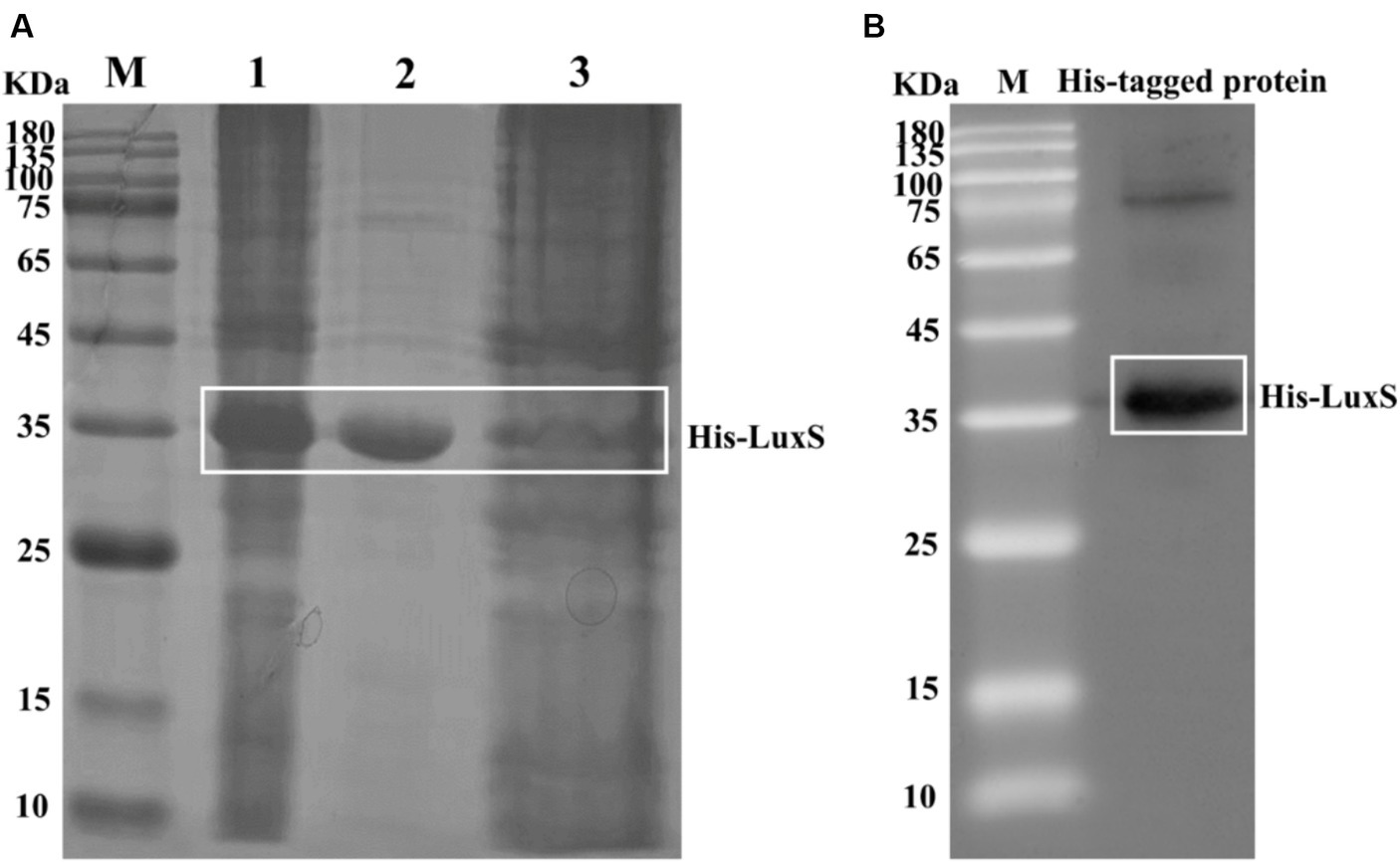
Figure 1. Induction of recombinant His-LuxS expression. The protein in extracts was obtained after the lysis of cells from induced E. coli BL21. SDS-PAGE analysis of the products expressed by E. coli BL21 harboring pET32a-LuxS (A). Lane 1, pET32a-LuxS/BL21 isopropyl β-d-1-thiogalactopyranoside induced; lane 2, the purified recombinant His-LuxS protein; lane 3, Ni-affinity filtered solution. Western blot analysis of the recombinant protein using an anti-His tag antibody (B).
3.2 Construction and verification of the LuxS mutant strain
Deletion of the luxS gene and complementation of the luxS gene were confirmed by PCR (Figure 2A) using primers luxS-F/R. The luxS gene was replaced by a chloramphenicol cassette; therefore, the chloramphenicol cassette in luxS mutants was identified by PCR using CM-F/R primers (Figure 2B). To ensure the mutation site was correct, the fragments were amplified from wild-type and mutant strains (Figure 2C). The predicted size of the LuxS protein is 17 kDa. Deletion of the LuxS protein was confirmed by Western blotting, showing no band in 17 kDa of the mutant strain (Figure 2D).
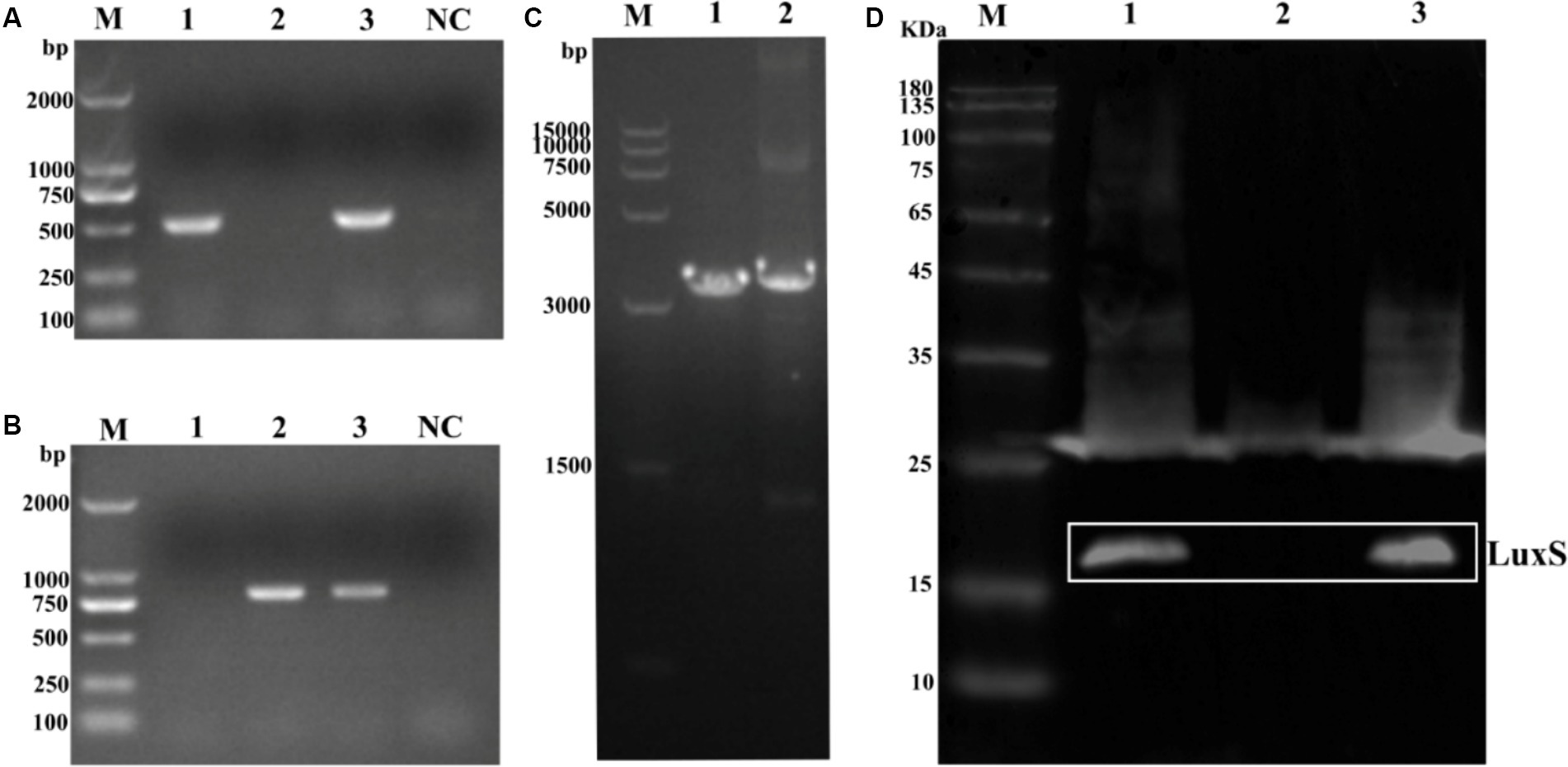
Figure 2. Identification of mutant strain and complemented strains of Av. paragallinarum. Agarose gel electrophoresis analysis of the luxS gene (A), chloramphenicol cassette (B), and the total fragments [(C), the chloramphenicol cassette is longer (294 bp) than the luxS sequence]. Western blot analysis of the LuxS protein deletion (D). The LuxS is 17 kDa. Lane 1, wild-type; lane 2, mutant strain; lane 3, complemented strain; NC, negative control.
3.3 Detection of AI-2 activity and expression of related quorum sensing genes
Test supernatants of AB medium washes of lawn plates of Av. paragallinarum (wild-type, mutant strain, and complemented strain) were measured, and there was a significant drop of AI-2 activity in the luxS mutant, and the activity was successfully recovered in the complemented strain (Figure 3). The results indicated that LuxS is necessary for AI-2 production in Av. paragallinarum.
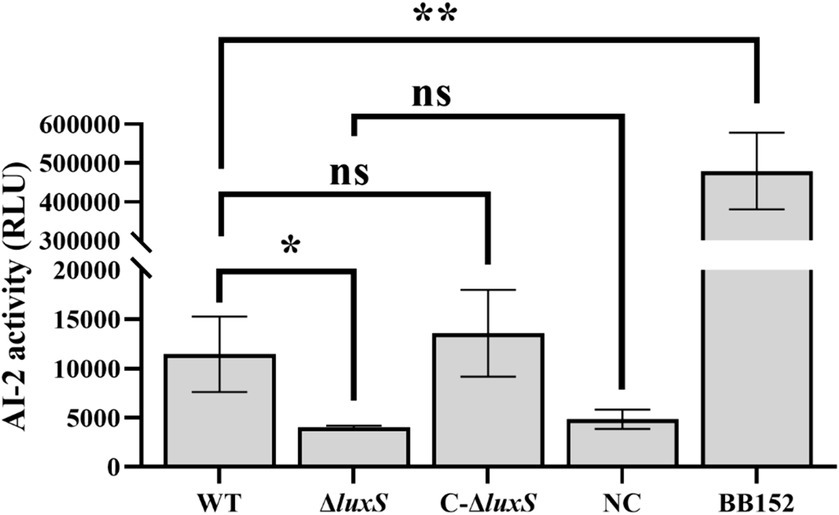
Figure 3. Bioluminosity values after 6 h of growth. AI-2 activity is represented by relative light units (RLU) to compare AI-2 molecule expression among strains. Vibrio harveyi BB152 served as a positive control, and AB medium was used as a negative control (NC). The error bars represent the standard deviation from three independent experiments. * represents p < 0.05, ** represents p < 0.01, and ns represents no significant difference.
In addition, real-time qPCR showed no significant differences in mRNA levels of related quorum sensing genes, lsrR, lsrB, and lsrK, between the wild-type strain, the mutant strain, and the complemented strain. However, the mRNA level of the pfs gene increased remarkably in the luxS mutant strain compared with the wild type, and the mRNA level of the pfs of the complemented strain was recovered to a level similar to that of the wild type (Figure 4). These results indicate that Av. paragallinarum LuxS is an important enzyme in the activated methyl cycle, but may not be involved in the conventional LuxS/AI-2 quorum-sensing system.
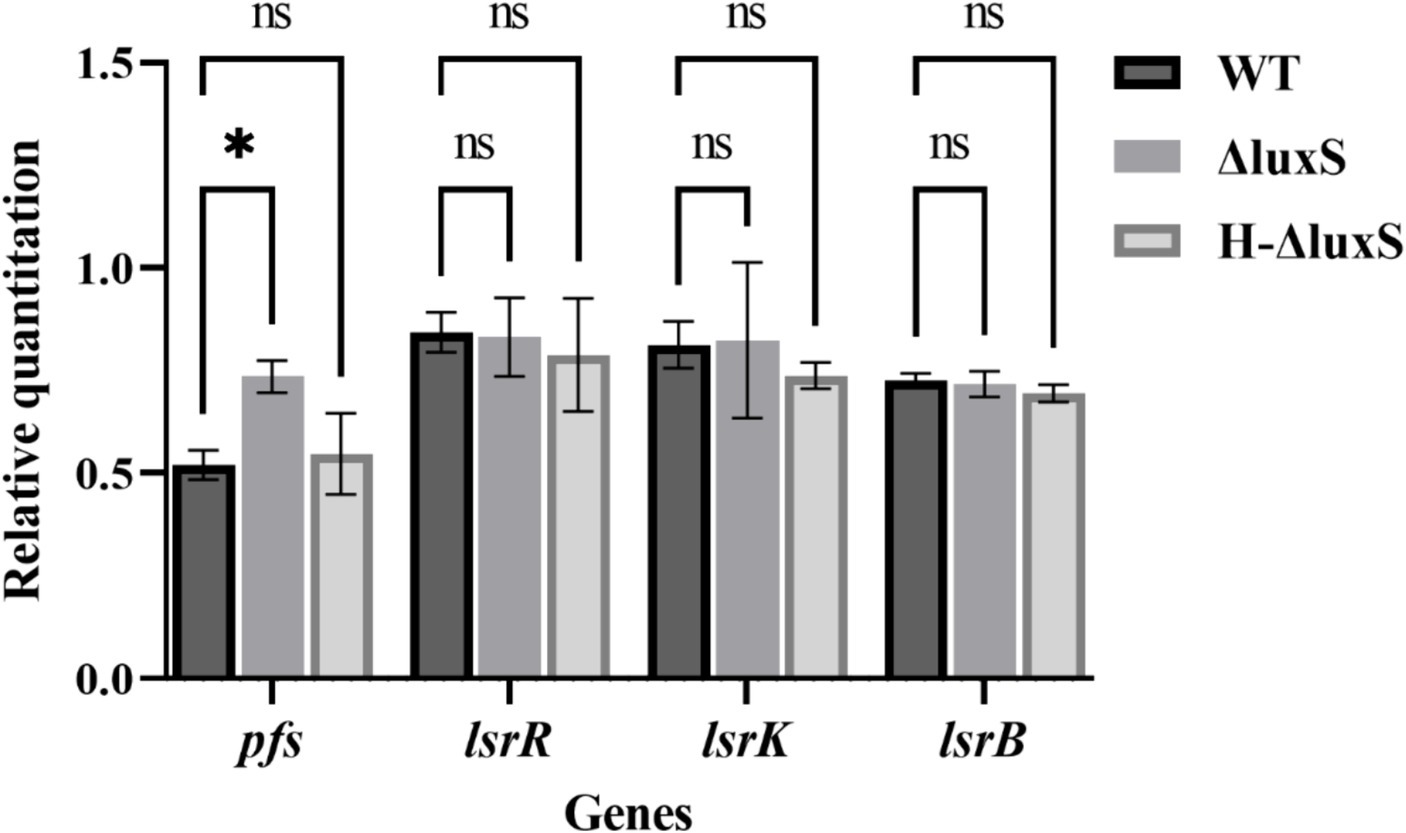
Figure 4. Effects of the luxS mutant on the expression of quorum sensing genes in Av. paragallinarum. qRT-PCR showed increased pfs expression (p = 0.0229) in the mutant compared with the wild-type strain, and recovered pfs expression to a level similar to that of the wild-type strain in the complemented strain. Other related genes did not show statistical differences in expression between the wild-type and the luxS mutant. * represents p < 0.05. Data represent means ± SD of three experiments conducted in triplicate.
3.4 Pathogenicity of the LuxS mutant strain
There was no distinct difference in adherence to HD11 and DF-1 cells between the wild type and the mutant. Deletion of the luxS gene, however, significantly reduced the capacity to invade HD11 and DF-1 cells (Figure 5A). The Av. paragallinarum invasion of DF-1 and HD11 cells by the wild type and the mutant was visualized by TEM (Figure 5B).
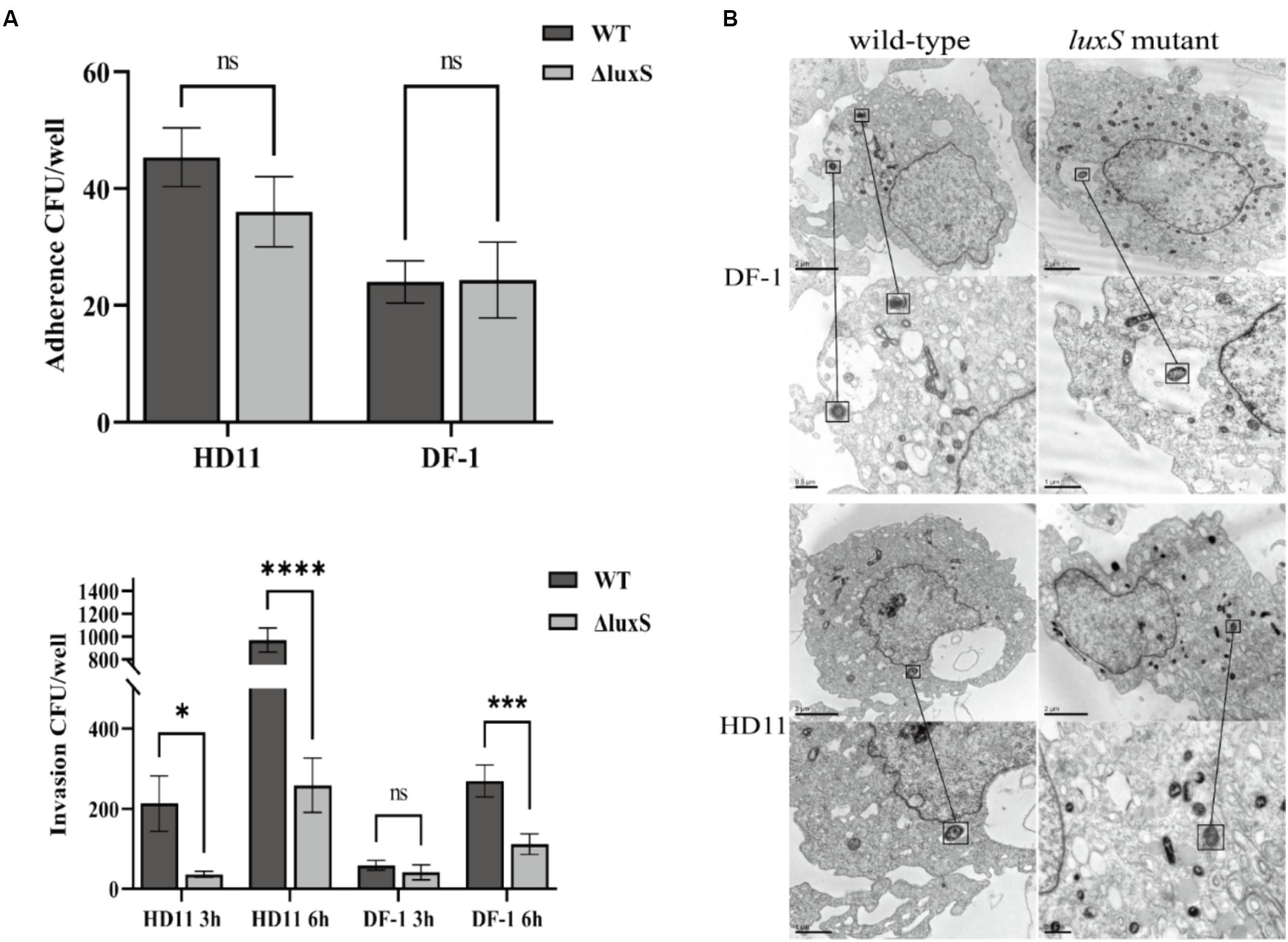
Figure 5. Cell adherence and invasion assay. Infection HD11 and DF-1 cells by wild-type or the luxS mutant. There was no statistical difference in adherence to HD11 or DF-1 cells between the wild-type and the luxS mutant. There was significantly reduced invasion of both HD11 and DF-1 cells by the luxS mutant. * represents p < 0.05, *** represents p < 0.001, **** represents p < 0.0001, and ns represents no significant difference. The columns represent the means and standard deviations of three experiments (A). Transmission electron microscopy of DF-1 and HD11 cells shows infection with wild-type and mutant Av. paragallinarum, and the bacteria was noted in the black box (B).
To evaluate the impact of the luxS gene on the virulence of Av. paragallinarum in vivo, we used a relatively low challenge dose to ensure the virulence of a low level of the mutant strain could be observed. Clinical signs of infectious coryza were recorded from days 1–7 post-infection. The chickens infected with wild-type and mutant strains at the dose of 1.5 × 105 CFU or 1.5 × 104 CFU all displayed serious facial and nasal discharge on day 1. However, there were some minor differences when the challenge dose was 1.5 × 103 CFU. The chickens infected with the mutant strain showed clinical symptoms on day 3 after the challenge, while the wild strain showed symptoms on day 1 (Figure 6A). Compared with wild strain infection, the chickens infected with the mutant strain of the lowest dose (1.5 × 103 CFU) showed milder clinical manifestations. One chicken displayed milder nasal discharge, one chicken showed moderate facial swelling, and four out of six chickens displayed serious facial swelling and nasal discharge, the same clinical signs as the wild strain (Figure 6B). These minor differences indicate that the luxS gene does not play an essential role in the pathogenesis of Avibacterium paragallinarum.

Figure 6. Pathogenicity tests of luxS mutant strain in vivo. Clinical signs of infectious coryza were recorded from days 1–7 post-infection. The number of animals infected with the bacteria of the lowest dose (1.5 × 103 CFU) is shown in (A), and the number of clinical scores was measured on infection day 3 (B).
4 Discussion
LuxS is widely distributed among bacteria. It is involved in the production of autoinducer (AI-2) and is an integral component of the activated methyl cycle in bacteria (29). A large number of Gram-negative and Gram-positive bacteria are universally present in the LuxS, and AI-2 is considered an inter-species quorum sensing signal (30). Quorum sensing is a communication method among bacteria (30). In the same environment, they exchange complex and precise information by releasing and receiving tiny chemical molecules as signals. In the previous study, LuxS was associated with many virulence features, including biofilm formation, toxin production, adherence, motility, and stress response, as well as being involved in metabolisms such as iron, sulfur, and carbon metabolism (7). The family of Pasteurellaceae strains Glaesserella parasuis, Haemophilus influenzae, Mannheimia haemolytica, and Actinobacillus pleuropneumoniae exist in the LuxS/AI-2 system, and the luxS mutant affected the physiological and virulence of the strains (14, 17, 31, 32). As a member of the Pasteurellaceae family, the role of the LuxS in Av. paragallinarum remains unclear. Therefore, the main focus of this study is to investigate whether the LuxS affects the pathogenicity of Av. paragallinarum, as well as its impact on cell adhesion and invasion, and the regulation of other related molecules. We generated the luxS mutant strain and evaluated its adhesion and invasion of HD11 and DF-1 cells, while also evaluating its virulence in chickens. Similar to the previous studies showing that LuxS is associated with pathogen virulence, this study showed delayed and milder clinical symptoms between animals challenged with luxS mutant Av. paragallinarum. These results indicate that the LuxS may not play an essential role in Av. paragallinarum infection.
In this study, different strains of Av. paragallinarum were used to construct the luxS mutant, including standard strains 221, 0222, and Modesto and isolate 3005. However, only 3005-ΔluxS was obtained. Previously, the same situation was found when constructing the HutZ mutant of Av. paragallinarum, which is involved in iron homeostasis in Av. paragallinarum (20). It seems that different strains had different mutation probabilities. Two isolates were used, and the capsular mutants of Av. paragallinarum were constructed by the inactivation of the hctA gene using the TargeTronH gene knockout system (33). In addition, to verify the conservation of the luxS gene in Av. paragallinarum, several luxS gene sequences of Av. paragallinarum and other Pasteurellaceae strains were selected from the NCBI database, and homology analysis was performed. A phylogenetic analysis shows the genetic relationships among Av. paragallinarum and other Pasteurellaceae strains. The homology of the luxS gene among reference strains and local isolates of Av. paragallinarum is over 99% and ranges from 69 to 78% with other Pasteurellaceae strains (Supplementary Figure 1). This analysis shows that the luxS gene sequence is highly conserved among Av. paragallinarum. Therefore, we used strain 3,005 as a representative to study the function of LuxS. In the future, more strains will be used to verify the difference in mutant rate between standard strains and isolates.
AI-2-like molecules can be detected in many bacterial species by the reporter strain, V. harveyi BB170 (32, 34, 35). Certain components in tryptic soy broth may interfere with AI-2 activity (19); therefore, AB medium wash supernatants from lawn plates of Av. paragallinarum were used in AI-2 detection. The AI-2 detection assay showed that the LuxS/AI-2 system exists in Av. paragallinarum and that LuxS is a key gene in AI-2 synthesis because the luminescence value of the luxS deletion group was almost the same as that of the negative control. Furthermore, some regulated genes are associated with AI-2 recognition and modification; therefore, we assessed the effect of the luxS mutant on the transcription of lsrB (the AI-2-binding protein gene) (11), lsrK (encoding a kinase responsible for AI-2 phosphorylation) (12), and lsrR (encoding a repressor for the luxS-regulated lsr operon) (13). However, there was no obvious difference in the transcription of lsrB, lsrK, and lsrR between the wild type and the luxS mutant, which indicates that the genes reported to relate to the recognition and modification of AI-2 molecules may not be applicable to Av. paragallinarum. Additionally, as LuxS and Pfs are the only two essential enzymes in the biosynthetic pathway for AI-2 (9), the significantly increased transcription of pfs in the luxS mutant indicates that LuxS may play an important role in the activated methyl cycle pathway of Av. paragallinarum. Together, these findings indicate that LuxS plays an essential role in the production of AI-2 in Av. paragallinarum and may also be involved in the activated methyl cycle pathway in Av. paragallinarum.
The luxS deficiency can reduce the ability of some bacteria to survive in macrophages, indicating that luxS plays an important role in immunological evasion after invasion (36, 37). We, therefore, evaluated the ability of wild-type and luxS mutant Av. paragallinarum to invade HD11 and DF-1 cells. We noted that the deletion of luxS resulted in a significant decrease in cell invasion. This showed that the absence of luxS may decrease the ability of Av. paragallinarum to survive in macrophages and that luxS deficiency has an adverse effect on the invasion of the pathogen. Bacterial invasion is an essential factor in bacterial infection and pathogenicity, however, the mechanism by which LuxS affects Av. paragallinarum invasion is not clear, and further studies are warranted.
In conclusion, this study showed that LuxS may not only be associated with the activated methyl cycle but is also involved in the AI-2 quorum sensing pathway in Av. paragallinarum. Additionally, the luxS mutant shows significantly decreased invasive capacity but is not essential for the virulence of Av. paragallinarum. Further research on the quorum-sensing system of pathogens may provide novel therapeutic strategies to prevent and control Av. paragallinarum in poultry.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by the Beijing Academy of Agriculture and Forestry Sciences Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
DL: Data curation, Formal analysis, Methodology, Writing – original draft. CH: Data curation, Formal analysis, Funding acquisition, Writing – review & editing. GL: Investigation, Resources, Writing – original draft. MZ: Investigation, Resources, Writing – original draft. FX: Resources, Writing – original draft. JQ: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. HS: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Reform and Development Project of the Beijing Academy of Agriculture and Forestry Sciences (XMS202409), the Beijing Municipal Natural Science Foundation (6182010), the National Natural Science Foundation of China (32102619), and the Earmarked Fund for Hebei Layer-Broiler Innovation Team of Modern Agro-industry Technology Research System (HBCT2018150101 and HBCT2018150207).
Acknowledgments
The authors gratefully thank Dr. Xiangan Han for providing Vibrio harveyi strains BB170 and BB152.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1427966/full#supplementary-material
References
1. Blackall, PJ. Infectious coryza: overview of the disease and new diagnostic options. Clin Microbiol Rev. (1999) 12:627–32. doi: 10.1128/CMR.12.4.627
2. Blackall, PJ, Christensen, H, Beckenham, T, Blackall, LL, and Bisgaard, M. Reclassification of Pasteurella gallinarum, [Haemophilus] paragallinarum, Pasteurella avium and Pasteurella volantium as Avibacterium gallinarum gen. nov., comb. nov., Avibacterium paragallinarum comb. nov., Avibacterium avium comb. nov. and Avibacterium volantium comb. nov. Int J Syst Evol Microbiol. (2005) 55:353–62. doi: 10.1099/ijs.0.63357-0
3. Xu, Y, Cheng, J, Huang, X, Xu, M, Feng, J, Liu, C, et al. Characterization of emergent Avibacterium paragallinarum strains and the protection conferred by infectious coryza vaccines against them in China. Poult Sci. (2019) 98:6463–71. doi: 10.3382/ps/pez531
4. Blackall, PJ, and Soriano-Vargas, E. Infectious coryza and related bacterial infections In: D Eswayne, M Boulianne, C Logue, L Mc Dougald, V Nair, and D Suarez, et al., editors. Diseases of poultry. 14th ed: Wiley-Blackwell (2020). 890–906.
5. Man, WH, de Steenhuijsen Piters, WA, and Bogaert, D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. (2017) 15:259–70. doi: 10.1038/nrmicro.2017.14
6. Wang, S, Payne, GF, and Bentley, WE. Quorum sensing communication: molecularly connecting cells, their neighbors, and even devices. Ann Rev Chem Biomol Eng. (2020) 11:447–68. doi: 10.1146/annurev-chembioeng-101519-124728
7. Vendeville, A, Winzer, K, Heurlier, K, Tang, CM, and Hardie, KR. Making 'sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. (2005) 3:383–96. doi: 10.1038/nrmicro1146
8. Williams, P, Winzer, K, Chan, WC, and Camara, M. Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond Ser B Biol Sci. (2007) 362:1119–34. doi: 10.1098/rstb.2007.2039
9. Schauder, S, and Bassler, BL. The languages of bacteria. Genes Dev. (2001) 15:1468–80. doi: 10.1101/gad.899601
10. Winzer, K, Hardie, KR, Burgess, N, Doherty, N, Kirke, D, Holden, MTG, et al. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology. (2002) 148:909–22. doi: 10.1099/00221287-148-4-909
11. Miller, ST, Xavier, KB, Campagna, SR, Taga, ME, Semmelhack, MF, Bassler, BL, et al. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. (2004) 15:677–87. doi: 10.1016/j.molcel.2004.07.020
12. Medarametla, P, Kronenberger, T, Laitinen, T, and Poso, A. Structural characterization of LsrK as a quorum sensing target and a comparison between X-ray and homology models. J Chem Inf Model. (2021) 61:1346–53. doi: 10.1021/acs.jcim.0c01233
13. Taga, ME, Semmelhack, JL, and Bassler, BL. The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol Microbiol. (2001) 42:777–93. doi: 10.1046/j.1365-2958.2001.02669.x
14. Daines, DA, Bothwell, M, Furrer, J, Unrath, W, Nelson, K, Jarisch, J, et al. Haemophilus influenzae luxS mutants form a biofilm and have increased virulence. Microb Pathog. (2005) 39:87–96. doi: 10.1016/j.micpath.2005.06.003
15. Fong, KP, Gao, L, and Demuth, DR. luxS and arcB control aerobic growth of Actinobacillus actinomycetemcomitans under iron limitation. Infect Immun. (2003) 71:298–308. doi: 10.1128/iai.71.1.298-308.2003
16. James, D, Shao, H, Lamont, RJ, and Demuth, DR. The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals. Infect Immun. (2006) 74:4021–9. doi: 10.1128/iai.01741-05
17. van der Vinne, AN, Lo, RY, and Shewen, PE. Construction and analysis of a Mannheimia haemolytica A1 luxS mutant. Vet Microbiol. (2005) 110:53–66. doi: 10.1016/j.vetmic.2005.06.011
18. Huo, C, Zeng, X, Xu, F, Li, F, Li, D, Li, G, et al. The transcriptomic and bioinformatic characterizations of iron acquisition and heme utilization in Avibacterium paragallinarum in response to iron-starvation. Front Microbiol. (2021) 12:610196. doi: 10.3389/fmicb.2021.610196
19. Han, X, Liu, L, Fan, G, Zhang, Y, Xu, D, Zuo, J, et al. Riemerella anatipestifer lacks luxS, but can uptake exogenous autoinducer-2 to regulate biofilm formation. Res Microbiol. (2015) 166:486–93. doi: 10.1016/j.resmic.2015.06.004
20. Huo, C, Jiao, L, Li, G, Li, D, Lin, W, Sun, Y, et al. HutZ is required for efficient heme utilization and contributes to the pathogenicity of Avibacterium paragallinarum. Microbiol Spectr. (2023) 11:e0397922. doi: 10.1128/spectrum.03979-22
21. Wen, S, Chen, X, Xu, F, and Sun, H. Validation of reference genes for real-time quantitative PCR (qPCR) analysis of Avibacterium paragallinarum. PLoS One. (2016) 11:e0167736. doi: 10.1371/journal.pone.0167736
22. Zhang, W, Zhao, Y, Hu, L, Guan, C, Xun, M, Wu, F, et al. Establishment of purification method for prokaryotic expression of serpin gene for Dermatophagoides farinae. Protein Expr Purif. (2022) 195-196:106080. doi: 10.1016/j.pep.2022.106080
23. Kristensen, BM, Sinha, S, Boyce, JD, Bojesen, AM, Mell, JC, and Redfield, RJ. Natural transformation of Gallibacterium anatis. Appl Environ Microbiol. (2012) 78:4914–22. doi: 10.1128/AEM.00412-12
24. Bassler, BL, Wright, M, Showalter, RE, and Silverman, MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. (1993) 9:773–86. doi: 10.1111/j.1365-2958.1993.tb01737.x
25. Wang, L, Li, J, March, JC, Valdes, JJ, and Bentley, WE. luxS-dependent gene regulation in Escherichia coli K-12 revealed by genomic expression profiling. J Bacteriol. (2005) 187:8350–60. doi: 10.1128/JB.187.24.8350-8360.2005
26. Li, Q, Li, Y, Xia, J, Wang, X, Yin, K, Hu, Y, et al. Virulence of Salmonella enterica serovar Pullorum isolates compared using cell-based and chicken embryo infection models. Poult Sci. (2019) 98:1488–93. doi: 10.3382/ps/pey482
27. Trujillo-Ruiz, HH, Shivaprasad, HL, Morales-Erasto, V, Talavera-Rojas, M, Salgado-Miranda, C, Salazar-Garcia, F, et al. Virulence of serovar C-1 strains of Avibacterium paragallinarum. Avian Dis. (2016) 60:837–40. doi: 10.1637/11421-040716-ResNote
28. Bragg, RR. Virulence of South African isolates of Haemophilus paragallinarum. Part 1: NAD-dependent field isolates. Onderstepoort J Vet Res. (2002) 69:163–9.
29. De Keersmaecker, SC, Sonck, K, and Vanderleyden, J. Let LuxS speak up in AI-2 signaling. Trends Microbiol. (2006) 14:114–9. doi: 10.1016/j.tim.2006.01.003
30. Waters, CM, and Bassler, BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. (2005) 21:319–46. doi: 10.1146/annurev.cellbio.21.012704.131001
31. Li, L, Zhou, R, Li, T, Kang, M, Wan, Y, Xu, Z, et al. Enhanced biofilm formation and reduced virulence of Actinobacillus pleuropneumoniae luxS mutant. Microb Pathog. (2008) 45:192–200. doi: 10.1016/j.micpath.2008.05.008
32. Zhang, B, Ku, X, Zhang, X, Zhang, Y, Chen, G, Chen, F, et al. The AI-2/luxS quorum sensing system affects the growth characteristics, biofilm formation, and virulence of Haemophilus parasuis. Front Cell Infect Microbiol. (2019) 9:62. doi: 10.3389/fcimb.2019.00062
33. Tu, TY, Hsieh, MK, Tan, DH, Ou, SC, Shien, JH, Yen, TY, et al. Loss of the capsule increases the adherence activity but decreases the virulence of Avibacterium paragallinarum. Avian Dis. (2015) 59:87–93. doi: 10.1637/10937-091414-reg
34. Bassler, BL, Greenberg, EP, and Stevens, AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. (1997) 179:4043–5. doi: 10.1128/jb.179.12.4043-4045.1997
35. Pan, Y, Siddaramappa, S, Sandal, I, Dickerman, A, Bandara, AB, and Inzana, TJ. The role of luxS in Histophilus somni virulence and biofilm formation. Infect Immun. (2021) 89:89. doi: 10.1128/IAI.00567-20
36. Lee, J, Remold, HG, Ieong, MH, and Kornfeld, H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. (2006) 176:4267–74. doi: 10.4049/jimmunol.176.7.4267
Keywords: quorum sensing, LuxS, autoinducer-2, Avibacterium paragallinarum , virulence
Citation: Li D, Huo C, Li G, Zhu M, Xu F, Qiao J and Sun H (2024) The absence of luxS reduces the invasion of Avibacterium paragallinarum but is not essential for virulence. Front. Vet. Sci. 11:1427966. doi: 10.3389/fvets.2024.1427966
Edited by:
Xiaorong Zhang, Yangzhou University, ChinaReviewed by:
Shaohui Wang, Chinese Academy of Agricultural Sciences, ChinaTang Fang, Nanjing Agricultural University, China
Patrick Blackall, The University of Queensland, Australia
Copyright © 2024 Li, Huo, Li, Zhu, Xu, Qiao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiling Sun, c3VuaHVpbGluZzAxQDE2My5jb20=
 Donghai Li1,2
Donghai Li1,2 Guiping Li
Guiping Li Huiling Sun
Huiling Sun