- 1Department of Parasitology, University of Agriculture, Faisalabad, Pakistan
- 2Department of Veterinary Preventive Medicine, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia
Infectious bursal disease (IBD) is one of the dangerous diseases of poultry that affects the bursa of Fabricius, which is an important organ of the bird’s immune system. IBD virus is resistant to many drugs, making its control difficult. Vaccination of IBD is in practice for a long time worldwide to control IBD, but secondary issues like vaccine failure and lower efficacy lead to their reduced use in the field. Multiple medicines are currently used, but the phytochemicals have emerged as promising agents for controlling IBD. The drugs to be developed should possess direct antiviral properties by targeting viral entry mechanisms, enhancing the host immune response, and inhibiting viral protein synthesis. Phytochemicals have potential to contribute to food security by minimizing the possibility of disease outbreaks and ensuring that consumers worldwide obtain healthy poultry products. It has been now claimed that direct and indirect activities of phytochemicals can be effective in the control of IBDV. Although available evidence suggest that the phytochemicals can contribute in controlling occurrence IBDV, there is a definite need of focused studies to gain more insight and develop rational strategies for their practical use. This review highlights the disease caused by IBDV, inhibition of viral replication, boosting the immune system, disruption of viral membrane, and important phytochemicals showing antiviral activities against IBDV.
Introduction
Poultry is a significant source of income and food that is essential for food security and global agriculture (1, 2). Poultry products like chicken meat and eggs are accessible sources of protein. Additionally, they are a good source of vital nutrients for human health, such as vitamins and minerals (3, 4). The largest and most significant source of animal protein for human consumption is the poultry industry (5, 6). More than 130 million metric tons of poultry meat and more than 86.67 million metric tons of eggs were produced worldwide in 2020 (7). Bacteria (Mycobacterium tuberculosis, coliform bacillary), ectoparasites and endoparasites, fungal pathogens, and eleven virus species can spread diseases horizontally and vertically in poultry (8, 9). One of the main reasons for financial losses in the global chicken industry is viral outbreaks (10, 11). Zootechnical performances, including body weight gain, feed conversion ratio, feed intake, and the quality of the eggs and meat produced, are significantly impacted by viral outbreaks in the poultry industry (12, 13). Avian influenza virus (AIV) (14), Newcastle disease virus (NDV) (15), Marek’s disease virus (MDV), infectious laryngotracheitis virus (ILTV), infectious bursal disease virus (IBDV) and infectious bronchitis virus (IBV) are the major pathogens that cause diseases in poultry (16).
IBDV is a highly contagious, positive sense, single-stranded, and RNA-based gammacoronavirus from the family of coronaviridae (17). IBD is a widespread infectious disease caused by a virus that infects young chickens and weakens their immune system (18, 19). It is caused by a birnavirus and mostly affects the bursa of Fabricius, an important organ in poultry for the growth of their immune systems (20, 21). Multiple IBDV serotypes are frequently observed co-circulating on farms, with little cross-protection from the vaccination strain against unrelated field strains. Multiple other species of IBDV cause diseases in various species of birds (Table 1).
IBDV has spread over South East Asia, the Middle East, Europe, Africa, and Latin America, causing high mortality in chickens (29, 30). IBDV is extremely infectious and spreads at a high rate in poultry (31). Young chicks are especially susceptible to IBDV, which causes immunosuppression and mortality often between 3 and 6 weeks of age (32, 33). The virus may remain in the environment for several weeks and is excreted in the feces of infected birds (34, 35). Although indirect transmission via contaminated feed, water, equipment, or employees and direct contact between sick and vulnerable birds is the main source of infection” the primary mode of infection is indirect transmission through contaminated water, feed, equipment, or personnel, as well as direct contact between infected and susceptible birds (15, 36). The virus may spread between farms through rodents, wild birds, and insects as carriers (37). The virus enters in a chicken body through the digestive and respiratory system where it infects the dendritic cells and macrophages that are present in the mucosal lining of the digestive and respiratory tract (38). After penetration into the cell, IBDV replicates quickly, facilitating its spread through the body via blood circulation and the lymphatic system. Afterward, the virus infects B-lymphocytes, vital for immune system development (39). IBDV destroys the B-lymphocytes, decreasing antibody production, and leading to immunosuppression (40). The circulating B-cells in the secondary lymphoid tissues and lymphoid follicles in the bursa of Fabricius are both destroyed by the virus. As a result, fewer antibodies are produced, cell-mediated immunity is compromised, and the body is more vulnerable to other diseases (41). The injured birds are more susceptible to secondary infections and immunosuppressive effects can last for several weeks (42). Chicken with immunosuppression and low immunity are more susceptible to disease and vaccine failure. Both layer and broiler flocks are susceptible to IBD which can result in high mortality rates, less production, and substantial financial loss for producers (43). The pathogenicity of IBDV can vary depending on the immune status, age of the infected birds, and the viral strain (44). The symptoms of IBD include lethargy, depression, exhaustion, decreased appetite, watery diarrhea, immune depression, less energy, and a drop in feed consumption (45). With decreased productivity, higher mortality rates, veterinary costs, decreased egg production, and trade restrictions, IBDV can significantly affect the economics of poultry production. This critical problem emphasizes the necessity for researchers to develop strategies to reduce IBDV. One of the most popular solutions for treating IBDV on a commercial level is vaccinations. There are several commercially available IBDV vaccinations (46). Vaccines are being developed to target various strains of the IBDV and are effective against multiple species of the parasite. Modified-live vaccine (MLV), IBD vectored vaccine (vHVT13), live attenuated vaccines, inactivated vaccines, immune-complex (Icx), and live recombinant vaccines expressing the capsid (VP2) antigen of IBDV vaccines are being used in routine farming (47). IBD vaccines may provide immunity, but some problems limit their use. Some commonly available vaccinations are no longer effective in protecting IBDV in many commercial poultry farms (48). The apparent inability to prevent IBDV infections through vaccination may occasionally be caused by improper vaccine virus administration, antigenic variations among the viruses, insufficient potency of the live attenuated vaccine virus, or interaction between the vaccine virus and any residual maternally derived antibodies (49). The traditional strain vaccine was not protective against variant IBDV strains. Additionally, IBDV vaccines provide only transient protection and require repeated application, especially in layer and breeder chicken (50). The expensive cost of the IBDV vaccine restricts its use in poultry. The virus is challenging to control since it can endure for a long time in the environment. Finding a suitable substitute to combat IBD is necessary due to these problems. Several approaches are being investigated, for the prevention and management of IBD, including the use of amino acids, organic acids, and their derivatives (51). Phytochemicals are also proposed alternatives for controlling IBD, as they are gaining attention from researchers for their antifungal (3), anti-infectious, anti-inflammatory, antioxidant, and immunomodulatory properties (52, 53). These investigations have generated valuable insights into how plants can efficiently mitigate various types of IBD (54). However, there is a need to further investigate the pharmacological properties and the mechanism of action of these botanicals. In this review, we have summarized the effective agents of phytochemicals and their modes of action, as well as the properties that make them beneficial for use against IBD.
Pathology and methods for controlling IBD
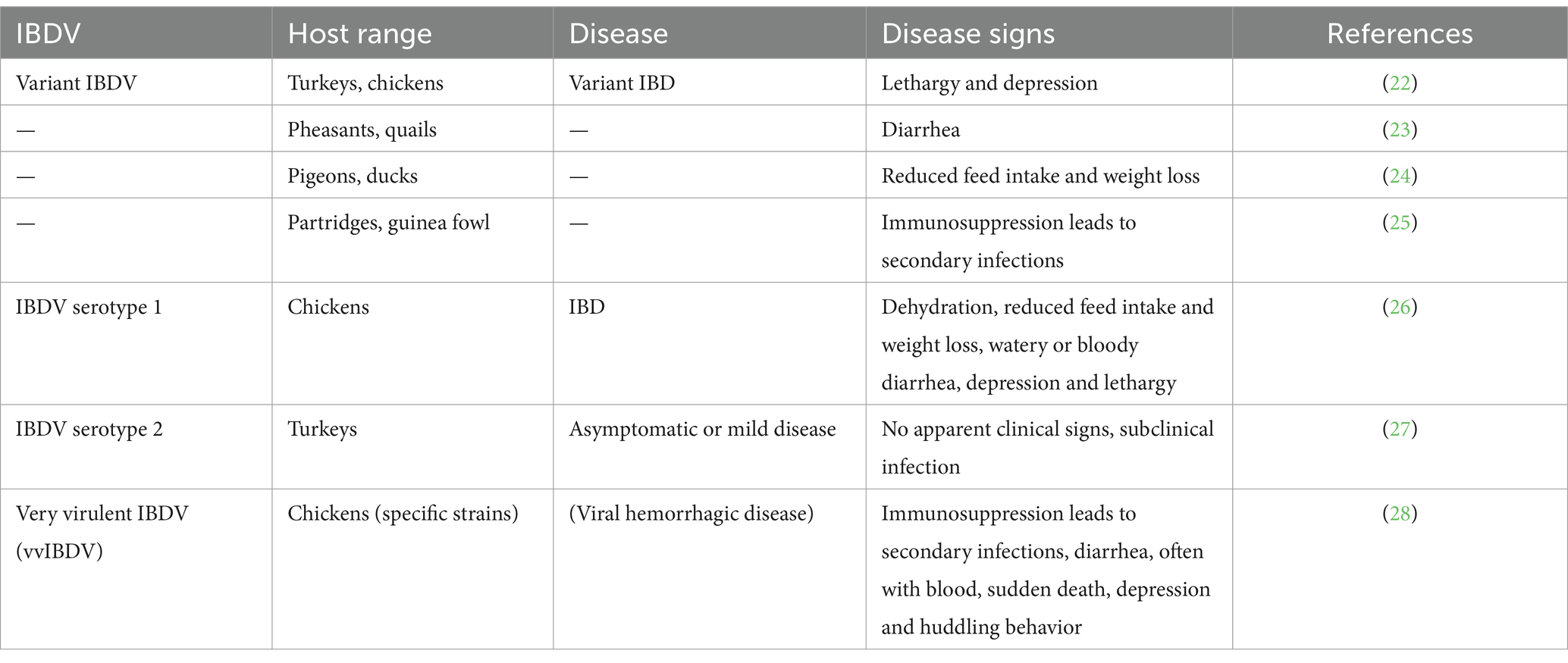
It is important to identify the points at which IBD can be effectively controlled before reviewing the plants and plant products used to control IBD. IBDV is transferred by infected macrophages to the bursa of Fabricius and experiences intracytoplasmic replication in IgM+ B lymphocytes (55). The stimulation of macrophages increases interferon- (IFN-) production in the bursa of Fabricius (56). Nitric oxide and other pro-inflammatory cytokines, including interleukin-6 (IL-6), are released simultaneously (57). The bursal lesions developed as a result of these cytokines. Additionally, the healthy B-cells around IBDV-infected cells may undergo apoptosis as a result of the IFN-γ produced during the infection (58) (Figure 1).
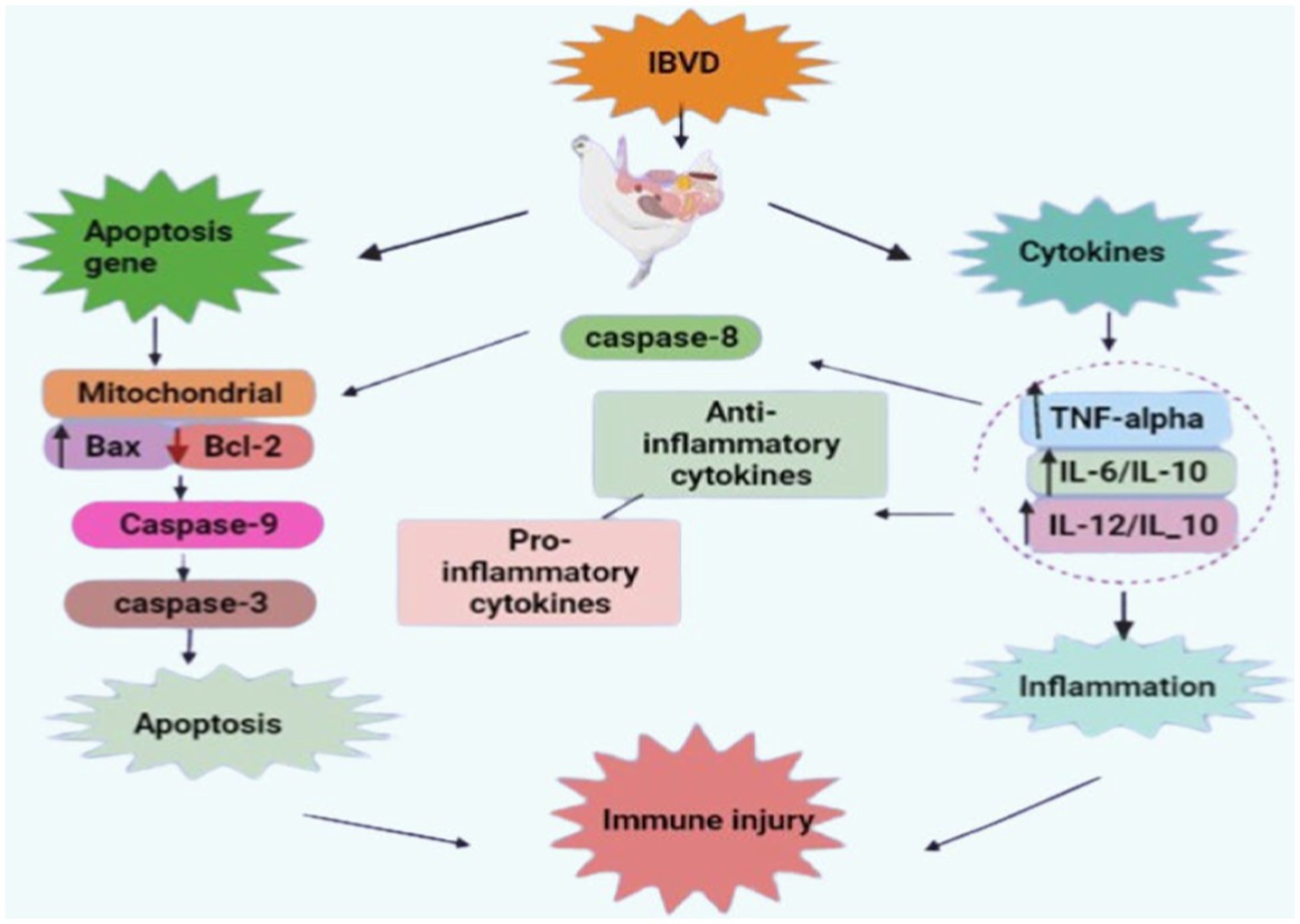
Figure 1. Pathogenesis of infectious bursal disease (retrieved from https://app.biorender.com/).
The bone marrow and caecal tonsils can help in the later replication of IBDV. According to flow cytometric studies, the IgM+ of the B-cell population is significantly lower than the IgA and IgG. IBDV has been reported to be generally unaffected by the mesenchymally derived reticular cells seen in the germinal center, bursal cortex, red pulp of the spleen, and periarteriolar lymphoid sheaths (59). The absence of B-cell proliferative environment is assumed to be the cause of the elimination of bursal follicular dendritic cells in IBDV infection. Some points where IBDV can be controlled by recognizing the stages of this cycle and its related diseases. To prevent IBD, strict hygiene standards and vaccination with traditional live, attenuated, and inactivated viral vaccines have been utilized (60). It is challenging to remove the persistent IBDV particles from the farm because the virus endures for 52 days in feed and water and 122 days in poultry farms (47). It is expensive to control IBDV in infected chickens and prevent the spread of the disease to other flocks. As a result, vaccination is still the preferred strategy for controlling IBD. Even with strict immunization procedures, vaccine failures still happen as a result of the virus evolution (61). To reduce the immunosuppressive effects of IBDV, it is crucial to prevent infection at a young age, but parent stock can be immunized to achieve this (25). Many researchers have explored the use of phytochemicals for controlling IBD. The effectiveness of the plant in combating the disease depends on its active constituents.
Phytochemicals
Phytochemicals, usually referred to as phytonutrients, are physiologically active substances that are present in plants in their natural state (62, 63). Fruits, vegetables, cereals, and other plant-based foods receive their colors, flavors, and aromas from phytochemicals (64). There are 1,000 different phytochemicals, each with its own special qualities and purposes (65).
Phytochemicals have a variety of bioactivities, including antiviral, antibacterial, antiparasitic, antioxidant, antivenin, larvicidal, antifungal, anti-inflammatory, anti-diabetic, anti-amoebic, and wound healing properties (Figure 2) (66). Numerous phytochemicals have antioxidant characteristics that can aid in preventing cellular damage and oxidative stress which are factors in a variety of health issues (67). In the body, dangerous free radicals that can harm cells and cause chronic inflammation and disease are neutralized by antioxidants (68). Additionally, phytochemicals control gene expression and have an impact on the activity of numerous proteins and enzymes in the body (69). Various phytochemicals can target different physiological pathways and activities that can have a wide range of impacts on health and the prevention of disease (70). Currently, the value of herbal medicine is promising. It is often employed in the treatment of numerous illnesses, even those that are incurable (71). The use of phytochemicals as natural alternatives to medicinal products for a variety of health issues has gained popularity in recent years (54). Different plants have been examined for their activity against IBD, which has been mentioned in Table 2.
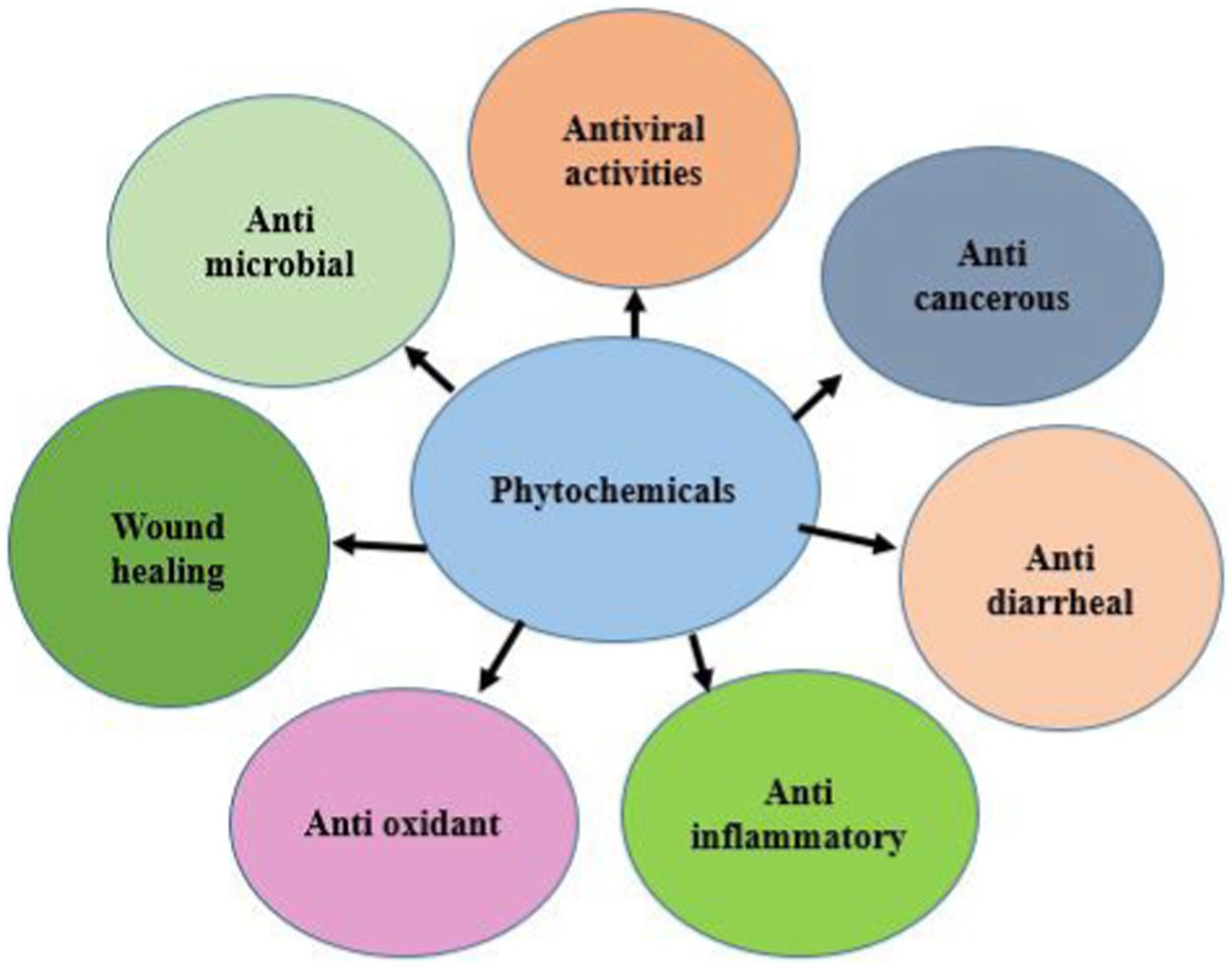
Figure 2. Therapeutics properties of phytochemicals (retrieved from https://app.biorender.com/).

Table 2. Exploring the efficacy of bioactive phytochemicals in controlling infectious bursal disease.
Mechanisms of action of phytochemicals
Phytochemicals can be used in the treatment of IBD due to their anti-inflammatory, antiviral, and immunomodulatory effects (91). Certain phytochemicals, including alkaloids, flavonoids, and polyphenols, prevent IBDV from replicating by preventing the virus from entering host cells (Figure 3) (86). Additionally, phytochemicals may alter the reaction of the host immune system to viral infection (92). Some flavonoids can increase the production of cytokines, which are essential for the immune response, while other flavonoids may suppress the production of pro-inflammatory cytokines, which can result in tissue damage (93). Essential oils and other phytochemicals have been demonstrated to strengthen the host immune system by boosting the generation of natural killer cells and other immune cells, which affect the strengthening of the host’s defense against viral infection (94). Phytochemicals can also use their antiviral benefits by lowering oxidative stress, which is a characteristic of viral infections (95). Phytochemicals with antioxidant characteristics, such as flavonoids and polyphenols can shield host cells from harm by reactive oxygen species produced during viral infection (96).
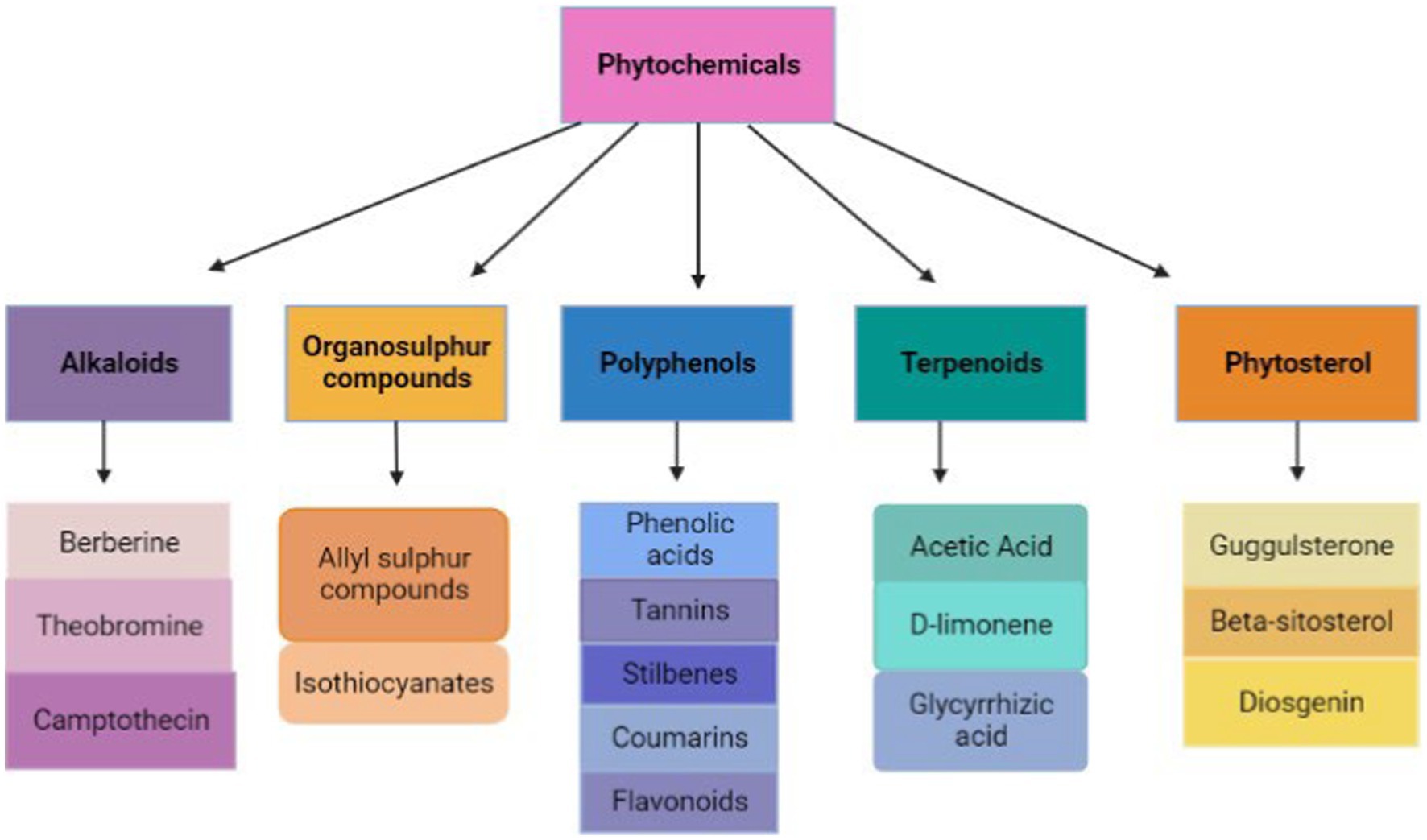
Figure 3. Types of phytochemicals (retrieved from https://app.biorender.com/).
Botanical compounds for controlling infectious bursal disease
Flavonoids
Flavonoids are a subclass of phenolic compounds, distinguished by having two phenolic rings joined by a three-carbon bridge (97). Flavonoids have two aromatic rings (A and B rings) joined by a heterocyclic ring (C ring) as its fundamental structure (98). The subclassification of flavonoids includes flavanols, flavanones, flavones, isoflavones, catechins, and anthocyanins (99). One of the main compounds that have been investigated for its antibacterial properties is flavonoids (100). Flavonoids and their potential chemo-preventive bioactivities have received a lot of interest in the past two decades, particularly their antiviral characteristics in viral infectious diseases (101). Currently, flavonoids have been investigated against a variety of RNA and DNA viruses. In humans, they exhibit a variety of physiological effects, including antiviral, cytotoxic, anti-inflammatory, antiallergic, antibacterial, antioxidant, and anticancer activities. Flavonoids are pleiotropic substances, which means that their functional groups can interact with a variety of cellular targets and obstruct a variety of pathways (102). They also lack systemic toxicity, and their capacity to work in concert with conventional medications has largely been established (103). These characteristics make flavonoid candidates to disrupt the viral life cycle. Flavonoids are chemicals that boost the immune system by increasing IgM and IgG antibody production, which supports the humoral immune system of broilers (104). Flavonoids stimulate the synthesis of cytokines, particularly interferon, by activating macrophages. By decreasing bursal lesions and viral protein expression, they are particularly efficient against infectious IBDV in broilers (105). Many researchers have suggested that flavonoids could be used to control IBDV because of these properties (104).
Saponins
Saponins are chemical compounds found in a wide range of plants, including many therapeutic herbs (106). A variety of biological properties, including anti-inflammatory, antibacterial, antiviral, anticancer, antifungal, and immune modulatory actions, are present in saponins (107). Saponins are presently receiving attention for their potential therapeutic applications due to their diverse actions (108). Saponins interact with cholesterol in the intestines to produce complexes that prevent absorption (109). Saponins have antioxidant qualities that can aid in oxidative stress defense and improve overall health. Saponins can serve as immunoadjuvants, compounds that strengthen the reaction of the immune system to antigens (110). Saponins may help to regulate or lessen the effects of IBD by enhancing the immune system (111). Saponin increases the numbers of IgA+ cells and Intraepithelial Lymphocytes in the ileum, duodenum, and jejunum, while bursal lesions decreased in poultry (87). Plants that contain saponins also serve as astringents, lowering surface tension within the body and facilitating nutrition uptake by cells (112). These actions have led to the use of numerous saponin-containing plants in the treatment of IBD (113).
Terpenes and terpene derivatives
Terpenes are secondary metabolites found in plants that have an isoprene (2-methylbuta-1,3-diene) unit as their carbon backbone (114). There are about 40,000 distinct molecules of primary and secondary metabolism that belong to the largest class of phytochemicals known as terpenoids (115). Terpenes undergo various metabolic changes, such as oxidation and rearrangement, to produce terpenoids (116). These biochemical changes form terpenoids of oxygenated compounds of terpenes such as ketones, alcohols, acids, esters, ethers, and aldehydes. The only adequate volatile components of essential oils are sesquiterpenoids, hemi terpenoids, and mono terpenoids (117). These substances have the ability to integrate into phospholipid membranes because they are lipophilic (118). Because of their structure, terpenoids are more effective than synthetic medications at incorporating lipid layers into lipid layers or disrupting the important sterol metabolism of numerous pathogens (119). Terpenes can prevent viral DNA replication and interfere with virus adhesion to cellular membranes (120). These substances prevented viral replication by blocking the synthesis of viral structural proteins and the genes that code for the viral nucleocapsid protein, viral membrane protein, and viral spike (121). Research describes that terpenes and essential oils rich in terpenes are effective against IBD (86, 122).
Alkaloids
A significant class of naturally occurring organic compounds known as alkaloids makes up the majority of the largest group of phytochemicals (123). Alkaloids are distinguished by having nitrogen atoms, which gives them their alkaline characteristics and therapeutic effects (124). The majority of alkaloids are produced through the synthesis of amino acids like tryptophan, tyrosine, phenylalanine, ornithine, and lysine (125). The alkaloids have been shown to have anti-fungal, anti-inflammatory, and antibacterial effects (126, 127). Several alkaloid phytochemicals have the ability to inhibit and interact with viruses. To restrict access to host cells, they can obstruct attachment or viral fusion with the receptors on the surface of the host cell (128).
They can also interact with protein, viral RNA and DNA synthesis, and viral protein assembly (129). By concentrating on transport pathways, they can also stop viruses from invading other normal cells in the host (130). These characteristics have made alkaloids the preferred compound to be searched for their medical use against IBD. Multiple alkaloids have been used by researchers and found effective in controlling IBD (131).
Sulfur compounds
Sulfur has anti-inflammatory, antibacterial, antifungal, and antioxidant effects (132). They can boost the overall health of the immune system by assisting in the neutralization of free radicals and the reduction of oxidative stress (133). By affecting the generation and function of immune cells including macrophages and lymphocytes, sulfur components have been identified to alter immunological responses (134). They may increase immune cell activity and proliferative capacity, strengthening the immunological response (135). Sulfur components have been found to regulate immune responses by affecting the activity and production of immune cells such as macrophages and lymphocytes (136). They may increase immune cell activity and proliferation, resulting in a stronger immunological response (137). Allicin, S-allyl cysteine, Diallyl trisulfide, and Diallyl disulfide are examples of sulfur compounds. Allicin has an immune-stimulatory effect on poultry (138). Allicin, alliin, and other sulfur-containing substances have a potently stimulating effect on poultry immune systems (139). Multiple sulfur compounds have been used by researchers and found effective in controlling IBD (140, 141).
Vitamins
Vitamins are produced from the fruit and leaves of plants and play an important role in boosting the immune system leading to the control of major pathogenic diseases. Vitamin C boosts cellular and humoral responses, as well as a birds resistance to infections including IBD illnesses (142). Vitamin C improved the antibody production against IBDV in poultry. These results can be attributed to the increased activity of B lymphocytes and T lymphocytes in poultry (143). Various parameters of the immune system including specific antibody production, resistance to infections, numbers of antibody-producing cells, phagocytic index, and in vitro mitogenic reactions to lymphocytes, are modified by vitamins and not with antioxidant compounds (144). ARG, an essential amino acid for poultry, and an important antioxidant, influence positively both the cell-mediated and humoral immune responses of poultry (145). Arginine strengthens the immune system against bacterial and viral infections (146). Vitamin E enhances the immune response by decreasing prostaglandin E2 production, boosting macrophage phagocytic function, and T-cell proliferation, enhancing IL-2 production, and increasing interleukin-1 secretion by macrophages (147). Both Arginine and vitamin E can enhance immunological responses and may have an impact on resistance to disease (148, 149).
Conclusion
Phytochemicals derived from plants are the largest source of production of medicines. Many years ago, medicinal plants have been used for the control and prevention of disease. In this review, we observed that phytochemicals have the potential to control IBD. Various compounds extracted from plants showed excellent results against IBD. Phytochemicals show potential treatment for IBD in poultry.
Further focused research aimed at identifying promising derivatives having significant targeted effects on IBDV is highly required to develop practical and rational strategies for use of phytochemicals.
Author contributions
IT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2024-9/1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Birhanu, MY, Osei-Amponsah, R, Yeboah Obese, F, and Dessie, T. Smallholder poultry production in the context of increasing global food prices: roles in poverty reduction and food security. Anim Front. (2023) 13:17–25. doi: 10.1093/af/vfac069
2. Rashid, MHU, Mehwish, WH, Ahmad, S, Ali, L, Ahmad, N, Ali, M, et al. Unraveling the combinational approach for the antibacterial efficacy against infectious pathogens using the herbal extracts of the leaves of Dodonaea viscosa and fruits of Rubus fruticosus. Agrobiol Rec. (2024) 16:57–66. doi: 10.47278/journal.abr/2024.012
3. Bangulzai, N, Ahmed, SF, Kashif, M, Fatima, M, Ahmed, M, and Mushtaq, N. Antifungal activity of essential oils extracted from different plants against Penicillium digitatum causing green mold of citrus. Int J Agric Biosci. (2022) 11:75–83. doi: 10.47278/journal.ijab/2022.011
4. Attia, YA, Rahman, MT, Hossain, MJ, Basiouni, S, Khafaga, AF, Shehata, AA, et al. Poultry production and sustainability in developing countries under the COVID-19 crisis: lessons learned. Animals. (2022) 12:644. doi: 10.3390/ani12050644
5. Zurisha, RANI, Abbas, RZ, Abbas, A, Saeed, Z, Ur Rehman, T, Hussain, R, et al. In vitro and in vivo anticoccidial effects of butyric acid and its impact on blood and serum chemistry of broiler chickens. Kafkas Univ Vet Fak Derg. (2021) 27:583–8. doi: 10.9775/kvfd.2021.25907
6. Dohouonan, D, Brice, OEJ, Julien, GK, and Yao, T. Effectiveness of Calotropis procera (Ait. R. Br.) and Cassia siamea (Lamk.) leave powders in the control of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Int J Agric Biosci. (2022) 11:84–9. doi: 10.47278/journal.ijab/2022.012
7. Ravikumar, R, Chan, J, and Prabakaran, M. Vaccines against major poultry viral diseases: strategies to improve the breadth and protective efficacy. Viruses. (2022) 14:1195. doi: 10.3390/v14061195
8. Qandoos, AZ, Alatfeehy, NM, and Abd El-Ghany, WA. Isolation, characterization and pathogenicity of the most common bacteria associated with gut health in Egyptian broiler chicken flocks. Int J Vet Sci. (2022) 11:7–15. doi: 10.47278/journal.ijvs/2021.069
9. Ahmad, S, Humak, F, Ahmad, M, Altaf, H, Qamar, W, Hussain, A, et al. Phytochemicals as alternative anthelmintics against poultry parasites: a review. Agrobiol Rec. (2023) 12:34–45. doi: 10.47278/journal.abr/2023.015
10. Zaheer, I, Saleemi, MK, Javed, MT, Rahman, SU, and Abubakar, M. Clinical investigation and molecular prevalence of fowl adenoviruses of commercial poultry from division Faisalabad, Pakistan. Pak Vet J. (2022) 42:352–7. doi: 10.29261/pakvetj/2022.056
11. Ahmed, HM, Amer, SA, Abdel-Alim, GA, Elbayoumi, KM, Kutkat, MA, and Amer, MM. Molecular characterization of recently classified Newcastle disease virus genotype VII.1.1 isolated from Egypt. Int J Vet Sci. (2022) 11:295–301. doi: 10.47278/journal.ijvs/2021.097b
12. Mubashir, A, Ghani, A, and Mubashar, A. Common medicinal plants effective in peptic ulcer treatment: a nutritional review. Int J Agric Biosci. (2022) 11:70–4. doi: 10.47278/journal.ijab/2022.010
13. Cagirgan, AA, Kaplan, M, Pekmez, K, Van Schalkwyk, A, Arslan, F, and Timurkan, MO. The status of bovine viral diarrhea virus (BVDV) in western Türkiye: detection of three subtypes. Kafkas Univ Vet Fak Derg. (2022) 28:709–15. doi: 10.9775/kvfd.2022.27881
14. Saira, R, Raheem, A, and Inayat, A. Preparation and evaluation of oil adjuvanted inactivated bivalent vaccine against Newcastle disease virus and infectious bronchitis virus. Cont Vet J. (2023) 3:119–25.
15. Fazlani, TA, Shoaib, M, Mahmood, MS, Javaid, A, Hao, R, Amjed, MN, et al. Preparation and evaluation of oil adjuvanted inactivated bivalent vaccine against Avian influenza virus (AIV) and Newcastle disease virus (NDV). Cont Vet J. (2023) 3:110–8.
16. Abu, SM, Monira, N, Asmaul, H, Mahbub, EATM, Mustafa, KAH, and Masudur, RM. Seroprevalence of Newcastle disease in layer chickens and pathology in clinically affected chickens at Gazipur. Bangladesh Cont Vet J. (2022) 2:35–41.
17. Ziaf, K, Ali, Z, Noor, A, Ghani, MA, Anwar, W, Majeed, Y, et al. Onion seed production as influenced by foliar application of thiourea and PGPRs at post-anthesis stage. Agrobiol Rec. (2024) 15:117–23. doi: 10.47278/journal.abr/2024.005
18. Raja, P . Infectious bursal disease In: Environmental technology and engineering techniques. Palm Bay, FL: Apple Academic Press (2020). 309–25.
19. Samad, A, Abbas, A, Mehtab, U, Ur Rehman Ali Khera, H, Rehman, A, and Hamza, M. Infectious bronchitis disease in poultry its diagnosis. Prevention and control strategies. Ann Agric Crop Sci. (2021) 6:1100.
20. Ghazwani, M, Hakami, AR, Sani, SS, Sultana, S, Sultana, T, Bashir, W, et al. Antibacterial activity of aqueous and methanolic extract of Mentha piperita against pervasive bacteria isolated from urial the Ovis vignei. Pak Vet J. (2023) 43:103–8. doi: 10.29261/pakvetj/2023.018
21. Mosa, MI, Salem, HM, Bastamy, MA, and Amer, MM. Pathogenic and non-pathogenic factors; especially infectious bursal disease viruses; affect chicken digestive system microbiota and methods of its evaluation and recovery: a review. Egypt J Vet Sci. (2023) 54:733–60. doi: 10.21608/ejvs.2023.203480.1476
22. Xu, T, Xiong, T, Xie, W, Wu, J, Liu, X, Li, G, et al. Construction and evaluation of the immunogenicity and protective efficacy of recombinant replication-deficient human Adenovirus-5 expressing genotype VII Newcastle disease virus F protein and infectious bursal disease virus VP2 protein. Vaccine. (2023) 11:1051. doi: 10.3390/vaccines11061051
23. Kegne, T, and Chanie, M. Review on the incidence and pathology of infectious bursal disease. Br J Poul Sci. (2014) 3:68–77. doi: 10.5829/idosi.bjps.2014.3.3.8556
24. Kapoor, S, Kaur, H, Rehman, AU, Kumar, V, and Gupta, A. Infectious bursal disease: overview. J Med Pharm Allied Sci. (2021) 11:4661–5. doi: 10.55522/jmpas.V11I2.2312
25. Ingrao, F, Rauw, F, Lambrecht, B, and van den Berg, T. Infectious bursal disease: a complex host-pathogen interaction. Dev Comp Immunol. (2013) 41:429–38. doi: 10.1016/j.dci.2013.03.017
26. Bughio, E, Jatoi, AS, Memon, M, Bughio, R, Khoso, PA, Khoso, ZA, et al. Effect of age and route of administration on the efficacy of live infectious bursal disease vaccines in broiler. Sarhad J Agric. (2017) 33:232–9. doi: 10.17582/journal.sja/2017/33.2.232.239
27. Jackwood, DJ, Sommer-Wagner, SE, Crossley, BM, Stoute, ST, Woolcock, PR, and Charlton, BR. Identification and pathogenicity of a natural reassortant between a very virulent serotype 1 infectious bursal disease virus (IBDV) and a serotype 2 IBDV. Virology. (2011) 420:98–105. doi: 10.1016/j.virol.2011.08.023
28. Taghavian, O . Expression and characterization of infectious bursal disease virus protein for poultry vaccine development and application in nanotechnology In: Doctoral dissertation. Aachen: Publikationsserver der RWTH Aachen University (2013)
29. Rehman, TU, El-Mansi, AA, Alhag, SK, Al-Shuraym, LA, Saeed, Z, Arif, M, et al. Antiparasitic activity of methanolic and ethyl acetate extracts of Azadirachta indica against Haemonchus contortus. Pak Vet J. (2023) 43:199–203. doi: 10.29261/pakvetj/2023.014
30. Aliy, A, Shiferaw, S, Shegu, D, Senbeta, B, Guyassa, C, and Bulbula, A. Diagnostic techniques of infectious bursal disease virus: a review. Life Sci J. (2020) 17:1–12. doi: 10.7537/marslsj170620.01
31. Mató, T, Tatár-Kis, T, Felföldi, B, Jansson, DS, Homonnay, Z, Bányai, K, et al. Occurrence and spread of a reassortant very virulent genotype of infectious bursal disease virus with altered VP2 amino acid profile and pathogenicity in some European countries. Vet Microbiol. (2020) 245:108663. doi: 10.1016/j.vetmic.2020.108663
32. O’Connor, TP Jr, Lawrence, J, Andersen, P, Leathers, V, and Workman, E. Immunoassay applications in veterinary diagnostics In: The immunoassay handbook. Oxford, UK: Elsevier. (2013). 623–45.
33. Ramon, G, Legnardi, M, Cecchinato, M, Cazaban, C, Tucciarone, CM, Fiorentini, L, et al. Efficacy of live attenuated, vector and immune complex infectious bursal disease virus (IBDV) vaccines in preventing field strain bursa colonization: a European multicentric study. Front Vet Sci. (2022) 9:978901. doi: 10.3389/fvets.2022.978901
34. Velázquez-Antunez, J, Olivares-Perez, J, Olmedo-Juárez, A, Rojas-Hernandez, S, Villa-Mancera, A, Romero-Rosales, T, et al. Biological activity of the secondary compounds of Guazuma ulmifolia leaves to inhibit the eggs Haemonchus contortus hatching. Pak Vet J. (2022) 43:55. doi: 10.29261/pakvetj/2022.075
35. Liaqat, I, Noor, S, Qureshi, AS, Ali, S, Liaqat, I, Al-Arifa, N, et al. Biosynthesis and evaluation of Cinnamomum zeylanicum nanomaterials for the treatment of polycystic ovary syndrome in mice. Pak Vet J. (2023) 43:118–24. doi: 10.29261/pakvetj/2023.004
36. Fujiwara, M, Auty, H, Brown, I, and Boden, L. Assessing the likelihood of high pathogenicity avian influenza incursion into the gamebird sector in Great Britain via designated hatcheries. Front vet Science. (2022) 9:877197. doi: 10.3389/fvets.2022.877197
37. Makovska, I, Dhaka, P, Chantziaras, I, Pessoa, J, and Dewulf, J. The role of wildlife and pests in the transmission of pathogenic agents to domestic pigs: a systematic review. Animals. (2023) 13:1830. doi: 10.3390/ani13111830
38. Li, C, Wang, L, and Zheng, S. Immunosuppressive disease in poultry. Front Immunol. (2023) 14:1215513. doi: 10.3389/fimmu.2023.1215513
39. Yuan, L, Hensley, C, Mahsoub, HM, Ramesh, AK, and Zhou, P. Microbiota in viral infection and disease in humans and farm animals. Prog Mol Biol Transl Sci. (2020) 171:15–60. doi: 10.1016/bs.pmbts.2020.04.005
40. Trapp, J, and Rautenschlein, S. Infectious bursal disease virus’ interferences with host immune cells: what do we know? Avian Pathol. (2022) 51:303–16. doi: 10.1080/03079457.2022.2080641
41. Dutt, TS, Karger, BR, Fox, A, Youssef, N, Dadhwal, R, Ali, MZ, et al. Mucosal exposure to non-tuberculous mycobacteria elicits B cell-mediated immunity against pulmonary tuberculosis. Cell Rep. (2022) 41:111783. doi: 10.1016/j.celrep.2022.111783
42. Madelaire, CB, Silva, DP, Titon, SC, Lamadrid-Feris, F, Floreste, FR, Titon, B Jr, et al. Contrasting effects of transdermal and implant corticosterone treatments in the American bullfrog wound healing. Philos Trans R Soc B. (2023) 378:20220119. doi: 10.1098/rstb.2022.0119
43. Shiferaw, J, Dego, T, Tefera, M, and Tamiru, Y. Seroprevalence of infectious bronchitis virus in broiler and layer farms of Central Ethiopia. Biomed Res Int. (2022) 2022:8915400. doi: 10.1155/2022/8915400
44. Mató, T, Medveczki, A, and Kiss, I. Research note:hidden infectious bursal disease virus infections in Central Europe. Poult Sci. (2022) 101:101958. doi: 10.1016/j.psj.2022.101958
45. Ahmed, J, Singh, YD, Rajkhowa, TK, Arya, RS, Roychoudhury, P, and Kalita, A. Molecular diagnosis of infectious bursal disease outbreak in chickens in and around Aizawl district of Mizoram, India. Indian J Vet Pathol. (2022) 46:333–8. doi: 10.5958/0973-970X.2022.00056.6
46. Gao, H, Wang, Y, Gao, L, and Zheng, SJ. Genetic insight into the interaction of IBDV with host—a clue to the development of novel IBDV vaccines. Int J Mol Sci. (2023) 24:8255. doi: 10.3390/ijms24098255
47. Liu, M . Development of an immune complex vaccine to control variant infectious bursal disease virus infection in the Canadian broiler chicken industry In: Doctoral dissertation. Saskatoon, Canada: University of Saskatchewan (2020).
48. Ghanem, IAE, Abdullatif, TM, and Hassanin, O. The protection conferred against virulent Newcastle disease virus (vNDV) genotype VII by commercial double recombinant HVT vaccines and NDV live-attenuated vaccine as prime/boost vaccination regimens in commercial broiler chickens carrying maternally-derived antibodies (MDAs) against NDV. Avian Pathol. (2023) 52:251–63. doi: 10.1080/03079457.2023.2211548
49. Nair, V, Gimeno, I, Dunn, J, Zavala, G, Williams, SM, Reece, RL, et al. Neoplastic diseases In: Diseases of poultry. Hoboken, NJ: Wiley-Blackwell (2020). 548–715.
50. Reddy, SM, Izumiya, Y, and Lupiani, B. Marek’s disease vaccines: current status, and strategies for improvement and development of vector vaccines. Vet Microbiol. (2017) 206:113–20. doi: 10.1016/j.vetmic.2016.11.024
51. Moryani, AA, Rajput, N, Naeem, M, Shah, AH, and Jahejo, AR. Screening of the herbs and evaluation of their combined effects on the health and immunity of coccidiosis challenged broiler chickens. Pak Vet J. (2021) 41:228–34. doi: 10.29261/pakvetj/2021.005
52. Mevlüt, D, Murat, B, Mehmet, D, Zümrüt, D, Bilge, AT, and Öznur, U. Protective and therapeutic effect of quercetin in hepatotoxicity induced by sepsis in rats. Kafkas Univ Vet Fak Derg. (2021) 27:699–706. doi: 10.9775/kvfd.2021.26135
53. Degla, LH, Kuiseu, J, Olounlade, PA, Attindehou, S, Hounzangbe-Adote, MS, Edorh, PA, et al. Use of medicinal plants as alternative for the control of intestinal parasitosis: assessment and perspectives. Agrobiol Rec. (2022) 7:1–9. doi: 10.47278/journal.abr/2021.011
54. Shahzad, MI, Hussain, MS, Saeed, I, Ashraf, H, Anwar, S, and Ramzan, M. Phytochemical and antimicrobial studies of Salvadora persica, Prosopis cineraria and Tamarix aphyla plants from Cholistan, Pakistan. Pak J Biochem Biotechnol. (2022) 3:49–60. doi: 10.52700/pjbb.v3i2.130
55. Orakpoghenor, O, Oladele, SB, and Abdu, PA. Infectious bursal disease: transmission, pathogenesis, pathology and control-an overview. Worlds Poult Sci J. (2020) 76:292–303. doi: 10.1080/00439339.2020.1716652
56. Huang, X, Liu, W, Zhang, J, Liu, Z, Wang, M, Wang, L, et al. Very virulent infectious bursal disease virus-induced immune injury is involved in inflammation, apoptosis, and inflammatory cytokines imbalance in the bursa of fabricius. Dev Comp Immunol. (2021) 114:103839. doi: 10.1016/j.dci.2020.103839
57. Tram, NDT, Tran, QTN, Xu, J, Su, JCT, Liao, W, Wong, WSF, et al. Multifunctional antibacterial nanonets attenuate inflammatory responses through selective trapping of endotoxins and pro-inflammatory cytokines. Adv Healthc Mater. (2023) 12:e2203232. doi: 10.1002/adhm.202203232
58. Franciosini, MP, and Davidson, I. A walk through Gumboro disease. Poultry. (2022) 1:229–43. doi: 10.3390/poultry1040020
59. Qaid, MM, Albatshan, HA, Al-Garadi, MA, and Hussein, EO. Effect of two brooding systems and four stocking densities on immune response and stress indicators of broiler chicks during the brooding period. Ital J Anim Sci. (2023) 22:615–25. doi: 10.1080/1828051X.2023.2224808
60. Müller, H, Mundt, E, Eterradossi, N, and Islam, MR. Current status of vaccines against infectious bursal disease. Avian Pathol. (2012) 41:133–9. doi: 10.1080/03079457.2012.661403
61. Singh, RK, Sharma, GK, Mahajan, S, Dhama, K, Basagoudanavar, SH, Hosamani, M, et al. Foot-and-mouth disease virus: immunobiology, advances in vaccines and vaccination strategies addressing vaccine failures—an Indian perspective. Vaccine. (2019) 7:90. doi: 10.3390/vaccines7030090
62. Frank, J, Fukagawa, NK, Bilia, AR, Johnson, EJ, Kwon, O, Prakash, V, et al. Terms and nomenclature used for plant-derived components in nutrition and related research: efforts toward harmonization. Nutr Rev. (2020) 78:451–8. doi: 10.1093/nutrit/nuz081
63. Ara, C, Arshad, A, Faheem, M, Khan, M, and Shakir, HA. Protective potential of aqueous extract of Allium cepa against synthetic food dye, tartrazine induced reproductive toxicity. Pak Vet J. (2022) 42:358–63. doi: 10.29261/pakvetj/2022.029
64. Anshuman, KP . Phytochemicals: an immune booster against the pathogens In: Recent frontiers of phytochemicals. Amsterdam, Netherlands: Elsevier (2023). 501–9.
65. Montenegro-Landívar, MF, Tapia-Quirós, P, Vecino, X, Reig, M, Valderrama, C, Granados, M, et al. Polyphenols and their potential role to fight viral diseases: an overview. Sci Total Environ. (2021) 801:149719. doi: 10.1016/j.scitotenv.2021.149719
66. Asala, TM, Rowaiye, AB, Salami, SA, Baba-Onoja, OM, Abatan, MO, Ocheja, BO, et al. The antioxidant and hematopoietic effects of the methanolic extract fractions of Ocimum basilicum in acetaminophen-induced albino rats. Int J Vet Sci. (2022) 11:289–94. doi: 10.47278/journal.ijvs/2021.112
67. Nawaz, M, Zhou, J, Khalid, I, Shamim, A, Hussain, A, Ahmed, Z, et al. Antiparasitic activity of plants extract against gastrointestinal nematodes and Rhipicephalus microplus. Int J Vet Sci. (2022) 11:474–8. doi: 10.47278/journal.ijvs/2022.147
68. Wajiha,, and Qureshi, NA. In vitro anticoccidial, antioxidant activities and biochemical screening of methanolic and aqueous leaves extracts of selected plants. Pak Vet J. (2021) 41:57–63. doi: 10.29261/pakvetj/2020.07
69. Hassan, Z, Tijani, Y, Abubakar, S, Modu, B, and Uzairua, SM. Anti-fungal screening of five medicinal plants used in Nigeria. Int J Agric Biosci. (2021) 10:65–8. Available at: www.ijagbio.com
70. Salman, M, and Imran, A. In-vitro anticoccidial evaluation of Citrus sinensis essential oil against Eimeria oocysts. Agrobiol Rec. (2022) 10:15–8. doi: 10.47278/journal.abr/2022.020
71. Fatemeh, B, Seyed Ebrahim, H, Mehrdad, S, and Mokhtar, M. Exposure to aqueous-alcoholic extract of parsley leaves (Petroselinum crispum) in lead-treated rats alleviate liver damage. Kafkas Univ Vet Fak Derg. (2021) 27:717–23. doi: 10.9775/kvfd.2021.26163
72. Sadique, MA, Yadav, S, Ranjan, P, Verma, S, Salammal, ST, Khan, MA, et al. High-performance antiviral nano-systems as a shield to inhibit viral infections: SARS-CoV-2 as a model case study. J Mater Chem B. (2021) 9:4620–42. doi: 10.1039/D1TB00472G
73. Umar, S, Shah, MAA, Munir, MT, Yaqoob, M, Fiaz, M, Anjum, S, et al. RETRACTED: synergistic effects of thymoquinone and curcumin on immune response and anti-viral activity against Avian influenza virus (H9N2) in turkeys. Poult Sci. (2016) 95:1513–20. doi: 10.3382/ps/pew069
74. Yasmin, AR, Chia, SL, Looi, QH, Omar, AR, Noordin, MM, and Ideris, A. Herbal extracts as antiviral agents In: Feed additives. Cambridge, Massachusetts: Academic Press (2020). 115–32.
75. Zhao, S, Jia, Y, Zhang, W, Wang, L, Ma, Y, and Teng, K. Oral administration of Allium sativum extract protects against infectious bursal disease in chickens. Front Agric Sci Eng. (2016) 2:318–26. doi: 10.15302/J-FASE-2015080
76. Rehman, ZU, Meng, C, Sun, Y, Safdar, A, Pasha, RH, Munir, M, et al. Oxidative stress in poultry: lessons from the viral infections. Oxid Med Cell Longev. (2018) 2018:1–14. doi: 10.1155/2018/5123147
77. Saadawy, AH, Khalil, AM, Sidarous, LR, Ibrahim, MS, and Salem, TZ. Voltage-dependent anion channels: key players in viral infection. Rev Med Virol. (2023) 33:e2453. doi: 10.1002/rmv.2453
78. Dhama, K, Karthik, K, Khandia, R, Munjal, A, Tiwari, R, Rana, R, et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-current knowledge and future prospects. Curr Drug Metab. (2018) 19:236–63. doi: 10.2174/1389200219666180129145252
79. Khan, T, Khan, MA, Ullah, N, and Nadhman, A. Therapeutic potential of medicinal plants against COVID-19: the role of antiviral medicinal metabolites. Biocatal Agric Biotechnol. (2021) 31:101890. doi: 10.1016/j.bcab.2020.101890
80. Nesari, T, and Kajaria, D. Combating COVID-19 with holistic approach of Ayurveda. Indian J Tradit Knowl. (2021) 19:S-37.
81. Al Khdri, AMA . Effect of ginger (Zingiber officinale) and thyme (Thymus vulgaris) dietary supplementation on productive and immunological performance of broiler In: Doctoral dissertation. Iraq: University of Mosul (2009).
82. Buzała, M . Variability of secondary hemostasis in broiler chickens administered in ovo with selected prebiotics In: Doctoral dissertation. Toruń, Poland: Nicolaus Copernicus University (2015)
83. Mahfuz, S, Shang, Q, and Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: a review. J Anim Sci Biotechnol. (2021) 12:1–18. doi: 10.1186/s40104-021-00565-3
84. Hassan, YI, Kosir, V, Yin, X, Ross, K, and Diarra, MS. Grape pomace as a promising antimicrobial alternative in feed: a critical review. J Agric Food Chem. (2019) 67:9705–18. doi: 10.1021/acs.jafc.9b02861
85. Mukherjee, C, and Chakraborty, S. Study of dietary polyphenols from natural herbal sources for providing protection against human degenerative disorders. Biocatal Agric Biotechnol. (2021) 33:101956. doi: 10.1016/j.bcab.2021.101956
86. Jumaa, RS, Abdulmajeed, DI, and Karim, AJ. Evaluation of secondary metabolites of herbal plant extracts as an antiviral effect on infectious bursal disease virus isolates in embryonated chicken eggs. Vet World. (2021) 14:2971–8. doi: 10.14202/vetworld.2021.2971-2978
87. Zhai, L, Wang, Y, Yu, J, and Hu, S. Enhanced immune responses of chickens to oral vaccination against infectious bursal disease by ginseng stem-leaf saponins. Poult Sci. (2014) 93:2473–81. doi: 10.3382/ps.2014-04056
88. Özek, K, Wellmann, KT, Ertekin, B, and Tarım, B. Effects of dietary herbal essential oil mixture and organic acid preparation on laying traits, gastrointestinal tract characteristics, blood parameters and immune response of laying hens in a hot summer season. J Anim Feed Sci. (2011) 20:575–86. doi: 10.22358/jafs/66216/2011
89. Saki, AA, Kalantar, M, and Khoramabadi, V. Effects of drinking thyme essence (Thymus vulgaris L.) on growth performance, immune response and intestinal selected bacterial population in broiler chickens. Poult Sci J. (2014) 2:113–23. doi: 10.22069/psj.2014.1960
90. Selegean, M, Putz, MV, and Rugea, T. Effect of the polysaccharide extract from the edible mushroom Pleurotus ostreatus against infectious bursal disease virus. Int J Mol Sci. (2009) 10:3616–34. doi: 10.3390/ijms10083616
91. Wang, M, Li, C, Li, J, Hu, W, Yu, A, Tang, H, et al. Extraction, purification, structural characteristics, biological activity and application of polysaccharides from Portulaca oleracea L.(purslane): a review. Molecules. (2023) 28:4813. doi: 10.3390/molecules28124813
92. Behl, T, Kumar, K, Brisc, C, Rus, M, Nistor-Cseppento, DC, Bustea, C, et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed Pharmacother. (2021) 133:110959. doi: 10.1016/j.biopha.2020.110959
93. Bayram, P, Aksak Karamese, S, Ozdemir, B, Salum, C, Erol, HS, and Karamese, M. Two flavonoids, baicalein and naringin, are effective as anti-inflammatory and anti-oxidant agents in a rat model of polymicrobial sepsis. Immunopharmacol Immunotoxicol. (2023) 45:597–606. doi: 10.1080/08923973.2023.2197143
94. Maheshwari, S, Kumar, V, Bhadauria, G, and Mishra, A. Immunomodulatory potential of phytochemicals and other bioactive compounds of fruits: a review. Food Front. (2022) 3:221–38. doi: 10.1002/fft2.129
95. Bandopadhyay, S, Anand, U, Gadekar, VS, Jha, NK, Gupta, PK, Behl, T, et al. Dioscin: a review on pharmacological properties and therapeutic values. Biofactors. (2022) 48:22–55. doi: 10.1002/biof.1815
96. Stiller, A, Garrison, K, Gurdyumov, K, Kenner, J, Yasmin, F, Yates, P, et al. From fighting critters to saving lives: polyphenols in plant defense and human health. Int J Mol Sci. (2021) 22:8995. doi: 10.3390/ijms22168995
97. Kauffmann, AC, and Castro, VS. Phenolic compounds in bacterial inactivation: a perspective from Brazil. Antibiotics. (2023) 12:645. doi: 10.3390/antibiotics12040645
98. Shahab, M, Roberto, SR, Adnan, M, Fahad, S, Koyama, R, Saleem, MH, et al. Phenolic compounds as a quality determinant of grapes: a critical review. J Plant Growth Regul. (2023) 42:5325–31. doi: 10.1007/s00344-023-10953-w
99. Woo, HD, and Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J Gastroenterol. (2013) 19:1011–9. doi: 10.3748/wjg.v19.i7.1011
100. Al-Rooqi, MM, Mughal, EU, Raja, QA, Hussein, EM, Naeem, N, Sadiq, A, et al. Flavonoids and related privileged scaffolds as potential urease inhibitors: a review. RSC Adv. (2023) 13:3210–33. doi: 10.1039/D2RA08284E
101. Russo, M, Moccia, S, Spagnuolo, C, Tedesco, I, and Russo, GL. Roles of flavonoids against coronavirus infection. Chem Biol Interact. (2020) 328:109211. doi: 10.1016/j.cbi.2020.109211
102. Yao, J, Zhang, Y, Wang, XZ, Zhao, J, Yang, ZJ, Lin, YP, et al. Flavonoids for treating viral acute respiratory tract infections: a systematic review and meta-analysis of 30 randomized controlled trials. Front Public Health. (2022) 10:124. doi: 10.3389/fpubh.2022.814669
103. Lalani, S, and Poh, CL. Flavonoids as antiviral agents for enterovirus A71 (EV-A71). Viruses. (2020) 12:184. doi: 10.3390/v12020184
104. Al-Kahtani, SN, Alaqil, AA, and Abbas, AO. Modulation of antioxidant defense, immune response, and growth performance by inclusion of propolis and bee pollen into broiler diets. Animals. (2022) 12:1658. doi: 10.3390/ani12131658
105. Shehata, AA, Yalçın, S, Latorre, JD, Basiouni, S, Attia, YA, Abd El-Wahab, A, et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms. (2022) 10:395. doi: 10.3390/microorganisms10020395
106. Sharma, P, Tyagi, A, Bhansali, P, Pareek, S, Singh, V, Ilyas, A, et al. Saponins: extraction, bio-medicinal properties and way forward to anti-viral representatives. Food Chem Toxicol. (2021) 150:112075. doi: 10.1016/j.fct.2021.112075
107. Patil, SM, Ramu, R, Shirahatti, PS, Shivamallu, C, and Amachawadi, RG. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon. (2021) 7:e07054. doi: 10.1016/j.heliyon.2021.e07054
108. Beura, SK, Dhapola, R, Panigrahi, AR, Yadav, P, Kumar, R, Reddy, DH, et al. Antiplatelet drugs: potential therapeutic options for the management of neurodegenerative diseases. Med Res Rev. (2023) 43:1835–77. doi: 10.1002/med.21965
109. Zhang, Y, Hao, R, Chen, J, Li, S, Huang, K, Cao, H, et al. Health benefits of saponins and its mechanisms: perspectives from absorption, metabolism, and interaction with gut. Crit Rev Food Sci Nutr. (2023) 63:1–22. doi: 10.1080/10408398.2023.2212063
110. de Paula Barbosa, A . Saponins as immunoadjuvant agent: a review. Afr J Pharm Pharmacol. (2014) 8:1049–57. doi: 10.5897/AJPP2014.4136
111. Dong, J, Liang, W, Wang, T, Sui, J, Wang, J, Deng, Z, et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol Res. (2019) 144:66–72. doi: 10.1016/j.phrs.2019.04.010
112. Thakur, A, Sharma, V, and Thakur, A. An overview of anti-nutritional factors in food. Int J Chem Stud. (2019) 7:2472–9.
113. Tatli Cankaya, II, and Somuncuoglu, EI. Potential and prophylactic use of plants containing saponin-type compounds as antibiofilm agents against respiratory tract infections. Evid Based Complement Alternat Med. (2021) 2021:1–14. doi: 10.1155/2021/6814215
114. Lichtfouse, E, and Goyal, A eds. Lichtfouse suistanable agriculture reviews. France: Springer Nature (2013). 233 p.
115. Mohammadi-Cheraghabadi, M, and Hazrati, S. Terpenoids, steroids, and phenolic compounds of medicinal plants In: Phytochemicals in medicinal plants: biodiversity, bioactivity and drug discovery. Boca Raton, FL: CRC Press (2023). 105.
116. Baharum, SN, Bunawan, H, Ghani, MA, Mustapha, WA, and Noor, NM. Analysis of the chemical composition of the essential oil of Polygonum minus Huds. Using two-dimensional gas chromatography-time-of-fligth mass spectrometry (GC-TOF MS). Molecules. (2010) 15:7006–15. doi: 10.3390/molecules15107006
117. Xavier, V, Spréa, R, Finimundy, TC, Heleno, SA, Amaral, JS, Barros, L, et al. Terpenes In: Natural secondary metabolites: from nature, through science, to industry. Cham: Springer International Publishing (2023). 107–56.
118. Castillo, JA, Pinazo, A, Carilla, J, Infante, MR, Alsina, MA, Haro, I, et al. Interaction of antimicrobial arginine-based cationic surfactants with liposomes and lipid monolayers. Langmuir. (2004) 20:3379–87. doi: 10.1021/la036452h
119. Ghosh, S, Roy, K, and Pal, C. Terpenoids against infectious diseases In: Terpenoids against human diseases. Boca Raton, FL: CRC Press (2019). 187–208.
120. Fayyad, AG, Ibrahim, N, and Yaakob, WA. Phytochemical screening and antiviral activity of Marrubium vulgare. Malays J Microbiol. (2014) 10:106–11. doi: 10.21161/mjm.58013
121. Yang, JL, Ha, TK, Dhodary, B, Pyo, E, Nguyen, NH, Cho, H, et al. Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J Med Chem. (2015) 58:1268–80. doi: 10.1021/jm501567f
122. Atriya, A, Majee, C, Mazumder, R, Choudhary, AN, Mazumder, A, Dahiya, A, et al. Insight into the various approaches for the enhancement of bioavailability and pharmacological potency of terpenoids: a review. Curr Pharm Biotechnol. (2023) 24:1228–44. doi: 10.2174/1389201024666221130163116
123. Rosales, PF, Bordin, GS, Gower, AE, and Moura, S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia. (2020) 143:104558. doi: 10.1016/j.fitote.2020.104558
125. Rao, MJ, Duan, M, Wang, J, Han, S, Ma, L, Mo, X, et al. Transcriptomic and widely targeted metabolomic approach identified diverse group of bioactive compounds, antiradical activities, and their associated genes in six sugarcane varieties. Antioxidants. (2022) 11:1319. doi: 10.3390/antiox11071319
126. Doughari, JH, and Saa-Aondo, M. Phytochemical analysis of crude methanol extracts and antimicrobial activity of n-hexane fractions of methanol seed and pod extracts of Prosopis Africana on some selected microrganisms. Archives. (2021) 2:121–37.
127. Akbar, A, Gul, Z, Chein, SH, and Sadiq, MB. Investigation of anti-inflammatory properties, phytochemical constituents, antioxidant, and antimicrobial potentials of the whole plant ethanolic extract of Achillea santolinoides subsp. wilhelmsii (K. Koch) Greuter of Balochistan. Oxid Med Cell Longev. (2023) 2023:2567333. doi: 10.1155/2023/2567333
128. Abookleesh, FL, Al-Anzi, BS, and Ullah, A. Potential antiviral action of alkaloids. Molecules. (2022) 27:903. doi: 10.3390/molecules27030903
129. Faisal, S, Badshah, SL, Kubra, B, Emwas, AH, and Jaremko, M. Alkaloids as potential antivirals. A comprehensive review. Nat Prod Bioprospect. (2023) 13:4. doi: 10.1007/s13659-022-00366-9
130. Dong, HJ, Wang, ZH, Meng, W, Li, CC, Hu, YX, Zhou, L, et al. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses. (2018) 10:601. doi: 10.3390/v10110601
131. Anyanwu, AA, Jimam, NS, Omale, S, and Wannang, NN. Antiviral activities of Cucumis metuliferus fruits alkaloids on infectious bursal disease virus (IBDV). Phytopharmacology. (2017) 6:98–101. doi: 10.31254/phyto.2017.6206
132. Abdalla, MA, Famuyide, I, Wooding, M, McGaw, LJ, and Mühling, KH. Secondary metabolite profile and pharmacological opportunities of lettuce plants following selenium and sulfur enhancement. Pharmaceutics. (2022) 14:2267. doi: 10.3390/pharmaceutics14112267
133. Akbari, B, Baghaei-Yazdi, N, Bahmaie, M, and Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors. (2022) 48:611–33. doi: 10.1002/biof.1831
134. Wang, Z, Sun, Y, Yao, W, Ba, Q, and Wang, H. Effects of cadmium exposure on the immune system and immunoregulation. Front Immunol. (2021) 12:695484. doi: 10.3389/fimmu.2021.695484
135. Maggini, S, Wintergerst, ES, Beveridge, S, and Hornig, DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. (2007) 98:S29–35. doi: 10.1017/S0007114507832971
136. Dilek, N, Papapetropoulos, A, Toliver-Kinsky, T, and Szabo, C. Hydrogen sulfide: an endogenous regulator of the immune system. Pharmacol Res. (2020) 161:105119. doi: 10.1016/j.phrs.2020.105119
137. Sikalidis, AK . Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol Oncol Res. (2015) 21:9–17. doi: 10.1007/s12253-014-9860-0
138. Imran, M, Umer, T, Rehman, HU, Saleem, M, Farooq, H, Bhutta, ZA, et al. Anti-inflammatory, immunomodulatory and antioxidant activities of allicin, vitamin C and doxycycline, or their combination against Pasteurella multocida infection in rabbits. Cont Vet J. (2022) 2:112–7.
139. Demir, E, Sarica, S, Ozcan, MA, and Suicmez, M. The use of natural feed additives as alternatives for an antibiotic growth promoter in broiler diets. Br Poult Sci. (2003) 44:S44–5. doi: 10.1080/00071660301944
140. Huang, X, Wang, D, Hu, Y, Lu, Y, Guo, Z, Kong, X, et al. Effect of sulfated astragalus polysaccharide on cellular infectivity of infectious bursal disease virus. Int J Biol Macromol. (2008) 42:166–71. doi: 10.1016/j.ijbiomac.2007.10.019
141. Ali, BH, Jalob, ZK, Ibrahim, ZY, Jarad, AS, and Hasan, MS. Effect of dates (Phoenix dactylifera L.) on liver of broiler chicks infected with infectious bursal disease virus. Biochemical and histological study. Indian J Forensic Med Toxicol. (2020) 14:2243–9. doi: 10.37506/ijfmt.v14i2.3353
142. Karthiyaini, K, and Philiomina, PT (2009). Effect of vitamin C supplementation on some biochemical profile stressed broiler, Proceedings of the National Seminar of Recent Trends in Animal Welfare and Sustainable Livestock Production. Thrissur, India.
143. Leshchinsky, TV, and Klasing, KC. Relationship between the level of dietary vitamin E and the immune response of broiler chickens. Poult Sci. (2001) 80:1590–9. doi: 10.1093/ps/80.11.1590
144. Vallverdú-Coll, N, Mateo, R, Mougeot, F, and Ortiz-Santaliestra, ME. Immunotoxic effects of lead on birds. Sci Total Environ. (2019) 689:505–15. doi: 10.1016/j.scitotenv.2019.06.251
145. Kalvandi, O, Sadeghi, A, and Karimi, A. Arginine supplementation improves reproductive performance, antioxidant status, immunity and maternal antibody transmission in breeder Japanese quail under heat stress conditions. Ital J Anim Sci. (2022) 21:8–17. doi: 10.1080/1828051X.2021.2013136
146. Tayade, C, Jaiswal, TN, Mishra, SC, and Madhuri, K. L-arginine stimulates immune response in chickens immunized with intermediate plus strain of infectious bursal disease vaccine. Vaccine. (2006) 24:552–60. doi: 10.1016/j.vaccine.2005.08.059
147. Al Mahmud, A, Siddiqui, SA, Karim, MR, Al-Mamun, MR, Akhter, S, Sohel, M, et al. Clinically proven natural products, vitamins and mineral in boosting up immunity: a comprehensive review. Heliyon. (2023) 9:e15292. doi: 10.1016/j.heliyon.2023.e15292
148. Ertekin, A, Yıldırım, BA, Yıldırım, S, Yıldırım, F, and Tütüncü, M. Investigation of the lipid peroxidation, antioxidant enzymes, antioxidant vitamins, oxidation products of nitric oxide and some biochemical parameters in chicken with infectious bursal disease (IBD). Eur Poult Sci. (2016) 80:164. doi: 10.1399/eps.2016.164
149. Jaime, J, Vargas-Bermúdez, DS, Yitbarek, A, Reyes, J, and Rodríguez-Lecompte, JC. Differential immunomodulatory effect of vitamin D (1,25 (OH)2 D3) on the innate immune response in different types of cells infected in vitro with infectious bursal disease virus. Poult Sci. (2020) 99:4265–77. doi: 10.1016/j.psj.2020.06.006
Keywords: plants, poultry, antioxidant, immune response, virus, polyphenol
Citation: Tahir I and Alsayeqh AF (2024) Phytochemicals: a promising approach to control infectious bursal disease. Front. Vet. Sci. 11:1421668. doi: 10.3389/fvets.2024.1421668
Edited by:
Izhar Hyder Qazi, Shaheed Benazir Bhutto University of Veterinary & Animal Sciences, PakistanReviewed by:
Muhammad Ijaz, University of Veterinary and Animal Sciences, PakistanAmjad Islam Aqib, Cholistan University of Veterinary and Animal Sciences, Pakistan
Copyright © 2024 Tahir and Alsayeqh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah F. Alsayeqh, YS5hbHNheWVxaEBxdS5lZHUuc2E=
 Ifrah Tahir
Ifrah Tahir Abdullah F. Alsayeqh
Abdullah F. Alsayeqh