- 1College of Life Science, Northeast Agricultural University, Harbin, China
- 2Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process, National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China
- 3School of Biology and Food Engineering, Fuyang Normal University, Fuyang, China
Background: Shuanghuanglian is a Chinese medicine composed of Honeysuckle Flower, Baical Skullcap Root, and Fructus Forsythiae. It has various effects, including anti-inflammatory, antibacterial, antiviral, and immunomodulatory effects. The fermented product of Shuanghuanglian can be used as an antibiotic alternative, as it has similar efficacy, which may improve the immunity, feed intake and utilization efficiency of laying hens, thus improving their production performance. The aim of this study was to optimize the fermentation conditions for Shuanghuanglian using single factor and response surface methodology, evaluate the chemical and microbial composition of the Shuanghuanglian fermentation liquor (SFL), and explore the effects of SFL on the production performance of laying hens.
Methods: A total of 288 Xinyang black-feather laying hens (50 week-old) were randomly allocated to four treatments with nine replicates, each replicate containing eight hens, for a total of 37 days trial (including a 7-day adaptation period). The treatments included a control group (0% SFL in drinking water) and drinking water supplemented with 0.3, 0.5, or 0.7% SFL.
Results: The fermentation optimization conditions for Shuanghuanglian were selected as a solid-to-liquid ratio at 1:7, 3% inoculation quantity, fermentation temperature at 28°C for 5 days, initial pH of 7, 60 mesh (sieved), and rotation speed of 150 r/min. Various bioactive compounds, such as myrtenol, 2-hexyn-1-ol, arsenous acid tris(trimethylsilyl) ester, 3(10)-caren-4-ol, and oxime-, methoxy-phenyl, were detected in SFL. The most abundant bacterial phyla in SFL were Proteobacteria and Firmicutes, with Acinetobacter being the most abundant genus. The most abundant fungal phyla were Phragmoplastophyta and Magnoliophyta. The 0.5 and 0.7% SFL supplementation in water increased egg weight and laying rate, while decreasing the feed-to-egg ratio of laying hens compared with the control group (p < 0.05). Additionally, 0.3, 0.5, and 0.7% SFL supplementation in water increased (p < 0.05) the Haugh unit, but there were no significant differences (p > 0.05) in albumen height, egg shape index, egg thickness, and yolk color of the eggs.
Conclusion: Supplementation of SFL under optimized conditions had a positive impact on the production performance of laying hens, especially when the supplementation amount reached 0.5%. This study provides a theoretical basis for the application of Shuanghuanglian in the commercial egg industry.
1 Introduction
Shuanghuanglian is a Chinese medicine that consists of Chinese natural herbs, including Honeysuckle Flower, Baical Skullcap Root, and Fructus Forsythiae in a 1:1:2 ratio (1). It has been found to have various biological functions, such as antipyretic, anti-inflammatory, antibacterial, antiviral, and immune-enhancing effects, and is widely used for respiratory tract infections, mild pneumonia, and common cold treatments caused by virus and bacterial infection (2, 3). Recent studies have also shown that Shuanghuanglian can regulate the structure and function of the intestinal microbiota in rats (3), indicating that it may have potential to improve the intestinal health of poultry and livestock and therefore influence their production performance. Although Shuanghuanglian is widely used as a safe drug in human medicine (4), there is limited information available regarding its use in animal husbandry.
A previous study found that supplementing drinking water with Shuanghuanglian liquid improved the immune performance of broilers (5). Therefore, we hypothesized that Shuanghuanglian might have positive effects on the laying performance and egg quality of laying hens by reducing diseases. Since Shuanghuanglian is commonly used as an oral liquid (2–5) and the cell walls of Chinese herbal medicines typically limit the release of bioactive components (6), we used lignin-degrading bacteria (Bacillus amyloliquefaciens-c4) to ferment Shuanghuanglian to release the bioactive components into Shuanghuanglian fermentation liquor (SFL). Consequently, we optimized the fermentation conditions of Shuanghuanglian using single factor and response surface methodology, evaluated the compound and microbial composition of SFL, and further supplemented SFL in drinking water for laying hens. Albumen height, egg shape index, eggshell thickness, Haugh unit, and yolk color were determined as important indexes of egg quality (7, 8). This study will provide a theoretical basis for the application of Shuanghuanglian in laying hens.
2 Materials and methods
2.1 Sources of Shuanghuanglian and microbial inoculum
Honeysuckle Flower, Baical Skullcap Root, and Fructus Forsythiae were provided by Fuyang Normal University, Fuyang, Anhui, China. The microbial inoculum (Bacillus amyloliquefaciens-c4; preservation number CGMCC No. 15178) was provided by the Biological Inoculation Research and Development Center of Northeast Agricultural University, Harbin, Heilongjiang, China.
2.2 Optimization of microbial fermentation conditions by single factor experiment
Shuanghuanglian [not sieved; solid-to-liquid ratio (SLR) of 1:5] was added with 5% brown sugar. It was then inoculated with 5% microbial inoculum and fermented at 28°C for 7 days using a constant temperature shaker (Shidan Equipment Inc., Shanghai, China) with a rotational speed of 180 r/min.
The following conditions were used to determine fermentation optimization were as follows: (1) the SLR was adjusted to 1:1, 1:3, 1:5, 1:7, and 1:9, while keeping other fermentation conditions unchanged, (2) the inoculation quantity of microbial inoculum was adjusted to 1, 3, 5, 7, and 9%, while keeping other conditions unchanged, (3) the fermentation temperature was set to 24°C, 28°C, 33°C, 37°C, and 42°C using the constant temperature shaker (Shidan Equipment Inc., Shanghai, China), while keeping other conditions unchanged, (4) the fermentation time was set to 1, 3, 5, 7, and 9 days, while keeping other conditions unchanged, (5) the initial pH value was adjusted to 5, 6, 7, 8, and 9 using a pH meter (Gaozhi Precision Instrument Inc., Shanghai, China), while keeping other conditions unchanged, (6) the particle size was set to dp (decoction pieces form without sieving), 20, 40, 60, and 80 mesh using sieves (ZhenXing Inc., Guangzhou, China), while keeping other conditions unchanged, and (7) the rotational speed was set to 0, 120, 150, 180, and 210 r/min using the constant temperature shaker (Shidan Equipment Inc., Shanghai, China), while keeping other conditions unchanged. Each fermentation condition was replicated three times. Finally, the surface morphology of the three component medicines, chemical and microbial compositions, and feeding effects of Shuanghuanglian on laying hens were evaluated based on the optimized fermentation conditions.
2.3 Chemical compound composition analysis
The dry matter content of the Shuanghuanglian was analyzed using previously described methods by Esfahani and Goli (9). Chlorogenic acid, baicalin, and phillyrin were extracted using the ChP method (1). Briefly, 1 mL of SFL was added to 5 mL of 50% (w/v) methanol and sonicated for 25 min using a multifunctional constant-temperature ultrasonic extractor (Scientz-1000C, Xinzhi Biotechnology Inc., Ningbo, China) to obtain the supernatant for analysis. The chlorogenic acid, baicalin, and phillyrin contents were measured as previously described by Li et al. (10) using an ultraviolet spectrophotometer (T6 New Century, Puxi General Instrument Inc., Beijing, China). Additionally, the supernatants obtained from sonication were concentrated 100 times using a rotary evaporator (Hei-VAP Advantage ML/HB/G3, Heidolph, Schwabach, Germany) and filtered through a 0.22 μm filter membrane to determine the chemical composition of the SFL using a gas chromatography–mass spectrometer (Agilent 7890A/5975C, Agilent, Santa Clara, United States). The chemical composition of the SFL was analyzed in triplicate.
2.4 Observation of surface morphology
The surface morphologies of Honeysuckle Flower, Baical Skullcap Root, and Fructus Forsythiae were examined using a field emission scanning electron microscopy (FESEM; model SU8010; Hitachi, Japan), as previously described by Yi et al. (11). Images representing the average characteristics of the surface morphology were screened at a magnification of 1,000×.
2.5 Analysis of microbiota composition
The composition of the microbiota was analyzed following the methods previously described by Yi et al. (11). Briefly, the total microbial DNA of the SFL was extracted using the TIANamp Stool DNA Kit (Tiangen Biochemical Technology Inc., Beijing, China). The concentration and purity of the extracted DNA were determined using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Inc., Waltham, MA, United States). The V3–V4 region of the 16S rRNA genes was amplified using the bacterial universal primers 341F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Similarly, the V4 region of the 18S rRNA genes was amplified using the fungal universal primers F (5′-CCAGCASCYGCGGTAATTCC-3′) and R (5′-ACTTTCGTTCTTGATYRA-3′). The sequencing of the 16S rRNA and 18S rRNA was performed using the Illumina NovaSeq platform (Illumina Inc., San Diego, CA, United States) by Boyuezhihe Biology Science and Technology Co., Ltd. (Wuhan, China). The analysis of the microbiota composition of the SFL was performed in triplicate.
2.6 Animals, treatment, and feeding
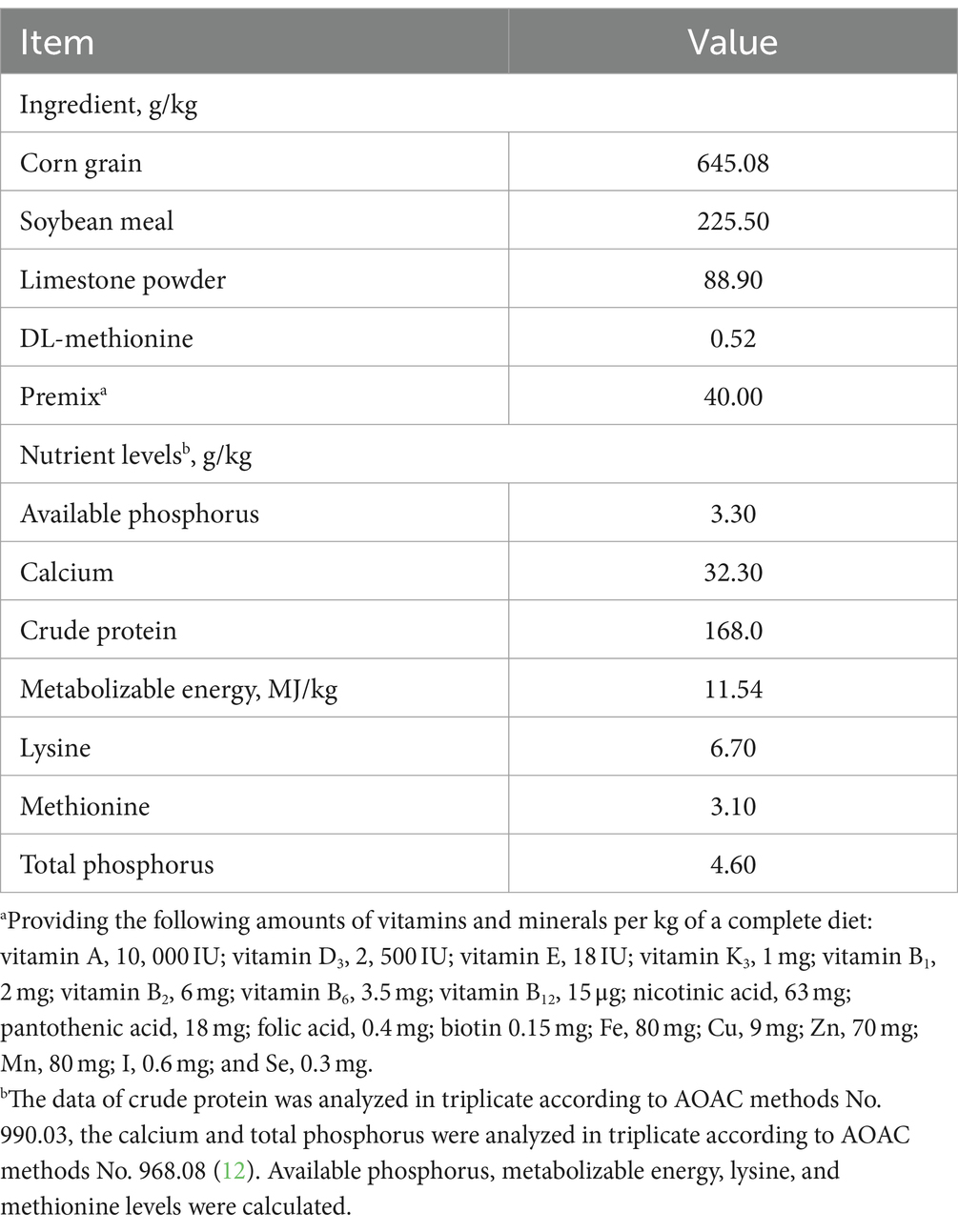
The animal trial was conducted at Duoduoli Agricultural Science and Technology Co., Ltd., Fuyang, China. A total of 288 healthy Xinyang black laying hens, 50 week-old (laying rate < 80%) and with similar body weight, were selected for the feeding trial. After a 7-days adaptation period, the laying hens were divided into four groups using a completely randomized block design. Each group was fed with different SFL levels in water: 0 (control), 0.3, 0.5, and 0.7%. Each group consisted of nine replicates with eight hens per replicate. The composition and nutrient levels of the basal diets are presented in Table 1. The experimental laying hens were reared at the Ancient West Lake Modern Agricultural Science and Technology Demonstration Park in Fuyang, China, and were fed twice daily at 06:00 and 18:00. All the hens had free access to water at all times. The SFL was mixed with drinking water before feeding. The experiment lasted for 37 days, including the adaptation period.
2.7 Determination of laying performance and egg quality
The egg production, egg weight, feed offered, and refusals were recorded daily to calculate average egg weight, average daily feed intake (ADFI), feed-to-egg ratio, and laying rate.
Eggshell thickness without egg membrane was measured as the mean of the thickness at either end and in the middle of the shell using a screw micrometer (211-115, Sanliang Measuring Tool Inc., Dongguan, China) to calculate the eggshell thickness. The albumin height and yolk color were measured with a multifunctional egg quality meter (EA-01, Tenovo International Inc., Beijing, China). The egg shape index was calculated from the longitudinal and transverse diameters of the eggs using Vernier calipers (171-502, Sanliang Measuring Tool Inc., Dongguan, China). The Haugh units of eggs were calculated using the albumen height and egg weight.
2.8 Response surface experimental design and statistical analysis
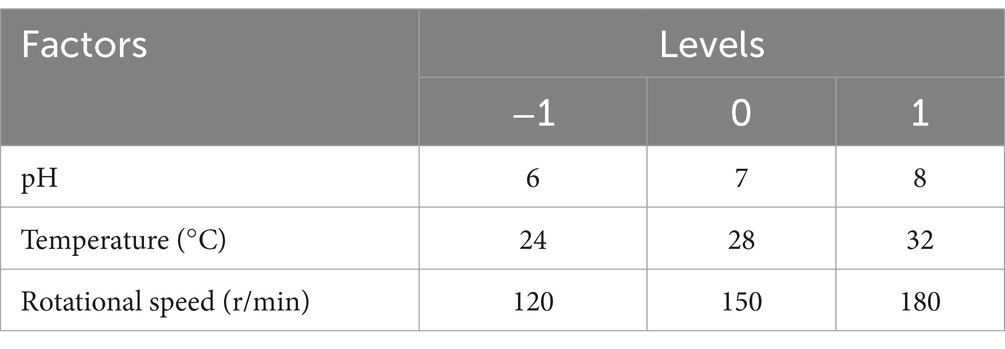
The response surface experiments were designed and analyzed using Design-Expert 13.0 software, following the methods described by Mohammadabadi et al. (13). The independent variables were the initial pH and temperature, initial pH and rotational speed, and temperature and rotational speed. The initial pH, temperature, and rotational speed ranges were 6–7, 24–32°C, and 120–180 r/min, respectively. The dependent variables included chlorogenic acid, baicalin, and phillyrin. Regression models for these variables were constructed based on the data obtained in this study. The accuracy of the regression models was assessed using R2, Radj, C.V.%, and Adep precision values.
The data for the optimized fermentation conditions and the production performance of laying hens were analyzed using one-way ANOVA, and the comparative analysis among different treatment groups was analyzed by Duncan’s multiple range test with SPSS software (version 26.0; SPSS Inc., Chicago, IL, United States). The data for the optimized fermentation conditions are presented as means and standard errors, and the data for the production performance of hens are presented as means and standard errors of the mean (SEM). Statistical significance was set at p < 0.05.
3 Results
3.1 Optimization of microbial fermentation conditions of Shuanghuanglian by single factor experiments
The results of the optimized fermentation conditions of Shuanghuanglian in a single-factor experiment are shown in Figure 1. Compared with the 1:5 and 1:7 SLR, Shuanghuanglian fermented at 1:1 and 1:3 SLR had a lower (p < 0.05) chlorogenic acid concentration, while Shuanghuanglian fermented at 1:1, 1:3, and 1:9 SLR had a lower (p < 0.05) baicalin concentration. Furthermore, Shuanghuanglian fermented at 1:7 SLR had a higher (p < 0.05) phillyrin concentration than the 1:1, 1:3, or 1:9 SLR (Figure 1A).
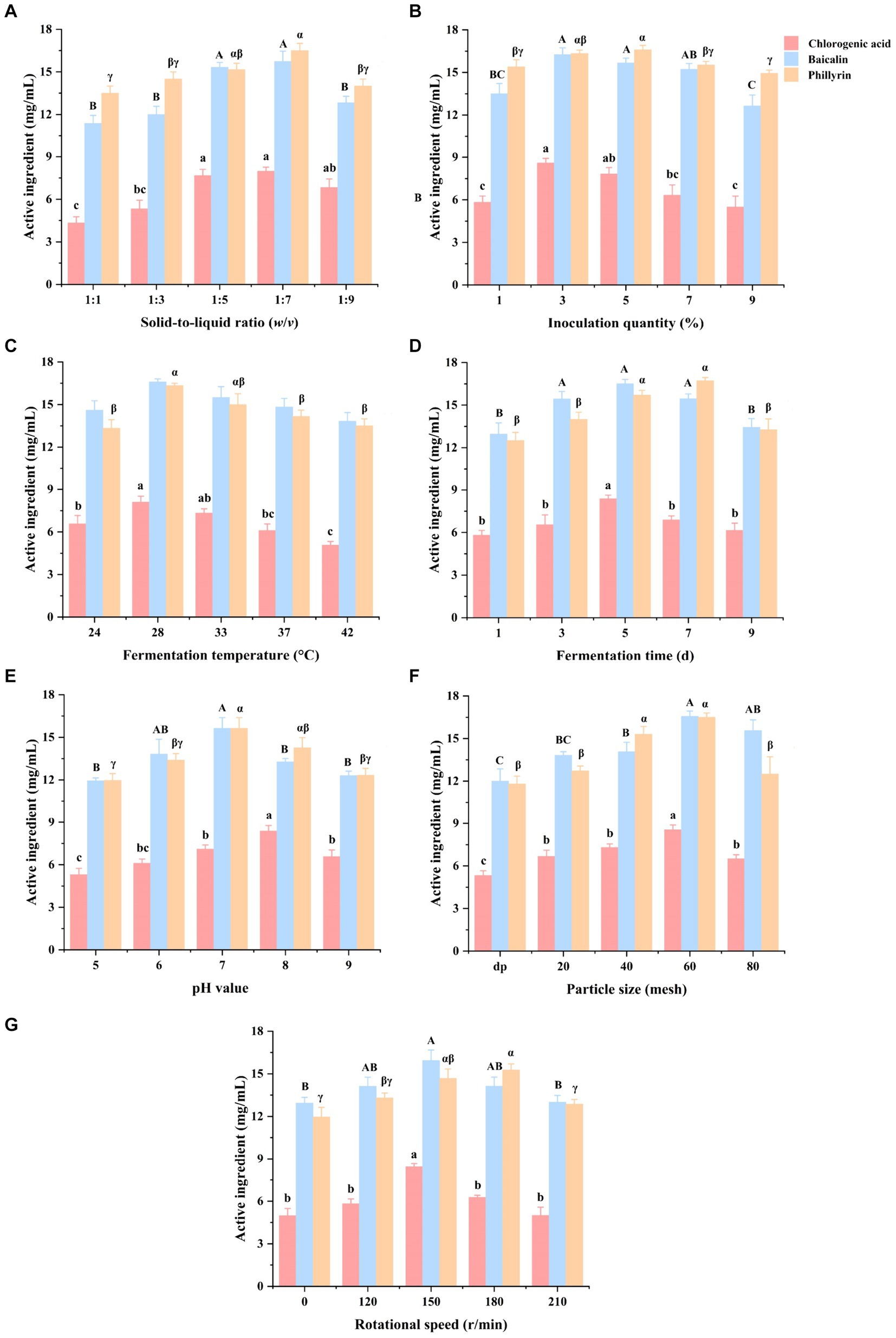
Figure 1. Effects of solid-to-liquid ratio (A), inoculation quantity (B), temperature (C), time (D), initial pH (E), particle size (F), and rotational speed (G) on chlorogenic acid, baicalin, and phillyrin concentrations of Shuanghuanglian fermentation liquor (n = 3). d, days; dp, decoction pieces form without sieving; data are presented as means ± standard error. Different letters in the same index indicate significant differences (p < 0.05).
In term of inoculation quantities, Shuanghuanglian fermented with 3% inoculation had a higher (p < 0.05) chlorogenic acid concentration than the 1, 7, and 9% inoculation. Shuanghuanglian fermented with 3 and 5% inoculation quantities had a higher (p < 0.05) baicalin concentration compared to the 1 and 9% inoculation. Moreover, Shuanghuanglian fermented with 5% inoculation had a higher (p < 0.05) phillyrin concentration than the 1, 7, and 9% inoculation (Figure 1B).
Compared with the fermentation temperatures at 24°C, 37°C, and 42°C, Shuanghuanglian fermented at 28°C had higher (p < 0.05) concentrations of chlorogenic acid and phillyrin. However, there was no significant difference (p > 0.05) in baicalin concentration among the different fermentation temperatures (including 24°C, 28°C, 33°C, 37°C, and 42°C) (Figure 1C).
Among the different fermentation times, Shuanghuanglian fermented for 5 days had a higher (p < 0.05) chlorogenic acid concentration compared to the other fermentation times (1, 3, 7, and 9 days). Shuanghuanglian fermented for 3, 5, and 7 days had a higher (p < 0.05) baicalin concentration compared to those fermented for 1 or 9 days. Furthermore, Shuanghuanglian fermented for 5 and 7 days had a higher (p < 0.05) phillyrin concentration than the 1, 3, and 9 days (Figure 1D).
When considering the initial pH values, Shuanghuanglian fermented with an initial pH of 8 had a higher (p < 0.05) chlorogenic acid concentration compared to the other initial pH values (5, 6, 7, and 9). Shuanghuanglian fermented with an initial pH of 7 had a higher (p < 0.05) baicalin concentration than the pH values of 5, 8, or 9. Moreover, Shuanghuanglian fermented with an initial pH value of 7 had a higher (p < 0.05) phillyrin concentration than the initial pH values of 5, 6, or 9 (Figure 1E).
Among the different particle sizes, Shuanghuanglian fermented with a particle size of 60 mesh had a higher (p < 0.05) chlorogenic acid concentration than other particle sizes (dp, 20, 40, and 80 mesh). Furthermore, Shuanghuanglian fermented with a particle size of 60 mesh had a higher (p < 0.05) baicalin concentration than the dp, 20, and 40 mesh, while Shuanghuanglian fermented with 40 and 60 mesh particle sizes had a higher (p < 0.05) phillyrin concentration compared to dp, 20, and 80 mesh (Figure 1F).
Compared with the 150 r/min, Shuanghuanglian fermented at 0, 120, 180, and 210 r/min had a lower (p < 0.05) chlorogenic acid concentration. Shuanghuanglian fermented at 150 r/min had a higher (p < 0.05) baicalin concentration than at the 0 and 210 r/min. Furthermore, Shuanghuanglian fermented at a rotational speed of 180 r/min had a higher (p < 0.05) phillyrin concentration than the 0, 120, and 210 r/min (Figure 1G).
3.2 Variance analysis of response surface model
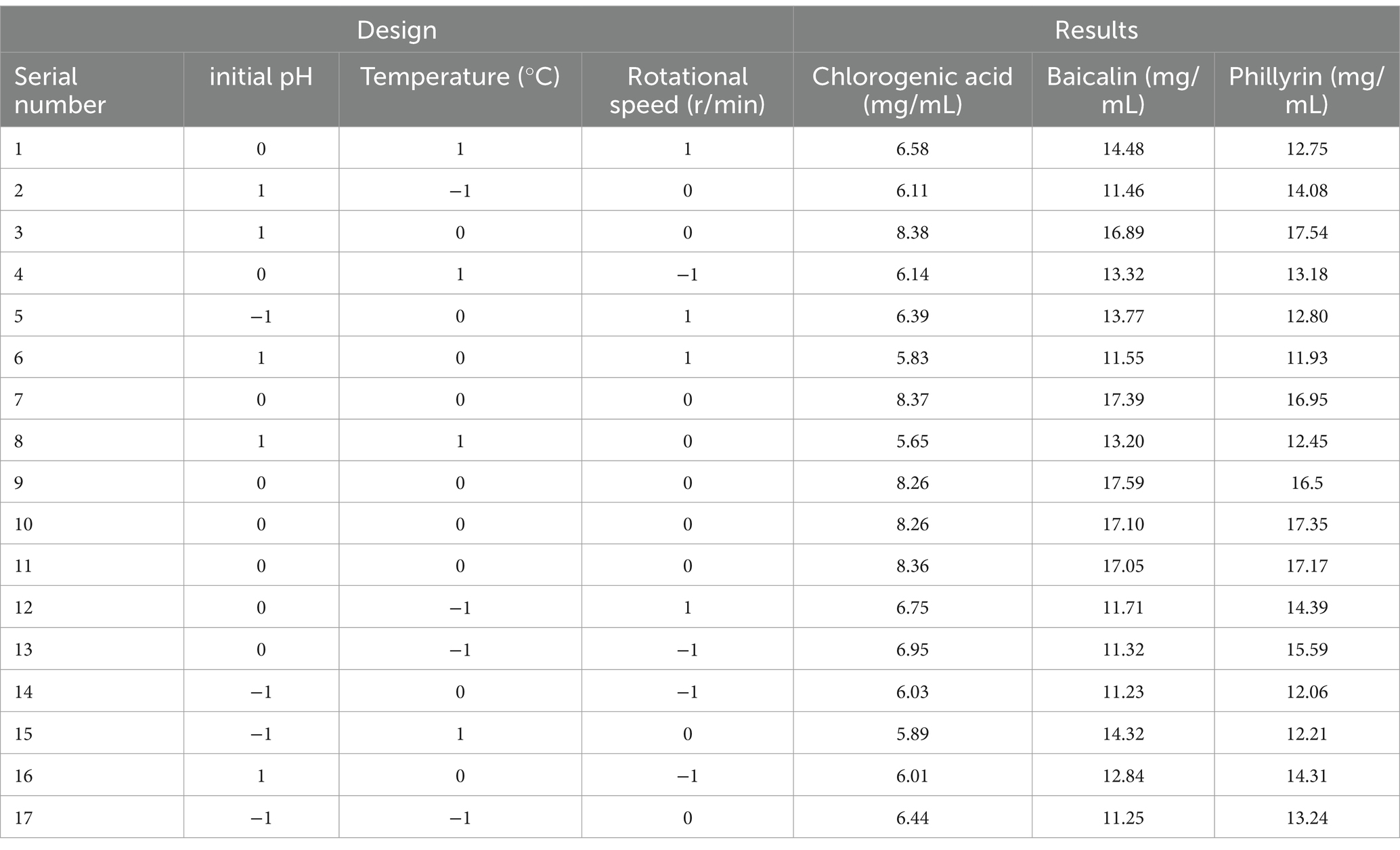
Based on the single-factor experiments, three factors (initial pH, temperature, and rotational speed) were considered to verify the response surface experiments with significant differences. The design and results of the optimized fermentation of Shuanghuanglian by the response surface experiments are shown in Tables 2, 3.
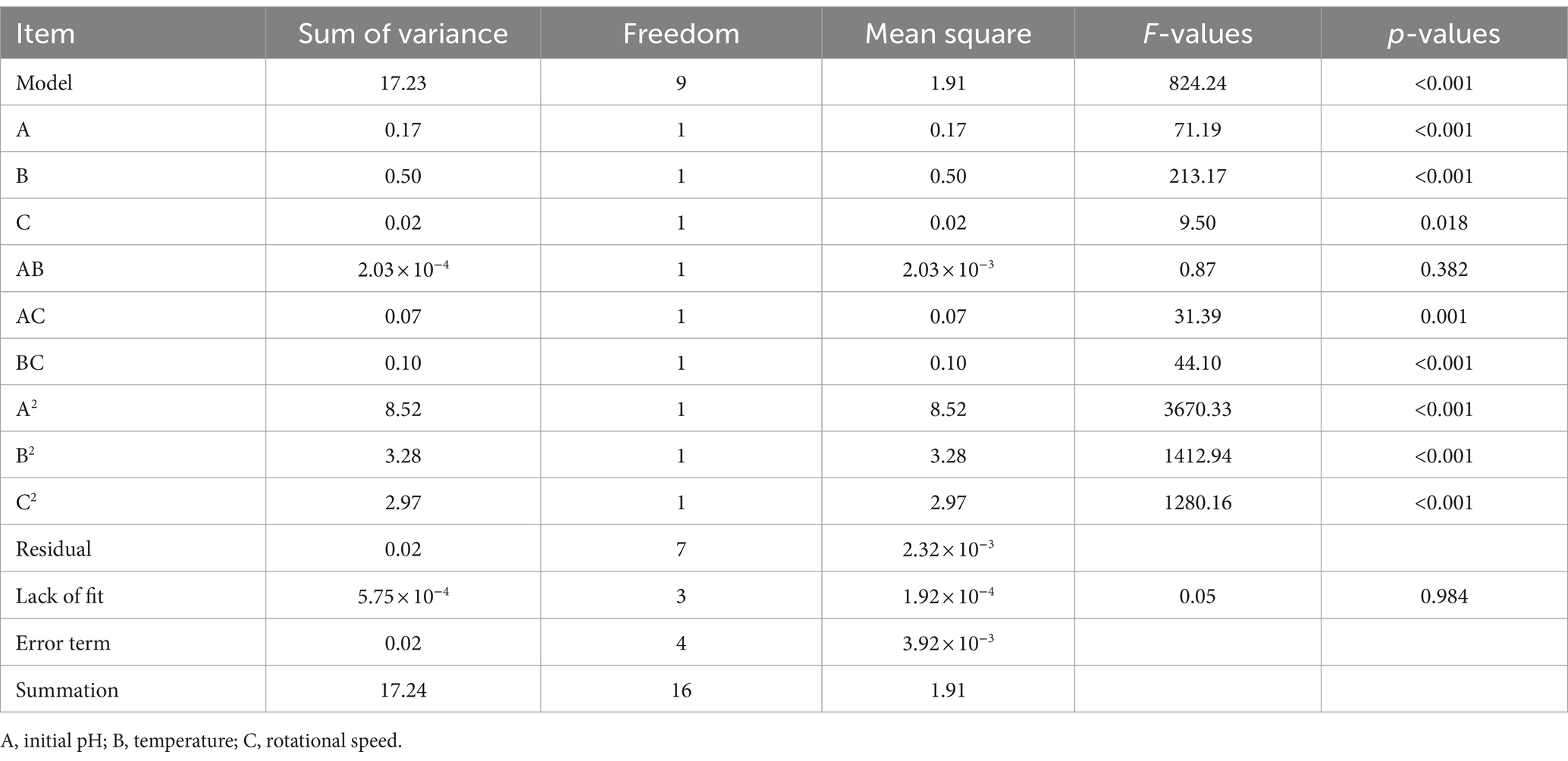
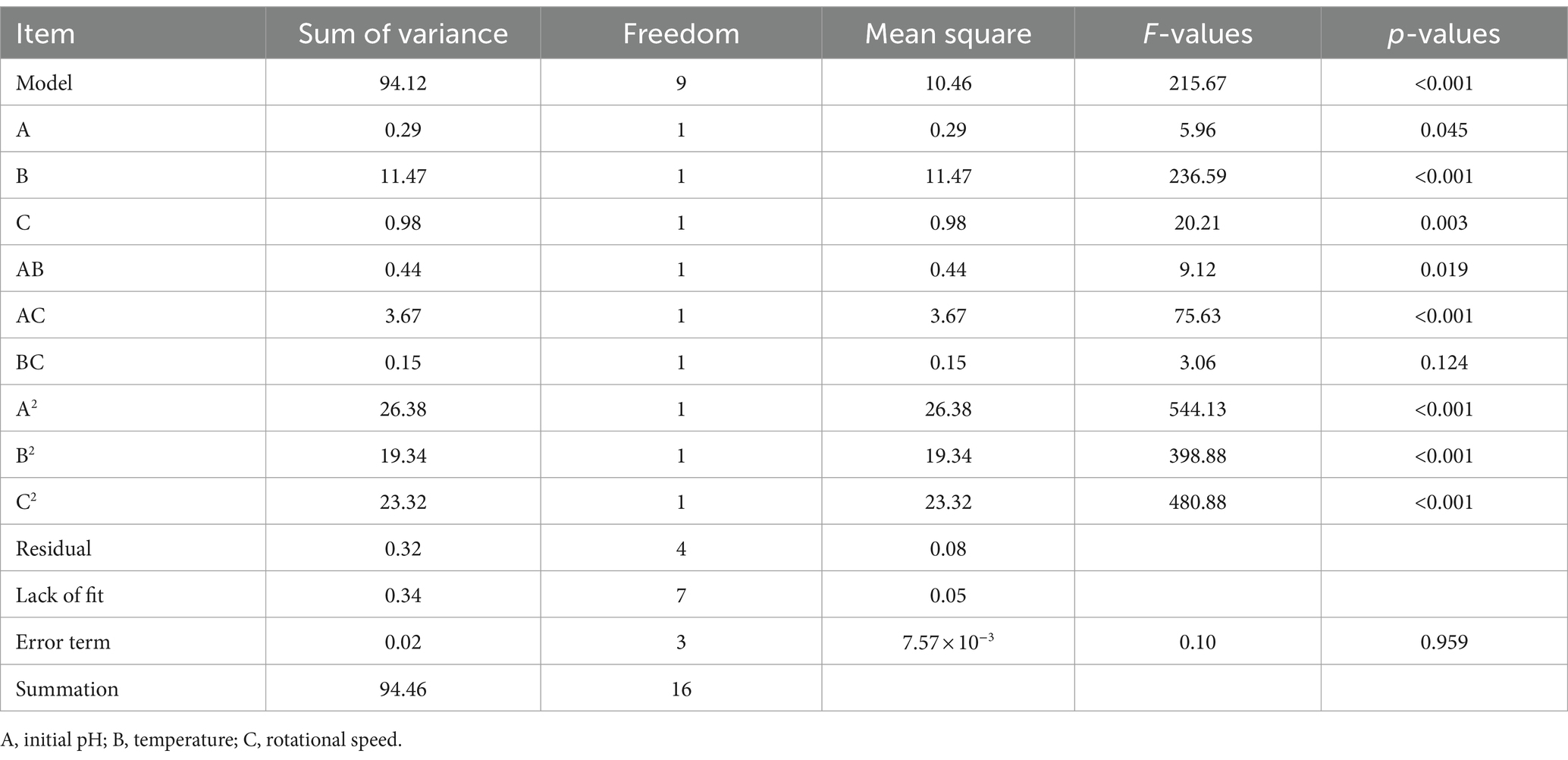
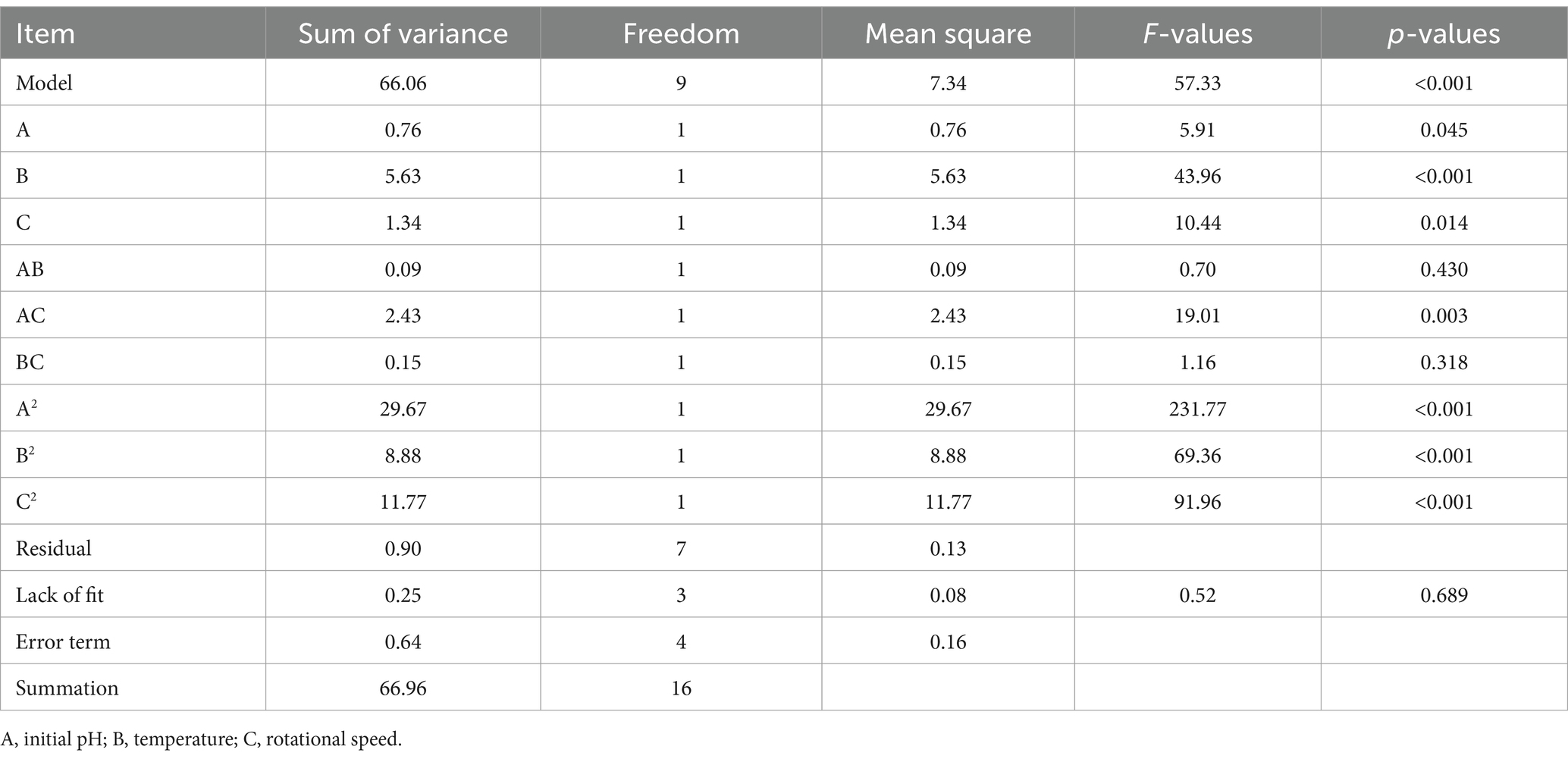
The variance analysis of chlorogenic acid is presented in Table 4. The regression model for chlorogenic acid is Y = 8.33 − 0.14A − 0.25B + 0.053C + 0.023AB − 0.14 AC + 0.16 BC − 1.42A2 − 0.88B2 − 0.84C2. Where Y represents the chlorogenic acid concentration (mg/mL), and A, B, and C represent initial pH, temperature (°C), and rotational speed (r/min), respectively. There was a significant difference (p < 0.05) in the regression model and no difference (p > 0.05) in the lack-of-fit. The factors A, B, C, AC, BC, A2, B2, and C2 had significant differences (p < 0.05), indicating that they had a significant influence on the chlorogenic acid concentration. The R2 and Radj values of the regression model were 0.999 and 0.998, respectively. The C.V.% and Adep Precision were 0.70 and 72.39, respectively, indicating that the model has high accuracy and credibility.
The variance analysis of baicalin is shown in Table 5. The regression model of baicalin is Y = 17.20 − 0.19A + 1.20B + 0.35C − 0.33AB − 0.96 AC + 0.19 BC − 2.50A2 − 2.14B2 − 2.35C2. Where Y represents the baicalin concentration (mg/mL), and A, B, and C represent initial pH, temperature (°C), and rotational speed (r/min), respectively. There was a significant difference (p < 0.05) in the regression model and no difference (p > 0.05) in the lack-of-fit. The factors A, B, C, AB, AC, A2, B2, and C2 had significant differences (p < 0.05), indicating that they had a great effect on the baicalin concentration. The R2 and Radj values of the regression model were 0.996 and 0.992, respectively. The C.V.% and Adep Precision were 1.58 and 35.45, respectively, indicating that the model has high accuracy and credibility.
The variance analysis of phillyrin is presented in Table 6. The regression model for phillyrin is Y = 17.10 + 0.31A − 0.84B − 0.41C − 0.15AB − 0.78 AC + 0.19 BC − 2.65A2 − 1.45B2 − 1.67C2. Where Y represents the phillyrin concentration (mg/mL), and A, B, and C represent initial pH, temperature (°C), and rotational speed (r/min), respectively. There was a significant difference (p < 0.05) in the model and no difference (p > 0.05) in the lack-of-fit. The factors A, B, C, AC, A2, B2, and C2 had significant differences (p < 0.05), indicating that they had great effects on the phillyrin concentration. The R2 and Radj values of the regression model were 0.987 and 0.969, respectively. The C.V.% and Adep Precision were 2.49 and 18.98, respectively, indicating that the model has high accuracy and credibility.
3.3 Optimization of microbial fermentation conditions of Shuanghuanglian by response surface experiment
The contour lines and response surface of the optimized fermentation of Shuanghuanglian are shown in Figures 2–4. The effects of the interaction between initial pH and temperature on the concentrations of chlorogenic acid, baicalin, and phillyrin in SFL are shown in Figure 2. The contour lines for chlorogenic acid were circular, indicating that the interaction between pH and temperature had no significant effect on chlorogenic acid. However, the concentration of chlorogenic acid reached its highest point, and pH and temperature had significant effects on the concentration of chlorogenic acid. The contour lines for baicalin and phillyrin were elliptical, and the interaction between pH and temperature significantly affected baicalin and phillyrin. Temperature and pH had more significant effects on the concentration of baicalin than the phillyrin. Compared with the effects of pH on baicalin and phillyrin concentrations, temperature had greater effects, and there was also a significant interaction between pH and temperature. When the pH value was fixed, the concentrations of chlorogenic acid, baicalin, and phillyrin increased initially and then decreased with increasing temperature. At the same temperature, the concentrations of the three bioactive components increased initially and then decreased, and the concentrations reached a maximum with increasing pH value. Overall, the highest concentrations of chlorogenic acid, baicalin, and phillyrin were observed within the temperature range of 27–30°C and the pH value of 6.5–7.5.
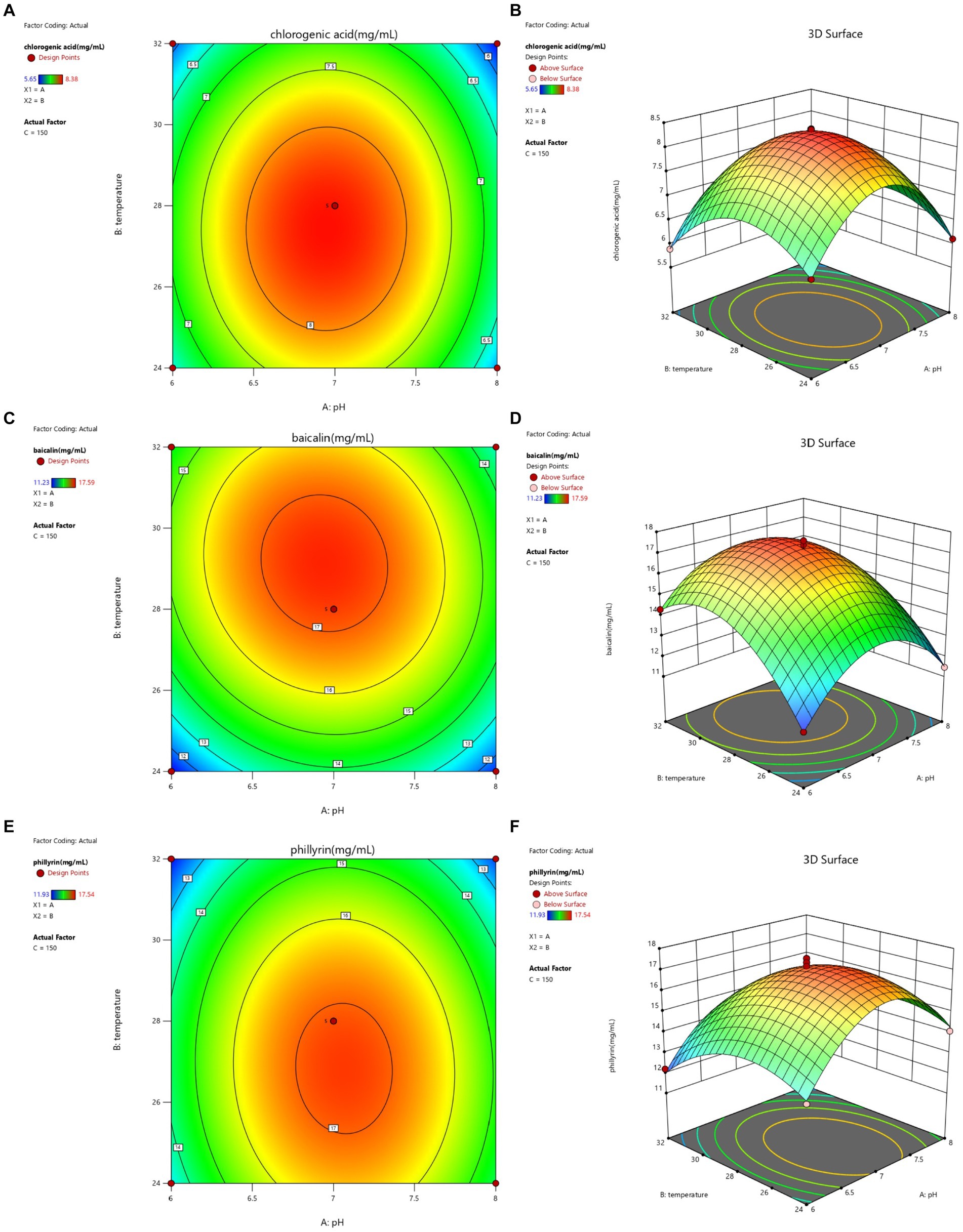
Figure 2. Contour lines and response surface of initial pH and temperature to chlorogenic acid (A,B), baicalin (C,D), and phillyrin (E,F) concentrations of Shuanghuanglian fermentation liquor.
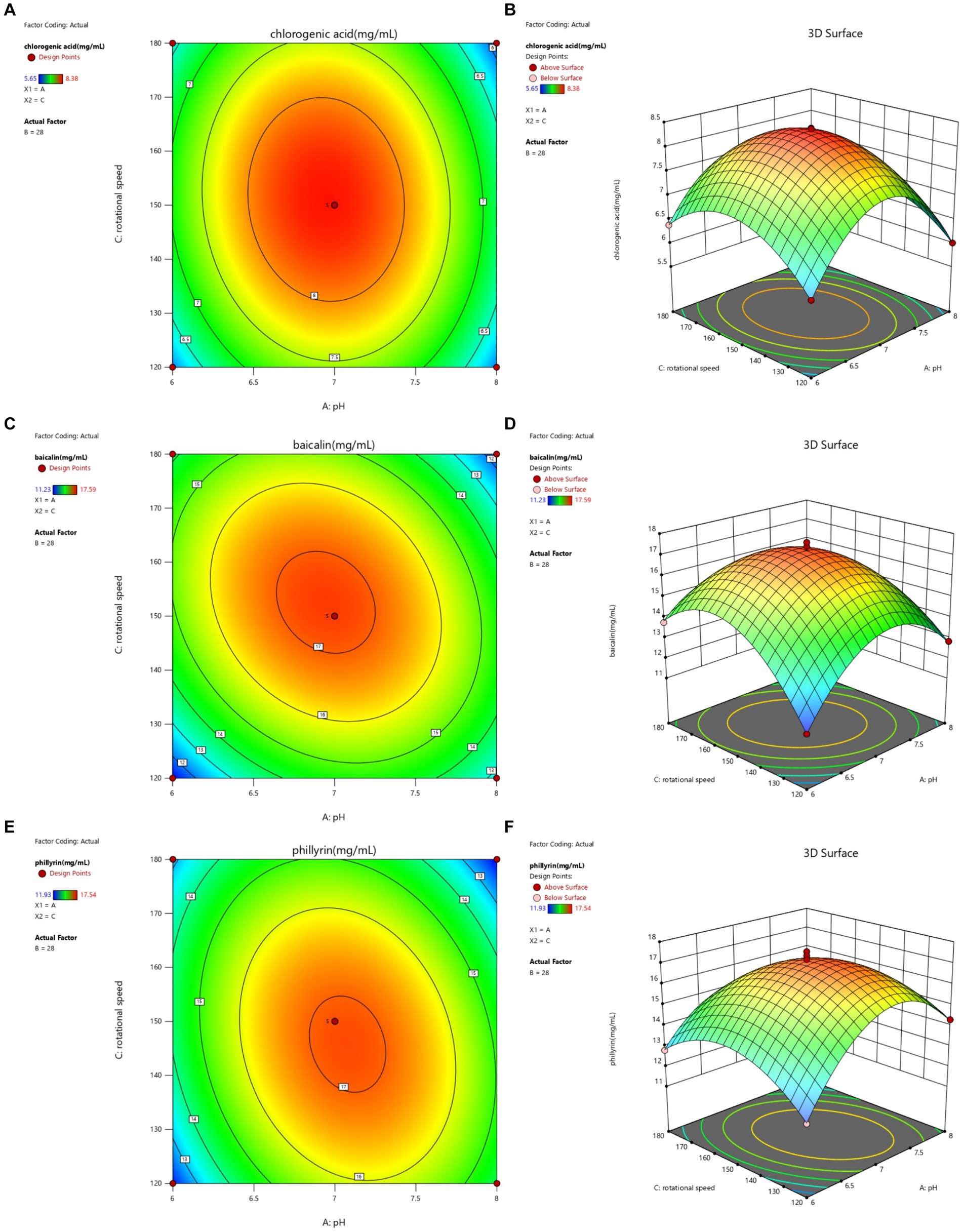
Figure 3. Contour lines and response surface of initial pH and rotational speed to chlorogenic acid (A,B), baicalin (C,D), and phillyrin (E,F) concentrations of Shuanghuanglian fermentation liquor.
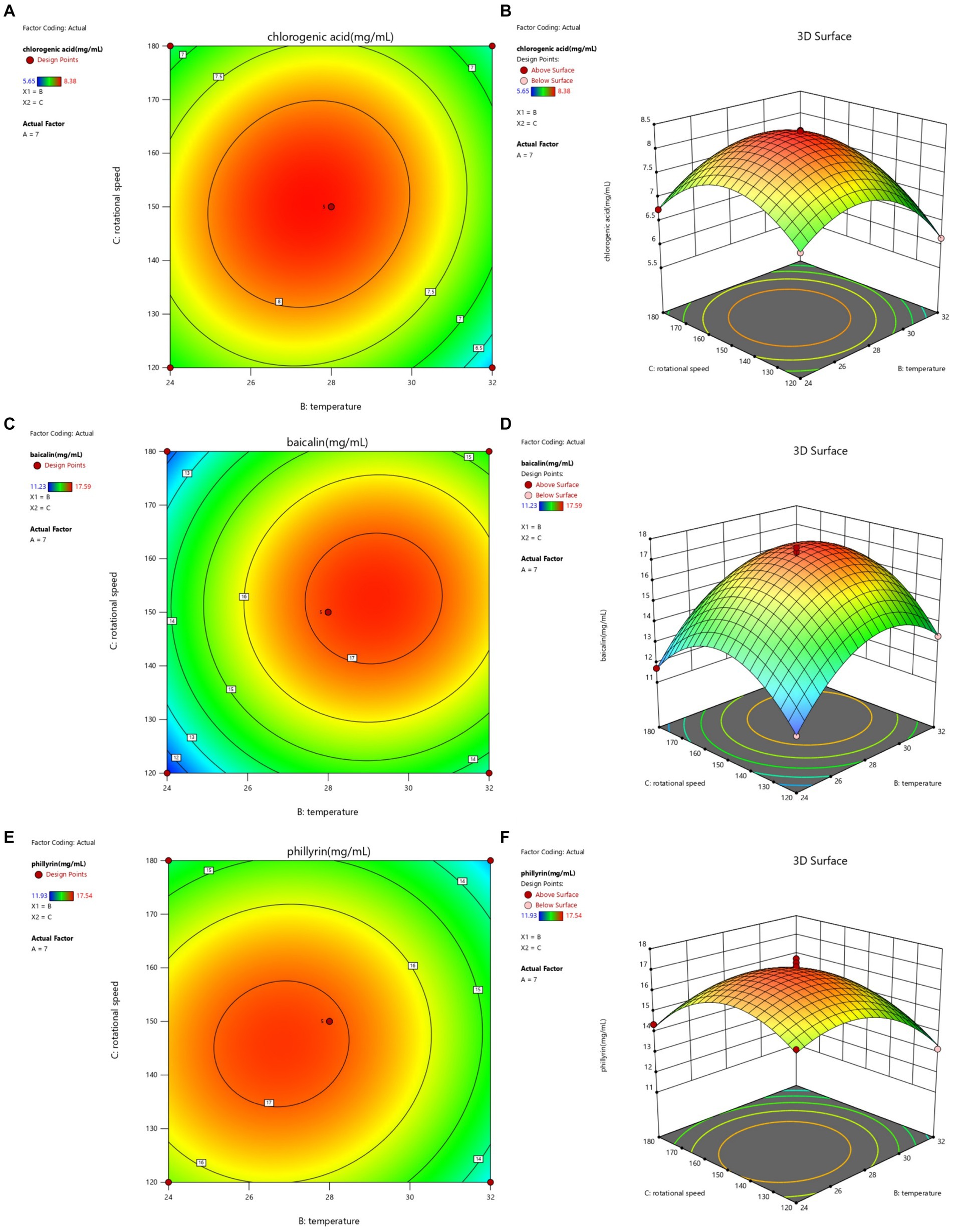
Figure 4. Contour lines and response surface of temperature and rotational speed to chlorogenic acid (A,B), baicalin (C,D), and phillyrin (E,F) concentrations of Shuanghuanglian fermentation liquor.
The interactions between initial pH and rotational speed on the chlorogenic acid, baicalin, and phillyrin concentrations in the SFL are shown in Figure 3. The contour lines for chlorogenic acid, baicalin, and phillyrin observed as oval shapes, and the interaction between pH and rotational speed had significant effects on the chlorogenic acid, baicalin, and phillyrin concentrations. Compared to the rotational speed on chlorogenic acid concentration, the pH value had a greater effect on its concentration. Compared with the effects of pH on baicalin and phillyrin concentrations, the effects of rotational speed on their concentrations were more significant. When the pH value was constant, the chlorogenic acid, baicalin, and phillyrin concentrations displayed an increasing trend initially and then decreased with increasing rotating speed, whereas when the rotational speed was constant, the three bioactive components displayed an increasing trend initially and then decreased with increasing pH value. Overall, the highest concentrations of chlorogenic acid, baicalin, and phillyrin were observed at a rotational speed of 145–155 r/min and the pH value of 6.5–7.5.
The interactions between temperature and rotational speed on chlorogenic acid, baicalin, and phillyrin concentrations in SFL are shown in Figure 4. The contours lines for baicalin and phillyrin concentrations were circular, and the interaction between temperature and rotational speed had no significant effects on baicalin and phillyrin concentrations. However, baicalin and phillyrin reached their highest concentration points, and the rotational speed and temperature had significant effects on baicalin and phillyrin concentrations. Temperature had a more significant effects on baicalin and phillyrin concentrations than the rotational speed. The contour line for chlorogenic acid concentration was elliptical, and the interaction between temperature and rotational speed had significant effects on the chlorogenic acid concentration. Temperature had a more significant effect on chlorogenic acid concentration than the rotational speed. When the temperature was constant, the chlorogenic acid, baicalin, and phillyrin concentrations displayed an increasing trend initially and then decreased with an increasing rotational speed. At a constant rotational speed, the three bioactive components initially increased and then decreased with the increasing temperature. Overall, the highest concentrations of chlorogenic acid, baicalin, and phillyrin appeared with a rotational speed of 145–155 r/min and temperature of 27–30°C.
The optimized fermentation conditions were determined as follows: an initial pH of 6.99, temperature of 27.89°C, and rotational speed of 149.99 r/min. Considering the application of production, the fermentation conditions were set with an initial pH of 7, temperature at 28°C, and rotational speed of 150 r/min. Under these conditions, the concentrations of chlorogenic acid, baicalin, and phillyrin were 9.36, 18.52, and 17.63 mg/mL, respectively. These concentrations were not significantly different from those predicted by the response surface model (concentrations of chlorogenic acid, baicalin, and phillyrin were 8.33, 17.17, and 17.12 mg/mL, respectively). Compared to Shuanghuanglian fermented under basic conditions, the concentrations of chlorogenic acid, baicalin, and phillyrin were increased by 75.60, 43.23, and 47.40%, respectively, indicating that the optimized technological parameters were accurate and reliable, and the response surface model could accurately predict the concentrations of chlorogenic acid, baicalin, and phillyrin in SFL.
Finally, the optimized fermentation conditions for Shuanghuanglian were selected as a 1:7 SLR, 3% inoculation quantity, 28°C fermentation for 5 days, initial pH of 7, 60 mesh particle size, and a rotational speed of 150 r/min.
3.4 Chemical composition of Shuanghuanglian fermentation liquor
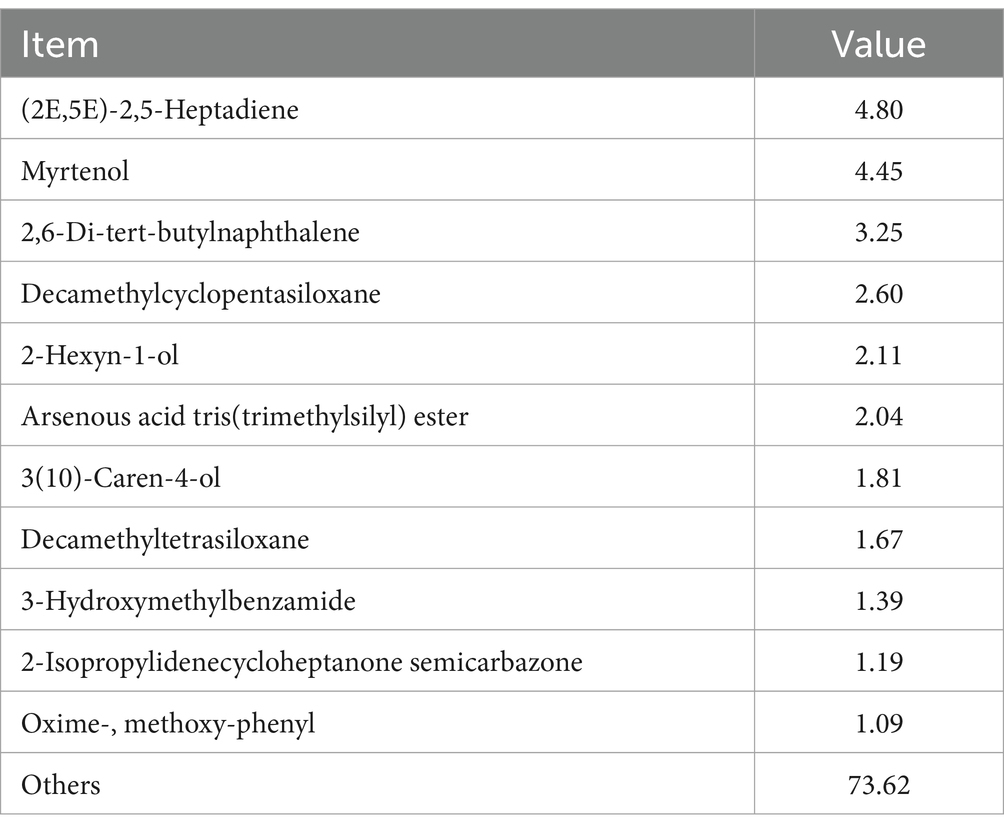
The chemical composition of SFL is presented in Table 7. Compounds with relative proportions ≤1.0% and qualitative values ≤50% were excluded from further analysis. The baicalin, phillyrin, and chlorogenic acid concentrations in SFL were 18.52, 17.63, and 9.36 mg/mL, respectively. Furthermore, (2E,5E)-2,5-heptadiene had the highest proportion (4.80%) in SFL, followed by myrtenol (4.45%), 2,6-di-tert-butylnaphthalene (3.25%), decamethylcyclopentasiloxane (2.60%), 2-hexyn-1-ol (2.11%), arsenous acid tris(trimethylsilyl) ester (2.04%), 3(10)-caren-4-ol (1.81%), decamethyltetrasiloxane (1.67%), 3-hydroxymethylbenzamide (1.39%), 2-isopropylidenecycloheptanone semicarbazone (1.19%), and oxime-, methoxy-phenyl (1.09%).
3.5 The surface morphology of honeysuckle flower, Baical skullcap root, and Fructus Forsythiae
Scanning electron microscopy analyses showed that the cell walls of Honeysuckle Flower (Figures 5A,B), Baical Skullcap Root (Figures 5C,D), and Fructus Forsythiae (Figures 5E,F) were more broken after fermentation compared to before fermentation.
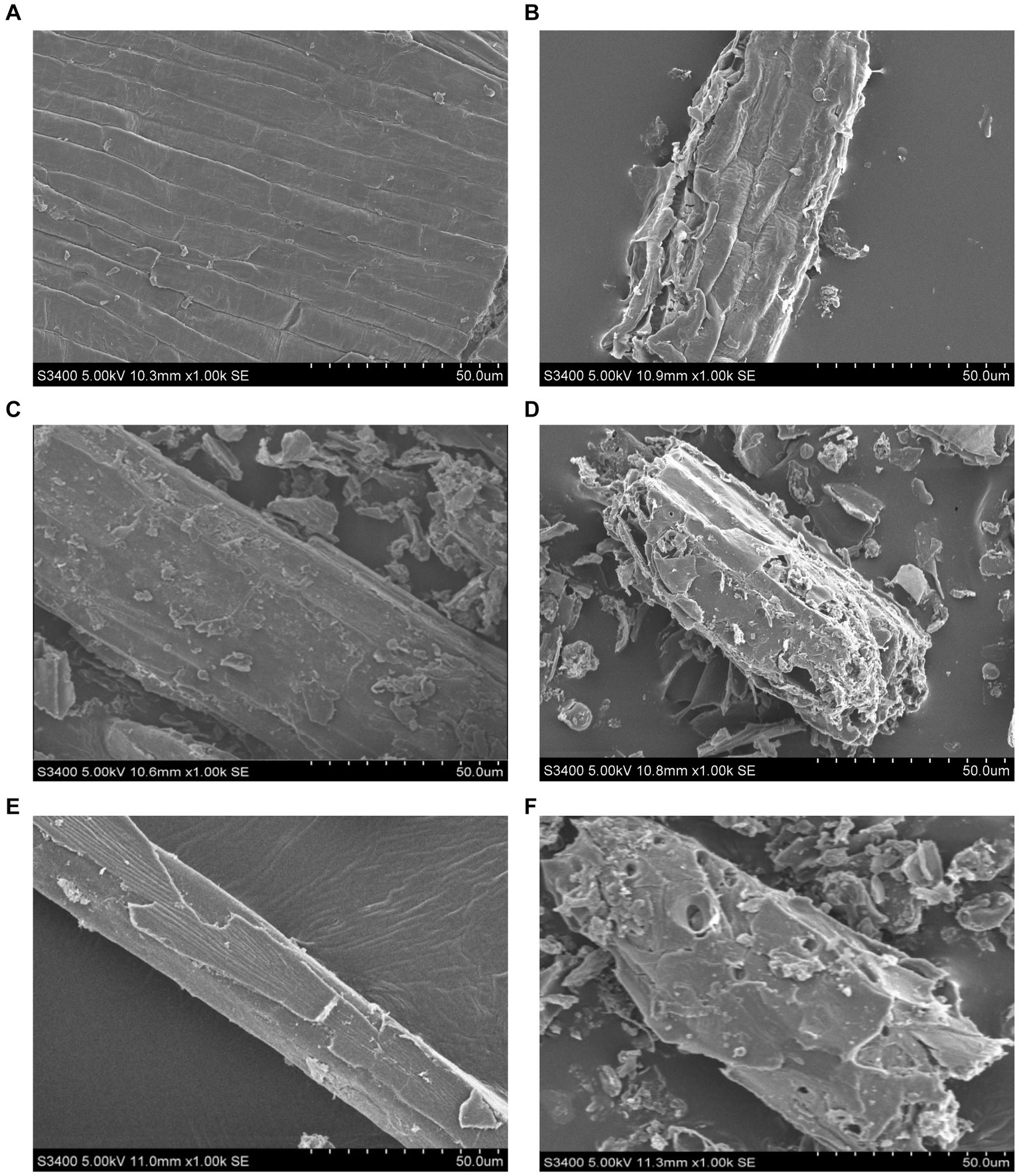
Figure 5. Scanning electron microscopy images of Honeysuckle Flower (A,B), Baical Skullcap Root (C,D), and Fructus Forsythiae (E,F) before and after fermentation.
3.6 Microbiota composition of Shuanghuanglian fermentation liquor
The bacterial composition in SFL is shown in Figure 6. Phyla with relative abundances ≤1.0% were not included in further analyses. Proteobacteria (58.27%) and Firmicutes (27.79%) were the most dominant phyla in SFL, followed by Bacteroidota (5.65%), Deinococcota (2.75%), Actinobacteria (2.13%), and Spirochaetota (2.08%) (Figure 6A). Genera with relative abundances ≤1.0% were excluded from further analysis. Acinetobacter (31.35%) was the most dominant genus in SFL, followed by Porphyrobacter (3.45%), Subdoligranulum (2.80%), Deinococcus (2.75%), Brevundimonas (2.65%), Lactobacillus (2.24%), Sphingomonas (2.15%), Treponema (2.08%), Enhydrobacter (2.08%), Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium (1.91%), Clostridia_UCG-014 (1.89%), Blautia (1.57%), Methylobacterium-Methylorubrum (1.53%), Sphingobium (1.44%), Muribaculaceae (1.39%), Blastomonas (1.24%), Agathobacter (1.10%), Streptococcus (1.08%), and Prevotella (1.01%) (Figure 6B).
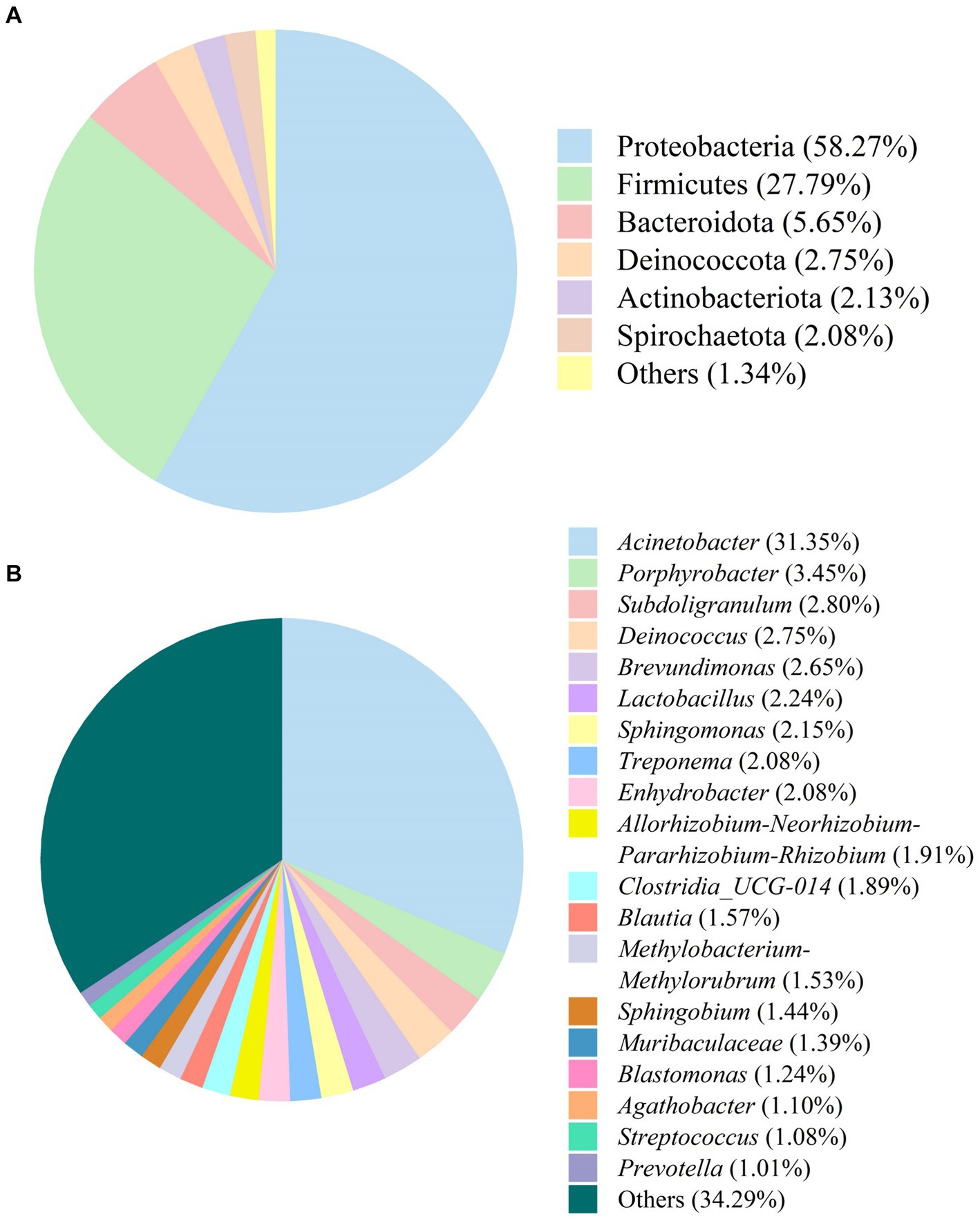
Figure 6. The bacteria composition of Shuanghuanglian fermentation liquor at the phylum (A) and genus (B) levels.
The fungal composition of SFL is shown in Figure 7. Phyla with relative abundances ≤1.0% were not considered for further analyses. The relative abundances of Phragmoplastophyta and Arthropoda in SFL were 97.48 and 1.04%, respectively (Figure 7A). Genera with relative abundances ≤1.0% were not included in further analyses. The relative abundances of Magnoliophyta and Dryopteris in SFL were 91.49 and 5.89%, respectively (Figure 7B).
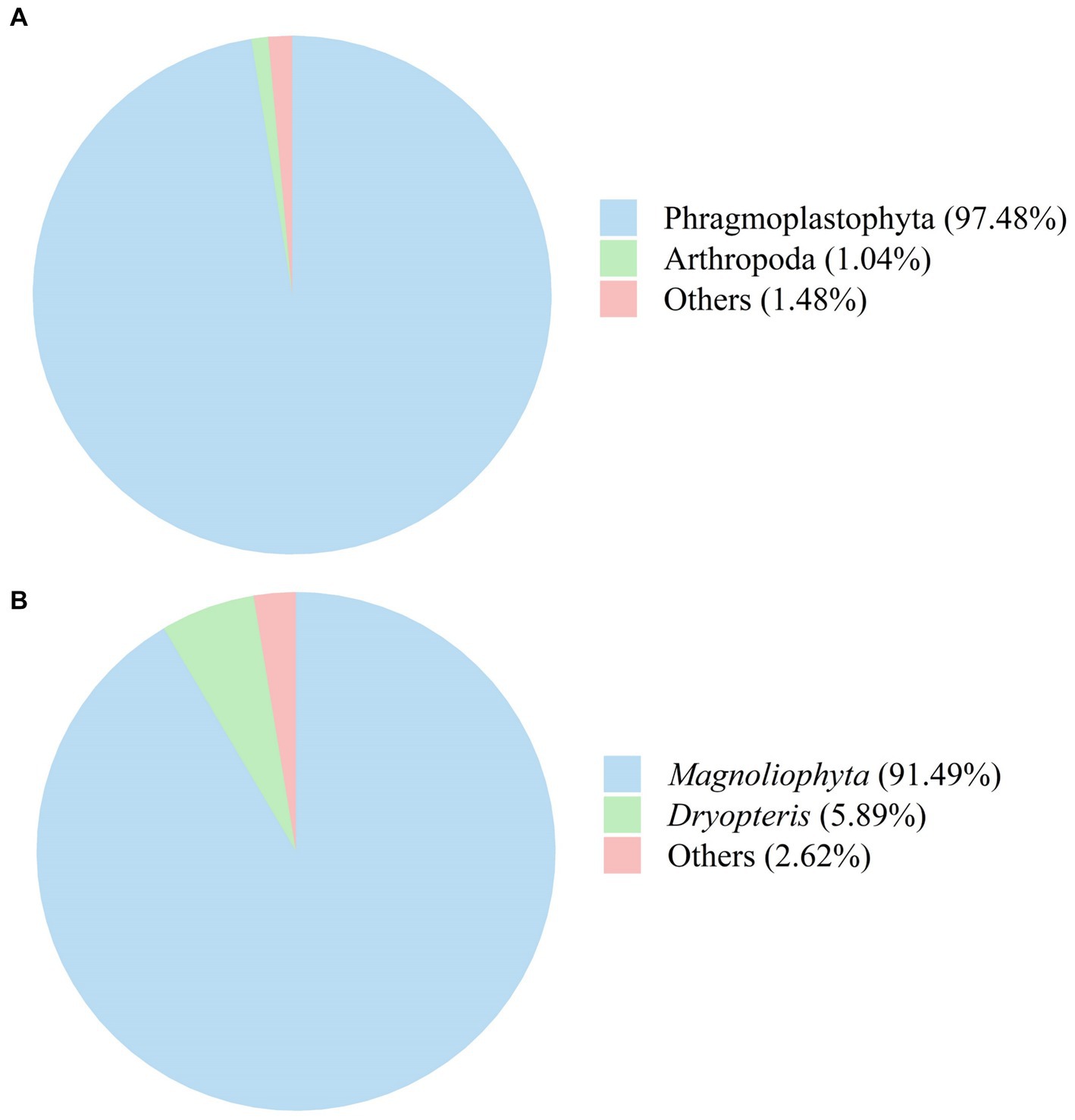
Figure 7. The fungi composition of Shuanghuanglian fermentation liquor at the phylum (A) and genus (B) levels.
3.7 Laying performance and egg quality
The effects of SFL on laying performance and egg quality are presented in Table 8. Supplementation of 0.5 and 0.7% SFL in drinking water increased (p < 0.05) egg weight and laying rate and decreased (p < 0.05) feed-to-egg ratio compared with the control group. However, there was no significant difference (p > 0.05) in the ADFI among different groups.
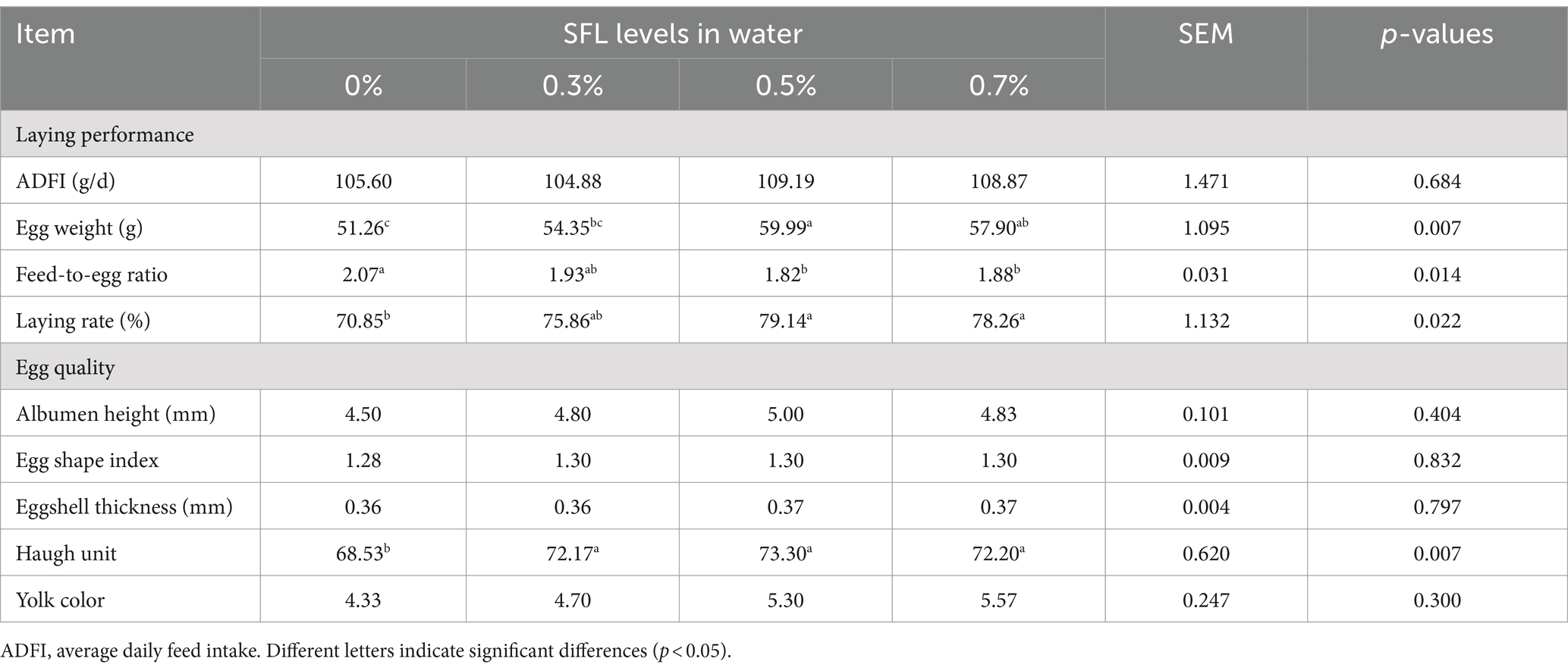
Table 8. Effects of Shuanghuanglian fermentation liquor (SFL) on laying performance and egg quality of laying hens.
Regarding egg quality traits, hens supplemented with 0.3, 0.5, and 0.7% SFL in drinking water had a higher (p < 0.05) Haugh unit than the control group, whereas there were no significant differences (p > 0.05) in albumen height, egg shape index, eggshell thickness, and yolk color among all groups.
4 Discussion
Shuanghuanglian has found to have anti-inflammatory, antibacterial, antiviral, and immunomodulatory effects (1–3). However, there is still a lack of research on the use of Shuanghuanglian in animal husbandry, particularly in the layer industry. In this study, fermentation was conducted with lignin-degrading bacteria (Bacillus amyloliquefaciens-c4) to enhance the bioactive components in Shuanghuanglian. This study optimized the fermentation conditions for Shuanghuanglian, investigated the compounds and microbial composition of SFL, and evaluated its effects on the production performance of laying hens. The results showed that supplementing SFL had positive effects on the production performance of laying hens. The bioactive components present in SFL, such as chlorogenic acid, baicalin, forsythin, myrtenol, 2-hexyn-1-ol, arsenous acid tris(trimethylsilyl) ester, 3(10)-caren-4-ol, and oxime-, methoxy-phenyl, may play a positive role in improving the production performance of laying. These components can also improve the immunity of laying hens due to their antibacterial, antiviral, and anti-inflammatory effects.
Chlorogenic acid, baicalin, and phillyrin are the main bioactive components of Honeysuckle Flower, Baical Skullcap Root, and Fructus Forsythiae (1), and have various biological effects such as antibacterial, antiviral, anti-inflammatory, anti-oxidative, and intestinal health-improving effects in laying hens (14–16). Thus, chlorogenic acid, baicalin, and phillyrin concentrations in SFL were assessed as representative indices to determine the optimal conditions for Shuanghuanglian. Moisture content, inoculation quantity, initial pH, particle size, rotational speed, fermentation time, and fermentation temperature are the main factors that could affect the concentrations of bioactive components present in Chinese medicine after fermentation (11, 17). Thus, in this study, we optimized these fermentation conditions. The concentrations of chlorogenic acid, baicalin, and phillyrin reached the highest values when the following parameters, including solid–liquid ratio, fermentation time, and particle size, were 1:7, 5 days, and 60 mesh. Therefore, the optimized fermentation conditions of solid–liquid ratio, fermentation time, and particle size for Shuanghuanglian were selected as a 1:7 SLR, 5 days, and 60 mesh through single factor experiments. However, Shuanghuanglian fermented with 3% inoculation had higher chlorogenic acid and baicalin concentrations, whereas fermented with 5% inoculation had higher baicalin and phillyrin concentrations in SFL. Shuanghuanglian fermented with 3% inoculation is more cost-effective than the 5% inoculation. Thus, this study used 3% inoculation for Shuanghuanglian fermentation. The optimized fermentation conditions of initial pH, temperature, and rotational speed were 6.99, 27.89°C, and 149.99 r/min, respectively, determined with response surface experiment. Considering the economical production, the optimal fermentation conditions of initial pH value, temperature, and rotational speed were set at 7, 28°C, and 150 r/min, respectively. Finally, the optimized fermentation conditions for Shuanghuanglian were selected as a 1:7 SLR, inoculation quantity 3%, initial pH 7, 60 mesh particle size, rotational speed 150 r/min, and 28°C fermentation for 5 days through single factor and response surface experiments.
We further determined the composition of SFL and identified various compounds with important biological efficacy. Among these compounds, myrtenol has been reported to have antibacterial, antibiofilm, anti-inflammatory, and antinociceptive effects (18, 19). In the present study, SFL contained higher myrtenol content, indicating that SFL may reduce disease incidence and damage to poultry. In addition, 2-hexyn-1-ol contributes to the antibacterial activity (20), and arsenous acid tris(trimethylsilyl) ester is a flavor-related compound (21, 22). Similar to the present study, arsenous acid tris(trimethylsilyl) ester and 3(10)-caren-4-ol have been detected in various Chinese herbal medicines in previous studies and exhibited antibacterial effects (23–26). Moreover, oxime-, methoxy-phenyl is also a flavor-related compound (27) and has been found to have antitumor and anticancer effects (28). Therefore, supplementing SFL to laying hens may increase disease resistance and feed intake, thereby enhancing production performance.
Proteobacteria and Firmicutes were the most abundant phyla in SFL, which is consistent with the bacterial phyla composition of Chinese herbal medicines fermented by Bacillus amyloliquefaciens-c4 (11). Acinetobacter was the most abundant genus detected in SFL and can produce various umami peptides (29). Thus, a higher relative abundance of Acinetobacter in SFL may increase the feed intake in poultry. However, there was no significant change in the abundance of Bacillus in the present study despite inoculation with 3% Bacillus amyloliquefaciens-c4, which is inconsistent with a previous study (11). This inconsistency may be due to Bacillus amyloliquefaciens-c4, being a lignin-degrading bacterium, attaching primarily to the cell wall of Shuanghuanglian rather than releasing it to SFL. This statement is also supported by the more disrupted surface morphology of the Honeysuckle Flower, Baical Skullcap Root, and Fructus Forsythiae after fermentation in the present study. In addition, Phragmoplastophyta was the main fungal phylum, and Magnoliophyta was the main fungal genus in SFL. The findings are consistent with the findings of Yi et al. (11), who reported that Chinese herbal medicines fermented with Bacillus amyloliquefaciens-c4 had higher abundances of Phragmoplastophyta and Magnoliophyta. The genera Magnoliophyta and Dryopteris belong to the phylum Phragmoplastophyta and exist widely in the air and soil environments (30), which may explain their abundance in SFL.
To evaluate the effects of SFL, different levels of SFL were supplemented with drinking water in laying hens. The results showed that 0.5 and 0.7% SFL supplementation increased egg weight and laying rate while decreasing the feed-to-egg ratio of laying hens. The increased production performance of laying hens is possibly due to the antibacterial and anti-inflammatory effects of SFL. In addition, supplementation of SFL increased the Haugh units of eggs, indicating that the bioactive components present in SFL improved the albumen and storage quality of eggs (31, 32). Wang et al. (14) also found that supplementation of Honeysuckle Flower and Baical Skullcap Root extracts in laying hens’ diet alleviated intestinal disorders and performance impairment caused by Salmonella pullorum infection. Interestingly, SFL supplementation had no effects on the ADFI of laying hens, although it included various flavor-related compounds, which may have been caused by the addition of SFL in drinking water rather than in the diet. Furthermore, supplementation of 0.3% SFL had no effects on the laying performance of hens, indicating that SFL supplementation in drinking water should be at least 0.5%. Overall, the findings indicate that SFL supplementation had beneficial effects on the production performance of laying hens, especially when supplementation reached 0.5%.
5 Conclusion
The optimal fermentation conditions for Shuanghuanlian were determined as follows: a SLR ratio of 1:7, 3% inoculation quantity, fermentation at 28°C for 5 days, initial pH of 7, a 60 mesh particle size, and a rotational speed of 150 r/min. These conditions are crucial in ensuring consistent product quality and composition for future testing and application. Supplementing at least 0.5% SFL in drinking water had positive effects on the production performance of laying hens, including increased egg weight, laying rate, and Haugh unit and decreased feed-to-egg ratio, which indicate that SFL based on the optimized fermentation conditions could positively influence the production performance of laying hens. Further in-depth studies are necessary to explore the long-term effects of SFL, the mechanisms of bioactive components in SFL, and the combination effects with other feed additives or drugs on the production performance of laying hens. Overall, these findings provide a solid foudation for further investigation into the use of Shuanghuanglian as a natural feed addition to improve the productivity and sustainability of egg production.
Data availability statement
The data presented in the study are deposited in the Figshare repository, accession number is doi: 10.6084/m9.figshare.27050017.v1.
Ethics statement
The animal study was approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China (ISA-2018-071). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YX: Data curation, Formal analysis, Visualization, Writing – review & editing. SY: Formal analysis, Visualization, Writing – original draft. XX: Data curation, Methodology, Writing – review & editing. MZ: Data curation, Methodology, Writing – review & editing. YC: Methodology, Writing – review & editing. WL: Methodology, Writing – review & editing. FL: Conceptualization, Funding acquisition, Writing – review & editing. XK: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was jointly supported by the City-School Cooperation Project of the Special Funds of Science and Technology in Fuyang City undertaken by Fuyang Normal University (SXHZ2020007), the Project of Provincial Research Institutes of Scientific Research Business Fee in Heilongjiang Province (CZKYF2023-1-B020), National Natural Science Foundation of China (U22A20443), and Key R&D projects in Heilongjiang Province (GY2023ZB0021).
Acknowledgments
We would like to thank the staff and postgraduate students from the College of Life Science, Northeast Agricultural University for collecting samples and Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. ChP. Pharmacopoeia of the People’s Republic of China. Part I, vol. 2015. Beijing, China: China Medical Science Press (2015).
2. Su, HX, Yao, S, Zhao, WF, Li, MJ, Liu, J, Shang, WJ, et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin. (2020) 41:1167–77. doi: 10.1038/s41401-020-0483-6
3. Gao, Y, Liu, L, Li, C, Liang, YT, Lv, J, Yang, LF, et al. Study on the antipyretic and anti-inflammatory mechanism of Shuanghuanglian oral liquid based on gut microbiota-host metabolism. Front Pharmacol. (2022) 13:843877. doi: 10.3389/fphar.2022.843877
4. Tang, Y, Wang, Z, Huo, C, Guo, X, Yang, G, Wang, M, et al. Antiviral effects of Shuanghuanglian injection powder against influenza a virus H5N1 in vitro and in vivo. Microb Pathog. (2018) 121:318–24. doi: 10.1016/j.micpath.2018.06.004
5. Yang, L, Ma, L, Guo, S, Xu, Q, Shen, Z, Wang, Y, et al. Effect of Shuanghuanglian propolis oral liquid on immune function of broilers. Anim Husbandry Feed Sci. (2016) 8:29–30. doi: 10.19578/j.cnki.ahfs.2016.01.009
6. Song, Z, Cheng, K, Zhang, L, and Wang, T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J Therm Biol. (2017) 69:184–90. doi: 10.1016/j.jtherbio.2017.07.015
7. Sarlak, S, Tabeidian, SA, Toghyani, M, Shahraki, ADF, Goli, M, and Habibian, M. Supplementation of two sources and three levels of iodine in the diet of laying hens: effects on performance, egg quality, serum and egg yolk lipids, antioxidant status, and iodine accumulation in eggs. Ital J Anim Sci. (2020) 19:974–88. doi: 10.1080/1828051X.2020.1810142
8. Sarlak, S, Tabeidian, SA, Toghyani, M, Shahraki, ADF, Goli, M, and Habibian, M. Effects of replacing inorganic with organic iron on performance, egg quality, serum and egg yolk lipids, antioxidant status, and iron accumulation in eggs of laying hens. Biol Trace Elem Res. (2021) 199:1986–99. doi: 10.1007/s12011-020-02284-8
9. Esfahani, DA, and Goli, M. The effect of Stevioside-Isomalt, whey protein concentrate, and Bacillus coagulans on the physicochemical and sensory properties of Iranian probiotic Masghati sweet. J Food Process Pres. (2022) 46:e16028. doi: 10.1111/jfpp.16028
10. Li, N, Du, YT, Ma, X, and Li, DM. Research progress in chromatographic fingerprints and spectrum-effect relationships of Shuanghuanglian. J Capital Normal Univ. (2021) 42:88–96. doi: 10.19789/j.1004-9398.2021.02.014
11. Yi, S, He, F, Azad, MAK, Zhu, Q, Zhang, M, Xu, X, et al. Optimal fermentation of Artemisia annua residues and its effects on production performance of laying hens. Fermentation. (2023) 9:456. doi: 10.3390/fermentation9050456
12. AOAC International ed. Official methods of analysis. 18th ed. Gaithersburg, MD, USA: AOAC International (2005).
13. Mohammadabadi, SS, Goli, M, and Tabasi, SN. Optimization of bioactive compound extraction from eggplant peel by response surface methodology: Ultrasound-assisted solvent qualitative and quantitative effect. Food Secur. (2022) 11:3263. doi: 10.3390/foods11203263
14. Wang, WW, Jia, HJ, Zhang, HJ, Wang, J, Lv, HY, Wu, SG, et al. Supplemental plant extracts from Flos lonicerae in combination with Baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front Microbiol. (2019) 10:1681. doi: 10.3389/fmicb.2019.01681
15. Chen, F, Zhang, H, Zhao, N, Yang, X, Du, E, Huang, S, et al. Effect of chlorogenic acid on intestinal inflammation, antioxidant status, and microbial community of young hens challenged with acute heat stress. Anim Sci J. (2021) 92:e13619. doi: 10.1111/asj.13619
16. Zhou, C, Lu, M, Cheng, J, Rohani, ER, Hamezah, HS, Han, R, et al. Review on the pharmacological properties of phillyrin. Molecules. (2022) 27:3670. doi: 10.3390/molecules27123670
17. Wang, S, Lu, L, Song, T, Xu, X, Yu, J, and Liu, T. Optimization of Cordyceps sinensis fermentation Marsdenia tenacissima process and the differences of metabolites before and after fermentation. Heliyon. (2023) 8:e12586. doi: 10.1016/j.heliyon.2022.e12586
18. Gomes, BS, Neto, BPS, Lopes, EM, Cunha, FVM, Araujo, AR, Wanderley, CWS, et al. Anti-inflammatory effect of the monoterpene myrtenol is dependent on the direct modulation of neutrophil migration and oxidative stress. Chem Biol Interact. (2017) 273:73–81. doi: 10.1016/j.cbi.2017.05.019
19. Cordeiro, L, Figueiredo, P, Souza, H, Sousa, A, Andrade-Junior, F, Barbosa-Filho, J, et al. Antibacterial and antibiofilm activity of myrtenol against staphylococcus aureus. Pharmaceuticals. (2020) 13:133. doi: 10.3390/ph13060133
20. Charles, JI, and Minakiri, IS. Evaluation of the antibacterial activity of Chromolaena Odorata in wistar rats and its chemical characterization. Am Sci Res J Eng Technol Sci. (2018) 42:111–29.
21. Nan, L, Guo, M, Chen, S, Li, Y, Cui, C, Song, Y, et al. Effect of peduncle on aroma of cabernet sauvignon dry red wine. AIP Conference Proceedings. (2019) 2079:020008. doi: 10.1063/1.5092386
22. Barido, FH, Kim, HJ, Shin, DJ, Kwon, JS, Kim, HJ, Kim, D, et al. Physicochemical characteristics and flavor-related compounds of fresh and frozen-thawed thigh meats from chickens. Food Secur. (2022) 11:3006. doi: 10.3390/foods11193006
23. Habibi, Z, Ghanian, S, Ghasemi, S, and Yousefi, M. Chemical composition and antibacterial activity of the volatile oil from seeds of Artemisia annua L. from Iran. Nat Prod Res. (2013) 27:198–200. doi: 10.1080/14786419.2012.662652
24. Hamedi, A, Pasdaran, A, and Pasdaran, A. Antimicrobial activity and analysis of the essential oils of selected endemic edible apiaceae plants root from Caspian Hyrcanian region (north of Iran). Pharm Sci. (2019) 25:138–44. doi: 10.15171/PS.2019.21
25. Navis, MS, and Saroja, RM. Studies on the isolation, identification and antibacterial activity of bioactive compound from Andrographis paniculata leaf extract. Ann Rom Soc Cell Bio. (2022) 26:3580–93.
26. Singh, A, Rai, G, Kumar, A, and Gautam, DNS. Pharmacognostical assessment, antioxidant ability and determination of phytomolecules using GC-MS in Gloriosa superba Linn. ResearchSquare. (2023) 3:20608. doi: 10.21203/rs.3.rs-2620608/v1
27. Sha, K, Zhang, ZJ, Sun, BZ, Li, HP, Song, HL, Lang, YM, et al. Investigation of physicochemical and textural characteristics and volatile compounds of Kazakh dry-cured beef. Food Sci Technol Res. (2017) 23:375–83. doi: 10.3136/fstr.23.375
28. Chipps, ES, Jayini, R, Ando, S, Protzman, AD, Muhi, M, Mottaleb, MA, et al. Cytotoxicity analysis of active components in bitter melon (Momordica charantia) seed extracts using human embryonic kidney and colon tumor cells. Nat Prod Commun. (2012) 7:1203–8. doi: 10.1177/1934578X1200700926
29. Yang, D, Li, C, Li, L, Wang, Y, Wu, Y, Chen, S, et al. Novel insight into the formation mechanism of umami peptides based on microbial metabolism in Chouguiyu, a traditional Chinese fermented fish. Food Res Int. (2022) 157:111211. doi: 10.1016/j.foodres.2022.111211
30. An, F, Sun, H, Wu, J, Zhao, C, Li, T, Huang, H, et al. Investigating the core microbiota and its influencing factors in traditional Chinese pickles. Food Res Int. (2021) 147:110543. doi: 10.1016/j.foodres.2021.110543
31. Lee, AR, Niu, KM, Lee, WD, Kothari, D, and Kim, SK. Comparison of the dietary supplementation of Lactobacillus plantarum, and fermented and non-fermented Artemisia annua on the performance, egg quality, serum cholesterol, and eggyolk-oxidative stability during storage in laying hens. Braz J Poult Sci. (2019) 21:1–8. doi: 10.1590/1806-9061-2018-0903
Keywords: bioactive compounds, egg quality, optimized fermentation, response surface methodology, Chinese medicine
Citation: Xu Y, Yi S, Xu X, Zhang M, Cui Y, Lan W, Li F and Kong X (2024) Optimal fermentation of Shuanghuanglian and its effects on production performance of laying hens. Front. Vet. Sci. 11:1415232. doi: 10.3389/fvets.2024.1415232
Edited by:
Moyosore Joseph Adegbeye, University of Africa, NigeriaReviewed by:
Xiaolong Ji, Zhengzhou University of Light Industry, ChinaRanran Hou, Qingdao Agricultural University, China
Copyright © 2024 Xu, Yi, Xu, Zhang, Cui, Lan, Li and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenglan Li, bGlmZW5nbGFuQG5lYXUuZWR1LmNu; Xiangfeng Kong, bm5reGZAaXNhLmFjLmNu
†These authors have contributed equally to this work
 Yongqing Xu1†
Yongqing Xu1† Xiangfeng Kong
Xiangfeng Kong