- 1ICMR-National Institute of Cholera and Enteric Diseases, Kolkata, India
- 2AMR Expert, Food and Agriculture Organization, New Delhi, India
The application of antibiotics in the poultry and veterinary sectors is very common practice in India. Owing to the seriousness of antimicrobial resistance (AMR), the present study has illustrated the overall scenario of AMR in the poultry and veterinary sectors in India through an in-depth scoping review and key informant interview (KII). In the poultry sector, most of the studies reviewed have reported resistant bacteria isolated from chicken meat, eggs, cloacal swabs, and fecal samples, and only a few have reported the presence of resistant bacteria in and around the environment of poultry farms. The major resistant bacteria that have been reported are E. coli, Salmonella spp., S. aureus, Campylobacter jejuni, and K. pneumoniae. These bacterial isolates exhibited resistance to various antibiotics, such as azithromycin (21.43%), tetracycline (11.30–100%), chloramphenicol (4.76–100%), erythromycin (75–83.33%), ciprofloxacin (5.7–100%), gentamicin (17–100%), amikacin (4.76%), cotrimoxazole (42.2–60%), trimethoprim (89.4%), ceftriaxone (80%), and cefotaxime (14.29–70%). Like the poultry sector, different antibiotics are also used for treating clinical and subclinical bovine mastitis, which is one of the major problems plaguing the dairy sector. Several AMR bacterial strains, such as E. coli, Staphylococcus aureus, S. epidermidis, and Klebsiella pneumoniae, have been reported by many researchers and showed resistance against tetracycline (74%), oxytetracycline (47.37%), ciprofloxacin (51%), streptomycin (57.89%), cephalosporin (100%), and trimethoprim (70%). The KIIs have revealed several reasons behind these AMR scenarios, of which the growing need for the production of food animals and their products with inadequate infrastructure and a lack of proper knowledge on farm management among the farmers are the major ones. Though several government legislations and policies have been laid down, proper implementation of these policies, strict surveillance on antibiotic application in the poultry and veterinary sectors, awareness generation among farmers, and infrastructure development can help minimize the development and transmission of AMR bacteria within and from these sectors.
1 Introduction
AMR is a global public health concern and is far more complex to address due to its multifactorial nature. Though the containment measures were focused on the human health sector, off late, the environmental factors contributing to the development of AMR, particularly in developing countries have been appreciated and the need for interventions in the context of One Health approach has been felt. The One Health approach toward mitigating AMR is also important to understand its complete phenomenology. Irrational antimicrobial uses in the human health sector, along with those in the veterinary and agriculture sectors, contribute heavily to the development of resistant organisms. The veterinary sector emerged as an important area of intervention in terms of AMR containment due to the increased incidence of identification of antibiotic-resistant bacteria from different specimens of sick farm animals. Usually, in developing countries, sick farm animals are treated without consulting veterinarians and as a result, they are often misdiagnosed and wrongly treated. Another pressing issue is the application/feeding of antibiotics to farm animals in sub-therapeutic doses. Moreover, not only in episodes of sickness, farm animals are been fed antibiotics as growth promoters to meet the needs of food animals in these highly populous countries. It is projected that by 2030 the global consumption of antibiotics in animal husbandry could be approximately 200,235 tons (1), and BRICS countries (Brazil, Russia, India, China, and South Africa) would experience a 99% rise in their usage during the same period (2). This shift from extensive to intensive farming in this sector involves high usage of antibiotics. Even the identification of resistant bacteria from farm environments has become common in LMICs. Frequent exposure to low dosages of antimicrobials results in favorable conditions for the emergence of antimicrobial-resistant bacteria (ARB) in animals (2). Inappropriate application dosages of antibiotics in food animals, non-adherence to prescription (3), over-the-counter (OTC) sale of antibiotics, and failure to identify drug withdrawal period in animal food (4) enhance the emergence, spread, and contamination of ARB in food animals, which can be disseminated to human either through contact with animals/food of animal origin directly or indirectly through AMR polluted environment (5).
Very recently a group of researchers conducted a strengths, weaknesses, opportunities, and threats (SWOT) Analysis on existing policies/guidelines/regulations/legislation in India (6) related to AMR containment in India and identified several opportunities in all the contributing sectors In this communication, we attempted to examine extensive empirical pieces of evidence from different parts of India to summarize knowledge on the magnitude and pattern of resistance of AMR bacteria in the livestock sector. In addition, we tried to find out better ways to cultivate animal food while minimizing the adverse impact on human health due to antibiotic-resistant pathogens through KII. We also tried to reflect on the problem of antibiotic use in animal farming as a contributor to the Global Problem of AMR from the perspective of One Health.
2 Methodology of the study
2.1 Study design
We conducted a sequentially explanatory mixed-method study.
2.2 Study duration
The research was carried out from April 2021 to March 2022.
2.3 Study population
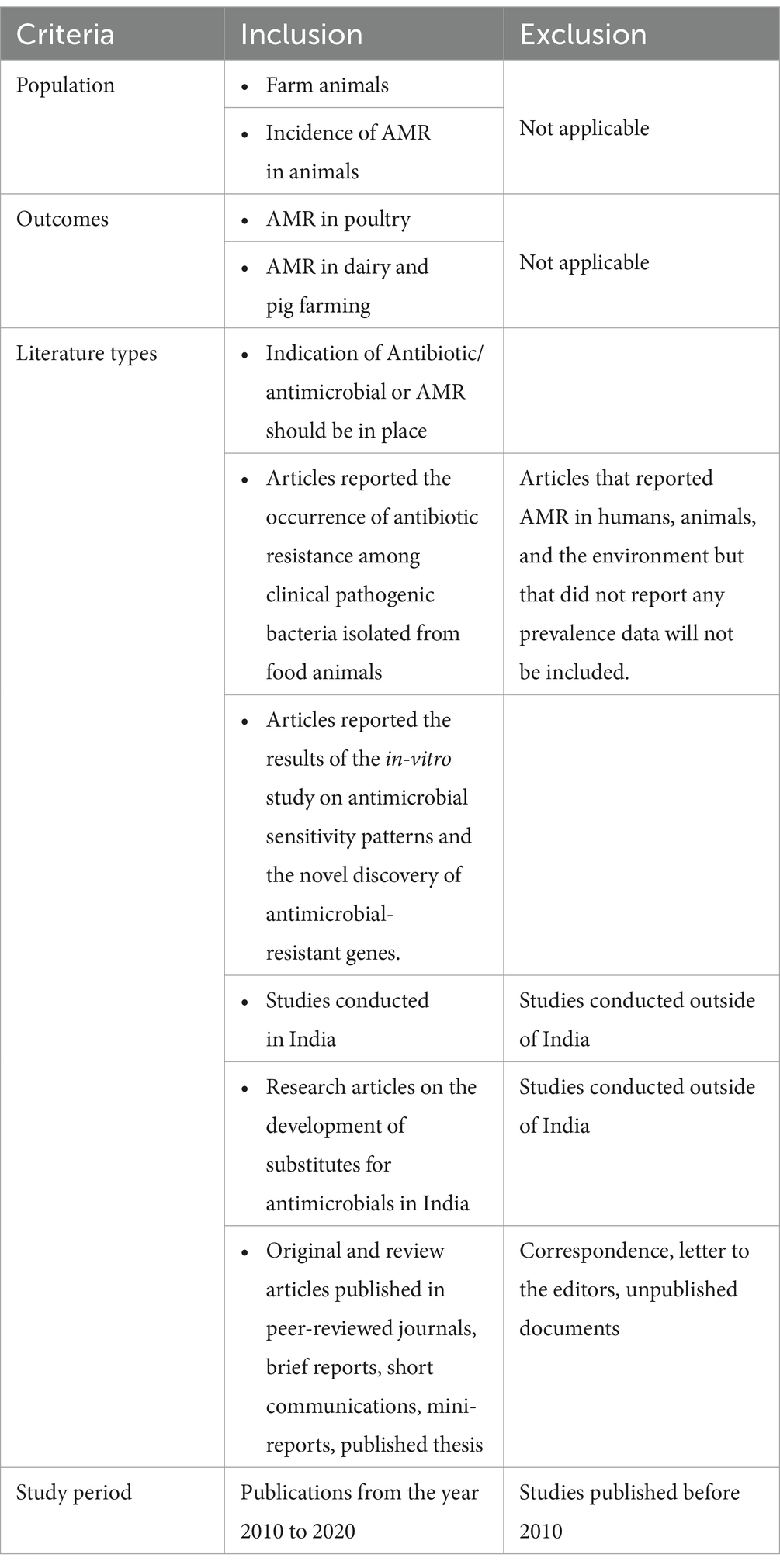
The articles on incidents and magnitude of AMR in the poultry/dairy/piggery sector in India, the use of antimicrobials, critically important antimicrobials in animal husbandry, and the implications of those antimicrobials on the environment published during 2010–2020 were included. KIIs were carried out with stakeholders of different strata in the animal husbandry sector.
2.4 Search string
(((((((((((antimicrobials) OR antibiotics) AND resistance) OR livestock rearing) OR poultry farming) OR cattle farming) OR dairy farming) OR swine farming) OR food animals) AND environment) AND India).
2.5 Literature review
A scoping review of the literature was conducted to map the available research on AMR based on the developed criteria (Table 1), followed by the development of a KII Guide for addressing the identified gaps. The following electronic databases of published literature were searched for relevant studies: PubMed/Medline, Google Scholar, Scopus, Embase, and Index Medicus, following strict inclusion and exclusion criteria. The synthesized literature was organized by using a PRISMA flow diagram (Figure 1). We considered 57 research articles in this study.
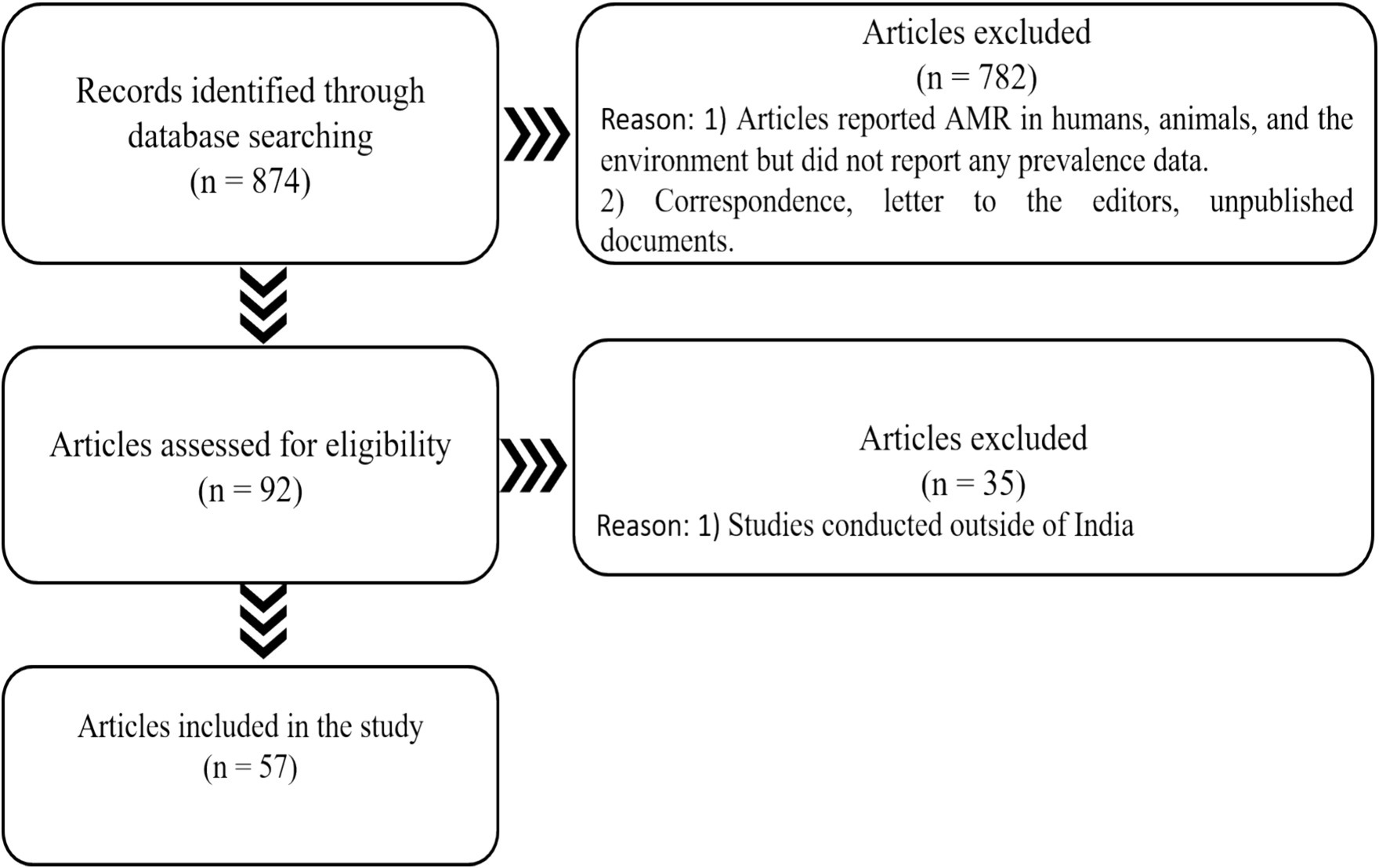
Figure 1. PRISMA displaying the number of papers searched, excluded, and included in the current study.
2.6 Interview with stakeholders
For conducting KIIs in the animal husbandry sector, stakeholders were chosen based on their proficiency in their field and readiness to share information. A total of three KIIs (two veterinarians and one extension officer) were conducted with an interview guide, which contained open questions with probes in the following segments: existing scenario of antibiotic application on farm animals; factors influencing AMR; ways to contain AMR in animal farming in India; and best practices in India to substitute antimicrobial application on animal farming. Each interview was audio-recorded and transcribed into text data in the local language first, then back-translated to English by the separate investigator. Afterward, all transcriptions were organized systematically and coded based on the predetermined gaps of the scoping review. The distinct codes were then assembled by removing redundancy to develop the key themes.
3 Results
3.1 Antimicrobial resistance in poultry in India
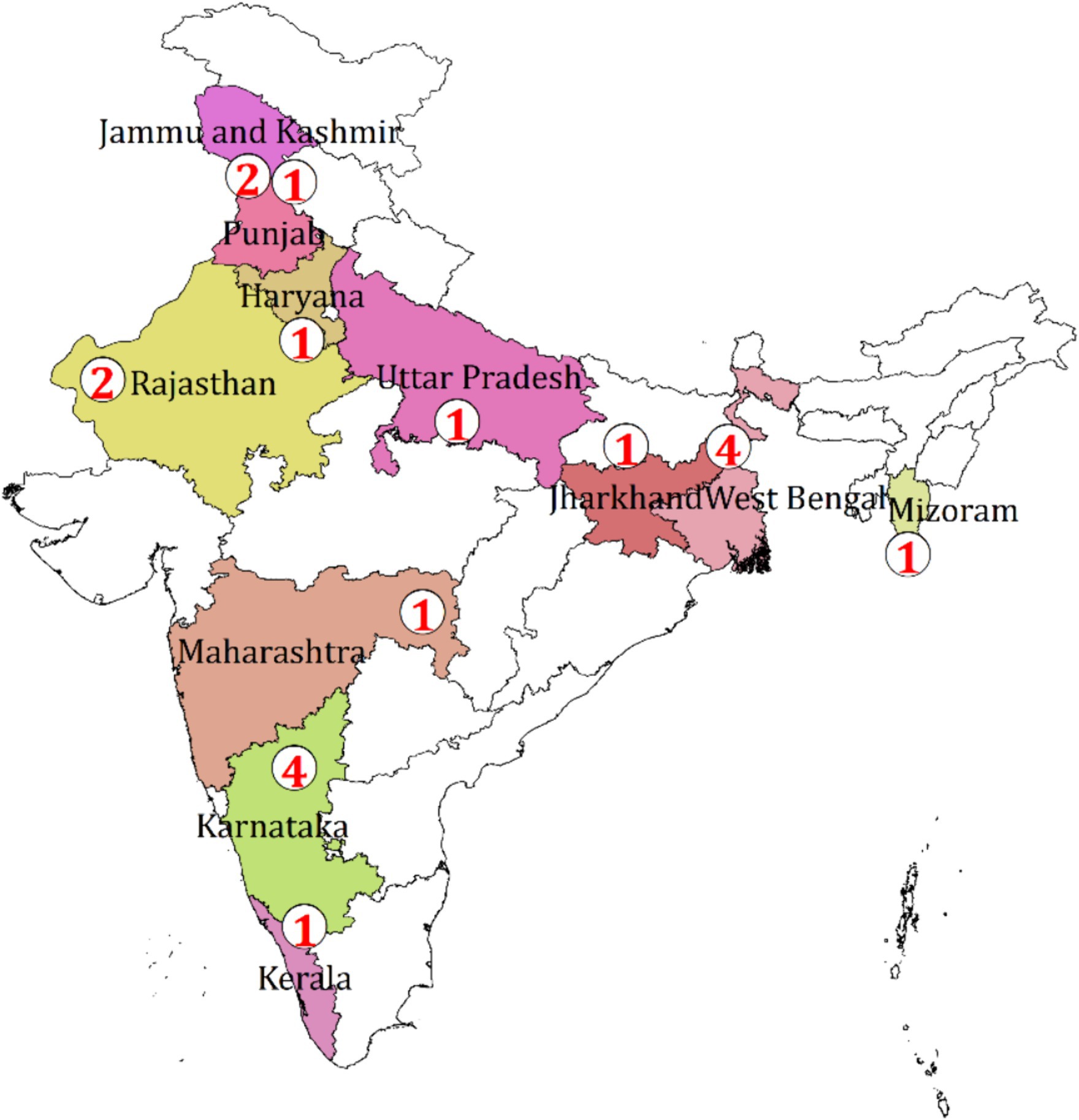
Among the available original research, most of them are concerned about bacteria collected from the samples of poultry birds including meat, eggs, cloacal swabs, and fecal samples, and few of them reported the presence of resistant bacteria in the drinking water of the farm. The spatial distribution of the study shows the evidence generated from almost all regions of India (Figure 2).

Figure 2. Spatial distribution of the number of studies on AMR from the poultry sector in India, 2010–2020.
Reported studies identified Salmonella sp. from various chicken organs in Kerala which was completely resistant to the antibiotic erythromycin. Singh et al. (15) discovered the prevalence of a resistant strain of Salmonella typhimurium with 100% resistance to clindamycin, oxacillin, penicillin, and vancomycin in the cloacal swab, feed, water, eggs, and feces of chickens in Bareilly, Uttar Pradesh. Studies carried out in states such as Tamil Nadu (7) and Kolkata (8) generated increased evidence of bacteria from poultry samples demonstrating high resistance to broad-spectrum antibiotics such as tetracycline, nalidixic acid, ciprofloxacin, levofloxacin, ofloxacin, and sulfamethoxazole-trimethoprim (Figure 3). Several genes have also been reported which are responsible for developing resistance among the bacterial strains. In Salmonella sp. tetA and tetB, blaTEM and CTX-M genes were reported to develop resistance against tetracycline, broad-spectrum β-lactamases, and β-lactamases with extended-spectrum, respectively (9). Similarly, for E. coli isolates some β-lactam genes such as blaTEM, blaCTX-M, and blaOXA were reported which showed resistance against a wide range of commonly used antibiotics such as nalidixic acid, trimethoprim, tetracycline, colistin, and ciprofloxacin, including β-lactam antimicrobials such as amoxicillin and ampicillin (10).
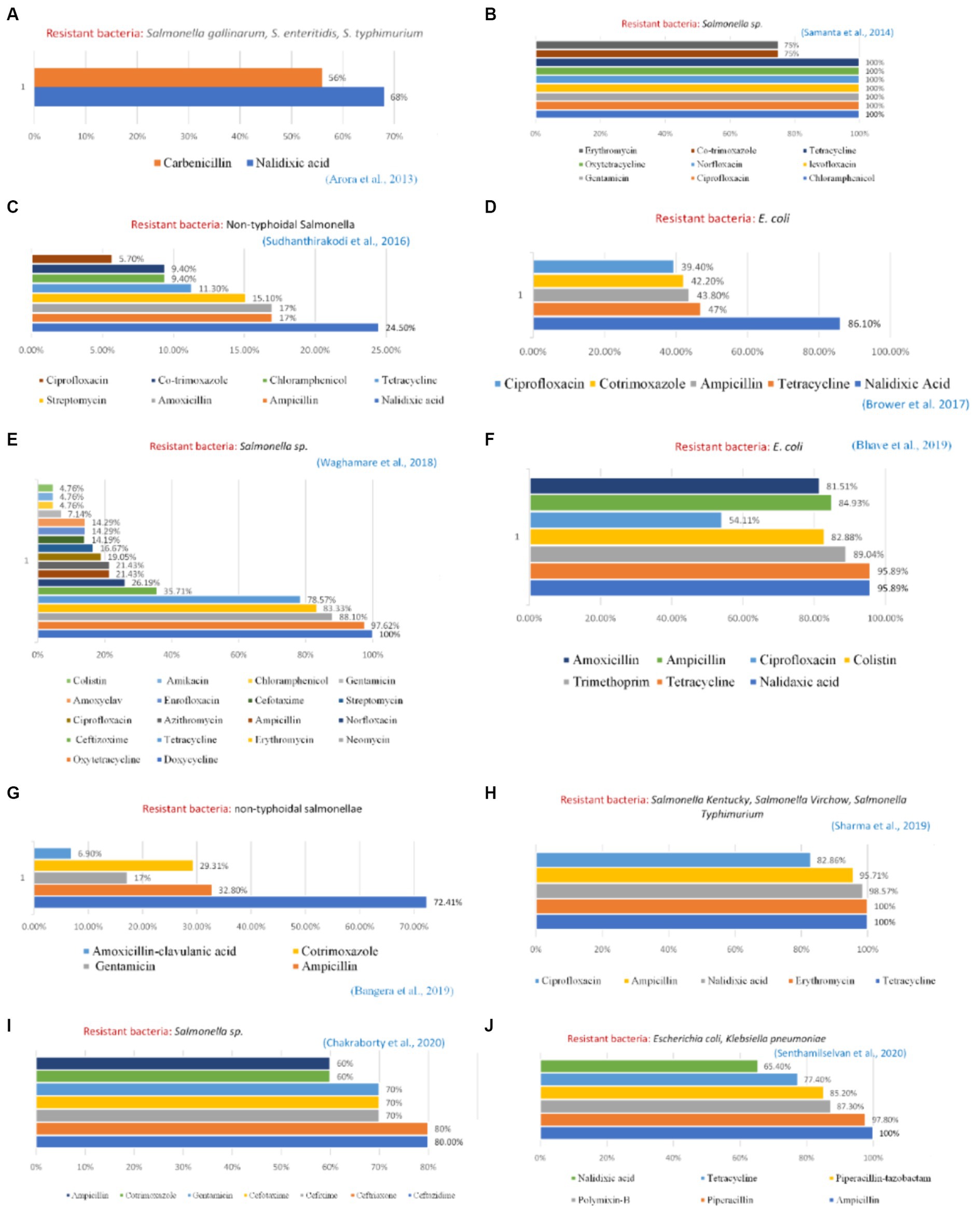
Figure 3. Profile of resistant bacteria from the poultry sector, India, 2010–2020. Amr instances reported from — (A) Haryana (B) West Bengal (C) West Bengal (D) Punjab (E) Maharashtra (F) Maharashtra (G) Karnataka (H) Uttrakhand and Uttar Pradesh (I) Mizoram (J) Tamil Nadu.
3.2 Antimicrobial resistance (AMR) in dairy and piggery in India
Figure 4 shows the distribution of the total number of collected original studies on AMR published in the dairy and piggery sectors in India. Mastitis has been the primary focus of most of the research on AMR in the dairy industry because of evidence of bacterial-resistant strains of mastitis in cow milk.
Escherichia coli resistance was reported by Thaker et al. (11) to ampicillin, streptomycin, and oxytetracycline (100, 57.89, and 47.37%, respectively) in cow milk. The presence of antimicrobial-resistant genes (ARGs) in bovine mastitis pathogens was the prime cause of such resistance. Bandyopadhyay et al. (12) in a West Bengal-based study explained intra-mammary infection of methicillin-resistant Staphylococcus epidermidis, Staphylococcus aureus, and ESBL-producing Escherichia coli in two Holstein Friesian (crossbred cows) with subclinical mastitis. West Bengal and Mizoram generated Klebsiella pneumonia in the milk of healthy cows having clinical and subclinical mastitis that are completely resistant to third-generation cephalosporin, 70% to sulpha/trimethoprim combination, 74% to tetracycline, and 51% to ciprofloxacin and piperacillin. In a study from West Bengal, S. aureus isolated from raw milk samples was found resistant to vancomycin (VRSA) (13). Besides slaughterhouse samples from Kerala, Mizoram, and West Bengal also reported increased resistant S. aureus. Several genes cause bovine mastitis, including β-lactamase genes (blaCTX-M, blaTEM, and blaSHV), plasmid-mediated quinolone resistance gene s (qnrS and qnrB), and sulfonamide resistance gene (sul1) (14).
3.3 Use of critically important antimicrobials in animal husbandry in India and their environmental implication
Critically important antimicrobials are those that are very frequently used for the treatment of both human and animal diseases, resulting in the emergence of ARGs, which eventually lose their effectiveness in treating people (16). The Critically Important Antimicrobials used for animal and human health issued by World Organisation for Animal Health (WOAH) and WHO (World Health Organization), has overlap of 47 antimicrobials. Many of the resistant bacteria predominant in animal reservoirs contaminate the environment through different pathways, can spread to humans, and pose a major threat to human health. The Centre for Science and Environment, Delhi, has made a list of critically important antimicrobials as well, administrated in animal husbandry in India (17).
3.4 Research conducted in India for developing alternatives to antibiotics application on food-producing animals
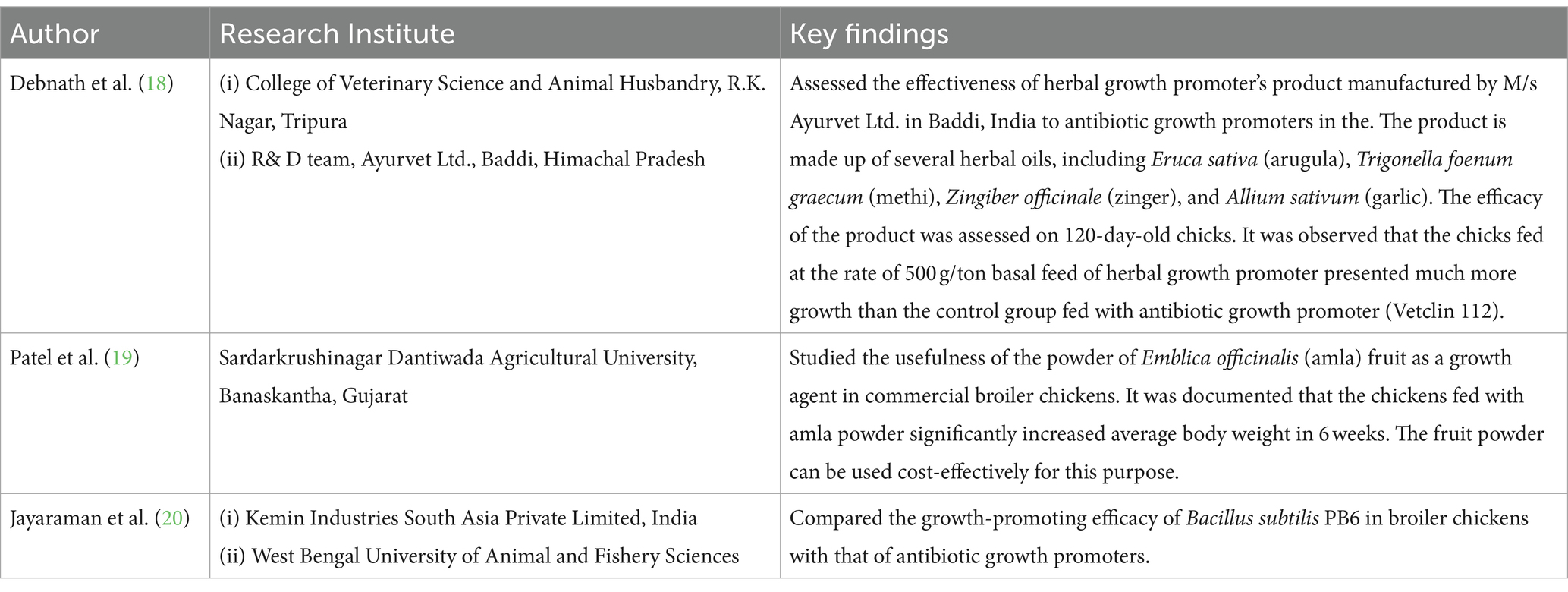
Various research institutes have attempted to develop antibiotic alternatives for producing animal food. Some salient findings of such research are summarized in Table 2.
3.5 Key informant interview (KII)
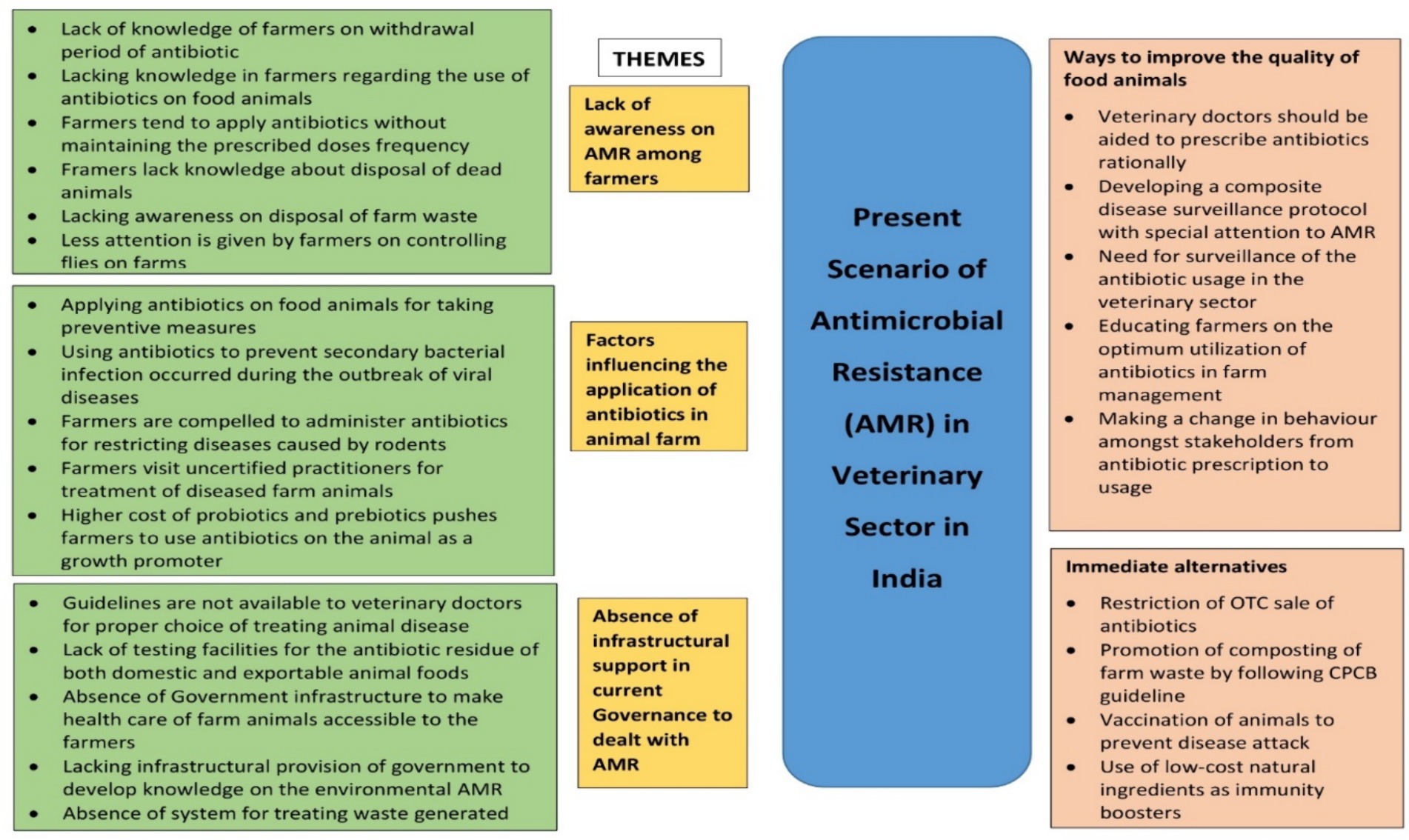
Lack of awareness of AMR among farmers, factors influencing the application of antibiotics in animal farms, and the absence of infrastructural support in current governance to deal with AMR emerged to be the major themes. These contributed immensely to the emergence and transmission of AMR bacteria in animals. Apart from having very limited awareness of the withdrawal period of antibiotics and application of them in appropriate doses farmers hardly had knowledge of farm management such as disposal of dead animals and farm waste and controlling of files. Inappropriately adopting preventive measures, restricting secondary bacterial diseases during viral outbreaks, visiting uncertified doctors to treat aliments of farm animals, preventing diseases caused by rodents, and excessive price of probiotics and prebiotics were identified as contributory factors. The stakeholders also pointed out the prerequisite of government infrastructural support including the need for guidelines to veterinarians for treating animal diseases, testing opportunities for antibiotic residue in animal food, provision by government in the development of knowledge on environmental AMR, inaccessibility of farmers to animal healthcare setup, and absence of west management in animal farms to mitigate problem such as AMR. According to the interviewees, some scopes could be created to tackle the spreading of AMR such as encouraging veterinaries to prescribe antibiotics rationally, developing a stringent disease surveillance protocol exclusively on AMR as well as the application of antibiotics in the veterinary sector, and awareness generation among farmers on the optimization of antibiotic administration in farm and making a desirable change in behavior among stakeholders toward antibiotics. To minimize the impact of AMR on the human body, some suitable alternatives were also suggested by the stakes such as checking of OTC sale of antibiotics, management of farm waste following the Central Pollution Control Board (CPCB), proper vaccination of animals, and utilization of cost-effective natural immunity boosters (Figure 5).
4 Discussion
The findings of the present study indicated the current scenario of AMR in the veterinary sector in India. A wide range of commonly used antibiotics such as tetracycline, erythromycin, gentamicin, chloramphenicol, ciprofloxacin, cotrimoxazole, amikacin, trimethoprim, ceftriaxone, and cefotaxime is rampantly used by the farmers in the veterinary sector. The use of antibiotic-based growth promoters in animal feed in India is a very common practice but often the farmers remain unaware of the presence of antimicrobials in the purchased feeds. Lack of proper knowledge of the farmers on antimicrobial usage is often misused by the agents and sales personnel of the different antimicrobial manufacturing companies promoting the use of antimicrobials both for prophylactic and therapeutic purposes (21). The scenario is becoming even more alarming as the critically important antimicrobials for human medicines are also being used for treating animal infections. This is the arena where AMR surveillance in the animal sector needs to be integrated with human health and antibiotic guidelines in both sectors may be made in harmony so there should not be any overlap in the use of critically important antibiotics across sectors. Despite having several guidelines and regulations on the usage of antimicrobials in animal farming in India, compliance with the same is a serious problem (5, 22–24). However, a synergy needs to be developed between different agencies such as the Bureau of Indian Standards (BIS) and the Food Standards and Safety Authority of India (FSSAI) for the reinforcement of the existing policies in controlling the unwise application of antibiotics and their residues in food animals. The practice of animal farming with less dependency on antimicrobials and shifting toward the use of India’s ethno-veterinary and traditional medicines can improve the present scenario in the long run (25, 26) if it becomes successful and economically viable, it can reduce the reliance on antimicrobials. Likewise, the ongoing research on developing herbal growth promoters in poultry can also be a future breakthrough. Investment may be made toward innovation of cost-effective alternatives to growth promoters and incentivization through subsidies may be recommended for costly alternatives at present. These initiatives will not only help to rationalize antibiotic use in animal farming but also reduce the selection pressure of antibiotics in the One Health Ecosystem Compendium.
Through KII several lacunae have been identified at the grass-root level. Farmers are randomly using antimicrobials in animal farming without proper knowledge, training, or even consulting veterinarians. Moreover, disposing of dead animals, farm manures, and litter unwisely also results in the transmission of AMR bacteria into the environment. Therefore, it is imperative to address this issue from the base level by generating awareness among the farmers about biosecurity practices such as restricted movements in the farms, quarantine and isolation of diseased or sick animals, fencing, cleaning and disinfection protocols, and diagnostic facilities (27). Animal health and AMR need to be perceived and recognized not in isolation but in the holistic and inclusive approach and as a part of global One Health initiative. The One Health approach is fully integrated into global efforts to address the problem of AMR. There are obstacles that need to be overcome including competing interests of multiple players and economic sectors including leading organizations and institutes working in animal, human, and environmental health. These actors should converge on key priorities and take calls for integrated action, find means to monitor AMR and control infections in multiple sectors, and develop cross-cutting and shared policies to govern the rational use of antimicrobials across all sectors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DC: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. FD: Writing – review & editing, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. SG: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. SS: Writing – review & editing, Methodology, Investigation, Data curation. SP: Writing – review & editing, Methodology, Investigation, Data curation. RC: Writing – review & editing, Methodology, Investigation, Data curation. AM: Writing – review & editing, Project administration, Methodology, Investigation, Conceptualization. AD: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization. RB: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. SD: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received funding from UNEP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Van Boeckel, TP, Glennon, EE, Chen, D, Gilbert, M, Robinson, TP, Grenfell, BT, et al. Reducing antimicrobial use in food animals. Science. (2017) 357:1350–2. doi: 10.1126/science.aao1495
2. Van Boeckel, TP, Brower, C, Gilbert, M, Grenfell, BT, Levin, SA, Robinson, TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. (2015) 112:5649–54. doi: 10.1073/pnas.1503141112
3. Chauhan, AS, George, MS, Chatterjee, P, Lindahl, J, Grace, D, and Kakkar, M. The social biography of antibiotic use in smallholder dairy farms in India. Antimicrob Resist Infect Control. (2018) 7:1–13. doi: 10.1186/s13756-018-0354-9
4. Sharma, C, Rokana, N, Chandra, M, Singh, B, Gulhane, R, Gill, J, et al. Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front Vet Sci. (2018) 4:237. doi: 10.3389/fvets.2017.00237
5. Muloi, D, Ward, MJ, Pedersen, AB, Fevre, EM, Woolhouse, ME, and van Bunnik, BA. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathog Dis. (2018) 15:467–74. doi: 10.1089/fpd.2017.2411
6. Debnath, F, Chakraborty, D, Giri, S, Saha, S, Pyne, S, Chakraverty, R, et al. Existing policies/guidelines on the environmental dimension of antimicrobial resistance in India: an insight into the key facets through review and SWOT analysis. Trop Med Infect Dis. (2022) 7:336. doi: 10.3390/tropicalmed7110336
7. Balasubramaniam, A, Arthanari Eswaran, M, Suresh, P, and Sukumar, K. Detection of tetracycline resistance determinant tetA gene and antimicrobial resistance pattern in Escherichia coli isolates recovered from healthy layer chickens. Vet World. (2014) 7:635–8. doi: 10.14202/vetworld.2014.635-638
8. Kabir, SL, Asakura, M, Shiramaru, S, Pal, A, Hinenoya, A, and Yamasaki, S. Molecular identification and antimicrobial resistance profiles of Campylobacter strains of poultry origin in India with special emphasis on fluoroquinolone resistance. Asian J Med Biol Res. (2015) 1:1–8.
9. Waghamare, RN, Paturkar, AM, Vaidya, VM, Zende, RJ, Dubal, ZN, Dwivedi, A, et al. Phenotypic and genotypic drug resistance profile of Salmonella serovars isolated from poultry farm and processing units located in and around Mumbai city, India. Vet World. (2018) 11:1682–8. doi: 10.14202/vetworld.2018.1682-1688
10. Bhave, S, Kolhe, R, Mahadevaswamy, R, Bhong, C, Jadhav, S, Nalband, S, et al. Phylogrouping and antimicrobial resistance analysis of extraintestinal pathogenic Escherichia coli isolated from poultry species. Turk J Vet Anim Sci. (2019) 43:117–26. doi: 10.3906/vet-1808-47
11. Thaker, HC, Brahmbhatt, MN, and Nayak, JB. Study on occurrence and antibiogram pattern of Escherichia coli from raw milk samples in Anand, Gujarat, India. Vet World. (2012) 5:556. doi: 10.5455/vetworld.2012.556-559
12. Bandyopadhyay, S, Samanta, I, Bhattacharyya, D, Nanda, PK, Kar, D, Chowdhury, J, et al. Co-infection of methicillin-resistant Staphylococcus epidermidis, methicillin-resistant Staphylococcus aureus and extended-spectrum β-lactamase producing Escherichia coli in bovine mastitis–three cases reported from India. Vet Q. (2015) 35:56–61. doi: 10.1080/01652176.2014.984365
13. Bhattacharyya, D, Banerjee, J, Bandyopadhyay, S, Mondal, B, Nanda, PK, Samanta, I, et al. First report on vancomycin-resistant Staphylococcus aureus in bovine and caprine milk. Microb Drug Resist. (2016) 22:675–81. doi: 10.1089/mdr.2015.0330
14. Koovapra, S, Bandyopadhyay, S, Das, G, Bhattacharyya, D, Banerjee, J, Mahanti, A, et al. Molecular signature of extended spectrum β-lactamase producing Klebsiella pneumoniae isolated from bovine milk in eastern and North-Eastern India. Infect Genet Evol. (2016) 44:395–402. doi: 10.1016/j.meegid.2016.07.032
15. Singh, R, Yadav, AS, Tripathi, V, and Singh, RP. Antimicrobial resistance profile of Salmonella present in poultry and poultry environment in north India. Food Control. (2013) 33:545–548.
16. Nobrega, DB, Tang, KL, Caffrey, NP, De Buck, J, Cork, SC, Ronksley, PE, et al. Prevalence of antimicrobial resistance genes and its association with restricted antimicrobial use in food-producing animals: a systematic review and meta-analysis. J Antimicrob Chemother. (2021) 76:561–75. doi: 10.1093/jac/dkaa443
17. Khurana, A., Sinha, R., and Bhati, D. (2021). Conserving the use of critically important antimicrobials in food-producing animals: Gaps and possibilities in global guidance and Indian policy framework. Centre for Science and Environment, New Delhi.
18. Debnath, BC, Choudhary, KBD, Ravikanth, K, Thakur, A, Maini, S, and Nagar, RK. Comparative efficacy of natural growth promoter (av/agp/10) with antibiotic growth promoter on overall growth performance and intestinal morphometry in broiler birds. Int J Pharm Sci Heal Care. (2014) 2:155–68.
19. Patel, AP, Bhagwat, SR, Pawar, MM, Prajapati, KB, Chauhan, HD, and Makwana, RB. Evaluation of Emblica officinalis fruit powder as a growth promoter in commercial broiler chickens. Vet World. (2016) 9:207–10. doi: 10.14202/vetworld.2016.207-210
20. Jayaraman, S, Das, PP, Saini, PC, Roy, B, and Chatterjee, PN. Use of Bacillus Subtilis PB6 as a potential antibiotic growth promoter replacement in improving performance of broiler birds. Poult Sci. (2017) 96:2614–22. doi: 10.3382/ps/pex079
21. Parkunan, T, Ashutosh, M, Sukumar, B, Chera, JS, Ramadas, S, Chandrasekhar, B, et al. Antibiotic resistance: a cross-sectional study on knowledge, attitude, and practices among veterinarians of Haryana state in India. Vet World. (2019) 12:258–65. doi: 10.14202/vetworld.2019.258-265
22. CDDEP (2016). A use and resistance in food animals. Current policy and recommendations. Center for Disease Dynamics, economics and policy, Washington, DC, USA. Available online at: https://www.cddep.org/wp-content/uploads/2017/06/india_abx_report-2.pdf (Accessed September 24, 2021).
23. Garg, A, and Mohanta, R. (2012). Antibiotic use in animal husbandry sector: What has to be done? Agriculture today. Available online at: https://www.researchgate.net/publication/275019219_ANTIBIOTICS_USE_IN_ANIMAL_HUSBANDRY_SECTOR_WHAT_HAS_TO_BE_DONE (Accessed September 18, 2021).
24. Singh, P. One health approach to tackle antimicrobial resistance in South East Asia. BMJ. (2017) 358:j3625. doi: 10.1136/bmj.j3625
25. Balaji, S, and Chakravarthi, P. Ethnoveterinary practices in India – a review. Vet World. (2010) 3:549–51. doi: 10.5455/vetworld.2010.549-551
26. Phondani, P, Maikhuri, R, and Kala, C. Ethnoveterinary uses of medicinal plants among traditional herbal healers in Alaknanda catchment of Uttarakhand, India. Afr J Trad CAM. (2010) 7:195–206. doi: 10.4314/ajtcam.v7i3.54775
27. Dhaka, P, Chantziaras, I, Vijay, D, Bedi, JS, Makovska, I, Biebaut, E, et al. Can improved farm biosecurity reduce the need for antimicrobials in food animals? A scoping review. Antibiotics. (2023) 12:893. doi: 10.3390/antibiotics12050893
28. Arora, D, Kumar, S, Singh, D, Jindal, N, and Mahajan, NK. Isolation, characterization and antibiogram pattern of Salmonella from poultry in parts of Haryana, India. Adv Anim Vet Sci. (2013) 1:161–3.
29. Bangera, SR, Umakanth, S, Chowdhury, G, Saha, RN, Mukhopadhyay, AK, and Ballal, M. Poultry: a receptacle for non-typhoidal salmonellae and antimicrobial resistance. Iranian J Microbiol. (2019) 11:31–8. doi: 10.18502/ijm.v11i1.702
30. Brower, CH, Mandal, S, Hayer, S, Sran, M, Zehra, A, Patel, SJ, et al. The prevalence of extended-Spectrum Beta-lactamase-producing multidrug-resistant Escherichia Coli in poultry chickens and variation according to farming practices in Punjab, India. Environ Health Perspect. (2017) 125:077015. doi: 10.1289/EHP292
31. Chakraborty, S, Roychoudhury, P, Samanta, I, Subudhi, PK, Das, M, De, A, et al. Molecular detection of biofilm, virulence and antimicrobial resistance associated genes of Salmonella serovars isolated from pig and chicken of Mizoram, India. Indian J Anim Res. (2020) 54. doi: 10.18805/ijar.B-3817
32. Rajagopal, R, and Mini, M. Outbreaks of salmonellosis in three different poultry farms of Kerala, India. Asian Pac J Trop Biomed. (2013) 3:496–500. doi: 10.1016/S2221-1691(13)60103-3
33. Samanta, I, Joardar, SN, Das, PK, Sar, TK, Bandyopadhyay, S, Dutta, TK, et al. Prevalence and antibiotic resistance profiles of Salmonella serotypes isolated from backyard poultry flocks in West Bengal, India. J Appl Poult Res. (2014) 23:536–45. doi: 10.3382/japr.2013-00929
34. Senthamilselvan, B. Antimicrobial resistance profile of bacterial isolates from fecal and cloacalswab samples collected from broiler chickens in Chennai, southern India. Ann Romanian Soc Cell Biol. (2020):892–8.
35. Sharma, J, Kumar, D, Hussain, S, Pathak, A, Shukla, M, Kumar, VP, et al. Prevalence, antimicrobial resistance and virulence genes characterization of nontyphoidal Salmonella isolated from retail chicken meat shops in northern India. Food Control. (2019) 102:104–11. doi: 10.1016/j.foodcont.2019.01.021
Keywords: antimicrobial resistance, veterinary sector, antibiotics, bovine mastitis, India
Citation: Chakraborty D, Debnath F, Giri S, Saha S, Pyne S, Chakraverty R, Majumdar A, Deb AK, Bhatia R and Dutta S (2024) Contribution of veterinary sector to antimicrobial resistance in One Health compendium: an insight from available Indian evidence. Front. Vet. Sci. 11:1411160. doi: 10.3389/fvets.2024.1411160
Edited by:
Harish Tiwari, The University of Sydney, AustraliaReviewed by:
Fanta Gutema, The University of Iowa, United StatesAdnan Mannan, University of Chittagong, Bangladesh
Copyright © 2024 Chakraborty, Debnath, Giri, Saha, Pyne, Chakraverty, Majumdar, Deb, Bhatia and Dutta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Falguni Debnath, ZmFsZ3VuaWRlYm5hdGhAeWFob28uaW4=
 Debjit Chakraborty
Debjit Chakraborty Falguni Debnath
Falguni Debnath Sandip Giri
Sandip Giri Shatabdi Saha1
Shatabdi Saha1 Raja Chakraverty
Raja Chakraverty Agniva Majumdar
Agniva Majumdar Shanta Dutta
Shanta Dutta