- 1Livestock and Forage Directorate, Somali Region Pastoral and Agro-Pastoral Research Institute, Jigjiga, Ethiopia
- 2College of Veterinary Medicine, Jigjiga University, Jigjiga, Ethiopia
Background: Subclinical mastitis in camels, an inflammation of the udder without visible signs, can reduce milk quality and raise bacteria levels. Regular monitoring of camel milk is crucial for consumer safety.
Methods: A cross sectional study was conducted in Jigjiga city, Ethiopia to investigate the prevalence and characteristics of subclinical mastitis in she-camels. The study included 244 lactating she-camels from three privately-owned camel dairy farms, and a questionnaire survey was conducted with 60 camel owners.
Results: The overall prevalence of subclinical mastitis in she-camels was 10.6% (26/244), with no significant difference among the studied dairy farms. Risk factors that influenced the result of California Mastitis Test (CMT) included age and udder and leg hygiene. The study revealed that S. aureus was the most prevalent bacterium among the isolated bacteria, with a prevalence rate of 34.5%. This was followed by S. agalactiae, S. dysgalactiae, and Pasteurella multocida, with prevalence rates of 29.8, 19.4, and 16.2%, respectively. Among the isolated bacteria, 84.5% were sensitive to Erythromycin, 60% to Streptomycin, 44.7% to Oxytetracycline, and 36.7% to Tetracycline. Interviews with camel owners revealed that 66.7% used mixed herd grazing methods and reported feed shortage. Treatment practices for sick camels included modern veterinary drugs, traditional medicines, or a combination of both. The owners of camel dairy farms did not maintain proper hygiene practices during milking, such as not using soap when washing hands.
Conclusion: Addressing camel mastitis necessitates access to alternative drugs, comprehensive herder training, and enhanced management practices.
1 Introduction
The one-humped camels (Camelus dromedarius) demonstrate remarkable adaptation to arid and semi-arid environments, enabling them to not only survive but also thrive. They possess the ability to produce milk even in the midst of severe droughts, a crucial advantage over cattle, sheep, and goats, which often suffer high mortalities under such conditions. Consequently, dromedaries play a vital role in sustaining the livelihoods of pastoralists, particularly in the pastoral areas of Africa (1). Milk is a very nutritional food that is rich in carbohydrate, proteins, fats, vitamins, and minerals. However, milk and milk products of dairy cows and camel can harbor a variety of microorganisms and can be important sources of food borne pathogens to humans (2). Mastitis, a prevalent disease among camels, poses a significant threat to both the health of these animals and the economic well-being of the pastoralists. As it can result in substantial financial losses, combatting mastitis becomes paramount in safeguarding the livelihoods of pastoralists (3).
Mastitis can occur in two main forms: clinical and subclinical. Clinical mastitis shows visible signs and abnormalities in the animal’s udder and milk, while subclinical mastitis leads to reduced milk production without obvious symptoms (4). Diagnosing subclinical mastitis can be particularly challenging because it often lacks visible signs, making it easy for the condition to go unnoticed. This can lead to a prolonged presence of the disease within the herd, causing higher economic losses compared to clinical mastitis. Farmers may not be aware of the issue until it has already had a significant impact on milk quality and production. Therefore, early and targeted testing of the milk is essential to detect and address subclinical mastitis promptly, ultimately minimizing economic losses and ensuring the overall health and productivity of the herd (5).
In Ethiopia, the dairy industry faces significant obstacles due to various diseases associated with livestock farming practices, with mastitis being the primary concern. The effects of mastitis on this sector are extensive and encompass several areas. These include the temporary or permanent impairment of milk production capacity, a decline in milk quality, milk wastage resulting from antibiotic drug residues, increased expenses related to veterinary care and labor, a reduced productive lifespan of the animals, diminished value of meat after slaughter, and losses arising from reduced overall dairy product output (6).
Reports of sub-clinical mastitis in camels have been documented in various regions of Africa, including Egypt (7), Somalia (8), and Kenya (9). Very recent studies conducted in two pastoral districts in southern Ethiopia, reported a prevalence of sub-clinical mastitis in dairy cows, camels, and goats as 33.3, 26.3, and 25.0%, respectively; and quarter-level prevalence of sub-clinical mastitis in cows, camels and goats as 17.6, 14.5, and 20%, respectively (10). Sub-clinical mastitis in Ethiopia has been over-looked, with limited research and information available on its prevalence, geographic distribution, impact on milk quality, and related consumer risks. There is a need to study and understand the risk factors and negative effects of mastitis on milk and milk products to improve prevention and control measures (11). Particularly, there is very limited knowledge about sub-clinical mastitis in camels, including its causes and occurrence. However, cases of mastitis in camel have recently been reported in Ethiopia (12).
Limited information exists on the antimicrobial resistance (AMR) of pathogens in camel milk, but a study conducted in southern Ethiopia found that S. aureus isolates in camel milk were resistant to multiple drugs. The use of antimicrobials in dairy farms, particularly in food animal production, has been connected to an increased resistance to tetracycline in S. aureus and E. coli strains causing mastitis. This practice is widely acknowledged as a contributing factor to the development of antimicrobial resistance (10). The objective of this study was to determine the prevalence of camel sub-clinical mastitis and identify the associated risk factors. With great emphasis on isolation, characterization, and determination of antibiotic sensitivity of the bacteria causing sub-clinical mastitis in and around Jigjiga City, Fafan Zone, Somali Region, Ethiopia.
2 Materials and methods
2.1 Description of the study area
The study was conducted in and around Jigjiga city, Fafan zone, Somali regional state. Fafan administrative zone, is located in the northern part of the Ethiopian Somali Region, at 9°20′ North latitude; 45°56′° East longitude, about 630 km East of Addis Ababa. It covers a total land area of 40.86 km2 with altitude ranging from 500 to 1,650 m above sea level (masl). The area receives a precipitation ranging from 300 to 600 mm per annum and an average daily temperature of 16–20°C. Agro-pastoralist is a dominant production system in Fafan zone. The estimated livestock population of the zone is 248,435 cattle, 666,130 sheep, 503,881 goats 72,390 camels, and 10,548 poultry (13) (Figure 1).
2.2 Study design
A cross-sectional study was conducted from October 2021 to June 2022, on lactating female camels of local breed (Camelus dromedarius) reared either under intensive or extensive systems.
2.3 Study population
The study population consisted of lactating female camels from Jigjiga’s commercial dairy farms. The camels included in the study were all indigenous breeds of one humped camel (Camelus dromedarius) selected from three privately owned camel dairy farms (Dhaygel: n = 81, Barkomal: n = 131, Suleka: n = 32) that followed a semi-intensive farming system. Semi-intensive camel dairy farming in the Somali region combines aspects of intensive and extensive farming practices to optimize milk production from camels. This system includes regular milking, supplementary feeding, proper housing and management, breeding management, healthcare and disease management, as well as water and pasture management to ensure high-quality milk production while maintaining the health and wellbeing of the camels. The age of the animal was determined by examining dentation and owners records. In addition, A group of 60 specifically chosen camel owners, along with three owners/managers/attendants of camel dairy farms, were interviewed for the study.
2.4 Questionnaire survey
During the farm visits, data were collected using a pretested semi-structured questionnaire administered through personal interviews at each time. The information gathered included farm biodata and herd management practices of camel owners, such as the production system (pastoral or agro-pastoral), the purpose of keeping livestock (milk, meat, income, or social prestige), camel herd grazing methods (mixed with other species or grazing separately), cleaning the house (yes or no), milking practices (with or without calf suckling), washing hands before milking (yes or no), and cleaning milking equipment (yes or no).
2.5 Sample size determination
The sample size was determined using the formula described by Thrusfield (14), considering an expected prevalence of 18.1%, an absolute precision of 5%, and a 95% confidence interval. However, to increase precision, the sample size was increased to 244 lactating camels that were sampled in this study.
Where: P = expected prevalence
n = required sample size
d = desired absolute precision
2.6 Sampling technique
First, the udder was cleaned to remove any dirt or debris. Following this, each teat was wiped with a clean cloth and disinfected using 70% alcohol. The initial streams of milk were discarded, and approximately 10–20 mL of milk was collected from each quarter (5–10 mL in each teat) in sterile universal bottles with unique labels. Risk factors, such as age, lactation stage, parity, production system, source of water, and udder and leg hygiene, were all documented.
2.7 California mastitis testing
To assess the clinical form of mastitis, milk from each quarter was examined using a strip cup, and any observable changes in color, odor, and consistency were recorded. Additionally, the presence of subclinical mastitis was determined using the California mastitis test (CMT) following the prescribed procedures outlined by Ferronatto et al. (15). The CMT results were interpreted subjectively according to the categories of negative, trace, 1+, 2+, or 3+, as outlined by Adkins and Middleton (16). Using the CMT, camels were classified as positive for SCM if they had readings of (1+, 2+, or 3+), while negative and trace readings were considered as negative.
2.8 Bacteriological laboratory examination and antimicrobial susceptibility testing
Bacteriological examination and AMR testing were conducted following the methods described by Quinn et al. (17) and the Clinical and Laboratory Standards Institute (CLSI) guidelines (18). For the analysis, a loopful of milk sample was streaked onto tryptose blood agar base supplemented with 5% sheep blood agar (Oxoid, United Kingdom) and MacConkey Agar using the quadrant streaking technique for each quarter. After incubation, the cultural growth macro-and micro-scale characteristics were evaluated, examining distinctions of each colony including colony morphology, hemolysis, and pigment production. Gram staining was performed on pure culture colonies to analyze their staining reaction and cellular morphology under a light microscope at 100× magnification. To avoid confusion in the Gram stain reaction, a potassium hydroxide (KOH) test was also conducted. For further examination, mixed colonies and Gram-negative bacteria were sub-cultured on sheep blood and MacConkey (Oxoid, Hampshire, United Kingdom) agar plates. Pure cultures of single colony types from both blood and MacConkey agar were transferred onto nutrient agar-medium for a series of primary tests including Catalase, Oxidase, Motility, and Fermentative-Oxidative tests, as well as secondary tests like triple sugar iron agar, citrate utilization test, methyl red test, and indole test, following standard procedures (19, 20).
Isolated bacterial colonies was transferred to a tube with sterile normal saline, creating a homogenous suspension adjusted to a turbidity equivalent to a 0.5 McFarland standard. The bacterial suspension were then inoculated onto Muller-Hinton agar using a sterile swab to cover the entire surface, with plates left to dry at room temperature. Prepared plates was checked for sterility and incubated overnight at 37°C before antimicrobial disks were placed on the media surface. A variety of commonly used antibiotics were selected for susceptibility testing in accordance with CLSI criteria. Standard antimicrobial impregnated disks (HiMedia Mumbai, India) were used, including Erythromycin 15 μg, Streptomycin 10 μg, Oxytetracycline 30 μg, and Tetracycline 30 μg. The zone of inhibition diameters around the disks were measured with rulers, and isolates were classified as susceptible, intermediate, or resistant following CLSI standards. Isolates resistant to three or more antimicrobial subclasses were considered multidrug resistant (21).
2.9 Data analysis
The data obtained from laboratory investigations and questionnaire survey were organized in a Microsoft Excel spreadsheet and analyzed using STATA (version 16; Stata Corp LP, College Station, TX, United States). Descriptive statistics were used to determine the prevalence of subclinical mastitis. The associations between subclinical mastitis and various factors (such as location/farm, age, lactation stage, parity, and production system, sources of water, and udder and leg hygiene) were evaluated using the chi-square test (χ2). The odds ratio (OR) was utilized to indicate the level of association between risk factors and SCM occurrence, with 95% confidence intervals provided. Variables with a p value <0.25 in univariate logistic regression analysis were included in a multivariate logistic regression analysis to control for potential confounding variables, and adjusted odds ratios were calculated. The model’s goodness-of-fit was assessed through backward elimination, where variables were sequentially removed starting from the least influential until the removal of a variable had a significant impact on the dependent variable. Collinearity between variables was checked using standard error, and model fitness was evaluated through the Hosmer and Lemeshow test and Omnibus test. A 95% confidence level was maintained throughout the data presentation, and a p value less than 0.05 (i.e., p < 0.05) was considered statistically significant.
3 Results
3.1 House hold questionnaires and dairy camel farm owners interview survey
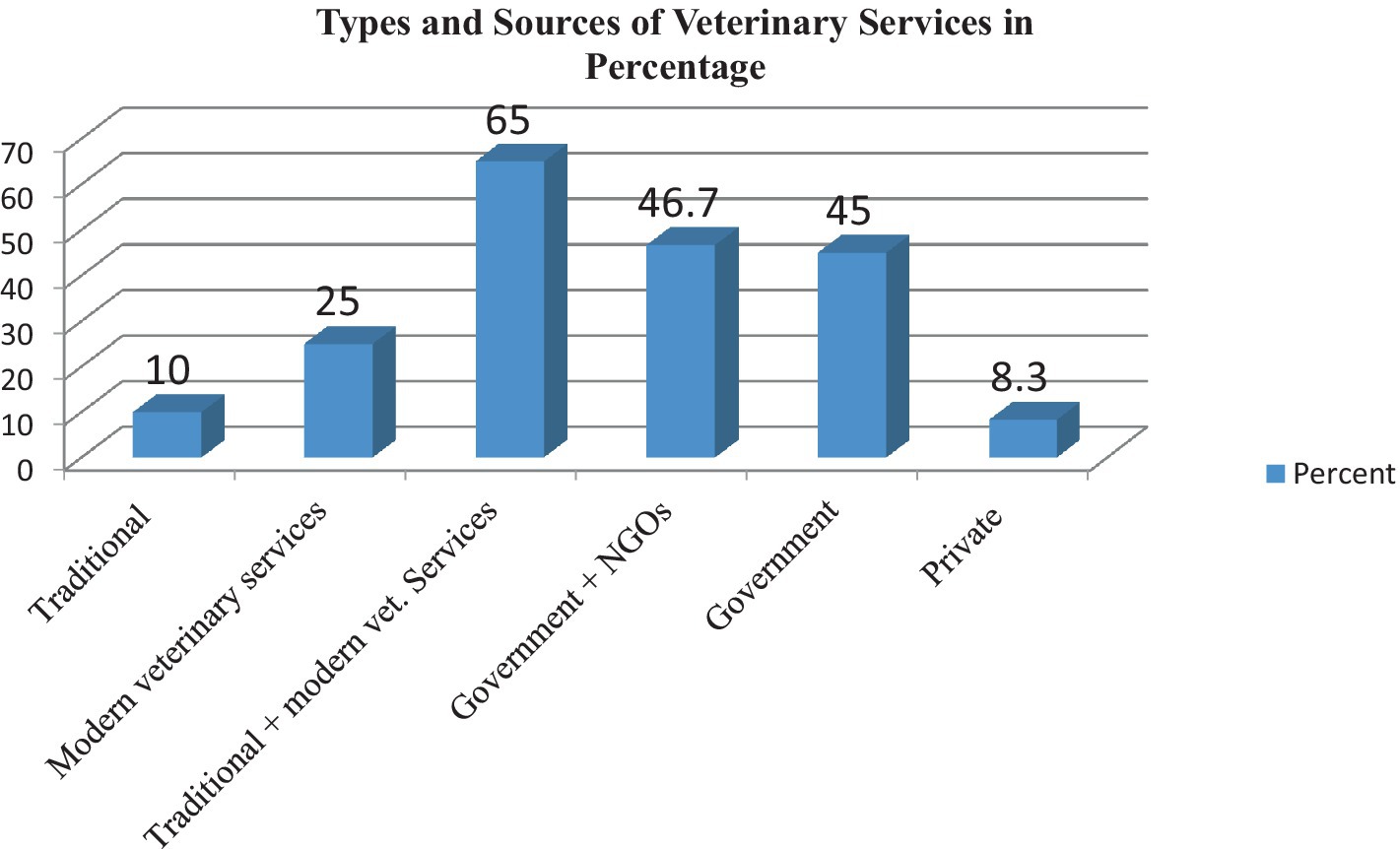
In the study area, the main feed sources for dairy camels were natural pasture and crop residue, as stated by 58.3% of respondents. Feed shortage problems were reported by 98.3% of dairy camel owners. Out of 60 respondents, 85% were aware of clinical mastitis in lactating she camels, but none were aware of subclinical mastitis in apparently health she camels. When treating mastitis, 65% used modern veterinary drugs and 25% relied on traditional medicines, particularly experienced camel owners. Most respondents (93.3%) had access to veterinary services. The summarized survey results are presented in Tables 1, 2, with a bar column chart (Figure 2).
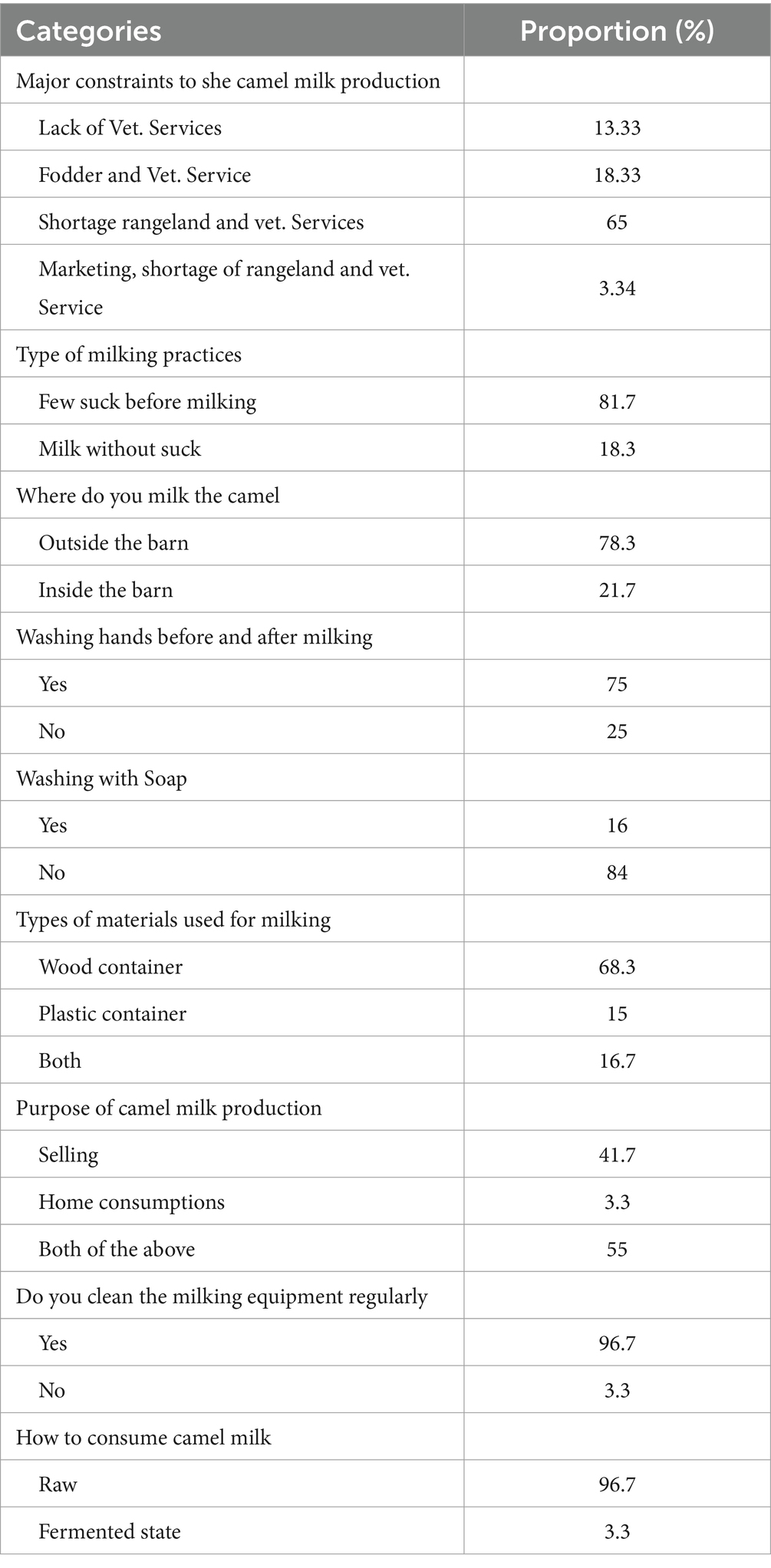
Table 2. Major constraints of camel milk production and handling practices in three dairy farm camels in Somali region, Ethiopia.
3.2 Prevalence of subclinical mastitis
In the study area, the overall prevalence of subclinical mastitis in she-camels was 10.6% (95% CI: 0.067–0.146) with 26 out of 244 individuals affected. Among the three camel dairy farms examined, Suleka had the highest prevalence at 12.5% (95% CI: 0.003–0.246), followed by Dhaygel and Barkomal with prevalence of 11.1% (95% CI: 0.041–0.181) and 9.9% (95% CI: 0.047–0.151), respectively. However, there were no significant differences in the prevalence of subclinical mastitis among the different dairy farms (p > 0.05) (Table 3).
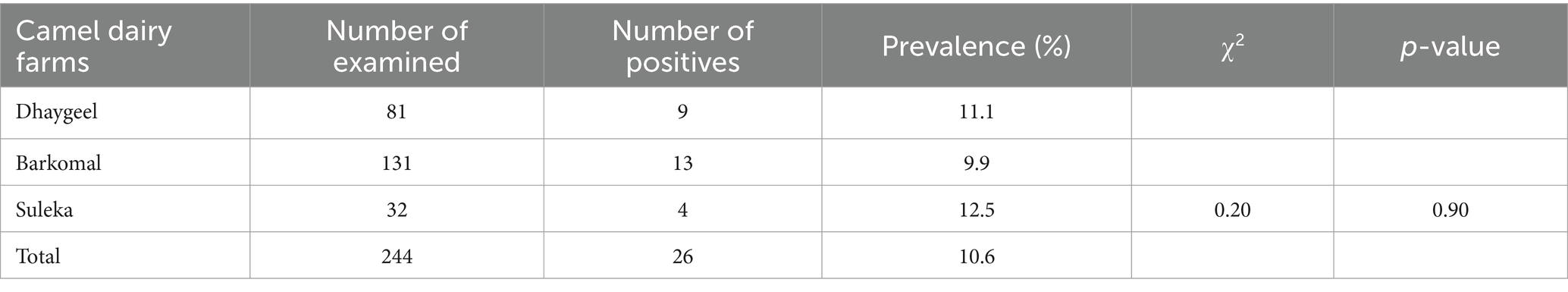
Table 3. Prevalence of sub-clinical mastitis in the she-camels from the three dairy farm camels in Somali region, Ethiopia.
She camels in the 5–7 years age category were found to be at a greater risk compared to those aged 2–4 and over 7 years. Poor hygiene of the udder and legs were identified as significant risk factors for subclinical mastitis in she-camels, with poor udder and leg hygiene being the most affected factors (p < 0.05). However, factors such as location/farm, lactation stage, parity production system, and sources of water for the farms did not display a statistically significant association with the occurrence of subclinical mastitis in the study areas (p > 0.05) (Table 4).
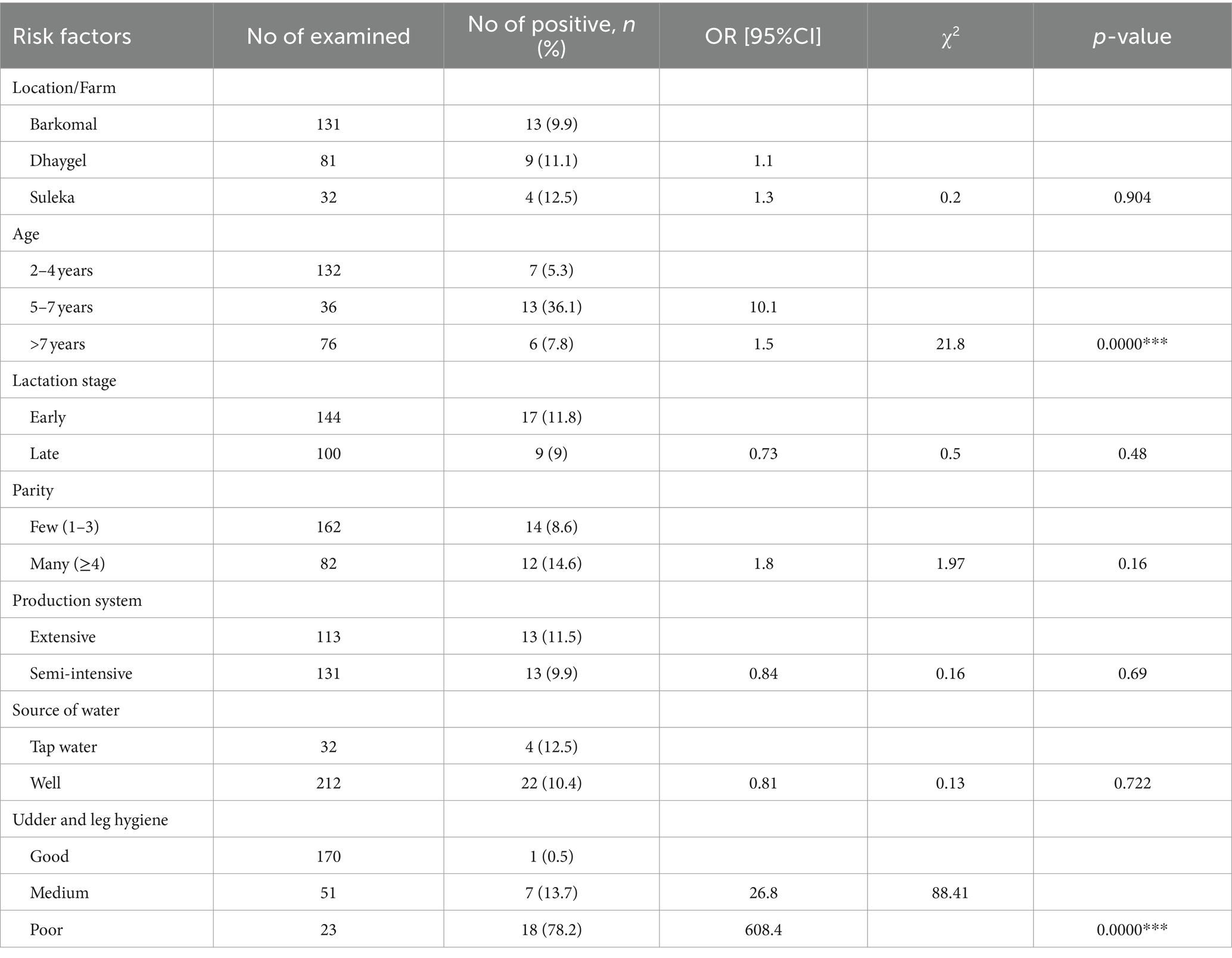
Table 4. Multivariable logistic regression analysis of factors associated with subclinical mastitis in lactating camels from three dairy farms in the Somali region of Ethiopia.
3.3 Bacterial species isolation analysis
In the 26 cultured samples, a total of 510 bacterial colonies were found. The most common species was Staphylococcus aureus, comprising 34.5% of the isolated bacteria. Other bacteria like Streptococcus agalactiae, Streptococcus dysgalactiae, and Pasteurella multocida were also present, with proportions of 29.8, 19.4, and 16.2%, respectively (Table 5).
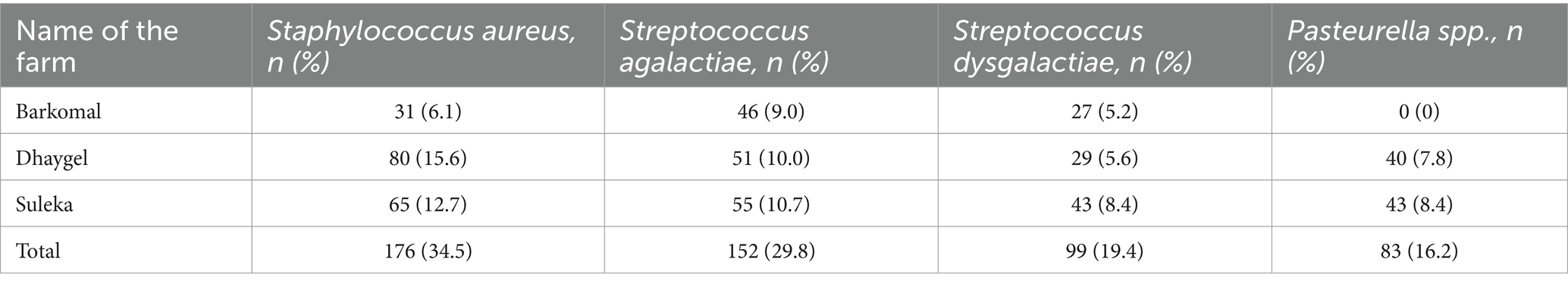
Table 5. Distribution of isolates and individual prevalence of bacterial species isolated from she camels in three dairy farm camels in Somali region, Ethiopia.
3.4 Antibiotic sensitivity test (Kirby-Bauer disk diffusion method)
The study found that Staphylococcus, Streptococcus, and Pasteurella species isolates had higher susceptibility to erythromycin compared to other antibiotics tested. Erythromycin was the most effective antibiotic, with high susceptibility among the identified bacterial species. Streptomycin was the second most effective antibiotic. In contrast, oxytetracycline and tetracycline antibiotics showed low sensitivity among the identified bacterial species. The resistance profile against tetracycline was relatively high among the bacteria tested (Table 6).
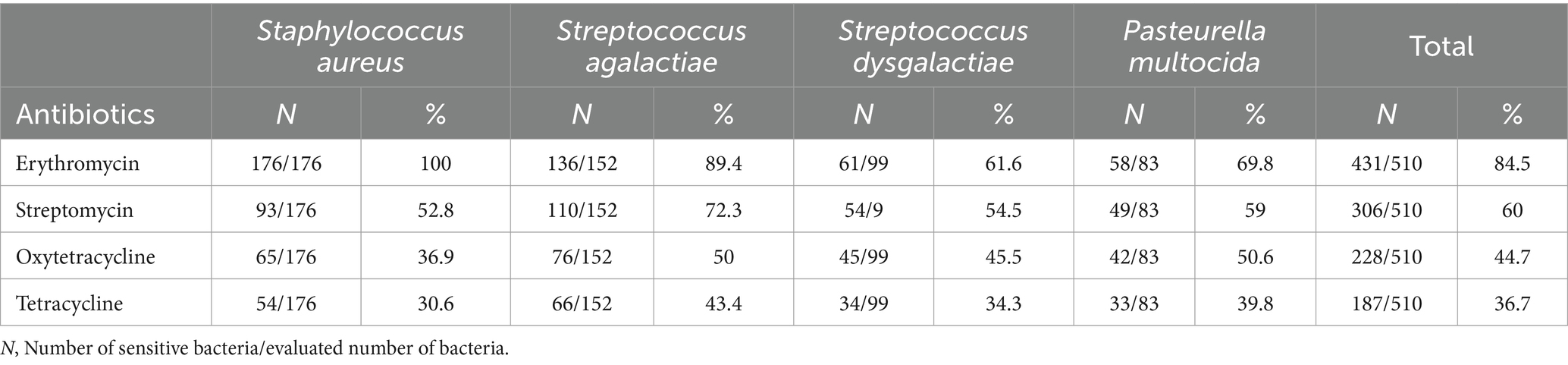
Table 6. In vitro susceptibility test of bacterial profiles from camel milk samples with subclinical mastitis in somali region dairy farms.
4 Discussion
The prevalence of subclinical mastitis in she camels in this study (10.6%) aligns with previous studies by Mohamud et al. (8) who reported 9.8% in Somalia, and Juboori et al. (22) who reported 11.67% in the UAE. Similarly, the findings are comparable to Balemi et al. (10) who reported 14.5% in Ethiopia. However, Geresu et al. (12) found a slightly higher prevalence of 18.1% in Southern Ethiopia. On the other hand, the camel mastitis prevalence found in the present study is relatively lower than the reported 59.8% in the Afar Region of Ethiopia (23), 76% in pastoral areas of eastern Ethiopia (24), 18.5% in Abu Dhabi, United Arab Emirates (25) and 34.7% in Borena zone of Oromia Regional State (26). Contrastingly, Almaw and Molla (27) reported a lower incidence of 2.1% for subclinical mastitis in lactating she camels in northeastern Ethiopia, which is below the prevalence indicated in the current study. The higher prevalence of those research studies may be attributed to differences in the management systems of camels.
The 5–7 years age group showed a significant difference in subclinical mastitis compared to other age groups, aligning with previous studies on the correlation between age of she camels and sub-clinical mastitis prevalence by Geresu et al. (12), which found a significant difference in sub-clinical mastitis among different age groups (p < 0.05). Additionally, our study found that udder and leg hygiene measures significantly influenced the prevalence of camel subclinical mastitis. Nevertheless, lactation stage is not significantly associated with the occurrence of camel mastitis in the study areas, with (p > 0.05) consistent with the findings of Mahboob et al. (28).
In addition, Alebie et al. (29) found that neither parity nor lactation stages were statistically significant in the occurrence of camel subclinical mastitis in lactating she camels from Dubti district, Afar Regional State, Northeastern Ethiopia, which is consistent with our current results. However, Mogeh et al. (30) reported a significant difference in camel subclinical mastitis across lactation stage and parity (p < 0.05) among lactating dromedary camels in and around Hargeisa, Somaliland. Variation in research methodologies and types of bacteria examined across previous studies may contribute to the differences in findings and discrepancies in reported rates of subclinical mastitis.
The findings of the study regarding the respondents’ knowledge and practices related to mastitis in she camels are worth discussing. It is noteworthy that a significant majority of respondents (85%) were aware of clinical mastitis in lactating she camels, indicating a basic understanding of this condition. However, the complete lack of familiarity with subclinical mastitis in seemingly healthy she camels among the respondents highlights a gap in knowledge that could potentially impact the health management of the camel herd. The treatment practices reported by the respondents also provide insights into the current approaches taken in managing mastitis. The fact that 65% of respondents turned to modern veterinary drugs for treating mastitis suggests a reliance on evidence-based interventions. On the other hand, the 25% of respondents who preferred traditional medicines and sought advice from experienced camel owners indicate a potential reliance on indigenous knowledge and practices. This brings to light the blending of modern and traditional approaches in managing mastitis in she camels within the community. Furthermore, the high percentage of respondents (93.3%) having access to veterinary services is an encouraging finding as it indicates the potential for professional guidance and support in managing mastitis cases. This accessibility to veterinary services can contribute to improved diagnostic and treatment outcomes for mastitis in she camels. These findings are consistent with the report by Seligsohn et al. (9).
The study findings indicate that a majority of farmers (75%) claim to wash their hands before milking. However, an even larger proportion (96.7%) regularly clean the milking equipment. It is worth noting that a significant number of farmers (84%) do not use soap during handwashing. This highlights a potential gap in hygiene practices that may require further attention and education among farmers. The absence of proper hygiene standards during milking processes could be a contributing factor to the spread of subclinical mastitis in camel herds Wang et al. (31).
The prevalence of Staphylococcus aureus and Streptococcus agalactiae in the current study, at 34.5 and 29.8% respectively, aligns with the results of Wubishet et al. (26), who reported 38.0 and 27.5% prevalence rates of Staphylococcus aureus and Streptococcus agalactiae isolates in mastitis-positive lactating she camels from the Borena Zone, Oromia Regional State, Ethiopia. Nonetheless, Compared to the 34.5% found in the current study, Eyassu and Bekele (32) reported a significantly lower percentage of Staphylococcus aureus (4.2%).
The present study has shown a high level of multi-drug resistance against commonly used drugs such as Tetracycline and Oxytetracycline. However, Erythromycin was found to be the most effective antibiotic, with high susceptibility among the isolated bacterial species, this is in line (33). Based on the results of the antibiotics sensitivity test in the current study, it can be concluded that the preferred antimicrobial drugs for treating mastitis in dairy camels should be Erythromycin first, followed by Streptomycin and Oxytetracycline in descending order.
5 Conclusion
The present study revealed a high prevalence of sub-clinical mastitis in dairy camels, indicating it as a significant health problem. Among the risk factors considered, age and hygienic measures such as udder and leg hygiene showed a significant association with the prevalence of sub-clinical mastitis in lactating she camels. The major bacterial species identified as the cause of mastitis in the study area were Staphylococcus aureus, Streptococcus agalactiae, Streptococcus dysgalactiae, and Pasteurella multocida. The antibiotic sensitivity test indicated that Erythromycin was the preferred drug for treatment, while Oxytetracycline and Tetracycline showed the least efficacy in the study area. Based on the findings, it is recommended to focus on various aspects to address camel mastitis. These include conducting further research, utilizing traditional knowledge, increasing the availability of alternative drugs, providing comprehensive training, and implementing improved management practices. These measures aim to reduce the prevalence and transmission of the disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by ethics committee of Somali region pastoral and agro-pastoral research institute (SORPARI). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HH: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. ZD: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AA: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The funding for the procurement of diagnostic reagents and equipment was provided jointly by Jigjiga University and SORPARI to carry out this research work.
Acknowledgments
The authors acknowledge SORPARI for funding this research. Special thanks to the Jigjiga University for providing access to microbiology laboratory. Finally, we acknowledge the pastoralists who are highly appreciated for their tremendous cooperation and allowing us to perform skin tests on their animals and interviews.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AMR, Antimicrobial resistance; CLSI, Clinical and Laboratory Standards Institute; CMT, California mastitis test; MASL, Meters above sea level; SORPARI, Somali Region Pastoral Agro-pastoral Research Institute
References
1. Adah, AS, Ayo, JO, and Adah, DA. Unique physiological and Behavioural adaptive features of the one-humped camel (Camelus dromedarius) to arid environments. J Appl Vet Sci. (2023) 8:57–64. doi: 10.21608/javs.2022.168375.1184
2. Nagpal, R, Behare, P, Kumar, M, Mohania, D, Yadav, M, Jain, S, et al. Milk, milk products, and disease free health: an updated overview. Crit Rev Food Sci Nutr. (2012) 52:321–33. doi: 10.1080/10408398.2010.500231
3. Iyer, AP, Albaik, M, and Baghallab, I. Mastitis in camels in African and Middle East countries. J Bacteriol Parasitol. (2014) 05:1. doi: 10.4172/2155-9597.1000188
4. Ruegg, PL. A 100-year review: mastitis detection, management, and prevention. J Dairy Sci. (2017) 100:10381–97. doi: 10.3168/jds.2017-13023
5. Abrahmsén, M, Persson, Y, Kanyima, BM, and Båge, R. Prevalence of subclinical mastitis in dairy farms in urban and peri-urban areas of Kampala. Uganda Trop Anim Health Product. (2014) 46:99–105. doi: 10.1007/s11250-013-0455-7
6. Guadu, T, and Abebaw, M. Challenges, opportunities and prospects of dairy farming in Ethiopia: a review. World J Dairy Food Sci. (2016) 11:01–9. doi: 10.5829/idosi.wjdfs.2016.11.1.10140
7. Jilo, K, Galgalo, W, and Mata, W. Camel mastitis: a review. MOJ Ecol Environ Sci. (2017) 2:00034. doi: 10.15406/mojes.2017.02.00034
8. Mohamud, A, Mohamed, Y, Jama, O, Mishra, P, and Mohamed, M. Prevalence and major pathogens associated with clinical and subclinical mastitis in dairy camel (Camelus dromedarius) in Benadir region of Somalia. Vet Sci Res Rev. (2020) 6:132–7. doi: 10.17582/journal.vsrr/2020.6.2.132.137
9. Seligsohn, D, Nyman, A, Younan, M, Sake, W, Persson, Y, Bornstein, S, et al. Subclinical mastitis in pastoralist dairy camel herds in Isiolo, Kenya: prevalence, risk factors, and antimicrobial susceptibility. J Dairy Sci. (2020) 103:4717–31. doi: 10.3168/jds.2019-17701
10. Balemi, A, Gumi, B, Amenu, K, Girma, S, Gebru, M, Tekle, M, et al. Prevalence of mastitis and antibiotic resistance of bacterial isolates from CMT positive milk samples obtained from dairy cows, camels, and goats in two pastoral districts in southern Ethiopia. Animals. (2021) 11:1530. doi: 10.3390/ani11061530
11. Girma, A, and Tamir, D. Prevalence of bovine mastitis and its associated risk factors among dairy cows in Ethiopia during 2005–2022: a systematic review and meta-analysis. Vet Med Int. (2022) 2022:1–19. doi: 10.1155/2022/7775197
12. Geresu, MA, Abera Leliso, S, and Liben, GW. Camel mastitis: prevalence, risk factors, and isolation of major bacterial pathogens in Gomole district of Borena zone, southern Ethiopia. Vet Med Int. (2021) 2021:1–11. doi: 10.1155/2021/9993571
13. CSA (2019). Agricultural Sample Survey, Volume II: Report on Livestock and livestock characteristics (Private peasant holdings). Statistical Bulletin 587. Central Statistical Agency (CSA).
15. Ferronatto, JA, Ferronatto, TC, Schneider, M, Pessoa, LF, Blagitz, MG, Heinemann, MB, et al. Diagnosing mastitis in early lactation: use of Somaticell®, California mastitis test and somatic cell count. Ital J Anim Sci. (2018) 17:723–9. doi: 10.1080/1828051X.2018.1426394
16. Adkins, PR, and Middleton, JR. Laboratory Handbook on Bovine Mastitis National Mastitis Council, Incorporated (2017).
17. Quinn, PJ, Markey, BK, Leonard, FC, Hartigan, P, Fanning, S, and Fitzpatrick, E. Veterinary Microbiology and Microbial Disease. Chichester, West Sussex, UK: John Wiley & Sons (2011).
18. CLSI (2018). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Clinical and Laboratory Standards Institute, Wayne, PA.
19. Noble, MA. Bailey and Scott’s diagnostic microbiology, Betty Forbes, Daniel F. Sahm, and Alice S. Weissfeld. St. Louis, MO: Mosby, 2002, 1069 pp. ISBN 0-323-01678-2. Clin Chem. (2002) 48:1816. doi: 10.1093/clinchem/48.10.1816
20. Green, LH. Diagnostic medical microbiology In: LH, Green and E, Goldman editors. Practical Handbook of Microbiology (4th edn) London, New York: CRC Press (2021). 103–14.
21. Clinical and Laboratory Standards Institute (2019). Understanding susceptibility test data as a component of antimicrobial stewardship in veterinary settings. Clinical and Laboratory Standards Institute, Wayne, PA, USA.
22. Al-Juboori, A, Kamat, N, and Sindhu, J. Prevalence of some mastitis causes in dromedary camels in Abu Dhabi, United Arab Emirates. Iraqi J Vet Sci. (2013) 27:9–14. doi: 10.33899/ijvs.2013.82861
23. Bekele, T, and Molla, B. Mastitis in lactating camels (Camelus dromedarius) in Afar region, North-Eastern Ethiopia. Berl Munch Tierarztl Wochenschr. (2001) 114:169–72.
24. Seifu, E, and Tafesse, B. Prevalence and etiology of mastitis in traditionally managed camels (Camelus dromedarius) in selected pastoral areas in eastern Ethiopia. Ethiopian Vet J. (2010) 14:103–14. doi: 10.4314/evj.v14i2.63887
25. Mehamud, J, Megersa, M, Abebe, Y, and Ahmed, M. Prevalence, risk factors and major bacterial causes of camel mastitis in Gursum district, eastern Hararghe. Ethiopia Glob Vet. (2017) 18:203–8. doi: 10.5829/idosi.gv.2017.203.208
26. Wubishet, Z, Dabaso, A, and Getachew, G. Prevalence, associated risk factors and bacterial pathogens of camel mastitis in Borena zone Oromia regional state. Ethiopia Int J Vet Sci. (2016) 5:280–4.
27. Almaw, G, and Molla, B. Prevalence and etiology of mastitis in camels (Camelus dromedarius) in eastern Ethiopia. J Camel Pract Res. (2000) 7:97–100.
28. Ali, M, Avais, M, Ijaz, M, Chaudhary, M, Hussain, R, Aqib, AI, et al. Epidemiology of subclinical mastitis in dromedary camels (Camelus dromedarius) of two distinct agro-ecological zones of Pakistan. Pak J Zool. (2019) 51:527–32. doi: 10.17582/journal.pjz/2019.51.2.527.532
29. Alebie, A, Molla, A, Adugna, W, Tesfaye, A, and Ejo, M. Prevalence, isolation, identification, and risk factors of major bacterial cause of camel subclinical mastitis. BioMed Res Int. (2021) 2021:5522331. doi: 10.1155/2021/5522331
30. Mogeh, A, Teklu, A, and Ogleh, M. The prevalence of mastitis and its associated risk factors in lactating dromedary camels in and around Hargesa, Somaliland. Int J Sci Eng Res. (2019) 10:201–11.
31. Wang, H, Wang, T, Zhang, B, Li, F, Toure, B, Omosa, IB, et al. Water and wastewater treatment in Africa–current practices and challenges. CLEAN Soil Air Water. (2014) 42:1029–35. doi: 10.1002/clen.201300208
32. Eyassu, S, and Bekele, T. Prevalence and etiology of mastitis in traditionally managed camels (Camelus dromedarius) in selected pastoral areas in eastern Ethiopia. Ethiopian Vet J. (2009) 13:69–79.
Keywords: antibiotic sensitivity, antimicrobial resistance, camel, California mastitis test, Staphylococcus aureus, udder health, Jigjiga, Ethiopia
Citation: Jama MM, Hussein HA, Darod ZA and Ahad AA (2024) Determination of prevalence of subclinical mastitis, characterization of intra-mammary infection-causing bacteria, and antibiotic susceptibility in dairy camels in Jigjiga City, Somali region, Ethiopia. Front. Vet. Sci. 11:1398118. doi: 10.3389/fvets.2024.1398118
Edited by:
Domenico Vecchio, Experimental Zooprophylactic Institute of Southern Italy (IZSM), ItalyReviewed by:
Shuvo Singha, Chattogram Veterinary and Animal Sciences University, BangladeshDinah Seligsohn, National Veterinary Institute, Sweden
Copyright © 2024 Jama, Hussein, Darod and Ahad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hassan Abdi Hussein, SGFzc2FuLmFiZGlAamp1LmVkdS5ldA==
 Mohamoud Mohamed Jama
Mohamoud Mohamed Jama Hassan Abdi Hussein
Hassan Abdi Hussein Ziad Abdulahi Darod
Ziad Abdulahi Darod Abdullahi Adan Ahad
Abdullahi Adan Ahad