
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 18 June 2024
Sec. Animal Behavior and Welfare
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1393236
This article is part of the Research Topic Animal Health and Production: Identifying Challenges and Finding a Way Forward View all 40 articles
 Chunli Yang1†
Chunli Yang1† Songhao Liu1†
Songhao Liu1† Cong Tao1†
Cong Tao1† Jing Yu1
Jing Yu1 Mengping Yang1
Mengping Yang1 Lijuan Guo1
Lijuan Guo1 Liya Bao1
Liya Bao1 Xiaobing Li1
Xiaobing Li1 Jing Yang1,2*
Jing Yang1,2* Kangfeng Jiang1*
Kangfeng Jiang1*Toxoplasma gondii (T. gondii) is a worldwide zoonotic parasite that can infect almost warm-blood animals, including humans, which seriously affect the health of host. Cats are known to be the only definitive host of T. gondii and continuously excrete highly infectious oocysts. This parasite carried by the companion animals leads to a great public health risk. However, there is little information on epidemiology of T. gondii in urban cats in Kunming, Southwest China. In the present study, a total of 231 serum and fecal samples were collected in Kunming aera, and then seroprevalence of T. gondii IgG antibodies in serum and molecular investigation in feces were analyzed to elucidate T. gondii infection in urban cats. The results revealed that 168 of 231 cats (72.7%) were positive for T. gondii antibodies, and 1 of 74 cat feces (1.4%) also showed a positive PCR for T. gondii DNA. The positive fecal sample was sequenced and then phylogenetically analyzed, and the isolate of T. gondii in the present study was closely related to T. gondii strain CN. In addition, the food, water and age of cats were identified as the risk factor for seropositivity. Overall, our findings indicate the widespread occurrence of T. gondii infection in urban cats in Kunming, Southwest China and identify food, water and age are the risk factors associated with T. gondii infection, which can provide effective information for developing strategies to prevent and control this zoonosis.
Toxoplasma gondii (T. gondii) is an obligate intracellular parasite with wide worldwide distribution and host range that can infect almost all warm-blooded animals, leading to severe zoonosis (1). This parasite infects one-third of the world population and numerous animals, which raises public health concern (2, 3). The ingestion of oocyst-contaminated water and undercooked food containing cysts are the main sources of T. gondii infection. As definitive hosts of this parasite, cats play an important role in life cycle and spread of T. gondii (4). Sexual reproduction of the parasite occurs in the intestinal epithelium of cats, resulting in the formation of oocysts. The oocysts eventually are excreted in cat feces, contaminating the environment and causing infections in other animals (5, 6). Primary infection is often subclinical, whereas in immunocompromised hosts, the latent infection can be activated and develop into life-threatening toxoplasmosis, including lethal encephalitis, ophthalmia and abortion (7–9).
Previous research has demonstrated that direct contact with cats is not considered a primary risk for human infection due to the short duration of oocyst shedding (10). However, cats could continue to shed millions of oocysts for about 1–2 weeks in their lifetime, which can survive for months in the environment and become a major contributor to human infection (11, 12). With the improvement of living standards, there is a raise in the number of urban cats (13). An increasing number of studies have reported the surveys of Toxoplasma seroprevalence and molecular detection in the world, including some areas of China (3, 11, 14). It is well known that seroepidemiological studies are useful for indicating infection in an area and a special population of cats, which can be used to assess the infection risk for the definitive and intermediate hosts (15). In recent years, seroprevalence of Toxoplasma in cats has been reported to distribute in 5 to 40% in mainland China, yet seroprevalence can be as high as 100% due to sample size and geography (16, 17). Molecular diagnostics of toxoplasmosis were generally based on detection of specific DNA sequences, and the detection of T. gondii DNA in cat feces was generally considered as the presence of oocysts. An earlier serological survey revealed that T. gondii IgG antibodies in pet dogs and pregnant women were 21.6 and 29.2%, respectively, which were speculated to be associated with the existence of a high density of urban cats, exposing people and animals to an elevated density of oocysts (18). However, there is still limited information on the prevalence of T. gondii in urban cats in Kunming, the capital of Yunan, Southwest China, and the risk factors of toxoplasmosis in cats in this region also remain unclear.
In the present study, the serological and molecular survey of T. gondii infection were determined in urban cats in Kunming, Southwest China. In addition, we analyzed the evolutionary relationship of the isolate of T. gondii in current study, and evaluated the associated risk factors exposure to the parasite, which could provide public health workers with useful information for the prevention and control of the zoonosis, and for the protection of human health.
A total of 231 serum and fecal samples were collected from the leg veins of cats between October 2022 and September 2023 in Kunming, Southwest China, with the geographical coordinates of 25°02′47″N and 102°42′34″E, to detect the seroprevalence and fecal DNA of T. gondii in urban cats. The blood samples were left at room temperature for 1 h, followed by centrifugation at 3000 rpm for 10 min to separate the serum, which was stored at −20°C for subsequent ELISA analysis. All feces from cats were harvested from rectum, and the fresh feces were immediately sent to laboratory for DNA determination.
The ELSIA assays were conducted to detect anti-T. gondii antibodies in the serum samples, and the methods were described previously (19, 20). Briefly, freshly egressed T. gondii ME49 tachyzoites were collected and lysed by sonication (21). Subsequently, the lysate was centrifuged at 12000 rpm for 10 min at 4°C, and the soluble Toxoplasma antigen (TSA) were determined by BCA Protein Assay Kit (Beyotime Biotechnology, Beijing, China). Next, the ELISA assays were conducted to investigate anti-T. gondii antibodies in urban cats (20). The 10 μg/mL TSA was diluted in coating buffer (0.05 M Carbonate–Bicarbonate, pH 9.6) at 4°C overnight to perform indirect ELISA analysis, and the serum samples diluted at 1:100 were added to each well. After washing, the HRP conjugated anti-cat IgG secondary antibodies (Solarbio, Rabbit-anti-Cat IgG: K0082R) were diluted 1:2000 and added to appropriate wells. The color was developed by the addition of substrate solution TMB, and the OD value was determined at 450 nm using a microplate reader. For the resulting judgment, the cut-off point of a positive sample was set to be at least two times higher than that of the negative sample.
DNA was extracted from the feces of urban cats via Fecal DNA Isolation Kit (Tiangen, China) according to the manufacture’s protocols. Subsequently, the fecal DNA was amplified with the Toxoplasma RE 529-bp sequence specific primers (forward primer: 5’-TGACTCGGGCCCAGCTGCGT-3’ and reverse primer: 5’-CTCCTCCCTTCGTCCAAGCCTCC-3’) by PCR. The primer pairs and PCR temperature cycling conditions were in according to a previous study (22, 23). The PRC reactions were carried out with a total of 25 μL reaction volume that contained 15 ng of template DNA, 12.5 μL of 2× Taq PCR mix (Vazyme Biotechnology Co., Ltd., Nanjing, China), 1 μL of each primer, and 9.5 μL of ddH2O. PCR program was initiated at 95°C for 5 min, followed by 35 cycles at 95°C for 45 s, 60°C for 45 s, 72°C for 45 s and finished with 72°C for 5 min. Extraction blanks (containing no DNA) were included as negative controls to test for contamination, and DNA was extracted from T. gondii type II strain as the positive control. Finally, the amplified products were loaded on 1.5% agar, and then evaluated under UV [GeL Logic 510-Imaging System (SHST, China)]. The length of the DNA fragment expected to be amplified was approximately 529 bp.
The PCR product was sequenced (Sangon Biotech, Shanghai, China), and sequence analysis of RE 529-bp gene was performed to determine the T. gondii strain. The sequences were analyzed by Basic Local Alignment Search Tool (BLAST), and compared with those of available sequences in the GenBank (24). Phylogenetic analysis was performed using MEGA software (Version: 11) through the neighbor-joining method with 1000 bootstraps to accomplish several sequence alignments (25).
The prevalence and 95% confidence intervals per pathogen species were calculated using the Vassarstats program.1 Differences in T. gondii prevalence for different variables such as gender, breed, food, water, age and lifestyle were analyzed using a chi square test. The differences were considered statistically significant when the resulting p < 0.05.
Of the 231 examined samples, 168 (72.7%) were positive for T. gondii antibodies, and only one (1.4%) sample examined by RE 529-bp PCR showed a positive for Toxoplasma DNA (Table 1 and Supplementary Figure S1). The PCR product of RE 529-bp gene was sequenced and the result was analyzed by BLAST program. The obtained oligonucleotide sequence was submitted to Genbank database, and the evolutionary tree was constructed by MEGA software. The alignment results revealed that the homology level of T. gondii repetitive sequencing products compare to other strains is varies ranging from 79 to 91%. The phylogenetic analysis revealed that the isolate was more closely linked to T. gondii strain CN, an isolate from China (DQ779193.1) (Figure 1).
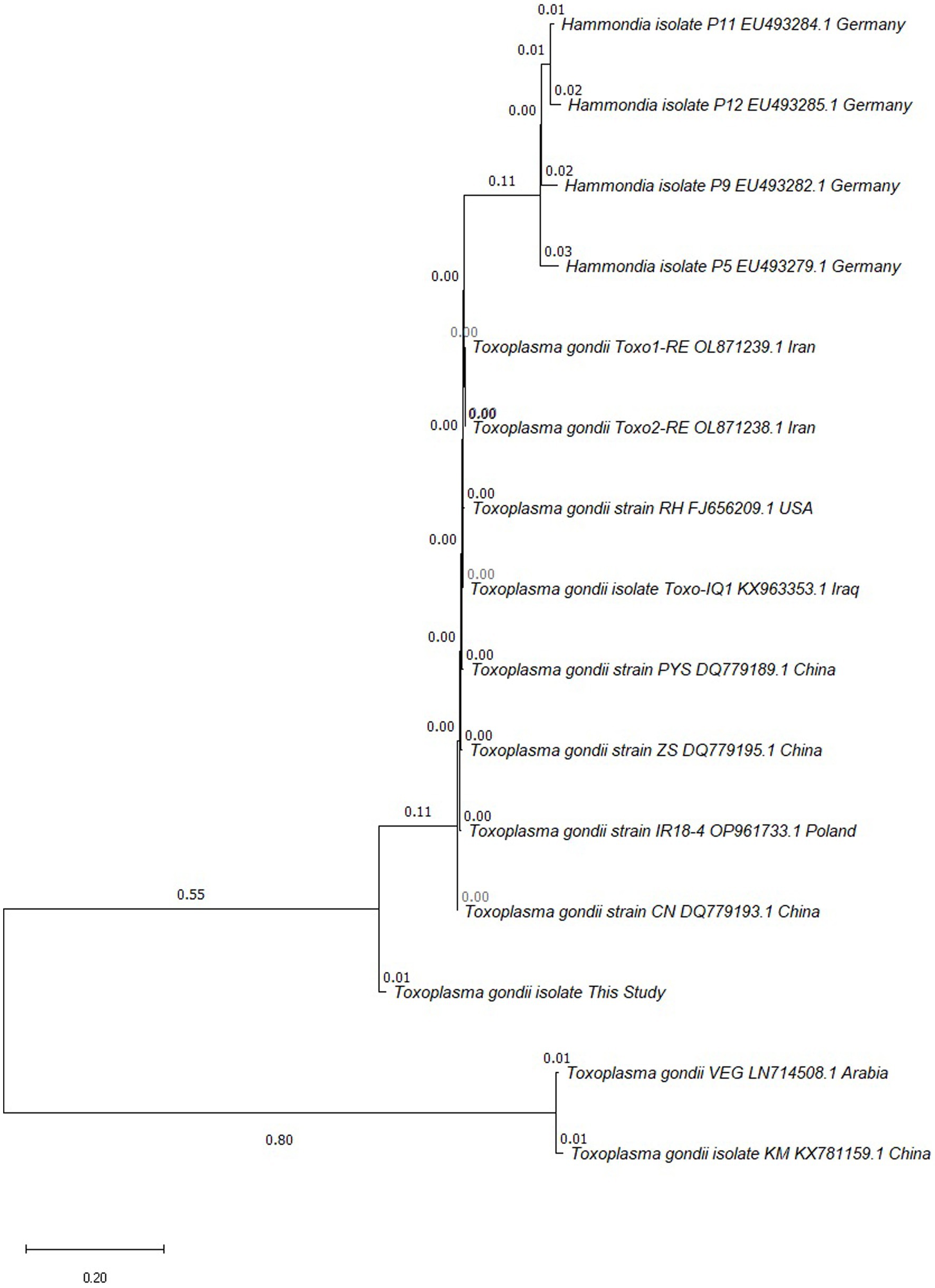
Figure 1. Phylogenetic tree analysis based on repetitive RE 529-bp gene partial sequence was used to confirm the identification of T. gondii isolates by neighbor-joining method.
Next, we analyzed the common potential risk factors for the infection of cats with T. gondii, including gender, breed, food, water, age and lifestyle. The results suggested that there was no significant association between gender or breed and T. gondii positivity (Table 2). Nevertheless, the seropositivity was found to be related to the diet of cats, with a higher positive rate in cats fed with animal organs (61.8%) and tap water (88.0%) than in cats fed with homemade cooked food (8.7%) and boiled water (52.0%). Furthermore, the seroprevalence of T. gondii was significantly associated with age of cats and increased with the ages of cats (Table 2). Unlike other studies, the present study identified no significant relationship between lifestyle and T. gondii positivity, although stray cats were more likely to be exposed to T. gondii oocysts.
T. gondii is a widespread zoonotic pathogen, and cats are the ultimate host of T. gondii and can excrete numerous oocysts. Therefore, cats play a crucial role in the transmission of T. gondii, and the serological and molecular survey of T. gondii infection in cats are essential for the development of toxoplasmosis prevention and control. Previous studies have reported the seroprevalence of T. gondii in cats in Southwest China, however, there was lack of information about the prevalence of toxoplasmosis in urban cats in Kunming, Southwest China (3, 26). In the present study, a total of 231 serum and 74 fecal samples of cats were detected, with positivity of 72.7 and 1.4%, respectively, which were the highest in China report so far. In China, seroprevalence of Toxoplasma infection in cats has been reported in some provinces, with a distribution of infection rates ranging from 2.5 to 60.0%, for an overall infection rate of 20.2% (14). Previous surveys reported varying seroprevalence of T. gondii infection in pet cats in Southwest China: 63.16% in Guizhou (26), 13.0% in Sichuan (3), and 12.8% in Chongqing (3). In addition, an earlier survey mentioned that the seroprevalence of T. gondii infection in cats in Kunimg was as high as 50.3%, although this data has not yet been published (18). Compared with the previous sero-epidemiological studies form these reported provinces, the frequency of Toxoplasma-seropositive cats in our study was significantly higher. The difference in seroprevalence for T. gondii may be relate to ecological and geographic factors, as well as the welfare status of urban cats in the areas. Another possible reason for the high T. gondii seropositivity is the dietary habit of eating raw meat in Kunming region, and the urban cats in Kunming are likely to be fed with undercooked food contaminated with cysts. Taken together, above data indicated that toxoplasmosis was widely spread cats in the region.
As the only terminal host of T. gondii, the oocysts excreted in the feces of cats are a huge threat to the environment and other animals (27). The molecular investigation of T. gondii infection is still limited, and our study is the first to report the Toxoplasma infection in cat feces in Kunming. The molecular prevalence of T. gondii infection in Kunming is 1.4%, which was different from 0% in Qinghai (14) and 52.63% in Guizhou (26). The high seropositivity rate of T. gondii but low PCR positivity rate in urban cats in Kunming is consistent with the results of some previous studies. Prevalence of anti-T. gondii antibodies was 14.8%, and only 0.7% positive rate for PCR (28). The seroprevalence of T. gondii infection in cats was as high as 41.4%, whereas the DNA positivity rate was 0% (14). It is generally believed that the definitive host sheds oocysts for about 2 weeks at the time of initial infection and thereafter no longer sheds oocysts upon secondary infections, due to long-term immunization preventing oocyst shedding (29, 30). Therefore, seropositivity of T. gondii does not mean that the cat is excreting oocysts, which requires further measurement by PCR (30, 31). In addition, it has been suggested that the detection of T. gondii DNA in the feces of cats does not necessarily imply oocyst shedding, and there may exist bradyzoites from prey passing through gut, which are also infective (32, 33). The strain identified in current study was closely related to the isolate from Guangdong area, while more distantly related to typical strains (types I and III strains), and to the strain previously reported in cat feces in Kunming (34).
Common potential risk factors for T. gondii infection in cats include gender, breed, food, water, age and lifestyle (35, 36). Similar to other studies, the present study also confirmed that gender was not a key factor for Toxoplasma infection (3, 37). However, there was no significant difference in T. gondii seropositivity between mixed breed and thoroughbred cats, which may imply that there was no preference for different cat breeds in Kunming area. In terms of age, the seropositive rate of T. gondii was 60.4% in cats under 1 year of age, while the seropositive rate was much higher in cats older than 1 year of age, which may be related to the increased probability of exposure to T. gondii through food, water, and outdoor activities as cats grow older (38). Furthermore, the lower seropositivity of T. gondii in cats fed with homemade cooked food and boiled water suggested that cats were less susceptible to T. gondii in the feeding model. However, fecal Toxoplasma positive rate was higher in younger cats, suggesting that cats have previously been infected with T. gondii, and that they no longer secrete oocysts with age, which is consistent with the serologic results. In addition, this study also found that there was no significant correlation between the seropositive rate of T. gondii and lifestyle, which was different from some previous reports (3, 11), suggesting that urban cats in the Kunming area are raised in a special way, especially domesticated cats, whose seropositive rate of T. gondii was not significantly different from that of stray cats, suggesting that pet owners in this area may often feed their cats undercooked meat or contaminated water. It is worth drawing attention to the fact that the high seropositive rate of domesticated cats, indicating that a large number of T. gondii oocysts may be present in the environment of the region, which may have contaminated the water source in the region.
In conclusion, our study reveals that the infection rate of T. gondii in cats in Kunming area is high, which implies there is a hidden danger of public health safety. As a common pet in people’s life, cats carry pathogens that pose a threat to human health. Therefore, the investigation and analysis of T. gondii infection in cats is of great significance for avoiding human infections and formulating scientific and reasonable strategies to control toxoplasmosis. More measures need to be taken in order to protect the public health. The proper disposal of cat litter, and feeding cats with cooked food and boiled water as much as possible to reduce the oocyst burden are recommended. In addition, given the high seroprevalence of T. gondii IgG antibodies, it is necessary to perform a screening test and determine the IgG antibody titer in pregnant women and immunocompromised humans in Kunming, Southwest China.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The animal studies were approved by the Life Scientific Ethic Committee of Yunnan Agricultural University (Approval number: 202207006). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
CY: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. SL: Methodology, Project administration, Writing – review & editing. CT: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. JYu: Data curation, Writing - review & editing. MY: Data curation, Writing - review & editing. LG: Data curation, Writing - review & editing. LB: Data curation, Writing - review & editing. XL: Project administration, Writing - review & editing. JYa: Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. KJ: Validation, Writing – review & editing, Funding acquisition, Methodology, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Yunnan Provincial Innovation Team of Key Technologies for Prevention and Control of Important Livestock and Poultry Diseases (grant No. 202405AS350004), Yunnan Education Department Research Projects (grant No. 2023J0487), and Longyan University & Fujian Provincial Key Laboratory for the Prevention and Control of Animal Infectious Diseases and Biotechnology (grant No. ZDSYS2022004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1393236/full#supplementary-material
PCR products of isolated T. gondii from cat feces based on RE 529-bp gene. M, 3000 bp molecular weight marker; P, positive control; N, negative control; lanes 1-22, PCR product of RE 529-bp gene.
2. Dubey, JP, Cerqueira-Cézar, CK, Murata, FHA, Kwok, OCH, Hill, D, Yang, Y, et al. All about Toxoplasma gondii infections in pigs: 2009-2020. Vet Parasitol. (2020) 288:109185. doi: 10.1016/j.vetpar.2020.109185
3. Xia, N, Ji, N, Li, L, Huang, Y, Yang, C, Guo, X, et al. Seroprevalence and risk factors of Toxoplasma gondii in urban cats from China. BMC Vet Res. (2022) 18:331. doi: 10.1186/s12917-022-03427-w
4. Dubey, JP . The history of Toxoplasma gondii--the first 100 years. J Eukaryot Microbiol. (2008) 55:467–75. doi: 10.1111/j.1550-7408.2008.00345.x
5. Hunter, CA, and Sibley, LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. (2012) 10:766–78. doi: 10.1038/nrmicro2858
6. Vilares, A, Gargaté, MJ, Ferreira, I, Martins, S, Júlio, C, Waap, H, et al. Isolation and molecular characterization of Toxoplasma gondii isolated from pigeons and stray cats in Lisbon, Portugal. Vet Parasitol. (2014) 205:506–11. doi: 10.1016/j.vetpar.2014.08.006
7. Elsheikha, HM, Marra, CM, and Zhu, XQ. Epidemiology, pathophysiology, diagnosis, and Management of Cerebral Toxoplasmosis. Clin Microbiol Rev. (2021) 34:e00115–9. doi: 10.1128/CMR.00115-19
8. Montoya, JG, and Liesenfeld, O. Toxoplasmosis. Lancet. (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
9. Tenter, AM, Heckeroth, AR, and Weiss, LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. (2000) 30:1217–58. doi: 10.1016/S0020-7519(00)00124-7
10. Elmore, SA, Jones, JL, Conrad, PA, Patton, S, Lindsay, DS, and Dubey, JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. (2010) 26:190–6. doi: 10.1016/j.pt.2010.01.009
11. Attipa, C, Yiapanis, C, Tasker, S, and Diakou, A. Seroprevalence of Toxoplasma gondii in cats from Cyprus. Pathogens. (2021) 10:882. doi: 10.3390/pathogens10070882
12. Dubey, JP, and Jones, JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. (2008) 38:1257–78. doi: 10.1016/j.ijpara.2008.03.007
13. Ding, H, Gao, YM, Deng, Y, Lamberton, PH, and Lu, DB. A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasit Vectors. (2017) 10:27. doi: 10.1186/s13071-017-1970-6
14. Yang, J, Ai, J, Qi, T, Ni, X, Xu, Z, Guo, L, et al. Toxoplasma gondii and Neospora caninum infections in stray cats and dogs in the Qinghai-Tibetan plateau area, China. Animals. (2022) 12:1390. doi: 10.3390/ani12111390
15. Bawm, S, Phyu, AZ, Chel, HM, Htun, LL, Nakao, R, and Katakura, K. Seroprevalence of Toxoplasma gondii in household cats in Myanmar and molecular identification of parasites using feline faecal oocysts. Food Waterborne Parasitol. (2020) 20:e00094. doi: 10.1016/j.fawpar.2020.e00094
16. Dubey, JP, Cerqueira-Cézar, CK, Murata, FHA, Kwok, OCH, Yang, YR, and Su, C. All about toxoplasmosis in cats: the last decade. Vet Parasitol. (2020) 283:109145. doi: 10.1016/j.vetpar.2020.109145
17. Zhou, S, Sang, Z, Wang, L, and Zhang, T. Seroprevalence of Toxoplasma gondii in cats in mainland China 2016-2020: a meta-analysis. J Vet Sci. (2022) 23:e13. doi: 10.4142/jvs.21209
18. Duan, G, Tian, YM, Li, BF, Yang, JF, Liu, ZL, Yuan, FZ, et al. Seroprevalence of Toxoplasma gondii infection in pet dogs in Kunming, Southwest China. Parasit Vectors. (2012) 5:118. doi: 10.1186/1756-3305-5-118
19. Gu, Y, Wang, Z, Cai, Y, Li, X, Wei, F, Shang, L, et al. A comparative study of Toxoplasma gondii seroprevalence in mink using a modified agglutination test, a Western blot, and enzyme-linked immunosorbent assays. J Vet Diagn Invest. (2015) 27:616–20. doi: 10.1177/1040638715596033
20. Sun, X, Wang, Z, Li, J, Wei, F, and Liu, Q. Evaluation of an indirect ELISA using recombinant granule antigen GRA1, GRA7 and soluble antigens for serodiagnosis of Toxoplasma gondii infection in chickens. Res Vet Sci. (2015) 100:161–4. doi: 10.1016/j.rvsc.2015.04.011
21. Yang, J, Yang, C, Qian, J, Li, F, Zhao, J, and Fang, R. Toxoplasma gondii α-amylase deletion mutant is a promising vaccine against acute and chronic toxoplasmosis. Microb Biotechnol. (2020) 13:2057–69. doi: 10.1111/1751-7915.13668
22. Arefkhah, N, Sarkari, B, Asgari, Q, Moshfe, A, Khalafi, MH, and Mohammadpour, I. Molecular genotyping of Toxoplasma gondii in sheep aborted fetuses reveals predominance of type I infection in southwest of Iran. Iran J Parasitol. (2020) 15:374–82. doi: 10.18502/ijpa.v15i3.4202
23. Edvinsson, B, Lappalainen, M, and Evengård, B. Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Clin Microbiol Infect. (2006) 12:131–6. doi: 10.1111/j.1469-0691.2005.01332.x
24. Altschul, SF, Gish, W, Miller, W, Myers, EW, and Lipman, DJ. Basic local alignment search tool. J Mol Biol. (1990) 215:403–10. doi: 10.1016/S0022-2836(05)80360-2
25. Tamura, K, Stecher, G, and Kumar, S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. (2021) 38:3022–7. doi: 10.1093/molbev/msab120
26. Li, YN, Nie, X, Peng, QY, Mu, XQ, Zhang, M, Tian, MY, et al. Seroprevalence and genotype of Toxoplasma gondii in pigs, dogs and cats from Guizhou province, Southwest China. Parasit Vectors. (2015) 8:214. doi: 10.1186/s13071-015-0809-2
27. Halonen, SK, and Weiss, LM. Toxoplasmosis. Handb Clin Neurol. (2013) 114:125–45. doi: 10.1016/B978-0-444-53490-3.00008-X
28. Galván-Ramírez, ML, Charles-Niño, C, Pedroza-Roldán, C, Salazar-Reveles, C, Ocampo-Figueroa, KL, Rodríguez-Pérez, LR, et al. Prevalence of Toxoplasma gondii measured by Western blot, ELISA and DNA analysis, by PCR, in cats of Western Mexico. Pathogens. (2022) 11:109. doi: 10.3390/pathogens11010109
29. Chi, X, Fang, K, Koster, L, Christie, J, and Yao, C. Prevalence of feline immunodeficiency virus and Toxoplasma gondii in feral cats on St. Kitts, West Indies. Vet Sci. (2021) 8:16. doi: 10.3390/vetsci8020016
30. Dubey, JP . Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J Parasitol. (1995) 81:410–5. doi: 10.2307/3283823
31. Sağlam, T, Düşen, S, Mete, E, and Karaman, Ü. Comparative evaluation of re 529-Bp sequence and B1 Gene in the detection of Toxoplasma gondii through PCR in water samples of Denizli, Turkey. Acta Parasitol. (2022) 67:555–9. doi: 10.1007/s11686-021-00494-1
32. Poulle, ML, Forin-Wiart, MA, Josse-Dupuis, É, Villena, I, and Aubert, D. Detection of Toxoplasma gondii DNA by qPCR in the feces of a cat that recently ingested infected prey does not necessarily imply oocyst shedding. Parasite. (2016) 23:29. doi: 10.1051/parasite/2016029
33. Zhu, S, Camp, L, Patel, A, Vanwormer, E, and Shapiro, K. High prevalence and diversity of Toxoplasma gondii DNA in feral cat feces from coastal California. PLoS Negl Trop Dis. (2023) 17:e0011829. doi: 10.1371/journal.pntd.0011829
34. Liang, Y, Chen, J, Meng, Y, Zou, F, Hu, J, and Esch, GW. Occurrence and genetic characterization of GRA6 and SAG2 from Toxoplasma gondii oocysts in cat feces, Kunming, China. Southeast Asian J Trop Med Public Health. (2016) 47:1134–42.
35. Metwally, S, Hamada, R, Sobhy, K, Frey, CF, and Fereig, RM. Seroprevalence and risk factors analysis of Neospora caninum and Toxoplasma gondii in cattle of Beheira, Egypt. Front Vet Sci. (2023) 10:1122092. doi: 10.3389/fvets.2023.1122092
36. Rafique, A, Nasir, S, Ashraf, A, Nawaz, Z, Zahid, FM, Abbas, A, et al. Sero-surveillance and risk factors analysis of caprine toxoplasmosis in Faisalabad Punjab, Pakistan. Pak Vet J. (2022) 42:102–6. doi: 10.29261/pakvetj/2021.020
37. Wang, S, Zhou, Y, Niu, J, Xie, Q, Xiao, T, Chen, Y, et al. Seroprevalence of Toxoplasma gondii infection in domestic cats in Central China. Parasite. (2017) 24:10. doi: 10.1051/parasite/2017010
Keywords: Toxoplasma gondii, cat, seroprevalence, molecular investigation, risk factor, zoonosis
Citation: Yang C, Liu S, Tao C, Yu J, Yang M, Guo L, Bao L, Li X, Yang J and Jiang K (2024) Serological and molecular survey of Toxoplasma gondii infection and associated risk factors in urban cats in Kunming, Southwest China. Front. Vet. Sci. 11:1393236. doi: 10.3389/fvets.2024.1393236
Received: 28 February 2024; Accepted: 22 April 2024;
Published: 18 June 2024.
Edited by:
Izhar Hyder Qazi, Shaheed Benazir Bhutto University of Veterinary & Animal Sciences, PakistanReviewed by:
Muhammad Kasib Khan, University of Agriculture, Faisalabad, PakistanCopyright © 2024 Yang, Liu, Tao, Yu, Yang, Guo, Bao, Li, Yang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yang, SmluZ3lhbmc3MjhAeW5hdS5lZHUuY24=; Kangfeng Jiang, a2FuZ2ZlbmdqaWFuZ0B5bmF1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.