
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 09 April 2024
Sec. Veterinary Epidemiology and Economics
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1380203
 Nisar Ahmad1,2,3*
Nisar Ahmad1,2,3* Saeed A. Khan4
Saeed A. Khan4 Hafiz A. Majid5
Hafiz A. Majid5 Rehman Ali1,2
Rehman Ali1,2 Riaz Ullah6
Riaz Ullah6 Ahmed Bari7
Ahmed Bari7 Noor Ul Akbar3
Noor Ul Akbar3 Abdul Majid3*
Abdul Majid3*Introduction: Haemonchus contortus (H. contortus) is a blood-feeding nematode causing infectious disease haemonchosis in small ruminants of tropical and subtropical regions around the world. This study aimed to explore the prevalence and phylogeny of H. contortus in small ruminants using the internal transcribed spacer-2 (ITS-2) gene. In addition, a comprehensive review of the available literature on the status of H. contortus in Pakistan was conducted.
Methods: Fecal samples were collected from sheep and goats (n = 180). Microscopically positive samples were subjected to DNA extraction followed by PCR using species-specific primers.
Results: The overall prevalence of H. contortus was 25.55% in small ruminants. The prevalence of H. contortus was significantly associated with months and area. The highest occurrence of haemonchosis was documented in July (38.70%), whereas the lowest occurred in December (11.11%), with significant difference. The prevalence was highest in the Ghamkol camp (29.4%) and lowest in the arid zone of the Small Ruminant Research Institute (17.5%) (p = 0.01). The results of the systematic review revealed the highest prevalence of haemonchosis (34.4%) in Khyber Pakhtunkhwa (p = 0.001).
Discussion: Phylogenetic analysis revealed a close relationship between H. contortus and isolates from Asia (China, India, Iran, Bangladesh, Malaysia, and Mongolia) and European countries (Italy and the United Kingdom). It has been concluded that H. contortus is prevalent in small ruminants of Kohat district and all over Pakistan, which could be a potential threat to food-producing animals, farmers, dairy, and the meat industry. Phylogenetic analysis indicates that H. contortus isolates share close phylogenetic relationships with species from Asia and Europe.
Haemonchus contortus (H. contortus) is a parasitic worm causing haemonchosis in ruminants of tropical and subtropical climates around the globe (1, 2). Adult worms suck blood from the abomasum of goats and sheep, triggering edema, anemia, diarrhea, and sometimes death (3). Gastrointestinal parasites are abundant, and a single female is capable of producing up to 10,000 eggs each day (4). Eggs are excreted in host feces and dispersed into infective L3s on pastures, where they infect new hosts when consumed, and in turn infect millions of sheep and goats throughout the world. Haemonchus infects a wide variety of hosts and quickly becomes resistant to most of the anthelminthic drugs to contain it (5). It causes substantial economic losses due to the cost of anthelmintic medications, body weight loss, decrease in milk, meat, wool, and overall growth, and sometimes causes death to infected animals, adversely affecting livestock production (6).
Haemonchus is the most economically important blood-feeding parasite in grazing ruminants worldwide (7), with ancestral roots in sub-Saharan Africa, where numerous species of native artiodactyl hosts exist (8). Three known sympatric species of Haemonchus infect ruminants across Asia. A greater number of H. contortus, H. longistipes, and H. placei have been documented due to the international movement of domesticated animals (7, 8). H. contortus is primarily a small ruminant parasite; H. longistipes is most frequently reported in camels; and H. placei infects cattle. In addition, H. similis, the fourth sympatric species, has been found to infect Latin American cattle (9).
Helminth resistance to numerous anthelmintic medications is rapidly growing, causing significant public health problems. In the near future, controlling some parasites with current anthelminthic medications like oxfendazole, levamisole, and ivermectin may become more challenging (10). H. contortus has evolved different strategies to evade host immune response during infection (12, 13). Parasitic nematodes cause a long-term infection in the host, generally with just a modest inflammatory response. This is attributed to the release of complex excretory/secretory protein (ESP) mixtures into host tissues that interfere with host signaling mechanisms and immune homeostasis. However, these molecular mechanisms are yet to be explored and need further in-depth research. Therefore, to control parasitic infections researchers are actively exploring alternative methods such as nanovaccine (14, 15), nanoparticle (16), and plant extracts have shown promising anti-parasitic activity to counter anthelminthic resistance in H. contortus (17–19).
Pakistan is mainly an agricultural country contributing 22.9% to the Gross Domestic Product (GDP), where livestock account for approximately 62.68% of agriculture and 14.36% of the national GDP (20). H. contortus infestation has become one of the biggest problems of sheep and goat husbandry in Pakistan. Aside from the lack of meat due to mortality and lower growth in small ruminants, there is an exceedingly high proclivity to gain immunity against anthelmintic medications, and a proven model for researching the genetics and population structure of resistant strains (9).
Environmental and geographical constraints, population growth, and living conditions are a few of the variables that alter a population’s genetic makeup (21). Haemonchus is favored by a high rate of gene flow across populations, providing an opportunity for the dissemination of genes conferring resistance to anthelmintics (22). Parasitic nematode ecology, epidemiology, and evolution can be better understood by studying genetic diversity and genetic relationships using phylogenetic trees (23). In addition, precise identification and genetic characterization are essential for a valid diagnosis and efficacy in the control programs of parasitic nematodes (3). The ribosomal ITS-2 gene is one of the most variable nuclear loci, and its high rate of evolution can be used to achieve intraspecific variation among H. contortus populations (24). This nematode marker has been used in species differentiation and genetic variability studies. Interpretation of the inherited differences within and among H. contortus species may promote a better understanding of transmission patterns and the establishment of a control strategy (22).
Controlling haemonchosis is essential to recouping losses, since it significantly harms Pakistan’s small ruminant industry. To ensure effective and reliable parasite control, it is necessary to have a thorough understanding of the current epidemiological parameters influencing the distribution of disease. Numerous studies have been conducted on H. contortus in ruminants around Pakistan, as shown in Table 1; however, no microscopic, molecular, or phylogenetic analysis study has been found in the province of Khyber Pakhtunkhwa, particularly district Kohat. Therefore, the current study was designed to investigate epidemiology and phylogeny of H. contortus species in small ruminants with a systematic review of Pakistan’s previously published studies on ruminants to provide baseline information. The paucity of molecular data on Haemonchus in the small ruminant population of Kohat district was the main impetus to carry out this investigation. The current study will advance our knowledge of Pakistan’s haemonchosis epidemiology, including its genesis, transmission patterns, and population structure, which might direct government actions to halt the spread of the disease.
The current study was approved from Kohat University of Science and Technology Kohat ethical approval committee for sample collection from animals. Written informed consent has been granted by the owners for their animal’s participation in the current study.
The present study was done at Kohat, Khyber Pakhtunkhwa. It lies 489 m above sea level between 32° 47′ and 33° 53′ north latitude and 70° 34′ and 72° 17′ east longitude (Figure 1). Approximately 5 g of feces was directly obtained from the rectum of 180 sheep and goats (90 each) under strict aseptic conditions in the study area. All information, including the animal type, sex, age, location, and collection month, was recorded on the mandated proforma for each sheep and goat. The Molecular Parasitology and Virology Laboratory, Department of Zoology, KUST, received appropriately labeled samples in cold boxes. Prior to further processing, the samples were preserved at −20°C.
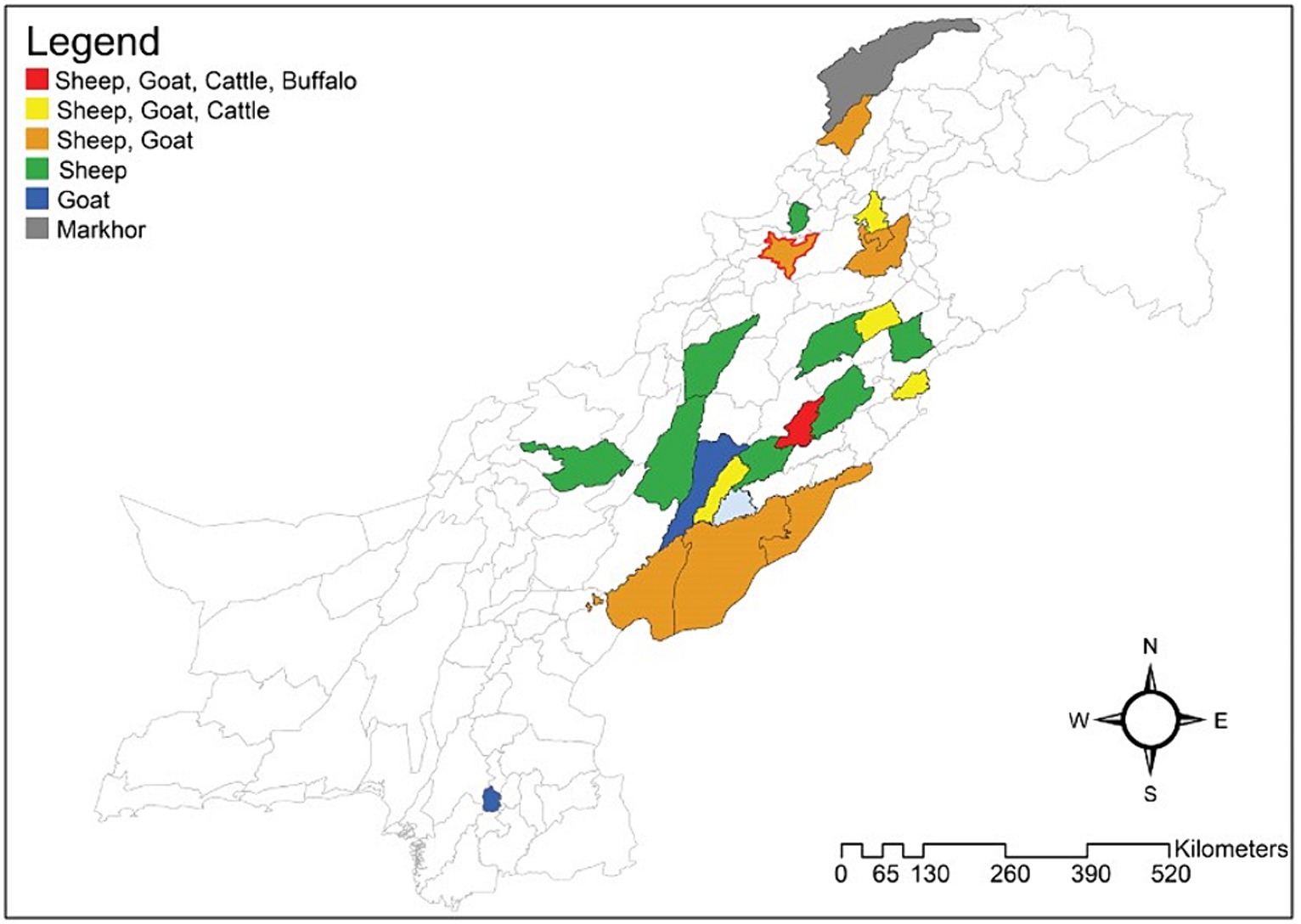
Figure 1. Pakistani map with colored areas displays epidemiological studies performed on Haemonchus contortus in different districts. 1) Red indicates studies performed on sheep, goats, cattle, and buffaloes in that area; 2) sheep, goat, and cattle investigations are shown in yellow. 3) The golden hue indicates that both sheep and goats were examined for H. contortus in certain areas. 4) Studies on sheep are indicated in green. 5) Studies involving goats are shown in blue. 6) Studies involving only markhor are displayed in gray, and 7) Red outline on golden hue color shows the current study sampling area district Kohat. (Drawn using the software “ArcGIS” (https://desktop.arcgis.com/en/)).
Three grams of feces were added to container 1 with 50 mL of PBS dispensed into the same container, and a tongue blade was used to mix the feces with the PBS solution. The fecal suspension was transferred from the jar to container 2, and finally to the test tube using a double layer of cheesecloth. A convex meniscus formed on top of the test tube after the solution was1 gently tapped off. A delicate coverslip was set atop the test tube and left undisturbed for 20 min. Twenty minutes later, the coverslip and drop of fluid attached to it were carefully removed from the test tube and placed on a clean slide. For microscopic positive samples, the tube was filled with 2 mL of PBS and spun in a microcentrifuge for 5 min at 3000 rpm to rinse the eggs. After the supernatant was removed, the rinsing procedure was repeated. Once the supernatant was removed, 300 𝜇l of the ova in the pellet was collected for genome extraction (46). Coverslips adhering to the slide were stained with iodine and examined for eggs and larvae using a light compound microscope under 10X and 40X objective lens, according to Ebrahim (47).
Haemonchus positive samples were extracted using the “QIAamp Fast DNA Stool Mini Kit” (Qiagen GmbH, Hilden, Germany). The manufacturer’s protocol was used to extract the complete genomic DNA. Amplification of the prepared DNA was carried out as described by Hussain et al. (9). The ITS2 gene was amplified using NC1F (5’-ACGTCTGGTTCAGGGTTGTT-3′) and NC1R (5’-TTAGTTTCTTTTCCTCCGCT-3′) with an amplicon size of 350 bp (23). To achieve the necessary gene amplification, a thermal cycler was utilized with a 25 𝜇l PCR reaction volume comprising of 13 𝜇l PCR Master mix (dNTPs, MgCl2, Taq DNA polymerase), 1 𝜇l forward and reverse primers, 5 𝜇l PCR water (dH2O), and 5 𝜇l extracted DNA. The initial temperature was 96°C for 7 min, followed by 40 cycles of 95°C for 45 s, 57.5°C for 45 s, and 72°C for 45 s, with a final extension temperature of 72°C for 7 min. A 1.5% agarose gel was used for DNA resolution. Bands (Fermentas, United States) were compared to a 2000 bp DNA ladder marker (23). A gel documentation device was used to resolve the bands under UV illumination.
Adherence to Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) was maintained throughout the execution of this review on articles related to H. contortus epidemiology in ruminants across Pakistan (10). Research articles published in English were retrieved from different databases, including ISI Web of Science, Mendeley, PubMed, ScienceDirect, EBSCHO, and Google Scholar. Databases were searched for articles using keywords such as prevalence, epidemiology, infection rate/infestation rate, Haemonchus contortus, Haemonchus, sheep, goat, ruminants, and Pakistan. Boolean operators “AND” and “OR” were also used to retrieve articles (“Prevalence” OR “epidemiology” OR “infection rate” OR “infected” AND “Haemonchus contortus” AND “Pakistan” AND “ruminants” OR “sheep” OR “goat”) on the H. contortus distribution rate across Pakistan from January 2000 till July 2023. The article references and PDFs were downloaded to a folder created using Mendeley reference manager software for the current study. Duplicate studies were removed from the folder after checking the title and references, followed by irrelevant article removal due to the non-availability of data in the abstract.
We checked the article titles and abstracts to determine whether they had any data on the prevalence of H. contortus in Pakistan. The inclusion criteria were as follows: i) articles written in English, ii) articles containing H. contortus distribution in ruminants, and iii) articles published between January 2000 and July 2023. However, we excluded reviews, duplicate articles, studies dealing with other species of Haemonchus prevalence data, articles involving two species without confirmation of a single species outcome, publications with vague data such as trichostrongylid/strongyles infection rate, or sources of samples without complete text.
The current systematic review included studies that were evaluated using the following criteria to ascertain quality scores: (a) random sampling/not, (b) sampling method clear/not, (c) sampling method detailed/not, (d) number of samples ≥80/not, and (e) ≥3 risk factors. By calculating the aforementioned answers, each study was given a score; a score of 0 for “no” and a score of 1 was given for “yes,” aggregating a total score of 5. Studies with a score of 0–1, 2–3, and 4–5 were classified as below average, average, and good quality, respectively.
To ensure that no papers were overlooked, two writers (NA and RA) separately searched the English databases for titles and abstracts of articles that met the inclusion criteria. The eligibility of the study for inclusion was determined by the same writers who independently reviewed the entire text. Potentially eligible studies were excluded if (i) the prevalence data results were not provided in terms of the number of samples and (ii) the dataset was ambiguously presented based on ruminants. Another author (AM) made the ultimate choice following a debate in which the two writers could not reach an agreement. Finally, the data were extracted by NA and RA, which included pertinent information about the initial author, publication year, host species, type of sample, method of study, number of examined samples, positive sample, prevalence rate, random sampling/not, clear sampling method/not, detailed sampling method/not, number of samples ≥80/not, and ≥ 3 risk factors studies/not to calculate the score and quality of included studies using a pre-designed Microsoft Excel spreadsheet (Figure 2).
After amplicon confirmation through gel electrophoresis, 4 randomly selected amplified DNA products (two each sheep and goat samples) were shipped to Macrogen Inc. (Seoul, South Korea) for purification and sequencing. The chromatograms of the sequenced samples were interpreted, and noise was removed at the start and end of each sequence using BioEdit software. The resulting sequences were subjected to BLAST in NCBI2 for confirmation of H. contortus. After confirmation of H. contortus, the reference sequences were retrieved and transferred to Mega11 for further processing. Sequences were trimmed and aligned using Clustal W, followed by the inclusion of an outgroup in the software, and the maximum composite likelihood technique was used for phylogenetic analysis. The sequences from this study and those from the NCBI gene repository were used to construct a phylogenetic tree. Through the addition of a maximal composite probability parameter and 1,000 bootstrap replications, an evolutionary tree was constructed using the maximum composite likelihood approach. Nucleotide sequences reported in the present study are available in GenBank™, an NCBI database, under the accession numbers OK447878, OK481181, OM276841, and OM276825.
To determine the differences between the observed and expected data, numerous factors, including type of ruminant, age, sex, month, and distribution by locality, were analysed in relation to the prevalence of haemonchosis using Chi-squared and Fisher exact tests in R software. A p-value of less than 0.05 was used to determine the statistical significance of the findings (11).
A total of 180 fecal samples were collected from both sheep and goats of different age groups, sex, months, and area from July to December, 2021. Forty-six (46) small ruminants were found positive for haemonchosis. The documented overall prevalence of H. contortus in small ruminants was 25.55%. However, sheep was non-significantly more susceptible (28.89%) to haemonchosis in comparison to goats (22.22%) p > 0.05 (Figure 3A). Both goats and sheep were divided into male and female categories based on sex. The sex-wise infection of H. contortus recorded in the current research work was higher in female sheep and goats (28.82%) as compared to male sheep and goats (20.28%) p > 0.05 (Figure 3B).
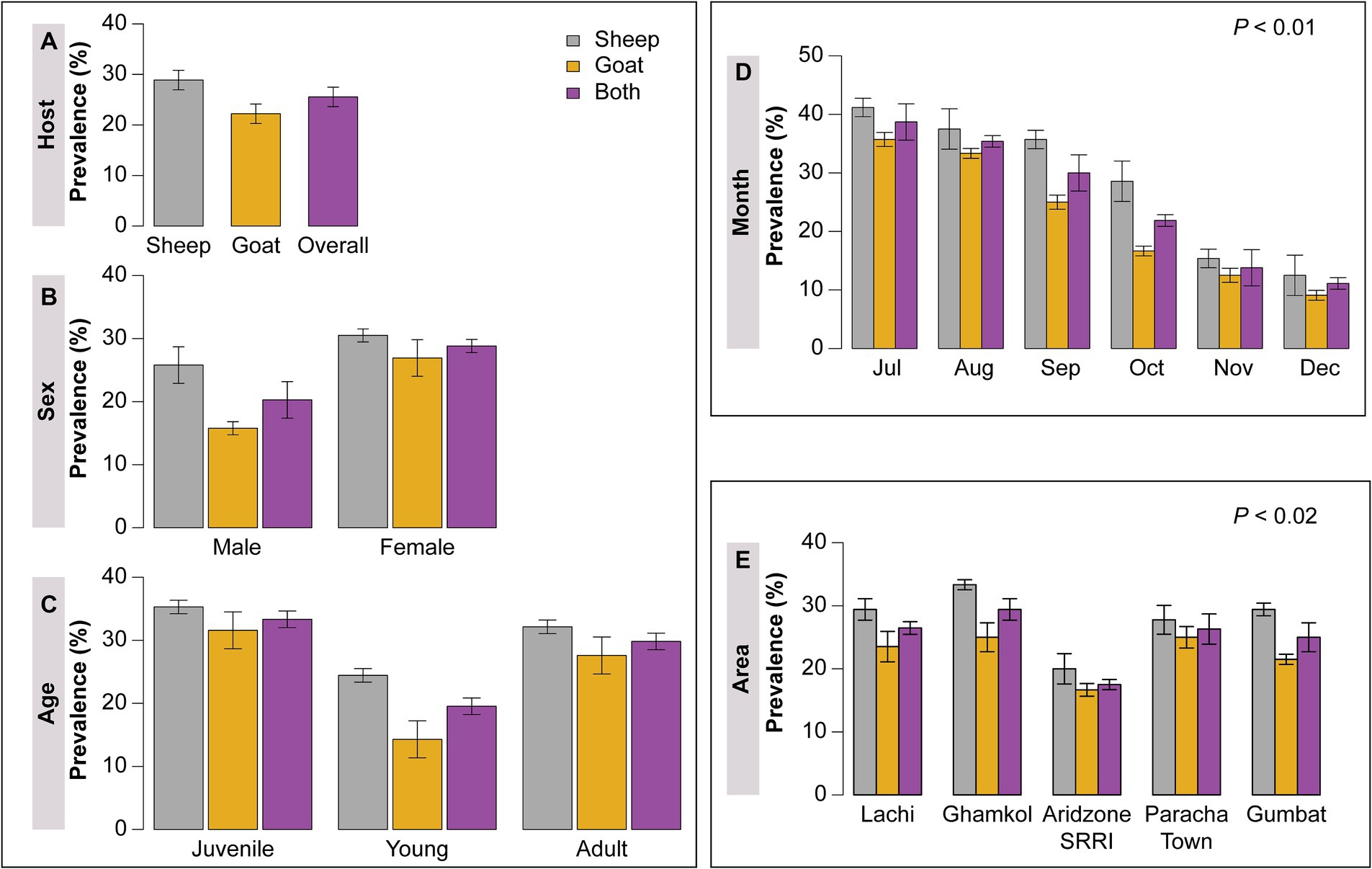
Figure 3. (A) Showing host based overall prevalence of H. contortus, (B) sex wise prevalence of sheep and goat, (C) H. contortus occurrence based on age group, (D) area wise prevalence, and (E) Month wise prevalence of H. contortus in sheep and goats.
Small ruminants were divided into three age groups: <1 year, 1–3 year, and > 3 years named as juvenile, young and adult, respectively. The highest prevalence rate was recorded in juvenile (33.33%) and adult age groups (29.82%), while the lowest was in the young age group (19.54%) however, the differences were non-significant p > 0.05 (Figure 3C). The present study was divided into 5 different localities of district Kohat named Lachi, Ghamkol Camp, Arid Zone Small Ruminant Research Institute (SRRI), Paracha Town, and Gumbat. The highest occurrence of Haemonchus was observed in Ghamkol camp (29.41%), while the lowest was in the Arid Zone (SRRI) (17.5%) of district Kohat p < 0.02 (Figure 3D). The current study was comprised of 6 months of sample data from July to December. The highest incidence of 38.70% of H. contortus was documented in the month of July, whereas the lowest was 11.11% in December p < 0.01 (Figure 3E).
We identified and added a total of 118 articles PDF with references from our searched databases using Mendeley Web Importer. We removed 35 duplicate articles from the Mendeley reference manager software dedicated folder for the current study. After removing duplicate articles, 83 articles remained. Primary screening of the title and abstract resulted in the exclusion of 24 papers not concerning with Pakistan; the remaining 59 articles were then chosen for full-text reading. In addition, 38 studies were removed according to our inclusion and exclusion parameters. Finally, 21 eligible papers based on H. contortus epidemiology in Pakistani ruminants were eventually included in the current systematic review, as shown in Table 1.
Out of 21 articles, the highest number of studies 13 (61.90%) were reported from Punjab, followed by Khyber Pakhtunkhwa 5(23.80%) and 1(4.76%) each from Baluchistan, Sindh, and Islamabad capital territory. The current systematic review consists of Haemonchus contortus studies on the ruminant population, which includes sheep, goats, cattle, buffalo, camels, and markhor. The most common types of samples used for H. contortus identification were fecal 17, followed by worm 3, and a single study collected both blood and fecal samples. Multiple methods were used for H. contortus evaluation, including direct smear microscopy, key identification, floatation, sedimentation, McMaster, and the Eggs per gram method. The most common evaluation method was direct microscopy, while the least common was key identification, as shown in Table 1. Based on risk factors 12 studies fall in the average category, followed by 6 in the good category and 3 in the below-average category.
The 21 selected Pakistani studies on ruminants show a 38.06% overall prevalence rate of H. contortus across Pakistan. Studies performed in all 5 regions of Pakistan, namely Khyber Pakhtunkhwa, Punjab, Baluchistan, Sindh, and Islamabad capital territory area, accounted for 34.49, 40.53, 10.41, 14.64, and 88.5%, respectively, p < 0.001. Based on the type of animal, sheep, goat, cattle, buffalo, and markhor show 44.99, 27.09, 15.08, 14.29, and 40% H. contortus with significant difference p < 0.001 respectively, Figure 4.
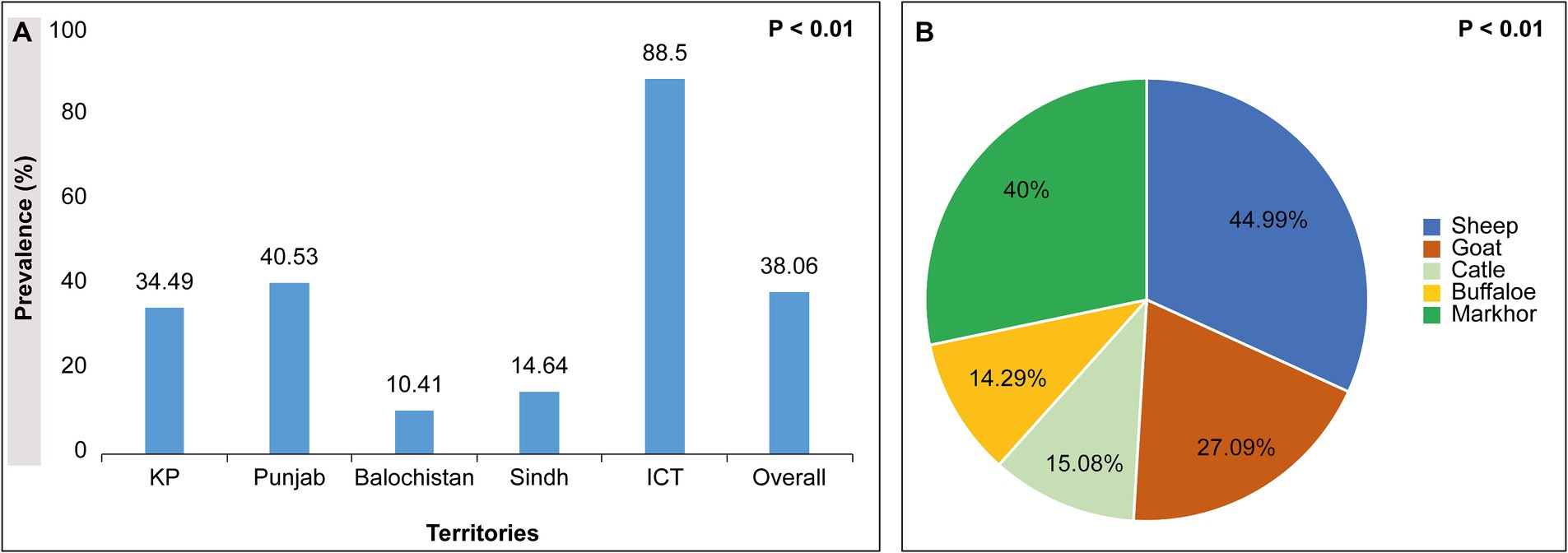
Figure 4. (A) showing territory wise prevalence of H. contortus; (B) showing animal wise prevalence of H. contortus in ruminants across Pakistan.
Followed by DNA extraction and amplification of the ITS-2 gene, a PCR product of approximately 350 bp was found under UV light. The top hits and highly similar sequences were retrieved for downstream phylogenetic tree construction. A dendrogram was constructed for the ITS-2 gene of Haemonchus spp. and other related nematode genera, including Marshallagia and Trichostrongylus. The dendrogram is comprised of 4 sequences from Kohat and 24 published sequences of different geographical localities, including Pakistan, China, India, Malaysia, Iran, Bangladesh, Thailand, Mongolia, Yemen, Italy, Denmark, and Japan, among others.
The phylogenetic analysis clustered H. contortus into a major clade with different subclades (Figure 5). H. contortus is grouped separately from other related species, i.e., H. bedfordi, H. longistipes, and H. placei. The H. contortus of the current study was closely related to the H. contortus of the neighboring Asian countries, including Iran, India, China, Mongolia, and Malaysia. This subclade also comprised a sequence from district Jhang of Pakistan, United Kingdom, Italy, and Mongolia. Surprisingly, our study’s neighbor joining dendrogram (Figure 5) showed that the isolates under investigation were quite closely related to those from Italy and the United Kingdom.
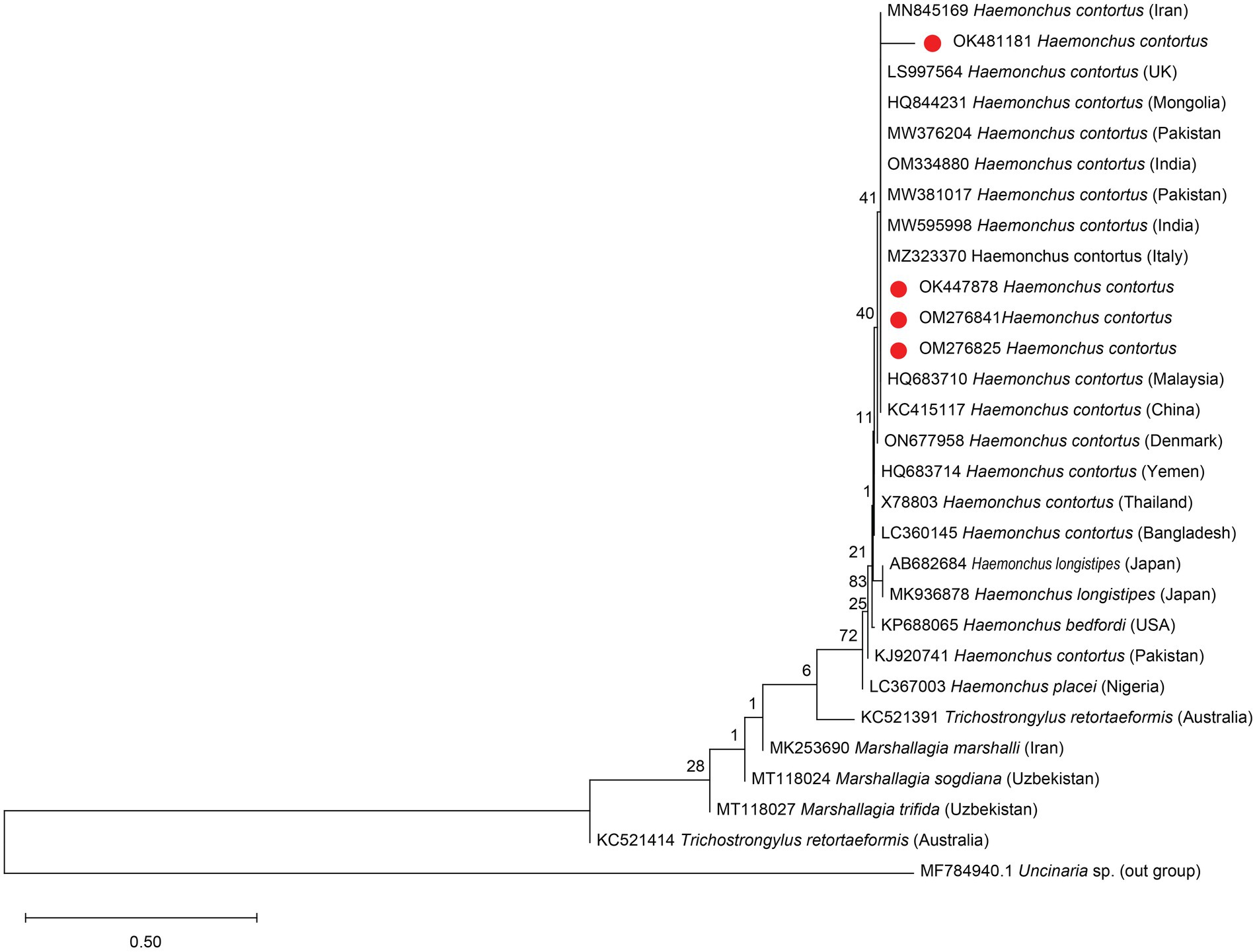
Figure 5. Phylogenetic tree of H. contortus in sheep and goat using ITS2 marker. The evolutionary history was inferred using the Neighbor-Joining method (48). The optimal tree is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches (49). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method (50) and are in the units of the number of base substitutions per site. This analysis involved 29 nucleotide sequences. All positions with less than 95% site coverage were eliminated, i.e., fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position (partial deletion option). There were total of 88 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 (51).
Haemonchus contortus is an efficient blood feeding parasite that causes significant economic losses to the livestock sector via decreased body weight, milk, meat, and wool production, in addition to other forms of harm and often the death of afflicted animals. Considering the significance of haemonchosis, the current study was carried out with the goal of determining the prevalence and phylogenetic analysis of H. contortus using microscopic and genetic marker ITS2 in small ruminants of district Kohat with a systematic review of Pakistan H. contortus prevalence studies to provide baseline information.
Our findings of 27.55% H. contortus in sheep and goats of district Kohat are lower than the total 38.06% prevalence rate in 21 selected studies across Pakistani ruminants, with 34.49% Haemonchus occurrence in our study area province Khyber Pakhtunkhwa, as shown in Table 1. Earlier, several studies indicated varying prevalence rates of haemonchosis in the small ruminants of Pakistan, with numbers ranging from 6 to 88%, as shown in Table 1. Researchers across the globe observed 3–90% of haemonchosis infection rate in small ruminants (52–59). Possible explanations for the observed variation in prevalence include differences in sample size, seasonality, environmental variability, and management practices in the research region. Several variables, including management practices, grazing patterns (such as mixed herds of male, female, juvenile, and adult animals), farmers incomes and education levels, and the irrational use of anthelmintics, might affect the parasite population density (31, 40).
The total recorded sex-wise prevalence of haemonchosis in small ruminants was greater in females (28.82%) than males (20.28%) p > 0.05. Our findings are consistent with those of Brik et al. (59) and Raza et al. (41), who found a greater rate of haemonchosis in females (30.98 and 35.19%) than in males (15.63 and 31.80%), respectively. Whereas, the present research contradicts Nabi et al. (60), and Tassawar et al. (2010) as they reportedhigher prevalence rate of H. contortus in male as compared to female population. The increased prevalence of haemonchosis may be attributed to female sensitivity to parasitism as a result of reproductive stress and a weakened immune system (61).
The age-specific prevalence rate of haemonchosis in small ruminants showed that juvenile age group is most vulnerable to H. contortus (33.33%), followed by adult age group (29.82%), with the lowest infection rates found in young age group (19.54%) p > 0.05. Our findings concur with those of (38, 62–64), as they also noted a higher H. contortus infection rate in the younger and older age groups. Higher infection rates in the younger and older age groups could be associated with reduced immunity in these animals since most cases of haemonchosis afflict either immunocompromised adults or non-immune young animals (i.e., during the first grazing season) (65).
Month-wise observed prevalence rates of H. contortus in small ruminants show that July and August had the highest rates of infection (38.71 and 35.48%, respectively), followed by September (30%), whereas December had the lowest rate of infection (11.11%) p < 0.01. Our results are in agreement with (43, 66–69) as they also reported a higher infection rate in humid conditions. The elevated biotic potential of H. contortus infection contributes to its swift predominance at a time when environments on pastures are favorable for free-living phases to develop and survive (69). Similar to our finding, Durrani et al. (70) and Rizvi et al. (71) also reported a higher prevalence of haemonchosis in the months of July and August, with the authors explaining that the presence of moisture favored the growth of the larvae.
The highest haemonchosis was documented in the localities of Ghamkol camp (29.41%), while the lowest prevalence was recorded in Arid zone (SSRI) 17.5% with p < 0.02. Variations in the prevalence rate of haemonchosis in sheep and goats might be influenced by different factors, including the grazing behavior, economic status, education level of the farmers, differential management practices, natural resistance, nutrition, and anthelmintics used (72).
The current study result is consistent with previous investigations as we found a single Haemonchus specie, H. contortus, in both sheep and goats, showing close association with the same geographical region and neighboring countries, including Iran (73), Pakistan (Jhang) (74), India (OM334880, MW595998), China (22), Malaysia (24), and Mongolia (844231). However, our H. contortus samples from both sheep and goat yielded an unexpected finding in the neighbor joining dendrogram (Figure 5), showing a strong correlation with the isolates from Italy (75) and the United Kingdom (LS997564) of distinct European continent. Troell et al. (76) and Dey et al. (23) also reported similar findings, where isolates from Greece overlapped with the obtained isolates of Australia and Malaysian isolates were closely related to American isolates, respectively. Since there is no proof of direct animal migration across two continents, the cause of these occurrences is the introduction of parasite populations via imported animals of the same provenance (23).
The primary limitation of the current research work was our inability to target multiple districts of Khyber Pakhtunkhwa province for sampling and sequenced limited samples for H. contortus identification and genetic variability in sheep and goats of district Kohat owing to limited time of study.
We concluded that H. contortus is prevalent across Pakistan, particularly in district Kohat and ITS2 genetic marker confirms our microscopic and molecular identification. The incidence of H. contortus is correlated with area and seasonal groups. Phylogenetic analysis shows a close association with Asian and European (Italian and UK) isolates. Haemonchus contortus must be further studied in unexplored areas in future studies, and other genes should be targeted for more diverse results.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The animal studies were approved by the Kohat University of Science and Technology Kohat ethical approval committee for sample collection from animals. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
NA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. SK: Funding acquisition, Resources, Supervision, Writing – review & editing. HM: Investigation, Supervision, Writing – review & editing. RA: Data curation, Investigation, Software, Writing – review & editing. RU: Funding acquisition, Methodology, Writing – review & editing. AB: Funding acquisition, Writing – review & editing. NUA: Investigation, Writing – review & editing. AM: conceptualization, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received researchers supporting grant from project number (RSP2024R346) at King Saud University Riyadh, Saudi Arabia for financial support.
We are grateful to livestock and dairy development research wing (Arid zone small ruminant research institute Kohat) Khyber Pakhtunkhwa, Pakistan for their cooperation in data sampling. NA is PhD student under CSC scholarship and the current research work is from his M.Phil. dissertation. Authors also thanks the reviewers and editors for their comments and valuable inputs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wen, Z, Aleem, MT, Aimulajiang, K, Chen, C, Liang, M, Song, X, et al. The GT1-TPS structural domain protein from Haemonchus contortus could be suppressive antigen of goat PBMCs. Front Immunol. (2022) 12:787091. doi: 10.3389/fimmu.2021.787091
2. Wen, ZH, Xie, XR, Aleem, MT, Aimulajiang, K, Chen, C, Liang, M, et al. In vitro characterization of Haemonchus contortus trehalose-6-phosphate phosphatase and its immunomodulatory effects on peripheral blood mononuclear cells (PBMCs). Parasit Vectors. (2021) 14:611. doi: 10.1186/s13071-021-05115-4
3. Gasser, RB, Bott, NJ, Chilton, NB, Hunt, P, and Beveridge, I. Toward practical, DNA-based diagnostic methods for parasitic nematodes of livestock—bionomic and biotechnological implications. Biotechnol Adv. (2008) 26:325–34. doi: 10.1016/j.biotechadv.2008.03.003
4. Prichard, R. Genetic variability following selection of Haemonchus contortus with anthelmintics. Trends Parasitol. (2001) 17:445–53. doi: 10.1016/S1471-4922(01)01983-3
5. Qamar, W, and Alkheraije, KA. Anthelmintic resistance in Haemonchus contortus of sheep and goats from Asia--a review of in vitro and in vivo studies. Pak Vet J. (2023):43, 376–387. doi: 10.29261/pakvetj/2023.088
6. Nazish, A, Fozia,, Khattak, B, Ali Khan, T, Ahmad, I, Ullah, R, et al. Antinematode activity of abomasum bacterial culture filtrates against Haemonchus contortus in small ruminants. Animals (Basel). (2021) 11:1843. doi: 10.3390/ani11061843
7. Gilleard, JS. Haemonchus contortus as a paradigm and model to study anthelmintic drug resistance. Parasitology. (2013) 140:1506–22. doi: 10.1017/S0031182013001145
8. Hoberg, EP, Lichtenfels, JR, and Gibbons, L. Phylogeny for species of Haemonchus (Nematoda: Trichostrongyloidea): considerations of their evolutionary history and global biogeography among Camelidae and Pecora (Artiodactyla). J Parasitol. (2004) 90:1085–102. doi: 10.1645/GE-3309
9. Hussain, T, Periasamy, K, Nadeem, A, Babar, ME, Pichler, R, and Diallo, A. Sympatric species distribution, genetic diversity and population structure of Haemonchus isolates from domestic ruminants in Pakistan. Vet Parasitol. (2014) 206:188–99. doi: 10.1016/j.vetpar.2014.10.026
10. Ali, R, Ahmad, N, Mussarat, S, Majid, A, Alnomasy, SF, and Khan, SN. Nanoparticles as alternatives for the control of Haemonchus contortus: a systematic approach to unveil new anti-haemonchiasis agents. Front Vet Sci. (2021) 8:789977. doi: 10.3389/fvets.2021.789977
11. R Core Team (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/
12. Liang, M, Lu, M, Aleem, MT, Zhang, Y, Wang, M, Wen, Z, et al. Identification of excretory and secretory proteins from Haemonchus contortus inducing a Th9 immune response in goats. Vet Res. (2022) 53:36. doi: 10.1186/s13567-022-01055-8
13. Wen, Z, Zhang, Y, Feng, J, Aimulajiang, K, Aleem, MT, Lu, M, et al. Excretory/secretory proteins inhibit host immune responses by downregulating the TLR4/NF-κB/MAPKs signaling pathway: a possible mechanism of immune evasion in parasitic nematode Haemonchus contortus. Front Immunol. (2022) 13:1013159. doi: 10.3389/fimmu.2022.1013159
14. Wang, Q, Muhammad, TA, Muhammad, WH, Muhammad, AM, Muhammad, H, Yan, R, et al. Hepatocellular carcinoma-associated antigen 59 and ADP-ribosylation factor 1 with poly (lactic-co-glycolic acid): a promising candidate as nanovaccine against haemonchosis. Microb Pathog. (2022) 168:105614. doi: 10.1016/j.micpath.2022.105614
15. Wang, Q, Muhammad, TA, Muhammad, WH, Muhammad, AM, Muhammad, H, Yan, R, et al. Haemonchus contortus hepatocellular carcinoma-associated antigen 59 with poly (lactic-co-glycolic acid): a promising nanovaccine candidate against Haemonchus contortus infection. Vet Parasitol. (2021) 292:109398. doi: 10.1016/j.vetpar.2021.109398
16. Kandeel, M, Akhtar, T, Zaheer, T, Ahmad, S, Ashraf, U, and Omar, M. Anti-parasitic applications of nanoparticles: a review. Pak Vet J. (2022):42, 2074–7764. doi: 10.29261/pakvetj/2022.040
17. Ur-Rehman, T, El-Mansi, AA, Alhag, SK, Al-Shuraym, LA, Saeed, Z, Arif, M, et al. Antiparasitic activity of methanolic and ethyl acetate extracts of Azadirachta indica against Haemonchus contortus. Pak Vet J. (2023) 43:199–203. doi: 10.29261/pakvetj/2023.014
18. Al-Saeed, FA, Ismael Bamarni, SS, Iqbal, KJ, Faruk, AZ, Mahmood, S, Şahin, T, et al. In vitro anthelmintic efficacy of Haloxylon salicornicum leaves extract using adult Heamonchus contortus Worms. Pak Vet J. (2023):43, 91–96. doi: 10.29261/pakvetj/2022.091
19. Velázquez-Antunez, J, Olivares-Perez, J, Olmedo-Juárez, A, Rojas-Hernandez, S, Villa-Mancera, A, and Romero, RT. Biological activity of the secondary compounds of Guazuma ulmifolia leaves to inhibit the hatching of eggs of Haemonchus contortus. Pak Vet J. (2023):43:55. doi: 10.29261/pakvetj/2022.075
20. Pakistan Economic Survey (2023). Government of Pakistan, finance division, economic advisor’s wing Islamabad. Available at: http://www.finance.gov.pk/survey_1819.html
21. Troell, K, Engström, A, Morrison, DA, Mattsson, JG, and Höglund, J. Global patterns reveal strong population structure in Haemonchus contortus, a nematode parasite of domesticated ruminants. Int J Parasitol. (2006) 36:1305–16. doi: 10.1016/j.ijpara.2006.06.015
22. Yin, F, Gasser, RB, Li, F, Bao, M, Huang, W, Zou, F, et al. Genetic variability within and among Haemonchus contortus isolates from goats and sheep in China. Parasit Vectors. (2013) 6:1–9. doi: 10.1186/1756-3305-6-279
23. Dey, AR, Zhang, Z, Begum, N, Alim, MA, Hu, M, and Alam, MZ. Genetic diversity patterns of Haemonchus contortus isolated from sheep and goats in Bangladesh. Infect Genet Evol. (2019) 68:177–84. doi: 10.1016/j.meegid.2018.12.021
24. Gharamah, AA, Azizah, MNS, and Rahman, WA. Genetic variation of Haemonchus contortus (Trichostrongylidae) in sheep and goats from Malaysia and Yemen. Vet Parasitol. (2012) 188:268–76. doi: 10.1016/j.vetpar.2012.04.003
25. Ali, M, Majid, HA, Khan, MR, Muhammad, D, Ullah, F, Shuaib, M, et al. (2022). Prevalence and drug efficacy against gastrointestinal nematodes particularly Haemonchus contortus in district Kohat, Pakistan. World Journal of Pharmacy and pharmaceutical sciences, 11:107–115.
26. Ruhoollah, KW, Al-Jabr, OA, Khan, T, Khan, A, El-Ghareeb, WR, Aguilar-Marcelino, L, et al. Prevalence of gastrointestinal parasite in small ruminants of district Dir upper Khyber Pakhtunkhwa Province of Pakistan. Braz J Biol. (2021) 83:e248978. doi: 10.1590/1519-6984.248978
27. Bibi, R, Afshan, K, Khan, IA, Iqbal, Z, Kayani, AR, Mushtaq, M, et al. Phenotyping and prevalence of Haemonchus contortus (Nematoda: Trichostongylidae) in ruminants from endemic areas of Pakistan: influence of host species and geographical area on phenotypic traits of Worms. Pak Vet J. (2017) 37:170–4.
28. Qasim, HM, Avais, M, Durrani, AZ, Khan, MA, and Shahzad, AH. Dynamic dispersal of haemonchosis, its treatment and effect on blood profile of small ruminants of Lodhran district, Punjab, Pakistan. Pak J Zool. (2016) 48:755–61.
29. Jamal, Q, Jafar, S, and Shah, A. Prevalence of Haemonchus contortus in markhor of chitral gol national park. J Sci Technol Univ Peshawar. (2016) 40:19–23.
30. Jamil, M, Mansoor, M, Latif, N, and Hussain, N. Infection rate and chemotherapy of various helminths in Ovis aries in Dera Ismail Khan KPK, Pakistan. Int J. (2016) 2:7–17.
31. Lashari, MH, and Tasawar, Z. Prevalence of some gastrointestinal parasites in sheep in southern Punjab, Pakistan. Pak Vet J. (2011) 31:295–8.
32. Raza, MA, Younas, M, and Schlecht, E. Prevalence of gastrointestinal helminths in pastoral sheep and goat flocks in the Cholistan desert of Pakistan. J Anim Plant Sci. (2014):24, 127–134.
33. Khalid, M, Muhammad, I, Durrani, AZ, Khan, MA, Sabir, AJ, and Saleem, MH. Infection rate and therapeutic trials on various gastrointestinal parasites in sheep and goats in and around Lahore, Pakistan. Pak J Zool. (2013) 45:489–94.
34. Razzaq, A, Ashraf, K, Maqbool, A, Khan, MA, Islam, M, and Khan, H. Epidemiology, serodiagnosis and therapeutic studies on ovine nematodes at district Loralai, Balochistan, Pakistan. J Anim Plant Sci. (2013) 23:–1559.
35. Ayaz, MM, Raza, MA, Murtaza, S, and Akhtar, S. Epidemiological survey of helminths of goats in southern Punjab, Pakistan. Trop Biomed. (2013) 30:62–71.
36. Ullah, N, Khan, MS, and Shah, M. Infestation of helminthes parasite in sheep, Ovis aries (L.) in district Peshawar, Pakistan. Int. J. Biosci. (2013) 3:28–34. doi: 10.12692/ijb/3.2.28-34
37. Akhter, N, Arijo, A, Phulan, M, Iqbal, Z, and Mirbahar, K. Prevalence of gastro-intestinal nematodes in goats in Hyderabad and adjoining areas. Pak Vet J. (2011) 31:287–90.
38. Tasawar, Z, Ahmad, S, Lashari, MH, and Chaudhary, SH. Prevalence of Haemonchus contortus in sheep at research Centre for conservation of Sahiwal cattle (RCCSC) Jehangirabad District Khanewal, Punjab, Pakistan. Pak J Zool. (2010) 42:735–9.
39. Khan, MN, Sajid, MS, Khan, MK, Iqbal, Z, and Hussain, A. Gastrointestinal helminthiasis: prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitol Res. (2010) 107:787–94. doi: 10.1007/s00436-010-1931-x
40. Gadahi, J, Arshed, M, Ali, Q, Javaid, S, and Shah, S. Prevalence of gastrointestinal parasites of sheep and goat in and around Rawalpindi and Islamabad, Pakistan. Vet World. (2009) 2:51–3.
41. Raza, MA, Murtaza, S, Bachaya, HA, Dastager, G, and Hussain, A. Point prevalence of haemonchosis in sheep and goats slaughtered at Multan abattoir. J Anim Plant Sci. (2009) 28:158–9.
42. Ijaz, M, Khan, MS, Avais, M, Ashraf, K, and Ali, MM. Infection rate and chemotherapy of various helminths in goats in and around Lahore. Pak Vet J. (2008) 28:167.
43. Lateef, M, Iqbal, Z, Jabbar, A, Khan, M, and Akhtar, M. Epidemiology of trichostrongylid nematode infections in sheep under traditional husbandry system in Pakistan. Int J Agric Biol. (2005) 20:596–600.
44. Jabeen, F, Ahmad, N, Ahmad, KM, Chaudhry, MA, and Ali, S. Studies on the epidemiology and chemotherapy of haemonchosis in sheep in the Punjab. Pak Vet J. (2000) 20:90–92.
45. Sajid, A, Khan, MQ, Qayyum, M, and Khan, MFU. Prevalence of gastrointestinal parasites in sheep and goats maintained at NARC, Islamabad. Pak Vet J. (2000) 20:157–8.
46. Jurasek, ME, Bishop-Stewart, JK, Storey, BE, Kaplan, RM, and Kent, ML. Modification and further evaluation of a fluorescein-labeled peanut agglutinin test for identification of Haemonchus contortus eggs. Vet Parasitol. (2010) 169:209–13. doi: 10.1016/j.vetpar.2009.12.003
47. Ebrahim, ZK. Effect of gastrointestinal parasites infestation on some hematological and biochemical parameters in sheep. Alex J Vet Sci. (2018):59, 44–47. doi: 10.5455/ajvs.1922
48. Saitou, N, and Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. (1987) 4:406–25.
49. Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution (N Y). (1985) 39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x
50. Tamura, K, Nei, M, and Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci. (2004) 101:11030–5. doi: 10.1073/pnas.0404206101
51. Tamura, K, Stecher, G, and Kumar, S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. (2021) 38:3022–7. doi: 10.1093/molbev/msab120
52. Kalule, F, Vudriko, P, Nanteza, A, Ekiri, AB, Alafiatayo, R, Betts, J, et al. Prevalence of gastrointestinal parasites and molecular identification of beta-tubulin mutations associated with benzimidazole resistance in Haemonchus contortus in goats from selected districts of Uganda. Vet Parasitol Reg Stud Reports. (2023) 42:100889. doi: 10.1016/j.vprsr.2023.100889
53. Mussa, SM. Study on the prevalence and associated risk factors of Haemonchus contortus infection in small ruminants in Mitto District, Silte zone, Ethiopia. J Vet Heal Sci. (2023) 4:46–53. doi: 10.33140/JVHS
54. Gareh, A, Elhawary, NM, Tahoun, A, Ramez, AM, El-Shewehy, DMM, Elbaz, E, et al. Epidemiological, morphological, and morphometric study on Haemonchus spp. recovered from goats in Egypt. Front Vet Sci. (2021) 8:705619. doi: 10.3389/fvets.2021.705619
55. Rinaldi, L, Catalan, D, Musella, V, Cecconi, L, Hertzberg, H, Torgerson, PR, et al. Haemonchus contortus: spatial risk distribution for infection in sheep in Europe. Geospat Health. (2015) 9:325–31. doi: 10.4081/gh.2015.355
56. Mushonga, B, Habumugisha, D, Kandiwa, E, Madzingira, O, Samkange, A, Segwagwe, BE, et al. Prevalence of Haemonchus contortus infections in sheep and goats in Nyagatare District, Rwanda. J Vet Med. (2018) 2018:1–9. doi: 10.1155/2018/3602081
57. Besier, RB, Kahn, LP, Sargison, ND, and Van Wyk, JA. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv Parasitol. (2016) 93:95–143. doi: 10.1016/bs.apar.2016.02.022
58. Akkari, H, Jebali, J, Gharbi, M, Mhadhbi, M, Awadi, S, and Darghouth, MA. Epidemiological study of sympatric Haemonchus species and genetic characterization of Haemonchus contortus in domestic ruminants in Tunisia. Vet Parasitol. (2013) 193:118–25. doi: 10.1016/j.vetpar.2012.12.014
59. Brik, K, Hassouni, T, Elkharrim, K, and Belghyti, D. A survey of Haemonchus contortus parasite of sheep from Gharb plain, Morocco. Parasite Epidemiol Control. (2019) 4:e00094. doi: 10.1016/j.parepi.2019.e00094
60. Nabi, H, Saeed, K, Shah, SR, Rashid, MI, Akbar, H, and Shehzad, W. Epidimiological study of gastrointestinal nematodes of goats in district swat, Khyber Pakhtunkhwa, Pakistan. Sci. Int. (2014) 26:283–6.
61. Urquhart, GM, Armour, J, Duncan, JL, and Dunn, AM and, Jennings, FW. 2nd Veterinary Parasitology, Blackwell Science Ltd. Oxford (1996).
62. Dorny, P, Symoens, C, Jalila, A, Vercruysse, J, and Sani, R. Strongyle infections in sheep and goats under the traditional husbandry system in peninsular Malaysia. Vet Parasitol. (1995) 56:121–36.
63. ACM, F, and Rajapakse, R. Prevalence of coccidia and gastrointestinal nematode infections in cross bred goats in the dry areas of Sri Lanka. Small Rumin Res. (2001) 40:233–8. doi: 10.1016/S0921-4488(01)00179-1
64. Horak, IG. Parasites of domestic and wild animals in South Africa. XLII. Helminths of sheep on four farms in the eastern Cape Province. Onderstepoort J Vet Res. (2003) 70:175–86.
65. Adduci, I, Sajovitz, F, Hinney, B, Lichtmannsperger, K, Joachim, A, Wittek, T, et al. Haemonchosis in sheep and goats, control strategies and development of vaccines against Haemonchus contortus. Animals. (2022) 12:2339. doi: 10.3390/ani12182339
66. Keyyu, JD, Kyvsgaard, NC, Monrad, J, and Kassuku, AA. Epidemiology of gastrointestinal nematodes in cattle on traditional, small-scale dairy and large-scale dairy farms in Iringa district, Tanzania. Vet Parasitol. (2005) 127:285–94. doi: 10.1016/j.vetpar.2004.10.014
67. Khajuria, JK, and Kapoor, PR. Prevalence of parasites in sheep and goats at Kathua-Jammu. J Vet Parasitol. (2003) 17:121–6.
68. Nwosu, CO, Madu, PP, and Richards, WS. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of North-Eastern Nigeria. Vet Parasitol. (2007) 144:118–24. doi: 10.1016/j.vetpar.2006.09.004
69. Qamar, MF, Maqbool, A, Khan, MS, Ahmad, N, and Muneer, MA. Epidemiology of Haemonchosis in sheep and goats under different managemental conditions. Vet World. (2009) 2:413–7.
70. Durrani, Z, Kamal, N, and Khan, S. Sero-diagnosis of Haemonchosis in small ruminants. Global Vet. (2007) 1:1–66.
71. Rizvi, AR, Magrey, TW, and Zia, EUH. Clinical epidemiology and chemotherapy of haemonchosis in goats in Faisalabad. Pak. Vet. J. (1999) 54:107–9.
72. Ouattara, L, and Dorchies, P. Gastro-intestinal helminths of sheep and goats in subhumid and sahelian areas of Burkina Faso. Rev. Med. Vet. (2001) 152:165–70.
73. Hosseinnezhad, H, Sharifdini, M, Ashrafi, K, Atrkar Roushan, Z, Mirjalali, H, and Rahmati, B. Trichostrongyloid nematodes in ruminants of northern Iran: prevalence and molecular analysis. BMC Vet Res. (2021) 17:371. doi: 10.1186/s12917-021-03086-3
74. Qamar, W, Zaman, MA, Faheem, M, Ahmed, I, Ali, K, Qamar, MF, et al. Molecular confirmation and genetic characterization of Haemonchus contortus isolates at the nuclear ribosomal ITS2 region: first update from Jhang region of Pakistan. Pak Vet J. (2022) 42:251–5. doi: 10.29261/PAKVETJ/2021.071
75. Knoll, S, Dessì, G, Tamponi, C, Meloni, L, Cavallo, L, Mehmood, N, et al. Practical guide for microscopic identification of infectious gastrointestinal nematode larvae in sheep from Sardinia, Italy, backed by molecular analysis. Parasit Vectors. (2021) 14:505. doi: 10.1186/s13071-021-05013-9
Keywords: Haemonchus contortus, prevalence, small ruminants, ITS-2, PCR, phylogenetic analysis, epidemiology, phylogeny
Citation: Ahmad N, Khan SA, Majid HA, Ali R, Ullah R, Bari A, Akbar NU and Majid A (2024) Epidemiology and phylogeny of Haemonchus contortus through internal transcribed spacer 2 gene in small ruminants. Front. Vet. Sci. 11:1380203. doi: 10.3389/fvets.2024.1380203
Received: 01 February 2024; Accepted: 26 March 2024;
Published: 09 April 2024.
Edited by:
Muhammad Kasib Khan, University of Agriculture, Faisalabad, PakistanReviewed by:
Sultan Ali, University of Agriculture, Faisalabad, PakistanCopyright © 2024 Ahmad, Khan, Majid, Ali, Ullah, Bari, Akbar and Majid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Majid, YWJkdWxtYWppZEBrdXN0LmVkdS5waw==; Nisar Ahmad, bmlzYXJiaGl0dGFuaUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.