- 1Division of Risk Analysis and Management, International Institute for Zoonosis Control, Hokkaido University, Sapporo, Japan
- 2Laboratory of Parasitology, Department of Disease Control, Faculty of Veterinary Medicine, Hokkaido University, Sapporo, Japan
- 3Laboratory of Veterinary Internal Medicine, Department of Clinical Sciences, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, Japan
- 4Veterinary Teaching Hospital, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, Japan
- 5Laboratory of Molecular Medicine, Department of Disease Control, Faculty of Veterinary Medicine, Hokkaido University, Sapporo, Japan
- 6Department of Infectious Diseases, Hokkaido Institute of Public Health, Sapporo, Hokkaido, Japan
Echinococcus multilocularis is a cestode that causes human alveolar echinococcosis, a lethal zoonotic disease distributed in the northern hemisphere. The life cycle of this parasite is maintained in nature by voles as intermediate hosts and foxes as definitive hosts in Hokkaido, Japan. Although dogs are also susceptible to the parasite, the infection has been considered typically asymptomatic. We report the detection of E. multilocularis eggs in the diarrheal feces of a dog with chronic gastrointestinal signs, which disappeared after anthelmintic treatment. The mitochondrial genome sequence constructed by sequencing of the overlapping PCRs using DNA from the eggs was identical to the most predominant haplotype previously reported in red foxes in Hokkaido. This case highlights that Echinococcus infection should be considered as a differential diagnosis for diarrheal dogs in the disease endemic areas. Further efforts are needed to accumulate parasite genotypes in domestic dogs as well as humans to assess the risk of human infection from dogs.
Introduction
Alveolar echinococcosis (AE) in humans, a potentially fatal zoonosis if left untreated is caused by the metacestode stage of the parasite Echinococcus multilocularis, which is widely distributed in the northern hemisphere. The life cycle of this parasite involves carnivores such as foxes and dogs as definitive hosts and voles as intermediate hosts. A total of 254 AE cases were reported between 2010 and 2020 in Japan, with Hokkaido having the highest burden of the disease (1). The prevalence of E. multilocularis infections in foxes was 30–40% in Hokkaido (2). Furthermore, studies of pet dogs in Hokkaido have estimated the infection rates to be 7.1% in a rural area (3) and 1.9% in an urban area (4). Thus, pet dogs in endemic areas may play an important role in the transmission of the parasite to humans.
The adult cestode resides in the small intestine of the definitive host, and infected hosts typically do not show clinical signs (5). However, there have been two reports of the incidental detection of E. multilocularis in dogs that exhibited severe gastrointestinal signs such as vomiting and diarrhea with mild hypoproteinemia (6, 7). Here, we report a case of a pet dog raised in an urban area of Hokkaido that showed intermittent gastrointestinal symptoms and was infected with E. multilocularis. When treated with an anthelmintic, the clinical signs of the dog disappeared.
Case description
An 8-year-old female spayed Jack Russell Terrier was presented to Hokkaido University Veterinary Teaching Hospital with a 2-month history of intermittent vomiting, anorexia, and borborygmi. These clinical signs were unresponsive to symptomatic therapy provided by the referring veterinarian in Sapporo, Hokkaido. The dog had no previous clinical history, and was prescribed ivermectin monthly for heartworm prevention. The owner always kept the dog indoors or on a leash when outdoors.
At presentation, the dog’s feces were watery, and blood and taeniid eggs were observed on direct fecal smear examination (Figure 1). Physical examination showed no abnormalities. A complete blood count demonstrated mild eosinophilia (1,820 cells/μL, reference range 170–1,570 cells/μL) and serum chemistry revealed no significant abnormalities. Abdominal ultrasonography revealed mild jejunal lymphadenopathy.
The dog was treated with praziquantel (6.4 mg/kg), pyrantel (18.5 mg/kg), and febantel (19.2 mg/kg) (Drontal Plus, Bayer Yakuhin, Osaka, Japan) as an oral single dose for the treatment of intestinal cestodiasis with suspected echinococcosis. The therapeutic response in the dog was carefully monitored, with chronic inflammatory enteritis (CIE) also considered as a differential diagnosis. Gastrointestinal signs resolved markedly within a few days after treatment and did not recur. A follow-up fecal examination with a flotation technique performed 5 days after treatment revealed no parasite eggs.
To identify the taeniid species of the eggs, we performed a multiplex PCR assay (8). DNA was extracted from 0.2 g of the fecal sample, according to the method previously described (9). The PCR performed using this DNA yielded a single band of ~400 bp, which was in accordance with the expected amplicon size for E. multilocularis.
We used an amplicon-based next-generation sequencing method to genotype the parasite, targeting mitochondrial protein-coding sequences (CDSs). The whole mitochondrial genome was amplified by overlapping PCRs using four sets of primers as reported previously (10). Illumina sequencing libraries were constructed and sequenced on an Illumina MiSeq platform using the MiSeq reagent kit v3 for 600 cycles (Illumina, Hayward, CA, United States). Read mapping against a reference mitogenome sequence of E. multilocularis (GenBank accession no. AB018440) (11) using CLC Genomics Workbench v20.0.4 (Qiagen, Hilden, Germany) generated a complete mitochondrial genome sequence for the parasite (accession no. LC744000). The sequence is 13,738 bp in length and consisted of 12 CDSs, 22 transfer RNA genes, and 2 ribosomal RNA genes as previously reported (10). The network analysis using PopART v1.7 (12) showed that the mitochondrial haplotype based on three complete CDSs (cytochrome b, NADH dehydrogenase subunit 2, cytochrome c oxidase subunit I) was genotype A4, which is predominantly detected in wild foxes in Hokkaido (Figure 2) (10, 13–16).
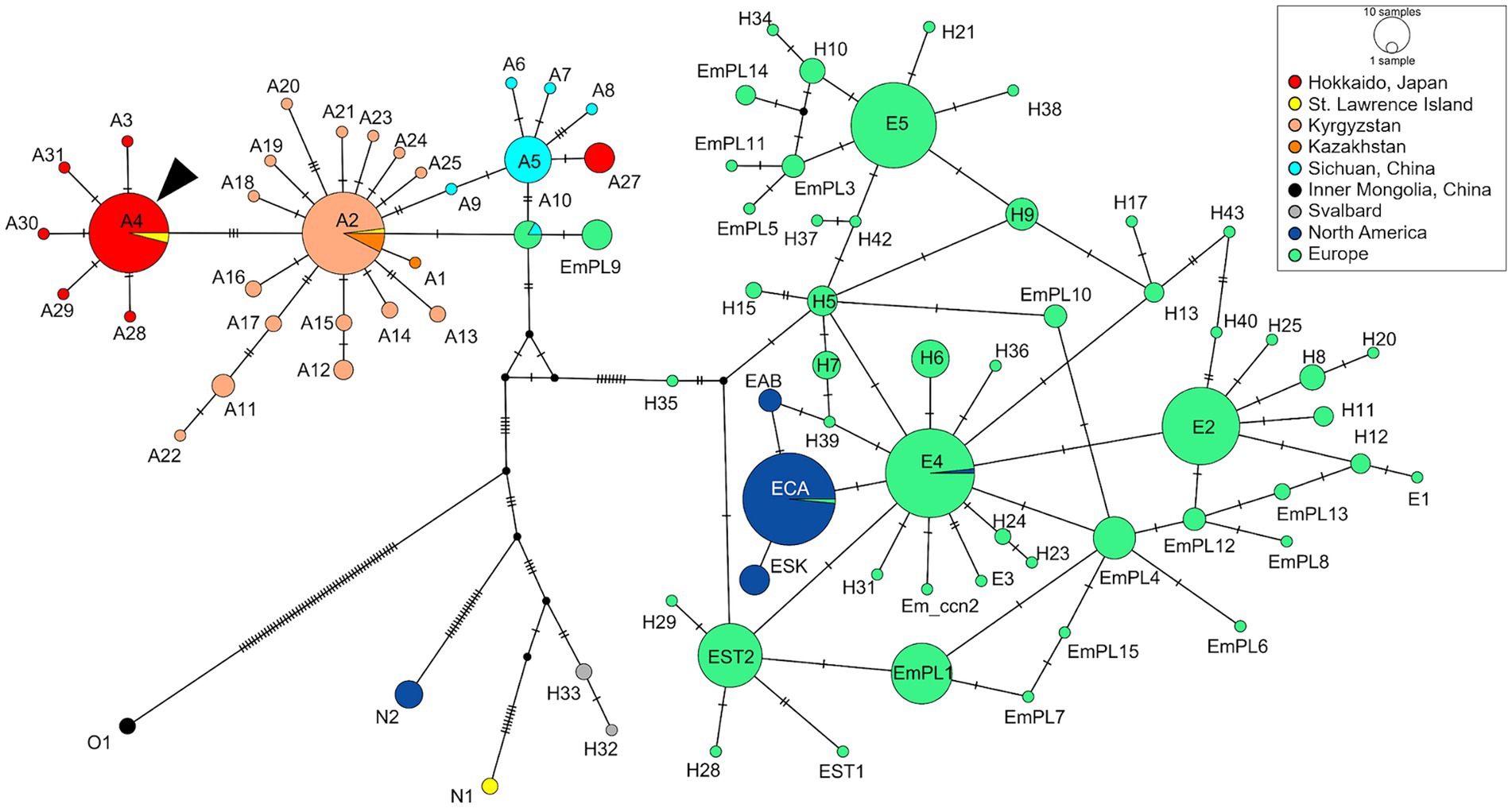
Figure 2. Median-joining haplotype network of Echinococcus multilocularis based on three mitochondrial protein-coding sequences. The analysis included haplotypes based on three mitochondrial genes (cytochrome b, NADH dehydrogenase subunit 2, and cytochrome c oxidase subunit I) previously reported in other endemic areas. The arrowhead indicates the haplotype obtained in this case (A4).
Discussion and conclusion
We have reported a rare case of intestinal E. multilocularis infection in a pet dog with gastrointestinal signs. Chronic intermittent or persistent diarrhea in dogs is commonly caused by gastrointestinal or extragastrointestinal disorders. The cause of gastrointestinal disorders includes infectious, neoplastic, mechanical, toxic, or noninfectious inflammatory such as CIE. CIE is the most common cause of chronic gastrointestinal disease in dogs (79%), followed by parasitic infection (12%) (17). Given the complete resolution of these signs following anthelmintic therapy, Echinococcus infection was thought to be the most likely cause of the disease. In fact, experimental oral infection of E. multilocularis protoscoleces into beagle dogs resulted in intermittent diarrhea in two out of four dogs (18). Moreover, upon reinfection all four dogs exhibited frequent diarrhea from the early stages of the infection. These experimental data, together with previous reports of symptomatic echinococcosis in dogs (6, 7), support the assumption that dogs could develop gastrointestinal signs including diarrhea upon E. multilocularis infection. Thus, this parasitic disease should be considered as one of the differential diagnoses in dogs with chronic gastrointestinal signs that live in endemic areas.
Although Hokkaido is an endemic region for AE, there are no national regulations for animal deworming. The lack of regulations poses not only the risk of transmission from pet dogs to humans, but also the risk of further expansion of the parasite distribution through animal transportation (10). The case presented here, where the indoor dog became infected, underscores such potential risks of diffusion by pet animals. It would be important to apply appropriate deworming programs for high-risk dogs and to use reliable diagnostic methods.
In certain areas of Hokkaido, the periodic distribution of praziquantel-containing baits has been provided to reduce the prevalence of E. multilocularis in foxes. Field studies have demonstrated the efficacy of anthelmintic baiting in reducing contamination by the eggs, thereby decreasing the risk of infection (19–21). It is worth mentioning here that mass drug administration for controlling malaria has been associated to the emergence of drug-resistant parasites (22–25). Although the drug resistance in E. multilocularis has never been reported, a recent study introduced the clinical resistance to praziquantel in another cestode, Dipylidium caninum (26). Therefore, it is important to monitor the drug resistance in clinical settings and explore alternative treatment options for canine echinococcosis.
Genotyping parasites is essential for a better understanding the epidemiological status of infectious diseases. For instance, a previous study in Kyrgyzstan found that haplotype A2 was the most common genotype in both humans and dogs, which is pivotal information to analyze the transmission dynamics and pathogenicity of the parasites (16). Previous research has revealed that an Asian haplotype A4 is the most predominant haplotype among red foxes in Hokkaido (10). The genotype detected in the current study was also assigned to the same haplotype, which may indicate that dogs are likely to have the same susceptibility to the genotype as foxes. Given the potential phenotypic differences among genotypes (27, 28), further studies genotyping parasites affecting both pet dogs and humans would be valuable to assess the risk of human infection from dogs.
In the current case, the animal was always kept indoors or on a leash when outdoors, and never observed any hunting behavior. These are contrary to the risk factors for canine echinococcosis, including roaming outdoors unattended and hunting/killing small animals (29, 30). Nevertheless, the dog likely became infected as a result of ingesting infected vole(s) during outdoor activities, such as preying or scavenging. This is supported by the facts that the detected parasite was the most dominant haplotype in Hokkaido, and the dog had no travel history outside Hokkaido. Taken together, this case emphasizes the importance for pet owners and animal care professionals to understand the risk of transmission of E. multilocularis even from indoor dogs in the endemic regions. It also highlights the need to employ proper biosafety protocols when handling suspect animals showing gastrointestinal signs.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The requirement of ethical approval was waived by Ethics Screening Committee of Hokkaido University Veterinary Teaching Hospital for the studies involving animals because this is a case report of examinations performed for the purpose of patient treatment, and no action contrary to treatment was performed. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners of the animals for the publication of this case report.
Author contributions
IK: Funding acquisition, Investigation, Writing – original draft. NH: Formal analysis, Investigation, Methodology, Writing – original draft. NY: Conceptualization, Supervision, Writing – review & editing. NoN: Resources, Writing – review & editing. KS: Resources, Writing – review & editing. NS: Resources, Writing – review & editing. KM: Resources, Writing – review & editing. KN: Resources, Writing – review & editing. HK: Resources, Writing – review & editing. KY: Funding acquisition, Resources, Writing – review & editing. RN: Conceptualization, Supervision, Writing – review & editing. MT: Resources, Supervision, Writing – review & editing. NaN: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Japan Society for the Promotion of Science KAKENHI (grant no. JP20K06402 and JP23H02369) and JST SPRING (grant no JPMJSP2119).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Institute of Infectious Diseases. Infectious diseases weekly report [internet]. (2022). Available at: https://www.niid.go.jp/niid/ja/idwr.html (Accessed February 24, 2024).
2. Kamiya, M. Collaborative control initiatives targeting zoonotic agents of alveolar echinococcosis in the northern hemisphere. J Vet Sci. (2007) 8:313–21. doi: 10.4142/jvs.2007.8.4.313
3. Irie, T, Yamada, K, Morishima, Y, and Yagi, K. High probability of pet dogs encountering the sylvatic cycle of Echinococcus multilocularis in a rural area in Hokkaido, Japan. J Vet Med Sci. (2019) 81:1606–8. doi: 10.1292/jvms.19-0307
4. Irie, T, Mukai, T, and Yagi, K. Echinococcus multilocularis surveillance using copro-DNA and egg examination of shelter dogs from an endemic area in Hokkaido, Japan. Vector Borne Zoonotic Dis. (2018) 18:390–2. doi: 10.1089/vbz.2017.2245
5. Deplazes, P, and Eckert, J. Veterinary aspects of alveolar echinococcosis – a zoonosis of public health significance. Vet Parasitol. (2001) 98:65–87. doi: 10.1016/S0304-4017(01)00424-1
6. Jenkins, EJ, Kolapo, TU, Jarque, MP, Ruschkowski, C, and Frey, C. Intestinal infection with Echinococcus multilocularis in a dog. J Am Vet Med Assoc. (2023) 261:1–3. doi: 10.2460/javma.23.02.0099
7. Kuroki, K, Morishima, Y, Neil, J, Beerntsen, BT, Matsumoto, J, and Stich, RW. Intestinal echinococcosis in a dog from Missouri. J Am Vet Med Assoc. (2020) 256:1041–6. doi: 10.2460/javma.256.9.1041
8. Trachsel, D, Deplazes, P, and Mathis, A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. (2007) 134:911–20. doi: 10.1017/S0031182007002235
9. Irie, T, Ito, T, Kouguchi, H, Yamano, K, Uraguchi, K, Yagi, K, et al. Diagnosis of canine Echinococcus multilocularis infections by copro-DNA tests: comparison of DNA extraction techniques and evaluation of diagnostic deworming. Parasitol Res. (2017) 116:2139–44. doi: 10.1007/s00436-017-5514-y
10. Hayashi, N, Nakao, R, Ohari, Y, Irie, T, Kouguchi, H, Chatanga, E, et al. Mitogenomic exploration supports the historical hypothesis of anthropogenic diffusion of a zoonotic parasite Echinococcus multilocularis. iScience. (2023) 26:107741. doi: 10.1016/j.isci.2023.107741
11. Nakao, M, Yokoyama, N, Sako, Y, Fukunaga, M, and Ito, A. The complete mitochondrial DNA sequence of the cestode Echinococcus multilocularis (Cyclophyllidea: Taeniidae). Mitochondrion. (2002) 1:497–509. doi: 10.1016/S1567-7249(02)00040-5
12. Leigh, JW, and Bryant, D. Popart: full-feature software for haplotype network construction. Methods Ecol Evol. (2015) 6:1110–6. doi: 10.1111/2041-210X.12410
13. Santoro, A, Santolamazza, F, Cacciò, SM, La Rosa, G, Antolová, D, Auer, H, et al. Mitochondrial genetic diversity and phylogenetic relationships of Echinococcus multilocularis in Europe. Int J Parasitol. (2024) 54:233–45. doi: 10.1016/j.ijpara.2024.01.003
14. Nakao, M, Xiao, N, Okamoto, M, Yanagida, T, Sako, Y, and Ito, A. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int. (2009) 58:384–9. doi: 10.1016/j.parint.2009.07.010
15. Bohard, L, Lallemand, S, Borne, R, Courquet, S, Bresson-Hadni, S, Richou, C, et al. Complete mitochondrial exploration of Echinococcus multilocularis from French alveolar echinococcosis patients. Int J Parasitol. (2023) 53:555–64. doi: 10.1016/j.ijpara.2023.03.006
16. Alvarez Rojas, CA, Kronenberg, PA, Aitbaev, S, Omorov, RA, Abdykerimov, KK, Paternoster, G, et al. Genetic diversity of Echinococcus multilocularis and Echinococcus granulosus sensu lato in Kyrgyzstan: the A2 haplotype of E. multilocularis is the predominant variant infecting humans. PLoS Negl Trop Dis. (2020) 14:e0008242. doi: 10.1371/journal.pntd.0008242
17. Volkmann, M, Steiner, JM, Fosgate, GT, Zentek, J, Hartmann, S, and Kohn, B. Chronic diarrhea in dogs – retrospective study in 136 cases. J Vet Intern Med. (2017) 31:1043–55. doi: 10.1111/jvim.14739
18. Kouguchi, H, Irie, T, Matsumoto, J, Nakao, R, Sugano, Y, Oku, Y, et al. The timing of worm exclusion in dogs repeatedly infected with the cestode Echinococcus multilocularis. J Helminthol. (2016) 90:766–72. doi: 10.1017/S0022149X15001169
19. Uraguchi, K, Irie, T, Kouguchi, H, Inamori, A, Sashika, M, Shimozuru, M, et al. Anthelmintic baiting of foxes against Echinococcus multilocularis in small public area, Japan. Emerg Infect Dis. (2022) 28:1677–80. doi: 10.3201/eid2808.212016
20. Inoue, T, Nonaka, N, Kanai, Y, Iwaki, T, Kamiya, M, and Oku, Y. The use of tetracycline in anthelmintic baits to assess baiting rate and drug efficacy against Echinococcus multilocularis in foxes. Vet Parasitol. (2007) 150:88–96. doi: 10.1016/j.vetpar.2007.08.027
21. Hegglin, D, and Deplazes, P. Control of Echinococcus multilocularis: strategies, feasibility and cost-benefit analyses. Int J Parasitol. (2013) 43:327–37. doi: 10.1016/j.ijpara.2012.11.013
22. Payne, D. Spread of chloroquine resistance in Plasmodium. Parasitol Today. (1987) 3:241–6. doi: 10.1016/0169-4758(87)90147-5
23. Verdrager, J. Epidemiology of the emergence and spread of drug-resistant falciparum malaria in South-East Asia and Australasia. J Trop Med Hyg. (1986) 89:277–89.
24. Verdrager, J. Localized permanent epidemics: the genesis of chloroquine resistance in Plasmodium falciparum. Southeast Asian J Trop Med Public Health. (1995) 26:23–8.
25. Wernsdorfer, WH. The biological and epidemiological basis of drug resistance in malaria parasites. Southeast Asian J Trop Med Public Health. (1992) 23:123–9.
26. Chelladurai, JJ, Kifleyohannes, T, Scott, J, and Brewer, MT. Praziquantel resistance in the zoonotic Cestode Dipylidium caninum. Am J Trop Med Hyg. (2018) 99:1201–5. doi: 10.4269/ajtmh.18-0533
27. Bartel, MH, Seesee, FM, and Worley, DE. Comparison of Montana and Alaska isolates of Echinococcus multilocularis in gerbils with observations on the cyst growth, hook characteristics, and host response. J Parasitol. (1992) 78:529–32. doi: 10.2307/3283660
28. Davidson, RK, Lavikainen, A, Konyaev, S, Schurer, J, Miller, AL, Oksanen, A, et al. Echinococcus across the north: current knowledge, future challenges. Food Waterborne Parasitol. (2016) 4:39–53. doi: 10.1016/j.fawpar.2016.08.001
29. Kern, P, Ammon, A, Kron, M, Sinn, G, Sander, S, Petersen, LR, et al. Risk factors for alveolar echinococcosis in humans. Emerg Infect Dis. (2004) 10:2088–93. doi: 10.3201/eid1012.030773
Keywords: Echinococcus multilocularis, gastrointestinal sign, dog, zoonosis, mitochondrial genome
Citation: Kida I, Hayashi N, Yokoyama N, Nagata N, Sasaoka K, Sasaki N, Morishita K, Nakamura K, Kouguchi H, Yagi K, Nakao R, Takiguchi M and Nonaka N (2024) Case report: Echinococcus multilocularis infection in a dog showing gastrointestinal signs in Hokkaido, Japan. Front. Vet. Sci. 11:1373035. doi: 10.3389/fvets.2024.1373035
Edited by:
Mughees Aizaz Alvi, University of Agriculture, PakistanReviewed by:
Zorica D. Dakić, University of Belgrade, SerbiaRanju Manoj, Cornell University, United States
Erica Marchiori, University of Padua, Italy
Copyright © 2024 Kida, Hayashi, Yokoyama, Nagata, Sasaoka, Sasaki, Morishita, Nakamura, Kouguchi, Yagi, Nakao, Takiguchi and Nonaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nozomu Yokoyama, y-nozomu@vetmed.hokudai.ac.jp; Nariaki Nonaka, nnonaka@vetmed.hokudai.ac.jp
†These authors have contributed equally to this work and share senior authorship
 Izumi Kida
Izumi Kida Naoki Hayashi2
Naoki Hayashi2 Nozomu Yokoyama
Nozomu Yokoyama Kazuyoshi Sasaoka
Kazuyoshi Sasaoka Noboru Sasaki
Noboru Sasaki Kensuke Nakamura
Kensuke Nakamura Hirokazu Kouguchi
Hirokazu Kouguchi Ryo Nakao
Ryo Nakao Mitsuyoshi Takiguchi
Mitsuyoshi Takiguchi Nariaki Nonaka
Nariaki Nonaka