- 1Biosafety Research Institute and Laboratory of Veterinary Deramtology, College of Veterinary Medicine, Jeonbuk National University, Iksan, Republic of Korea
- 2Biosafety Research Institute and Laboratory of Veterinary Internal Medicine, College of Veterinary Medicine, Jeonbuk National University, Iksan, Republic of Korea
The development of a non-invasive method to analyze cytokine expression in the skin will provide further understanding of inflammatory skin disorders. This study aimed to evaluate cytokine expression in the skin through cerumen swabbing in dogs with otitis externa (OE) and to investigate whether increased cytokine expression in infected OE reflects the inflammatory status of the ear canal. Three groups consisting of control dogs (n = 24), dogs with ceruminous Malassezia OE (n = 25), and dogs with suppurative bacterial OE (n = 15) were included in the study. The concentrations of keratinocyte-derived cytokines including Interleukin (IL)-8/chemokine ligand (CXCL)8, IL-10, IL-6, Tumor necrosis factor (TNF)-α, and IL-1ß in the cerumen of the ear canal of the included patients were analyzed using commercial ELISA kits. Additionally, correlations between cytokine levels and cytology scores (of Malassezia yeasts, cocci/rod-shaped bacteria, and inflammatory cells) were assessed. IL-8/CXCL8 concentrations were significantly higher in dogs with ceruminous Malassezia OE and dogs with suppurative bacterial OE than in control dogs. Furthermore, IL-8/CXCL8 concentrations positively correlated with Malassezia scores in dogs with ceruminous OE (r = 0.630) and with bacterial scores in dogs with suppurative OE (r = 0.601). In addition, increased expression of IL-6 and IL-1ß were detected in dogs with suppurative bacterial OE compared to those with Malassezia OE and control dogs, and showed positive correlation with inflammatory cell scores IL-6 r = 0.520, IL-1ß; r = 0.680). Therefore, keratinocyte-derived cytokines could be evaluated using non-invasive methods such as cerumen swabbing in dogs with OE.
1 Introduction
Cytokines are key cell-signaling proteins in various immune and homeostatic pathways and has contributed to the understanding of the pathophysiology of immune diseases in both human and veterinary medicine (1, 2). In the epidermis, keratinocytes produce a vast repertoire of cytokines, including interleukins, growth factors, colony-stimulating factors, and chemokines, in response to external stimuli such as allergens, bacterial infections, or chemical substances (3). Cytokine expression has been investigated in inflammatory skin disorders to gain insights into the physiological and pathological processes of the disease (3). Previous studies on cytokine analysis in inflammatory skin disorders have used serum cytokines or skin samples collected by biopsy (3, 4). However, the serum only reflects changes in cytokine expression throughout the body and does not reflect cytokine expression in the skin itself. Additionally, because skin biopsy is an invasive method, there are limitations in its use for collecting data from many patients. Therefore, research on non-invasive methods for analyzing skin cytokine expression has recently become of interest. These methods employ collecting samples from the cerumen, ocular surface, and stratum corneum by tape stripping (3, 5–7). These non-invasive methods allow sampling from many patients and analysis of the expression of cytokines in the skin.
Among these non-invasive methods, cerumen is a biological substance composed of lipids, proteins, amino acids, and carbohydrates produced by keratinocytes and ceruminous and reflects the pathophysiological status of the ear canal (5, 8). In addition, when inflammation occurs in the ear canal, the cerumen contains antimicrobial defense molecules, including lysozyme and immunoglobulins, as well as additional proteins with antimicrobial functions (8). A previous study investigating miRNA expression in dogs with otitis externa (OE) demonstrated miRNA profile changes between healthy and otitis-affected dogs, and bio-informatics showed that altered miRNAs in the cerumen may be involved in the modulation of the host immune response (5). In addition, a previous study using multiplex cytokine analyses in the ear canals of dogs with OE caused by atopic dermatitis showed increased levels of interleukin (IL)-8 and IL10 (6). However, the previous study selected only non-infected OE to avoid the production of pro-inflammatory cytokines related to microorganism contamination/proliferation. Malassezia and Staphylococcus spp. Stimulate cytokine production by epidermal keratinocyte (9, 10). Therefore, evaluating cytokine expression in patients with OE infected with these microorganisms provides further information of cytokine analysis using the cerumen in both primary and secondary OE infections.
The aim of this study was to determine the cytokine concentrations that can be detected using non-invasive techniques for skin surface cerumen collection. For this purpose, we selected infected OE cells that induced pro-inflammatory cytokine secretion in keratinocyte cell lines upon exposure to microbial agents. Infected OE was further divided by ceruminous Malassezia OE and suppurative bacterial OE and the concentration of keratinocyte-derived cytokines including IL-8/chemokine ligand (CXCL)8, IL-10, IL-6, tumor necrosis factor (TNF)-α, and IL-1ß were analyzed. In addition, to investigate whether increased cytokine expression in infected OE reflects the inflammatory status of the ear canal, cytokine concentrations were analyzed in relation to the number of infectious agents (Malassezia yeasts and cocci/rod-shaped bacteria) and inflammatory cells.
2 Materials and methods
2.1 Dogs
Client-owned dogs, regardless of their breed or sex, were enrolled in this study. Written informed consent from both owners and approval from the Institutional Animal Care and Use Committee of Jeonbuk National University (NON2022-101) were obtained before the study began. The dogs were divided into three groups, namely dogs with ceruminous Malassezia OE (n = 25) suppurative bacterial OE (n = 15), and controls (n = 24). Dogs with ceruminous Malassezia OE group had erythema and ceruminous ear discharge in the ear canal. On the cytology of ear canal swabs, Malassezia yeasts (more than five organisms/immersion oil field) and keratin were mainly observed (Figure 1A). The suppurative bacterial OE group included dogs that showed erosion to ulcerative lesions and purulent discharge in the ear canal. On the cytology of ear canal swabs, inflammatory cells, such as degenerative neutrophils, macrophages, and bacteria, including rods and cocci (more than 10 organisms/immersion oil field), were mainly observed, while Malassezia organisms were rarely seen (Figure 1B). Mixed infections with Malassezia yeasts and bacteria were excluded. Healthy dogs with no history of OE were included in the control group. Dogs undergoing treatment with topical or systemic antibacterial, antifungal, or anti-inflammatory therapies in the 30 days prior to the study were excluded.
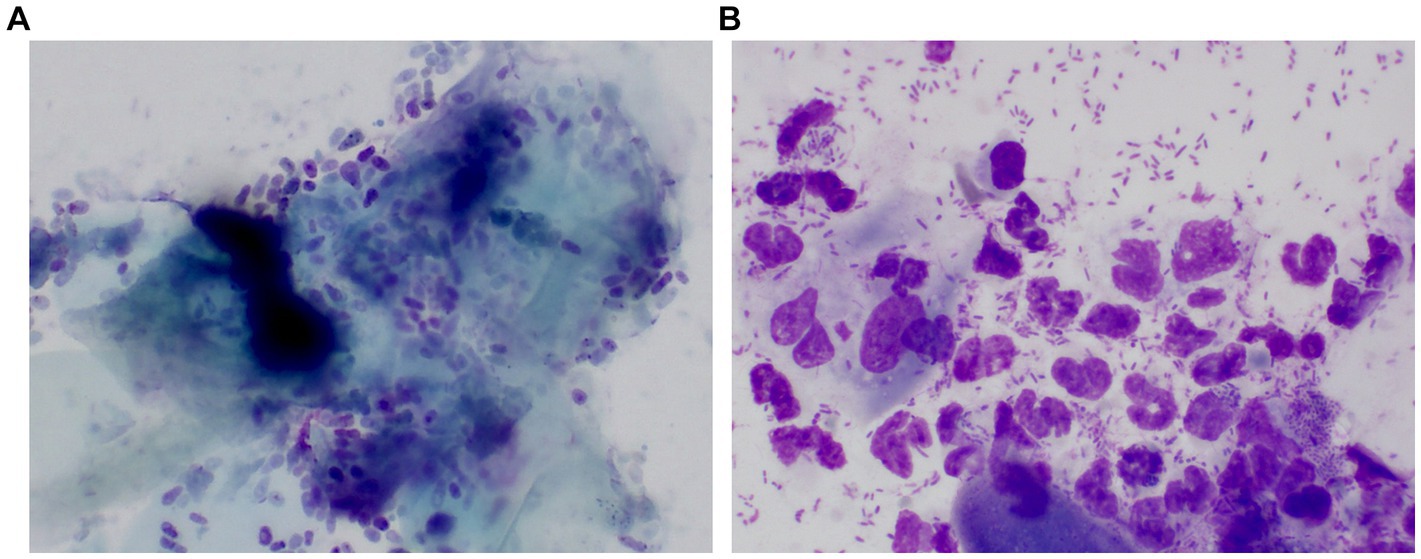
Figure 1. Representative pictures of cytologic examination in a dog with ceruminous Malassezia OE and a dog with suppurative bacteria OE The cytology of the dog with ceruminous Malassezia OE revealed numerous Malassezia yeasts and keratin were mainly observed (A). One dog with suppurative bacterial OE showed large numbers of cocci/rod-shaped bacteria and inflammatory cells, such as degenerative neutrophils and macrophages. Malassezia was rarely observed (B).
2.2 Clinical and cytological evaluation
The ears were examined using a video-otoscope and a 0–3 Otitis Index Score (OTIS)3 was used to evaluate the severity of otitis, as previously reported (11). Erythema, hyperplasia, erosions/ulcerations, and exudates of both the vertical and horizontal ear canals were assessed on a scale of 0–3 to give a total score of 0–12. Cytology was performed by collecting the cerumen using a standard dry non-sterile cotton swab from the junction of the vertical and horizontal ear canals. The slides were dried and stained using Diff Quick staining. Cytology scores from 0 to 4 were assigned based on the presence of Malassezia, cocci/rod-shaped bacteria, and inflammatory cells such as neutrophils and macrophages, using modified methods of previously reported (12). Accordingly, for Malassezia scores in dogs with ceruminous Malassezia OE, a score of 0 was assigned when no round to oval yeasts of Malassezia were observed: 1 for 1–5, 2 for 5–10, 3 for 10–20 and 4 for >20 under immersion oil. For bacterial scores in suppurative bacteria OE, a score of 0 was given when neither cocci nor rod-shaped bacteria were observed, 1 for 1–10, 2 for 10–20, 3 for 20–40 and 4 for >40 in an immersion oil field. In addition, for the inflammatory cell score in the suppurative bacteria OE, a score of 0 was assigned where no inflammatory cells were seen: 1 for 1–5, 2 for 5–10, 3 for 10–20 and 4 for >20 within the immersion oil field. In addition, to identify bacteria agents, bacterial culture were performed in eight of 15 dogs with suppurative bacterial OE. Ear swab samples were cultured in blood agar and the colonies were identified by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (VITEK MS v.2; bioMérieux, Inc., Durham, NC, USA).
2.3 Analysis of chemokine and cytokine expression in cerumen of ear canal
The cerumen was obtained by inserting dry sterile swabs into the vertical and horizontal ear canals. Ear swab samples were treated with 0.05% Tween 20 in phosphate-buffered saline for 30 min with sonication. The extracts were then centrifuged for 5 min at 2100 × g to remove skin solids, and aliquots of the extracts were collected. The aliquots were placed in dry Eppendorf tubes and frozen at −20°C until analysis. Total soluble protein concentrations were analyzed using a BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) with bovine serum albumin as the reference standard. Immunoassays to detect IL-8/CXCL8 (DY1608, R&D Systems, Minneapolis, MN, USA), IL-10 (DY735, R&D Systems), IL-6 (DY1609, R&D Systems), TNF-α (DY1507, R&D Systems), and IL-1ß (DY3747, R&D Systems) in the cerumen were performed according to the manufacturer’s instructions. The concentrations of canine IL-8/CXCL8, IL-10, IL-6, TNF-α and IL-1ß were calculated as pg/mg total soluble protein.
2.4 Statistical analysis
Statistical analyses were performed using the SPSS statistics (Version 29.0. Armonk, NY, IBM Corp.). The cytokine concentrations in the three groups were statistically analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test. In addition, Pearson’s correlation test was used to analyze the correlation between cytokine concentrations and cytology scores. Correlation coefficients (r) of <−0.4 and > 0.4 indicate significant negative and positive correlations, respectively. Statistical significance was set at p < 0.05.
3 Results
3.1 Clinical manifestations of OE groups and control dogs
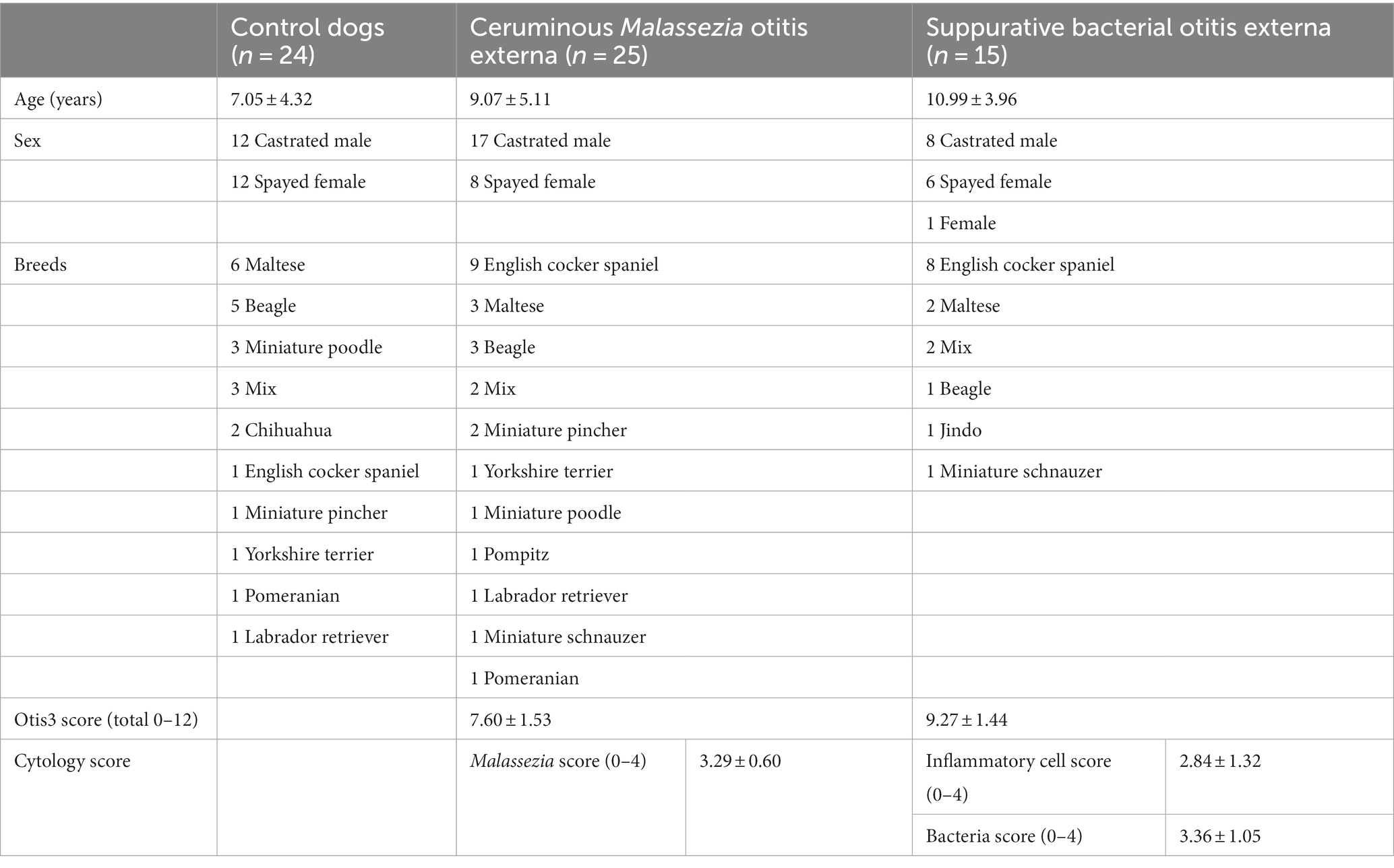
The participant’s age, sex, breed, and OTIS3 and cytology scores of dogs with Malassezia OE and dogs with suppurative bacterial OE are summarized in Table 1. In dogs with ceruminous Malassezia OE, the average OTIS3 scores were 7.60 ± 1.53, 2.04 ± 0.54, 2.28 ± 0.46, 0.92 ± 0.57, and 2.36 ± 0.46 for the total, erythema, hyperplasia, erosion/ulceration, and exudate scores, respectively. In addition, the average OTIS3 scores in dogs with suppurative bacterial OE were 9.27 ± 1.44, 2.47 ± 0.52, 1.80 ± 0.86, 2.47 ± 0.52, and 2.71 ± 0.47 for the total, erythema, hyperplasia, erosion/ulceration, and exudate scores, respectively. The average Malassezia cytology score in dogs with ceruminous Malassezia OE was 3.29 ± 0.60 and inflammatory cell score and bacterial score in dogs with suppurative bacterial OE were 2.84 ± 1.32 and 3.36 ± 1.05, respectively. In addition, cytology of suppurative bacterial OE revealed that all 15 dogs with suppurative bacterial OE showed mixed cytological infections with rods and cocci. In addition, we performed bacterial culture in eight of 15 dogs with suppurative bacterial OE, which revealed a mixed infection of gram-negative bacilli and gram-positive cocci in all eight dogs. The details of isolated bacteria are present in Supplementary Table S1.
3.2 Cytokine expression in cerumen of OE groups and control dogs
To analyze cytokine and chemokine expression in cerumen of dogs with OE, concentrations of keratinocytes-derived cyotkiens of IL-8/CXCL8, IL-10, IL-6, TNF-α, and IL-1ß were investigated using commercially available ELISA kits and calculated as pg/mg soluble protein. In both groups of ceruminous Malassezia OE (p < 0.01) and suppurative bacterial OE (p < 0.01), IL-8/CXCL8 concentrations were significantly higher than those in the control dogs (Figure 2A). Furthermore, IL-8/CXCL8 concentrations in subjects with suppurative bacterial OE were significantly higher than those in subjects with ceruminous Malassezia OE (p < 0.01, Figure 2A). In addition, increased expression of IL-1ß was detected in dogs with suppurative bacterial OE, compared to those with Malassezia OE and control dogs (p < 0.01, Figure 2B). IL-6 concentrations were significantly higher in dogs with suppurative bacterial OE than in those Malassezia OE and control dogs (p < 0.01, Figure 2C). In contrast, in Malassezia OE and control dogs, IL6 was barely detected using the immunoassay used in this study (Figure 2C). In addition, expression of IL-10 and TNF-α were undetectable in all subjects using commercially available immunoassay kit used in this study.
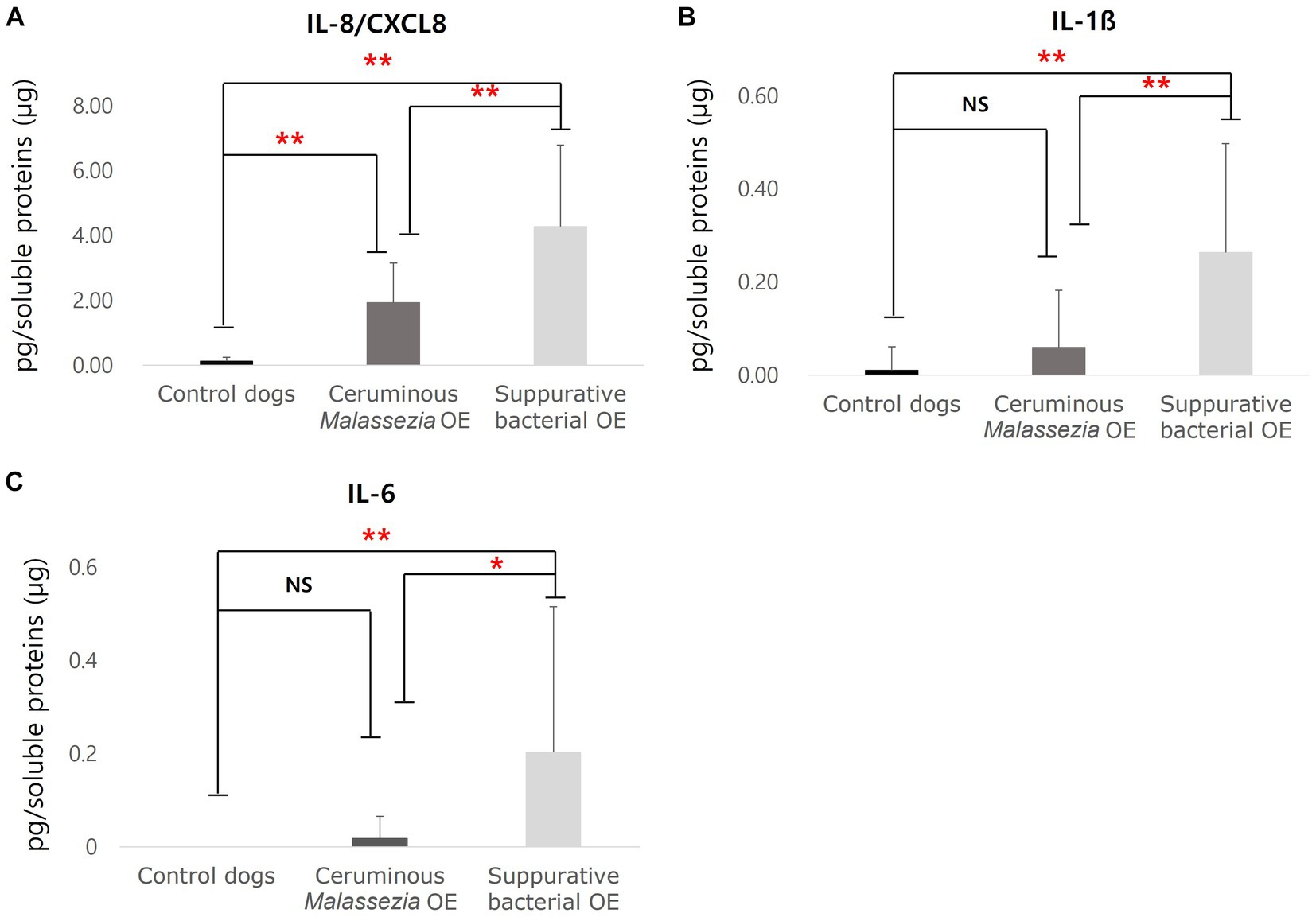
Figure 2. Cytokines concentrations in cerumen of control dogs, dogs with ceruminous Malassezia OE and dogs with suppurative bacteria OE IL-8/CXCL8 concentrations were significantly increased in ceruminous Malassezia OE and suppurative bacterial OE than those in control dogs, with the levels being higher in suppurative bacterial OE (A). In addition, increased concentrations of IL-1ß were detected in dogs with suppurative bacterial OE, comparing those in Malassezia OE and control dogs (B). IL-6 concentrations were significantly higher in dogs with suppurative bacterial OE than in those Malassezia OE and control dogs (C) (NS, no significant difference; **p < 0.01).
3.3 Correlation of cytokines concentrations with cytology scores
The correlation of Malassezia score with IL-8/CXCL8 concentrations in dogs with ceruminous Malassezia OE, and the correlation of inflammatory cell scores and bacterial scores with IL-8/CXCL8, IL-6, and IL-1ß concentration in dogs with suppurative bacterial OE were investigated. In dogs with ceruminous Malassezia OE, IL-8/CXCL8 concentrations showed positive correlation with Malassezia score (Figure 3A, r = 0.630, p < 0.01). In dogs with suppurative bacterial OE, the IL-8/CXCL8 concentration showed a positive correlation with the bacterial score (Figure 3B, r = 0.601, p = 0.018), whereas no significant correlation was noted with the neutrophil score (data not shown). Additionally, IL-6 and IL-1ß concentrations showed positive correlation with inflammatory cell scores (Figures 3C,D, IL6; r = 0.520, p = 0.047, IL-1ß; r = 0.680, p < 0.01). However, no significant correlations were observed between bacteria scores and IL-6 and IL-1ß concentrations (data not shown).
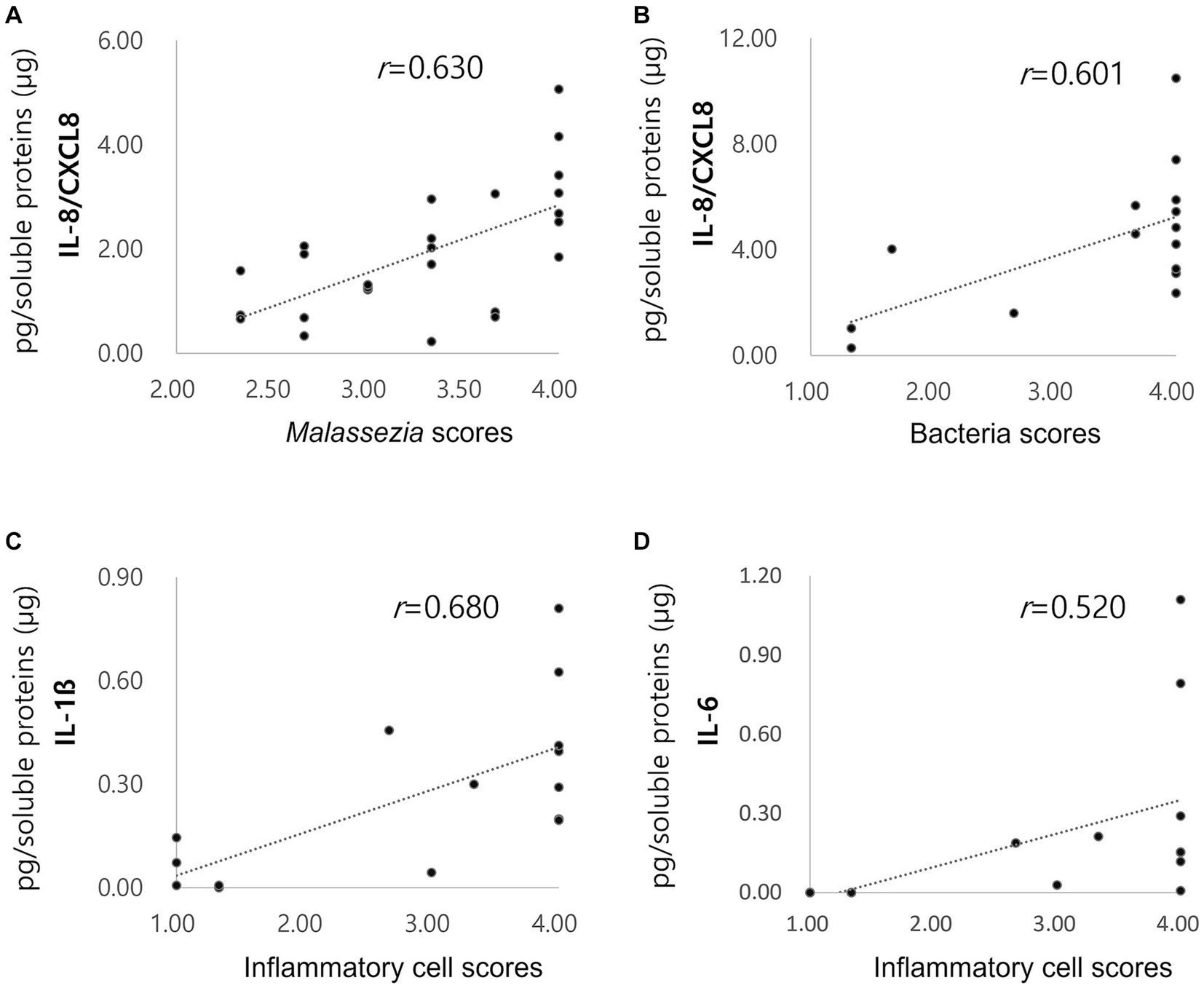
Figure 3. Correlations of cytokine concentrations with Malassezia score, bacteria score and inflammatory score. Increased concentrations of acute phase proteins in dogs with pyometra The IL-8/CXCL8 concentration in ceruminous Malassezia OE positively correlated with Malassezia scores ((A), r = 0.630, p < 0.01). In suppurative bacterial OE, the IL-8/CXCL8 concentration positively correlated with the bacterial scores ((B), r = 0.601, p = 0.018). IL-6 (C) and IL-1ß (D) concentrations showed positive correlation with inflammatory cell scores (IL6; r = 0.520, p = 0.047, IL-1ß; r = 0.680, p < 0.01).
4 Discussion
Cerumen analysis is a non-invasive method that is able to reflect the inflammatory status of dogs (8). The present study evaluated skin cytokine expression in dogs infected with OE using non-invasive cerumen swabbing methods. Cytokines in the cerumen are differentially expressed between ceruminous Malassezia OE and suppurative bacterial OE. Accordingly, ceruminous Malassezia OE showed increased IL-8/CXCL8 concentrations than those of the control, while suppurative bacterial OE showed significantly increased levels of IL-6, IL-1ß, and IL-8/CXCL8. IL-8/CXCL8 levels are higher in suppurative bacterial OE than in ceruminous Malassezia OE. Furthermore, increased cytokine levels positively correlated with the number of infectious agents or inflammatory cells. The present study indicates that non-invasive methods of cerumen swabbing in dogs with OE allow the analysis of cytokine expression, which might reflect the pathophysiological status of the ear canal. Keratinocyte-derived cytokines may be differentially expressed depending on the presence or absence of pathogens or inflammatory cells.
IL-8/CXCL8 is a member of the supergene family of pro-inflammatory and chemotactic cytokines, and is produced by macrophages and other cell types, such as the epithelial cells of keratinocytes (13). In this study, both ceruminous Malassezia OE and suppurative bacterial OE showed significantly increased IL-8/CXCL8 concentrations. Furthermore, IL-8/CXCL8 concentrations were positively correlated with Malassezia infectious organisms and bacteria. Similar findings have been reported with IL-8 concentrations in the ear canal of ceruminous in dogs with OE were significantly decreased with the reduction of Malassezia organisms due to the use of ear cleaners (12). Previous studies have shown that Malassezia species induce IL-8 secretion by human keratinocytes (9, 14-16). Therefore, the increased IL-8/CXCL8 concentrations in ceruminous Malassezia OE may be related to the high number of Malassezia organisms that induce IL-8/CXCL8 secretion from epidermal keratinocytes. In addition, it has been reported that bacteria such as Staphylococcus spps. Directly induce IL-8 production in human keratinocytes (10). In this study, the increased IL-8/CXCL8 concentrations in suppurative bacterial OE might be associated with increased bacterial colonization in the ear canal. IL-8 concentrations were significantly higher in dogs with suppurative bacterial OE than in those with ceruminous Malassezia OE. These results were due to higher numbers of bacteria in suppurative OE than Malassezia yeast in ceruminous OE and/or the presence of other inflammatory cells in suppurative OE.
Similarly, significantly increased IL-8/CXCL8 concentrations have been reported in previous studies that analyzed cytokines by swabbing the cerumen or occlusal surface of dogs with atopic dermatitis (6, 7). In an analysis of cytokine expression in conjunctival swabs, IL-8 was the only cytokine that was significantly increased in atopic dogs, showing a positive correlation with conjunctival and pruritus scores (7). In addition, the concentration of IL-8 is also significantly higher in the ears of atopic dogs than in healthy dogs (6). Interestingly, in contrast to the present study, the previous study only selected non-infected otitis patients to avoid the production of pro-inflammatory cytokines related to microbes. As IL-8/CXCL8 concentrations are affected by infectious organisms, including Malassezia and bacterial agents, infectious organisms should be excluded when studying IL-8 as a biomarker of atopic dermatitis.
IL-1β and IL-6 are pro-inflammatory cytokines that are crucial for host-defense responses to infection and injury. They are produced and secreted by various cell types, particularly those of the innate immune system, such as monocytes and macrophages (17, 18). In addition, it has been reported that Malassezia yeasts and bacteria stimulate IL-1β and IL-6 production from epidermal keratinocytes (10, 19–21). However, in this study, no significant increases in these cytokines were observed in the dogs with ceruminous Malassezia OE; specifically, IL-6 was barely detected in these samples. A previous study using the Luminex® assay demonstrated that IL-6 was undetectable in the ear canal of dogs with atopic OE (6). In contrast, this study showed significantly increased concentrations of IL-1ß and IL-6 in the cerumen of dogs with suppurative bacterial OE. In addition, the concentrations of these cytokines were positively correlated with inflammatory cell scores. Therefore, it is possible that in the case of OE with purulent inflammation, the expression of IL6 is increased to recruit neutrophils and/or to further increase cytokine production by inflammatory cells. Therefore, when studying cytokines through skin surface swabbing, IL-1ß and IL-6 can be selected as targets when purulent inflammation is present.
The expressions of IL-10 and TNF-α was not detected on the ELISA essay used on all subjects. In a previous study using Luminex® technology, IL-10 concentration was significantly increased in the ear canal of dogs with atopic OE (6). The assay range of the IL-10 ELISA kits used in this study was 31.2 to 2,000 pg/mL,while predefined kits using Luminex® technology in the previous study had a range of 12 to 50,000 pg/mL (6). Therefore, the lack of detectable IL-10 in this study might be related to the analytical method in which the commercially available ELISA kits used in this study has a relatively lower sensitivity for IL-10 in the cerumen of dog ear canals. Additionally, since TNF-α was not detectable in a study using Luminex® assay (6), TNF-α might be minimally expressed in cerumen.
The potential limitations of this study are as follows. (1) Because the primary factors for OE have not been fully identified, the effects of primary factor on cytokine expressions have not been completely ruled out. Primary factor of OE such as allegic skin disease and eondocrinopaty could affect cytokine expressions. There might be cytokines that could not be detected because the cytokine analysis was performed using commercially available ELISA kits. Large-scale studies of several types of OE using other assays such as the Luminex® assay are required to further understand cytokine expression as biomarkers in dogs with OE.
In conclusion, the present study demonstrated that increased IL-8/CXCL8, IL-6, and IL-1ß levels were detected using non-invasive cerumen swabbing in dogs with OE infected with Malassezia and bacteria. The present study provides fundamental data for future analysis of skin cytokine expression, especially using non-invasive methods such as swabbing the cerumen, ocular surface, and tape stripping of the stratum corneum. Target cytokines can be eliminated depending on the presence or absence of infectious agents and inflammatory cells.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Institutional Animal Care and Use Committee of Jeonbuk National University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
J-SY: Writing – original draft, Investigation. JP: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Project No. PJ01690702 from the Rural Development Administration, Republic of Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor DY declared a past co-authorship with the authors JP and J-SY.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1355569/full#supplementary-material
References
1. Liu, TJ, Lin, LL, McMeniman, E, Wu, J, Kao, YC, Kumari, S, et al. Cytokine/chemokine assessment as a complementary diagnostic tool for inflammatory skin diseases. Front Immunol. (2022) 13:1028435. doi: 10.3389/fimmu.2022.1028435
2. Tamamoto-Mochizuki, C, Santoro, D, Saridomikelakis, MN, Eisenschenk, MNC, Hensel, P, and Pucheu-Haston, C. International committee on allergic diseases of animals (ICADA). Update on the role of cytokines and chemokines in canine atopic dermatitis. Vet Dermatol. (2023) 35:25–39. doi: 10.1111/vde.13192
3. Portugal-Cohen, M, and Kohen, R. Non-invasive evaluation of skin cytokines secretion: an innovative complementary method for monitoring skin disorders. Methods. (2013) 61:63–8. doi: 10.1016/j.ymeth.2012.10.002
4. Fedenko, ES, Elisyutina, OG, Filimonova, TM, Boldyreva, MN, Burmenskaya, OV, Rebrova, OY, et al. Cytokine gene expression in the skin and peripheral blood of atopic dermatitis patients and healthy individuals. Self Nonself. (2011) 2:120–4. doi: 10.4161/self.2.2.16939
5. Lecchi, C, Zamarian, V, Borriello, G, Galiero, G, Grilli, G, Caniatti, M, et al. Identification of altered miRNAs in cerumen of dogs affected by otitis externa. Front Immunol. (2020) 11:914. doi: 10.3389/fimmu.2020.00914
6. Lecru, LA, Combarros, D, Moog, F, Marinovic, L, Kondratjeva, J, Amalric, N, et al. Multiplex cytokine analyses in ear canals of dogs suggest involvement of IL-8 chemokine in atopic otitis and Otodectic mange-preliminary results. Animals. (2022) 12:575. doi: 10.3390/ani12050575
7. Pressanti, C, Ravailhe, E, Castellote-Brun, J, Amalric, N, Lecru, LA, Kondratjeva, J, et al. Survey of cytokines on ocular surfaces of atopic dogs by multiplex analysis using two sampling methods -a pilot study. Vet Dermatol. (2021) 32:625–e167. doi: 10.1111/vde.13010
8. Shokry, E, and Filho, NRA. Insights into cerumen and application in diagnostics: past, present and future prospective. Biochem Med. (2017) 27:030503. doi: 10.11613/BM.2017.030503
9. Sparber, F, and LeibundGut-Landmann, S. Host responses to Malassezia spp. in the mammalian skin. Front Immunol. (2017) 8:1614. doi: 10.3389/fimmu.2017.01614
10. Sasaki, T, Kano, R, Sato, H, Nakamura, Y, Watanabe, S, and Hasegawa, A. Effects of staphylococci on cytokine production from human keratinocytes. Br J Dermatol. (2003) 148:46–50. doi: 10.1046/j.1365-2133.2003.05017.x
11. Nuttall, T, and Bensignor, E. A pilot study to develop an objective clinical score for canine otitis externa. Vet Dermatol. (2014) 25:530–7. doi: 10.1111/vde.12163
12. King, SB, Doucette, KP, Seewald, W, and Forster, SL. A randomized, controlled, single-blinded, multicenter evaluation of the efficacy and safety of a once weekly two dose otic gel containing florfenicol, terbinafine and betamethasone administered for the treatment of canine otitis externa. BMC Vet Res. (2018) 14:307. doi: 10.1186/s12917-018-1627-5
13. Kondo, S, Kono, T, Sauder, DN, and McKenzie, RC. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol. (1993) 101:690–4. doi: 10.1111/1523-1747.ep12371677
14. Moog, F, Mivielle, J, Dumitrache, MO, Amalric, N, Lecru, LA, et al. Clinical and Microbiological Performances and Effects on Lipid and Cytokine Production of a Ceruminolytic Ear Cleaner in Canine Erythemato-Ceruminous Otitis Externa. Vet Sci. (2022) 9:185. doi: 10.3390/vetsci9040185
15. Buommino, E, De Filippis, A, Parisi, A, Nizza, S, Martano, M, Iovane, G, et al. Innate immune response in human keratinocytes infected by a feline isolate of Malassezia pachydermatis. Vet Microbiol. (2013) 163:90–6. doi: 10.1016/j.vetmic.2012.12.001
16. Park, HR, Oh, JH, Lee, YJ, Park, SH, Lee, YW, Lee, S, et al. Inflammasome-mediated inflammation by Malassezia in human keratinocytes: a comparative analysis with different strains. Mycoses. (2021) 64:292–9. doi: 10.1111/myc.13214
17. Lopez-Castejon, G, and Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. (2011) 22:189–95. doi: 10.1016/j.cytogfr.2011.10.001
18. Tanaka, T, Narazaki, M, and Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
19. Zhang, YJ, Han, Y, Sun, YZ, Jiang, HH, Liu, M, Qi, RQ, et al. Extracellular vesicles derived from Malassezia furfur stimulate IL-6 production in keratinocytes as demonstrated in in vitro and in vivo models. J Dermatol Sci. (2019) 93:168–75. doi: 10.1016/j.jdermsci.2019.03.001
20. Watanabe, S, Kano, R, Sato, H, Nakamura, Y, and Hasegawa, A. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J Invest Dermatol. (2001) 116:769–73. doi: 10.1046/j.1523-1747.2001.01321.x
Keywords: dog, otitis externa, cytokine, cerumen, Malassezia, bacteria
Citation: Yoon J-S and Park J (2024) Non-invasive evaluation of cytokine expression using the cerumen of dogs with otitis externa. Front. Vet. Sci. 11:1355569. doi: 10.3389/fvets.2024.1355569
Edited by:
DoHyeon Yu, Gyeongsang National University, Republic of KoreaReviewed by:
Byeongteck Kang, Chungbuk National University, Republic of KoreaPiera Anna Martino, University of Milan, Italy
Copyright © 2024 Yoon and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinho Park, anBhcmtAamJudS5hYy5rcg==
 Ji-Seon Yoon
Ji-Seon Yoon Jinho Park
Jinho Park