- 1Animal Health Section, The 4th Regional Livestock Office, Department of Livestock Development, Khon Kaen, Thailand
- 2Nawa District Livestock Office, Department of Livestock Development, Nakhon Phanom, Thailand
- 3Buriram Provincial Livestock Office, Department of Livestock Development, Buriram, Thailand
- 4Animal Health Section, The 7th Regional Livestock Office, Department of Livestock Development, Nakhon Pathom, Thailand
- 5Regional Field Epidemiology Training Program for Veterinarian, Bureau of Disease Control and Veterinary Services, Department of Livestock Development, Bangkok, Thailand
- 6Bureau of Disease Control and Veterinary Services, Department of Livestock Development, Bangkok, Thailand
- 7Akkhararatchakumari Veterinary College, Walailak University, Nakhon Si Thammarat, Thailand
- 8College of Veterinary Science and Medicine, Central Luzon State University, Science City of Muñoz, Philippines
- 9Veterinary Public Health and Food Safety Centre for Asia Pacific (VPHCAP), Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand
- 10Research Center for Veterinary Biosciences and Veterinary Public Health, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand
Introduction: Thailand experienced a nationwide outbreak of lumpy skin disease (LSD) in 2021, highlighting the need for effective prevention and control strategies. This study aimed to identify herd-level risk factors associated with LSD outbreaks in beef cattle herds across different regions of Thailand.
Methods: A case–control study was conducted in upper northeastern, northeastern, and central regions, where face-to-face interviews were conducted with farmers using a semi-structured questionnaire. Univariable and multivariable mixed effect logistic regression analyses were employed to determine the factors associated with LSD outbreaks. A total of 489 beef herds, including 161 LSD outbreak herds and 328 non-LSD herds, were investigated.
Results and discussion: Results showed that 66% of farmers have operated beef herds for more than five years. There were very few animal movements during the outbreak period. None of the cattle had been vaccinated with LSD vaccines. Insects that have the potential to act as vectors for LSD were observed in all herds. Thirty-four percent of farmers have implemented insect control measures. The final mixed effect logistic regression model identified herds operating for more than five years (odds ratio [OR]: 1.62, 95% confidence interval [CI]: 1.04–2.53) and the absence of insect control management on the herd (OR: 2.05, 95% CI: 1.29–3.25) to be associated with LSD outbreaks. The implementation of insect-vector control measures in areas at risk of LSD, especially for herds without vaccination against the disease, should be emphasized. This study provides the first report on risk factors for LSD outbreaks in naïve cattle herds in Thailand and offers useful information for the development of LSD prevention and control programs within the country’s context.
1 Introduction
Lumpy skin disease (LSD) is a highly contagious viral disease that primarily affects cattle. It is caused by the lumpy skin disease virus (LSDV), a member of the Capripoxvirus genus (1). Clinical manifestations of LSD can include fever, loss of appetite, and general weakness. The most notable feature, however, is the appearance of the characteristic skin nodules. These nodules can occur on various parts of the body, including the head, neck, limbs, and genital areas (2). In severe cases, the nodules may become ulcerated, leading to secondary bacterial infection. LSD poses significant economic implications for cattle populations. In affected herds, the morbidity rate can vary widely, ranging from 3 to 85%, depending on the susceptibility of cattle and other factors (3, 4). The mortality rate is typically lower than 3% (3), but in some cases, it may exceed 40% (5). The disease can have devastating effects on the livestock industry, leading to substantial economic losses. The World Organization for Animal Health (WOAH) has defined LSD as a disease requiring notification due to the potential for rapid virus propagation in susceptible cattle populations and the consequential considerable economic effects in affected herds (6, 7).
While LSD was previously confined to Africa with occasional incursions into the Middle East, recent outbreaks have raised concerns about its emergence and rapid spread in Asia (4, 8–14). Thailand, being a significant hub for livestock production and trade in Southeast Asia, has also experienced the impact of LSD outbreaks since March 2021 (15). It was initially detected in the cattle farming regions located in the northeastern part of Thailand (9). Later, outbreaks of LSD were reported across the country. There were 283,213 affected herds with 628,089 cases across 64 provinces as of June 30, 2022 (12).
Various risk factors associated with LSD outbreaks in endemic settings have been identified (16, 17). The movement of infected animals is considered as a significant factor in facilitating long-range transmission, whereas arthropod-borne transmission is likely to be the primary mechanism responsible for the rapid and aggressive spread of the disease over short distances (18). The predominant blood-feeding arthropod vectors for LSD are stable flies (Stomoxys calcitrans), mosquitoes (Aedes aegypti), and hard ticks (Rhipicephalus and Amblyomma species) (1). Furthermore, cattle breed, source of replacement stock, introduction of new animals, herd size, communal grazing and watering management, and housing were identified as potential risk factors for the LSD outbreak in previous studies (16, 19–22). Moreover, management type, gender, age, precipitation, and intake of community water sources have been determined to be risk factors for LSD (23). However, there is a notable research gap regarding the specific risk factors for LSD in the context of Thailand.
Understanding the risk factors associated with the occurrence of LSD is crucial for effective prevention and control strategies. Identifying and quantifying these factors can aid in the development of targeted interventions, including vaccination campaigns, vector control measures, and improved biosecurity practices. Therefore, this study aims to determine the risk factors contributing to the occurrence of LSD outbreaks in naïve cattle herds in various regions of Thailand. The finding from this study has the potential to significantly advance the development of targeted control measures and policies. Ultimately, this will lead to improved management and prevention of the disease. The outcomes of this study may also contribute to the existing body of knowledge on LSD risk factors, potentially benefiting other countries facing similar challenges.
2 Materials and methods
2.1 Study population and sampling
This case–control study was conducted in three provinces of Thailand: Nakhon Phanom, Buriram, and Prachuap Khiri Khan (Figure 1). The study took place from July to September in Nakhon Phanom, and from August to September in both Buriram and Prachuap Khiri Khan, all in the year 2021. It is important to note that the questionnaire survey was not conducted during the outbreak period, as the primary investigation prioritized the outbreak investigation protocol carried out by livestock authorities in each area. It is noteworthy that the surveys in all three provinces were carried out approximately 2 months after the latest herd had confirmed the LSD outbreak. Furthermore, the study focused on households that owned cattle as the primary unit of analysis. To ensure representative samples, a multi-stage sampling technique was employed.
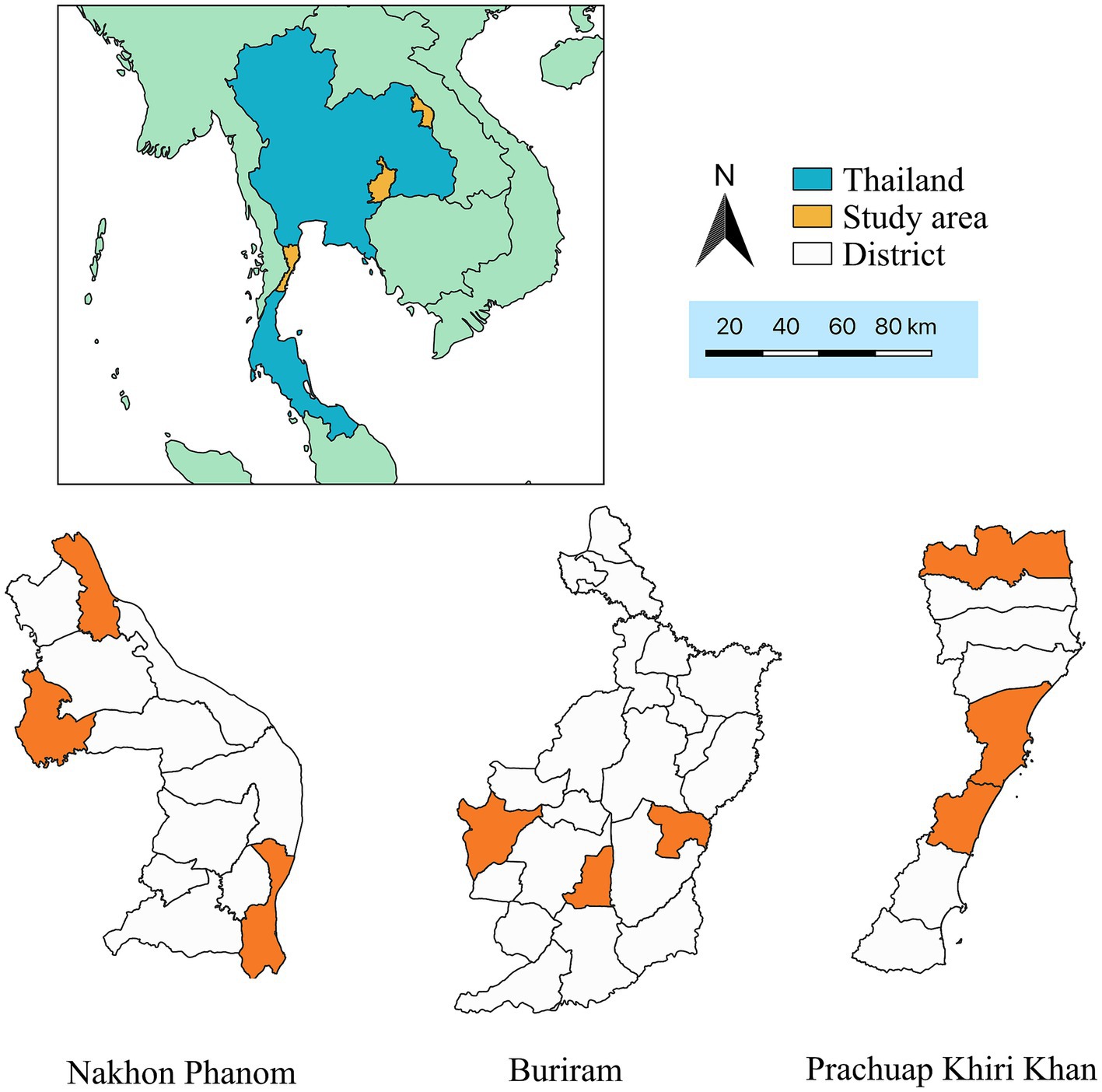
Figure 1. Map of Thailand displaying study areas (orange color) which are located in Nakhon Phanom, Buriram, and Prachuap Khiri Khan provinces.
Initially, the selection of provinces was purposive and based on collaboration between central and local veterinary authorities. Subsequently, within each province, three districts were chosen using a simple random sampling approach. Furthermore, subdistricts within each district were randomly selected. The case herds in this study were identified based on the official outbreak investigation reports issued by local veterinary authorities in each subdistrict. In each sub-district area, all LSD outbreak herds were included in the study. Control herds were randomly selected from herds located in the same sub-village as the case herds. An approximately 1:2 ratio for case and control herds, respectively was applied. As a result, the total number of herds included in this study for Nakhon Phanom, Buriram, and Prachuap Khiri Khan provinces was 159, 180, and 150, respectively.
2.2 Case and control definitions
Cattle herd served as the epidemiological unit. A case herd, or an LSD-outbreak herd, was defined as a herd with at least one individual cattle showing the LSD clinical signs, which include raised, circular, firm, nodules varying from 1 to 7 cm diameter, as observed by investigators from the Department of Livestock Development (DLD) (9). Confirmation of the disease could be through laboratory testing using the polymerase chain reaction (PCR) method (12), although it was not always a prerequisite. A control herd, or a non-LSD outbreak herd, was defined as a beef cattle herd located in the same village and/or subdistrict as the case herds. The control herds must not have any history of clinical LSD among their animals. The historical records of LSD outbreaks were cross-checked with information provided by farmers and local veterinary authorities during the questionnaire survey.
2.3 Questionnaire survey
The semi-structured questionnaire utilized in this study was developed collaboratively by veterinary experts from the DLD and epidemiologists from the Regional Field Epidemiology Training Program for Veterinarians (R-FETPV), supported by the Food and Agriculture Organization of the United Nations (FAO). Several questions in the questionnaire were adopted from the official outbreak investigation form employed for nationwide investigations of LSD outbreaks. The questionnaire covered various relevant variables including the owner’s profile, farm characteristics, biosecurity, and other management practices.
Data collection was carried out by livestock and veterinary authorities. In cases where data were incomplete, follow-up telephone interviews were conducted to gather the necessary information.
2.4 Hierarchical structure of the data
The data is organized into a hierarchical structure, wherein it is structured into multiple levels or layers, with each level representing distinct units of study. Within the study’s dataset, farms are grouped into clusters within districts, and these districts, in turn, are clustered within provinces. This hierarchical arrangement facilitates statistical analyses.
2.5 Statistical analysis
2.5.1 Descriptive analysis
Descriptive statistics, including the mean and standard deviation for quantitative variables, as well as frequencies (expressed as percentages) for qualitative variables, were calculated using R version 3.6.2 (https://www.r-project.org).
2.5.2 Univariable mixed effect logistic regression analysis
The mixed effect univariable logistic regression model used in this study incorporated both fixed and random effects. Each potential risk factor is defined as a fixed effect, while the individual district was included as a random effect (24). To account for the clustering of districts within provinces, a factor named “province” was included in the univariable and multivariable logistic models as a fixed effect (25). The odds ratio and p-value were determined based on Wald’s test.
Subsequently, risk factors with a p-value less than 0.2 were selected for further analysis using a mixed effect multivariable logistic regression. The objective of this step was to select factors that have a significant association with the outcome while accounting for potential confounding variables. Multicollinearity between variables was also examined using a Cramer’s π-prime statistics. A pair of categorical variables was considered collinear if Cramer’s π-prime statistics was greater than 0.7 (24, 26).
2.5.3 Multivariable mixed effect logistic regression analysis
2.5.3.1 Model
In the mixed effect multivariable logistic regression model, the potential risk factors were considered as fixed effects, while the individual district was defined as a random effect, similar to a previous study (21). The models also incorporated the variable “province” as a fixed effect as suggested in the literature (25). The statistical model can be expressed as follows (27):
where is the outbreak status (1 = outbreak or 0 = non-outbreak) of a herd clustered in district . The term represents the intercept, is the regression coefficient for the fixed effect factors and is a set of fixed effect factors . The term is the random effects on the intercept for the district which includes herd . It was assumed that . The error terms are assumed to follow a logistic distribution with mean zero and variance .
2.5.3.2 Model selections
Model selection was performed using a backward stepwise method. Akaike’s Information Criteria (AIC) was utilized as the criterion for selecting the most appropriate model (23, 28–31). The interaction between variables was also examined during the model selection process. If the inclusion of an interaction term did not improve the model, the interaction term was removed from the model.
Confounding was assessed by examining the change in estimated coefficients of the variables that remained in the final model upon the addition of a non-selected variable. If the inclusion of this new variable resulted in a change of >25% in any parameter estimate, that variable was deemed a confounder and retained in the model (24, 26).
2.5.3.3 Evaluation of multicollinearity and model assumptions
After identifying the final model, an assessment of multicollinearity was conducted by examining the variance inflation factors (VIF) values. The VIF represents the ratio of the overall variance in the model to the variance when a specific single variable is included. A VIF value below 5 indicates no evidence of multicollinearity among the variables included in the final model (32). Additionally, residual diagnostics for the final mixed effect model were evaluated.
2.5.3.4 In the final model, odds ratios and their corresponding 95% confidence intervals were calculated for each variable intra-class correlations
For the final model, we considered the variance components as a random effect, dividing them into two levels based on their origin. The first level variance is equivalent to on the logit scale, and this represents the error variance in the binary model. The second level variance symbolizes the random intercept that changes based on the district’s effect, symbolized as . As a result, to illustrate these variances, we calculated the intra-class correlation (ICC). The formula used for calculating the ICC is as follows (25):
A low ICC indicates minimal clustering as most of variance is found within individual districts. In contrast, a high ICC means that there is less variation within a district when compared to the variation observed between the different districts (33).
The mixed effect logistic regression was conducted using the “glmer” function from the “lme4” package. To assess the variance inflation factors (VIF), the “vif” function from the “car” package was employed. The diagnostics of residuals were carried out using “DHAMa” package. The ICC was obtained from “mlmhelpr” package.
3 Results
3.1 Respondent and management practices
A total of 161 LSD-outbreak herds and 328 non-LSD outbreak herds from three provinces in Thailand participated in this study. The provinces included Buriram (n = 180), Nakhon Phanom (n = 159), and Prachuap Khiri Khan (n = 150). The average age of the participants was 54 in the case group and 53 in the control group. Males constituted approximately 72% of the respondents in both groups (Table 1). Most respondents in both groups had a primary education. The average duration of herd operation was 7.7 years with a median of 5 years, The average number of cattle per herd was 4.6 with a median of 5 animals. The majority of herds (90%) had facilities for keeping cattle in stalls.
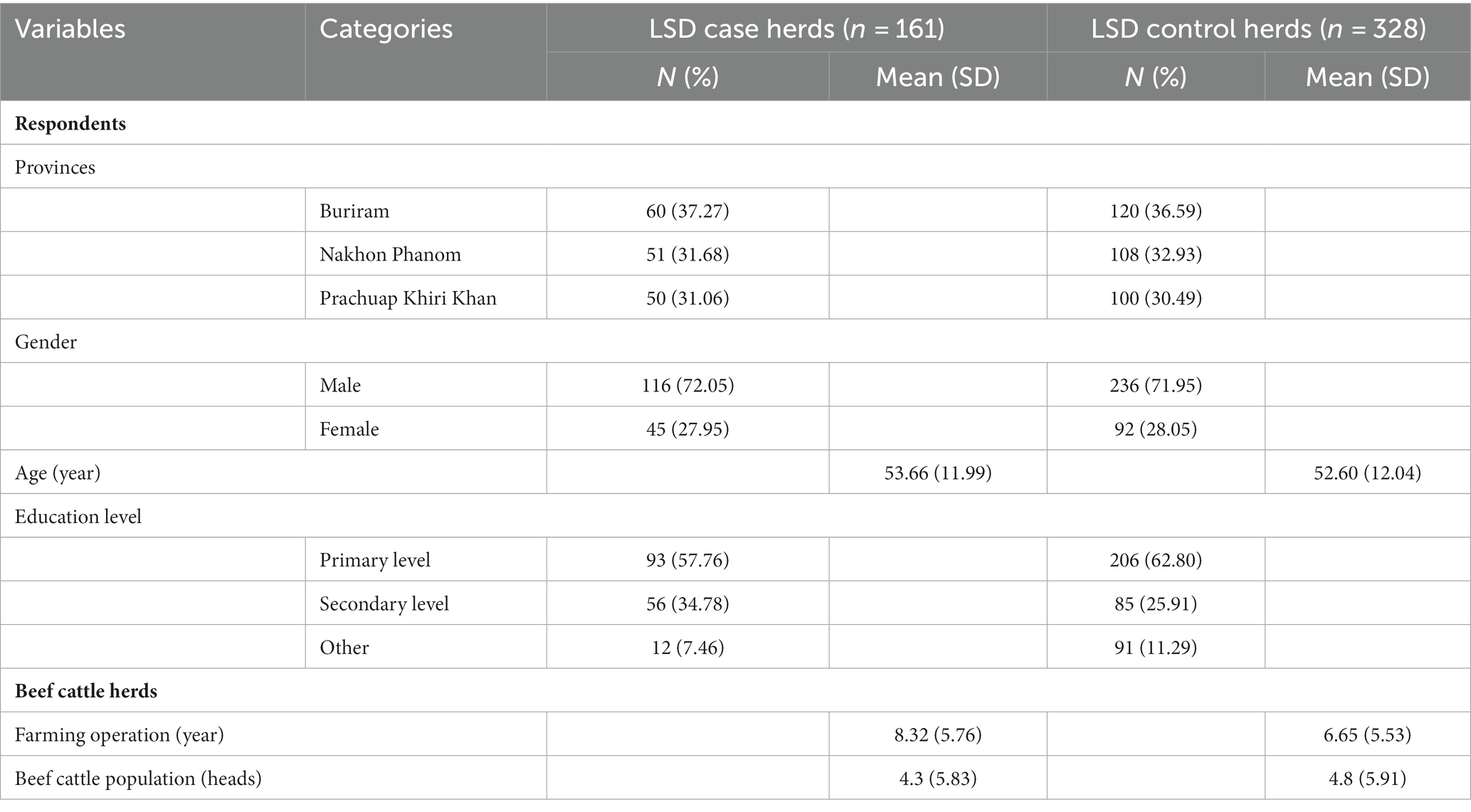
Table 1. Characteristics of respondents, and LSD case (herd with LSD outbreak) and control (herd without LSD outbreak) herds enrolled in a case–control study of risk factors associated with lumpy skin disease outbreaks in beef herds in Thailand.
Farm characteristics and management practices for the herds included in this study are summarized in Table 1. Out of all the herds investigated, only eleven herds had a history of purchasing cattle from other herds and transporting them to their own facilities. All herds examined reported the presence of stable flies or mosquitoes or both. Notably, none of the herds had a history of using LSD vaccines. Additionally, the data highlights that 36% of herds with an operational history exceeding 5 years experienced LSD outbreaks, while the percentage was lower at 25% for herds operated for 5 years or less (Table 2). Insect control measures have been adopted by 34% of farmers. Among those who did not implement these measures, 40% experienced an LSD outbreak, while only 28% of farmers who employed such control measures encountered outbreaks (Table 2). Risk factors associated with LSD outbreaks.
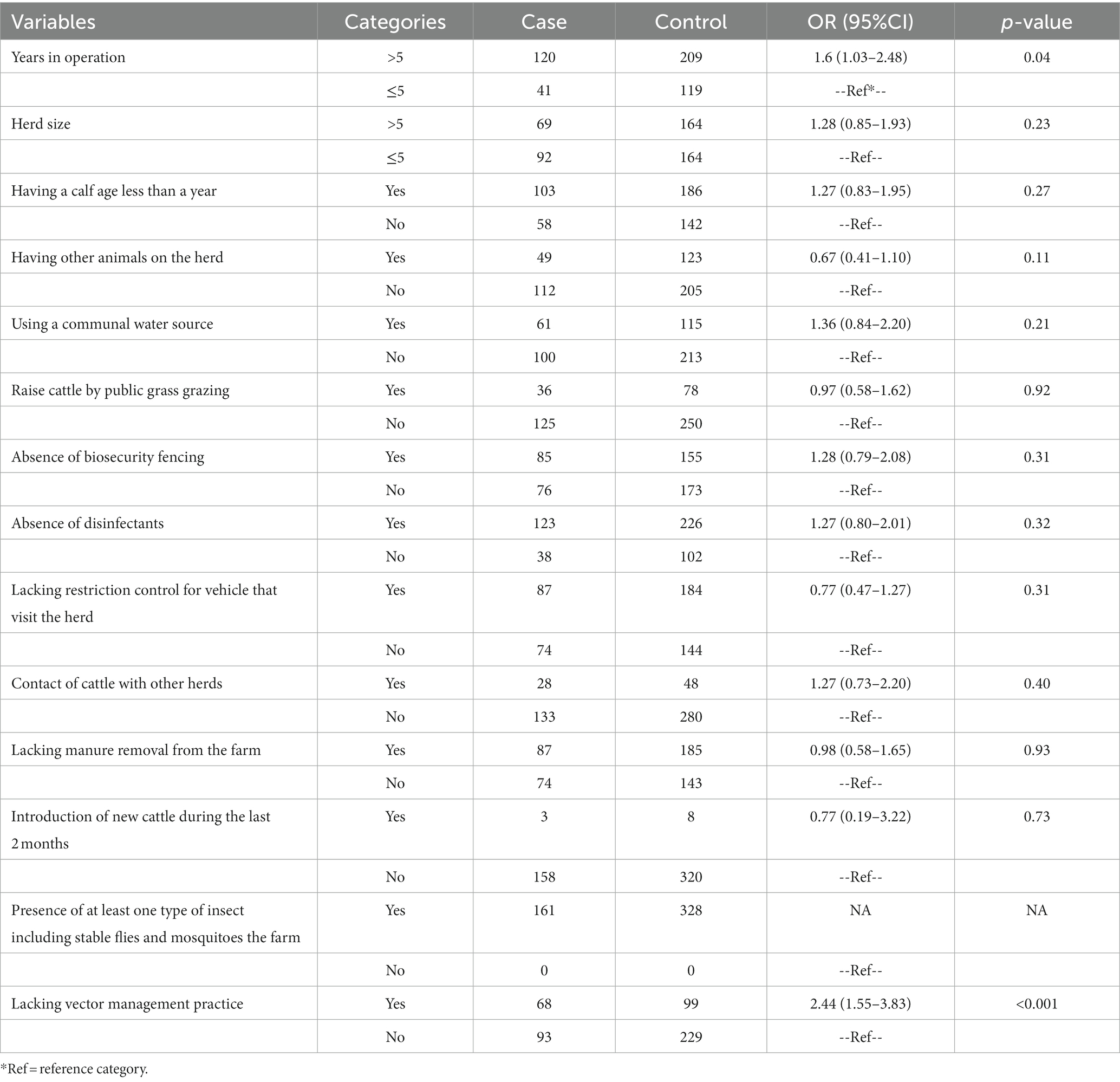
Table 2. Summary of associated risk factors related to lumpy skin disease in cattle of herd level based on univariable logistic regression analysis in 3 provinces (n = 489).
3.2 Risk factors
The risk factors for LSD outbreaks identified in this investigation, as determined by univariable logistic regression, are presented in Table 2. The analysis revealed that the number of years in operation and the absence of vector management on the herd were associated with the LSD outbreak status.
In the final multivariable mixed effect logistic regression model (Table 3), results showed that cattle herds operating for more than five years had 1.62 times greater odds of experiencing an LSD outbreak (OR = 1.62; 95%CI = 1.04–2.53) than those operating for fewer years. Furthermore, herds that did not implement insect vector control measures had 2.05 times greater odds of being affected by LSDV (OR = 2.05; 95%CI = 1.29–3.25) compared to those implementing these control measures.
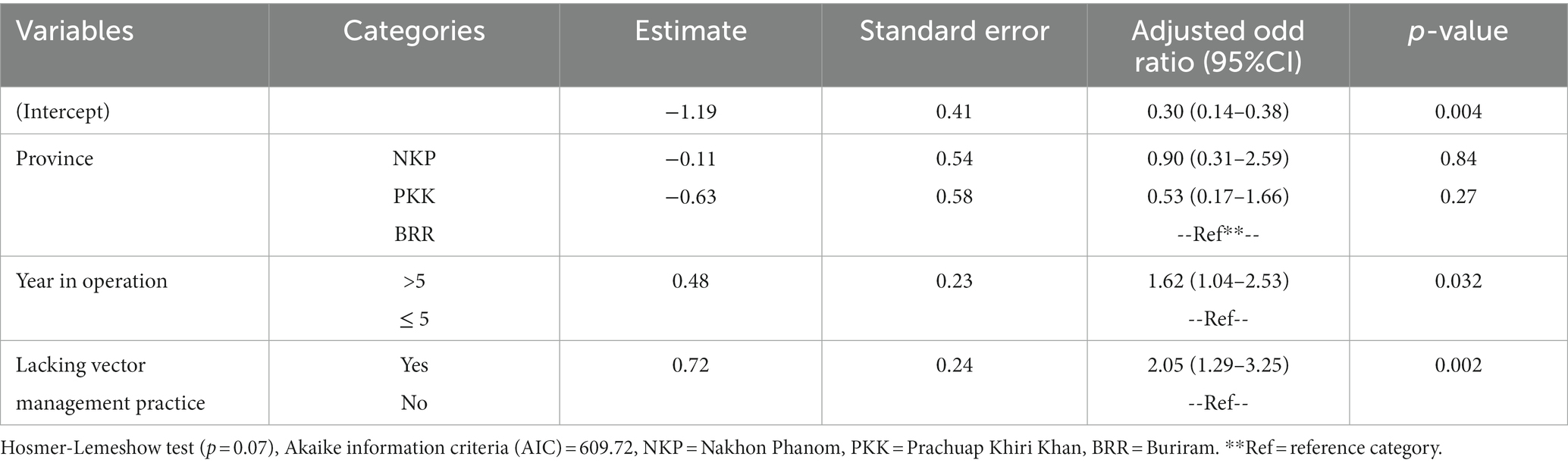
Table 3. Risk factors from the final multivariable logistic regression model* for the lumpy skin disease outbreak in cattle herds at the herd level.
During the model selection step, no significant interaction term was identified in the final model. Furthermore, there was no evidence of multicollinearity among the variables included in the final model, as all variables included in the final model had VIF values of less than 1.04. The ICC from the final model was equal to 0.09, indicating that the effects of the variation observed within the district were smaller compared to the variation between the different districts.
Results related to the residual diagnostics for the final mixed effect model, including QQ plot residuals and a plot between residuals and predicted values, are displayed in the Supplementary Figure S1. The results demonstrate a lack of violations in the model assumptions.
4 Discussion
This study aimed to identify the risk factors associated with LSD outbreaks in naïve beef cattle herds located in the upper northeast, northeast, and central regions of Thailand. This research is an integral component of a national project that seeks to comprehend the epidemiology of LSDV, which has caused a significant outbreak in the country. The findings from this study hold the potential to contribute valuable insights to the national strategy for disease prevention and control.
Blood-sucking insects play a significant role in the mechanical transmission of LSDV (34–36). Various bloodsucking arthropods, such as mosquitoes (Aedes aegypti), stable flies (Stomoxys calcitran), horn flies (Haematobia irritans), house flies (Musca domestica), and hard ticks (Dermacentor marginatus, Hyalomma asiaticum, Rhipicephalus appendiculatus, Rhipicephalus decoloratus, and Amblyomma hebraeum), have been previously identified as potential transmitters of LSDV (37–39). Additionally, recent studies have confirmed that LSDV can be transmitted by insect vectors from animals infected with LSDV to animals that are susceptible to the disease (34, 40, 41). Based on mixed effect logistic regression analysis, this study determined that lack of vector control on the herds was identified as a significant risk factor for LSD outbreaks. In other words, herds of farmers who did not apply insect vectors control practices had 2.05 times greater odds for LSD outbreak than herds of farmers who did apply such practices. This finding provides support for the results from previous investigation conducted in other areas in Thailand (9), which reported that naïve cattle herd affected by LSD were primarily characterized by suboptimal insect control measures. Furthermore, all cattle herds in the present study were found to harbor insects that could potentially act as vectors for LSD. Thus, with inefficient insect vector control, it was revealed that the transmission of LSD in the naïve herds in this study is likely due to insect vectors. This speculation is supported by previous spatial epidemiological studies conducted in Thailand reporting that insect vectors play a crucial role in LSD outbreaks in cattle farming areas where herds are closely situated or in regions with a high concentration of cattle herds (9, 42, 43). In addition to the findings of the current study, a study conducted in Thailand, employing transmission kernel analysis, similarly affirms that herd-to-herd transmission in LSD outbreak areas occurs within short distances, with the estimated range falling between 0.2 and 0.8 kilometers (44). This discovery emphasizes the pivotal role that insects may play as significant vectors in the transmission among cattle herds. Furthermore, aligning with the outcomes of our study, the absence of insect vector control measures on farms emerges as a notable risk factor for LSD outbreaks in Indonesia. This investigation demonstrates that farms without insect vector control measures had 8.6 times (OR = 8.6) greater odds for experiencing an LSD outbreak compared to those implementing such measures (45). The impact of insect vectors on LSD transmission has been also observed in different settings. For example, in Sub-Saharan Africa, LSD outbreaks are typically observed following the rainy season when insect populations increase (46). A study conducted in Israel also demonstrated a correlation between the relative abundance of insect vectors in December and April and LSD outbreaks (47). Similarly, in various regions of Nepal, LSD outbreaks were reported during the rainy season (June to August), indicating a link to the increased population of arthropods in the area (48). Furthermore, in terms of implications, eliminating insects on a large scale is deemed impossible due to the common abundance of insect vectors in cattle farming areas throughout the year in Thailand (9). We recommend concentrating on measures to manage and mitigate the role of disease-transmitting vectors. This includes controlling breeding sites for insects, such as standing water and cattle manure. Additionally, the application of insecticides for vector control may be considered, but caution is advised, taking into account potential impacts on human health and the environment. The present study also showed that herds operating for more than five years had higher odds of experiencing LSD outbreaks compared to herds operating for less than five years. However, it is challenging to explain this finding. Although we examined the association between the total years of operation and other variables such as insect control and farm biosecurity, none of the pairs demonstrated a significant association. We hypothesize that farmers who possess over five years of experience may exhibit different farming practices in comparison to other groups of farmers. For example, individuals may exhibit a decreased propensity to obtain news or updates through online channels, which serve as a primary means of disseminating information regarding the LSD outbreaks in Thailand (12). To address this knowledge gap, a follow up study should be conducted to investigate this factor. Additionally, further investigation is necessary to investigate other risk factors that were not considered in this study.
Purchasing and selling animals during LSD outbreaks are determined as important risk factors of LSD outbreaks according to the study in Kazakhstan (21) and Indonesia (45). These factors were not identified as risk factors in this study. Strict animal movement restriction to mitigate LSD spread in Thailand was implemented during the course of this research. Only 2% of cattle herds included in this study have a history of animal movement limiting the evaluation of its impact to the occurrence of LSD. Another risk factor linked to the incidence of LSD was the size of the herd. Larger herds were found to have a higher risk of LSD infection, which can be attributed to factors such as stressful conditions, increased likelihood of exposure to the LSD virus, and greater possibilities for disease transmission (49). However, in this study, herd size was found to be less significant, mainly because most herds were small, typically consisting of around 5 cattle each, as they were predominantly owned by small-scale farmers.
Based on the findings of this study, it is recommended to implement insect control measures in LSD outbreak areas where no LSD vaccine is available, particularly for naïve herds. For herds that have been vaccinated against LSD, the use of insecticides can be an additional option, taking into account factors such as the abundance of insect vectors, the effectiveness of insecticide application, and economic considerations (7). It’s also important to point out that the source of the LSD outbreaks in the study areas was not determined. While the results suggest no correlation between LSD outbreaks and animal movements such as buying animals from other herds, it is crucial to remember that a small number of herds included in the study did have a history of animal movement. Thus, the sample size might not be large enough to fully examine the impact of this variable. In the study areas, we hypothesize that the origin of LSD outbreaks could be due to unauthorized movement of LSDV-infected cattle into the affected regions. Alternatively, the insects carrying the LSDV might have been introduced to the study areas either by flying or being transported by vehicles from other outbreak areas. Once an outbreak occurred, the spread of LSDV was likely aided by the high abundance of insect vectors in the outbreak regions, as suggested by previous studies (9, 12).
This study is subject to certain limitations. As it relied on a questionnaire survey, there is a possibility of recall bias and information bias, which are inherent to this type of study design. Furthermore, the presence of similar management factors in both outbreak and non-outbreak herds, as these practices were implemented in both types of herds, poses challenges in conducting statistical comparisons. Moreover, it should be noted that the diagnosis of LSD is primarily based on clinical signs, and as a result, subclinical cases may be included in the control group. However, given that most herds are naïve, cattle affected with LSDV would likely exhibit clinical signs of the disease (9). Therefore, the occurrence of subclinical cases in the control herd is less likely, but it should still be acknowledged as a limitation. Furthermore, it is important to note that the study was only conducted in a naïve herd. Therefore, interpretations of the results should take this condition into consideration.
Despite certain limitations, this study represents the first investigation of potential risk factors for LSD outbreaks in Thailand. The research was conducted across multiple sites throughout the country, providing a more comprehensive understanding compared to a study limited to a single area. Also, the sample size falls within the range of previously reported studies, being larger than some conducted to determine risk factors for LSD (16, 19, 20, 45, 50). Additionally, the statistical models employed in this study accounted for the hierarchical effects of herds nested within each site or district.
5 Conclusion
This study investigated the risk factors associated with LSD outbreaks in beef cattle herds in Thailand. The results revealed that herds operating for more than five years had a higher likelihood of experiencing LSD outbreaks. Additionally, herds without effective vector management practices were found to be at a greater risk of LSD outbreaks. These findings highlight the importance of implementing insect-vector control measures in LSD-risk areas, especially for herds that have not been vaccinated against LSD. This study is a significant contribution to the understanding of LSD outbreaks in Thailand. It was conducted across multiple sites. The findings can serve as guidance for managing LSD in naïve cattle herds in various settings.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the data used in this study is derived from lumpy skin disease outbreak investigations carried out by the Department of Livestock Development (DLD), Thailand, and therefore, it’s not publicly accessible. Requests to access these datasets should be directed to Department of Livestock Development (DLD), Thailand, email: ZGxkLmluZm9AYWMudGg=.
Ethics statement
This study was granted ethical approval by the Walailak University Ethics Committee (WUEC-23-085-01) and was conducted in compliance with applicable guidelines and regulations. The questionnaire survey was conducted by livestock and/or veterinary authorities from the Department of Livestock Development, Ministry of Agriculture and Cooperatives. Informed consent was obtained from all subjects involved in the study.
Author contributions
OA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. WW: Investigation, Validation, Writing – review & editing. BP: Investigation, Validation, Writing – review & editing. SJ: Investigation, Validation, Writing – review & editing. MS: Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. SP: Conceptualization, Data curation, Supervision, Writing – review & editing. TP: Conceptualization, Supervision, Writing – review & editing. TD: Conceptualization, Writing – review & editing. CS: Formal analysis, Validation, Visualization, Writing – review & editing. RS: Data curation, Validation, Visualization, Writing – review & editing. CJ: Data curation, Validation, Visualization, Writing – review & editing. VP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project received support from the Food and Agriculture Organization of the United Nations (FAO) and the Regional Field Epidemiology Training Program for Veterinarians (R-FETPV). This work was also funded by Chiang Mai University (grant: R66IN00356). The article processing charge was supported by the National Research Council of Thailand and Chiang Mai University (grant: FF66/021 and R000029530). The funders had no role in the study design, data analysis, decision to publish, or manuscript preparation.
Acknowledgments
The authors extend their gratitude to the farmers who took part in this study and the local livestock or veterinary authorities from the Department of Livestock Development who conducted the questionnaire survey.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1338713/full#supplementary-material
References
1. Tuppurainen, ES , and Oura, CA . Review: lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transbound Emerg Dis. (2012) 59:40–8. doi: 10.1111/j.1865-1682.2011.01242.x
2. Tuppurainen, ES , Venter, EH , and Coetzer, JA . The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort J Vet Res. (2005) 72:153–64. doi: 10.4102/ojvr.v72i2.213
4. Badhy, SC , Chowdhury, MGA , Settypalli, TBK , Cattoli, G , Lamien, CE , Fakir, MAU, et al. Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains. BMC Vet Res. (2021) 17:61. doi: 10.1186/s12917-021-02751-x
5. Kayesh, MEH , Hussan, MT , Hashem, MA , Eliyas, M , and Anower, AM . Lumpy skin disease virus infection: an emerging threat to cattle health in Bangladesh. Hosts Viruses. (2020) 7:97. doi: 10.17582/journal.hv/2020/7.4.97.108
6. Bowden, TR , Babiuk, SL , Parkyn, GR , Copps, JS , and Boyle, DB . Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology. (2008) 371:380–93. doi: 10.1016/j.virol.2007.10.002
7. Roche, X , Rozstalnyy, A , Tagopacheco, D , Kamata, A , Claudia, P , Beltran-Alcrudo, D, et al. Introduction and spread of lumpy skin disease in south, east and Southeast Asia: qualitative risk assessment and management. Rome: FAO (2020).
8. Acharya, KP , and Subedi, D . First outbreak of lumpy skin disease in Nepal. Transbound Emerg Dis. (2020) 67:2280–1. doi: 10.1111/tbed.13815
9. Arjkumpa, O , Suwannaboon, M , Boonrod, M , Punyawan, I , Liangchaisiri, S , Laobannue, P, et al. The first lumpy skin disease outbreak in Thailand (2021): epidemiological features and spatio-temporal analysis. Front Vet Sci. (2022) 8:799065. doi: 10.3389/fvets.2021.799065
10. Lu, G , Xie, J , Luo, J , Shao, R , Jia, K , and Li, S . Lumpy skin disease outbreaks in China, since 3 august 2019. Transbound Emerg Dis. (2021) 68:216–9. doi: 10.1111/tbed.13898
11. Saltykov, YV , Kolosova, AA , and Feodorova, VA . Update of lumpy skin disease: emergence in Asian part of Eurasia. Acta Vet Brno. (2022) 72:287–99. doi: 10.2478/acve-2022-0023
12. Suwankitwat, N , Songkasupa, T , Boonpornprasert, P , Sripipattanakul, P , Theerawatanasirikul, S , Deemagarn, T, et al. Rapid spread and genetic characterisation of a recently emerged recombinant lumpy skin disease virus in Thailand. Vet Sci. (2022) 9:542. doi: 10.3390/vetsci9100542
13. Haider, A , Farhan, A , Nawaz, A , Ali, A , Abbas, Z , and Mehmood, A . The financial toll of lumpy skin disease in Pakistan, and whether or not vaccination is worth it for preventing future outbreaks. Ann PIMS. (2023) 19:187–93. doi: 10.48036/apims.v19i2.788
14. Punyapornwithaya, V , Arjkumpa, O , Buamithup, N , Kuatako, N , Klaharn, K , Sansamur, C, et al. Forecasting of daily new lumpy skin disease cases in Thailand at different stages of the epidemic using fuzzy logic time series, NNAR, and ARIMA methods. Prev Vet Med. (2023) 217:105964. doi: 10.1016/j.prevetmed.2023.105964
15. Arjkumpa, O , Suwannaboon, M , Boonrawd, M , Punyawan, I , Laobannu, P , Yantaphan, S, et al. First emergence of lumpy skin disease in cattle in Thailand, 2021. Transbound Emerg Dis. (2021) 68:3002–4. doi: 10.1111/tbed.14246
16. Gari, G , Waret-Szkuta, A , Grosbois, V , Jacquiet, P , and Roger, F . Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiol Infect. (2010) 138:1657–66. doi: 10.1017/s0950268810000506
17. Molla, W , Frankena, K , Gari, G , Kidane, M , Shegu, D , and de Jong, MC . Seroprevalence and risk factors of lumpy skin disease in Ethiopia. Prev Vet Med. (2018) 160:99–104. doi: 10.1016/j.prevetmed.2018.09.029
18. Sprygin, A , Pestova, Y , Wallace, DB , Tuppurainen, E , and Kononov, AV . Transmission of lumpy skin disease virus: a short review. Virus Res. (2019) 269:197637. doi: 10.1016/j.virusres.2019.05.015
19. Hailu, B , Tolosa, T , Gari, G , Teklue, T , and Beyene, B . Estimated prevalence and risk factors associated with clinical lumpy skin disease in North-Eastern Ethiopia. Prev Vet Med. (2014) 115:64–8. doi: 10.1016/j.prevetmed.2014.03.013
20. Kiplagat, SK , Kitala, PM , Onono, JO , Beard, PM , and Lyons, NA . Risk factors for outbreaks of lumpy skin disease and the economic impact in cattle farms of Nakuru county. Kenya Front Vet Sci. (2020) 7:259. doi: 10.3389/fvets.2020.00259
21. Issimov, A , Kushaliyev, K , Abekeshev, N , Molla, W , Rametov, N , Bayantassova, S, et al. Risk factors associated with lumpy skin disease in cattle in West Kazakhstan. Prev Vet Med. (2022) 207:105660. doi: 10.1016/j.prevetmed.2022.105660
22. Uddin, MA , Islam, MA , Rahman, A , Rahman, MM , Khasruzzaman, AKM , Ward, MP, et al. Epidemiological investigation of lumpy skin disease outbreaks in Bangladeshi cattle during 2019-2020. Transbound Emerg Dis. (2022) 69:3397–404. doi: 10.1111/tbed.14696
23. Ochwo, S , Vander Waal, K , Munsey, A , Nkamwesiga, J , Ndekezi, C , Auma, E, et al. Seroprevalence and risk factors for lumpy skin disease virus seropositivity in cattle in Uganda. BMC Vet Res. (2019) 15:236–9. doi: 10.1186/s12917-019-1983-9
24. Islam, MN , Khan, MK , Khan, MFR , Kostoulas, P , Rahman, AA , and Alam, MM . Risk factors and true prevalence of bovine tuberculosis in Bangladesh. PLoS One. (2021) 16:e0247838. doi: 10.1371/journal.pone.0247838
25. Dohoo, I , Martin, W , and Stryhn, H . Mixed models for discrete data In: S Margaret McPike editor. Veterinary epidemiologic research. Charlottetown, P.E.I.: University of Prince Edward Island (2003). 500–3.
26. Noman, Z , Anika, T , Haque, Z , Rahman, A , Ward, M , and Martínez-López, B . Risk factors for rabid animal bites: a study in domestic ruminants in Mymensingh district, Bangladesh. Epidemiol Infect. (2021) 149:e76. doi: 10.1017/S095026882100056X
27. Colella, V , Wongnak, P , Tsai, YL , Nguyen, VL , Tan, DY , Tong, KBY, et al. Human social conditions predict the risk of exposure to zoonotic parasites in companion animals in east and Southeast Asia. Commun Med. (2022) 2:144. doi: 10.1038/s43856-022-00210-8
28. Kobayashi, S , Tsutsui, T , Yamamoto, T , Hayama, Y , Kameyama, K-i , Konishi, M, et al. Risk factors associated with within-herd transmission of bovine leukemia virus on dairy farms in Japan. BMC Vet Res. (2010) 6:1–6. doi: 10.1186/1746-6148-6-1
29. Graham, D , Clegg, T , Thulke, H-H , O’sullivan, P , McGrath, G , and More, S . Quantifying the risk of spread of bovine viral diarrhoea virus (BVDV) between contiguous herds in Ireland. Prev Vet Med. (2016) 126:30–8. doi: 10.1016/j.prevetmed.2016.01.017
30. Singhla, T , Boonyayatra, S , Punyapornwithaya, V , Vander Waal, KL , Alvarez, J , Sreevatsan, S, et al. Factors affecting herd status for bovine tuberculosis in dairy cattle in northern Thailand. Vet Med Int. (2017) 2017:2964389. doi: 10.1155/2017/2964389
31. Manlove, K , Branan, M , Baker, K , Bradway, D , Cassirer, EF , Marshall, KL, et al. Risk factors and productivity losses associated with Mycoplasma ovipneumoniae infection in United States domestic sheep operations. Prev Vet Med. (2019) 168:30–8. doi: 10.1016/j.prevetmed.2019.04.006
32. Akinwande, MO , Dikko, HG , and Samson, A . Variance inflation factor: as a condition for the inclusion of suppressor variable (s) in regression analysis. Open J Stat. (2015) 5:754–67. doi: 10.4236/ojs.2015.57075
33. Dohoo, I , Martin, W , and Stryhn, H . Mixed models for continuous data In: S Margaret McPike editor. Veterinary epidemiologic research. Charlottetown, P.E.I: University of Prince Edward Island (2003). 478.
34. Sohier, C , Haegeman, A , Mostin, L , De Leeuw, I , Campe, WV , De Vleeschauwer, A, et al. Experimental evidence of mechanical lumpy skin disease virus transmission by Stomoxys calcitrans biting flies and Haematopota spp. horseflies. Sci Rep. (2019) 9:20076. doi: 10.1038/s41598-019-56605-6
35. Akther, M , Akter, SH , Sarker, S , Aleri, JW , Annandale, H , Abraham, S, et al. Global burden of lumpy skin disease, outbreaks, and future challenges. Viruses. (2023) 15:1861. doi: 10.3390/v15091861
36. Bianchini, J , Simons, X , Humblet, M-F , and Saegerman, C . Lumpy skin disease: a systematic review of mode of transmission, risk of emergence and risk entry pathway. Viruses. (2023) 15:1622. doi: 10.3390/v15081622
37. Annandale, CH , Holm, DE , Ebersohn, K , and Venter, EH . Seminal transmission of lumpy skin disease virus in heifers. Transbound Emerg Dis. (2014) 61:443–8. doi: 10.1111/tbed.12045
38. Gubbins, S . Using the basic reproduction number to assess the risk of transmission of lumpy skin disease virus by biting insects. Transbound Emerg Dis. (2019) 66:1873–83. doi: 10.1111/tbed.13216
39. Sultankulova, KT , Shynybekova, GO , Issabek, AU , Mukhami, NN , Melisbek, AM , Chervyakova, OV, et al. The prevalence of pathogens among ticks collected from livestock in Kazakhstan. Pathogens. (2022) 11:1206. doi: 10.3390/pathogens11101206
40. Sanz-Bernardo, B , Haga, IR , Wijesiriwardana, N , Basu, S , Larner, W , Diaz, AV, et al. Quantifying and modeling the acquisition and retention of lumpy skin disease virus by hematophagus insects reveals clinically but not subclinically affected cattle are promoters of viral transmission and key targets for control of disease outbreaks. J Virol. (2021) 95:e02239–20. doi: 10.1128/jvi.02239-20
41. Haegeman, A , Sohier, C , Mostin, L , De Leeuw, I , Van Campe, W , Philips, W, et al. Evidence of lumpy skin disease virus transmission from subclinically infected cattle by Stomoxys calcitrans. Viruses. (2023) 15:1285. doi: 10.3390/v15061285
42. Punyapornwithaya, V , Seesupa, S , Phuykhamsingha, S , Arjkumpa, O , Sansamur, C , and Jarassaeng, C . Spatio-temporal patterns of lumpy skin disease outbreaks in dairy farms in northeastern Thailand. Front Vet Sci. (2022) 9:957306. doi: 10.3389/fvets.2022.957306
43. Modethed, W , Singhla, T , Boonsri, K , Pringproa, K , Sthitmatee, N , Vinitchaikul, P, et al. Identifying the patterns and sizes of the first lumpy skin disease outbreak clusters in northern Thailand with a high degree of dairy farm aggregation using spatio-temporal models. PLoS One. (2023) 18:e0291692. doi: 10.1371/journal.pone.0291692
44. Punyapornwithaya, V , Salvador, R , Modethed, W , Arjkumpa, O , Jarassaeng, C , Limon, G, et al. Estimating the transmission kernel for lumpy skin disease virus from data on outbreaks in Thailand in 2021. Viruses. (2023) 15:2196. doi: 10.3390/v15112196
45. Susanti, T , Susetya, H , Widayani, P , Fitria, Y , and Pambudi, GT . Risk factors, logistic model, and vulnerability mapping of lumpy skin disease in livestock at the farm level in Indragiri Hulu District, Riau Province, Indonesia, in 2022. Vet World. (2023) 16:2071–9. doi: 10.14202/vetworld.2023.2071-2079
46. Mulatu, E , and Feyisa, A . Review: lumpy skin disease. J Vet Sci Technol. (2018) 9:1–8. doi: 10.4172/2157-7579.1000535
47. Kahana-Sutin, E , Klement, E , Lensky, I , and Gottlieb, Y . High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Med Vet Entomol. (2017) 31:150–60. doi: 10.1111/mve.12217
48. Gautam, M , Kattel, P , and Kaphle, K . Review on lumpy skin disease and its emerging threat to livestock in Nepal. Vet Sci Res Rev. (2022) 8:43–51. doi: 10.17582/journal.vsrr/2022.8.1.43.51
49. Macpherson, CNL . The effect of transhumance on the epidemiology of animal diseases. Prev Vet Med. (1995) 25:213–24. doi: 10.1016/0167-5877(95)00539-0
Keywords: lumpy skin disease, risk factors, cattle herds, control measures, Thailand
Citation: Arjkumpa O, Wachoom W, Puyati B, Jindajang S, Suwannaboon M, Premashthira S, Prarakamawongsa T, Dejyong T, Sansamur C, Salvador R, Jainonthee C and Punyapornwithaya V (2024) Analysis of factors associated with the first lumpy skin disease outbreaks in naïve cattle herds in different regions of Thailand. Front. Vet. Sci. 11:1338713. doi: 10.3389/fvets.2024.1338713
Edited by:
Beatriz Martínez-López, University of California, Davis, United StatesReviewed by:
Marta Martinez Aviles, Instituto Nacional de Investigación y Tecnología Agroalimentaria (INIA), SpainAbdul Rehman, University of California, Davis, United States
Copyright © 2024 Arjkumpa, Wachoom, Puyati, Jindajang, Suwannaboon, Premashthira, Prarakamawongsa, Dejyong, Sansamur, Salvador, Jainonthee and Punyapornwithaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Veerasak Punyapornwithaya, dmVlcmFzYWsucEBjbXUuYWMudGg=
 Orapun Arjkumpa
Orapun Arjkumpa Wanwisa Wachoom2
Wanwisa Wachoom2 Sirima Jindajang
Sirima Jindajang Sith Premashthira
Sith Premashthira Tippawon Prarakamawongsa
Tippawon Prarakamawongsa Tosapol Dejyong
Tosapol Dejyong Roderick Salvador
Roderick Salvador Chalita Jainonthee
Chalita Jainonthee Veerasak Punyapornwithaya
Veerasak Punyapornwithaya