- 1Avon Ridge Equine Veterinary Services, Brigadoon, WA, Australia
- 2School of Veterinary Medicine, Murdoch University, Murdoch, WA, Australia
- 3School of Population and Global Health, University of Western Australia, Crawley, WA, Australia
- 4EMT Consulting, Tiverton, United Kingdom
Introduction: Dopaminergic agonists are accepted as the most effective treatment for pituitary pars intermedia dysfunction. However, some horses are refractory to daily oral pergolide, the recommended registered treatment. Extended-release cabergoline (ERC) injection may offer an alternative. The objective of this retrospective case series was to describe clinical and endocrinological responses to ERC.
Methods: Medical records of horses treated with weekly intramuscular injections of ERC (5 mg/mL, BOVA Aus) at either 0.01 mg/kg (high dose, HD) (n = 10) or 0.005 mg/kg (low dose, LD) (n = 30) were reviewed. Short-term ACTH responses were assessed at 5–8 days using a Wilcoxon signed ranked test. Longer-term ACTH responses (30 to 365 days) were assessed using generalised estimating equations.
Results: Five to eight days after the first dose of LDERC, median adrenocorticotropic hormone (ACTH) concentration was lower (p = 0.001), changing from 153 pg/mL (IQR: 78, 331) to 57 pg/mL (IQR: 30, 102). With HDERC, median ACTH concentration was also 153 pg/mL (IQR: 96, 185) before and then 56 pg/mL (IQR: 29, 86) after 5–8 days of treatment (p = 0.047). Over 12 months of treatment, ACTH concentration ranged from 14 to >1,250 pg/mL (median: 51 pg/mL) in horses treated with LDERC and 20 to 472 pg/mL (median: 50 pg/mL) in horses treated with HDERC. Measurements remained above the seasonal reference range in 39.3 and 52.3% of horses treated with LDERC and HDERC, respectively. Clinical improvement was reported by owners in 78.3 and 100% of horses treated with LDERC and HDERC, respectively. Partial, self-limiting inappetence was reported in 30.0% of LDERC and 60% HDERC cases. Seven horses exhibited lethargy (5 LDERC, 2 HDERC). Insulin concentrations measured 30 days post-ERC treatment were no different from baseline.
Discussion: Clinical and endocrinological responses were consistent with results of previous reports of oral pergolide treatment. Weekly injection of ERC may be an effective alternative to pergolide; the 0.005 mg/kg dose appeared to be as effective, with less risk of inappetence, than the 0.01 mg/kg dose that has been reported previously.
Introduction
Pituitary pars intermedia dysfunction (PPID) is a neurodegenerative condition that results in loss of dopaminergic inhibition of the pars intermedia and leads to an overproduction of peptides, including adrenocorticotrophic hormone (ACTH) (1). PPID is the most common endocrine condition in geriatric horses and is encountered frequently in clinical practice (2). Epidemiological studies have demonstrated a disease prevalence of approximately 15–30% in horses over the age of 15 yrs (3). Clinical signs associated with PPID include hypertrichosis, laminitis, polyuria, polydipsia, lethargy, muscle wastage, and delayed wound healing (1, 4). In addition to good husbandry, the treatment of PPID involves the use of dopaminergic agonists, which reduce peptide secretion from the pars intermedia (1, 2, 5).
Pergolide, a dopaminergic agonist, has been used widely for the treatment of PPID for over two decades (5, 6). Pergolide is currently registered as tablet and (in some countries) liquid preparation; however, some horses are refractory to daily oral administration, and previous studies have reported that owners might be less committed to lifelong therapy in older animals (7–9). A recent study demonstrated poor compliance with the administration of pergolide tablets, and this may have implications for the control of clinical signs even though it did not appear to affect ACTH responses (9).
Cabergoline is a dopaminergic agonist that has the same mechanism of action as pergolide but is available as an extended-release intramuscular injection, alleviating the need for daily oral administration of medication (10, 11). For several decades, cabergoline was used to treat functional pituitary adenomas in humans, including cases refractory to pergolide therapy, as it has a high affinity for dopaminergic receptors in the hypothalamus and pituitary gland (12–16). Previous studies in horses have investigated the effects of cabergoline on prolactin, MSH and insulin concentrations (10, 11, 17) and shown that these hormones have reduced consistent with the drug having an inhibitory effect on both melanotrophs and lactotrophs in the equine pituitary gland.
Anecdotally, extended-release cabergoline (ERC) administered as a low-volume intramuscular injection is an effective alternative to pergolide. The objectives of the current study were to: (1) review and describe the initial (5–8 days) and longer-term (12 months) clinical and endocrinological responses to two dose rates of an intramuscular extended release cabergoline (ERC) injection that is being used widely in clinical practice for the treatment of PPID.
Materials and methods
Horses
Clinical records of privately-owned horses treated by Avon Ridge Equine Clinic (anonymised for peer review) between June 2021 and October 2022 were reviewed to identify horses that had been treated with ERC for the management of PPID. Horse weight was estimated using a validated weigh tape.1
Treatment
In accordance with previous reports (10, 11, 17), horses initially received treatment with ERC as an intramuscular injection (5 mg/mL)2 at a “high” dose of 0.01 mg/kg (HDERC). Some horses were reported by owners to experience in appetence at this higher dose, and prompting a change in the practice protocol to use a “low” dose of 0.005 mg/kg (LDERC) for subsequent cases in an attempt to mitigate issues with appetite reduction.
Site selection and correct intramuscular injection technique were demonstrated by the treating veterinarian when administering the first dose. The injection site was cleaned with isopropyl alcohol3 prior to injection, and the total dose of cabergoline (maximum 1 mL) was administered at a single injection site in either the neck or gluteal musculature. Owners administered subsequent doses and were advised to record clinical changes and to contact their veterinarian immediately if any adverse effects were observed. Cabergoline is not currently registered for use in horses; thus, informed client consent for the use of an unregistered medicine was obtained prior to commencing treatment. Clinical signs were assessed and recorded at repeat veterinary examinations in consultation with the owners responsible for day-to-day care of the horses.
Laboratory investigations
ACTH data was collated and grouped relative to cabergoline administration. For all cases ACTH results were available from up to 3 days prior to treatment, 5 to 8 days after the first injection of ERC, and thereafter at less consistent but regular intervals as dictated by the clinical progress of each case. Post-prandial insulin concentrations were also available from up to 3 days prior to, and after 30 (+/−3), days of treatment. Insulin was measured approximately 90 min after the provision of the horse’s normal feed and forage in the morning. Insulin and ACTH concentrations were measured using an Immulite 2000.4
Data analysis
Signalment and clinical data were transposed from case records to Microsoft Excel5 for subsequent analysis. Age, sex, and baseline ACTH were compared between high and low doses of ERC using Wilcoxon sign-rank or Chi-squared tests. Median ACTH concentration and interquartile range (IQR) were calculated for baseline (up to 30 days prior to treatment), 5–8 days, 30–90 days, 91–180 days, and 181–365 days post treatment. Where horses had had ACTH concentration measured more than once during a period, the mean of the results was used. Each ACTH concentration measurement was also classified as being within or above the geographical and seasonal reference range (18) for the month of collection. The percentage of horses with one or more ACTH concentrations above the normal range was reported for each period.
Efficacy of HDERC and LDERC were examined by comparing the ACTH responses at different time points with baseline ACTH concentration. Comparisons between measures taken at baseline and 5–8 days were made using a Wilcoxon signed ranked test, overall and stratified by high and low dose. Comparison between ACTH concentration at each longer-term follow-up period and baseline was performed using generalised estimating equations, using a negative binomial distribution for ACTH values and a binomial distribution for normal/high ACTH values. Analysis was carried out in StataMP version 17.6
Animal ethics approval
An ethics committee was consulted, and it was determined that ethics committee oversight was not required for the retrospective review of clinical data.
Results
Horses
Data from 40 horses were included. Mean (±standard deviation) age was 22.4 (±5.1 years), with 25 geldings (62.5%) and 15 mares (37.5%). The average weight of horses at baseline was 344 ± 146 kg. A variety of breeds were represented, including Welsh and Welsh crosses (n = 18, 45.0%), Thoroughbreds (n = 4, 10.0%), Standardbreds (n = 3, 7.5%), Quarter horses (n = 3, 7.5%), Shetlands (n = 2, 5.0%), Miniatures and Miniature crosses (n = 2, 5.0%), Warmblood and Warmblood crosses (n = 2, 5.0%), and a range of other mixed breeds (n = 6, 15.0%). There were insufficient data to compare groups but there were no obvious differences in breed distributions between the HDERC and LDERC groups. Horses started treatment at all times of year: January 9, February 2, March 0, April 1, May 3, June 0, July 7, August 7, September 1, October 2, November 2, and December 6, with no apparent difference between groups.
Clinical signs of PPID at initial evaluation included hypertrichosis (n = 28, 70.0%), weight loss (n = 10, 25.0%), laminitis (or a history of laminitis) (n = 10, 25.0%), muscle atrophy (n = 9, 22.5%), lethargy (n = 4, 10.0%), recurrent infections (n = 2, 5.3%), polyuria and polydipsia (n = 1, 2.6%) and poor wound healing (n = 2, 5.3%). Fifteen horses had previously received treatment with pergolide with a mean of 15.2 days (range 1–60 days) between receiving their last dose of pergolide and commencing treatment with ERC. Seven had received pergolide within a week of starting pergolide treatment (3 HDERC, 4 LDERC).
Treatment
The most common reason for commencing ERC was actual or anticipated difficulty administering daily oral medication (n = 38, 97.4%). Three horses also displayed signs of partial anorexia whilst on treatment with pergolide at 2 mcg/kg. One horse demonstrated high ACTH concentration, complete anorexia and somnolence when treated with pergolide at 2 mcg/kg. Ten horses (25.0%) were treated with 0.01 mg/kg HDERC, 30 (75.0%) with 0.005 mg/kg LDERC. There was no significant difference between the two groups in terms of age (p = 0.253), sex (p = 0.187), or baseline ACTH (p = 0.179). Owners did not report missing any treatment doses.
At the end of the study period, 25 horses continued on treatment (5 HDERC, 20 LDERC) and seven horses were lost to follow up (3 HDERC, 4 LDERC). Of the horses that discontinued treatment, 2 (LDERC) stopped due to marked anorexia or colic, 2 due to owner finances (LDERC), 2 became refractory to injections (LDERC), 3 were euthanased for unrelated accidents or illness (2 LDERC, 1 HDERC), and 1 was switched to pergolide (HDERC).
Short-term ACTH responses
Measurements of ACTH concentration were available for 32 horses on day 0 and after a single dose of cabergoline at days 5–8. The median ACTH concentration reduced from 153 pg/mL (IQR: 79, 245) to 57 pg/mL (30, 96) (p < 0.001). All horses had an ACTH concentration above the seasonal reference range prior to treatment; 19 horses (59.4%) remained above the seasonal reference after a single dose of cabergoline. Short-term ACTH responses stratified by dose are shown in Table 1; the reduction in ACTH concentration was significant in both groups.
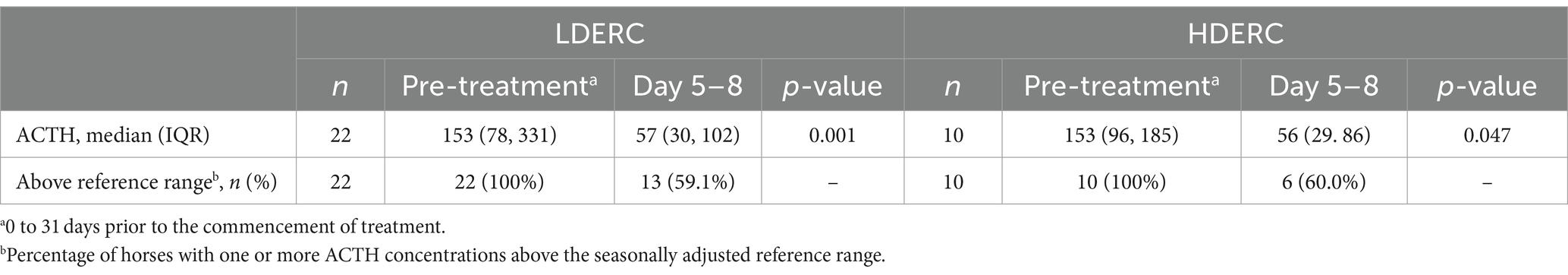
Table 1. Short-term adrenocorticotropic hormone responses following a single dose of extended release cabergoline, stratified by low (LDERC) and high dose (HDERC).
Longer-term ACTH responses
In horses with longer-term follow-up data available, ACTH concentration ranged from 59 to >1,250 pg/mL (median: 162 pg/mL) prior to treatment with all horses having an ACTH concentration above the seasonal reference range (Table 2). In the year following the commencement of treatment, ACTH concentrations ranged from 13 to 1,250 pg/mL (median: 50 pg/mL), with 46.4% of measurements remaining above the seasonal reference range. Longer-term ACTH responses are shown in Table 2 and are stratified by dose in Table 3. Reduction in ACTH concentration was significant with both doses at all time points, with the exception of the day 181–365 HDERC group.
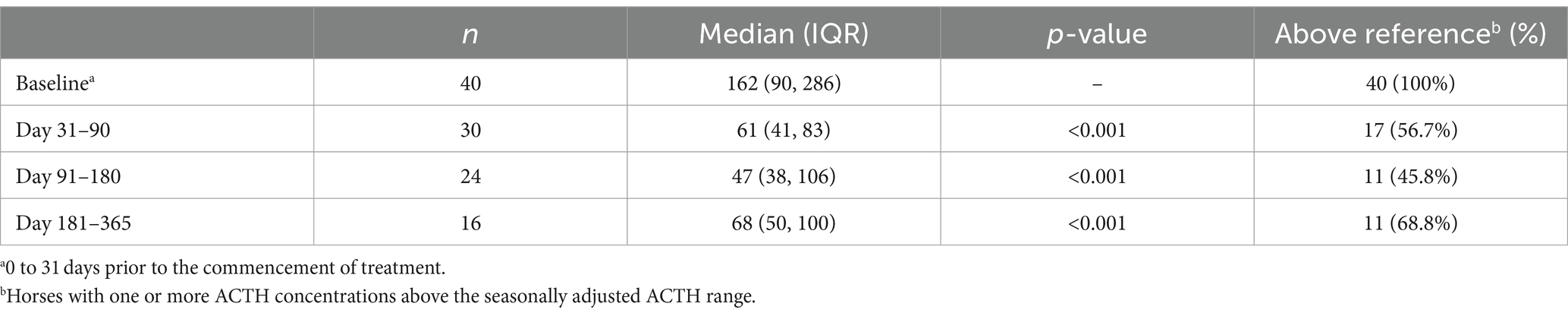
Table 2. Adrenocorticotropic hormone concentration (pg/mL) in 40 horses treated with extended release cabergoline.
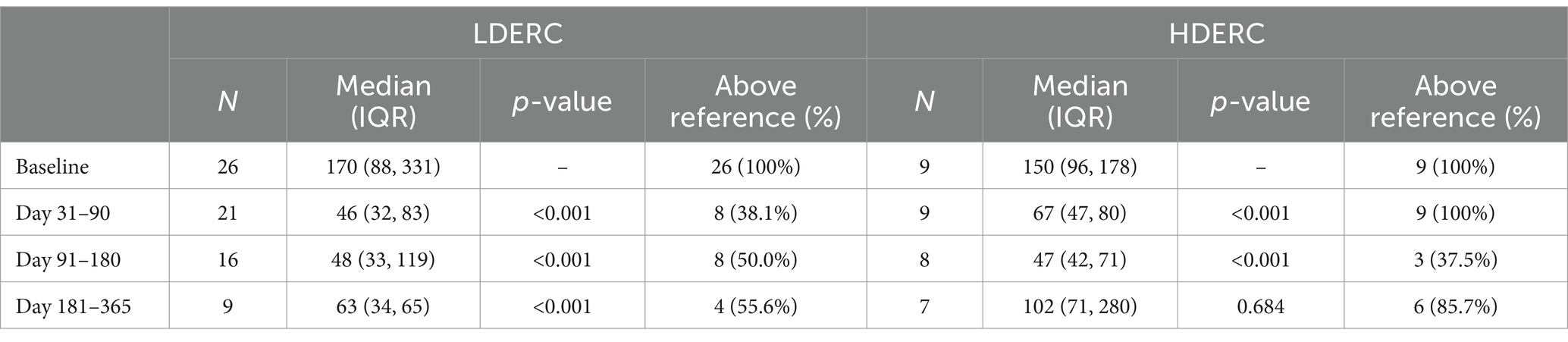
Table 3. Longer-term adrenocorticotropic concentration (pg/mL) following treatment with extended release cabergoline, stratified by low (LDERC) and high dose (HDERC).
Insulin responses
Measurement of insulin concentration was performed at day 0 and day 30 in seven horses in the HDERC group. All horses were already being managed for equine metabolic syndrome with a diet consisting of <10% NSC for at least one week prior to measurement of insulin concentration. The diet of the horses remained consistent at both testing timepoints. Median insulin concentration prior to treatment was 185 μu/mL (IQR: 113, 279) and after treatment was 241 μu/mL (IQR: 113, 284) (p = 0.563).
Longer-term clinical response to treatment
The owners’ perceived response to treatment was recorded in 31 of the 40 horses (23 LDERC and 8 HDERC). In the horses treated with LDERC, 78.3% reported an improvement (n = 17), while no change was reported by 13.0% of owners (n = 3) and 8.7% reported worsening of clinical signs (n = 2). Of the horses which improved, 77.8% demonstrated improved coat shedding (n = 14), 66.7% increased energy levels (n = 12), and 22.2% showed signs of improved body condition (n = 4). All owners reported good compliance with the weekly injection of ERC. Improvement was reported in 100% of the horses treated with HDERC (n = 8). Of these, 87.5% demonstrated improved coat shedding (n = 7), 25% increased energy levels (n = 2), and 25% showed signs of improved body condition (n = 2).
Adverse reactions
Adverse events including self-limiting lethargy (HDERC: n = 2, 20%; LDERC: n = 5, 16.7%), partial anorexia (HDERC: n = 6, 60%; LDERC: n = 9, 30.0%), and mild colic (LDERC: n = 2, 6.7%) occurred within 12–36 h following the injection of ERC. Where partial anorexia was observed, horses displayed a preference for hay and grass over concentrate feed for approximately 12–24 h following injection with ERC. No reactions were reported at the injection sites in any of the horses that were treated.
The owners of one horse receiving HDERC opted to switch to pergolide after two injections, as anorexia and lethargy were observed for 12 h following each dose. The horse displayed no adverse effects when treated with pergolide. One horse receiving LDERC displayed lethargy and inappetence for 72 h. Treatment with LDERC was discontinued after the third dose, and the horse commenced treatment with pergolide at 2 mcg/kg. No adverse events were observed on pergolide. Of the three horses which demonstrated anorexia on pergolide, only one showed signs of partial anorexia (for 12 h) following treatment with LDERC. The horse which demonstrated marked anorexia and became an obtunded following each daily dose of pergolide displayed signs of partial anorexia for 18 h following each weekly injection with LDERC but remained clinically normal between doses after this time. Three horses that displayed partial anorexia on ERC (HDERC: n = 2; LDERC: n = 1) did not previously demonstrate any adverse effects on pergolide. One horse treated with LDERC was partially anorexic and displayed signs of low-grade colic for 6 days following the first dose. No further doses were administered, and no further episodes of colic or anorexia were reported.
Discussion
In the horses studied, once weekly injection of cabergoline was associated with a reduction in ACTH concentration and an improvement in clinical signs of PPID at the previously reported 0.01 mg/kg dose and also at a lower 0.005 mg/kg dose.
Although median ACTH concentration decreased using both doses of ERC, ACTH concentration remained above the seasonal reference range in around half the treated horses. Whilst this appears disappointing, similar responses are identified in response to treatment of PPID with pergolide, with only 30% of horses having seasonally normal ACTH concentrations following treatment with this oral dopaminergic agonist (19, 20). Season has a profound effect on ACTH concentrations (21–23); however, it remains unclear whether the return of ACTH concentrations to within seasonal reference ranges should be an objective of treatment to optimise equine welfare in PPID (24). Inconsistent responses to treatment, as observed among horses treated with ERC in the present study, has also been reported following pergolide treatment (20). Inconsistency of response has been attributed both to inter-horse variability in pharmacokinetics and pharmacodynamics of dopaminergic agonists and to the heterogeneous and progressive nature of PPID (20, 25).
Whilst it can be challenging to assess objectively, clinical response to PPID treatment is ultimately more important than endocrine response (2, 24), and the owners of 83.9% of horses in this study reported improvement in clinical signs. However, owners were not blinded and were therefore subject to bias. Hypertrichosis, weight loss, history of laminitis, and muscle atrophy were the most common presenting signs in this study, consistent with previous reports (24, 26, 27). Decreases in hypertrichosis, laminitis occurrence and the reversal of weight loss and muscle atrophy have been used to assess clinical response to pergolide (24, 26, 27), with time to the improvement of clinical signs ranging from 2 months to 3–4 years (20, 26, 27). Clinical responses observed in this study are therefore consistent with those reported with pergolide. Treatment with ERC was not associated with post-prandial insulin responses in the small subset of horses where insulin measurements were performed. This is consistent with a previous study in horses which demonstrated that cabergoline does not affect the insulin response to a glucose challenge (17), and similar findings have also been reported with pergolide (28).
All but one owner in the current study cited difficulty in administering a daily oral medication as a reason to commence ERC treatment. All owners reported good compliance with ERC treatment. A study in Australia estimated that almost 70% of horses over 15 years of age lived exclusively at pasture, suggesting ageing horses are managed less intensively, which may make it more challenging and less appealing to medicate them daily in feed or by mouth (3). The extended-release nature of cabergoline offers an advantage in cases where daily administration of pergolide presents challenges with practicality and, therefore, compliance (29). Compliance in human and veterinary medicine is an emerging area of research in which equine medicine lags behind, with few studies having been performed. One report found that horse owners were less compliant compared to small animal owners, and veterinarians significantly overestimated client compliance (30). This is further supported by a recent survey from the UK, which compared the amount of pergolide used with the amount that should have been dispensed and showed that compliance was very poor, with only 48% of owners purchasing ≥90% of the amount required to supply the prescribed dose (9). This study also found that age and breed had a significant effect, with compliance being extremely low in owners of Shetland ponies and horses ≥26 yrs old (9). Previous studies have also demonstrated that routine health care was less frequently performed in aged animals (7, 8), suggesting that owners may be less committed to, and compliant with, healthcare recommendations in older horses (9). In addition to reducing the time and effort involved in treating PPID, the ERC injection allows for precise dosing, which may also offer advantages over pergolide tablets in smaller ponies where splitting of tablets may pose challenges for owners and potentially reduce compliance (9).
The HDERC initially used in this study was based on previous studies in horses (10, 11, 17). One report compared the effects of this 0.01 mg/kg dose of cabergoline (a different formulation to the one used in the current study) and pergolide on prolactin concentration and demonstrated that the suppressive effects of cabergoline lasted at least 10 days compared to an intra-muscular injection of pergolide, which only produced 24 h of suppression (11). Following injection every 10 days, cabergoline has also been shown to suppress plasma MSH concentrations (17). The authors’ anecdotal experience of using the ERC preparation used in the current study suggests a rapid onset of action and variable duration of effect, with ACTH concentration dropping within a few days and remaining suppressed for up to 2 weeks. In most horses, ACTH concentration appears to start to increase after 7 to 10 days hence the recommended 7 days treatment interval. Pharmacokinetic and pharmacodynamic studies of ERC in horses are required to determine the optimal dosing regimen. Preliminary observations from this study suggest the LDERC might be more appropriate for further study than the HDERC, as clinical responses were similar and there were less unwanted effects. Adverse events were noted in both groups; however, fewer cases of anorexia were reported in the LDERC compared with HDERC (30.0% vs. 60%), albeit case numbers were small. Anorexia is also reported following pergolide administration (1, 31); however, in this study, some horses demonstrated anorexia following pergolide administration but not ERC and vice versa. It is unclear why this is the case. Despite the variability which exists, ERC may offer an alternative treatment for horses which are unable to tolerate pergolide due to adverse effects.
Previous studies investigating the use of ERC in normal horses have not reported any adverse effects (10, 11, 17). In all cases where partial anorexia was reported in the current study, the owners observed that horses had a preference for long-stem forage (hay or grass) over cereal-based feeds. In humans, the use of dopaminergic agonists has been associated with feelings of nausea (32, 33), which may explain the reduction in feed intake in horses. A significant reduction in feed intake has also been observed in dairy cows following a single injection of ERC (34). The reduction in prolactin concentration that occurs with cabergoline administration (10) may suppress appetite, as prolactin has been shown to stimulate food intake in other species (34, 35). The two horses that suffered from anorexia for longer than 24 h had significant dental disease, both having lost several molars and having had incisors extracted for the treatment of equine odontoclastic tooth resorption and hypercementosis, a common disorder affecting older horses (36, 37). As a result, neither were able to chew long-stem forage. Whilst anorexia from pergolide administration might be resolved by abruptly reducing the dose (1), the long-acting nature of ERC does not allow for such rapid drug withdrawal. Until further investigations are performed, veterinary surgeons should be cognisant of the possibility that ERC may have a more profound effect on feed intake in horses with significant dental pathology.
This study provides preliminary data and is limited by retrospective data collection, lack of an untreated control or placebo group, small sample size, and short follow-up period. However, the results suggest that once weekly injection of extended release cabergoline may be an effective treatment for horses with PPID and provides a basis for designing more robust investigations. A dose of 0.005 mg/kg may be more appropriate for the treatment of PPID than the 0.01 mg/kg dose that has been reported in horses previously. Larger, blinded, randomised clinical trials and studies on the pharmacokinetics and pharmacodynamics of ERC are warranted.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by University of Murdoch Animal Ethics Committee, Perth for the studies involving animals because collation of clinical data was retrospective. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
TS: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. EK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GR: Conceptualization, Formal analysis, Writing – review & editing. DR: Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. BOVA Aus subsidised the costs of the extended release cabergoline and some laboratory testing.
Acknowledgments
The authors are grateful to the owners of the horses for sharing their clinical data.
Conflict of interest
DR provides consultancy services to BOVA Aus, BOVA UK and Luoda Pharma, who have developed and produced the extended release cabergoline preparation that was investigated. TS has received subsidised travel expenses from BOVA Aus for attending CPD events.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Virbac, 361 Horsley Road, Milperra, NSW 2214, Australia.
2. ^BOVA Aus, Suite 1, 304-318 Kingsway Caringbah, NSW 2229.
3. ^Isopropyl Alcohol; PharmAust Manufacturing, Malaga, WA, Australia.
4. ^Siemens, 885 Mountain Highway, Bayswater, Victoria 3,153, Australia.
5. ^Microsoft corporation, Redmond, USA.
6. ^StataCorp, Texas, USA.
References
1. McFarlane, D. Equine pituitary pars intermedia dysfunction. Vet Clin North Am Equine Pract. (2011) 27:93–113. doi: 10.1016/j.cveq.2010.12.007
2. Durham, AE. Endocrine disease in aged horses. Vet Clin North Am Equine Pract. (2016) 32:301. doi: 10.1016/j.cveq.2016.04.007
3. McGowan, T, Pinchbeck, G, Phillips, C, Perkins, N, Hodgson, D, and McGowan, C. A survey of aged horses in Queensland, Australia. Part 1: management and preventive health care. Aust Vet J. (2010) 88:420–7. doi: 10.1111/j.1751-0813.2010.00637.x
4. Secombe, C, Bailey, S, Laat, M d, Hughes, K, Stewart, A, Sonis, J, et al. Equine pituitary pars intermedia dysfunction: current understanding and recommendations from the Australian and New Zealand equine endocrine group. Aust Vet J. (2018) 96:233–42. doi: 10.1111/avj.12716
5. Durham, A, McGowan, C, Fey, K, Tamzali, Y, and Van Der, KJ. Pituitary pars intermedia dysfunction: diagnosis and treatment. Equine vet Educ. (2014) 26:216–23. doi: 10.1111/eve.12160
6. Tatum, R, McGowan, C, and Ireland, J. Efficacy of pergolide for the management of equine pituitary pars intermedia dysfunction: a systematic review. Vet J. (2020) 266:105562–2. doi: 10.1016/j.tvjl.2020.105562
7. Ireland, JL, Clegg, PD, McGowan, CM, McKane, SA, and Pinchbeck, GL. A cross-sectional study of geriatric horses in the United Kingdom. Part 2: health care and disease. Equine Vet J. (2011) 43:37–44. doi: 10.1111/j.2042-3306.2010.00142.x
8. Ireland, JL, McGowan, CM, Clegg, PD, Chandler, KJ, and Pinchbeck, GL. A survey of health care and disease in geriatric horses aged 30 years or older. Vet J. (2010) 192:57–64. doi: 10.1016/j.tvjl.2011.03.021
9. Hague, N, Durham, AE, and Menzies-Gow, NJ. Pergolide dosing compliance and factors affecting the laboratory control of equine pituitary pars intermedia dysfunction. Vet Rec. (2021) 189:e142. doi: 10.1002/vetr.142
10. Hebert, R, Thompson, D, Mitcham, P, Lestelle, J, Gilley, R, and Burns, P. Inhibitory effects of Pergolide and Cabergoline formulations on daily plasma prolactin concentrations in geldings and on the daily prolactin responses to a small dose of Sulpiride in mares. J Equine Vet. (2013) 33:773–8. doi: 10.1016/j.jevs.2012.12.006
11. Valencia, NA, Thompson, DL, and Oberhaus, EL. Long-term and short-term dopaminergic (Cabergoline) and Antidopaminergic (Sulpiride) effects on insulin response to glucose, glucose response to insulin, or both in horses. J Equine Vet. (2017) 59:95–103. doi: 10.1016/j.jevs.2017.10.008
12. Curran, MP, and Perry, CM. Cabergoline. Drugs. (2004) 64:2125–41. doi: 10.2165/00003495-200464180-00015
13. Ferriere, A, Cortet, C, Chanson, P, Delemer, B, Caron, P, Chabre, O, et al. Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur J Endocrinol. (2017) 176:305–14. doi: 10.1530/eje-16-0662
14. Alexandraki, KI, and Grossman, AB. Pituitary-targeted medical therapy of Cushing’s disease. EOIDER. (2008) 17:669. doi: 10.1517/13543784.17.5.669
15. Contin, M, Riva, R, Albani, F, and Baruzzi, A. Pharmacokinetic optimisation of dopamine receptor agonist therapy for Parkinson’s disease. CNS Drugs. (2000) 14:439–55. doi: 10.2165/00023210-200014060-00003
16. Shaojian, L, Anke, Z, Xun, Z, and Zhe, BW. Treatment of pituitary and other tumours with Cabergoline: new mechanisms and potential broader applications. Neuroendocrinology. (2020) 110:477–88. doi: 10.1159/000504000
17. Valencia, NA, Thompson, DL, Oberhaus, EL, and Gilley, RM. Long-term treatment of insulin-insensitive mares with Cabergoline: effects on prolactin and melanocyte stimulating hormone responses to sulpiride and on indices of insulin sensitivity. J Equine Vet. (2014) 34:680–6. doi: 10.1016/j.jevs.2013.12.015
18. Secombe, CJ, Tan, RHH, Perara, DI, Byrne, DP, Watts, SP, and Wearn, JG. The effect of geographic location on circannual adrenocorticotropic hormone plasma concentrations in horses in Australia. J Vet Intern Med. (2017) 31:1533–40. doi: 10.1111/jvim.14782
19. Schott, H, Coursen, C, Sw, E, Nachreiner, R, Refsal, K, Ewart, S, et al. The Michigan Cushing’s project. Proceedings of the annual convention of the Association of American Equine Practitioners. (2001) 22–24.
20. Durham, AE. Therapeutics for equine endocrine disorders. Vet Clin North Am: Equine Pr. (2017) 33:127–39. doi: 10.1016/j.cveq.2016.11.003
21. McFarlane, D, Banse, H, Knych, HK, and Maxwell, LK. Pharmacokinetic and pharmacodynamic properties of pergolide mesylate following long-term administration to horses with pituitary pars intermedia dysfunction. J Vet Pharmacol Ther. (2017) 40:158–64. doi: 10.1111/jvp.12339
22. McFarlane, D, Donaldson, MT, McDonnell, SM, and Cribb, AE. Effects of season and sample handling on measurement of plasma α-melanocyte-stimulating hormone concentrations in horses and ponies. Am J Vet Res. (2004) 65:1463–8. doi: 10.2460/ajvr.2004.65.1463
23. Schreiber, CM, Stewart, AJ, Kwessi, E, Behrend, EN, Wright, JC, Kemppainen, RJ, et al. Seasonal variation in results of diagnostic tests for pituitary pars intermedia dysfunction in older, clinically normal geldings. J Am Vet Med Assoc. (2012) 241:241–8. doi: 10.2460/javma.241.2.241
24. Perkins, GA, Lamb, S, Erb, HN, Schanbacher, B, Nydam, DV, and Divers, TJ. Plasma adrenocorticotropin (ACTH) concentrations and clinical response in horses treated for equine Cushing’s disease with cyproheptadine or pergolide. Equine Vet J. (2002) 34:679–85. doi: 10.2746/042516402776250333
25. Meyer, JC, Hunyadi, LM, and Ordóñez-Mena, JM. The accuracy of ACTH as a biomarker for pituitary pars intermedia dysfunction in horses: a systematic review and meta-analysis. Equine Vet J. (2022) 54:457–66. doi: 10.1111/evj.13500
26. Rohrbach, BW, Stafford, JR, Clermont, RSW, Reed, SM, Schott, HC, and Andrews, FM. Diagnostic frequency, response to therapy, and long-term prognosis among horses and ponies with pituitary par intermedia dysfunction, 1993–2004. J Vet Intern Med. (2012) 26:1027–34. doi: 10.1111/j.1939-1676.2012.00932.x
27. Spelta, C, and Axon, J. Case series of equine pituitary pars intermedia dysfunction in a tropical climate. Aust Vet J. (2012) 90:451–6. doi: 10.1111/j.1751-0813.2012.00997.x
28. Gehlen, H, May, A, and Bradaric, Z. Comparison of insulin and glucose metabolism in horses with pituitary pars intermedia dysfunction treated versus not treated with Pergolide. J Equine Vet. (2014) 34:508–13. doi: 10.1016/j.jevs.2013.11.001
29. Furtado, T, and Rendle, D. To improve welfare in the equine species should we place greater emphasis on understanding our own? Equine Vet J. (2022) 54:1001–4. doi: 10.1111/evj.13869
30. Verker, M, van Stokrom, M, and Endenburg, N. How can veterinarians optimise owner compliance with medication regimes. Eur J Companion Anim Pract. (2008) 18:73–7.
31. Schott, HC. Pituitary pars intermedia dysfunction: equine Cushing’s disease. Vet Clin North Am Equine Pract. (2002) 18:237–70. doi: 10.1016/s0749-0739(02)00018-4
32. Pivonello, R, Martino, MCD, Cappabianca, P, Leo, MD, Faggiano, A, Lombardi, G, et al. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist Cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. (2009) 94:223–30. doi: 10.1210/jc.2008-1533
33. Martins, D, Mehta, MA, and Prata, D. The “highs and lows” of the human brain on dopaminergics: evidence from neuropharmacology. Neurosci Biobehav Rev. (2017) 80:351–71. doi: 10.1016/j.neubiorev.2017.06.003
34. Larsen, M, Franchi, G, Herskin, M, Foldager, L, Larsen, M, Hernández-Castellano, L, et al. Effects of feeding level, milking frequency, and single injection of cabergoline on feed intake, milk yield, milk leakage, and clinical udder characteristics during dry-off in dairy cows. J Dairy Sci. (2021) 104:11108–25. doi: 10.3168/jds.2021-20289
35. Woodside, B. Prolactin and the hyperphagia of lactation. Physiol Behav. (2007) 91:375–82. doi: 10.1016/j.physbeh.2007.04.015
36. Henry, TJ, Puchalski, SM, Arzi, B, Kass, PH, and Verstraete, FJM. Radiographic evaluation in clinical practice of the types and stage of incisor tooth resorption and hypercementosis in horses. Equine Vet J. (2017) 49:486–92. doi: 10.1111/evj.12650
Keywords: horse, endocrine, laminitis, geriatric, dopamine, ACTH
Citation: Sundra T, Kelty E, Rossi G and Rendle D (2024) Retrospective assessment of the use of extended-release cabergoline in the management of equine pituitary pars intermedia dysfunction. Front. Vet. Sci. 11:1332337. doi: 10.3389/fvets.2024.1332337
Edited by:
Valentina Meucci, University of Pisa, ItalyReviewed by:
Victor Alejandro Castillo, University of Buenos Aires, ArgentinaDianne McFarlane, University of Florida, United States
Teresa Burns, The Ohio State University, United States
Harold Schott, Michigan State University, United States
Copyright © 2024 Sundra, Kelty, Rossi and Rendle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Rendle, ZGF2ZXJlbmRsZUBtZS5jb20=
 Tania Sundra
Tania Sundra Erin Kelty
Erin Kelty Gabriele Rossi
Gabriele Rossi David Rendle
David Rendle