- 1U.S. Navy Marine Mammal Program, Naval Information Warfare Center Pacific, San Diego, CA, United States
- 2Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, University of Missouri, Columbia, MO, United States
- 3Department of Anesthesiology, College of Medicine, University of Florida, Gainesville, FL, United States
- 4Innovative Veterinary Medicine, Ponte Vedra, FL, United States
- 5Department of Clinical Sciences, College of Veterinary Medicine, Kansas State University, Manhattan, KS, United States
- 6Department of Statistics, College of Arts and Science, University of Missouri, Columbia, MO, United States
- 7U.S. Navy Marine Mammal Program, National Marine Mammal Foundation, San Diego, CA, United States
Introduction: Use of mechanical ventilation during general anesthesia is a necessary practice in the anesthetization of small cetaceans as spontaneous ventilation fails to provide adequate gas exchange. Currently available methods of ventilation do not account for the intermittent breathing strategy of representative species within this infraorder of fully aquatic mammals and may have a significant effect on cardiac and respiratory physiology.
Methods: To understand the impact of mechanical ventilation on cardiopulmonary function in one small species of cetacean, the bottlenose dolphin (Tursiops truncatus), we compared controlled mechanical ventilation (CMV) to a novel ventilation method known as apneustic anesthesia ventilation (AAV). AAV simulates the normal inspiratory breath-hold pattern of dolphins. Ten anesthetic procedures (dental procedure, n = 9; bronchoscopy, n = 2) were performed on nine dolphins (age range: 10–42 years; mean = 32 years; median = 37 years; female = 3, 40%; male = 6, 60%). In a cross-over study design, dolphins were instrumented and randomly assigned to AAV or CMV as the initial mode of ventilation, then switched to the alternate mode. Baseline cardiopulmonary data were collected and again after 30 min on each mode of ventilation. Cardiac index, stroke volume index, systemic vascular resistance, alveolar dead space, alveolar-arterial oxygen tension gradient, arterial oxygen content, oxygen delivery index, and dynamic respiratory system compliance index were calculated at each of the four time points.
Results: During AAV, dolphins had higher arterial oxygen tension, higher mean airway pressure, reduced alveolar dead space ventilation and lower alveolar-arterial oxygen difference. Cardiovascular performance was not statistically different between the two modes.
Discussion: Our study suggests AAV, which more closely resembles the conscious intermittent respiratory pattern phenotype of dolphins, improves ventilation and pulmonary function in the anesthetized dolphin. Future studies should evaluate the cardiopulmonary effects of neutral buoyancy and cardiopulmonary sparing drug protocols to reduce the need for hemodynamic support of current protocols.
1 Introduction
Within the infraorder of fully aquatic mammals, Cetacea, the bottlenose dolphin (Tursiops truncatus) has been well represented under the care of humans for over 100 years (1). Despite the large number of dolphins under human care, relatively few have been safely anesthetized for clinical procedures and recovered (2–5). The current limited body of literature on cetacean anesthesia focuses on drug combinations and techniques for rapid induction of anesthesia but does not address the physiologic effects of these approaches (4–12). Specifically, no literature exists on the influences of current ventilation protocols on cetacean cardiopulmonary physiology. This data gap is largely due to the paucity of controlled physiologic studies and the stagnated development of advanced methods for supportive anesthetic care in dolphins. These critical factors have been a remarkable hurdle in furthering our knowledge, understanding, and optimization of dolphin anesthesia.
As a completely aquatic marine mammal, the bottlenose dolphin evolved specialized cardiovascular and pulmonary features to facilitate breath-hold diving and underwater foraging activities (13–15). Such adaptations include, but are not limited to, periarterial venous rete for efficient thermoregulation, valve-less peripheral veins, rete mirabilia surrounding central nervous system structures, an intermittent, breath-holding breathing pattern, and reinforcement of pulmonary bronchioles with cartilage (16–20). These cardiopulmonary adaptations should be considered when performing general anesthesia, whereby ventilation and pharmacologic strategies may modulate gas-exchange mechanisms, autonomic nervous system function and responses, and vital organ perfusion states.
In our clinical experience, similar to large terrestrial mammals, cardiopulmonary derangements such as hypoventilation, ventilation-perfusion mismatch, vasodilation, and depression of cardiac contractility likely occur in anesthetized dolphins when applying commonly accepted anesthetic drugs (e.g., opioids, propofol, benzodiazepines, and inhalation anesthetics) and conventional ventilation strategies (2). It would then follow that these effects may lead to arterial hypoxemia, hypercapnia, hypotension, and decreased cardiac output. For example, respiratory depression is a characteristic of dolphin sedation and anesthesia that demands rapid intubation and respiratory support in the form of mechanical ventilation. The use of propofol and inhalation anesthetics are known to contribute to profound vasodilation and hypotension in various species and, thus, may impair circulation in the dolphin (21, 22). If not properly mitigated, these derangements can affect organ perfusion, reduce oxygen delivery, and predispose the animal to organ injury (e.g., acute kidney injury, myocardial injury, hypoxic brain injury), and may even contribute to neuropathic and myopathic conditions (23, 24). In large, completely aquatic mammals, like the dolphin, little is known about the pathophysiologic influences of anesthesia on respiratory gas exchange efficiency and organ perfusion. Thus, there is a need to understand the physiologic impacts of anesthesia in a species with unique and specialized structural and physiologic adaptations, as well as to develop strategies to alleviate and reduce anesthesia-associated physiologic derangements.
Controlled mechanical ventilation (CMV) with supplemental oxygen is a well-defined strategy used for minimizing the impact of anesthesia-induced respiratory abnormalities (e.g., hypoventilation, hypercapnia, and hypoxemia) in large aquatic mammals (2, 4, 5, 9, 25–30). While various approaches to mechanical ventilation are used within the field of human anesthesiology, available large animal veterinary anesthesia equipment has limited options. Herein, we define CMV (also referred to as conventional mechanical ventilation) as intermittent positive pressure ventilation in the absence of positive end-expiratory pressure and alveolar recruitment maneuvers (31). As defined by the authors, CMV most closely resembles the normal respiratory pattern of most non-hibernating terrestrial mammals. The dolphin, on the contrary, has an intermittent, inspiratory breath-hold respiratory pattern with significant heart rate variation during each inspiratory-to-expiratory cycle, also referred to in clinical medicine as respiratory sinus arrhythmia (32–36). While this cardiopulmonary coupling strategy may improve gas exchange in the conscious diving dolphin, the heart rate variation associated with intermittent breathing converts to a normal sinus rhythm following induction of anesthesia and initiation of mechanical ventilation (33, 37). In addition, dolphin alveoli readily collapse, pushing gases into the proximal reinforced terminal bronchioles as a proposed strategy to reduce gas exchange and oxygen consumption when diving (38). Mechanical ventilation with 100% oxygen can also promote alveolar collapse over time via absorption atelectasis (39–41). Thus, the dolphin may be more likely to experience atelectasis from alveolar collapse when anesthetized with high fractions of inspired oxygen (FIO2) and ventilated using traditional CMV paradigms. For these reasons, we sought to evaluate a novel mechanical ventilation approach, known as apneustic anesthesia ventilation (AAV), that may address physiologically appropriate ventilation needs of the dolphin, thereby supporting optimal cardiopulmonary function (31, 42). This mechanical ventilation strategy more closely resembles the intermittent respiratory pattern phenotype of the dolphin and therefore is worthy of investigation in the target population (26, 30, 43).
CMV increases airway pressure from ambient to higher pressures in order to produce tidal ventilation. In doing so, lung volume at the start of inspiration is typically below functional residual capacity (FRC; e.g., the lung volume below which alveoli are prone to collapse). In contrast, AAV maintains airway pressure above ambient, and therefore above FRC and produces tidal ventilation by decreasing pressure from this continuously applied, elevated baseline pressure (Phigh) to a lower pressure (Plow) with the goal of maintaining lung volume at or slightly above FRC (Figure 1) (31, 43). The time at Phigh, referred to as Thigh, is significantly longer than the time at Plow (Tlow). This strategy promotes maintenance of lung volume and alveolar recruitment to minimize the progressive formation of anesthesia-associated atelectasis while optimizing respiratory system compliance. In human patients, AAV was shown to result in significant reduction in dead-space ventilation, improved respiratory system compliance and a lower arterial to alveolar carbon dioxide tension difference (43). In dorsally recumbent, anesthetized horses inspiring 38%–47% O2, respiratory system dynamic compliance was higher and venous admixture was lower with AAV compared to CMV. However, no notable improvement in cardiovascular performance was evident in this model (31). In human patients, AAV may exhibit improved cardiovascular performance compared to CMV by augmenting venous return secondary to the use of lower mean airway pressures, while minimizing ventilation-perfusion mismatch (43).
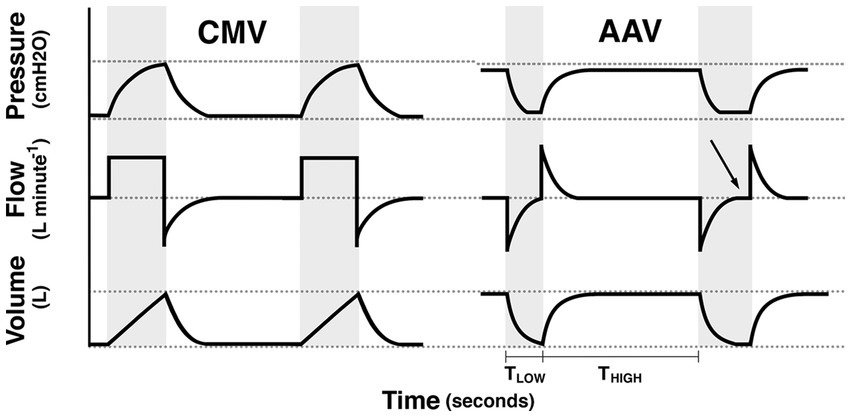
Figure 1. Schematic illustration of the pressure, flow, and volume vs. time waveforms for controlled mechanical ventilation (CMV) and apneustic anesthesia ventilation (AAV). Gray background indicates periods of tidal ventilation corresponding to inspiratory time during CMV and the time (TLOW) at PLOW during AAV. White background indicates expiratory time during CMV and the time (THIGH) of PHIGH during AAV. For AAV waveforms, the first release, illustrates proper limitation of release time whereas the second release illustrates a long release time with flow stagnation (arrow). Reproduced with permission from Innovative Veterinary Medicine, Inc.
The objective of the study described here was to compare the cardiopulmonary effects of AAV to CMV, while using a repeatable anesthetic drug combination, in anesthetized bottlenose dolphins undergoing clinically indicated medical or surgical procedures. Based on the equine ventilation investigative model and clinical experience with cetacean anesthesia, we hypothesized the use of AAV would result in improved indices of pulmonary function in dolphins when compared to a traditional implementation of CMV.
2 Materials and methods
2.1 Animals
Nine (age range: 10–42 yrs.; mean: 32 yrs.; median: 37 yrs.; 3 female, 6 male) bottlenose dolphins, housed and cared for by the U.S. Navy Marine Mammal Program (MMP), Naval Information Warfare Center (NIWC) Pacific, in San Diego Bay (CA, United States), were anesthetized in a randomized, crossover design without a washout period to compare the cardiopulmonary effects of the two mechanical ventilation modes. Ventilation mode starting order was randomly assigned using a binary random number generator (www.random.org; Ireland). One female dolphin was anesthetized on 2 separate occasions separated by approximately 1 year, for a total of 10 anesthetic procedures on 9 dolphins.
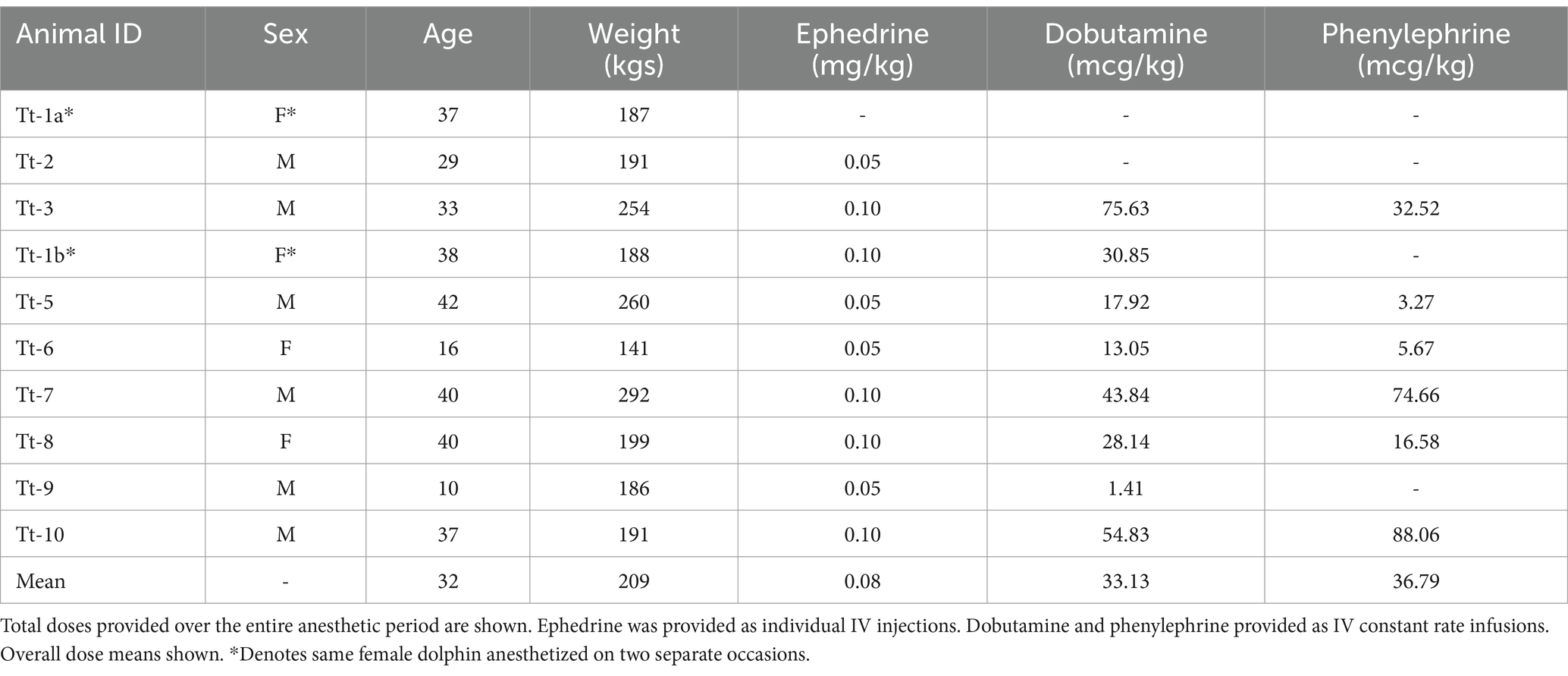
As an index of general health and a prognostic tool for anesthesia-related complications, the physical status of individual dolphins were classified using an adapted version of the American Society of Anesthesiologists (ASA) physical status classification system (Table 1) (44). All animals were anesthetized for clinically indicated procedures and received comprehensive health evaluations by a veterinarian at least 7–10 days prior to the anesthetic procedures (Table 2). Health evaluations consisted of physical examinations including weight, clinicopathologic analyses (complete blood count with differential, plasma biochemical profile, and blood gas analysis sampled from the fluke periarterial venous rete), thoracic and abdominal ultrasound examinations, and electrocardiographic tracing evaluation. Animals were either excluded from the study or the procedure was delayed, if health evaluations exhibited any of the following: clinicopathologic abnormalities for the population, abnormal electrocardiographic tracings, and/or abnormal thoracic and abdominal ultrasound findings (34, 45–48). In a previous study in horses, statistically significant differences were found with an a priori power analysis of variance (ANOVA) design with α = 0.05, 1 − β = 0.8, effect size of 0.5 and repeated-measures correlation of 0.5 suggesting a sample size of 10 (31). For this reason, as well as the limited availability of dolphins requiring general anesthesia to address clinical disease, 10 dolphins were included in this study.
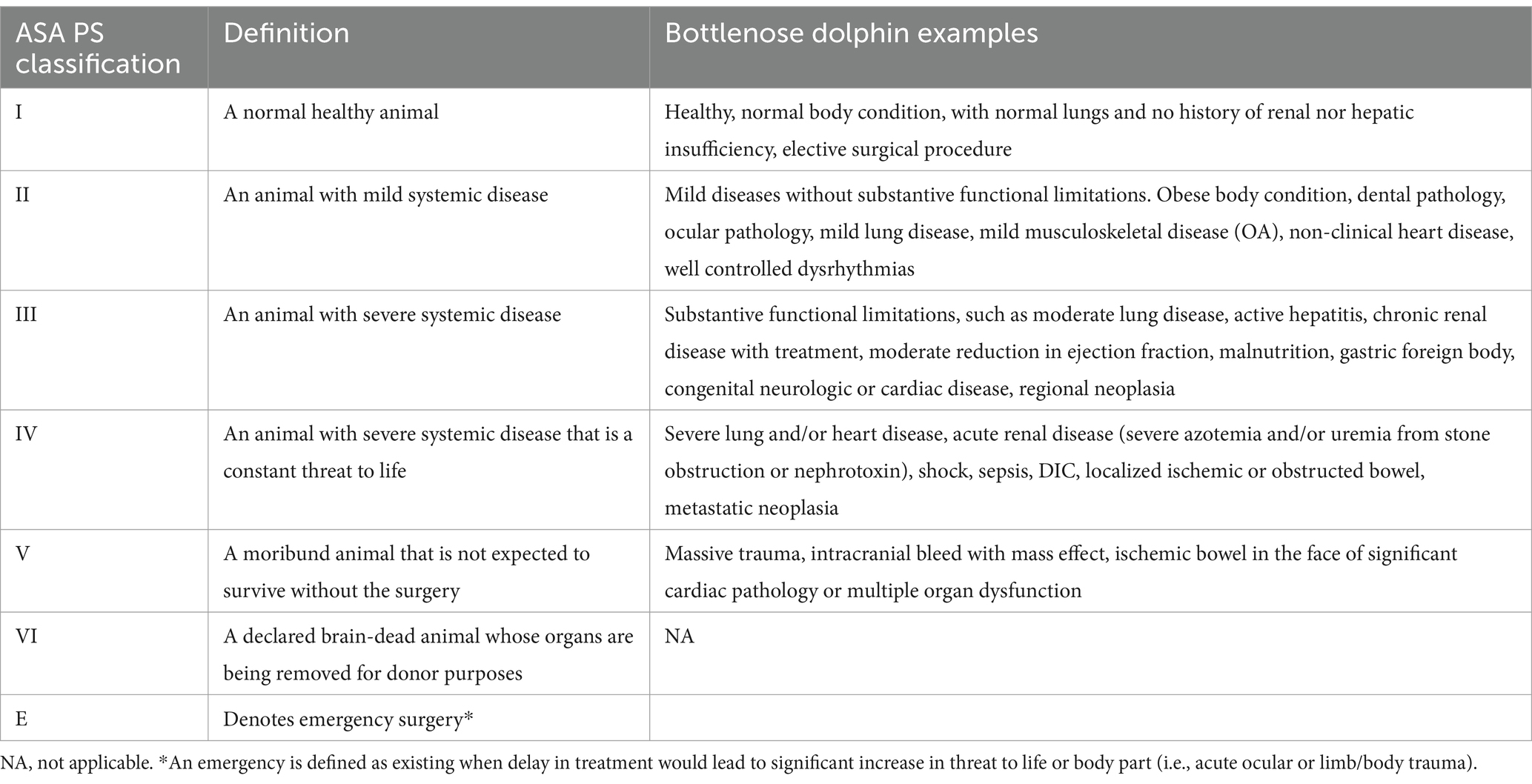
Table 1. Physical status classification with definitions for adult bottlenose dolphins enrolled in this study and adapted from the American Society of Anesthesiologists physical status (ASA PS) classification score for humans.
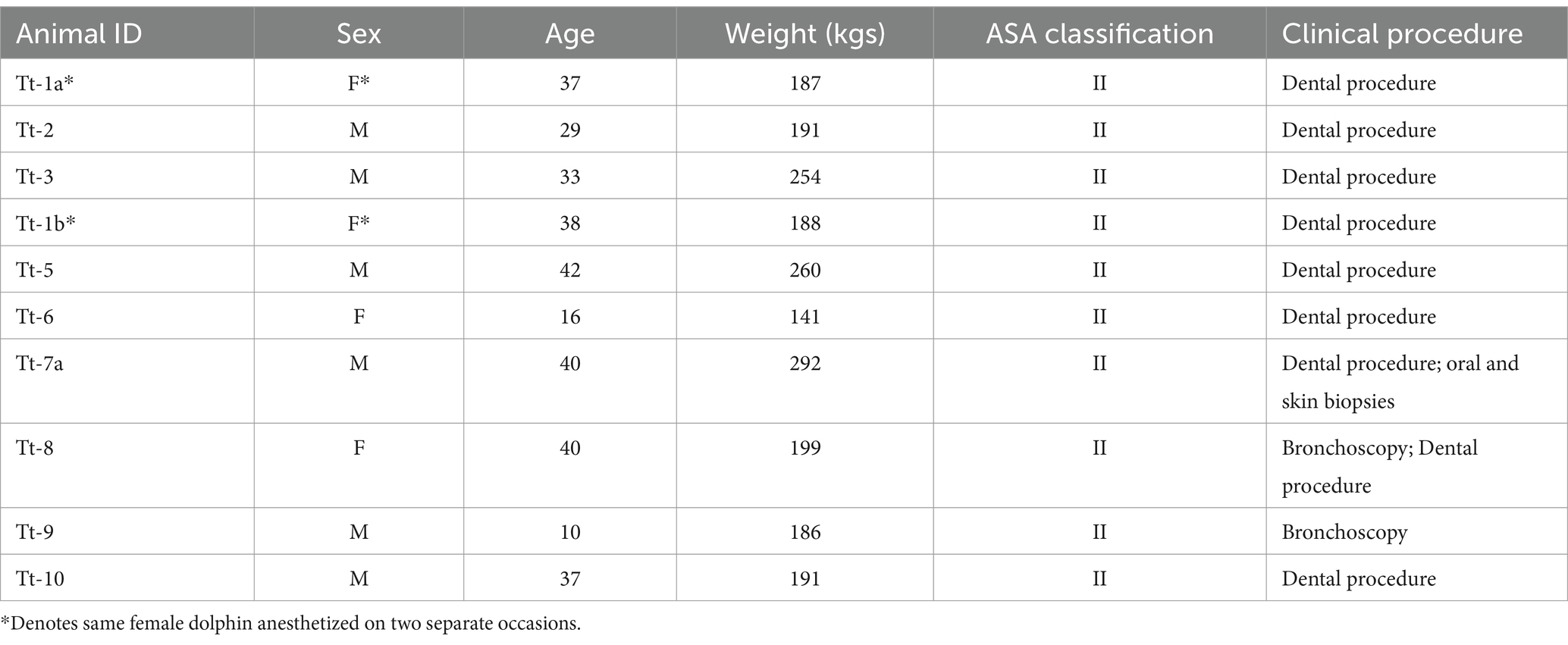
Table 2. Signalment, American Society of Anesthesiologists (ASA) physical status classification, and clinical procedures for bottlenose dolphins, Tursiops truncatus, enrolled in the study.
Secretary of Navy Instruction 3900.41H directs that Navy marine mammals be provided the highest quality of care. NIWC Pacific is accredited by AAALAC International and adheres to the national standards of the U.S. Public Health Service policy on the Humane Care and Use of Laboratory Animals and the Animal Welfare Act. NIWC Pacific’s animal care and use program is routinely reviewed by an institutional animal care and use committee and the U.S. Navy Bureau of Medicine. The animal use and care protocol for Navy dolphins in support of this study was approved by NIWC Pacific’s Institutional Animal Care and Use Committee (No. 134-2019) and the U.S. Navy Bureau of Medicine (NRD-1198).
2.2 Anesthesia
Food was withheld from dolphins for at least 18 h prior to each anesthetic procedure. Two dolphins, however, received oral diazepam sedation (5 mg tablets, Teva Pharmaceuticals United States, Inc., Parsippany, NJ 07054, United States; 0.08–0.30 mg/kg PO) in one capelin (Mallotus villosus) 1–2 h prior to injectable pre-medication. All dolphins were pre-medicated with a combined midazolam (West-Ward, Eatontown, NJ 07724, USA; 0.08–0.1 mg/kg IM) and meperidine (Hikma Pharmaceuticals, Berkeley Heights, NJ 07922 United States; 0.1–0.2 mg/kg IM) injection. A 5 Fr, 10 cm catheter (Micro-introducer Kit, Innovative Veterinary Medicine, Inc., Ponte Vedra, FL 32081, United States) was aseptically placed, using ultrasound guidance, into a lateral caudal subcutaneous vein (49). Immediately prior to and during induction of anesthesia, a 5 mL/kg IV bolus of lactated Ringer’s solution (Baxter Healthcare Corporation, Deerfield, IL 60015, United States) was administered. General anesthesia was induced with midazolam (0.02 mg/kg IV) and propofol (Hospira, Inc., Lake Forest, IL 60045 USA; 2–4 mg/kg IV) to allow for orotracheal intubation (16–18 mm internal diameter, cuffed silicone tracheal tube). Anesthesia was maintained in sternally-recumbent dolphins using sevoflurane (Baxter Healthcare Corporation, Deerfield, IL 60015, United States) and 100% oxygen on a circle breathing system. Anesthetic gas delivery was adjusted to maintain 1.8%–2.0% end-tidal sevoflurane concentration (FEsevo). Intravenous lactated Ringer’s solution infusion was maintained at 5 mL/kg/h throughout the anesthetic procedure. All dolphins were temporarily placed into lateral, oblique position to allow placement of a 4 Fr, 10 cm palmar median artery catheter (Innovative Veterinary Medicine, Inc., FL, USA) for continuous direct blood pressure measurement, arterial blood gas sampling, and cardiac output determination via lithium dilution (LiDCO Unity; LiDCO, London, N17 0QJ, United Kingdom) (50).
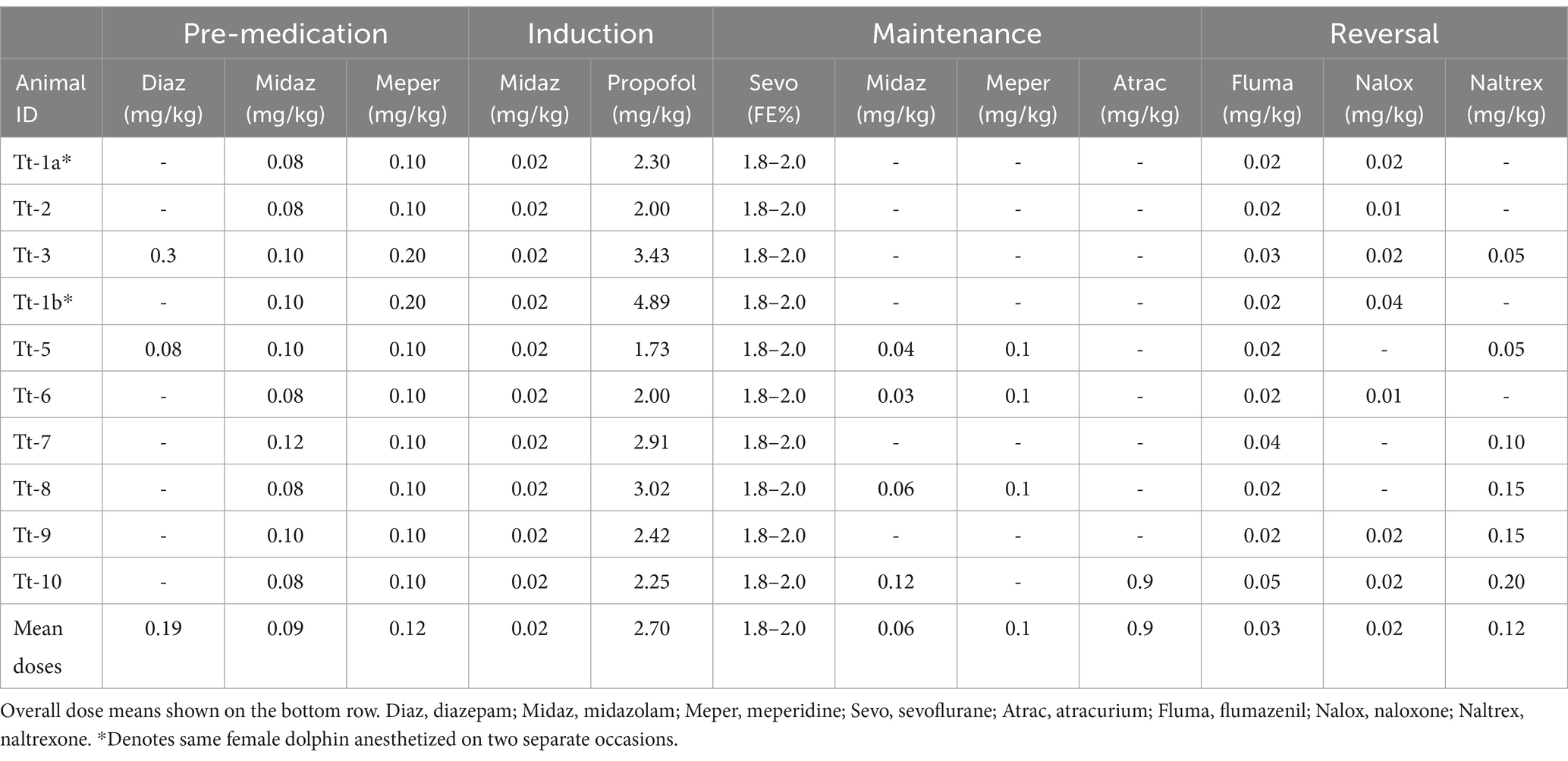
Additional anesthesia and analgesia were provided, when clinically indicated, in the form of regional or local nerve blocks (lidocaine, 2% inj, Hospira, Inc.; bupivacaine, 0.5% inj, Hospira, Inc.) and/or midazolam (0.02 mg/kg) and/or meperidine (0.1–0.2 mg/kg) IV boluses. In one dolphin, neuromuscular blockade (atracurium besylate, Hospira, Inc., 0.1 mg/kg IV, PRN q20 mins) was used to provide muscle relaxation. Table 3 provides a summary of anesthetic protocols used on individual animals.
2.3 Study design and criteria
The two modes of ventilation were applied to each dolphin in a cross-over design. CMV was applied using a commercially available large animal anesthesia machine with a descending bellows ventilator (Surgivet LDS3000 with DHV1000; Smiths Medical ASD Inc., OH, United States). AAV was applied using a custom-built ventilator (DolVent; Innovative Veterinary Medicine, LLC., FL, United States) adapted to drive the same commercially available bellows assembly. Ventilation modes were applied based on previously published implementation strategies and estimates of tidal volumes for dolphins—maximum 20 mL/kg tidal volume (VT) and an arterial carbon dioxide tension (PaCO2) within 50–55 mmHg (31, 51). Mean arterial blood pressure (MAP) was maintained between 65 and 80 mmHg using ephedrine (Akorn, Inc., Lake Forest, IL 60045, United States; 0.05 mg/kg IV) followed by a constant rate infusion (CRI) of dobutamine (Hospira, Inc., 0.1–2 mcg/kg/min IV). Phenylephrine (Avadel Legacy Pharmaceuticals, LLC, Chesterfield, MO 63005, United States; 0.01–1 mcg/kg/min IV) CRI was supplemented to manage hypotension when the dobutamine CRI was insufficient. Table 4 provides a summary of hemodynamic support used for managing blood pressure in individual dolphins.
Four phases occurred in all dolphin procedures: Phase 1—instrumentation; Phase 2A—first ventilation mode; Phase 3—transition; Phase 2B—alternate ventilation mode; Phase 4—recovery (Figure 2). During Phase 2A and 2B, data were collected at baseline (T0) and at 30 min (T1) after baseline. A 30-min transition interval (Phase 3) was allotted between Phases 2A and 2B for switching ventilation modes and adjusting ventilatory settings to meet study criteria. Surgical and medical procedures occurred throughout the study period but were temporarily halted to allow for steady-state data collection and physiologic measurements at each T0 and T1. During Phase 4, reversal agents flumazenil (Fresenius Kabi, Lake Zurich, IL 60047, United States; 1:13 IV) and naloxone (International Medication Systems Limited, El Monte, CA 91733, United States; 10 mcg/kg IV) were administered once FEsevo consistently measured less than 0.8% (Carescape B650; GE Healthcare, Chicago, IL 60661, United States). All dolphins recovered from general anesthesia without complications.
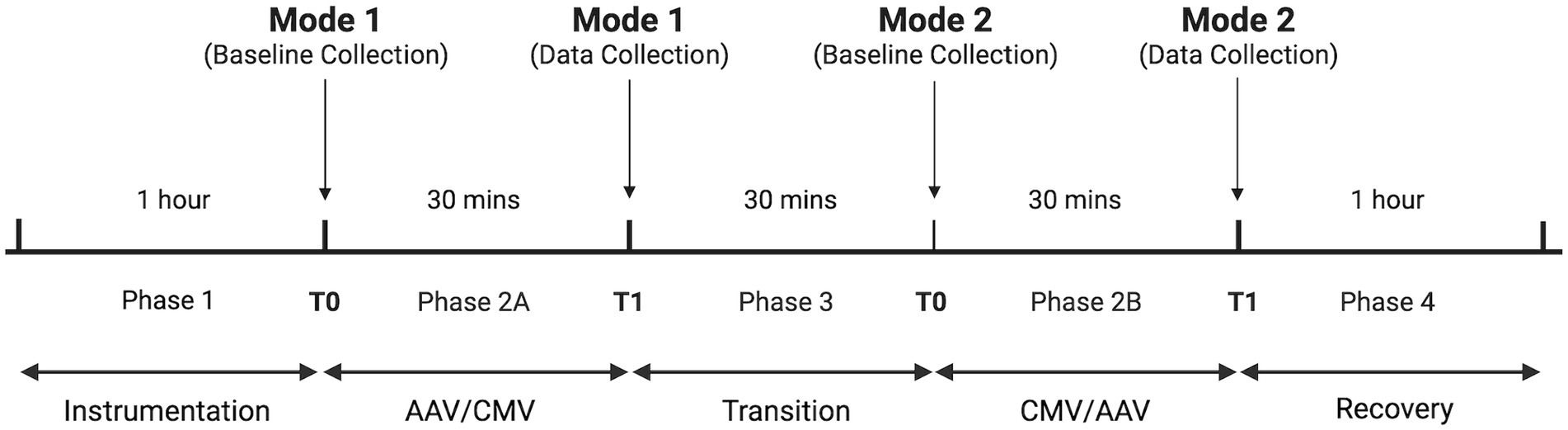
Figure 2. Cross-over study design to compare two ventilation modes in bottlenose dolphins. Order of ventilation mode was randomly determined. Times in each phase are approximate. Figure created using Biorender.com.
2.4 Monitoring and data collection
A multiparameter monitor (Carescape B650, GE Healthcare) was used for monitoring base-apex electrocardiogram, pulse oximetry, side-stream airway gases (FEsevo and ETCO2) and MAP. Calibration of the airway gas module (755571-HEL, GE Healthcare) according to manufacturer specifications was performed prior to each anesthetic procedure.
A calibrated, pitot tube differential pressure spirometer (DolVent EUI; Innovative Veterinary Medicine, Inc., FL, United States) was placed between the breathing system and the tracheal tube for measurement of respiratory variables. Spirometer accuracy was assessed prior to each anesthetic procedure using a 7 L calibrated syringe (Model 4,900; Hans Rudolph Inc., KS, United States) to ensure measured volumes were within +/− 100 mL of delivered volumes (<2%). Measured and calculated variables common to both ventilation modes included VT, respiratory rate (fR), mean airway pressure (Paw), and total respiratory system dynamic compliance (CRS). CMV specific variables included inspiratory-to-expiratory ratio (I:E), peak inspiratory pressure (PIP), and end-expiratory pressure (EEP). AAV specific variables included Phigh, Plow, Thigh, and Tlow. For CMV and AAV, CRS was computed as VT/(PIP − EEP) and VT/(Phigh − Plow), respectively.
To avoid the need for right heart catheterization in the study dolphins, cardiac output (CO) was measured via lithium dilution (LiDCO Unity) administered in a peripheral vein (31, 50, 52). A sterile lithium chloride solution (lithium chloride, powder, Sigma-Aldrich Inc., St. Louis, MO 63103, United States; sterile water, inj, APP Pharmaceuticals, LLC, Schaumburg, IL 60173, United States) was prepared prior to each anesthetic procedure and a 0.01 mmol/kg IV bolus given for each cardiac output determination.
At each data collection time point, cardiopulmonary data were recorded, systemic arterial (pHa, PaO2, PaCO2, hemoglobin, and oxyhemoglobin saturation) and venous (pHv, PvO2, PvCO2, oxyhemoglobin saturation) blood samples were simultaneously collected (safePICO Aspirator; Radiometer Medical ApS, Copenhagen 2700, Denmark) from the lateral caudal subcutaneous vein and the palmar median artery for immediate analysis (ABL90 Flex, Radiometer Medical ApS), and CO was measured.
2.5 Calculated data
Data calculated using standard formulae included stroke volume (SV = CO/HR), alveolar dead space as a fraction of alveolar ventilation [VDalv/VTalv = (PaCO2 − ETCO2)/PaCO2], alveolar-arterial oxygen tension difference [PAO2 – PaO2, where PAO2 = (PB – PH20) – PACO2/R]; PB is barometric pressure, PH20 is the saturated vapor pressure of water at 37°C, PACO2 is alveolar PCO2 tension (approximated by PaCO2), R is the respiratory quotient and assumed equal to 0.8, systemic vascular resistance (SVR = MAP/CO), arterial blood oxygen content (CaO2 = 1.36 × [Hb] × SaO2 + 0.003 × PaO2), and oxygen delivery (DO2 = CaO2 × CO). CO, SV, DO2, VT, and CRS were indexed to body mass and denoted by an appended I.
2.6 Statistical analysis
Statistical modeling of the cardiopulmonary data utilized repeated measures three-way ANOVA with main factors mode of ventilation, time point, and order (SAS Version 9.4; SAS Institute, NC, United States). The full model, with specified covariance structure, was fit and assumptions were checked, including residual diagnostics and the assumption of normality. To examine if order of ventilation mode was statistically significant, a re-parameterized two-way ANOVA model with two main factors (order, 2 levels, and time point-by-mode, 4 levels) was used to facilitate contrast estimation. When interactions were not significant, main effects were interpreted using the overall F-statistic and p-value, followed by post hoc mean comparisons and contrast estimation using Tukey’ simultaneous inference p-value adjustment. Statistical significance was set at p < 0.05.
3 Results
Comprehensive health evaluations indicated the physical health statuses of all dolphins were appropriate for general anesthesia and participation in the study. Descriptive statistics for variables specific to each mechanical ventilation mode are provided in Table 5. Three factors included in the final statistical model were order, time point, and ventilation mode. No significant differences were found within a mode between time points for all variables.
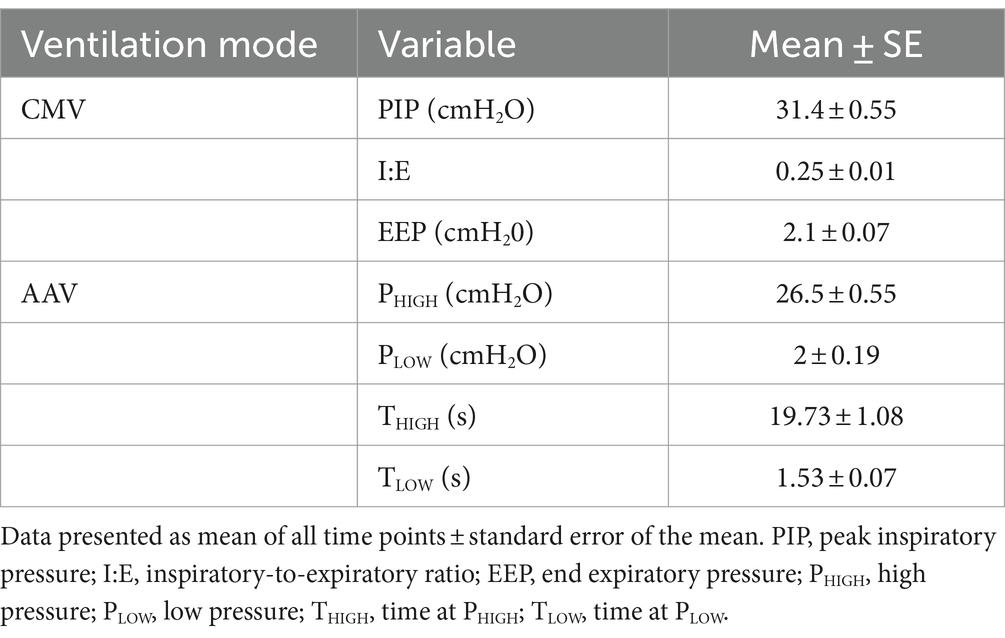
Table 5. Descriptive statistics of variables recorded during conventional mechanical ventilation (CMV) and apneustic anesthesia ventilation (AAV) modes in nine sternally recumbent anesthetized dolphins (10 anesthetic procedures).
An effect of mode of ventilation at all time points was found for fR, PaO2, PaCO2, pHa, ETCO2, venous oxygen tension (PvO2), VDalv/VTalv, PAO2-PaO2, mean Paw, CRSI, and systolic arterial pressure (SysAP; Tables 6, 7). Respiratory rate (fR; p < 0.0001) and PaCO2 (p = 0.022) were lower and ETCO2 (p < 0.0001) was higher in AAV compared to CMV. pHa was higher (i.e., closer to normal, physiologic pH) on AAV compared to CMV (p = 0.0121). PaO2 and PvO2 were significantly higher on AAV compared to CMV (p < 0.0001 and p = 0.039, respectively). Mean airway pressure (Paw) and respiratory system dynamic compliance index (CRSI) were significantly higher on AAV (p < 0.0001). Alveolar dead space ventilation (VDalv/VTalv) and alveolar-arterial oxygen tension difference (PAO2-PaO2) were lower on AAV compared to CMV (p < 0.0001). While SysAP was significantly lower on AAV (p = 0.0021), MAP and diastolic arterial pressure (DiasAP) were not statistically significant at p = 0.0504 and 0.0610, respectively. The statistically significant effects of ETCO2 were not considered clinically significant, as the study criteria aimed to maintain a narrow range of PaCO2 by titrating ETCO2 using respiratory rate. Additionally, inherent differences in fR between the two ventilation modes likely affected accurate comparison of ETCO2. No statistically significant differences between modes were found for HR, MAP, DiasAP, CI, pHv, venous carbon dioxide tension (PvCO2), pulse oximetry (SpO2), SV index (SVI), and SVR.
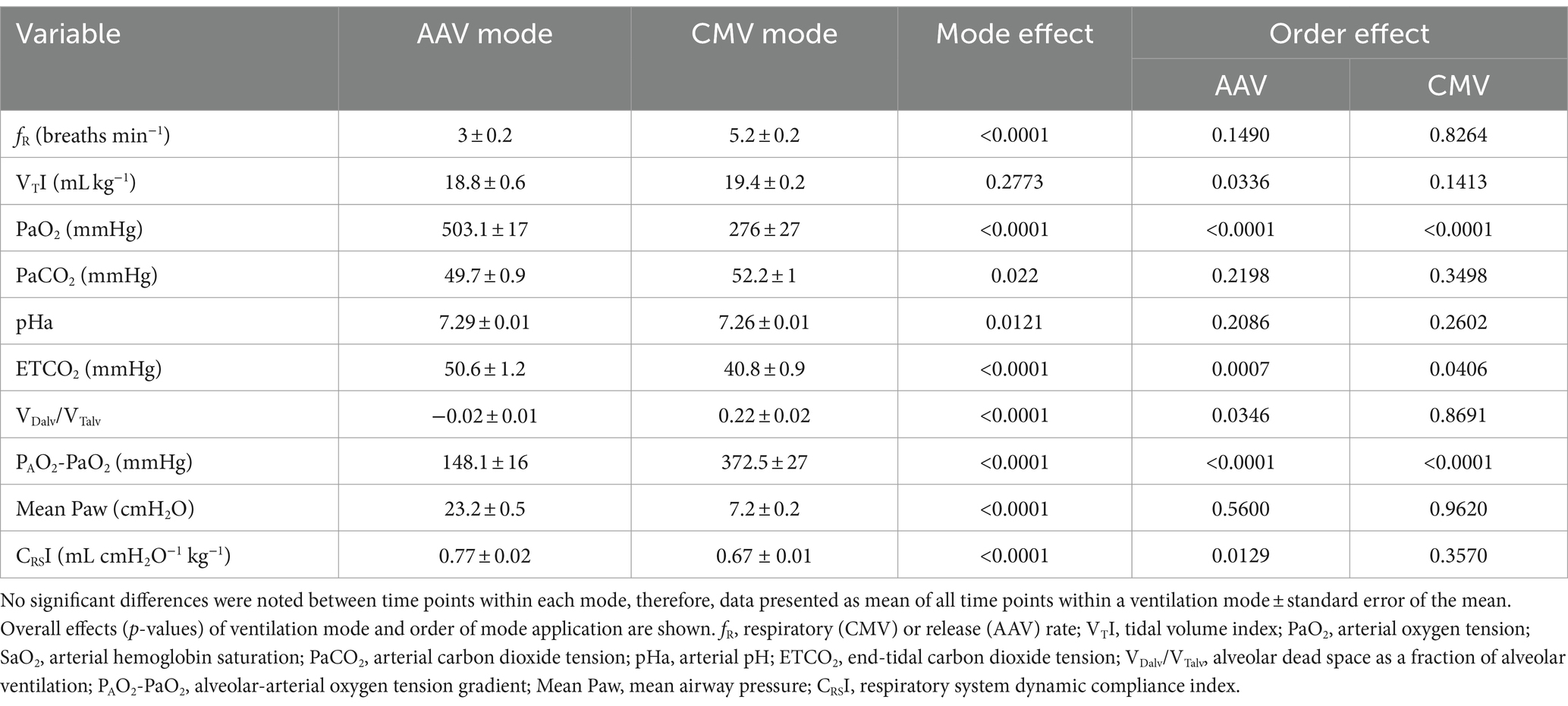
Table 6. Measured and calculated respiratory variables in nine sternally recumbent anesthetized dolphins (10 anesthetic procedures) ventilated with either apneustic anesthesia ventilation (AAV) or conventional mechanical ventilation (CMV).
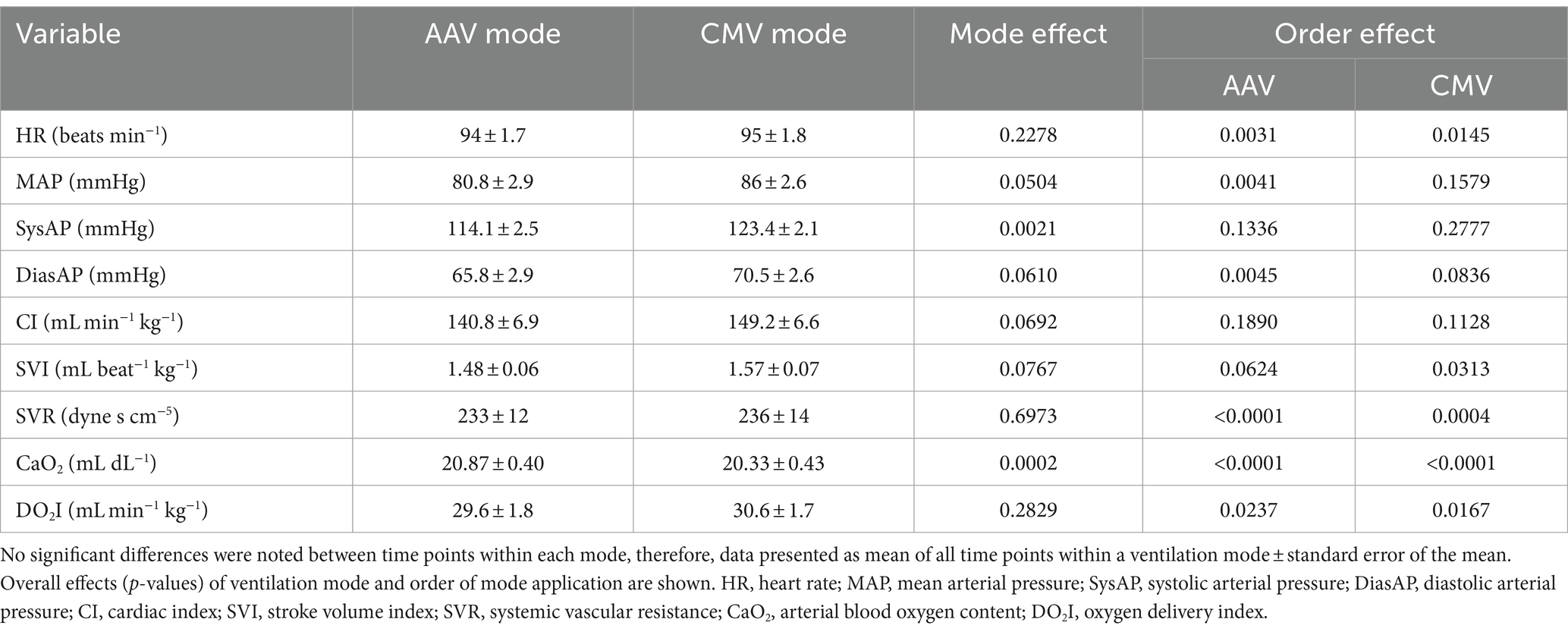
Table 7. Measured and calculated cardiovascular variables in nine sternally recumbent anesthetized dolphins (10 anesthetic procedures) ventilated with either apneustic anesthesia ventilation (AAV) or conventional mechanical ventilation (CMV).
An effect of order of ventilation mode was found for VTI, PaO2, ETCO2, VDalv/VTalv, PAO2-PaO2, CRSI, MAP, DiasAP (Tables 6, 7). When AAV was applied as the first ventilation mode (Phase 2A), VTI and PaO2 were significantly higher compared to when CMV was the first mode applied (p = 0.0335 and p < 0.0001, respectively). VDalv/VTalv and PAO2-PaO2 were significantly lower (p = 0.035 and p < 0.0001, respectively) and CRSI was significantly higher when AAV was applied as the first mode (p = 0.0129). When AAV was the first mode used, MAP and DiasAP were significantly lower than when CMV was applied first (p = 0.0041 and 0.0045, respectively). Finally, SVI was significantly higher when CMV was applied first (p = 0.0313).
4 Discussion
In this study, we assessed the cardiopulmonary effects of two distinct modes of mechanical ventilation on anesthetized bottlenose dolphins to determine if a novel mode, AAV, may provide enhanced cardiopulmonary performance compared to CMV.
Central to this study are the significant differences in clinically relevant pulmonary variables when comparing the two ventilation modes. Despite provision of ~98% inhaled oxygen and targeting a PaCO2 range between 50 and 55 mmHg via continuous ETCO2 measurements during both ventilation modes, AAV exhibited significantly higher PaO2, significantly less alveolar dead space ventilation (VDalv/VTalv), and lower alveolar-arterial oxygen tension differences (PAO2-PaO2) than CMV. This would imply improved pulmonary function during AAV and less ventilation-perfusion scatter, whereby most of the dolphin’s lung volume would be adequately perfused and able to effectively participate in gas exchange. Additionally, respiratory system compliance index (CRSI) was significantly higher on AAV, supporting larger tidal volumes over smaller changes in airway pressure, thus improving gas exchange as evidenced from the lower alveolar dead space ventilation (i.e., the proportion of alveolar ventilation not participating in gas exchange).
Improved assessment of ventilation-perfusion matching in the study dolphins would require venous admixture calculation with pulmonary arterial blood and measurement of pulmonary arterial and pulmonary arterial occlusion pressures, increasing the risk of adverse events and adding the complexity of pulmonary arterial vascular access in this species (53, 54). For this reason, central cannulation was not performed. Furthermore, current methods of estimating alveolar dead space in ventilated patients, as performed in this study, are based on end-tidal CO2 concentrations as approximations of mean alveolar CO2 in healthy subjects, and therefore, less accurate measurements of Bohr dead space (55). While all dolphins in this study received comprehensive health evaluations, presence of subclinical, or asymptomatic, pulmonary disease cannot be completely excluded.
The effects of positive pressure ventilation in either mode of mechanical ventilation would be expected to increase intrathoracic pressures and contribute to reduced cardiac preload, cardiac output, blood pressure, and subsequently reduced oxygen delivery to tissues. An important determinant of the cardiovascular effects of positive pressure ventilation is mean airway pressure (mean Paw), whereby increasing airway pressures can contribute to decreasing preload. During implementation of AAV in the study dolphins, mean Paw was significantly higher due to the proportion of time spent at PHIGH (approximately 16 mmHg higher than CMV Paw). However, overall, PHIGH on AAV was lower than PIP on CMV. In a clinical setting, PHIGH and PLOW should be applied to optimize dynamic compliance of the respiratory system to prevent progression of atelectasis. The cardiovascular effects of the higher mean Paw were mostly observed when AAV was the first mode implemented in the study dolphins. For example, when AAV was the first mode of mechanical ventilation, MAP and SVR were significantly lower. AAV displayed lower SV while CMV showed higher SVI with no significant differences in the other cardiovascular variables. However, these findings are likely attributable to the low number of animals enrolled in the study or to the pre-established criteria of a narrow MAP maintenance range, most often requiring the use of inotropic and vasopressive agents to modulate cardiovascular responses. Additionally, the intentional hypercapnia component of the current study (50–55 mmHg) may have contributed to physiologic advantages, such as increased circulating catecholamine concentrations, which may support cardiovascular function and may have contributed to non-significance in most of the cardiovascular variables (56). Overall, from a clinical perspective, AAV did not appear to negatively affect the hemodynamic status of the study dolphins.
While this study provides evidence of improved pulmonary function in dolphins ventilated using the AAV strategy, a comprehensive understanding of normal, in-water dolphin physiology is critical to the understanding of the overall influences of general anesthesia on dolphin cardiopulmonary physiology. Having evolved and adapted to aquatic environments, dolphin circulation, perfusion, and efficiency of gas exchange are likely altered by the removal of minimal to neutral buoyancy for an anesthetized procedure. In one study with 4 out-of-water dolphins anesthetized with a nitrous oxide gas mixture, the neuromuscular blocking agent succinyldicholine, and intraperitoneal or intravenous thiopental and mechanically-ventilated, SVI and CI directly measured between 0.4–0.8 mL/kg and 47–105 mL/min/kg, respectively (57). Directly measured MAP ranged between 122 and 142 mmHg (systolic: 142–160 mmHg; diastolic: 111–130 mmHg). In another study using halothane gas and 10 mg/kg intravenous thiopental, MAP in the study dolphins averaged 115 mmHg, where normal MAP was considered 120–140 mmHg (37). Dolphins in these studies were ventilated using a modified large animal ventilator with a ventilation strategy termed apneustic plateau ventilation, whereby rapid inflation was followed by an inspiratory breath hold and rapid pressure release (37, 58, 59). This mode did not include end expiratory pressure control methods and may have contributed to progressive atelectasis in the study dolphins.
In other studies, using non-invasive methods on non-anesthetized in-water dolphins, average calculated SVI and CI were 0.8 mL/kg and 32.2 mL/min/kg, respectively (60). The dolphins in the current study had SVI measuring 1.5 ± 0.1 mL/kg and CI measurements between 139 and 154 mL/min/kg, with no significant differences observed between the two ventilation modes. Average MAP was between 80.8 and 86 mmHg throughout all phases of the study. Although limited data is available for comparison, the increased SVI and CI and lower MAP measured in anesthetized out-of-water dolphins compared to non-anesthetized in-water dolphins could be attributable to differences in measurement techniques or anesthesia-associated modulation of normal circulation (i.e., post-intubation hypotension, hyperoxia, baroreceptor reflex, differences in mechanical ventilation strategies, and/or anesthetic agents). While these measurements were acquired under general anesthesia and out-of-water conditions, it is difficult to discern the effects of removal from a neutrally buoyant condition from the effects of anesthesia on the measured cardiovascular indices. The comparably lower arterial blood pressure readings in the current study, however, may elucidate an anesthesia-associated hypotensive state of current protocols when compared to anesthetized dolphins from earlier studies, where MAP ranged between 115 and 142 mmHg (37, 57).
While this is the first controlled study comparing to two distinct modes of ventilation in dolphins, several study limitations may have influenced measured and calculated variables, including lack of age and sex matching in the study population, measurement errors, and large gaps in the pharmacokinetic and pharmacodynamic understanding of the anesthetic drugs used. Additionally, interpretation of the cardiovascular variables subjected to statistical analysis may have been confounded by the use of ephedrine boluses and dobutamine and phenylephrine constant rate infusions to maintain the study’s target MAP range of 65–80 mmHg.
5 Conclusion
General anesthesia of this completely aquatic mammal is constrained by several logistical and physiologic complexities, briefly described herein. This study aimed to evaluate one such physiologic complexity, the ventilation-induced cardiopulmonary responses of dolphins, using two distinct ventilation strategies, while attempting to control for variables that would inherently confound interpretation of these responses (i.e., targeted MAP range, pain-associated cardiovascular modulation). While both modes of ventilation provided adequate ventilation-perfusion matching and tissue oxygen delivery at 100% inspired oxygen, AAV resulted in a lower alveolar to arterial oxygen gradient, higher dynamic respiratory system compliance index, significantly higher mean airway pressures, and less alveolar dead space compared to CMV. Additionally, AAV resulted in significantly decreased indices of some parameters of cardiovascular performance (MAP and DiasAP) when implemented as the first mode of ventilation. However, regardless of order of implementation, there were no significant differences in cardiovascular variables between the modes.
Clinical application of mechanical ventilation in dolphins should, therefore, prioritize individual patient needs when possible. Additional complexities of dolphin anesthesia and physiology to consider and mitigate include modulation of core body temperature, patient padding to reduce myopathic and neuropathic morbidities, physiologic effects of removal from neutral buoyancy, and logistics of anesthetic emergence and surgical wound healing. Future studies should, therefore, assess the effects of the out-of-water condition on dolphin circulation and pulmonary function, to include establishment of normal in-water mean arterial pressures, in order to better inform veterinarians on physiologically appropriate anesthetic approaches.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Naval Information Warfare Center Pacific, Institutional Animal Care and Use Committee. The study was conducted in accordance with institutional requirements (No. 134-2019) and the U.S. Navy Bureau of Medicine (NRD-1198).
Author contributions
CL-B: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. JD: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Visualization, Writing – review & editing. DH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. LT: Formal analysis, Methodology, Validation, Writing – review & editing. SR: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. JB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Office of Naval Research, contract number N68335-16-C-0020 (Anesthesia Ventilator for Atlantic Bottlenose Dolphins and California Sea Lions), awarded to JB. Additional support was provided to CL-B through Naval Information Warfare Center Pacific, P-NISE-AR-23-230205;76725.
Acknowledgments
The authors thank the efforts of the veterinary, training, and facilities staff of the U.S. Navy Marine Mammal Program. The veterinarians and veterinary technicians of the National Marine Mammal Foundation and Army Veterinary Corps participated and contributed, in some way great or small, to every anesthetic procedure performed on the Navy marine mammals included in this dataset. The cognizant biotechnicians responsible for the daily care and welfare of Navy marine mammals provided logistical support for this project. Brittany Novick, Celeste Parry, Mark Baird, Veronica Cendejas, and Erin Brodie of the National Marine Mammal Foundation and MAJ Kyle Ross of the Army Veterinary Corps were instrumental in the technical and logistical efforts of executing this study.
In memoriam
The authors would like to acknowledge a co-author, SR, a remarkable scientist and the original dolphin anesthesiologist who, unfortunately, passed away in 2022 prior to completion of this manuscript. Thank you for entrusting us with this work. We dedicate this manuscript in your honor.
Conflict of interest
JB is the owner of Innovative Veterinary Medicine, Inc. (IVM). IVM manufactured the DolVent System through funding provided by the Office of Naval Research, contract number N68335-16-C-0020. JD was an employee of IVM during research execution and is the inventor of both airway pressure release ventilation (APRV; U.S. patent 4773411) and apneustic anesthesia ventilation (AAV; U.S. patent 6123072).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Townsend, CH. The porpoise in captivity. Zool Sci Contrib N Y Zool Soc. (1914) 1:289–99. doi: 10.5962/p.203801
2. Bailey, JE. Cetacean Anesthesia: A review of 34 general Anesthesia events, lessons learned, and future plans International Association of Aquatic Animal Medicine (2021).
3. Jones, BL, McClain, AM, Sportelli, JJ, and Le-Bert, CR. Return of sound production as a biomarker of bottlenose dolphin emergence from Anesthesia. Animals. (2023) 13:2531. doi: 10.3390/ani13152531
4. Russell, J, Jeffery, N, Bailey, J, Osborn, S, Le-Bert, C, Whitehead, H, et al. Cerebrospinal fluid sampling in a bottlenose dolphin (Tursiops Truncatus) under general Anesthesia. J Zoo Wildl Med. (2021) 51:148. doi: 10.1638/2019-0148
5. Lee, C, Jensen, ED, Meegan, J, Ivancic, M, Bailey, J, Hendrickson, D, et al. Surgical Management of a Chronic Neck Abscess in a U.S. navy bottlenose dolphin. Mil Med. (2019) 184:e360–4. doi: 10.1093/milmed/usy316
6. Schmitt, TL, Knych, HK, Mama, KR, Nollens, HH, Croft, L, DiRocco, S, et al. Plasma concentrations and disposition of propofol in bottlenose dolphins (Tursiops Truncatus) during general Anesthesia International Association of Aquatic Animal Medicine (2018).
7. Walsh, MT, Reidarson, T, McBain, J, Dalton, L, Gearhart, S, Chittick, E, et al. Sedation and Anesthesia techniques in cetaceans American Association of Zoo Veterinarians (2006).
8. Ridgway, S, Houser, D, Finneran, J, Carder, D, Keogh, M, Van Bonn, W, et al. Functional imaging of dolphin brain metabolism and blood flow. J Exp Biol. (2006) 209:2902–10. doi: 10.1242/jeb.02348
9. Rosenberg, JF, Haulena, M, Bailey, JE, Hendrickson, DA, Ivancic, M, and Raverty, SA. Emergency Anesthesia and exploratory laparotomy in a compromised Pacific white-sided dolphin (Lagenorhynchus Obliquidens). J Zoo Wildl Med. (2017) 48:581–5. doi: 10.1638/2016-228R1.1
10. Ponganis, PJ, McDonald, BI, Tift, MS, Gonzalez, SC, DaValle, B, Gliniecki, RA, et al. Effects of inhalational Anesthesia on blood gases and Ph in California Sea lions. Mar Mamm Sci. (2017) 33:726–37. doi: 10.1111/mms.12388
11. McCormick, JG, and Ridgway, SH. History of the development of Anesthesia for the dolphin: a quest to study a brain as large as Man's. Anesthesiology. (2018) 129:11–21. doi: 10.1097/ALN.0000000000002213
12. Higgins, JL, and Hendrickson, DA. Surgical procedures in pinniped and cetacean species. J Zoo Wildl Med. (2013) 44:817–36. doi: 10.1638/2012-0286R1.1
13. Piscitelli, MA, Raverty, SA, Lillie, MA, and Shadwick, RE. A review of cetacean lung morphology and mechanics. J Morphol. (2013) 274:1425–40. doi: 10.1002/jmor.20192
14. Williams, T, Friedl, W, and Haun, J. The physiology of bottlenose dolphins (Tursiops Truncatus): heart rate, metabolic rate and plasma lactate concentration during exercise. J Exp Biol. (1993) 179:31–46. doi: 10.1242/jeb.179.1.31
15. Fahlman, A. Cardiorespiratory adaptations in small cetaceans and marine mammals. Exp Physiol. (2023). Epub 20231115) 109:324–34. doi: 10.1113/EP091095
16. Meagher, EM, McLellan, WA, Westgate, AJ, Wells, RS, Frierson, J, and Pabst, DA. The relationship between heat flow and vasculature in the dorsal fin of wild bottlenose dolphins Tursiops Truncatus. J Exp Biol. (2002) 205:3475–86. doi: 10.1242/jeb.205.22.3475
17. Rowlands, CE, McLellan, WA, Rommel, SA, Costidis, AM, Yopak, KE, Koopman, HN, et al. Comparative morphology of the spinal cord and associated vasculature in shallow versus deep diving cetaceans. J Morphol. (2021) 282:1415–31. Epub 20210714. doi: 10.1002/jmor.21395
18. Bonato, M, Bagnoli, P, Centelleghe, C, Maric, M, Brocca, G, Mazzariol, S, et al. Dynamics of blood circulation during diving in the bottlenose dolphin (Tursiops Truncatus): the role of the retia mirabilia. J Exp Biol. (2019) 222:jeb198457. doi: 10.1242/jeb.198457
19. Fahlman, A, Moore, MJ, and Garcia-Parraga, D. Respiratory function and mechanics in pinnipeds and cetaceans. J Exp Biol. (2017) 220:1761–73. doi: 10.1242/jeb.126870
20. Kooyman, GL, Castellini, MA, and Davis, RW. Physiology of diving in marine mammals. Annu Rev Physiol. (1981) 43:343–56. doi: 10.1146/annurev.ph.43.030181.002015
21. Berry, SH. Injectable Anesthetics In: KA Grimm, LA Lamont, WJ Tranquilli, SA Greene, and SA Robertson, editors. Veterinary Anesthesia and analgesia: The fifth edition of Lumb and Jones. Ames, IA: John Wiley & Sons, Inc. (2015). 277–97.
22. Steffey, EP, Mama, KR, and Brosnan, RJ. Inhalation Anesthestics In: KA Grimm, LA Lamont, WJ Tranquilli, SA Greene, and SA Robertson, editors. Veterinary Anesthesia and analgesia: The fifth edition of Lumb and Jones. Ames, IA: John Wiley & Sons, Inc. (2015). 297–331.
23. Klein, L. Anesthetic complications in the horse. Vet Clin North Am Equine Pract. (1990) 6:665–92. doi: 10.1016/s0749-0739(17)30537-0
24. Duke, T, Filzek, U, Read, MR, Read, EK, and Ferguson, JG. Clinical observations surrounding an increased incidence of Postanesthetic myopathy in halothane-anesthetized horses. Vet Anaesth Analg. (2006) 33:122–7. doi: 10.1111/j.1467-2995.2005.00189.x
25. Haulena, M, and Schmitt, T. Anesthesia In: FMD Gulland, LA Dierauf, and KL Whitman, editors. CRC handbook of marine mammal medicine. 3rd ed. Boca Raton, FL: CRC Press (2018). 567–606.
26. Le-Bert, CR, Ridgway, S, and Bailey, J. Apneustic Anesthesia ventilation: A modified approach for marine mammal ventilatory support International Association of Aquatic Animal Medicine (2021).
27. Meegan, JM, Miller, R, Ross, K, Gildea, T, McClain, A, Bailey, J, et al. Multidisciplinary Management of Complex Airway Stenosis in a bottlenose dolphin (Tursiops Truncatus) International Association of Aquatic Animal Medicine (2021).
28. Colitz, CMH, Bailey, J, and Mejia-Fava, J. Cetacean and pinniped ophthalmology In: FMD Gulland, LA Dierauf, and KL Whitman, editors. Crc handbook of marine mammal medicine. 3rd ed. Boca Raton, FL: CRC Press (2018). 517–36.
29. Dold, C, and Ridgway, S. Cetaceans In: G West, D Heard, and N Caulkett, editors. Zoo animal and wildlife immobilization and Anesthesia. 2nd ed: John Wiley & Sons, Inc (2014). 679–91. doi: 10.1002/9781118792919.ch49
30. Le-Bert, C, Bukoski, A, Downs, J, Hodgson, D, Euliano, N, Blanch, P, et al., Comparison of cardiopulmonary effects of controlled mechanical ventilation and apneustic Anesthesia ventilation in bottlenose dolphins (Tursiops Truncatus) and California Sea lions (Zalophus Californianus). In: American Association of Zoo Veterinarians Conferenece; (2020). Veterinary Information Network.
31. Bukoski, A, Hodgson, D, Downs, J, LeBert, C, Thombs, L, and Bailey, J. An implementation of apneustic Anesthesia ventilation in the horse: comparison with conventional mechanical ventilation. Vet Anaesth Analg. (2022) 49:372–81. doi: 10.1016/j.vaa.2022.04.002
32. McCormick, JG. Relationship of sleep, respiration, and Anesthesia in the porpoise: a preliminary report. Proc Natl Acad Sci USA. (1969) 62:697–703. doi: 10.1073/pnas.62.3.697
33. Fahlman, A, Miedler, S, Marti-Bonmati, L, Ferrero Fernandez, D, Muñoz Caballero, P, Arenarez, J, et al. Cardiorespiratory coupling in cetaceans; a physiological strategy to improve gas exchange? J Exp Biol. (2020) 223:jeb226365. doi: 10.1242/jeb.226365
34. Harms, CA, Jensen, ED, Townsend, FI, Hansen, LJ, Schwacke, LH, and Rowles, TK. Electrocardiograms of bottlenose dolphins (Tursiops Truncatus) out of water: habituated collection versus wild Postcapture animals. J Zoo Wildl Med. (2013) 44:972–81. doi: 10.1638/2013-0093.1
35. Cauture, F, Sterba-Boatwright, B, Rocho-Levine, J, Harms, C, Miedler, S, and Fahlman, A. Using respiratory sinus arrhythmia to estimate inspired tidal volume in the bottlenose dolphin (Tursiops Truncatus). Front Physiol. (2019) 10:128. doi: 10.3389/fphys.2019.00128
36. Hayano, J, Yasuma, F, Okada, A, Mukai, S, and Fujinami, T. Respiratory sinus arrhythmia: a phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation. (1996) 94:842–7. doi: 10.1161/01.CIR.94.4.842
37. Ridgway, SH, and McCormick, JG. Anesthesia of the porpoise In: Textbook of veterinary Anesthesia. ed. L. R. SOMA. Baltimore, MD: The Williams and Wilkins Co. (1971). 394–403.
38. Garcia Parraga, D, Moore, M, and Fahlman, A. Pulmonary ventilation-perfusion mismatch: a novel hypothesis for how diving vertebrates may avoid the bends. Proc Biol Sci. (2018) 285:482. doi: 10.1098/rspb.2018.0482
39. Hubbell, JAE, Aarnes, TK, Bednarski, RM, Lerche, P, and Muir, WW. Effect of 50% and maximal inspired oxygen concentrations on respiratory variable in isoflurane-anesthetized horses. BMC Vet Res. (2011) 7:23. doi: 10.1186/1746-6148-7-23
40. Schauvliege, S, Savvas, I, and Gasthuys, F. The effect of the inspired oxygen fraction on arterial blood oxygenation in spontaneously breathing, isoflurane anaesthetized horses: a retrospective study. Vet Anaesth Analg. (2015) 42:280–5. doi: 10.1111/vaa.12208
41. Hol, L, Nijbroek, S, and Schultz, MJ. Perioperative lung protection: clinical implications. Anesth Analg. (2020) 131:1721–9. doi: 10.1213/ANE.0000000000005187
42. Downs, JB. Method and apparatus for breathing during Anesthesia. United States of America: U.S. Patent No. 6,123,072 (1998).
43. Bratzke, E, Downs, JB, and Smith, RA. Intermittent CPAP: a new mode of ventilation during general Anesthesia. Anesthesiology. (1998) 89:334–40. doi: 10.1097/00000542-199808000-00008
44. Portier, K, and Ida, KK. The Asa physical status classification: what is the evidence for recommending its use in veterinary Anesthesia?-a systematic review. Front Vet Sci. (2018) 5:204. doi: 10.3389/fvets.2018.00204
45. Venn-Watson, S, Jensen, ED, and Ridgway, SH. Effects of age and sex on clinicopathologic reference ranges in a healthy managed Atlantic bottlenose dolphin population. J Am Vet Med Assoc. (2007) 231:596–601. doi: 10.2460/javma.231.4.596
46. Smith, CR, Solano, M, Lutmerding, BA, Johnson, SP, Meegan, JM, Le-Bert, CR, et al. Pulmonary ultrasound findings in a bottlenose dolphin Tursiops Truncatus population. Dis Aquat Org. (2012) 101:243–55. doi: 10.3354/dao02537
47. Seitz, KE, Smith, CR, Marks, SL, Venn-Watson, SK, and Ivancic, M. Liver Ultrasonagraphy in dolphins: use of Ultrasonagraphy to establish a technique for hepatobiliary imaging and to evaluate metabolic disease-associated liver change in bottlenose dolphins (Tursiops Truncatus). J Zoo Wildl Med. (2016) 47:1034–43. doi: 10.1638/2015-0173.1
48. Fiorucci, L, Garcia-Parraga, D, Macrelli, R, Grande, F, Flanagan, C, Rueca, F, et al. Determination of the Main reference values in ultrasound examination of the gastrointestinal tract in clinically healthy bottlenose dolphins (Tursiops Truncatus). Aquat Mamm. (2015) 41:284–94. doi: 10.1578/am.41.3.2015.284
49. Rommel, S, Pabst, D, and McLellan, W. Functional anatomy of the cetacean reproductive system, with comparisons to the domestic dog In: Reproductive biology and phylogeny of Cetacea. Whales, Porpoises and Dolphins. 1st ed. Boca Raton: CRC Press. (2007). 127–45.
50. Mason, DJ, O’Grady, M, Woods, JP, and McDonell, W. Assessment of lithium dilution cardiac output as a technique for measurement of cardiac output in dogs. Am J Vet Res. (2001) 62:1255–61. doi: 10.2460/ajvr.2001.62.1255
51. Noren, SR, Williams, TM, Ramirez, K, Boehm, J, Glenn, M, and Cornell, L. Changes in partial pressures of respiratory gases during submerged voluntary breath hold across odontocetes: is body mass important? J Comp Physiol B. (2012) 182:299–309. doi: 10.1007/s00360-011-0612-0
52. Linton, RA, Young, LE, Marlin, DJ, Blissitt, KJ, Brearley, JC, Jonas, MM, et al. Cardiac output measured by lithium dilution, thermodilution, and transesophageal doppler echocardiography in anesthetized horses. Am J Vet Res. (2000) 61:731–7. doi: 10.2460/ajvr.2000.61.731
53. Araos, JD, Larenza, MP, Boston, RC, De Monte, V, De Marzo, C, Grasso, S, et al. Use of the oxygen content–based index, Fshunt, as an indicator of pulmonary venous admixture at various inspired oxygen fractions in anesthetized sheep. Am J Vet Res. (2012) 73:2013–20. doi: 10.2460/ajvr.73.12.2013
54. Bigeleisen, PE. Models of venous admixture. Adv Physiol Educ. (2001) 25:159–66. doi: 10.1152/advances.2001.25.3.159
55. Robertson, HT. Dead space: the physiology of wasted ventilation. Eur Respir J. (2015) 45:1704–16. Eur Respir J (2015) 46(4):1226. doi: 10.1183/09031936.50137614
56. Wagner, AE, Bednarski, RM, and Muir, WW 3rd. Hemodynamic effects of carbon dioxide during intermittent positive-pressure ventilation in horses. Am J Vet Res. (1990) 51:1922–9. doi: 10.2460/ajvr.1990.51.12.1922
57. Sommer, L, McFarland, W, Galliano, R, Nagel, E, and Morgane, P. Hemodynamic and coronary angiographic studies in the bottlenose dolphin (Tursiops Truncatus). Am J Phys. (1968) 215:1498–505. doi: 10.1152/ajplegacy.1968.215.6.1498
58. Nagel, EL, Morgane, PJ, and McFarland, WL. Anesthesia for the bottlenose dolphin, Tursiops Truncatus. Science. (1964) 146:1591–3. doi: 10.1126/science.146.3651.1591
59. Nagel, EL, Morgane, PJ, and McFarland, WL. Anesthesia for the bottlenose dolphin. Vet Med Small Anim Clin. (1966) 61:229–33.
60. Miedler, S, Fahlman, A, Valls Torres, M, Alvaro Alvarez, T, and Garcia-Parraga, D. Evaluating cardiac physiology through echocardiography in bottlenose dolphins: using stroke volume and cardiac output to estimate systolic left ventricular function during rest and following exercise. J Exp Biol. (2015) 218:3604–10. doi: 10.1242/jeb.131532
Keywords: bottlenose dolphin, mechanical ventilation, apneustic anesthesia ventilation, pulmonary physiology, anesthesia, physical status classification
Citation: Le-Bert CR, Bukoski A, Downs J, Hodgson DS, Thombs L, Ridgway SH and Bailey J (2024) Apneustic anesthesia ventilation improves pulmonary function in anesthetized bottlenose dolphins (Tursiops truncatus). Front. Vet. Sci. 11:1287478. doi: 10.3389/fvets.2024.1287478
Edited by:
Pamela Murison, University of Glasgow, United KingdomReviewed by:
Ismael Hernández Avalos, National Autonomous University of Mexico, MexicoAndreas Fahlman, Fundación Oceanogràfic de la Comunitat Valenciana, Spain
Copyright © 2024 Le-Bert, Bukoski, Downs, Hodgson, Thombs, Ridgway and Bailey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolina R. Le-Bert, Y2Fyb2xpbmEuci5sZS1iZXJ0LmNpdkB1cy5uYXZ5Lm1pbA==
†Deceased
 Carolina R. Le-Bert
Carolina R. Le-Bert Alex Bukoski
Alex Bukoski John Downs
John Downs David S. Hodgson
David S. Hodgson Lori Thombs6
Lori Thombs6 Sam H. Ridgway
Sam H. Ridgway James Bailey
James Bailey