
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 22 December 2023
Sec. Animal Nutrition and Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1301542
In this study, the effects of quercetin and daidzein on egg quality, lipid metabolism, and cecal short-chain fatty acids (SCFAs) were compared in layers. Hyline brown layers at 385 days of age with a similar laying rate (81.36% ± 0.62%) and body weight (2.10 kg ± 0.04 kg) were randomly divided into three treatments, six replicates per treatment, and 20 layers per replicate. Layers in control, quercetin, and daidzein treatment were fed by a basal diet supplemented with 0 mg/kg, 500 mg/kg quercetin, and 30 mg/kg of daidzein for 10 weeks. Results showed that eggshell strength and albumen height in week 4, egg yolk diameter in week 10, and eggshell thickness and egg yolk height in weeks 4 and 10 were significantly increased in the quercetin treatment (P ≤ 0.05); contents of phospholipid (PL) and lecithin (LEC) in egg yolk and high-density lipoprotein (HDL) content in serum were significantly increased; however, contents of malondialdehyde (MDA), total cholesterol (TC), and triglyceride (TG) in egg yolk, contents of TC, TG, low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) in serum, and contents of TC and TG in the liver were significantly decreased in the quercetin treatment (P ≤ 0.05); contents of isobutyric acid and valeric acid were significantly increased in the cecum of the quercetin treatment (P ≤ 0.05), compared with control. Moreover, egg yolk height in week 10 and eggshell thickness in weeks 4 and 10 were significantly increased in the daidzein treatment (P ≤ 0.05); contents of MDA, TC, and TG in egg yolk, TC, TG, and VLDL in serum, and TC and TG in liver were significantly decreased in the daidzein treatment (P ≤ 0.05); and HDL content was significantly increased in serum of the daidzein treatment (P ≤ 0.05) compared with control. However, daidzein did not affect SCFA content in the cecum. In conclusion, egg quality was improved by quercetin and daidzein by increasing the antioxidant ability of egg yolk and by regulating lipid metabolism in layers. Quercetin worked better than daidzein in improving egg quality under this experimental condition.
Eggs have a high nutritive value and are one of the important food sources for humans (1, 2). Human demand for eggs has been increasing as the world's population increases. Highly intensive farming meets the demand of consumers; however, long-term and high-intensive laying may affect the egg quality, decrease the nutritive value of eggs, increase the number of broken eggs, and cause economic loss. Therefore, it is crucial to improve egg quality (3). For the past decades, the application of different safe feed additives has worked very well for reducing cholesterol deposition in egg yolk, improving eggshell thickness and egg quality, including vitamins and minerals (4), probiotics (5), amino acids (6), and flavonoids (7). Recently, flavonoids caught much attention as functional additives to improve product quality and enhance the economic benefit of animal husbandry.
Quercetin, a flavonoid rich in apples, onions, sea buckthorn, and hawthorn, may exhibit anti-inflammatory, anti-viral, and anti-oxidative effects (8, 9). Moreover, quercetin may regulate lipid metabolism and reduce hepatic lipid deposition in mice fed with a high-fat diet (10). Our previous studies found that 0.04% quercetin significantly decreased the contents of TC and TG and increased the contents of PL and LEC in egg yolk of laying hens, and 0.02% of quercetin increased eggshell thickness, thus demonstrating the positive effect of quercetin on egg quality (11–13). Daidzein is an isoflavone found in alfalfa, red clover, white clover, soybean, and other legumes (14). For the past few years, daidzein has been used in stock farming, because it acts like estrogen, increasing animal fertility and improving the quality of animal products. Dietary supplementation with 200 mg/kg of daidzein increased reproductive performance, antioxidant ability, and serum hormones of sows (15, 16). Dietary supplementation with daidzein enhanced production performance and serum antioxidant levels in cattle; moreover, daidzein also improved the meat and milk quality in cattle (17). A diet supplemented with daidzein improved follicle development and increased egg weight, eggshell strength and thickness, and calcium content in the eggshell of layers (18, 19).
Egg quality may be improved by quercetin and daidzein in layers. However, our previous study found that the price of daidzein limited its uses, and quercetin increased production performance and economic returns better than daidzein in laying hens (20). Meanwhile, few studies compare the effects of quercetin and daidzein on the egg quality of layers. Therefore, this study further investigated the effects of a diet supplemented with separate quercetin and daidzein on egg quality, lipid metabolism, and cecal short-chain fatty acids in aged layers based on the above-mentioned research (20). This will provide a theoretical foundation for using quercetin to improve the egg quality of aged layers.
All procedures used in this study were approved by the Animal Care and Use Committee of the Northeast Agricultural University (NEAUEC20200203). Housing, management, and care of the birds conformed to the guidelines of Agricultural Animal in Agricultural Research and Teaching of Heilongjiang Province (HEI Animal Management Certificate No. 11928).
After 1 week of adaptation, Hyline Brown layers at 385 days of age with a similar laying rate (81.36% ± 0.62%) and body weight (2.10 kg ± 0.04 kg) were randomly divided into three treatments, six replicates per treatment, 20 layers each replicate. A single-factor experimental design was used in this study; layers in control, quercetin, and daidzein treatment were fed by a basal diet supplemented with 0 mg/kg, 500 mg/kg quercetin, and 30 mg/kg daidzein for 10 weeks. The basal diet was prepared by referring to the GB/T 5916-2020 Chinese Layers Feeding Standards (Table 1). Quercetin (CAS: 6151-25-3, purity ≥97%) was purchased from Nanjing Dulai Biotechnology Co. Ltd. (Nanjing, China), and daidzein (CAS: 468-66-8, purity ≥98%) was bought from Meryer (Shanghai) Chemical Technology Co. Ltd. (Shanghai, China). All layers were raised in triple wiry cages (526 × 423 × 381 mm; two birds in each cage) with 16-h natural light and artificial light per day in the experimental site of Northeast Agricultural University (Harbin, China) and were maintained with optimal ventilation during the experimental period. Layers had access to water and feed ad libitum during the 10-week experimental period.
At the end of weeks 4 and 10 of the experiment, 30 eggs/treatment were randomly collected to determine egg quality. The egg weight (g), albumen height (mm), egg yolk color, and Haugh unit were determined using an egg multi-tester (EMT-5200, Robotmation, Japan). The eggshell strength (N) was determined using eggshell strength meter (FHK-700 IIDP, Japan). The egg yolk height (mm) and diameter (mm) were determined using vernier calipers. The eggshell thickness was measured by the average of the sharp end, middle section, and blunt end of the eggshell using micrometer calipers.
At the end of the experiment, 30 eggs/treatment were randomly broken, and egg yolks were separated and stored at −80°C. The blood samples were collected from the jugular vein of six layers selected randomly and euthanized by cervical dislocation in each treatment (one layer per replicate) (21), and then centrifuged at 3,000 r/min for 15 min to obtain the serum and stored at −80°C. After blood collection, the layers were dissected, and the liver and fresh cecal digesta were taken, quickly frozen in liquid nitrogen and stored at −80°C for the following analysis.
The contents of malondialdehyde (MDA), total cholesterol (TC), triglyceride (TG), phospholipid (PL), lecithin (LEC) in egg yolk; the contents of TC, TG, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and very-low-density lipoprotein (VLDL) in serum; and the contents of TC and TG in the liver were determined using enzyme-linked immunosorbent assay (ELISA) following the kit instructions purchased from Jiangsu Baolai Biotechnology Co. Ltd. (Jiangsu, China).
Appropriate samples were put in 2-ml centrifuge tubes; 50 μl of 15% phosphoric acid, 100 μl of 125 μg/ml isocaproic acid, and 40 μl of ether were added in sequence for 1 min and then centrifuged at 12,000 r/min for 10 min at 4°C. The supernatant was taken for further analysis. The content of cecal SCFAs was determined by gas chromatography (GC) using Agilent HP-INNOWAX column (30 m × 0.25 mm × 0.25 μm) in Wuhan GeneCreate Biological Engineering Co. Ltd. (Wuhan, China). The GC conditions were as follows: injector temperature of 250°C; ion source temperature of 300°C; and transmission temperature of 250°C. The temperature program was as follows: initial temperature was set at 90°C, increasing 10°C/min to 120°C; increasing 5°C/min to 150°C; increasing 25°C/min to 250°C; and 250°C for 2 min. Carrier gas, He; flow rate, 1.0 ml/min.
All the results in this experiment were expressed as the means ± SEM. The data processing proceeded using SPSS 26.0 software, and variance analysis and the difference between treatments were performed using one-way ANOVA and Duncan's method, respectively. P ≤ 0.05 was considered a significant difference. The GraphPad Prism 9.5 software was used to draw the histogram.
Eggshell thickness and egg yolk height in weeks 4 and 10, eggshell strength and albumen height in week 4, and egg yolk diameter in week 10 were significantly increased in the quercetin treatment (P ≤ 0.05); eggshell thickness in weeks 4 and 10 and egg yolk height in week 10 were significantly increased in the daidzein treatment (P ≤ 0.05), compared with control. Egg yolk height and eggshell thickness in week 4 were significantly increased in the quercetin treatment (P ≤ 0.05; Table 2), compared with the daidzein treatment.
The contents of MDA, TC, and TG in the egg yolk of the quercetin and daidzein treatments were significantly decreased (P ≤ 0.05), the contents of PL and LEC in the egg yolk of the quercetin treatment were significantly increased (P ≤ 0.05), compared with control. The contents of TC, TG, and PL, LEC in egg yolk of the quercetin treatment were significantly decreased and increased, respectively, compared with the daidzein treatment (P ≤ 0.05; Table 3, Figure 1).
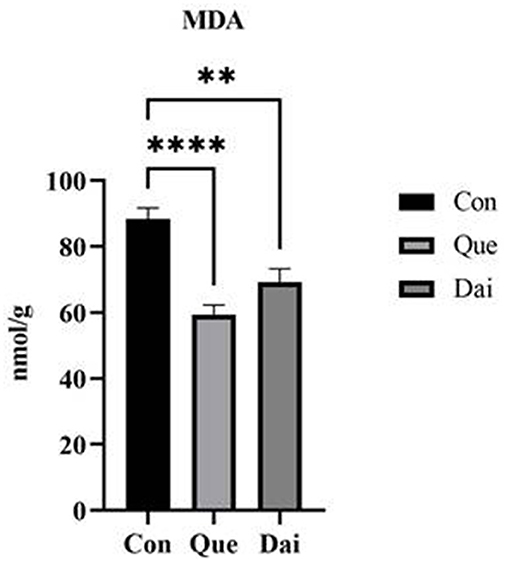
Figure 1. Effects of quercetin and daidzein on MDA content in egg yolk of layers. The results are expressed as mean ± SEM (n = 6). ** and **** indicate extremely significant difference (P < 0.01). MDA, malondialdehyde.
The contents of TC and TG in the serum and liver of the quercetin and daidzein treatments were significantly decreased (P ≤ 0.05); the VLDL content was significantly decreased and HDL content was significantly increased in the serum of the quercetin and daidzein treatments (P ≤ 0.05), and LDL content was significantly decreased in the serum of the quercetin treatment (P ≤ 0.05), compared with the control. Contents of TG, VLDL, and LDL were significantly decreased in the serum of the quercetin treatment (P ≤ 0.05), and TC content was significantly decreased in the serum and liver of the quercetin treatment, compared with the daidzein treatment (P ≤ 0.05; Table 4, Figure 2).
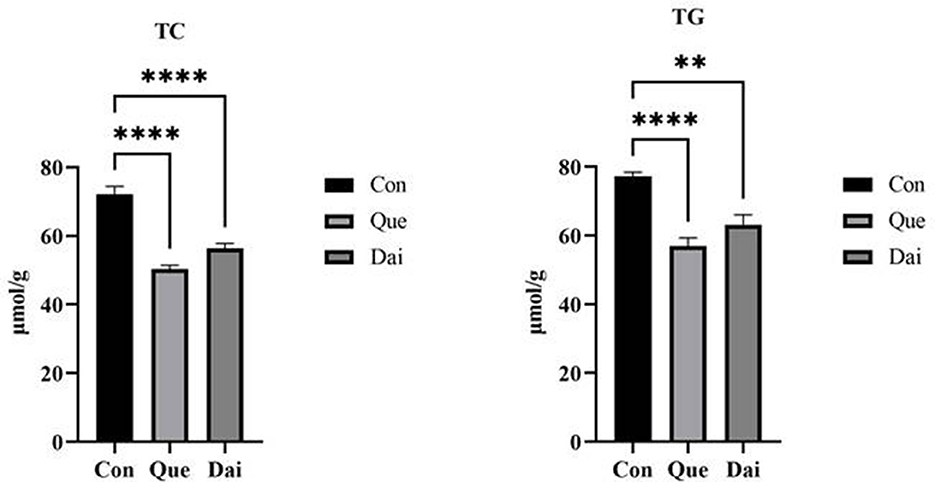
Figure 2. Effects of quercetin and daidzein on lipid content in liver of layers. The results are expressed as mean ± SEM (n = 6). ** and **** indicate extremely significant difference (P < 0.01). TC, total cholesterol; TG, triglyceride.
The contents of isobutyric acid and valeric acid in the cecum of the quercetin treatment were significantly increased (P ≤ 0.05); however, the content of short-chain fatty acids was not affected by daidzein (P > 0.05), compared with the control. The content of valeric acid was significantly increased in the cecum of the quercetin treatment (P ≤ 0.05), compared with the daidzein treatment (Table 5).
Egg quality including eggshell quality and nutrient content in eggs decreased with age in laying hens after the peak laying period (22). Egg quality includes intrinsic quality (egg yolk height, egg yolk diameter, albumen height, egg yolk color, Haugh unit, etc.) and extrinsic quality (eggshell strength, egg weight, eggshell thickness, etc.), which affects the commercial and edible value of eggs. Eggshell strength and thickness are important indicators of eggshell quality. However, the eggshell quality decreases rapidly with age, which results in huge economic losses (23). Albumen height and Haugh unit are the main indicators of albumen quality, the higher the albumen height and the larger the Haugh unit, the better the albumen quality and the fresher the eggs (24, 25). The egg yolk color is related to the pigment component in feed, and the deep color of the egg yolk means the health of the eggs (26). The egg yolk index refers to the ratio of egg yolk height to egg yolk diameter. The larger the egg yolk diameter, the smaller the egg yolk height, and the more fragile the egg yolk.
In the present study, quercetin increased eggshell strength, eggshell thickness, egg yolk height, and albumen height, and daidzein increased eggshell thickness and egg yolk height. Other studies have also shown that flavonoids may improve the egg quality of birds. A previous study on Lohmann Silver laying hens showed that a diet supplemented with 12 g/kg of Mulberry leaf flavonoids significantly increased eggshell strength and egg yolk color (27). Dietary supplementation with 400 mg/kg of quercetin increased the eggshell thickness, egg yolk height, albumen height, and Haugh unit in Tianfu laying hens during the late laying period (28). Our previous studies showed that dietary supplementation with 0.04% quercetin increased eggshell strength, eggshell thickness, and Haugh unit in Hessian laying hens at 39 weeks old (12). Dietary supplementation with 10 mg/kg of daidzein improved eggshell quality by regulating the shell gland genes (29). The eggshell thickness and eggshell strength tend to increase in laying hens fed by a diet supplemented with daidzein (10, 50, and 100 mg/kg) during the late laying period (30, 31). Dietary supplementation with daidzein also increased egg quality in quail during the late laying period (32). These results further confirmed the positive effect of flavonoids on egg quality in laying hens. Additionally, our results indicated that the effect of quercetin on egg quality was better than daidzein in layers.
Lipid peroxidation is a process in which biofilms are attacked by reactive oxygen species, and oxidation of polyunsaturated fatty acids produces harmful lipid peroxides including aldehydes, ketones, and acids (33). Lipid peroxidation reduces the quality and nutritive value of eggs and destroys vitamins, essential amino acids, and other nutrients in egg yolk, resulting in undesirable changes during storage and consumption (34). MDA is one of the end products in the process of lipid peroxidation; therefore, the MDA content may reflect the degree of lipid peroxidation in egg yolk (35). Our study found both quercetin and daidzein significantly decreased MDA content and improved the oxidation stability in egg yolk layers. It indicated that quercetin and daidzein inhibited the production of MDA in egg yolk and reduced the degree of lipid peroxidation, thus increasing albumen height and maintaining the freshness and nutritive value of eggs. It was consistent with other research studies, which reported that flavonoids may prevent the formation of hydroxyl radicals and lipid peroxidation, thus reducing MDA content. A diet supplemented with 1 and 3 g/kg of hesperidin (a bio-flavonoid) significantly reduced the MDA content in egg yolk and improved the antioxidant properties of fresh and stored eggs (36). Dietary supplementation with different doses of quercetin (200, 400, and 800 mg/kg) significantly decreased the MDA content in egg yolk with increasing amounts of quercetin; meanwhile, quercetin also improved the oxidative stability of eggs stored for 28 days at room temperature; and the research findings indicated that quercetin may maintain freshness and extend shelf life of eggs (37, 38). Furthermore, improving the serum and liver antioxidant ability of laying hens in our previous study also supported the reduction of egg yolk MDA content in this study (20).
There are almost all lipids in egg yolk (39). The content of phospholipids accounts for approximately 30% of lipids of egg yolk, and phospholipids are the basic components of biofilms (40), mainly including lecithin, cephalin, and phosphoinositide. Lecithin, known as the “third nutrient,” is a polyunsaturated phosphatidylcholine, which is a structural component of biofilms and may promote brain development and improve memory (41). Egg yolk also contains large amounts of cholesterol. Cholesterol is needed by humans for producing cell membranes and manufacturing the bile acids, vitamin D, and sex hormones; however, excessive intake of cholesterol may induce a series of cardiovascular diseases, including hyperlipidemia, hypertension, and heart disease, which damages health (42). Therefore, it is necessary to reduce cholesterol content in egg yolk. Our study found that both quercetin and daidzein decreased the contents of TC and TG, and quercetin also increased the PL and LEC content in egg yolk. These results indicated that flavonoids regulated lipid metabolism, enhanced lipolysis, and reduced cholesterol deposition in egg yolk, thus inhibiting oxidation of lecithin and improving egg nutritive value. Other researchers have found the same results in flavonoids. A diet supplemented with 0.4%−1.2% of Mulberry leaf flavonoids reduced the contents of TC and TG in egg yolk (27). TC content in egg yolk was decreased by dietary supplementation with quercetin and hesperidin (0.5 g/kg) in laying hens at 28 weeks old (43). Our previous studies also found that 0.04% of quercetin significantly decreased the contents of TC and TG and increased the contents of PL and LEC in the egg yolk of laying hens (11–13). Dietary supplementation with 10, 20, and 40 mg/kg of daidzein decreased TC content in egg yolk with increasing dietary daidzein in Hisex laying hens (44).
Poor egg quality resulting from lipid metabolism disorders reduced economic returns in laying hens. The liver synthesizes more than 90% of the cholesterol, and it is the main organ for cholesterol synthesis and transports cholesterol rapidly to blood in laying hens (45). Most of the serum cholesterol is transferred to the egg yolk by lipoproteins. LDL may transport cholesterol to tissues around the liver and cholesterol and triglyceride from the serum to the liver for re-circulation or to form bile acids excreted by the body. Lipids are not synthesized in the ovaries of laying hens. VLDL is the main carrier of cholesterol in the serum, takes charge of transferring cholesterol from the serum to the ovary, and then is absorbed by the oocyte to form egg yolk, thus synthesizing 95% of the cholesterol in egg yolk (46, 47). Therefore, the contents of TG, TC, HDL, LDL, and VLDL in the serum were used to estimate whether lipid metabolism is normal in laying hens. In the present study, both quercetin and daidzein decreased the contents of TC and TG in the serum and liver, decreased the contents of LDL and VLDL in the serum, and increased the HDL content in the serum, which suggested that quercetin and daidzein improved lipid metabolism of layers. Studies have shown that dietary supplementation with flavonoids may regulate lipid deposition in the liver, enhance immunity, and improve egg quality of layers (48). Flavonoids may form insoluble complexes with cholesterol in digesta, thereby inhibiting the intestinal absorption of endogenous and exogenous cholesterol. A diet supplemented with 0.5% fermented Ginkgo biloba leaves (flavonoids are the main active components) decreased the contents of TC, TG, and LDL-C and increased the HDL-C content in the serum of 49-week-old laying hens, thus reducing cholesterol content in eggs and improving the egg quality (7). Our previous studies found that 0.04 and 0.06% quercetin decreased the contents of TC, TG, and LDL in the serum, regulated cholesterol metabolism, and reduced abdominal fat percentage in broilers (49). Moreover, the contents of the serum and liver TC and TG tend to decrease with dietary supplementation with 10 mg/kg of daidzein in rats (50). A diet supplemented with 0.05% daidzein decreased the contents of TC, TG, and LDL-C in the serum and improved the meat quality of feeder cattle (51). These results indicated that flavonoids inhibited the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, whereby cholesterol content was reduced in the serum and liver, thus decreasing cholesterol deposition in egg yolk (52).
The cecum provides a relatively stable environment and has the largest and most complex microbial community in the intestines of poultry. SCFAs are the end product of microbial fermentation in the cecum, protect intestinal health, and serve as a source of energy for the host (5). A healthy intestinal environment may improve intestinal absorption of Ca; dietary Ca is absorbed by the intestines and deposited on the eggshell gland to form eggshells in laying hens (53). Furthermore, SCFAs regulate the balance among fatty acid synthesis, fatty acid oxidation, and lipolysis in the body (54). Almost all SCFAs are rapidly absorbed by enterocytes and transported to the liver and systemic circulation through the portal vein, affecting lipid metabolism in tissues (55). Butyrate regulated lipid metabolism in the liver and acetate reduced cholesterol levels in the serum of humans (56). Propionic acid may reduce cholesterol synthesis in human Caco-2/TC-7 enterocyte cells by inhibiting HMG-CoA reductase (57). In the present study, quercetin significantly increased the contents of isobutyric acid and valeric acid in the cecum, daidzein did not affect the content of cecal SCFAs. Dietary supplementation with 200 mg/kg of baicalin (a kind of flavonoid) reduced the accumulation of epididymal and perirenal fat in mice, produced SCFAs that are good for the intestines, and improved abnormal lipid metabolism (58). Dietary supplementation with genistein increased the content of intestinal SCFAs in mice, which play a key role in maintaining intestinal epithelial barrier homeostasis (59). A diet supplemented with 0.4 mg/kg of quercetin increased the content of SCFAs in the cecum and improved the intestinal health of laying hens challenged by lipopolysaccharide (LPS) (60). Quercetin increased the content of intestinal SCFAs and protected against antibiotic-induced intestinal dysregulation in mice (61). Moreover, the relative abundance of Lactobacillus was positively correlated with SCFA content in the cecum of birds (62), this result is consistent with a previous study in the laboratory that the relative abundance of cecal Lactobacillus in the quercetin group was higher than that in the daidzein group of laying hens (20). Therefore, we speculated that quercetin regulated lipid metabolism and protected intestinal health, thus increasing intestinal absorption of Ca and improving eggshell quality in layers, and that daidzein was not as effective as quercetin in improving egg quality, which possibly resulted from unaltered SCFA content in the cecum.
In conclusion, quercetin and daidzein improved egg quality by decreasing MDA content and cholesterol deposition and regulating lipid metabolism in egg yolk of layers. Quercetin worked better than daidzein in improving egg quality under this experimental condition.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
All procedures used in this study were approved by the Animal Care and Use Committee of the Northeast Agricultural University (NEAUEC20200203). Housing, management and care of the birds confirmed to the guidelines of Agricultural Animal in Agricultural Research and Teaching of Heilongjiang Province (HEI Animal Management Certificate No. 11928). The study was conducted in accordance with the local legislation and institutional requirements.
JiL: Writing—original draft, Data curation, Formal analysis, Software. JuL: Data curation, Writing—review & editing. SZ: Data curation, Writing—review & editing. YF: Writing—review & editing, Data curation. QY: Writing—review & editing, Data curation. YL: Writing—review & editing, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by National Natural Science Foundation of China (32072749).
The authors thank the National Natural Science Foundation of China for funding this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nimalaratne C, Wu J. Hen egg as an antioxidant food commodity: a review. Nutrients. (2015) 7:8274–93. doi: 10.3390/nu7105394
2. Romero C, Arija I, Viveros A, Chamorro S. Productive performance, egg quality and yolk lipid oxidation in laying hens fed diets including grape pomace or grape extract. Animals. (2022) 12:1076. doi: 10.3390/ani12091076
3. Sehirli E, Arslan K. An application for the classification of egg quality and haugh unit based on characteristic egg features using machine learning models. Expert Syst Appl. (2022) 205:117692. doi: 10.1016/j.eswa.2022.117692
4. Alagawany M, Elnesr SS, Farag MR, Tiwari R, Yatoo MI, Karthik K, et al. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health - a comprehensive review. Vet Quart. (2020) 41:1–29. doi: 10.1080/01652176.2020.1857887
5. Khan S, Moore RJ, Stanley D, Chousalkar KK. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl Environ Microbiol. (2020) 86:00600–20. doi: 10.1128/AEM.00600-20
6. Khattak F, Helmbrecht A. Effect of different levels of tryptophan on productive performance, egg quality, blood biochemistry, and caecal microbiota of hens housed in enriched colony cages under commercial stocking density. Poult Sci. (2019) 98:2094–104. doi: 10.3382/ps/pey562
7. Zhao L, Zhang X, Cao F, Sun D, Wang T, Wang G. Effect of dietary supplementation with fermented Ginkgo-leaves on performance, egg quality, lipid metabolism and egg-yolk fatty acids composition in laying hens. Livest Sci. (2013) 155:77–85. doi: 10.1016/j.livsci.2013.03.024
8. Azeem M, Hanif M, Mahmood K, Ameer N, Chughtai FRS, Abid U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: a review. Polym Bull. (2022) 80:241–62. doi: 10.1007/s00289-022-04091-8
9. Nguyen TLA, Bhattacharya D. Antimicrobial activity of quercetin: an approach to its mechanistic principle. Molecules. (2022) 27:2494. doi: 10.3390/molecules27082494
10. Hoek-van den Hil EF, van Schothorst EM, van der Stelt I, Swarts HJ, van Vliet M, Amolo T, et al. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. (2015) 10:23. doi: 10.1007/s12263-015-0469-z
11. Hu LL. Effects of Quercetin on Performance, Egg Quality, Cholesterol Content in Laying Hens. Harbin: Northeast Agricultural University. (2014) (in Chinese).
12. Liu Y, Li Y, Liu HN, Suo YL, Hu LL, Feng XA, et al. Effect of quercetin on performance and egg quality during the late laying period of hens. Br Poult Sci. (2013) 54:510–4. doi: 10.1080/00071668.2013.799758
13. Zhang L. Effects of Quercetin on Performance and Lipids Metabolism in Laying Hens. Harbin: Northeast Agricultural University (2013) (in Chinese).
14. Krizova L, Dadakova K, Kasparovska J, Kasparovsky T. Isoflavones. Molecules. (2019) 24:1076. doi: 10.3390/molecules24061076
15. Li Y, He G, Chen D, Yu B, Yu J, Zheng P, et al. Supplementing daidzein in diets improves the reproductive performance, endocrine hormones and antioxidant capacity of multiparous sows. Anim Nutr. (2021) 7:1052–60. doi: 10.1016/j.aninu.2021.09.002
16. Chen J, Huang Z, Cao X, Zou T, You J, Guan W. Plant-derived polyphenols in sow nutrition: an update. Anim Nutr. (2023) 12:96–107. doi: 10.1016/j.aninu.2022.08.015
17. Orzuna-Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Chay-Canul AJ, Miranda-Romero LA, Mendoza-Martínez GD. Meta-analysis of flavonoids use into beef and dairy cattle diet: performance, antioxidant status, ruminal fermentation, meat quality, and milk composition. Front Vet Sci. (2023) 10:1134925. doi: 10.3389/fvets.2023.1134925
18. Etxeberria U, Fernandez-Quintela A, Milagro FI, Aguirre L, Alfredo Martinez J, Portillo MP. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J Agric Food Chem. (2013) 61:9517–33. doi: 10.1021/jf402506c
19. Liu HY, Zhang CQ. Effects of daidzein on messenger ribonucleic acid expression of gonadotropin receptors in chicken ovarian follicles. Poult Sci. (2008) 87:541–5. doi: 10.3382/ps.2007-00274
20. Liu JY, Fu YX, Zhou SS, Zhao PY, Zhao J, Yang QL, et al. Comparison of the effect of quercetin and daidzein on production performance, anti-oxidation, hormones, and cecal microflora in laying hens during the late laying period. Poult Sci. (2023) 102:102674. doi: 10.1016/j.psj.2023.102674
21. Guo Y, Zhao ZH, Pan ZY, An LL, Balasubramanian B, Liu WC. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult Sci. (2020) 99:2100–7. doi: 10.1016/j.psj.2019.12.032
22. Bain MM, Nys Y, Dunn IC. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br Poult Sci. (2016) 57:330–8. doi: 10.1080/00071668.2016.1161727
23. Wistedt A, Ridderstrale Y, Wall H, Holm L. Effects of phytoestrogen supplementation in the feed on the shell gland of laying hens at the end of the laying period. Anim Reprod Sci. (2012) 133:205–13. doi: 10.1016/j.anireprosci.2012.06.020
24. Cao Y, Xun M, Ren S, Wang J. Effects of dietary organic acids and probiotics on laying performance, egg quality, serum antioxidants and expressions of reproductive genes of laying ducks in the late phase of production. Poult Sci. (2022) 101:102189. doi: 10.1016/j.psj.2022.102189
25. Wang XC, Wang XH, Wang J, Wang H, Zhang HJ, Wu SG, et al. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of Hy-Line Brown hens during the late laying period. J Anim Sci. (2018) 96:225–35. doi: 10.1093/jas/skx007
26. Zhang Q, Zhang K, Wang J, Bai S, Zeng Q, Peng H, et al. Effects of coated sodium butyrate on performance, egg quality, nutrient digestibility, and intestinal health of laying hens. Poult Sci. (2022) 101:102020. doi: 10.1016/j.psj.2022.102020
27. Zhang B, Wang Z, Huang C, Wang D, Chang D, Shi X, et al. Positive effects of Mulberry leaf extract on egg quality, lipid metabolism, serum biochemistry, and antioxidant indices of laying hens. Front Vet Sci. (2022) 9:1005643. doi: 10.3389/fvets.2022.1005643
28. Amevor FK, Cui Z, Ning Z, Du X, Jin N, Shu G, et al. Synergistic effects of quercetin and vitamin E on egg production, egg quality, and immunity in aging breeder hens. Poult Sci. (2021) 100:101481. doi: 10.1016/j.psj.2021.101481
29. Ni YD, Zhu Q, Zhou ZL, Grossmann R, Chen J, Zhao RQ. Effect of dietary daidzein on egg production, shell quality, and gene expression of ER-α, GH-R, and IGF-IR in shell glands of laying hens. J Agric Food Chem. (2007) 55:6997–7001. doi: 10.1021/jf071085r
30. Cai J, Gu H, Shi SR, Tong HB. Effects of high-dose daidzein on laying performance, egg quality and antioxidation in laying hens. J Poult Sci. (2013) 50:237–41. doi: 10.2141/jpsa.0120118
31. Gu H, Shi SR, Chang LL, Tong HB, Wang ZY, Zou JM. Safety evaluation of daidzein in laying hens: part II. Effects on calcium-related metabolism. Food Chem Toxicol. (2013) 55:689–92. doi: 10.1016/j.fct.2012.12.064
32. Sahin N, Onderci M, Balci TA, Cikim G, Sahin K, Kucuk O. The effect of soy isoflavones on egg quality and bone mineralisation during the late laying period of quail. Br Poult Sci. (2007) 48:363–9. doi: 10.1080/00071660701341971
33. Ahmad H, Tian J, Wang J, Khan MA, Wang Y, Zhang L, et al. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J Agric Food Chem. (2012) 60:7111–20. doi: 10.1021/jf3017207
34. Casagrande AC, Machado GC, Brunetto AL, Galli GM, Rosa GD, Araujo DN, et al. The addition of green propolis to laying hens had positive effects on egg quality: lower bacteria counts in the shell and lipid peroxidation in the yolk. An Acad Bras Cienc. (2021) 93:e20210315. doi: 10.1590/0001-3765202120210315
35. Muhammad AI, Mohamed DAA, Chwen LT, Akit H, Samsudin AA. Effect of sodium selenite, selenium yeast, and bacterial enriched protein on chicken egg yolk color, antioxidant profiles, and oxidative stability. Foods. (2021) 10:871. doi: 10.3390/foods10040871
36. Goliomytis M, Orfanou H, Petrou E, Charismiadou MA, Simitzis PE, Deligeorgis SG. Effect of hesperidin dietary supplementation on hen performance, egg quality and yolk oxidative stability. Br Poult Sci. (2014) 55:98–104. doi: 10.1080/00071668.2013.870328
37. Amevor FK, Cui Z, Du X, Ning Z, Deng X, Xu D, et al. Synergy between dietary quercetin and vitamin E supplementation in aged hen's diet improves hatching traits, embryo quality, and antioxidant capacity of chicks hatched from eggs subjected to prolonged storage. Front Physiol. (2022) 13:873551. doi: 10.3389/fphys.2022.873551
38. Simitzis P, Spanou D, Glastra N, Goliomytis M. Impact of dietary quercetin on laying hen performance, egg quality and yolk oxidative stability. Anim Feed Sci Tech. (2018) 239:27–32. doi: 10.1016/j.anifeedsci.2018.03.004
39. Wilson PB. Recent advances in avian egg science: a review. Poult Sci. (2017) 96:3747–54. doi: 10.3382/ps/pex187
40. Zhao YY, Xiong Y, Curtis JM. Measurement of phospholipids by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry: the determination of choline containing compounds in foods. J Chromatogr A. (2011) 1218:5470–9. doi: 10.1016/j.chroma.2011.06.025
41. Herron KL, Fernandez ML. Are the current dietary guidelines regarding egg consumption appropriate? J Nutr. (2004) 134:187–90. doi: 10.1093/jn/134.1.187
42. Kovacs-Nolan J, Phillips M, Mine Y. Advances in the value of eggs and egg components for human health. J Agric Food Chem. (2005) 53:8421–31. doi: 10.1021/jf050964f
43. Iskender H, Yenice G, Dokumacioglu E, Kaynar O, Hayirli A, Kaya A. Comparison of the effects of dietary supplementation of flavonoids on laying hen performance, egg quality and egg nutrient profile. Br Poult Sci. (2017) 58:550–6. doi: 10.1080/00071668.2017.1349297
44. Yun J, Qi G, Zhang P, Huo Q. Effect of dietary daidzein on egg cholesterol content and its antioxidant property in layers. Sci Agric Sinica. (2004) 37:756–61 (in Chinese).
45. Li P, Gao M, Fu J, Yan S, Liu Y, Mahmood T, et al. Dietary soya saponin improves the lipid metabolism and intestinal health of laying hens. Poult Sci. (2022) 101:101663. doi: 10.1016/j.psj.2021.101663
46. Ding XM, Mu YD, Zhang KY, Wang JP, Bai SP, Zeng QF, et al. Vitamin E improves antioxidant status but not lipid metabolism in laying hens fed a aged corn-containing diet. Anim Biosci. (2021) 34:276–84. doi: 10.5713/ajas.19.0934
47. Zhang J, Geng X, Zhang Y, Zhao X, Zhang P, Sun G, et al. Interaction between cecal metabolites and liver lipid metabolism pathways during induced molting in laying hens. Front Physiol. (2022) 13:862721. doi: 10.3389/fphys.2022.862721
48. Wei Y, Liu Y, Li G, Guo Y, Zhang B. Effects of quercetin and genistein on egg quality, lipid profiles, and immunity in laying hens. J Sci Food Agric. (2023). doi: 10.1002/jsfa.12910
49. Wang M, Wang B, Wang S, Lu H, Wu H, Ding M, et al. Effect of quercetin on lipids metabolism through modulating the gut microbial and AMPK/PPAR signaling pathway in broilers. Front Cell Dev Biol. (2021) 9:616219. doi: 10.3389/fcell.2021.616219
50. Bhattarai K, Adhikari S, Fujitani M, Kishida T. Dietary daidzein, but not genistein, has a hypocholesterolemic effect in non-ovariectomized and ovariectomized female Sprague-Dawley rats on a cholesterol-free diet. Biosci Biotech Bioch. (2017) 81:1805–13. doi: 10.1080/09168451.2017.1350562
51. Zhao XH, Yang ZQ, Bao LB, Wang CY, Zhou S, Gong JM, et al. Daidzein enhances intramuscular fat deposition and improves meat quality in finishing steers. Exp Biol Med. (2015) 240:1152–7. doi: 10.1177/1535370214564755
52. Rasouli E, Jahanian R. Comparative effects of genistein and antibiotics on performance, meat oxidative stability, jejunal morphology, and ileal microbial community in broiler chicks. Anim Feed Sci Tech. (2019) 256:114153. doi: 10.1016/j.anifeedsci.2019.03.005
53. Lv ZP, Yan SJ Li G, Liu D, Guo YM. Genistein improves the reproductive performance and bone status of breeder hens during the late egg-laying period. Poult Sci. (2019) 98:7022–9. doi: 10.3382/ps/pez367
54. Unno T, Hisada T, Takahashi S. Hesperetin modifies the composition of fecal microbiota and increases cecal levels of short-chain fatty acids in rats. J Agric Food Chem. (2015) 63:7952–7. doi: 10.1021/acs.jafc.5b02649
55. Coppola S, Avagliano C, Calignano A, Canani RB. The protective role of butyrate against obesity and obesity-related diseases. Molecules. (2021) 26:682. doi: 10.3390/molecules26030682
56. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
57. Alvaro A, Sola R, Rosales R, Ribalta J, Anguera A, Masana L, et al. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short-chain fatty acids. IUBMB Life. (2008) 60:757–64. doi: 10.1002/iub.110
58. Ju M, Liu Y, Li M, Cheng M, Zhang Y, Deng G, et al. Baicalin improves intestinal microecology and abnormal metabolism induced by high-fat diet. Eur J Pharmacol. (2019) 857:172457. doi: 10.1016/j.ejphar.2019.172457
59. Hou Q, Huang J, Zhao L, Pan X, Liao C, Jiang Q, et al. Dietary genistein increases microbiota-derived short chain fatty acid levels, modulates homeostasis of the aging gut, and extends healthspan and lifespan. Pharmacol Res. (2023) 188:106676. doi: 10.1016/j.phrs.2023.106676
60. Feng J, Li Z, Ma H, Yue Y, Hao K, Li J, Xiang Y, Min Y. Quercetin alleviates intestinal inflammation and improves intestinal functions via modulating gut microbiota composition in LPS-challenged laying hens. Poult. Sci. (2023) 102:102433. doi: 10.1016/j.psj.2022.102433
61. Shi T, Bian X, Yao Z, Wang Y, Gao W, Guo C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. (2020) 11:8003–13. doi: 10.1039/D0FO01439G
Keywords: quercetin, daidzein, layer, egg quality, nutritive value
Citation: Liu J, Liu J, Zhou S, Fu Y, Yang Q and Li Y (2023) Effects of quercetin and daidzein on egg quality, lipid metabolism, and cecal short-chain fatty acids in layers. Front. Vet. Sci. 10:1301542. doi: 10.3389/fvets.2023.1301542
Received: 25 September 2023; Accepted: 27 November 2023;
Published: 22 December 2023.
Edited by:
Arda Yildirim, Gaziosmanpaşa University, TürkiyeReviewed by:
Birendra Mishra, University of Hawaii at Manoa, United StatesCopyright © 2023 Liu, Liu, Zhou, Fu, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Li, bGl5YW9sendAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.