
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 21 November 2023
Sec. Veterinary Epidemiology and Economics
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1301316
 Yun-Yan Luo1†
Yun-Yan Luo1† Dan Yu1†
Dan Yu1† Hong-Ze Zhang1
Hong-Ze Zhang1 Zheng-Xiang Liu2
Zheng-Xiang Liu2 Ru-Dan Hong1
Ru-Dan Hong1 Mei Hong2
Mei Hong2 Zhi-Qiong Ai1
Zhi-Qiong Ai1 Jun-Jie Zhu1
Jun-Jie Zhu1 Jia-Xiang Yin1*
Jia-Xiang Yin1*Background: Small mammals serve as the main reservoir for Bartonella and as a proxy indicator of the potential risk of Bartonella transmission from nature to humans. They offer a valuable early warning for human infection. Nevertheless, geographical variations in the impact of the host on the occurrence of Bartonella infection are underestimated. This study was designed to investigate the infection characteristics of Bartonella and explore its species diversity in wild small mammals in western Yunnan Province, China.
Methods: Wild small mammals were captured from Yulong, Jianchuan, and Lianghe counties in western Yunnan Province between 2015 and 2016. Real-time quantitative PCR (qPCR) was used to detect Bartonella infection, and the Bartonella species were identified by phylogenetic analysis. The factors associated with Bartonella infection in small mammals were analyzed by the Chi-square Test.
Results: The prevalence of Bartonella in small mammals was 47.85% (768/1605). Lianghe County had the highest Bartonella infection rate, with 56.27% of the samples tested positive, followed by a rate of 50.91% was tested in Yulong County, and 39.97% in Jianchuan County (p < 0.001). Bartonella was detected positive in a total 25 small mammal species, with infection rates ranging from 2.17% to 100%. Niviventer fulvescens had the highest Bartonella infection rate. In comparison with the dominant small mammal species, Eothenomys mileyus had the lowest Bartonella infection rate than that in Apodemus chevrieri, Rattus tanezumi, and Apodemus draco (p < 0.001). Male small mammals had a higher infection rate than females (p < 0.05). The prevalence of Bartonella in small mammals during the summer season was higher compared to the other three seasons (p < 0.001). Woodland landscape had the highest Bartonella infection rate (p < 0.001). Bartonella rochalimae, B. japonica, B. tribocorum, B. washoensis, B. sylvatica, and B. rattimassiliensis were obtained from infected small mammals.
Conclusion: This study showed a high prevalence of Bartonella was detected with various Bartonella species in small mammals in Yulong, Jianchuan, and Lianghe counties of western Yunnan Province. These findings hold significant scientific clues, providing valuable reference points for further research of Bartonella natural foci in Yunnan or other analogues environments.
Bartonella is the causative agent of Bartonellosis, a facultative intracellular, fastidious, gram-negative bacterium, typically transmitted by parasites that bite, such as fleas, mites, and ticks (1). Humans and vertebrate animals (small mammals, dogs, pets, etc.) are the main reservoir hosts. Currently, there are 39 Bartonella species and three subspecies found in different small mammals, horses, cattle, deer, sheep, birds, canids, felids, marine mammals, and reptiles (source: https://lpsn.dsmz.de/genus/bartonella) (2). Previous studies have reported Bartonella positive rates in small mammals of 18% to 78% in European (1, 3–7), 20% to 52% in Asia (8–13), and 9.35% in Senegal (Africa) (14). Additionally, one study showed a 50% infection rate in marsupials in Brazil (15). These findings imply that Bartonella infection in small mammals might exhibit high adaptability across different geographic region.
Small mammals, as one of the most abundant species in nature, can harboring various pathogens or viruses. Rodent-borne diseases can easily be transmitted to humans when they come into contact with infected small mammals in nature. Therefore, small mammals can act as effective sentinels of natural disease transmission to humans. Especially rodents, as the main hosts of Bartonella, can carry up to 20 Bartonella species, but the infection rate is varied by region. Of the 20 Bartonella species, Bartonella henselae, B. tribocorum, B. rochalimae, B. elizabethae, B. grahamii, B. washoensis, B. tamiae, and B. vinsonii subsp. arupensis have been responsible for hazarding human health (5, 9, 16). Moreover, the increasing proximity of human activities to natural environments in recent years has led to greater human exposure to small mammals and vectors, indirectly elevating the risk of Bartonella infections among people from the natural environment. Thus, it is imperative to pay sustained attention to detecting Bartonella in small mammals in various habitats, which can provide effective scientific clues for the etiology and epidemiological research of Bartonella. There have been long-lasting reports of Bartonella detection in small mammals in Yunnan Province. In western Yunnan Province, the prevalence of Bartonella was 5% in small mammals reported by one study in Yulong county in the past decades (17). In Jianchuan county, Bartonella was isolated from blood in small mammals in 2017 with a 55.91% positive rate (18). Yang et al. previously detected Bartonella in small mammals in Dehong Dai and Jingpo Autonomous Prefecture (Lianghe county was one of the sample sites) with a 24.86% positive rate. However, there was no prevalence of Bartonella in Lianghe county (19). Still, it is insufficiently characterized for host-pathogens association of Bartonella in western Yunnan Province (18–21), even though there has an ideal environment for small mammals and vectors to survive. This study aimed to investigate the prevalence of Bartonella in small mammals collected from Yulong, Jianchuan, and Lianghe counties. Real-time quantitative PCR (qPCR) was used to detect Bartonella in small mammals, and phylogenetic analysis was performed to identify the diversity of Bartonella species. Additionally, the study examined the relationship between associated factors and Bartonella infection in small mammals. This effort is essential to gain a more profound insight into the complex interactions between Bartonella, its host species, and the environment, which in turn can inform strategies for disease prevention and management.
The study was carried out in three counties in western Yunnan Province. Yulong and Jianchuan counties are located in the middle of the Hengduan mountain but are separated by low-lying valleys. Yulong county has a low-latitude highland South Asian monsoon climate. Jianchuan county has a low-latitude highland monsoon climate and is concentrated growing pine forests. Lianghe county is located in the southwestern of the Hengduan Mountain and is characterized by the south subtropical monsoon climate. The three counties could be a potential Bartonella natural reservoir because the diverse climates and rich vegetation growths benefit small mammals and ectoparasites to survive and reproduce, contributing to unneglected concerns for the residents and travelers.
The study was carried out in four seasons, including winter (which started from December in 2015), spring (Mar in 2016), summer (July to August in 2016), and autumn (October in 2016).
Small mammals were captured by dead traps (15 × 8 cm). The species were identified by key morphology characteristics of small mammals, spleen or liver tissue of them have been collected under the aseptic condition and stored at −40°C. The type of landscapes was recorded in the fieldwork. In each county, landscapes where small mammals were most likely to be captured were selected based on the annual small mammals monitoring data, including bush, cultivation, and woodland. The details of small mammals captured and tissue collected were described in previous publication (22). The sampling sites of capturing small mammals in the three counties are shown in Figure 1.
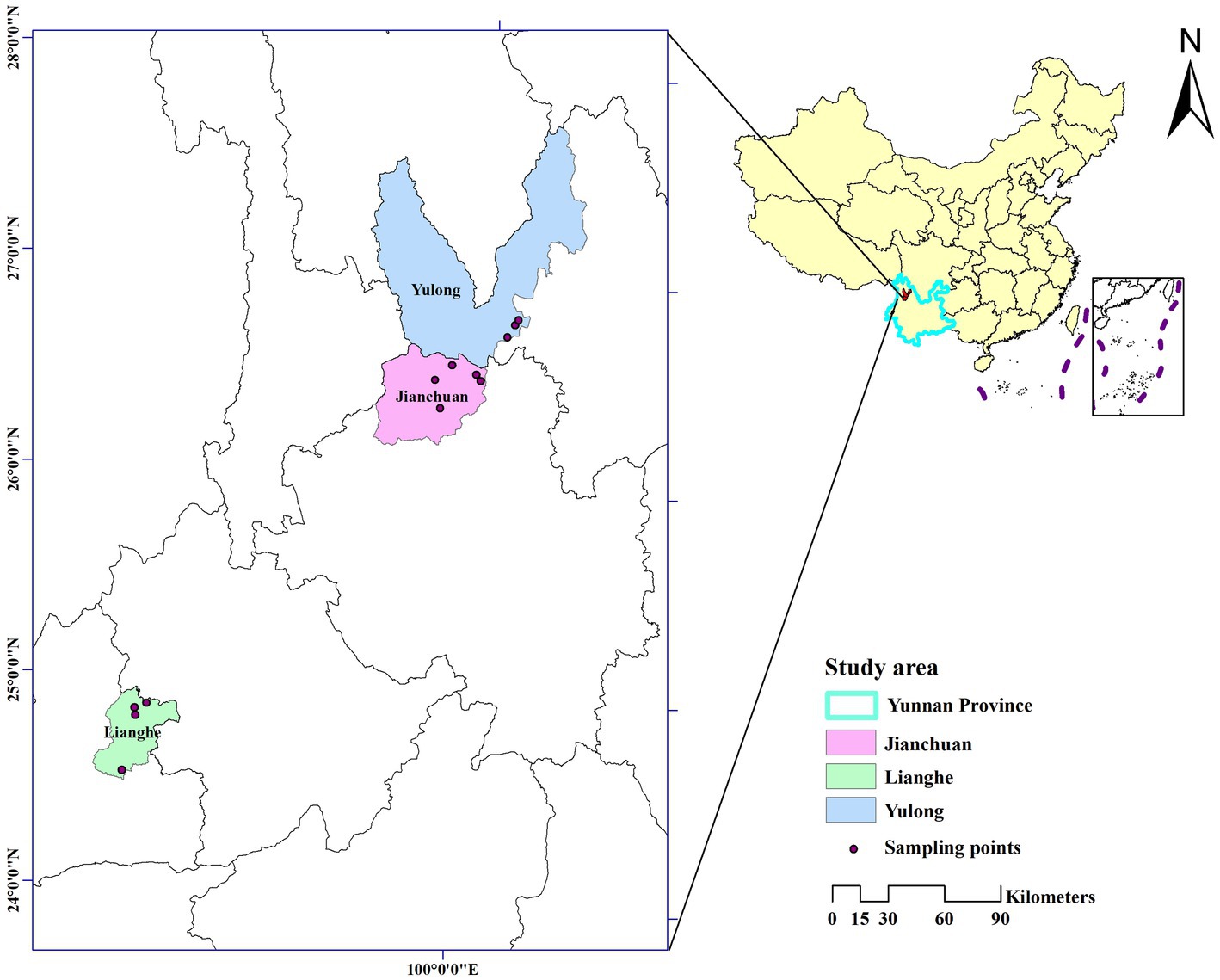
Figure 1. Sampling sites of capturing wild small mammals in the three counties of western Yunnan Province, China. The map was created by ArcGIS 10.2 to show the geographic area of Jianchuan, Yulong, and Lianghe counties in western Yunnan Province.
Deoxyribonucleic acid (DNA) from the spleen and liver of small mammals was extracted by the BioTeke Whole blood genomic DNA Kit (AU19014-16, BioTeke Corporation, Beijing, China), according to the manufacturer’s instruction and then stored at −40°C until subsequent molecular laboratory.
The concentration and purity of the extracted DNA were initially assessed. When the extract concentration was ≥50 μg/mL and A260/A280 was between 1.8 to 2.1, it was selected as the detection template. Real-time Quantitative Polymerase Chain Reaction (qPCR) was performed to amplify the ssrA gene sequence according to previous study (16). One set of primers with 10 μM (AuGCT Biotech, Beijing, China) (F: 5’-GCTATGGTAATAAATGGACAATGAAATAA-3′; R: 5’-GCTTCTGTTGCCAGGTG-3′) and a probe (P: FAM-ACCCCG CTTAAACCTGCGACG-BHQ1) were used under below conditions.
The reaction mixture (20 μL) contains the following components: 0.8 μL each of outer primers F and R, 0.4 μL of the probe, 10 μL of HR qPCR Master Mix (Huirui Biotechnology Co., Ltd., Shanghai, China), and 3 μL of the template. The qPCR condition consisted of pre-denaturation at 94°C for 5 min, denaturation at 94°C for 15 s, and annealing at 60°C for 45 s, followed by 40 cycles. The plasmid standards were used to draw the standard curve. The amplified products were determined according to the standard curve and limit of detection (Cq = 35), if the Cq <35 was positive.
The sequencing of positive samples was generated by conventional PCR according to the above reaction conditions. The amplified products were collected and electrophoresed in 1.5% agarose gel containing Gelview (BioTeke Corporation, Beijing, China) and visualized under the Gel imaging system (G: BOX F3, Syngene, American). The amplified products (269 bp) were confirmed as Bartonella positive. Following 10%–15% of the positive samples from each county, which included positive samples from different areas and landscapes whenever possible, were randomly selected to sequence in both directions (Sangon Biotech, Shanghai, China).
Sequences of successfully detected were edited and trimmed by DNASTAR (7.1 version). Reference complete or partial sequences encoding ssrA of Bartonella were retrieved from GenBank by the Blast program of the National Center for Biotechnology Information.1 Sample sequences were aligned with reference sequences using Sequence distance in Meglign of DNASTAR. Phylogenetic analysis was performed with the Clustal W protocol (default parameters) by Mega software (7.0 version). Phylogenetic trees were constructed by the Neighbor-joining method after 1,000 bootstrapped replicates.
Demographic, geographic, and laboratory parameters were recorded in Epidata. Wild small mammals were classified as dominant species (>10%) and other species (≤ 10%) according to the constituent ratio. The Bartonella infection rate in wild small mammals was calculated by wild small mammal samples infected with Bartonella over the number of wild small mammal DNA successfully extracted.
The number of positive were summarized and counted across different species, genus, area, and season using the “tidyverse” package in R software (4.0.2 version). The Bartonella infection in different genera, species, county, and season were compared using Chi-square Test. p values less than 0.05 were considered statistical significance.
The study has been approved the Medical Ethics Committee of Dali University (no. MECDU-201507-21). The small mammals captured were allowed and no painful.
A total of 1,605 wild small mammal specimens were taken across the three counties (648 in Jianchuan county, 550 in Yulong county, and 407 in Lianghe county), covering 30 different species. There are 22 species of Rodentia, 7 species of Insectivora, and one species of Scandentia. In total, Apodemus chevrieri was the dominant species, which accounted for 29.35% (471/1605), followed by Eothenomys miletus was 19.00% (305/1605) and Apodemus draco was 9.78% (157/1605). In Yulong county, the dominant species were A. chevrieri (33.64%), E. miletus (16.55%), and A. draco (16.00%). In Jianchuan county, the dominant species were A. chevrieri (44.14%), E. miletus (31.94%), and A. draco (10.65%). In Lianghe county, Rattus tanezumi (24.57%) was the dominant species, followed by Rattus rattus (19.90%), Mus pahari (10.81%), and Niviventer fulvescens (10.07%). The distribution of wild small mammals across the three counties is shown in Table 1.
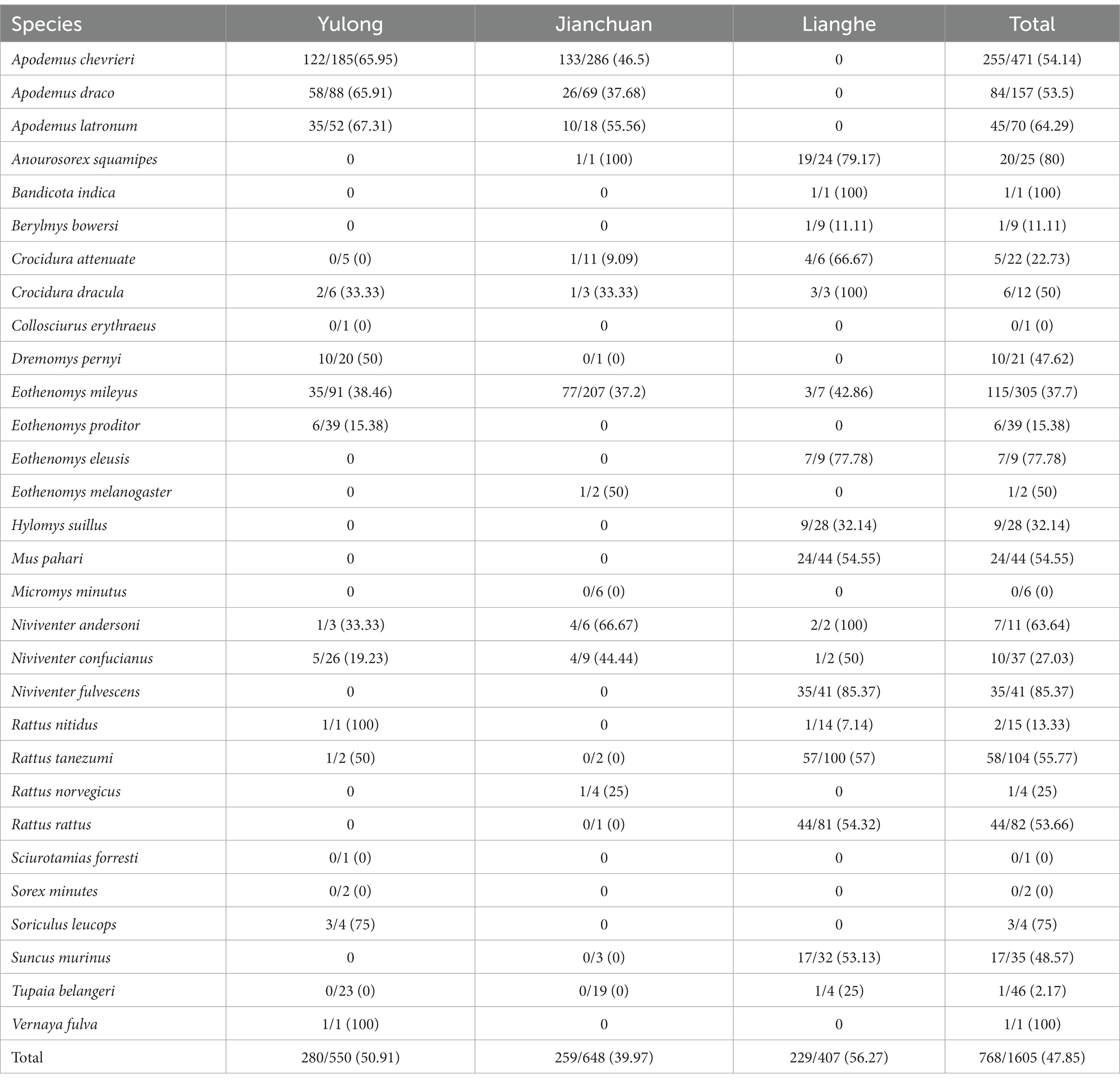
Table 1. Prevalence of Bartonella in wild small mammals captured from the three counties of western Yunnan Province [positive/n (%)].
Twenty-five various small mammal species were shown positive for Bartonella detection. The overall prevalence of Bartonella in wild small mammals was 47.85% (768/1605). The result of partial samples of qPCR and agarose gel electrophoresis are shown in Supplementary Figures S1, S2. The Bartonella was detected varied from 2.17 to 100% by different species. The prevalence of Bartonella was highest in N. fulvescens (85.37%, 35/41) and was lowest in Tupaia belangeri (2.17%, 1/46). Although Eothenomys eleusis, Soriculus leucops, Vernaya fulva, and Bandicota indica had a high prevalence (>60%), the sample size was too small to represent. Seven small mammal species were captured in all three counties, and Bartonella was detected in four of them, including Crocidura dracula, E. mileyus, Niviventer andersoni, and Niviventer confucianus, with E. mileyus had the highest infection rate (37.7%, 115/305), followed by N. confucianus (27.03%, 10/37). The infection rates of C. dracula and N. andersoni were more than 50%, but the sample size was too small to represent. Among the dominant small mammal species across the different counties, A. chevrieri had a prevalence of 65.95% (n = 185), followed by A. draco at 65.91% (n = 88), and E. mileyus at 38.46% (n = 91) in Yulong county. In Jianchuan county, A. chevrieri had a prevalence of 46.50% (n = 286), followed by A. draco at 37.68% (n = 69), and E. mileyus at 37.20% (n = 207). However, in Lianghe, N. fulvescens had a prevalence of 85.37% (n = 41), followed by M. pahari at 54.55% (n = 44), R. tanezumi at 57.00% (n = 100), and R. rattus at 54.32% (n = 81), the prevalence of Bartonella in the dominant small mammal species across different areas is shown in Figure 2.
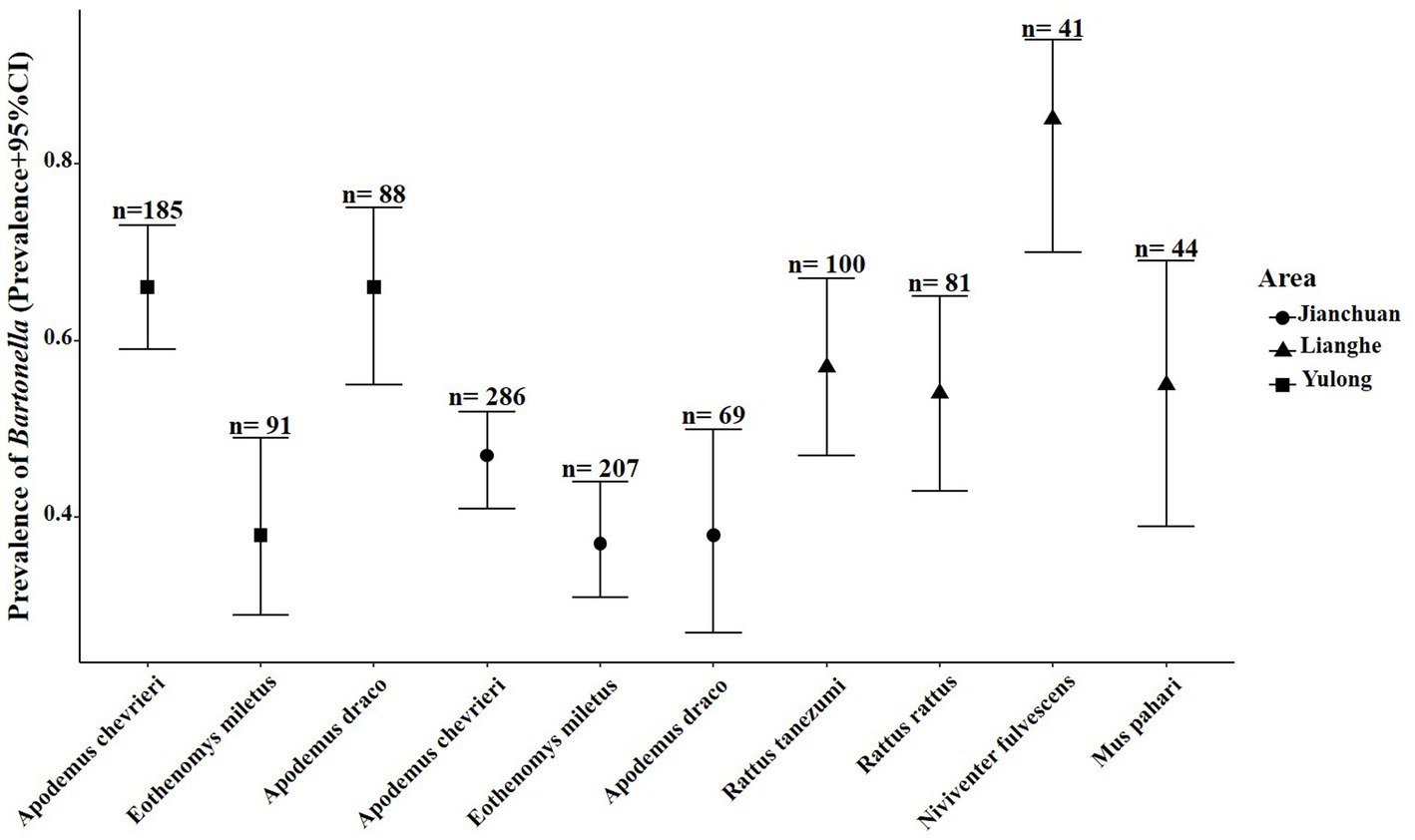
Figure 2. Prevalence of Bartonella in the dominant small mammal species in the three counties of western Yunnan Province. Circle represented the prevalence of Bartonella in the dominant small mammal species in Jianchuan county, triangle represented the prevalence of Bartonella in the dominant small mammal species in Lianghe county, square represented the Bartonella positive samples in Yulong county.
Eighteen small mammal species were captured in all four seasons, and Bartonella was detected in 14 of these species. Among the four seasons, summer exhibited the highest infection rate for Bartonella in small mammals (56.52%, 208/368), followed by spring (47.63%, 171/359) and autumn (44.99%, 211/469), while winter had the lowest rate (43.52%, 178/409). A. chevrieri, A. draco had a higher infection rate in spring and summer, E. mileyus had a higher infection rate in summer and winter. In addition, R. rattus had higher Bartonella infection in autumn and winter (Shown in Table 2). A total of 18 genera of small mammals were captured, of which 14 genera were infected Bartonella. The genus of Anourosorex had the highest Bartonella prevalence (80.00%, 20/25), followed by the genus of Niviventer (58.43%, 52/89) and Apodemus (55.01%, 384/698). The Soriculus genus had 75% Bartonella prevalence, but the sample was too small to represent. The prevalence of Bartonella distribution of small mammal species and small mammal genera by regions or season are shown in Tables 1, 2.
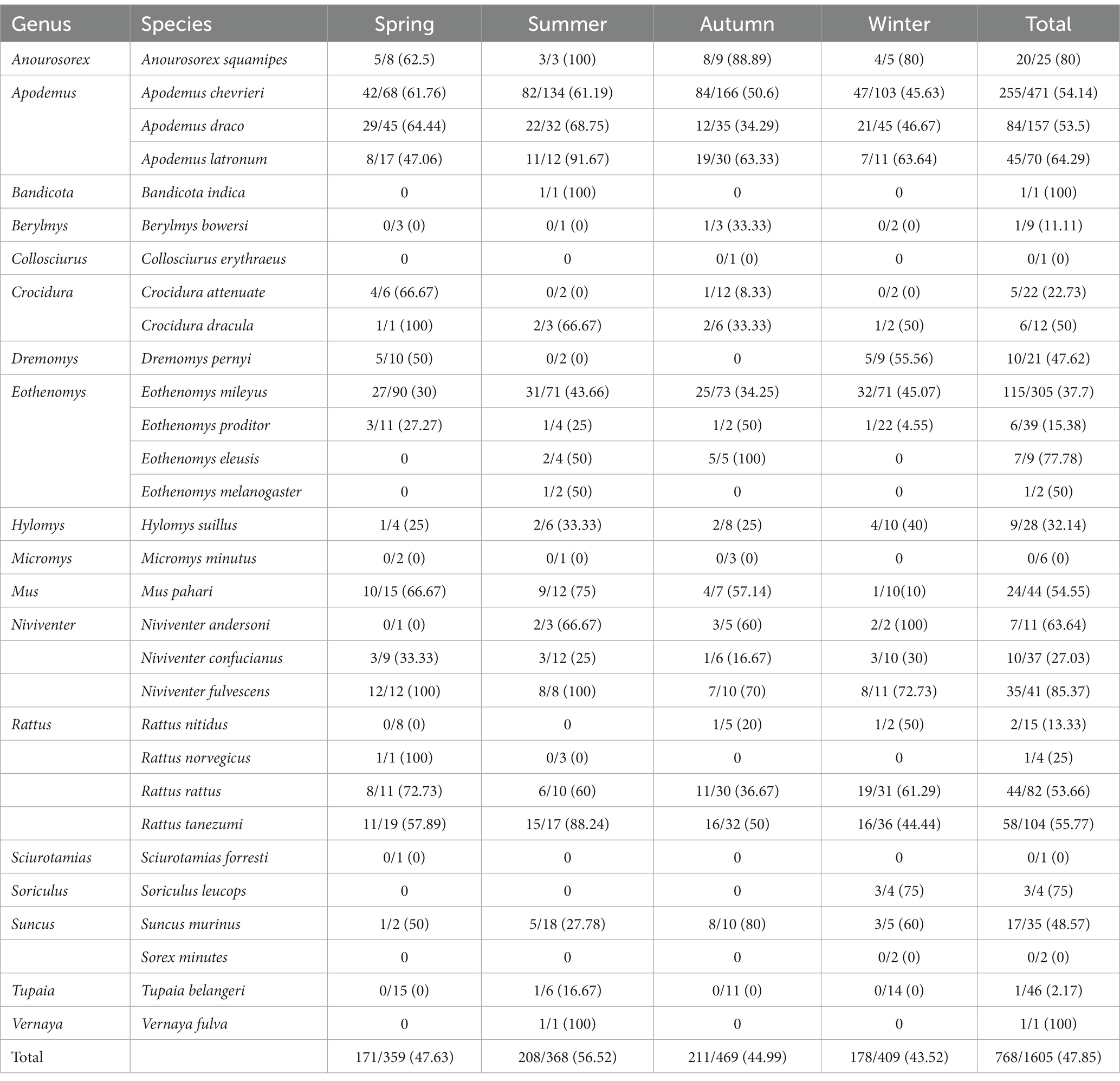
Table 2. Prevalence of Bartonella in wild small mammals captured across four seasons in the three counties of western Yunnan Province [positive/n (%)].
Comparing Bartonella infection in small mammals across the different species, E. mileyus had the lowest Bartonella infection rate than that in A. chevrieri, R. tanezumi, A. draco (p < 0.001). Still, the other three species (A. chevrieri, R. tanezumi, A. draco) had no significant differences from one another. Small mammals that were male had higher infection rate than females (p < 0.05). Comparing the prevalence of Bartonella in small mammals from the three counties, the prevalence of Bartonella in small mammals from Lianghe county had the highest infection rate, followed by those in Yulong, the lowest infection was in Jianchuan (p < 0.001). Among the four seasons, Bartonella infection of small mammals in summer had the highest infection than that in other three seasons (p = 0.001). In addition, Bartonella infection in small mammals in woodland had the highest infection than that in bush and cultivation (p = 0.001). The relationship between the associated factors and the infection of Bartonella in small mammals is shown in Table 3.
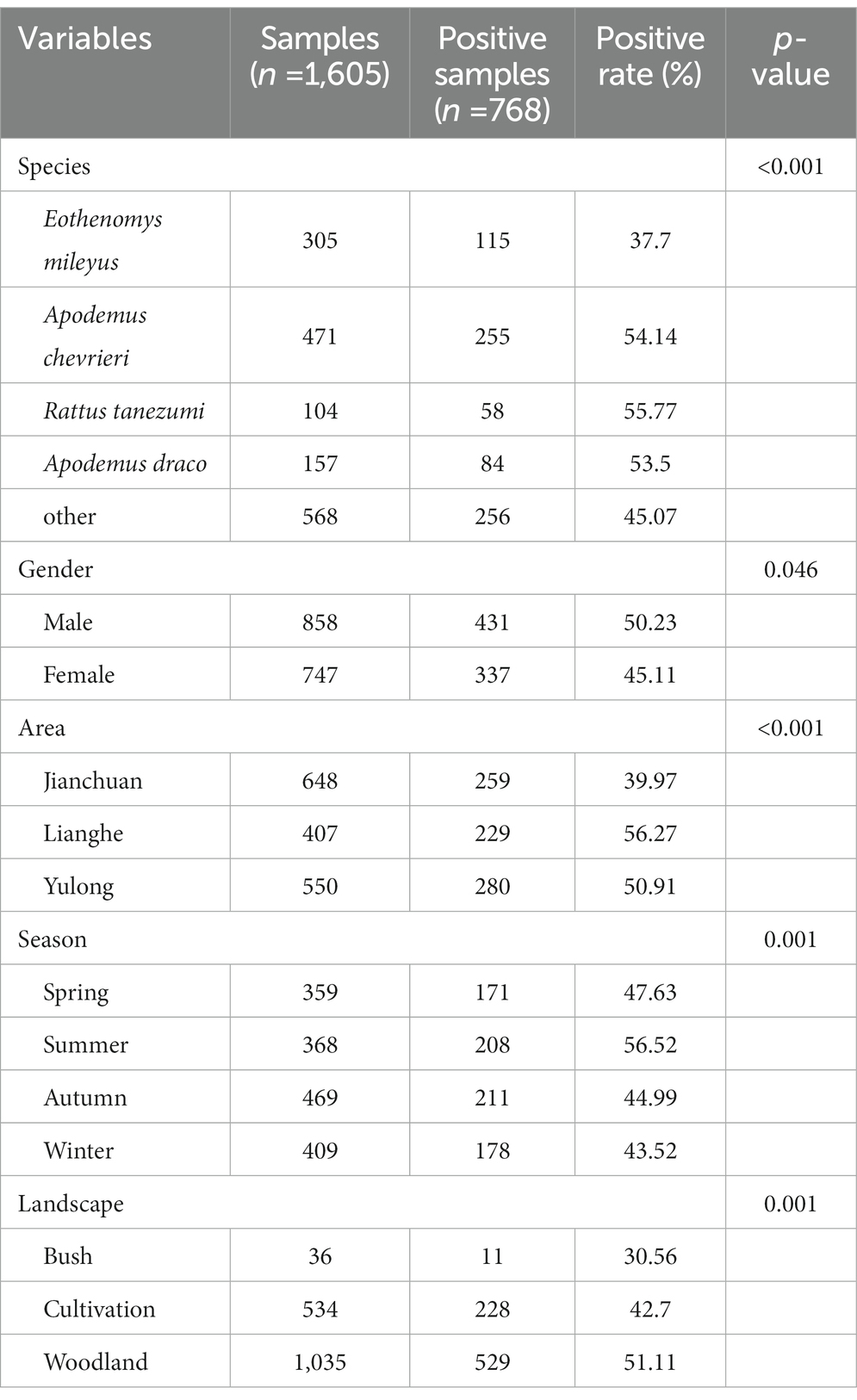
Table 3. Factor analysis of Bartonella infection rates in wild small mammals collected from the three counties of western Yunnan Province.
Out of 768 positive samples (280 were in Yulong, 259 were in Jianchuan, and the rest were 229 in Lianghe county), 96 positive sample were randomly selected for further sequence analysis. 33 samples (YL-2-181, YL-3-202, JC-3-011, LH-1-012, et al.) shared more than 96% identity to Bartonella tribocorum, where LH-1-068 and LH-1-115 were homologous up to 100%, JC-3-051, LH-1-012, LH-1-079, LH-1-098, LH-1-103, LH-3-015, LH-3-088, LH-3-198, and LH-4-139 (9 samples) were also shared more than 98% identity with Bartonella elizabethae, LH-3-058 shared 96% with B. tribocorum, but shared a higher identity with Bartonella rattimassiliensis at 99.6%. 31 samples (YL-3-147, JC-1-076, LH-1-046 et al.) shared more than 96% identity to Bartonella rochalimae, where LH-3-206 were homologous up to 100%. 18 samples (YL-3-101, JC-2-035, LH-1-026, et al.) shared more than 99% identity to B. japonica. YL-3-230, LH-1-094, and YL-1-011 (3 samples) shared more than 98% identity to Bartonella taylorii, and LH-1-094 also shared 97.2% identity to Bartonella sylvatica. YL-1-094 shared 96.8% identity with Bartonella grahamii, but shared a higher identity with B. washoensis at 98%.
The phylogenetic tree constructed with ssrA gene sequence homologies of 96 sample sequences and 27 reference sequences of Bartonella is shown in Figure 3. Phylogenetic analysis of sequences showed that the Bartonella strains detected in small mammals belonged to multiple clusters, including B. rochalimae, Bartonella japonica, B. tribocorum, B. sylvatica, B. rattimassiliensis, and Bartonella washoensis. Bartonella species in small mammals from Lianghe county belonged to five different strains, and it was more diverse than the strains of Bartonella identified from Yulong and Jianchuan counties, where only two and one strains were separately detected. The majority of sequences were ascribable to B. japonica. Among these positive samples, A. chevrieri, E. mileyus, and A. draco were detected with Bartonella japonica, R. rattus with B. rattimassiliensis, Dremomys pernyi with B. washoensis, M. Pahari with B. sylvatica, N. fulvescens with B. tribocorum, in addition, R. tanezumi was detected with both B. tribocorum and B. rochalimae. Sequence details from this study are shown in Supplementary Tables S1, S2.
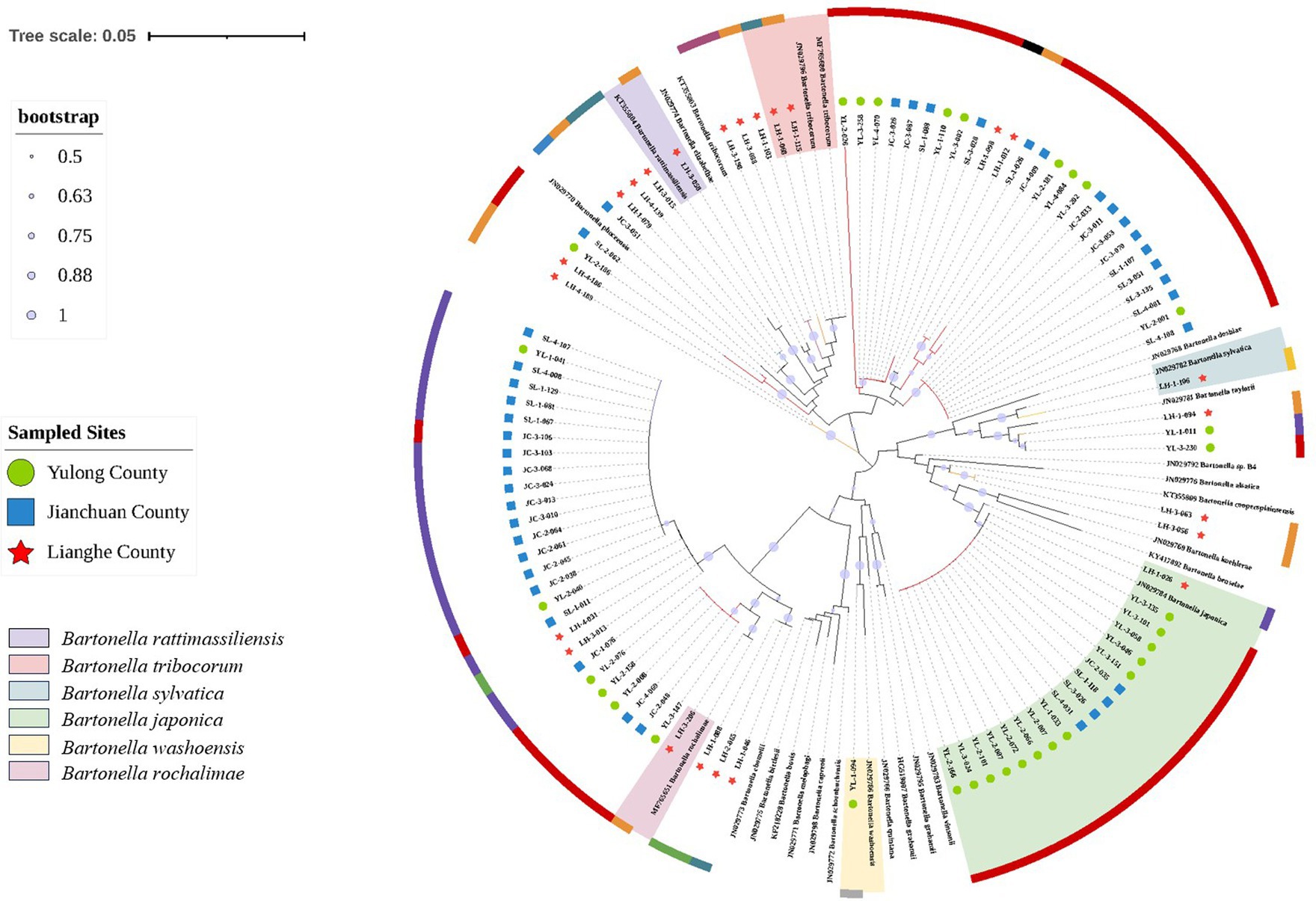
Figure 3. Neighbor-joining phylogenetic tree of Bartonella based on ssrA gene. Sequences from this study, green circle represented the positive samples in Yulong county, blue square represented the positive samples in Jianchuan county, red pentagram represented the positive samples in Lianghe county. The different colors of the outer circles represented the detection of Bartonella in different genera of small mammals: red represented Apodemus genera. Dark orange represented Rattus genera. Light orange represented Mus genera. Ligh green represented Hylomys genera. Dark green represented Niviventer genera. Blue represented Anourosorex genera. Purple represented Eothenomys genera. Dark pink represented Suncus genera. Gray represented Dremomys genera. Black represented Crocidura genera. The branch color was same represented the detection of Bartonella in different genera of small mammals. The different color shades represented the different Bartonella species.
Bartonella has gained recognition as an emerging zoonotic pathogen. It has been detected in various small mammals in China, particular exhibiting a high infection rate in the northern and southwestern regions (23). In Yunnan Province, Bartonella infection in small mammals has been studied across diverse climates and habitats. Moreover, the presence of Bartonella natural foci in northwestern Yunnan has already been confirmed, indicating the remarkable adaptability of Bartonella in various small mammals by different geographic regions (20). Despite these advancements, however, a critical gap remains in our understand of the epidemiology and species diversity of Bartonella in nature. Thus, it is of great important need for systematic and comprehensive investigations into the epidemiological and ecological characteristics of Bartonella infection in small mammals in different geographic areas. In this study, 1,605 small mammals were tested. The overall prevalence of Bartonella was 47.85%, B. rochalimae, B. japonica, B. tribocorum, B. washoensis, B. sylvatica, and B. rattimassiliensis were the main species. The Bartonella infection rate in small mammals was aligned with previous studies in Yunnan, and the infection rate exhibited a range of 15.84% to 55.91% in the small mammal population (17–19, 24). However, the infection rate in small mammals was higher than that of 37.41% in Shanxi province (13), 30.1%–38.61% in Qinghai province (11, 12), but slightly lower than that of 59.09% in Tibet (25), this may be due to the influence of sampling sites, captured small mammals species, or differences in detection methods. Additionally, in Lianghe county, a notably high prevalence of Bartonella infection was detected, reaching 56.27% in small mammals. Interestingly, it is worth noting that one previous study in Lianghe county, which detected a limited set of species, including R. tanezumi, Hylomys suillus, and Berylmys manipulus, did not detect Bartonella infections (19). In contrast, the present study expanded its scope by testing Bartonella in a broader ranges of small mammal species, encompassing a total of 30 small mammal species. This broader species detection suggests an increase likelihood of detecting Bartonella infection in small mammals, enhancing the potential influence of species richness on Bartonella detection rates.
A variety of small mammals can carry Bartonella in nature. Generally, small mammals are the main hosts of Bartonella. Diverse dominant small mammal species in different areas would lead to various primary hosts of Bartonella. The infection rate of Bartonella among small mammals in Vietnam was 14.9%, and the infection rate of R. tanezumi was the highest (49.2%), followed by Rattus norvegicus (20.7%) (26). In this study, the prevalence of Bartonella was highest in N. fulvescens (85.37%, 35/41), followed by A. latronum. Small mammals that survive in different areas determine the possibility of species carrying Bartonella. In this study, a slightly higher Bartonella infection was detected in male small mammals compared to females. This finding was similar to the previous study and could be attributed to the more vigorous activity of male small mammals, which can elevate their chances for exposure to pathogens and enhance the potential roles serving as a host for transmission to other small mammals (27).
Yulong and Jianchuan counties have similar dominant hosts and environments. Still, the rates of Bartonella infection in the two areas were different, and this could be due to variations in the number of dominant small mammal species infected with Bartonella, suggesting differences in how small mammals adapt to the pathogens in similar environments. In addition, the dominant small mammal species in Lianghe county also differed from those in Yulong and Jianchuan counties, with higher infection rates in R. tanezumi. This finding was in line with previous studies that showed R. tanezumi was the main host for Bartonella (7, 16, 17). In addition, Apodemus and Eothenomys genera had higher infection rates of Bartonella in Yulong and Jianchuan counties, and this finding also was in line with previous studies showed that Apodemus and Eothenomys genera were the main genera for Bartonella in small mammals (28, 29). The genera of Rattus, Apodemus, and Eothenomys are Yunnan’s the dominant small mammal species. Thus, it holds significant importance to maintain sustained surveillance of the genera of Rattus, Apodemus, and Eothenomys in small mammals. This proactive approach is crucial for preventing Bartonella infections from spilling over from natural reservoirs to humans, particularly when infected small mammal populations surpass a critical threshold.
The prevalence of Bartonella in small mammals had the highest rate in summer than in the other three seasons. This result was consistence with previous study, which showed that Bartonella infection of small mammals was higher in summer in Vietnam, Laos, and Thailand, suggesting that Bartonella infection status could be affected by the dynamics distribution of hosts, the abundance of vector parasite on small mammals in different seasons (30). It has been reported that temperature has a strong influence on the growth of fleas, indirectly affecting the infected flea transmission when seasons change, therefore indirectly affecting the spread of Bartonella (31, 32). Bartonella infection in small mammals inhabit in woodland and cultivation was higher, which was similar to previous study, such as a higher Bartonella infection rate in small mammals inhabited in forest and farmland in Shangdang Basin, China (13), indicating that the higher risk infection of Bartonella when people go to the woodland or farmland, especially travelers are more likely closer to the natural environments.
Based on ssrA gene, 768 positive samples were detected in small mammals, and the sequencing of positive samples was generated by conventional PCR, 96 samples were randomly selected and sequenced for molecular analysis. Six Bartonella species were obtained from small mammals based on the neighbor-joining phylogenetic tree, including B. rochalimae, B. japonica, B. tribocorum, B. sylvatica, B. rattimassiliensis, and B. washoensis. In addition, B. tribocorum could detect in N. fulvescens and R. tanezumi. B. japonica could detect in A. chevrieri, E. mileyus, and A. draco. Other strains of Bartonella were detected in one small mammal species, suggesting that highly adaptability and species diversity of Bartonella in small mammals, thus, long-term surveillance of Bartonella infection in small mammal is necessary to prevent the transmission of pathogens from nature to humans by infected small mammals. Moreover, R. tanezumi can detect B. rochalimae expect B. tribocorum, the two R. tanezumi in the same landscape and county were not completely the same, one R. tanezumi infected B. tribocorum were male captured in spring, but another R. tanezumi infected B. rochalimae were female captured in autumn, indicating that the diversity of same small mammal species in similar environments, and high adaptability of Bartonella to host.
This study has some limitations. First, there are twenty counties in western Yunnan Province, and this study only selected three counties as the sample sites. However, a large number of small mammals were captured in each county (550 were in Yulong County, 648 were in Jianchuan County, and 407 were in Lianghe County), which could improve the representativeness of the composition and distribution of small mammals in western Yunnan Province. Second, only 96 out of the 768 positive samples were sequenced due to inadequate research project funding, but the randomly selected samples included different counties and landscapes whenever possible. The above selection criteria could be biased, but the sequencing results of positive samples should be as representative as possible. As a result, there might be an insufficient number of Bartonella species identified in wild small mammals.
A high prevalence of Bartonella was detected in Yulong, Jianchuan, and Lianghe counties of western Yunnan Province. Six Bartonella species (B. rochalimae, B. japonica, B. tribocorum, B. sylvatica, B. rattimassiliensis, and B. washoensis) were obtained, with B. japonica being the main species. The rates of Bartonella infection were influenced by small mammal species, the gender of small mammals, geographical areas, seasons, and landscapes. Therefore, ongoing surveillance for Bartonella infection in small mammals is crucial, and efforts to identify Bartonella species should be maximized to prevent human infections caused by infected small mammals.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was approved by Medical Ethics Committee of Dali University. The study was conducted in accordance with the local legislation and institutional requirements.
Y-YL: Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. DY: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. H-ZZ: Data curation, Formal analysis, Methodology, Writing – original draft. Z-XL: Investigation, Writing – original draft. R-DH: Investigation, Methodology, Writing – original draft. MH: Investigation, Writing – original draft. ZQ-A: Methodology, Writing – original draft. J-JZ: Methodology, Writing – original draft. J-XY: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was funded by the National Natural Science Foundation of China (no. 81860565), the project of “Talent Support Program in Yunnan” (no. YNWR-MY-2019-008), the Science and Technology Innovation Team of Natural Focal Diseases Epidemiology in University of Yunnan Province (Yunnan Provincial Department of Education issued [2020] no. 102).
The authors would like to thank Jianchuan Station for Endemic Disease Control and Prevention, Lianghe Center for Disease Control and Prevention, and Yulong Center for Disease Control and Prevention for assistance in the progress of the project. The author would like to disclose that the content of the manuscript has previously appeared in Yu’s thesis (33).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1301316/full#supplementary-material
1. Spitalska, E, Minichova, L, Kocianova, E, Skultety, L, Mahrikova, L, Hamsikova, Z, et al. Diversity and prevalence of Bartonella species in small mammals from Slovakia, Central Europe. Parasitol Res. (2017) 116:3087–95. doi: 10.1007/s00436-017-5620-x
2. Parte, AC, Sardà Carbasse, J, Meier-Kolthoff, JP, Reimer, LC, and Göker, M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. (2020) 70:5607–12. doi: 10.1099/ijsem.0.004332
3. Abreu-Yanes, E, Martin-Alonso, A, Martin-Carrillo, N, Livia, KG, Marrero-Gagliardi, A, Valladares, B, et al. Bartonella in rodents and Ectoparasites in the Canary Islands, Spain: new insights into host-vector-pathogen relationships. Microb Ecol. (2018) 75:264–73. doi: 10.1007/s00248-017-1022-y
4. Galfsky, D, Król, N, Pfeffer, M, and Obiegala, A. Long-term trends of tick-borne pathogens in regard to small mammal and tick populations from Saxony, Germany. Parasit Vectors. (2019) 12:131. doi: 10.1186/s13071-019-3382-2
5. Obiegala, A, Jeske, K, Augustin, M, Król, N, Fischer, S, Mertens-Scholz, K, et al. Highly prevalent bartonellae and other vector-borne pathogens in small mammal species from the Czech Republic and Germany. Parasit Vectors. (2019) 12:332. doi: 10.1186/s13071-019-3576-7
6. Polat, C, Celebi, B, Irmak, S, Karatas, A, Colak, F, Matur, F, et al. Characterization of Bartonella taylorii strains in small mammals of the Turkish Thrace. EcoHealth. (2020) 17:477–86. doi: 10.1007/s10393-021-01518-y
7. Szewczyk, T, Werszko, J, Slivinska, K, Laskowski, Z, and Karbowiak, G. Molecular detection of Bartonella spp. in rodents in Chernobyl exclusion zone, Ukraine. Acta Parasitol. (2021) 66:222–7. doi: 10.1007/s11686-020-00276-1
8. Neves, ES, Mendenhall, IH, Borthwick, SA, Su, YCF, and Smith, GJD. Detection and genetic characterization of diverse Bartonella genotypes in the small mammals of Singapore. Zoonoses Public Health. (2018) 65:e207–15. doi: 10.1111/zph.12430
9. Böge, I, Pfeffer, M, Htwe, NM, Maw, PP, Sarathchandra, SR, Sluydts, V, et al. First detection of Bartonella spp. in small Mammals from Rice storage and processing facilities in Myanmar and Sri Lanka. Microorganisms. (2021) 9:658. doi: 10.3390/microorganisms9030658
10. Ansil, BR, Mendenhall, IH, and Ramakrishnan, U. High prevalence and diversity of Bartonella in small mammals from the biodiverse Western Ghats. PLoS Negl Trop Dis. (2021) 15:e0009178. doi: 10.1371/journal.pntd.0009178
11. Rao, H, Li, S, Lu, L, Wang, R, Song, X, Sun, K, et al. Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China. Sci Rep. (2021) 11:1735. doi: 10.1038/s41598-021-81508-w
12. Yu, J, Li, Q, Lu, L, Li, S, Song, X, Li, D, et al. Detection and genetic diversity of Bartonella species in small mammals from the central region of the Qinghai-Tibetan plateau, China. Sci Rep. (2022) 12:6996. doi: 10.1038/s41598-022-11419-x
13. Yu, J, Xie, B, Bi, G-Y, Zuo, H-H, Du, X-Y, Bi, L-F, et al. Prevalence and diversity of small rodent-associated Bartonella species in Shangdang Basin, China. PLoS Negl Trop Dis. (2022) 16:e0010446. doi: 10.1371/journal.pntd.0010446
14. Dahmana, H, Granjon, L, Diagne, C, Davoust, B, Fenollar, F, and Mediannikov, O. Rodents as hosts of pathogens and related zoonotic disease risk. Pathogens. (2020) 9:202. doi: 10.3390/pathogens9030202
15. Do Amaral, RB, Cardozo, MV, Varani, ADM, Furquim, MEC, Dias, CM, Assis, WOD, et al. First report of Bartonella spp. in marsupials from Brazil, with a description of Bartonella harrusi sp. nov. and a new proposal for the taxonomic reclassification of species of the genus Bartonella. Microorganisms. (2022) 10:1609. doi: 10.3390/microorganisms10081609
16. Mardosaitė-Busaitienė, D, Radzijevskaja, J, Balčiauskas, L, Bratchikov, M, Jurgelevičius, V, and Paulauskas, A. Prevalence and diversity of Bartonella species in small rodents from coastal and continental areas. Sci Rep. (2019) 9:12349. doi: 10.1038/s41598-019-48715-y
17. Du, C, Yin, J, Li, D, Wang, X, Cheng, X, Yang, G, et al. Investigation of Bartonella infection in small rodents in households in western Yunnan. Dis Surveill. (2016) 31:220–4.
18. Dong, S, Li, Y, Duan, C, Guo, Y, Shi, L, Zhong, Y, et al. Investigation of the status of Bartonella infection among rodents in Jianchuan county of Yunnan, 2017. Dis Surveill. (2019) 34:1022–5.
19. Yang, W, Song, X, Liang, W, Feng, Y, Zhang, Y, Zhang, Y, et al. Investigation of natural infection status of Bartonella in house rodents in Dehong prefecture of Yunnan province. Dis Surveill. (2018) 33:20–5.
20. Zhang, Y, Li, H, Gong, Z, Zhao, X, and Liu, W. Investigation of Bartonella infections among small mamamls in parts of northwestern Yunnan province. Acta Parasitol Med Entomol Sin. (2013) 20:110–4. doi: 10.3969/j.issn.1005-0507.2013.02.007
21. Bai, Y, Kosoy, MY, Maupin, GO, Gage, KL, Dong, X, and Ma, Y. Discovery of Bartonella species in rodents in Yunnan. Chinese J Zoonoses. (2002) 3:5–9.
22. Luo, YY, Zhao, QF, Wei, ZF, Hong, RD, Liu, ZX, Hong, M, et al. Comparative analysis of small mammal and parasitic flea composition in Yulong and Jianchuan plague foci in Yunnan Province, China: a cross-sectional study. Biomedical Journal of Scientific & Technical Research. (2021) 37:29901–11. doi: 10.26717/BJSTR.2021.37.006076
23. Yu, D, and Yin, J. Progress in research of natural infection status of Bartonella in rodents. Dis Surveill. (2018) 33:15–9.
24. Li, D, Yu, D, Liu, Q, and Gong, Z. Study on the prevalence of Bartonella species in rodent hosts from different environmental areas in Yunnan. Chin J Epidemiol. (2004) 11:20–3.
25. Rao, H, Yu, J, Li, S, Song, X, and Li, D. Gene polymorphisms of Bartonella species in small mammals in Maixiu National Forest Park in the Qinghai-Tibet plateau, China. Chin J Vector Biol Control. (2021) 32:398–403.
26. Loan, HK, Cuong, NV, Takhampunya, R, Klangthong, K, Osikowicz, L, Kiet, BT, et al. Bartonella species and trombiculid mites of rats from the Mekong Delta of Vietnam. Vector Borne Zoonotic Dis. (2015) 15:40–7. doi: 10.1089/vbz.2014.1604
27. Welc-Faleciak, R, Bajer, A, Behnke, JM, and Siński, E. The ecology of Bartonella spp. infections in two rodent communities in the Mazury Lake District region of Poland. Parasitology. (2010) 137:1069–77. doi: 10.1017/S0031182009992058
28. Kraljik, J, Paziewska-Harris, A, Miklisová, D, Blaňarová, L, Mošanský, L, Bona, M, et al. Genetic diversity of Bartonella genotypes found in the striped field mouse (Apodemus agrarius) in Central Europe. Parasitology. (2016) 143:1437–42. doi: 10.1017/S0031182016000962
29. Ma, J, Li, D, Chen, Z, and Liu, Q. Epidemiological characteristics of rodent-borne Bartonella. Dis Surveill. (2018) 33:7–14+2.
30. Jiyipong, T, Morand, S, Jittapalapong, S, and Rolain, JM. Bartonella spp. infections in rodents of Cambodia, Lao PDR, and Thailand: Identifying risky habitats. Vector Borne Zoonotic Dis. (2015) 15:48–55. doi: 10.1089/vbz.2014.1621
31. Jones, RT, Borchert, J, Eisen, R, MacMillan, K, Boegler, K, and Gage, KL. Flea-associated bacterial communities across an environmental transect in a plague-endemic region of Uganda. PLoS One. (2015) 10:e0141057. doi: 10.1371/journal.pone.0141057
32. Rothenburger, JL, Himsworth, CG, Nemeth, NM, Pearl, DL, and Jardine, CM. Beyond abundance: how microenvironmental features and weather influence Bartonella tribocorum infection in wild Norway rats (Rattus norvegicus). Zoonoses Public Health. (2018) 65:339–51. doi: 10.1111/zph.12440
Keywords: Bartonella, small mammals, geographical variations, phylogenetic analysis, western Yunnan
Citation: Luo Y-Y, Yu D, Zhang H-Z, Liu Z-X, Hong R-D, Hong M, Ai Z-Q, Zhu J-J and Yin J-X (2023) Molecular detection of Bartonella species in wild small mammals in western Yunnan Province, China. Front. Vet. Sci. 10:1301316. doi: 10.3389/fvets.2023.1301316
Received: 25 September 2023; Accepted: 09 November 2023;
Published: 21 November 2023.
Edited by:
Alexandro Guterres, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Goncalves-Oliveira J., Hebrew University of Jerusalem, IsraelCopyright © 2023 Luo, Yu, Zhang, Liu, Hong, Hong, Ai, Zhu and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia-Xiang Yin, Y2hpbmF5anhAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.