- 1Department of Veterinary Paraclinical Sciences, College of Veterinary Medicine, University of Southern Mindanao, Cotabato City, Philippines
- 2Department of Tropical Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Hookworm infections caused by Ancylostoma caninum, A. ceylanicum, A. braziliense, and Uncinaria stenocephala continue to be a significant threat to canine health globally (1, 2). In Asia, it is estimated that hookworm infections have a prevalence of around 35% among different species of canids endemic to the continent, with approximately 41% of domesticated dogs infected (3). Moreover, in Southeast Asia, recent reports have proven that the most common dog hookworm is the zoonotic A. ceylanicum, with A. caninum being a close second (4, 5). Conversely, A. ceylanicum is now regarded as the region's second most prevalent human hookworm, next only to Necator americanus (4, 6). Canine hookworms are of One Health significance since they have been noted to cause deleterious infection consequences in humans: A. caninum causes eosinophilic enteritis, A. braziliense is associated with “creeping eruptions,” and A. ceylanicum infections is a zoonosis that can circulate between humans and companion animals (1, 7, 8). This presents a One Health scenario where zoonotic hookworms from dogs cause a significant public health concern among neglected, impoverished communities (9).
In the Philippines, historical accounts have reported that A. caninum, A. ceylanicum, and A. braziliense have infected dogs and inhabitants in the country (10–12). Among these, only A. ceylanicum has been molecularly confirmed to infect both dogs and humans from the same community (13). Unfortunately, there are only five articles that have been published in the peer-reviewed and online gray literature from 2000 to 2023 regarding the epidemiology of canine hookworms in the Philippines. The reported prevalence ranges from 8% in urban areas such as Manila and Laguna Province to 48% in rural endemic areas of Mindanao (14, 15). For a country that has an estimated dog population of 23 2,900, 000 (16), roughly 11 million dogs would potentially be affected by hookworm infections. Figure 1 summarizes the results and geographic distribution of these studies. Aside from these recent reports, we know nothing about the epidemiology of canine hookworm infections.
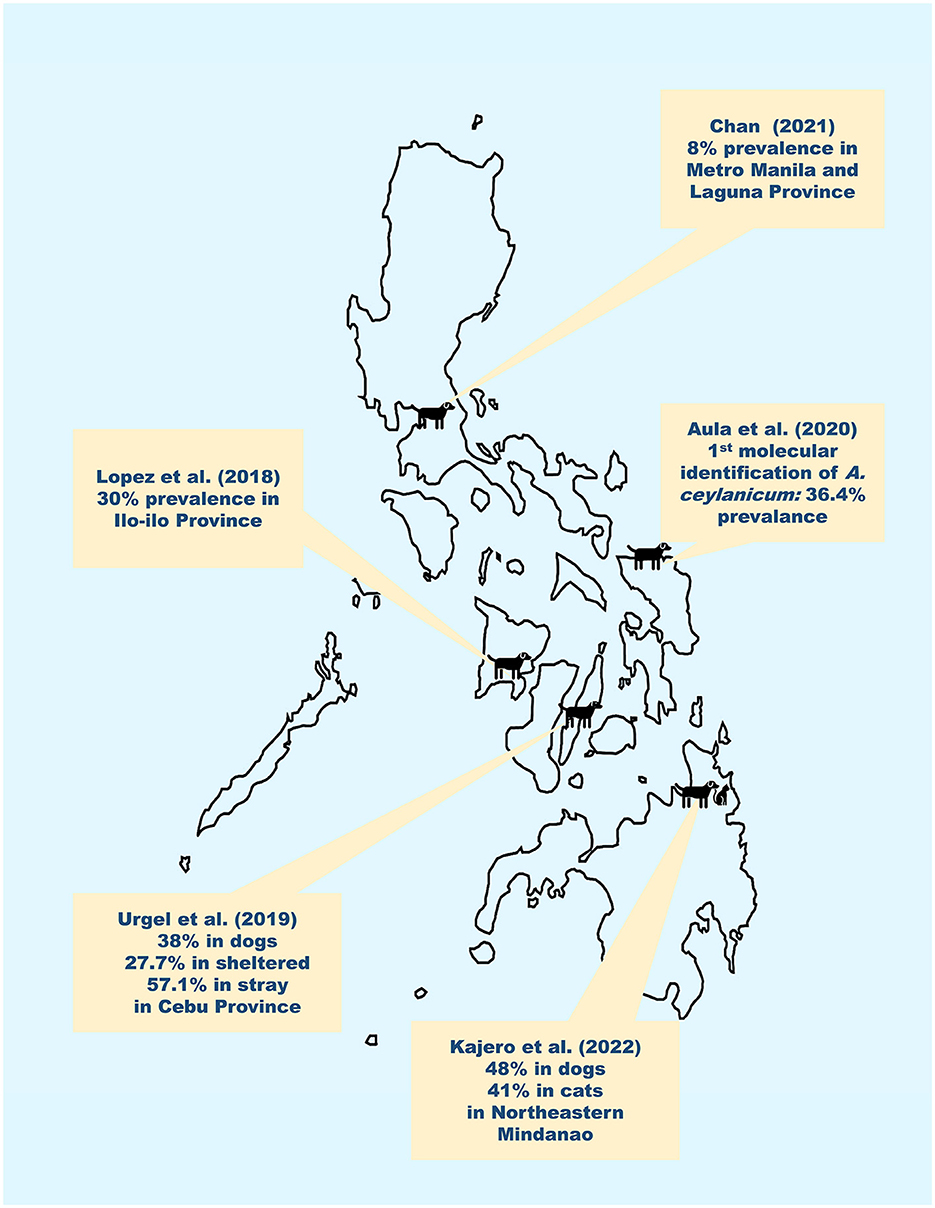
Figure 1. Results and geographical distribution of recent published reports (2000–2023) of canine hookworm infections in the Philippines. Recent reports by Chan (15), Lopez et al. (17), Urgel et al. (18), Aula et al. (13) and Kajero et al. (14) indicate that canine hookworm infections are between 8% to 48% in the Philippines.
I posit several possible reasons as to why there is a paucity of peer-reviewed literature regarding canine hookworm infections in the Philippines, which include the following:
1. Hookworm infections are neglected because these are not seen as a pressing canine health concern compared to other prevalent infectious diseases (e.g., canine ehrlichiosis and canine parvoviral enteritis).
2. The clinical management of canine gastrointestinal parasites is relatively straightforward due to broad-spectrum, combination anthelmintics. Resistance to any anthelmintic among canine hookworms has yet to be reported in the country, at least in the peer-reviewed literature. However, multidrug-resistant hookworms have already been noted in the United States, Australia, Brazil, New Zealand, and Canada (19–23).
3. Most cases of infections do not present overt clinical signs; hence, most owners are not aware that their pets are infected, which adds to the general neglect of canine hookworms. However, it must be noted that severe acute infections among puppies cause significant pathology (e.g., anemia and bloody diarrhea) and even death (24).
4. The unequal access to veterinary care among financially challenged dog owners results in poor knowledge of the damage caused by canine hookworms and the neglect of infections. This is of particular concern since hookworm infections are most likely to occur in resource-lacking endemic areas (25).
5. The notion that canine hookworm infections are ubiquitous undermines the value of researching their occurrence, risk factors, and control. This notion of undervaluing results in numerous gaps in research (e.g., drug resistance, infection epidemiology, and transmission dynamics) that continue to be unexplored in the country.
6. Student theses that investigate this problem are often never published in peer-reviewed journals; most Filipino universities do not have online repositories that store and make these research results available.
The dearth of research on canine hookworms in the Philippines presents some important research gaps and opportunities, which include the following:
1. There is a need to molecularly confirm the species of hookworm that currently affect dogs in the country, so we know which hookworm species occur in which area.
2. The epidemiology and risk factors of canine ancylostomiasis in most areas of the Philippines remain poorly understood. Epidemiological data will enable clinical practitioners to develop health programs that consider factors affecting hookworm infections, which will be effective for their canine patients.
3. The zoonotic nature of A. ceylanicum presents a One Health risk among Filipinos and companion animals that should be studied. The transmission dynamics and occurrence of this important hookworm are still poorly understood in the country. Research results addressing this issue can be used to optimize the Integrated Helminth Control Program of the Philippine Department of Health.
4. Research on soil contamination of canine hookworms and other geohelminths (i.e., Toxocara and Trichuris) is lacking in many endemic areas in the country. This research gap may be of importance since high Toxocara seroprevalence occurs among children exposed to areas with contaminated soils, which have been previously reported in the Philippines (26, 27).
5. The efficacy of treatment and control plans should be re-evaluated as unforeseen resistance to commonly used anthelmintics may have already occurred. Anthelmintic drug efficacy determination can be done based on the reduction of fecal egg count reduction test (FECRT) using parasitological assays (e.g., McMaster or Mini FLOTAC techniques) or molecular techniques (e.g., quantitative PCR) (28, 29). Moreover, specific gene mutations involved in resistance can be assessed, such as single nucleotide polymorphisms in the β-tubulin isotype 1 gene for benzimidazole resistance. SNPs reported among populations of A. caninum include those that occur at positions 134 (CAA/glutamine → CAT/histidine), 167 (TTC, TTT/phenylalanine → TAC, TAT/tyrosine), 198 (GAG, GAA/glutamic acid → GCG, GCA/alanine), and 200 (TTC/ phenylalanine → TAC/tyrosine or TTC/ phenylalanine → TTA/leucine) (23, 30–32).
6. Owner awareness of the risks and harms of canine hookworms should be assessed and subsequently improved. Barriers to accessing essential veterinary care for dog owners should also be investigated.
To end, research on the epidemiological features of canine hookworms on the national scale remains wanting in the Philippines. The impact of this important parasitosis is also poorly understood, leading to neglect of its potential One Health consequences. Therefore, it is the intent of this piece to present the dearth of veterinary research in canine hookworms in the country and the opportunities it presents in the hopes that actions can be sparked among the Filipino veterinary community.
Author contributions
JT: Conceptualization, Investigation, Writing – original draft, Visualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author would like to acknowledge the Postgraduate Scholarship for International Students of the Faculty of Medicine, Khon Kaen University, for funding his doctoral studies. Similarly, he sends his gratitude to his awesome supervisor, Associate Professor Sutas Suttiprapa, Ph.D.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. (2010) 26:162–7. doi: 10.1016/j.pt.2010.01.005
2. Pal M, Tolawak D, Garedaghi Y. A comprehensive review on major zoonotic parasites from dogs and cats. Int J Med Parasitol Epidemiol Sci. (2023) 4:1. doi: 10.34172/ijmpes.2023.02
3. Zibaei M, Nosrati MRC, Shadnoosh F, Houshmand E, Karami MF, Rafsanjani MK, et al. Insights into hookworm prevalence in Asia: a systematic review and meta-analysis. Trans Royal Soc Trop Med Hygiene. (2020) 114:141–54. doi: 10.1093/trstmh/trz115
4. Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis. (2014) 20:976–82. doi: 10.3201/eid2006.131770
5. Kladkempetch D, Tangtrongsup S, Tiwananthagorn S. Ancylostoma ceylanicum: the neglected zoonotic parasite of community dogs in Thailand and Its Genetic Diversity among Asian Countries. Animals. (2020) 10:2154. doi: 10.3390/ani10112154
6. Bui K-L, Nguyen T-H, Duong HD, Nguyen V-L, Nguyen T-N, Le L-A, et al. Ancylostoma ceylanicum infections in humans in Vietnam. Parasitol Int. (2021) 84:102405. doi: 10.1016/j.parint.2021.102405
7. Prociv P, Croese J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet (London, England). (1990) 335:1299–302. doi: 10.1016/0140-6736(90)91186-E
8. Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. (2013) 43:1009–15. doi: 10.1016/j.ijpara.2013.07.006
9. Traub RJ, Zendejas-Heredia PA, Massetti L, Colella V. Zoonotic hookworms of dogs and cats – lessons from the past to inform current knowledge and future directions of research. Int J Parasitol. (2021) 51:1233–41. doi: 10.1016/j.ijpara.2021.10.005
10. Wharton LD. The intestinal worms of dogs in the Philippine Islands. J Parasitol. (1917) 4:80–2. doi: 10.2307/3270819
11. Africa CM. Studies on Experimental Creeping Eruption in the Philippines. Philipp J Sci. (1932) 48:89–101.
12. Velasquez CC, Cabrera BC. Ancylostoma ceylanicum (Looss, 1911) in a Filipino woman. J Parasitol. (1968) 54:430–1.
13. Aula OP, McManus DP, Weerakoon KG, Olveda R, Ross AG, Rogers MJ, et al. Molecular identification of Ancylostoma ceylanicum in the Philippines. Parasitology. (2020) 147:1718–22. doi: 10.1017/S0031182020001547
14. Kajero OT, Janoušková E, Bakare EA, Belizario V, Divina B, Alonte AJ, et al. Co-infection of intestinal helminths in humans and animals in the Philippines. Trans R Soc Trop Med and Hyg. (2022) 116:727–35. doi: 10.1093/trstmh/trac002
15. Chan JMP. Detection of Intestinal Helminth Infections Among Domesticated and Sheltered Dogs in Selected Areas of Metro Manila and Laguna (Master's Thesis). Manila, Philippines: De La Salle University. (2021).
16. Chaudhari A, Kartal T, Brill G, Amano KJ, Lagayan MG, Jorca D. Dog ecology and demographics in several areas in the philippines and its application to anti-rabies vaccination programs. Animals. (2022) 12:105. doi: 10.3390/ani12010105
17. Lopez JC, Aguirre MJ, Dalisay J. Occurrence of Intestinal Helminth Parasites in Domestic Dogs (Canis familiaris domesticus) in Arevalo, Iloilo City, Philippines Using the Parasep Fecal Parasite Concentration Tenchnique. Publiscience. (2018) 1:95–9.
18. Urgel MFM, Ybañez RHD, Ybañez AP. The detection of gastrointestinal parasites in owned and shelter dogs in Cebu, Philippines. Vet World. (2019) 12:372–6. doi: 10.14202/vetworld.2019.372-376
19. Castro PDJ, Durrence K, Durrence S, Gianechini LS, Collins J, Dunn K, et al. Multiple anthelmintic drug resistance in hookworms (Ancylostoma caninum) in a Labrador breeding and training kennel in Georgia, USA. J Am Vet Med Assoc. (2023) 261:342–7. doi: 10.2460/javma.22.08.0377
20. Evason MD, Weese JS, Polansky B, Leutenegger CM. Emergence of canine hookworm treatment resistance: novel detection of Ancylostoma caninum anthelmintic resistance markers by fecal PCR in 11 dogs from Canada. Am J Vet Res. (2023) 84:9. doi: 10.2460/ajvr.23.05.0116
21. Furtado LFV, Bello ACPDP, Dos Santos HA, Carvalho MRS, Rabelo ÉML. First identification of the F200Y SNP in the β-tubulin gene linked to benzimidazole resistance in Ancylostoma caninum. Vet Parasitol. (2014) 206:313–6. doi: 10.1016/j.vetpar.2014.10.021
22. Jimenez Castro PD, Mansour A, Charles S, Hostetler J, Settje T, Kulke D, et al. Efficacy evaluation of anthelmintic products against an infection with the canine hookworm (Ancylostoma caninum) isolate Worthy 4.1F3P in dogs. Int J Parasitol. (2020) 13:22–27. doi: 10.1016/j.ijpddr.2020.04.003
23. Stocker T, Scott I, Šlapeta J. Unambiguous identification of Ancylostoma caninum and Uncinaria stenocephala in Australian and New Zealand dogs from faecal samples. Austral Vet J. (2023) 11:avj13272. doi: 10.1111/avj.13272
24. Hawdon JM, Wise KA. Ancylostoma caninum and Other Canine Hookworms. In:Strube C, Mehlhorn H, , editors. Dog Parasites Endangering Human Health. Cham: Springer International Publishing. (2021). p. 147–193.
25. Zendejas-Heredia PA, Colella V, Huggins LG, Schaper R, Schunack B, Traub RJ. An Integrated Coproscopic and Molecular Method Provides Insights into the Epidemiology of Zoonotic Intestinal Helminths of Dogs across Cambodia. Transbound Emerg Dis. (2023) 2023:e2001871. doi: 10.1155/2023/2001871
26. Abadilla MEG, Paller VGV. Toxocara canis prevalence in soil, dog stool, and human serum samples from a rural village in Los Baños, Laguna, Philippines. J Parasitic Dis. (2022) 46:889–95. doi: 10.1007/s12639-022-01507-0
27. Fajutag AJM, Paller VGV. Toxocara egg soil contamination and its seroprevalence among public school children in los baños, laguna, philippines. Southeast Asian J Trop Med Public Health. (2013) 44:4.
28. Geurden T, Smith ER, Vercruysse J, Yazwinski T, Settje T, Nielsen MK. World association for the advancement of veterinary parasitology (WAAVP) guideline for the evaluation of the efficacy of anthelmintics in food-producing and companion animals: General guidelines. Vet Parasitol. (2022) 304:109698. doi: 10.1016/j.vetpar.2022.109698
29. Jimenez Castro PD, Kaplan RM. Persistent or suspected- resistant hookworm infections. Clini Brief. (2020) 18:59–68.
30. Furtado LFV, De Paiva Bello ACP, Rabelo ÉML. Benzimidazole resistance in helminths: from problem to diagnosis. Acta Trop. (2016) 162:95–102. doi: 10.1016/j.actatropica.2016.06.021
31. Leutenegger CM, Lozoya CE, Tereski J, Savard C, Ogeer J, Lallier R. Emergence of Ancylostoma caninum parasites with the benzimidazole resistance F167Y polymorphism in the US dog population. Int J Parasitol. (2023) 21:131–40. doi: 10.1016/j.ijpddr.2023.01.001
32. Venkatesan A, Castro PDJ, Morosetti A, Horvath H, Chen R, Redman E, et al. Molecular evidence of widespread benzimidazole drug resistance in Ancylostoma caninum from domestic dogs throughout the USA and discovery of a novel β-tubulin benzimidazole resistance mutation. PLoS Pathog. (2023) 19:e1011146. doi: 10.1371/journal.ppat.1011146
Keywords: Ancylostoma, One Health, parasitology, soil-transmitted helminths, neglected tropical diseases
Citation: Tenorio JCB (2023) Canine hookworms in the Philippines—Very common but very much neglected in veterinary research. Front. Vet. Sci. 10:1297962. doi: 10.3389/fvets.2023.1297962
Received: 20 September 2023; Accepted: 01 November 2023;
Published: 29 November 2023.
Edited by:
Maria Paola Maurelli, University of Naples Federico II, ItalyReviewed by:
Pablo Jimenez Castro, Antech Diagnostics, United StatesCopyright © 2023 Tenorio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Clyden B. Tenorio, amNidGVub3Jpb0B1c20uZWR1LnBo
 Jan Clyden B. Tenorio
Jan Clyden B. Tenorio