- 1Business Economics Group, Wageningen University and Research, Wageningen, Netherlands
- 2School of Business, IPB University, Bogor, Indonesia
- 3Quantitative Veterinary Epidemiology, Wageningen University and Research, Wageningen, Netherlands
- 4Department of Animal Diseases and Veterinary Public Health, School of Veterinary Medicine and Biomedical Science, IPB University, Bogor, Indonesia
- 5Department of Economics, Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden
- 6Royal GD, Deventer, Netherlands
Increasing milk quality in smallholder dairy farms will result in a greater quantity of milk being delivered to milk collection centers, an increased milk price for farmers and consequently an improved farmers’ livelihood. However, little research on milk quality has been performed on smallholder farms in Southeast Asia. The objective of this study was to identify risk factors associated with somatic cell count (SCC) and total plate count (TPC) in Indonesian smallholder dairy farms. One dairy cooperative in West Java, Indonesia was selected based on its willingness to participate. All 119 member farmers in the cooperative, clustered in six groups, were interviewed and a bulk milk sample from all farms was collected in April 2022. Risk factors associated with dairy farms’ SCC and TPC were investigated using multivariable population-averaged generalized estimating equations (GEE) models. The mean geometric SCC and TPC from these farms were 529,665 cells/mL of milk and 474,492 cfu/mL of milk, respectively. Five risk factors including manure removal frequency, receiving mastitis treatment training, washing the udder using soap, number of workers, and ownership of the pasture area were associated with SCC. Two risk factors, manure removal frequency and dairy income contribution, were associated with TPC. These findings can therefore be used as a starting point to improve udder health and milk quality in Indonesia and other countries where smallholder farmers play a significant role in milk production.
1 Introduction
The dairy sector plays an important role in the economic development of several Southeast Asian nations, such as Indonesia, Malaysia, Vietnam, and Thailand (1). It provides income and employment opportunities for farmers and contributes to food security and nutrition (1–4). The vast majority of milk in Southeast Asia is produced on smallholder dairy farms. In recent years, the sector has experienced substantial growth due to population expansion, increasing per capita income, the rise of the middle class, and growing awareness of the health benefits of milk and dairy products (5, 6).
Consumption of milk per capita in Southeast Asian countries was projected to increase by 3% annually between 1997 and 2020 (7, 8). However, microbial contamination remains a significant concern for milk spoilage, food safety and can pose health risks to consumers (9–12). Dealing with these risks requires monitoring and managing milk quality parameters in dairy farms to ensure milk quality and food safety.
The primary indicators used to measure the quality and hygiene of bulk milk are somatic cell count (SCC) and total plate count (TPC; 13, 14). Monitoring SCC values provides an understanding of milk quality, udder health status, and the presence of subclinical mastitis (15, 16). On the other hand, TPC values in milk indicate predominantly bacterial contamination resulting from dairy farm practices such as milking, handling, and milk storage (17, 18).
Despite its importance in world-wide milk production, the quality of milk produced by smallholder dairy farmers is often suboptimal due to poor cleaning and disinfection practices, the suboptimal storage of milk, inadequate sanitation and poor hygiene in the milking environment (5, 19, 20). Elevated levels of SCC and bacterial contaminants in milk can have adverse effects on its quality and safety, leading to reduced milk yield and decreased shelf-life of milk and its derived products (21, 22). Moreover, high SCC and TPC levels can result in the rejection of milk-by-milk collection centers and processors because it fails to meet the required milk quality standards, resulting in substantial economic losses for dairy farmers (23, 24).
Creating knowledge on SCC and TPC risk factors is crucial in assisting farmers to decrease SCC and TPC to meet milk quality standards. Numerous risk factor studies have been conducted worldwide, with a significant emphasis on regions such as Europe and North America (25–28). Findings of such studies have contributed substantially to the realization of the National Mastitis Council’s ten-point mastitis control program, shedding light on critical risk factors and effective management practices. However, such knowledge is limited available for smallholder dairy systems in Southeast Asia (29–31).
The aim of this paper is therefore to determine milk quality and identify risk factors associated with SCC and TPC levels in bulk tank milk of smallholder dairy farms in Indonesia. The study sheds light on the challenges faced by smallholder dairy farmers in improving milk quality.
2 Materials and methods
2.1 Study design, area, and population
A cross-sectional study among smallholder dairy farmers in Cianjur District, West Java, Indonesia was conducted in April 2022. In Indonesia, dairy cooperatives are formal collective organizations formed by local dairy farmers, serving pivotal roles such as operating milk collection centers, providing market access, facilitating input supply, offering training, extension services, and animal health services, and advocating for their members’ interests at the local, regional, and national level. Relevant stakeholders, experts, and representatives from several dairy cooperatives were consulted to select one dairy cooperative from several potential candidates in West Java, Indonesia. After careful consideration of factors such as willingness to collaborate, a well-established reputation, convenient proximity to the laboratory facility in Bogor, and the size of the cooperative which allows for comprehensive farm visits, and the alignment of its activities with the current study, KPS Cianjur Utara cooperative was chosen. In the chosen cooperative, the payment scheme for milk currently does not incorporate SCC and TPC values. All of its 119 milk producing member farms were included in the study. The geographical distribution of the dairy farmers included in the study is displayed in Figure 1 and indicates the clustering of farmers. These clusters were used to form six geographically connected farmers groups. In Cianjur District, farmers’ groups play an important role in facilitating assistance and extension programs by the cooperatives and the local government. Farmers’ groups, in collaboration with the cooperative, also provide services to farmers (e.g., the facilitation of a milk collection point, veterinary services) and collectively provide and distribute feed and dairy farm inputs among their members.
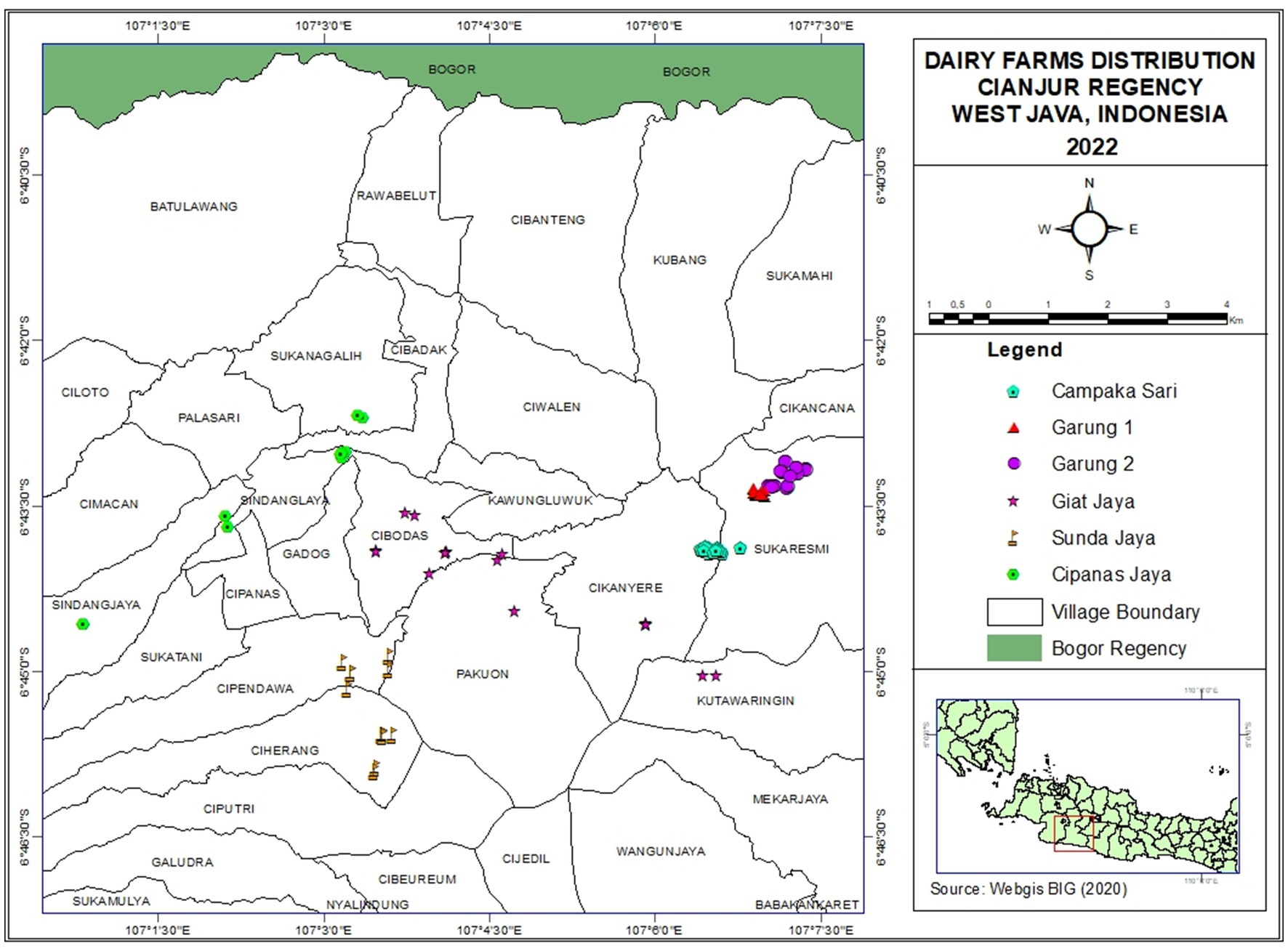
Figure 1. Geographical location of the 119 smallholder dairy farms included in the survey. Different colors and symbols indicate the six farmers’ groups.
2.2 Method of data collection
Ethical approval for this study was obtained from the Social Sciences Ethics Committee at Wageningen University and Research (WUR), Netherlands.
Farmers were interviewed using a paper-based questionnaire which was translated to and paraphrased in Bahasa Indonesia from English. Before administration, the questionnaire was pre-tested on 6 farmers and adapted according to their feedback. The data was collected by a trained team of 3 enumerators and 3 paramedics of the cooperative that visited the selected farms. Enumerators are individuals tasked with data collection from smallholder dairy farms through surveys and interviews. For this study, we involved 3 enumerators, all of whom possessed university degrees—2 in veterinary science and 1 in social science. Paramedics, on the other hand, are healthcare staff hired by the dairy cooperative to provide animal health services to dairy cattle and offer assistance to smallholder farmers who are cooperative members, particularly in matters related to dairy cattle health and management. To support our survey efforts, animal data recording, and milk sampling, we involved 3 paramedics from the selected dairy cooperative. Enumerators and paramedics were fluent in Bahasa Indonesia and the local language, Sundanese. To improve the quality of data collection, each filled questionnaire was immediately checked by a research data collection supervisor. If there were missing or illogical data, confirmation was sought with the dairy farmers, paramedics, or cooperative staff. The participating farmers were compensated to cover the opportunity cost of their time spent during interviews, as well as to enhance participation rates and ensure data accuracy.
2.3 Survey data
Information on 55 variables putatively associated with farmers’ milk quality parameters (SCC and TPC) was collected during the farm visits. It included data on socio-demographics, farm characteristics, dairy farm management practices, as well as risk factors regarding milk quality. The selection of these variables was based on a literature review of factors affecting milk quality in smallholder settings and the experience of the current research team with milk quality programs and udder health. The variables for this study were divided into 5 categories:
1. socio-demographics (age, gender, education, number of family members, main occupation, second occupation, dairy income contribution, and dairy business experience),
2. farm characteristics (livestock unit, barn size, barn ownership, land area for growing grass, pasture area ownership, total labor, family labor, paid labor, village, subdistrict, farmers’ group identification, milk production, water source, and type of bedding),
3. animal health services and training frequency (paramedic visits, frequency of animal health trainings, udder health trainings, and mastitis treatment trainings),
4. dairy management practices (cleaning stall, frequency of manure removal, using and changing of bedding, number of mastitis treatment days, visually checking the udder before milking, testing of new cows with the Californian mastitis test, pre-stripping, checking temperature and swelling of the udder before milking, checking the willingness of the cow for eating and drinking, being aware of subclinical mastitis, treating mastitis cows by themselves, using antibiotics at dry off, using a towel for cleaning the udder, post milking teat disinfection, milking mastitis cows at the end, isolating mastitis cows, washing of cows, washing the udder using soap, cleaning towel, number of cows per towel, washing hands, cleaning of milking equipment, milking methods, and level of feeding),
5. animal disease or mastitis impact (reduction of milk yield, number of days with reduced milk yield, return to pre-treatment milk yield levels after cure, and mastitis production impact).
2.4 Milk sampling and laboratory analyses
After milking their cows, farmers bring the milk to a milk collection center, where the sample was taken. In the milk collecting centers, 50 mL of bulk milk from each farm was stored in labeled tubes. Tubes were then placed on ice in a cooling box and delivered to the laboratory on the same day to prevent milk spoilage and the growth of bacteria.
Bulk milk samples were tested in the Laboratory of Veterinary Public Health at the School of Veterinary Medicine and Biomedical Science of IPB University (Indonesia). Samples were analyzed for somatic cell count (SCC), total plate count (TPC) and six other milk composition parameters including fat, solids nonfat, total solids, lactose, and protein content and milk density.
2.4.1 Somatic cell count
SCC was determined using the Breed method (32, 33). Briefly, after homogenization, 0.01 mL of milk was pipetted on an object glass using a Breed pipette and was spread to form a 1 cm2 square using an elbow-tipped loop. The object glass was fixed with a Bunsen flame after which a Breed staining was performed. The object glass was first immersed in an ether alcohol solution for 2 min, then in Löffler’s methylene blue solution for 1–2 min, and lastly in 96% ethanol for 1 min to remove the remaining attached dyes. After drying, somatic cells were counted using a light microscope. The number of somatic cells was counted using 30 viewpoints, summed and divided by the number of viewpoints to determine the average number of somatic cells for the sample. The final SCC was determined by multiplying the average number of somatic cells with the microscopy factor (400,000).
2.4.2 Total plate count
TPC was determined using the pour plate method (34). A total of 1 mL milk was added to a solution of 9 mL 0.1% buffer peptone water (BPW) to obtain a 10−1 dilution. After homogenization, serial dilutions of 10−2, 10−3, 10−4, 10−5, and 10−6 were prepared to select 3 consecutive dilutions for cultured. A total of 1 mL of milk sample from 3 selected dilutions was transferred into a petri dish after which 10 mL to 15 mL of plate count agar was poured. After homogenization and solidification, the agar culture was incubated at 35°C for 24 to 48 h in an inverted position. The calculation was carried out for all microorganisms (both large and small) that grew in a petri dish. Petri dishes containing 25 to 250 colonies were recorded along with the number of dilutions made. Colony counts were determined according to the rules of the American Public Health Association (APHA):
2.4.3 Milk composition
Fat, solids nonfat, lactose, and protein content and milk density were determined using the Lactoscan SP milk analyzer (Milkotronic LTD., Nova Zagora, Bulgaria) following the guidelines of the manufacturer. The total solid parameter was accumulated from the fat and solid-non-fat components.
2.5 Data management and statistical analysis
Data management and statistical analysis were conducted using Stata/SE version 17.0 (StataCorp LLC, Texas, United States). The quality and composition (SCC, TPC, fat, solids nonfat, total solids, lactose, and protein content and milk density) of the bulk milk sample, overall and within each farmers’ group, was determined using descriptive statistics. The median differences of fat, solids nonfat, total solids, lactose, and protein content and milk density among farmers’ group were tested using Kruskal-Wallis’s equality of populations rank test.
To identify risk factors associated with milk quality, SCC and TPC were in focus. Given their skewed distributions, the natural logarithm of SCC (LnSCC) and TPC (LnTPC) was determined. Factors associated with LnSCC and LnTPC were separately analyzed using multivariable population-averaged generalized estimating equations (GEE) models in complete-case analyses. Initial data exploration using null mixed-effects models identified that there was substantial clustering of farms with farmers’ groups since their intraclass correlation coefficients were 25.9 and 13.3% for LnSCC and LnTPC, respectively. To correct for this clustering of farms within farmers’ groups in relation to dairy management practices, animal health services, and geographic location, farmers’ group identification was the clustering variable in the GEE models. The model further incorporated the exchangeable correlation structure (35), assuming an equable level of correlation across farms. In the model, the outcome variables, LnSCC and LnTPC, were continuous variables, normally distributed, and analyzed using the identity link function. The GEE model was defined as follows:
Linear relationships between the continuous variables and both outcome variables were checked using scatterplots based on 10-percentile data. Variable selection started with univariable regression models in which 55 variables were individually tested for their association with LnSCC or LnTPC. Variables that had a p-value below 0.20 in the Type III test were selected for further analysis. Correlation among pairs of selected explanatory variables was assessed thereafter to avoid multicollinearity. The Cramers’ V correlation test was used to determine the correlation between two categorical variables while the Spearman’s correlation test was used to investigate correlations between two numerical variables with nonlinear data and correlations between one categorical variable with one numerical variable. If two variables had a correlation coefficient higher than 0.5, one of the variables was selected in our analysis. This included the frequency of farmers’ training on topics of animal health and udder health. These two variables had a strong correlation with the frequency of training on mastitis treatment (Cramers’s V = 0.8 and 0.9, respectively) in the analysis for LnSCC. The latter was selected for the multivariable analysis as it represented training in mastitis treatment. Dairy income contribution and second occupation were also strongly correlated (Cramers’s V = 0.8) of which dairy income contribution was included in the analysis for LnTPC. Thereafter, a backward selection process was used for model specification until all selected variables significantly contributed to the model (p < 0.5), based on the Type III test, or were considered confounders. The latter was defined when effect estimates changed more than 25% when removing a variable from the model. Interaction terms were not evaluated. The quasi-likelihood under the independence model criterion (QIC) was employed as a goodness-of-fit measure to evaluate the final GEE model (36). A post-hoc power analysis was performed using the observed mean and standard deviation values for LnSCC and LnTPC. Statistical significance was defined at p < 0.05 and the observable difference in outcome values was calculated at a power of 80%.
3 Results
3.1 Description of study population and milk quality
The general demographic and farm characteristics of the 119 participating farms are provided in Table 1. Dairy farming in Cianjur, West Java, Indonesia is dominated by smallholders who have an average livestock unit of 5.44 and dairy labor of 2 persons per farm. The average age, education, and dairy business experience of dairy farmers were 44 years, 8 years, and 16 years, respectively.
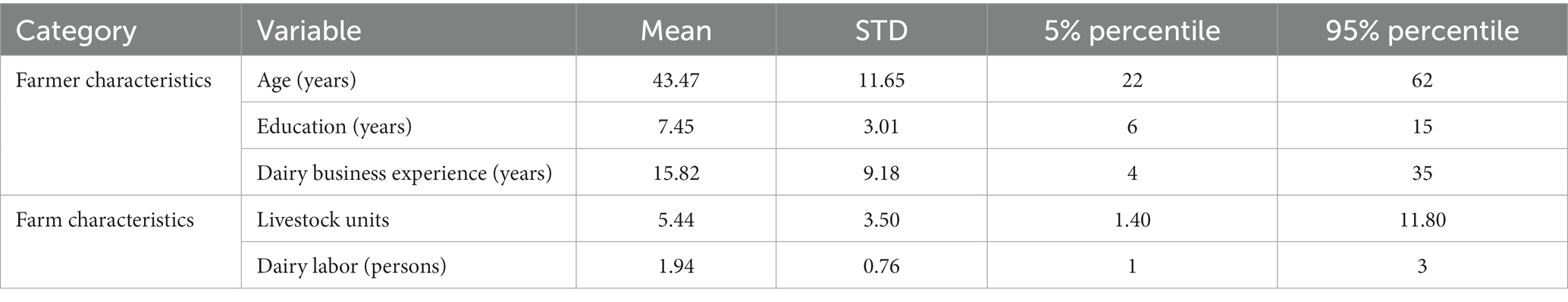
Table 1. Characteristics of 119 smallholder dairy farms located in Cianjur District, West, Java, Indonesia that were included in the survey.
The average value of milk quality parameters was 3.04% (SD = 0.99), 5.72% (SD = 1.05), 8.76% (SD = 1.66), 3.14% (SD = 0.57), 2.09% (SD = 0.38), and 1.02 gr/cm3 (SD = 0.00), for fat, solids nonfat, total solids, lactose, and protein content and milk density, respectively. There were no significant differences in the median of the six milk quality parameters among farmers’ groups.
The descriptive statistics of the LnSCC and LnTPC per farmers’ group are presented in Table 2. The mean overall LnSCC was 13.18, which corresponds with a geometric SCC of 529,665 cells/ml. Giat Jaya and Garung 2 had the highest and lowest average LnSCC with levels of 14.12 and 12.59, respectively. The mean overall LnTPC was 13.07, which is a geometric TPC of 474,492 cfu/mL. Garung 2 and Sunda Jaya had the highest and the lowest LnTPC levels with 14.47 and 12.10, respectively. Post-hoc power calculation indicated that with a power of 80% and a significance value of 5%, a difference of 0.5 LnSCC and 1.0 LnTPC units would be observable.
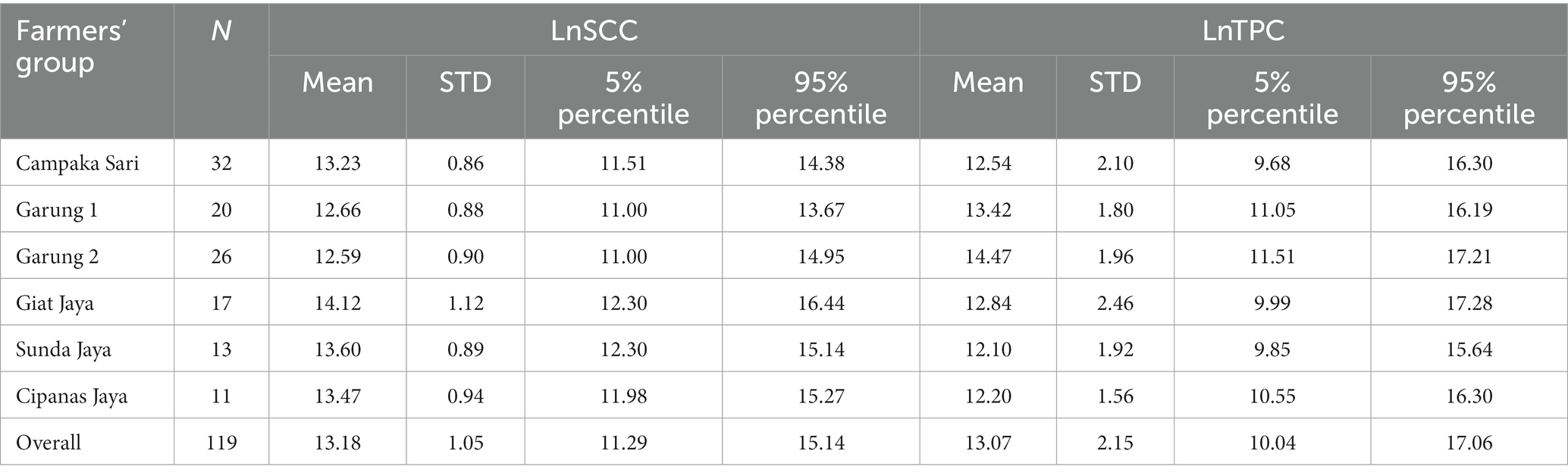
Table 2. Distribution of the natural logarithm of SCC (LnSCC) in cells/mL and the natural logarithm of TPC (LnTPC) in cfu/mL per farmers’ group.
3.2 Factors associated with LnSCC
The results of the GEE models for LnSCC are presented in Table 3. Five variables were significantly associated with LnSCC in the model considering all variables. Farmers who removed manure 3 times per day had a LnSCC that was 0.78 (95% CI: −1.11 to −0.44) units lower compared to those who removed manure 1 to 2 times per day. Farmers who received one mastitis treatment training in the last 12 months had 0.71 units lower LnSCC (95% CI: −1.14 to −0.28) compared to farmers that did not receive any mastitis treatment training. Those who received 3 to 6 mastitis treatment training had a LnSCC that was 0.77 units lower (95% CI: −1.20 to −0.33). Farmers who wash the udder of their cows after milking using soap had a LnSCC that was 1.73 (95% CI: −3.34 to −0.13) units lower compared to those who do not use soap. Furthermore, farmers who have 3–6 workers had a LnSCC that was 0.47 (95% CI: 0.00 to 0.94) units higher compared to those who have only 1 worker. Finally, ownership of the pasture area was also associated with LnSCC. Its value was 0.98 (95% CI: −1.74 to −0.21) units lower when farmers borrowed land for growing grass and 1.08 (95% CI: −1.75 to −0.42) units lower when they used communal or public land for growing their grass, in comparison to farmers who do not own any land for growing grass.
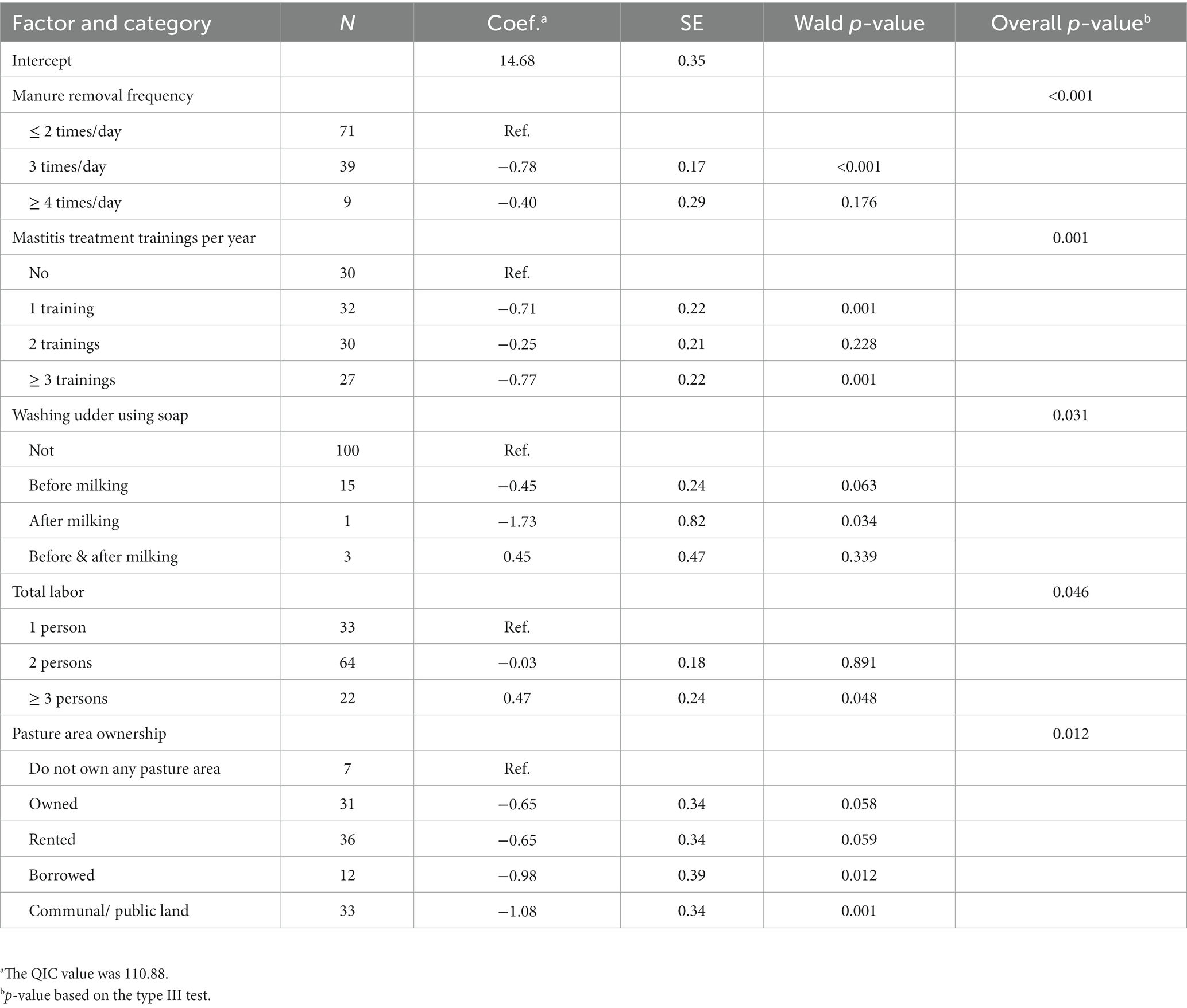
Table 3. Final generalized estimating equations models explaining the natural logarithm of somatic cell count (SCC) of 119 smallholder dairy farms in Cianjur District, West Java, Indonesia.
3.3 Factors associated with LnTPC
Two factors were associated with dairy farmers’ LnTPC in the final GEE model as shown in Table 4. First, farmers who removed manure 4–6 times per day had LnTPC levels that were 1.46 (95% CI: 0.07 to 2.84) units higher compared to farmers who removed manure 1–2 times per day. Second, herds in which the dairy income contributed 50 to 75% to their total income were associated with LnTPC levels that were 1.01 (95% CI: 0.22 to 1.80) units higher compared to those herds where the dairy income contributed more than 75% of the total income.
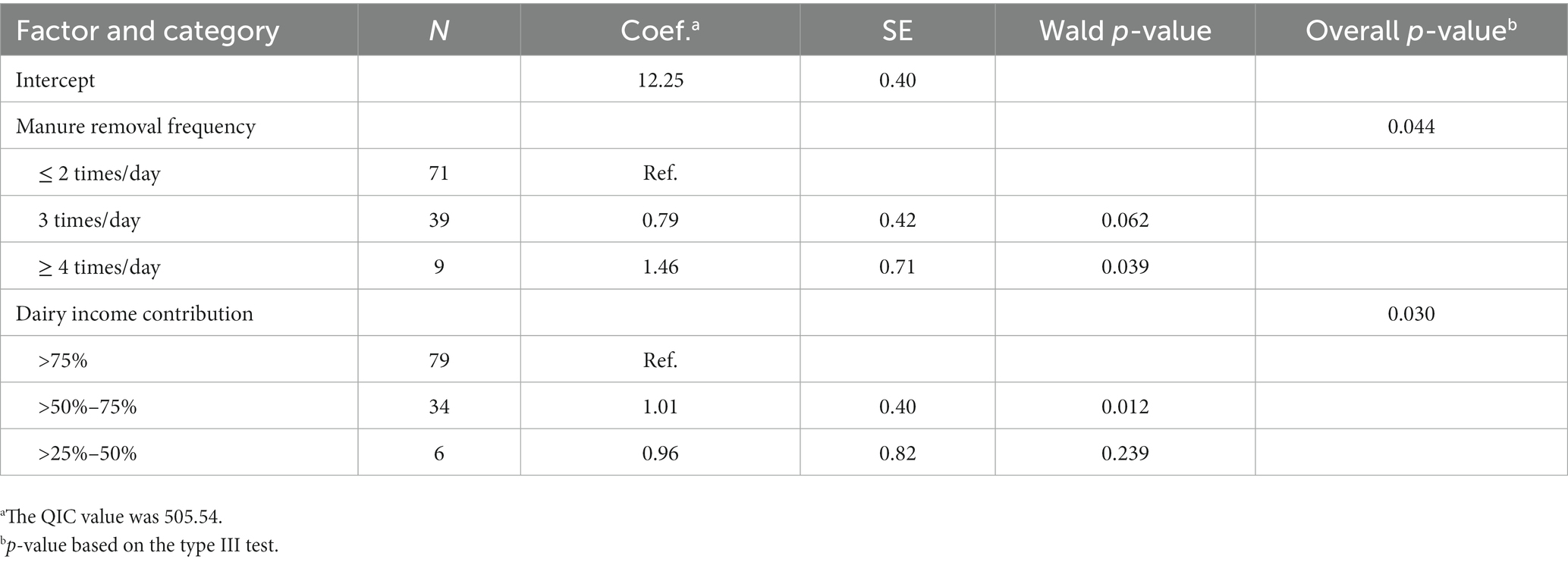
Table 4. Final generalized estimating equations models explaining the natural logarithm of total plate count (TPC) of 119 smallholder dairy farms in Cianjur District, West Java, Indonesia.
4 Discussion
This study assessed the SCC and TPC levels, as well as identified the factors associated with these milk parameters in the context of smallholder dairy farming in the Cianjur District of West Java, Indonesia. In this regard, we examined the geometric mean SCC of the Cianjur dairy farmers in our study, which was found to be 529,665 cells/ml. This finding emphasizes the importance of enhancing udder health management practices to align SCC levels with acceptable standards, specifically adhering to the maximum limit of 400,000 cells/ml of milk stipulated in the Indonesian National Standard (No. SNI 3141.1:2011) for fresh milk (37). A high SCC is not only a milk quality problem, but high SCC values also indicate udder health problems (15, 38). In general, a threshold SCC value of 200,000 cells/ml of milk is used to identify a cow with subclinical mastitis (16, 39–45). A high bulk milk SCC indicates towards a high prevalence of subclinical mastitis leading to reduced productivity, profitability and animal welfare. The geometric mean Total Plate Count (TPC) among Cianjur dairy farmers was found to be 480,000 cfu/mL, which falls below the maximum permissible limit of 1,000,000 cfu/mL of milk as set by the Indonesian National Standard (No. SNI 3141.1:2011) for fresh milk (37). However, there is still room for improvement given the variation seen between farms. Also, it cannot be ruled out that TPC and other milk quality parameters levels may change over time given the cross-sectional design of our study. Currently, TPC is not a factor considered by Cianjur dairy cooperative in determining the milk price for farmers. Introducing payment schemes that incentivize smallholder farmers to increase the hygienic quality of milk potentially improves the overall quality of milk in the supply chain (46, 47). Additionally, no statistically significant differences were observed in the median values of the six milk quality parameters (fat, solids nonfat, total solids, lactose, protein content, and milk density) among the farmers’ groups.
As expected, our study found that management practices, such as manure removal frequency and udder washing with soap were significantly associated with SCC levels. Accumulation of manure in the barn can create a favorable environment for mastitis pathogens, which can cause subclinical mastitis (48–50). Hence, frequent removal of manure is critical for improving milk quality and reducing SCC levels (19, 51–53). However, we found that frequent removal of manure was associated with increased levels of TPC. This could be attributed to the practice used by smallholder farmers in West Java, Indonesia to remove manure in the barn by hosing of water (wet cleaning). Such wet conditions are conducive to bacterial growth and the spread of bacteria around the barn, cows, and milk (54, 55). These findings suggest that there may be a need for alternative practices of manure removal (such as dry cleaning) to minimize the risk of bacterial contamination in the barn, cows, and milk.
Pre-milking and post-milking procedures are critical in minimizing bacterial contaminants on the cow’s udder and teats. Washing the udder with soap before milking and post milking teat disinfection are effective practices in reducing bacterial contamination. Proper washing and drying of the udder and teat are also important in maintaining udder health, reducing mastitis risk, and minimizing SCC levels in milk (56, 57).
Effective mastitis control and treatment are crucial for maintaining milk quality and preventing economic losses for smallholder dairy farmers. Several studies have shown that mastitis control and treatment training can improve the knowledge and practices of farmers in preventing and managing intramammary infections (25, 58). Enhancing milk quality and effectively managing mastitis begins by ensuring that farmers possess a thorough awareness of the milk quality parameters (59). In Cianjur, dairy farmers receive mastitis control and treatment training from various organizations, including dairy cooperatives, the local government, dairy companies, and universities collaborating with farmers’ groups. Dairy cooperatives and farmer groups in Indonesia play complementary roles in supporting dairy farming. Cooperatives are more formalized entities, primarily focused on marketing, processing, and providing various services to their members. In contrast, farmer groups are informal associations that facilitate collaborative farming, knowledge sharing, and community support among individual farmers. The training covers various topics such as milking hygiene, proper milking procedures, early detection of mastitis infections, udder health management, and appropriate treatment of infected cows, which can improve dairy farmers’ knowledge and practices in preventing and managing mastitis infections (60).
The study identified some factors associated with SCC. First, a positive association between total labor and increasing SCC in dairy farms was found. This is in line with previous studies that found that hiring more labor on farms was associated with increasing SCC levels (61, 62). The lack of standardized practices and variation in routines among hired labor highlight the need for implementing standard operating procedures on the farm. Second, our research revealed that farmers who lack land for growing grass had higher levels of SCC. As an alternative, farmers frequently used vegetable waste sourced from traditional markets as a substitute for feeding their dairy cows. However, heavy metal contamination, such as lead (Pb), copper (Cu), and mercury (Hg), has been found in traditional market vegetable waste in Yogyakarta, Indonesia (63). It is therefore important to properly process and check vegetable waste for physical contamination before being fed to the cows.
Predictably, better general hygiene practices were associated with a lower number of bacteria in bulk tank milk. This study found that farmers who used and/or changed their bedding regularly had lower total plate counts in their milk samples compared to farmers who did not use bedding in their barns. This finding is consistent with previous studies that have shown the importance of managing bedding to control bacterial growth and udder infections (64–68).
Smallholder farmers who had a higher dairy income contribution had a lower level of TPC in their bulk tank milk, indicating that they may have better knowledge of hygiene practices. This suggests that improving the knowledge of smallholder farmers regarding milk quality and hygiene practices can lead to greater implementation of these practices, resulting in an improved milk quality (20). Additionally, smallholder farmers who implement good hygiene practices may produce higher quality milk, which in turn may contribute to higher revenues or milk prices. The implementation of an incentive based on milk quality by the cooperative could serve as an additional motivating factor for farmers to adopt and maintain these essential hygiene practices.
Due to our cross-sectional study design, we are unable to prove causal effects and caution is required when interpreting our results. Rather, our results should be interpreted as associations. Another limitation pertains to the relatively modest sample size of dairy farms present in the cooperative that we studied. However, census data from one dairy cooperative was available and post-hoc power calculations indicated that an observed difference in 0.5 LnSCC units and 1.0 LnTPC units would be detectable. This was also the approximate size of effects present in the final models (Tables 3, 4). Furthermore, we applied the GEE population-averaged model in the analysis to provide robust and reliable inferences about associations between explanatory variables and the outcome variables at the population level (69–71). Therefore, this study’s findings can be used to make inferences about the wider population of smallholder dairy farms in tropical Southeast Asian countries, in which dairy farms have similar characteristics as in the study area.
5 Conclusion
This study identified milk quality parameters and examined the various risk factors that were associated with levels of bulk milk somatic cell count (SCC) and total plate count (TPC) smallholder dairy farms in Indonesia. To achieve lower levels of SCC and TPC, several policy recommendations can be implemented. Firstly, it is crucial to encourage smallholder dairy farmers to adopt good hygiene practices, such as regular and thorough manure removal at least three times per day, proper udder washing using soap before and after milking, and the regular utilization and replacement of bedding in the barn. Secondly, it is recommended that farmers’ groups, cooperatives, and local government bodies collaborate to develop effective extension and training programs aimed at enhancing farmers’ knowledge and skills in mastitis management and milk quality. Lastly, cooperatives and dairy companies should establish a comprehensive system for monitoring milk quality parameters, including SCC and TPC, at milk collecting centers. This system can involve regular inspections, sample testing, and compliance checks to ensure consistent adherence to the recommended practices. Therefore, these findings and recommendations can also serve as an initial reference for enhancing udder health and milk quality in other countries where smallholder farmers play a crucial role in milk production. By implementing these measures, it is possible to significantly improve the overall quality of milk and udder health, benefiting both smallholder farmers and the dairy sector as a whole.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Social Sciences Ethics Committee at Wageningen University and Research (WUR), Netherlands. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AF: Conceptualization, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. BB: Conceptualization, Formal analysis, Methodology, Writing – review & editing. OP: Methodology, Writing – review & editing. HH: Conceptualization, Methodology, Supervision, Writing – review & editing. TS: Project administration, Writing – review & editing. HP: Methodology, Writing – review & editing. YS: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Smart Indonesian Agriculture (Smart-In-Ag) project through the Interdisciplinary Research and Education Fund (INREF) of Wageningen University & Research, Wageningen, Netherlands (grant number 210095560).
Acknowledgments
The authors would like to thank the Indonesian smallholder dairy farmers, cooperative staffs, and enumerators for participating and involving in the study. The Grammarly software version 1.0.48.1091 was used to enhance the writing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Oliveros, MCR. The dairy industry in South East Asia: perspective, challenges and opportunities. IOP Conf Ser Earth Environ Sci. (2019) 372:1–9. doi: 10.1088/1755-1315/372/1/012068
2. Devendra, C. Smallholder dairy production systems in developing countries: characteristics, potential and opportunities for improvement – review. Asian Australas J Anim Sci. (2001) 14:104–13. doi: 10.5713/ajas.2001.104
3. Knips, V. Developing countries and the global dairy sector part I global overview. Rome (2005). Available at: https://www.fao.org/3/bp204e/bp204e.pdf
4. Bao, KLN, Sandjaja, S, Poh, BK, Rojroongwasinkul, N, Huu, CN, Sumedi, E, et al. The consumption of dairy and its association with nutritional status in the south east Asian nutrition surveys (SEANUTS). Nutrients. (2018) 10:759. doi: 10.3390/nu10060759
5. Moran, J. Tropical dairy farming: Feeding management for small holder dairy farmers in the humid tropics. Collingwood: Landlinks Press (2005).
6. Gerosa, S, and Skoet, J. Milk availability: trends in production and demand and medium-term outlook. ESA-FAO. (2012). 1–40 p.
7. Moran, J, and Morey, P. Strategies to increase the domestic production of raw milk in Indonesia and other south east Asian countries. Integrated Approach in Developing Sustainable Tropical Animal Production. (2015). p. 1–11. Available at: https://jurnal.ugm.ac.id/istapproceeding/article/view/30539/18422
8. Delgado, CL. Rising consumption of meat and milk in developing countries has created a new food revolution. J Nutr. (2003) 133:3907S–10S. doi: 10.1093/jn/133.11.3907s
9. Mózsik, G, and Figler, M. In Nutrition in Health and Disease—Our Challenges Now and Forthcoming Time. London, UK: IntechOpen, (2019) 2(3951):611–5.
10. Ahmedsham, M, Amza, N, and Tamiru, M. Review on milk and milk product safety, quality assurance and control. Int J Livestock Prod. (2018) 9:67–78. doi: 10.5897/ijlp2017.0403
11. Pyz-Łukasik, R, Paszkiewicz, W, Tatara, MR, Brodzki, P, and Bełkot, Z. Microbiological quality of milk sold directly from producers to consumers. J Dairy Sci. (2015) 98:4294–301. doi: 10.3168/jds.2014-9187
12. Girma, K, Tilahun, Z, and Haimanot, D. Review on milk safety with emphasis on its public health. World J Dairy Food Sci. (2014) 9:166–83. doi: 10.5829/idosi.wjdfs.2014.9.2.85184
13. Jayarao, BM, Pillai, SR, Sawant, AA, Wolfgang, DR, and Hegde, NV. Guidelines for monitoring bulk tank milk somatic cell and bacterial counts. J Dairy Sci. (2004) 87:3561–73. doi: 10.3168/jds.S0022-0302(04)73493-1
14. Barkema, HW, Schukken, YH, and Lam, TJGM. Management practices associated with low, medium, and high somatic cell counts in bulk milk. J Dairy Sci. (1998) 81:1917–27. doi: 10.3168/jds.S0022-0302(98)75764-9
15. Schukken, Y, Wilson, D, Welcome, F, Garrison-Tikofsky, L, and Gonzalez, R. Monitoring udder health and milk quality using somatic cell counts. Vet Res. (2003) 34:579–96. doi: 10.1051/vetres:2003028
16. Ruegg, APL, and Pantoja, JCF. Understanding and using somatic cell counts to improve milk quality. Irish J Agric Food Res. (2013) 52:101–17.
17. Chye, FY, Abdullah, A, and Ayob, MK. Bacteriological quality and safety of raw milk in Malaysia. Food Microbiol. (2004) 21:535–41. doi: 10.1016/j.fm.2003.11.007
18. Molineri, AI, Signorini, ML, Cuatrín, AL, Canavesio, VR, Neder, VE, Russi, NB, et al. Association between milking practices and psychrotrophic bacterial counts in bulk tank milk. Rev Argent Microbiol. (2012) 44:187–94.
19. Didanna, HL, Mengistu, A, Kuma, T, and Kuma, B. Improving milk safety at farm-level in an intensive dairy production system: relevance to smallholder dairy producers. Food Qual Safety. (2018) 2:135–43. doi: 10.1093/fqsafe/fyy009
20. Nyokabi, S, Luning, PA, de Boer, IJM, Korir, L, Muunda, E, Bebe, BO, et al. Milk quality and hygiene: knowledge, attitudes and practices of smallholder dairy farmers in Central Kenya. Food Control. (2021) 130:108303. doi: 10.1016/j.foodcont.2021.108303
21. Hagnestam-Nielsen, C, Emanuelson, U, Berglund, B, and Strandberg, E. Relationship between somatic cell count and milk yield in different stages of lactation. J Dairy Sci. (2009) 92:3124–33. doi: 10.3168/JDS.2008-1719
22. Ma, Y, Ryan, C, Barbano, DM, Galton, DM, Rudan, MA, and Boor, KJ. Effects of somatic cell count on quality and shelf-life of pasteurized fluid milk. J Dairy Sci. (2000) 83:264–74. doi: 10.3168/jds.S0022-0302(00)74873-9
23. Potter, TL, Arndt, C, and Hristov, AN. Increased somatic cell count is associated with milk loss and reduced feed efficiency in lactating dairy cows. J Dairy Sci. (2018) 101:9510–5. doi: 10.3168/jds.2017-14062
24. Hadrich, JC, Wolf, CA, Lombard, J, and Dolak, TM. Estimating milk yield and value losses from increased somatic cell count on US dairy farms. J Dairy Sci. (2018) 101:3588–96. doi: 10.3168/jds.2017-13840
25. Schewe, RL, Kayitsinga, J, Contreras, GA, Odom, C, Coats, WA, Durst, P, et al. Herd management and social variables associated with bulk tank somatic cell count in dairy herds in the eastern United States. J Dairy Sci. (2015) 98:7650–65. doi: 10.3168/jds.2014-8840
26. DeLong, KL, Lambert, DM, Schexnayder, S, Krawczel, P, Fly, M, Garkovich, L, et al. Farm business and operator variables associated with bulk tank somatic cell count from dairy herds in the southeastern United States. J Dairy Sci. (2017) 100:9298–310. doi: 10.3168/jds.2017-12767
27. Schwarz, D, Kleinhans, S, Reimann, G, Stückler, P, Reith, F, Ilves, K, et al. Investigation of dairy cow performance in different udder health groups defined based on a combination of somatic cell count and differential somatic cell count. Prev Vet Med. (2020) 183:105123. doi: 10.1016/j.prevetmed.2020.105123
28. Mondini, S, Gislon, G, Zucali, M, Sandrucci, A, Tamburini, A, and Bava, L. Risk factors of high somatic cell count and differential somatic cells in early lactation associated with selective dry cow therapy. Animal. (2023) 17:100982. doi: 10.1016/j.animal.2023.100982
29. Vu, NH, Lambertz, C, and Gauly, M. Factors influencing milk yield, quality and revenue of dairy farms in southern Vietnam. Asian J Anim Sci. (2016) 10:290–9. doi: 10.3923/ajas.2016.290.299
30. Naing, YW, Wai, SS, Lin, TN, Thu, WP, Htun, LL, Bawm, S, et al. Bacterial content and associated risk factors influencing the quality of bulk tank milk collected from dairy cattle farms in Mandalay region. Food Sci Nutr. (2019) 7:1063–71. doi: 10.1002/fsn3.945
31. Leelahapongsathon, K, Schukken, YH, and Suriyasathaporn, W. Quarter, cow, and farm risk factors for intramammary infections with major pathogens relative to minor pathogens in Thai dairy cows. Trop Anim Health Prod. (2014) 46:1067–78. doi: 10.1007/s11250-014-0603-8
32. Prescott, SC, and Breed, RS. The determination of the number of body cells in milk by a direct method. J Infect Dis. (1910) 7:632–40. doi: 10.1093/infdis/7.5.632
33. Sudarwanto, MB In: H Pisestyani, editor. Inspection of quality and safety of milk and its processed products. 2nd ed. Bogor: PT Penerbit IPB Press (2020)
34. Beuchat, LR, Copeland, F, Curiale, MS, Danisavich, T, Gangar, V, King, BW, et al. Comparison of the simplate(TM) total plate count method with petrifilm(TM), redigel(TM), and conventional pour-plate methods for enumerating aerobic microorganisms in foods. J Food Prot. (1998) 61:14–8. doi: 10.4315/0362-028X-61.1.14
35. Zorn, CJW. Generalized estimating equation models for correlated data: a review with applications. Am J Pol Sci. (2001) 45:470–90. doi: 10.2307/2669353
36. Cui, J. QIC program and model selection in GEE analyses. Stata J. (2007) 7:209–20. doi: 10.1177/1536867X0700700205
37. Miskiyah Study of Indonesian National Standard for liquid Milk in Indonesia. Jurnal Standardisasi (2011) 13:1–7. Available at: https://js.bsn.go.id/index.php/standardisasi/article/download/3/pdf
38. Bortolami, A, Fiore, E, Gianesella, M, Corro, M, Catania, S, and Morgante, M. Evaluation of the udder health status in subclinical mastitis affected dairy cows through bacteriological culture, somatic cell count and thermographic imaging (2015) 18:799–805. doi: 10.1515/pjvs-2015-0104,
39. Torres, AH, Rajala-schultz, J, Degraves, FJ, and Hoblet, KH. Using dairy herd improvement records and clinical mastitis history to identify subclinical mastitis infections at dry-off. J Dairy Res. (2008) 75:240–7. doi: 10.1017/S0022029908003257
40. Leach, KA, Green, MJ, Breen, JE, Huxley, JN, Macaulay, R, Newton, HT, et al. Use of domestic detergents in the California mastitis test for high somatic cell counts in milk. Vet Rec. (2008) 163:566–70. doi: 10.1136/vr.163.19.566
41. Kandeel, SA, Megahed, AA, Ebeid, MH, and Constable, PD. Ability of milk pH to predict subclinical mastitis and intramammary infection in quarters from lactating dairy cattle. J Dairy Sci. (2019) 102:1417–27. doi: 10.3168/jds.2018-14993
42. Busanello, M, Rossi, RS, Cassoli, LD, Pantoja, JCF, and Machado, PF. Estimation of prevalence and incidence of subclinical mastitis in a large population of Brazilian dairy herds. J Dairy Sci. (2017) 100:6545–53. doi: 10.3168/jds.2016-12042
43. El, AA. Correlation between some direct and indirect tests for screen detection of subclinical mastitis. Int Food Res J. (2014) 21:1249–54.
44. Skrzypek, R, Wójtowski, J, and Fahr, R. Factors affecting somatic cell count in cow bulk tank milk – a case study from Poland. J Vet Med. (2004) 51:127–31. doi: 10.1111/j.1439-0442.2004.00611.x
45. Ramírez, NF, Keefe, G, Dohoo, I, Sánchez, J, Arroyave, O, Cerón, J, et al. Herd- and cow-level risk factors associated with subclinical mastitis in dairy farms from the High Plains of the northern Antioquia, Colombia. J Dairy Sci. (2014) 97:4141–50. doi: 10.3168/jds.2013-6815
46. Treurniet, M. The potency of quality incentives: evidence from the Indonesian dairy value chain. Am J Agric Econ. (2021) 103:1661–78. doi: 10.1111/ajae.12176
47. Saenger, C, Qaim, M, Torero, M, and Viceisza, A. Contract farming and smallholder incentives to produce high quality: experimental evidence from the Vietnamese dairy sector. Agric Econ. (2013) 44:297–308. doi: 10.1111/agec.12012
48. Rajesh, R, Bong, K, Park, J, and Young, J. Application and environmental risks of livestock manure. J Korean Soc Appl Biol Chem. (2013) 56:497–503. doi: 10.1007/s13765-013-3184-8
49. Manyi-loh, CE, Mamphweli, SN, Meyer, EL, Makaka, G, Simon, M, and Okoh, AI. An overview of the control of bacterial pathogens in cattle manure. Int J Environ Res Public Health. (2016) 13:843. doi: 10.3390/ijerph13090843
50. Bicudo, JR, and Goyal, SM. Pathogens and manure management systems: a review. Environ Technol. (2003) 24:115–30. doi: 10.1080/09593330309385542
51. Dohmen, W, Neijenhuis, F, and Hogeveen, H. Relationship between udder health and hygiene on farms with an automatic milking system. J Dairy Sci. (2010) 93:4019–33. doi: 10.3168/jds.2009-3028
52. Zigo, F, Vasil', M, Ondrašovičová, S, Výrostková, J, Bujok, J, and Pecka-Kielb, E. Maintaining optimal mammary gland health and prevention of mastitis. Front Vet Sci. (2021) 8:1–17. doi: 10.3389/fvets.2021.607311
53. Robles, I, Kelton, DF, Barkema, HW, Keefe, GP, Roy, JP, Von, KMAG, et al. Bacterial concentrations in bedding and their association with dairy cow hygiene and milk quality. Animal. (2020) 14:1052–66. doi: 10.1017/S1751731119002787
54. Suranindyah, Y, Wahyuni, E, Bintara, S, and Purbaya, G. The effect of improving sanitation prior to milking on milk quality of dairy cow in farmer group. Procedia Food Sci. (2015) 3:150–5. doi: 10.1016/j.profoo.2015.01.016
55. Islam, MA, Islam, MN, Khan, MAS, Rashid, MH, and Obaidullah, SM. Effect of different hygienic condition during milking on bacterial count of cows’ milk. Bangladesh J Anim Sci. (2009) 38:108–14. doi: 10.3329/bjas.v38i1-2.9919
56. Gleeson, D, Brien, OB, Flynn, J, Callaghan, OE, and Galli, F. Effect of pre-milking teat preparation procedures on the microbial count on teats prior to cluster application. Ir Vet J. (2009) 62:461–7. doi: 10.1186/2046-0481-62-7-461
57. Ingawa, KH, Adkinson, RW, and Gough, RH. Evaluation of a gel teat cleaning and sanitizing compound for premilking hygiene. J Dairy Sci. (1992) 75:1224–32. doi: 10.3168/jds.S0022-0302(92)77871-0
58. Brightling, PB, Dyson, RD, Hope, AF, and Penry, J. A national programme for mastitis control in Australia: countdown Downunder. Ir Vet J. (2009) 62:S52–8. doi: 10.1186/2046-0481-62-S4-S52
59. Fadillah, A, van den Borne, B, Poetri, ON, Hogeveen, H, Umberger, W, Hetherington, JB, et al. Smallholders’ milk quality awareness in Indonesian dairy farms. J Dairy Sci. (2023) 106:7965–73. doi: 10.3168/jds.2023-23267
60. Hetherington, J, Umberger, W, Akzar, R, Granzin, B, Ritchie, Z, Daryanto, A, et al. Improving milk supply, competitiveness and livelihoods of smallholder dairy chains in Indonesia (IndoDairy). Canberra: ACIAR (2023). 1–116 p.
61. Bartlett, PC, Miller, GY, Lance, SE, and Heider, LE. Environmental and managerial determinants of somatic cell counts and clinical mastitis incidence in Ohio dairy herds. Prev Vet Med. (1992) 14:195–207. doi: 10.1016/0167-5877(92)90016-9
62. Dong, F, Hennessy, DA, and Jensen, HH. Factors determining milk quality and implications for production structure under somatic cell count standard modification. J Dairy Sci. (2012) 95:6421–35. doi: 10.3168/jds.2012-5522
63. Al, AM, Hasanah, H, and Agus, A. The potency of traditional market vegetable waste as ruminant feed in the special region of Yogyakarta. Adv Anim Vet Sci. (2021) 9:1416–23. doi: 10.17582/journal.aavs/2021/9.9.1416.1423
64. Bradley, AJ, Leach, KA, Green, MJ, Gibbons, J, Ohnstad, IC, Black, DH, et al. The impact of dairy cows’ bedding material and its microbial content on the quality and safety of milk – a cross sectional study of UK farms. Int J Food Microbiol. (2018) 269:36–45. doi: 10.1016/j.ijfoodmicro.2017.12.022
65. Metzger, SA, Hernandez, LL, Skarlupka, JH, Suen, G, Walker, TM, and Ruegg, PL. Influence of sampling technique and bedding type on the milk microbiota: results of a pilot study. J Dairy Sci. (2018) 101:6346–56. doi: 10.3168/jds.2017-14212
66. Zehner, MM, Farnsworth, RJ, Appleman, RD, Larntz, K, and Springer, JA. Growth of environmental mastitis pathogens in various bedding materials. J Dairy Sci. (1986) 69:1932–41. doi: 10.3168/jds.S0022-0302(86)80620-8
67. Rowbotham, RF, and Ruegg, PL. Association of bedding types with management practices and indicators of milk quality on larger Wisconsin dairy farms. J Dairy Sci. (2015) 98:7865–85. doi: 10.3168/jds.2015-9866
68. Hogan, JS, Smith, KL, Hoblet, KH, Todhunter, DA, Schoenberger, PS, Hueston, WD, et al. Bacterial counts in bedding materials used on nine commercial dairies. J Dairy Sci. (1989) 72:250–8. doi: 10.3168/jds.S0022-0302(89)79103-7
69. Burton, P, Gurrin, L, and Sly, P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. (1998) 17:1261–91. doi: 10.1002/(SICI)1097-0258
70. Neuhaus, JM, Kalbfleisch, JD, and Hauck, WW. A comparison of cluster-specific and population-averaged approaches for analyzing correlated binary data. Int Stat Rev. (1991) 59:25–35. doi: 10.2307/1403572
Keywords: dairy cattle, udder health, milk quality, total plate count, somatic cell count, generalized estimating equations
Citation: Fadillah A, van den Borne BHP, Poetri ON, Hogeveen H, Slijper T, Pisestyani H and Schukken YH (2023) Evaluation of factors associated with bulk milk somatic cell count and total plate count in Indonesian smallholder dairy farms. Front. Vet. Sci. 10:1280264. doi: 10.3389/fvets.2023.1280264
Edited by:
Chong Wang, Iowa State University, United StatesReviewed by:
Haben Fesseha Gebremeskel, Wolaita Sodo University, EthiopiaAlda F. A. Pires, University of California, Davis, United States
Copyright © 2023 Fadillah, van den Borne, Poetri, Hogeveen, Slijper, Pisestyani and Schukken. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Achmad Fadillah, YWNobWFkLmZhZGlsbGFoQHd1ci5ubA==;YWNobWFkZmFkaWxsYWhAYXBwcy5pcGIuYWMuaWQ=
 Achmad Fadillah
Achmad Fadillah Bart H. P. van den Borne
Bart H. P. van den Borne Okti Nadia Poetri
Okti Nadia Poetri Henk Hogeveen
Henk Hogeveen Thomas Slijper
Thomas Slijper Herwin Pisestyani
Herwin Pisestyani Ynte H. Schukken
Ynte H. Schukken