
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 28 November 2023
Sec. Comparative and Clinical Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1275964
 Jessica L. Graves1†
Jessica L. Graves1† Brennen A. McKenzie1*†
Brennen A. McKenzie1*† Zane Koch1
Zane Koch1 Alexander Naka1
Alexander Naka1 Nathaniel Spofford2
Nathaniel Spofford2 JoAnn Morrison2
JoAnn Morrison2Objective: The aim of this study is to evaluate age, sex, body weight, breed, neuter status, and age at neutering as risk factors for diagnosis of osteoarthritis in companion dogs.
Animals: Dogs seen as patients at Banfield Pet Hospital in the United States from 1998 to 2019 with a date of death in 2019. The final cohort consisted of 131,140 dogs.
Methods: In this retrospective cohort study, Cox proportional hazard models were used to test for associations between osteoarthritis incidence and age at baseline, sex, maximum body weight, maximum body condition score, neuter status, and age at neutering. The same model was used to test these associations in 12 representative breeds, chosen based on breed weight and sample size.
Results: Older age, higher adult body weight, gonadectomy, and younger age at gonadectomy were significantly associated with higher risks of osteoarthritis in the total cohort and in all 12 breeds evaluated. Higher body condition scores and sex were also significantly associated with osteoarthritis but with minimal effect sizes in the overall cohort, and these risk factors were not consistently significant in all breeds tested.
Clinical relevance: These results will assist veterinarians in identifying dogs at higher risk for osteoarthritis and applying appropriate diagnostic, preventative, and treatment interventions. An understanding of potentially modifiable risk factors, such as body condition and neutering, will support evidence-based discussions with dog owners about risk management in individual patients.
Musculoskeletal pain and lameness are among the most common clinical problems seen in companion dogs (1–3). A large proportion of these cases involve osteoarthritis (OA), the most common joint disorder in dogs. There is inconsistency in the literature regarding the terminology for this condition. Terms such as “osteoarthritis,” osteoarthrosis, and “degenerative joint disease” may be used interchangeably, or some authors may use different terms for conditions with specific etiology or pathogenesis. In this report, we use the term “osteoarthritis” to refer to a diagnosis identified in the sample dataset by any of the following structured diagnostic codes: osteoarthritis, arthritis or degenerative joint disease—excluding rheumatoid, septic, or immune-mediated arthritic conditions.
Prevalence of OA ranges from 2.5% to over 80%, depending on study methods and population characteristics (1, 4, 5). This variation likely reflects true differences in prevalence between populations, related to differences in breed, age, husbandry, and other causal factors, as well as differences in diagnostic methods and case definitions between studies (6).
A progressive, incurable condition, OA can affect comfort and quality of life for a substantial proportion of a patient's life (4). This condition compromises the welfare of affected dogs and places a significant burden on human caregivers (7). Musculoskeletal pain and locomotor dysfunction, often due to OA, are also among the most frequent reasons for euthanasia in dogs (8–11).
Currently, the primary approach to mitigating the negative impact of canine OA is treatment once clinical signs have manifested. Earlier detection and interventions to prevent or delay the development of OA could have significant benefits. Effective approaches for preventing or delaying the development of OA depend on a clear understanding of relevant risk factors and identifying individuals at increased risk.
Many putative risk factors have been associated with the development and progression of canine OA (6). There is strong evidence for the role of genetic factors influencing both individual risk and breed differences in susceptibility to OA. These factors are often associated indirectly with OA, causing predisposition conditions such as hip or elbow dysplasia or a propensity for cranial cruciate ligament rupture, which then leads to the development of arthritis (6, 12).
Body weight is another factor associated with OA risk. However, studies often do not clearly distinguish between body size and body condition. Larger breeds appear to be at greater risk, as do individuals who are overweight or obese, but the relationship between these different body-size variables is not always clear (6, 13–16).
Osteoarthritis is considered a disease of aging, and increased age is often associated with increased OA prevalence. This association is potentially complicated, however, by the lack of surveillance and diagnostic markers of early, pre-clinical joint disease. Predisposing conditions and early OA may be present undetected in young dogs, while older individuals may be more likely to be diagnosed with OA because of greater diagnostic attention or because the condition has progressed to more apparent clinical signs.
The evidence is limited and conflicting for many potential OA risk actors. Sex, for example, is often associated with OA prevalence, but both male and female dogs have been reported to be at increased risk, and the potential for confounding by body size, activity, and neuter status is high (6).
One of the most debated risk factors for OA is neuter status (6, 17–20). While most reports indicate neutered dogs are at higher risk than intact dogs, the details of the relationship between neuter status and OA are unclear. For example, this association seems to be consistently true for large-breed dogs and is less often found in smaller breeds (18). Neutered dogs are also at greater risk for obesity, and the degree to which the relationship between neutering and OA is mediated or confounded by body condition is often uncertain (6, 16, 21–23).
Some studies find that age at neutering influences the impact of gonadectomy on the risk of OA and important predisposing conditions, such as cruciate ligament disease (16, 20, 24). This suggests that gonadal hormones are protective primarily through effects of skeletal development, and neutering after puberty or skeletal maturity may be less likely to promote OA.
However, other studies report residual increased risk in dogs neutered after skeletal maturity and suggest that gonadal hormones may have an ongoing protective effect (16, 25). There is also significant variation in the existence and strength of associations between neutering and orthopedic disease found in different breeds and research studies (18). Many other factors, including diet, activity patterns, and even birth month, have been associated with the risk of OA, but detailed causal links have not been clearly identified (6).
From a preventative-medicine perspective, risk factors for OA can be considered modifiable or non-modifiable. Most genetic factors are not directly modifiable in individuals, and the risk presented by specific genotypes, breed, and conformation is fixed at birth or during development. Recent advances in the study of epigenetics suggest that it may be possible to mitigate the impact of some genes through environmental modification of the regulation of gene activity, but clinical interventions for doing so have not yet been validated (21, 26). On a population level, genetic factors which influence the occurrence of OA can potentially be modified by selective breeding targeting both individual genes associated with increased OA risk as well as conformations that predispose to the disease (26, 27).
Other factors associated with OA are clearly modifiable in individuals, including body weight and body condition, diet, activity patterns, and neutering practices.
Common dietary recommendations to delay or prevent OA include reduced feeding to prevent obesity and modulate skeletal development in growing puppies. Lifelong caloric restriction in a cohort of Labrador retrievers delayed the onset of hip OA and reduced the severity of the disorder (28, 29). Less definitive effects were reported for elbow and shoulder OA (30, 31). Whether these effects were due solely to differences in body condition or other influences of caloric restriction is uncertain.
Experimental studies have also shown that reduction in caloric and calcium content of diets can reduce the risk of developmental abnormalities in giant-breed dogs, such as hip dysplasia, that frequently lead to OA later in life (32, 33). Other nutritional interventions may also influence the development of OA and predisposing conditions (34), but there is still significant uncertainty about the efficacy of most dietary approaches.
Greater clarity about the role of key risk factors in the development of canine OA would be useful for informing preventative strategies. The purpose of this study was to examine selected risk factors for the development of OA in a large, retrospective cohort study of companion dogs using medical record data from primary care veterinary practices. We examined previously reported risk factors, including age, sex, breed, body weight, and body condition. We also sought to further investigate the relationships among OA, neuter status, and age at neutering, as well as the variability of these relationships among breeds.
By the end of 2019, the Banfield Pet Hospital network included 1,084 primary care small animal hospitals in 42 U.S. states, the District of Columbia, and Puerto Rico. All hospitals used the same proprietary practice information management system (PIMS; PetWare®) to enter patient and visit information, and the resulting electronic medical records were uploaded nightly to a central data warehouse. The PIMS contained both structured and unstructured fields for data entry. Structured data included diagnoses and clinical signs, physical examination findings, and invoiced services and medications; unstructured data included narrative text related to subjective and objective observations, patient assessment, and treatment plan.
All in-network visits from dogs with a death date in 2019 were extracted from the data warehouse, with visit information ranging from 1998 to 2019. Patient information for each visit included breed, sex, neuter status, visit age, and visit weight. Visit information included the location and reason for the visit, all structured clinical signs and diagnoses, examination findings related to musculoskeletal issues and body condition, and any invoiced items or services related to the diagnosis or treatment of osteoarthritis. Invoiced items or services related to in-hospital euthanasia were also extracted to more accurately identify the patient death date.
To improve data quality, the visit-level data were cleaned and evaluated for data entry errors. First, only visits in which the dog received a physical examination by a veterinarian were included. Some data were removed due to likely data entry errors. These include visits in which there were discrepant entries, visit dates occurring after 2019, weights recorded over 300 lbs, or dogs that had implausible data values (e.g., negative age). To minimize cohort effects, only dogs born after 1997 were included for analysis. Finally, the body condition score (BCS) assessment has changed over time (e.g., the use of 3-point, 5-point, and 9-point BCS scales), and scores were harmonized and converted into a single 9-point scale (Table 1).
Osteoarthritis was defined as the earliest diagnosis of the following structured diagnostic codes: osteoarthritis, arthritis, or degenerative joint disease—excluding rheumatoid, septic, or immune-mediated arthritic conditions. The diagnosis of OA was at the discretion of the individual clinician, without prespecified or standardized criteria or requirements for imaging or other specific criteria.
Follow-up time was calculated as the number of years between a dog's age at their first visit and their age at OA diagnosis or death, whichever occurred first—dogs with follow-up times >20 years were removed. To capture the incidence of aging-related OA, dogs that were not observed into maturity (2 years and up) or who already had OA at their first visit were not included in the analysis.
Desexing status was defined based on veterinarian assessment and if the dog was gonadectomized at a Banfield clinic. Age at desexing was defined as age during a visit with a desexing procedure. Age at desexing was only ascertained for dogs desexed at a Banfield clinic. To preserve temporality between desexing as a risk factor and OA incidence, dogs desexed after being diagnosed with OA were treated as intact. Dogs desexed after 5 years of age were not included in age at desexing analyses, as desexing later has a higher likelihood of being a therapeutic rather than an elective procedure.
Multiple weight and BCS metrics were created. To estimate the effects of maximum body size or condition, the 75th percentile of adult (2+ years of age) body weights and BCS were used to approximate a “maximum” weight or BCS. The 75th percentiles were used to minimize the influence of outliers. Median weight and BCS between ages 1.5 and 2.5 years of age were used to approximate the weight and BCS at developmental maturity. Not all dogs were seen between 1.5 and 2.5 years of age. In these cases, weight or BCS at desexing (so long as desexing occurred after full development, e.g., 2+ years of age) was used.
To estimate the effects of weight gain after desexing, the 75th percentiles of adult weight and BCS were used. As many dogs are desexed before 2 years of age, this value is equivalent to maximum body weight defined above. However, in the case where desexing occurred after 2 years of age, the 75th percentile was calculated using only observations. Percent change in weight and BCS after desexing was calculated as 100*(observation after desexing—observation at maturity)/observation at maturity.
The majority of dogs seen at Banfield are on a wellness plan (a set of pre-paid services meant to enhance the provision of preventive care services). Wellness plan data are structured within the system, so the wellness plan variable was converted to a binary variable encoding if a dog ever was on a wellness plan. Dogs ∧whose wellness plan started at or after OA diagnosis were treated as “never” having been on a plan.
Mean, standard deviation (SD), median, 25th and 75th percentiles, and ranges were calculated for continuous measures: age at baseline (the age at first visit on record), mature and maximum weight, age at OA or death, and duration of follow-up (years). Observation counts and proportions were calculated for categorical variables: sex (Female or Male), mature and maximum BCS (1–3 = Too thin; 4–5 = Ideal; 6–7 = Overweight, 8–9 = Obese), gonadectomy status (desexed or intact), age at desexing (<6 months, 6–12 months, 12–24 months, 24–60 months), and ever had a Banfield wellness plan (Yes or No). For all variables, the number of missing values was reported. All descriptive statistics are reported for the total cohort and stratified by OA diagnosis.
Survival analysis was used to characterize the incidence of OA in companion dogs. The analyses were conducted using the duration of follow-up as the measure of survival time, calculated as the years between a dog's first visit and their OA onset or death. This choice of follow-up time as the survival time scale enables easier estimation of age as a risk factor for OA onset. Dogs were right-censored at death.
A Cox proportional hazards model (35) was used to test joint associations of OA risk factors. In particular, age at baseline, max weight, max BCS, desexing status, and sex were used as risk factors. The Cox model was stratified, separating dogs on (76.3%) and not on (23.7%) wellness plans (Table 2). As the dogs on wellness plans were more closely observed on average, the likelihood of OA diagnosis in these dogs was higher (Supplementary Figure 1), mostly likely due to potential detection bias, and for this reason, a stratified Cox proportional hazards model was used. This stratified model accounts for this wellness plan-associated surveillance bias by allowing the baseline hazard of dogs in these groups to be different (36). On average, dogs on wellness plans visited Banfield 25.06 times (SD 18.54), while dogs not on a wellness plan visited only 4.67 times (SD 6.85).
After fitting the Cox proportional hazards model, using the coxph function from the survival package (37) in R, the proportional hazards assumption was assessed using cox.zph and visualization of residuals. The assumption being met, hazard ratios (HR), and their 95% confidence intervals (CI) were estimated to test the magnitude and direction of each risk factor on time to OA diagnosis. To account for the risk factors having varying scales of values, standardized HRs were calculated for continuous risk factors (e.g., age at baseline) by standardizing the risk factor (mean centering and dividing by the standard deviation). For all models, both the natural scale and standardized HRs and 95% CIs are reported, along with the concordance index and number of observations.
The marginal effects of each risk factor on OA-free survival time were plotted to better understand the magnitude of effect sizes. Marginal effects reflect the average effect of a given risk factor, holding all other covariates in the model constant. To visualize the effects of continuous risk factors, predicted OA-free survival curves were estimated based on quartile values or clinical relevance. To visualize the marginal effects of categorical variables, each level was used to predict OA-free survival curves. For risk factors not being directly visualized, continuous values were assigned as cohort median values (e.g., median weight) and categorical variables were assigned as a proportion (e.g., proportion male). As the majority of dogs were on wellness plans, strata were assigned to being on a wellness plan. For all interaction models, median survival times and their 95% CIs are also reported.
For breeds with at least 500 observations, binomial tests of proportion were used to compare breed-specific OA incidence rates against the cohort-wide incidence rate, using Bonferroni adjusted p-value to adjust for multiple comparisons. Given the diversity and size of the cohort, risk factors of OA were also evaluated in 12 representative breeds, chosen based on breed weight and sample size. First, the maximum body weight of all dogs in the cohort was split into quartiles, and then breeds were assigned to a weight quartile based on the mean maximum weight of all dogs of that breed. Then, the top three breeds within each quartile with the most samples were chosen for further analysis. This ensures the representation of a wide range of dog weights while not including breeds lacking sufficient sample size to make substantive conclusions. The same stratified Cox regression model used to test primary risk factors of OA was applied to each of these 12 breeds.
The analytic cohort included all dogs with complete data for age at baseline and maximum body weight observations. As there were many variables of interest and differential patterns of missingness across those variables, the data were not further restricted to complete data only. Instead, all available data for relevant variables tested were included in the modeling.
All analyses were performed in R version 4.1.2. Type I error was set to α = 0.05 for all statistical tests.
Descriptive statistics of the final cohort resulting from the data processing steps outlined are reported in Table 2. The final cohort included 131,140 dogs, 31,365 (23.9%) of which were diagnosed with OA during the course of the study. The cohort of dogs had an average age entering the study of 5.12 years (SD = 4.33), average mature weight of 41.8 lbs (SD = 31.0), and 63,541 (48.5%) dogs were female. There were 318 unique breeds present in the study, with Labrador Retrievers (n = 11,718), Chihuahuas (n = 10,147), and Yorkshire Terriers (n = 6,570) as the most frequent breeds observed.
Not all variables generated during the data processing steps outlined above contain observable data. The relatively high proportion of missing data in mature BCS is due to strict construct definitions to generate this variable. Mature BCS was defined as the median BCS score between 1.5 and 2.5 years—many dogs did not have visits during this window in which BCS was assessed. Similarly, age at desexing could only be quantified for dogs gonadectomized at a Banfield hospital, which could be confidently matched to a visit date. Approximately 20% of dogs in this cohort were desexed at Banfield, of which 1% were desexed after receiving an OA diagnosis, and 9.4% were desexed > 5 years of age. To preserve temporality and capture patterns of elective desexing, age at desexing > 5 years of age or after OA were set to missing and are therefore not included in age at desexing analyses.
To explore if the effects of previously reported risk factors were recapitulated in this expansive cohort, we fit the stratified Cox proportional hazards model using age at baseline, desexed status (desexed/intact), sex (male/female), adult weight, and adult BCS as our primary risk factors, and treated wellness plan as a stratifying variable. Stratification and the presence of being on a wellness plan were supported by evidence of a surveillance (or detection) bias in dogs that were on wellness plans (Supplementary Figure 1).
Table 3 and Figure 1 show that older age [Std. HR = 3.70 (3.64–3.76)], higher adult body weight (Std. HR = 1.83 (1.81–1.85)], and desexing [Std. HR = 1.37 (1.31–1.43)] are statistically significantly associated with higher risks of OA in this cohort. While hazard ratios for adult BCS and sex were both statistically significant, these effect sizes are minimal (Figure 1).
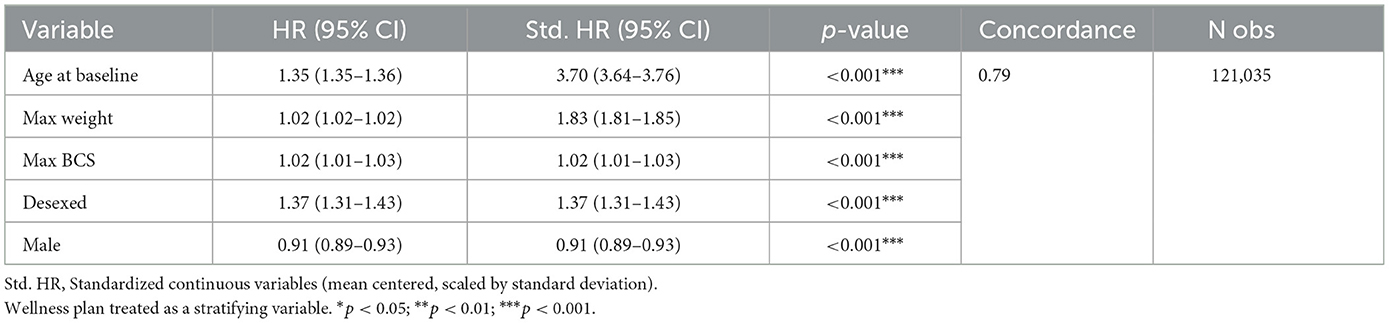
Table 3. Stratified Cox proportional hazard model testing the associations of primary risk factors on OA-free survival.
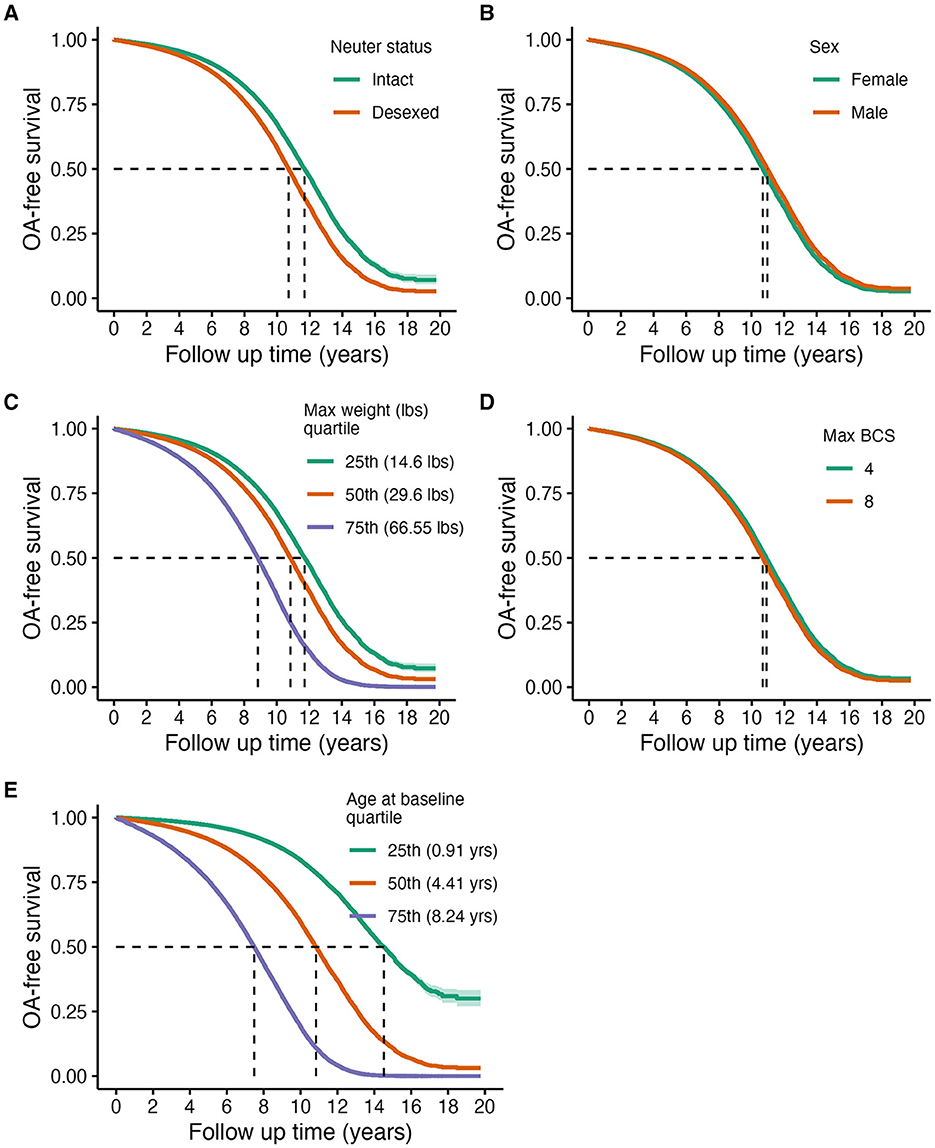
Figure 1. Predicted marginal OA-free survival curves of primary risk factors of OA: neuter status (A), sex (B), maximum weight (C), maximum BCS (D), and age at baseline (E). Assignments for generating predicted survival curves: median values for continuous covariates not being directly tested, proportions for binary variables, and assuming the presence of a wellness plan. Quantiles were used for continuous variable groupings being visualized, except for BCS, which were determined based on clinical relevance.
To explore if the effects of these risk factors were consistent across breeds, the same primary risk factor model was fit to a subset of breeds. The top three most frequent breeds within each weight quartile were selected as an analysis subset (see Statistical Methods: Breed-Specific Analyses section for more information). These breeds include Chihuahua, Yorkshire Terrier, Maltese, Shih Tzu, Pug, Dachshund, Beagle, Pit Bull, Siberian Husky, Labrador Retriever, German Shepherd, and Golden Retriever (Supplementary Table 1 and Supplementary Figure 2). A comparison of OA rates across all breeds with at least 500 observations can be found in Supplementary Figure 3. Notably, compared to the overall incidence rate of OA in the cohort, Chow Chow's had the largest OA risk, while French Bulldogs had the lowest.
Figure 2 summarizes these results using a dot-and-whisker plot of the standardized HR and 95% CI for each of the risk factors within each breed. Higher weight and older age are consistently associated with higher risks of OA across breeds. However, the effect of desexing on OA incidence shows variability across breeds, where desexing does not appear to be a risk factor for Yorkshire Terriers, Maltese, Shih Tzus, Pugs, Dachshunds, and Beagles. The effects of BCS also differ across breeds, where effects are more pronounced in smaller breeds. HRs and 95% CIs are reported in Supplementary Table 2.
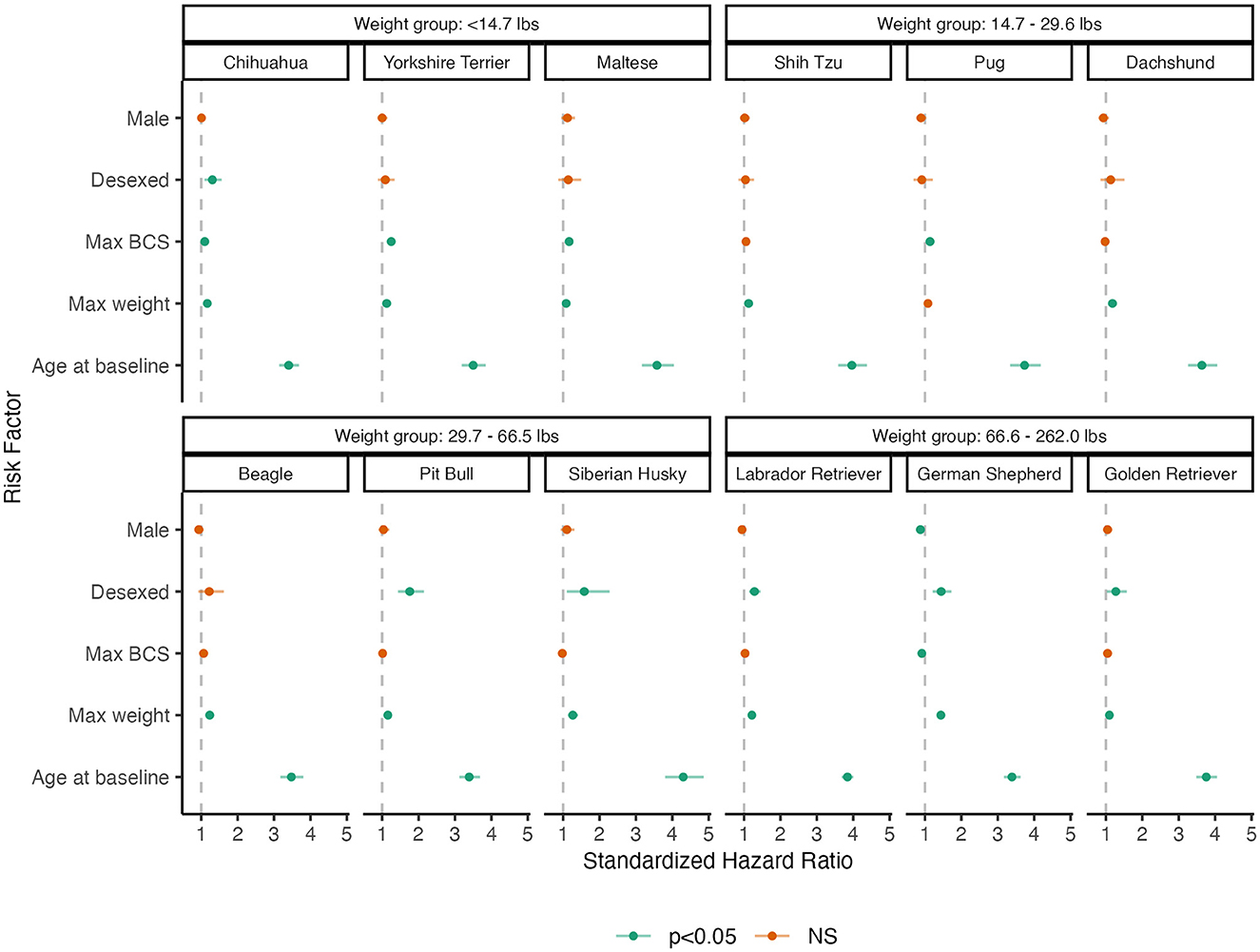
Figure 2. Dot-and-whisker plot results of breed-specific analysis of primary risk factors of OA. Dots and whiskers reflect the standardized HR and 95% CIs associated with each risk factor, respectively. Each panel reflects the model fit to an individual breed, where panels are grouped by weight groups. Color assignments designate the statistical significance of the HR.
Both weight and desexing are independent risk factors of OA. However, it has been reported that dogs that are desexed may be more likely to develop obesity (22, 38), which may increase the risk of OA. To test if the influence of higher body weight on OA-free survival differed by desexing status, the interaction between adult weight and desexing status was tested.
There was a statistically significant interaction between desexing and adult weight [Std. HR = 1.07 (1.03, 1.11), Table 4], suggesting that the increased risk of OA in larger dogs is greater in those that are desexed compared to intact dogs. Figure 3 shows the predicted survival curves as well as the predicted median survival times for each weight class and desexing group. Both desexed and intact dogs show that higher body weight is associated with higher OA risk; however, this risk appears slightly larger in desexed dogs than intact dogs.

Table 4. Stratified Cox proportional hazard model testing the interaction between desexing and maximum adult weight and BCS.
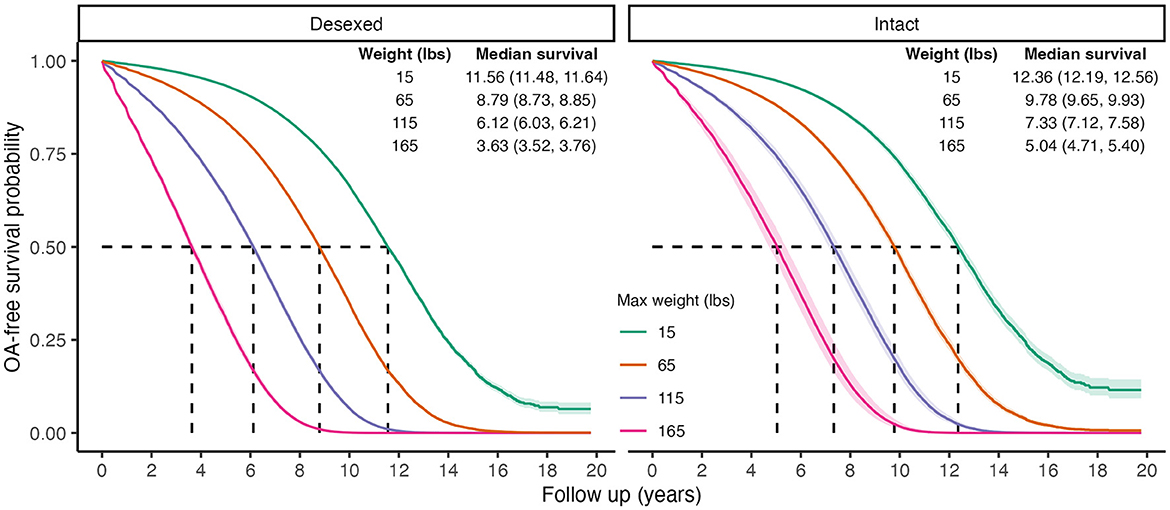
Figure 3. Predicted marginal OA-free survival curves to visualize interaction effects between weight and desexing status. Color indicates maximum weight values. Median survival times and 95% CIs are reported. To generate predictions, median values were used for continuous covariates, proportions for binary variables, and the presence of wellness plan were used.
Limitations of the modeling approach used in Table 2 and Figure 1 include the lack of temporal precedence—specifically, the inability to test subsequent weight gain after desexing and its influence on OA incidence. To test this hypothesis, percent changes in maximum weight within desexed dogs (calculated as 100*[maximum weight—mature weight]/mature weight) was tested as a risk factor for OA.
Both main effects of percent weight change as well as the interaction with mature body weight were tested in the full cohort and in a sensitivity analysis restricted to dogs whose age at desexing was ≤2 years of age. Mature body weight was treated as a covariate as larger-breed dogs are less likely to have larger percent changes in body weight compared to smaller-breed dogs. To determine if weight changes were independent of desexing status, these analyses were performed in intact dogs as well, where percent changes in weight reflected overall changes from mature body weight to maximum body weight.
Results in Table 5 show that a positive percent change in body weight was associated with a higher risk of OA [full cohort Std. HR = 1.12 (1.09–1.15); age desexing ≤ 2 Std. HR = 1.12 (1.09–1.15)]. There was also a significant interaction effect between the percent change in body weight after desexing and mature body size [Std. HR = 1.09 (1.05–1.12)], which was held in the dogs desexed ≤2 years of age. These results suggest that percent increases in body weight after desexing are a risk factor for dogs of all sizes, and these same increases may have more deleterious effects in larger dogs compared to smaller dogs.
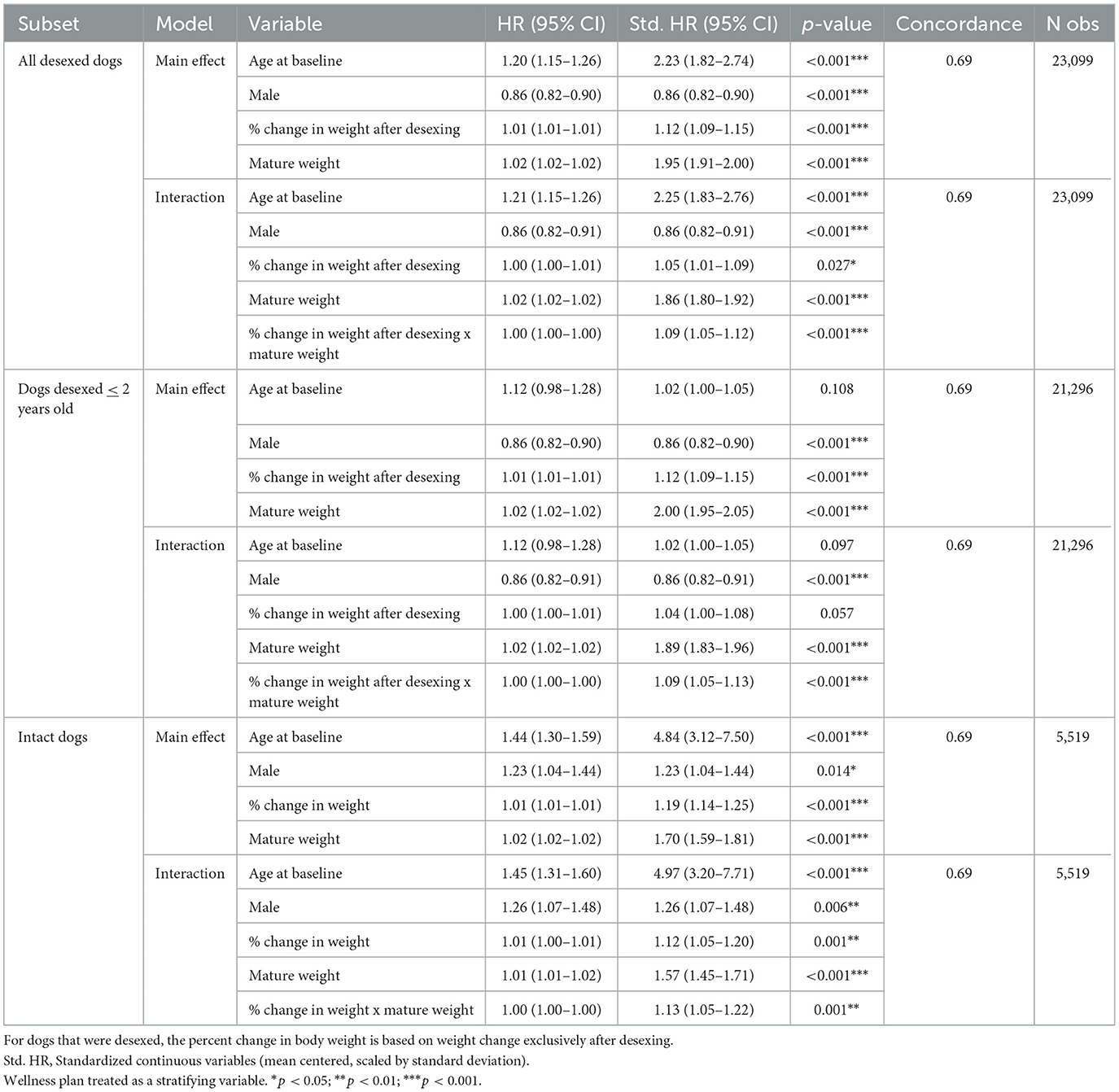
Table 5. Stratified Cox proportional hazard model testing both the main effects and interaction effects of percent change in body weight in desexed dogs, dogs desexed ≤2 years of age, and intact dogs, respectively.
Figure 4 visualizes the finding that percent increases in body weight after desexing were associated with higher OA risks in larger dogs compared to smaller dogs. It is important to note that 50% increases in body weight become less likely as mature body size increases, and the use of 50% increments is used only to facilitate interpretation of the direction and magnitude of effects. In small dogs, a 50% increase is associated with approximately a 9-month decrease in median time to OA. In contrast, in large dogs, a 50% increase in weight is associated with a 16-month decrease in time to OA.

Figure 4. Predicted marginal OA-free survival curves illustrating the interaction effect between percent weight change after desexing and mature body weight. Color indicates percent change values. Median survival times and 95% CIs are reported. To generate predictions, median values were used for continuous covariates, proportions for binary variables, and the presence of wellness plan were used.
As BCS is a distinct construct from weight or body size, percent changes in body condition score after desexing were also tested as a risk factor for OA. Paralleling the approach used in the percent change in weight models, both main effects of percent BCS change and the interaction with mature BCS were tested in the full cohort and in a sensitivity analysis restricted to dogs whose age at desexing was ≤2 years of age. Mature body BCS was treated as a covariate as dogs with higher BCS at maturity are less likely to have larger BCS changes in BCS than dogs with lower BCS at maturity. To determine if weight changes were independent of desexing status, these analyses were performed in intact dogs as well, where percent changes in BCS reflected overall changes from mature BCS to maximum BCS.
Results indicate that increases in percent change in BCS are associated with a higher risk of OA [Std. HR = 1.12 (1.05–1.20), Table 6] and that these effects may be stronger in dogs with higher mature BCS [Std. HR = 1.16 (1.04–1.29), Table 6]. However, limited variability in BCS in this cohort makes determining the clinical significance of these effects difficult. As seen in Supplementary Figures 5, 6, standard errors from model predictions show significant overlap, except in dogs that have higher mature BCS, where effects begin to appear strongest. However, it is important to note that due to there being a ceiling in the BCS tool, dogs with mature BCS of 7 can only experience at most a percent change increase of 28% [28% = 100*(9–7)/7].
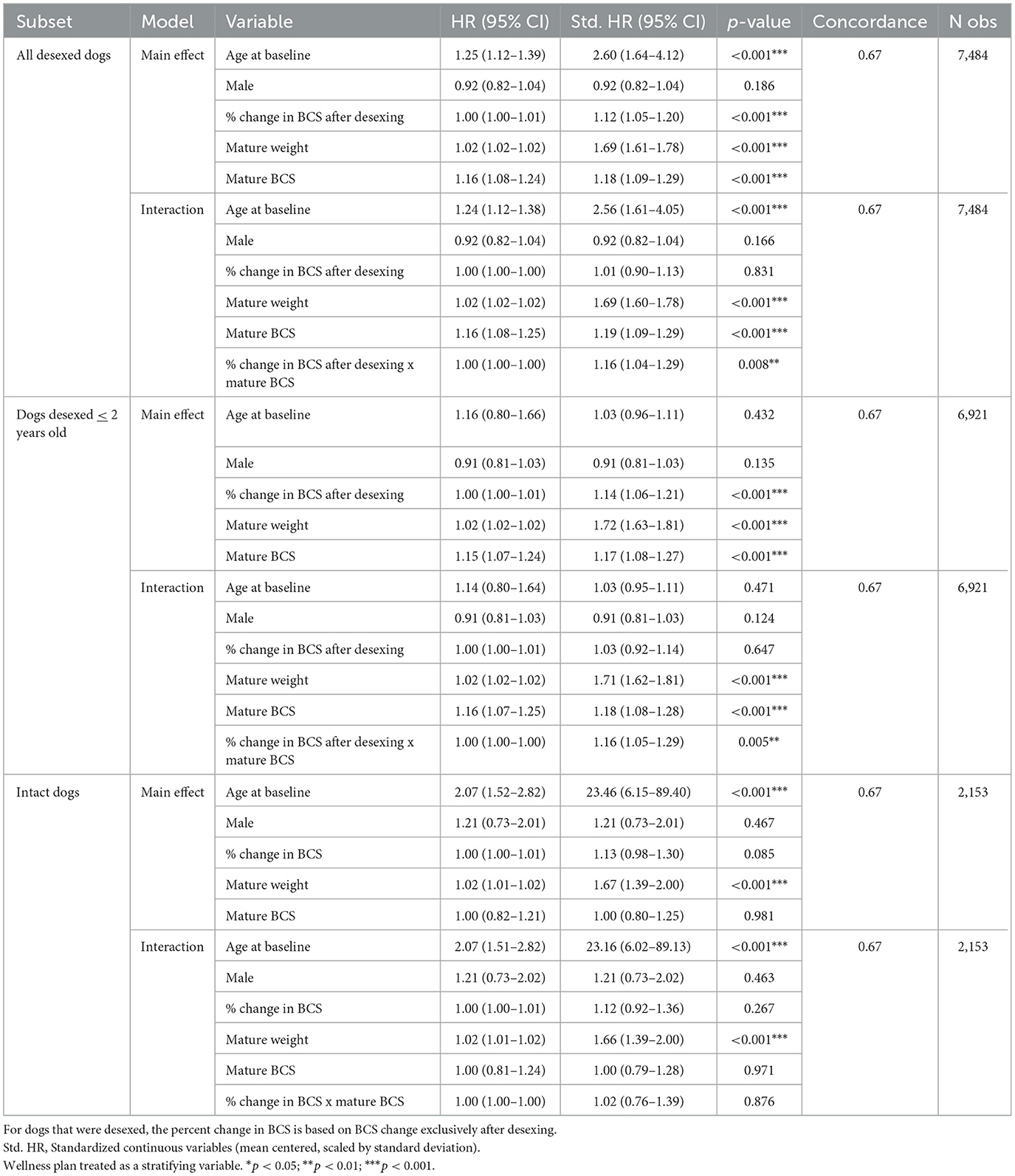
Table 6. Stratified Cox proportional hazard model testing both the main effects and interaction effects of percent change in BCS in desexed dogs, dogs desexed ≤2 years of age, and intact dogs, respectively.
These analyses were also performed in intact dogs, where percent changes in BCS reflect overall changes from mature BCS to maximum BCS to identify any changes across desexing status, where percent changes in BCS were not statistically significantly associated with OA incidence.
To test the hypothesis that age at desexing is a risk factor for OA, age at desexing was added to the primary risk factor model. Age at desexing was tested as a continuous variable and a categorical variable (<6 months, 6–12 months, 12–24 months, and 24–60 months). Age at desexing was significantly inversely associated with OA risk [Std. HR = 0.90 (0.86–0.94) Table 7]. Categorical analysis shows that relative to dogs desexed before 6 months of age, dogs desexed 6–12 months are 13% less likely to develop OA, while dogs that are desexed 12–24 or 24–60 months are 28 and 32% less likely to develop OA, respectively. This categorical model suggests a potential non-linear relationship between age at desexing and OA risk, such that the effects of age at desexing diminish as dogs get older. To test this, a sensitivity analysis was performed, adding a quadratic term in the continuous age at desexing model. The quadratic term for age at desexing was statistically significant [Std. HR = 1.35 (1.22–1.49) Table 7], and predictions are visualized in Figure 5. Results from this quadratic model more closely relate to the categorical model, providing additional evidence that the magnitude of the OA-related risk associated with age at desexing diminishes.
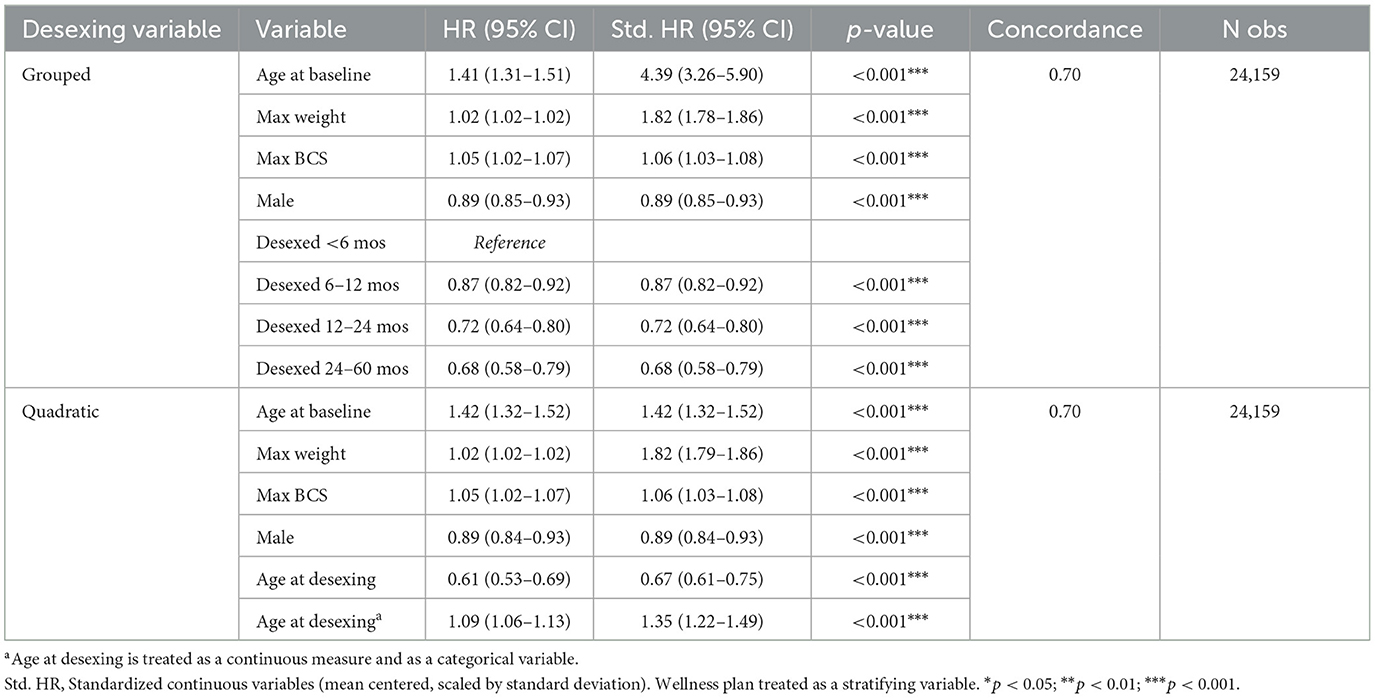
Table 7. Stratified Cox proportional hazard model testing the role of age at desexing as a risk factor for OA.
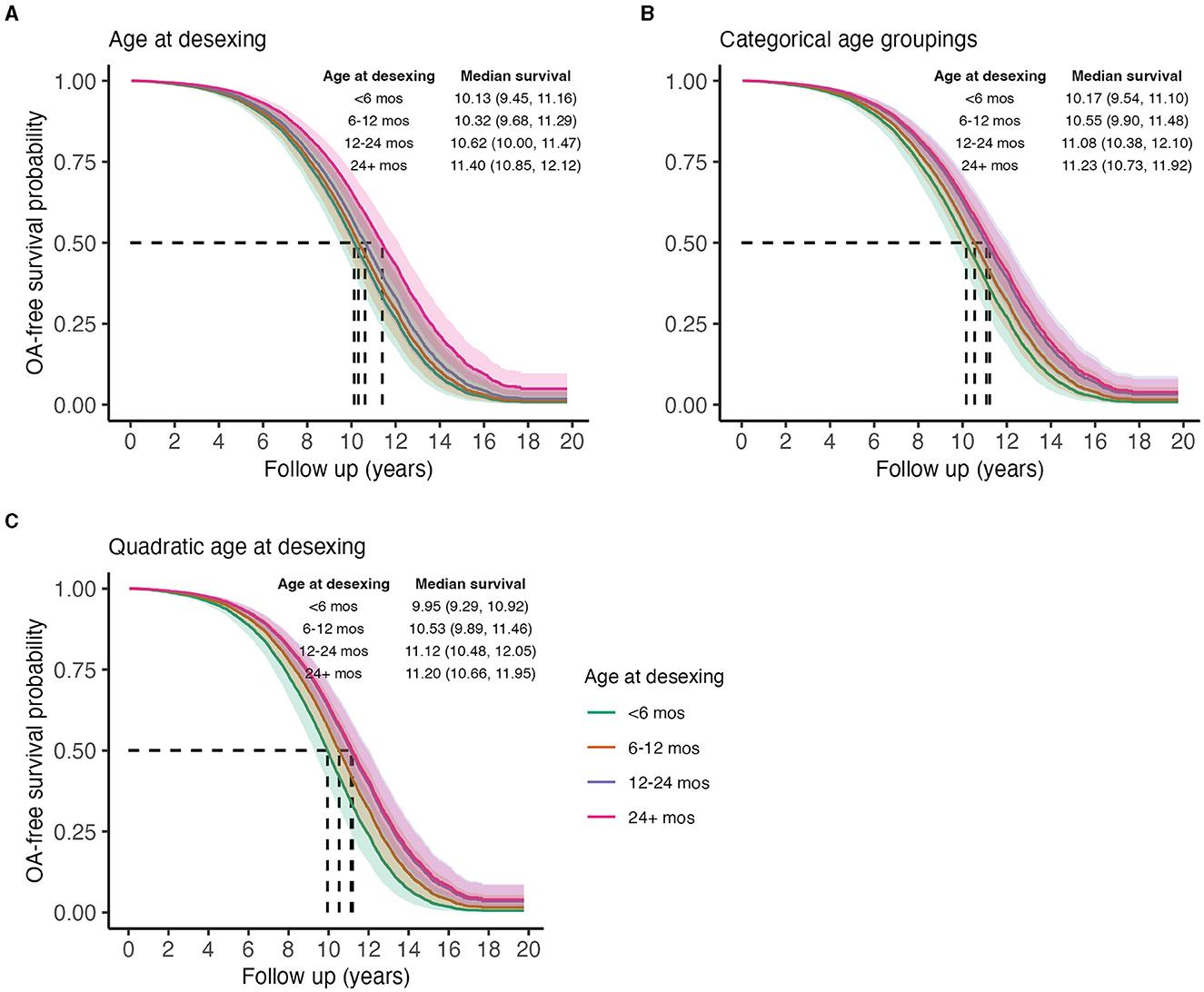
Figure 5. Predicted marginal OA-free survival curves illustrating the effect of age at desexing on OA incidence, treating age as a linear continuous variable (A), a categorical variable (B), or a quadratic variable (C). Color indicates ages at desexing. Median survival times and 95% CIs are reported. To generate predictions, median values were used for continuous covariates, proportions for binary variables, and the presence of wellness plan were used.
The proportion of dogs in this population that developed OA was 24.9%. It is difficult to compare this to occurrence data from other studies due to differences in study populations, case definitions, and other methodological factors. Previous analyses of primary practice databases in the UK, for example, reported a 1-year prevalence of OA in dogs of any age of 2.34% (1) and 2.5% (4). An owner survey, also in the UK, reported an overall prevalence of 2.23% (39).
In contrast, a proprietary industry survey of 200 veterinarians in the U.S. reported an overall prevalence of 20% (5) in dogs over 1 year of age, a figure frequently cited in reviews and other articles discussing the condition. A more recent study involving dogs 5–12 years of age and over 11 kg in weight presenting for dental prophylaxis found that 68% of dogs had radiographic evidence of OA in one or more joints (40). However, owner surveys from a subset of the population reported no clinical signs in 71% of the dogs, and these would likely not have received a diagnosis of OA in routine clinical practice. Such variability creates significant uncertainty about the true rate of occurrence of OA in dogs.
In this study, the incidence of 24.9% represents the clinical diagnosis of OA at any age and by any criteria in dogs from a primary care population who died in 2019, with no fixed interval or duration of follow-up. Diagnoses could be based on clinical impression, history and physical examination, radiographic interpretations, or other diagnostic procedures. Given the large size and broad geographic distribution of the study population, this should be reasonably representative of the occurrence of OA as routinely identified by general practice clinicians in companion dogs in the U.S.
Detection bias in retrospective cohort studies can influence the apparent prevalence of a condition and the apparent impact of risk factors for the disease (41). Owners of dogs in this population were offered various wellness plans, which consist of prepaid packages of services that can reduce the cost of clinic visits and some diagnostic tests, including radiographs. Enrollment in such a wellness plan has previously been suggested to be a risk factor for detection bias (38), and in this study, it was strongly associated with OA diagnosis. Dogs with a wellness plan had far more clinic visits and were more likely to have radiographs than dogs not enrolled in such a plan (data not shown), likely leading to a higher rate of detection of OA. This factor was controlled for by stratification in the analysis of other putative risk factors.
Age was by far the strongest risk factor for OA diagnosis, consistent with the established understanding of OA as a disease of aging. This effect was consistent across breeds of different sizes and with different rates of OA occurrence (Figure 2 and Supplementary Table 2). Diagnosis of OA in individual patients is more likely as time passes, because it is an incurable condition and so will be present and available for diagnosis longer and because it is progressive and so more likely to become clinically apparent as a dog ages.
In previous studies, sex has been inconsistently identified as a risk factor for OA (6). In this study, male dogs were at lower risk, though the size of this effect in the overall population was small. Within breeds, male dogs were at lower risk in some breeds but not others, independent of body weight and BCS (Figure 2 and Supplementary Table 2).
All types of OA were included in the case definition for this study, regardless of which joint was affected or whether a predisposing condition, such as joint dysplasia or cranial cruciate ligament rupture, was identified. Previous evaluations of sex as a risk factor for OA have focused more narrowly on particular joints, predisposing conditions, or breeds, and it is likely that the role of sex as a risk factor may vary among these (4). The small overall risk difference between male and female dogs suggests that diagnostic and preventative measures should be directed equally at dogs of both sexes.
Previous studies have shown that OA risk differs markedly between breeds (6). Breed is a complex risk factor involving differences in genetic makeup, body size and conformation, and likely also lifestyle variables influenced by owners, such as feeding practices and the type and intensity of activity. Because of the enormous phenotypic variability among dogs, evaluation of the role of body weight in OA risk can easily confound the effects of body size and breed with those of overweight.
In general, larger body size is often identified as a risk factor for OA in dogs. This pattern was confirmed in this study, with increased mature body weight positively associated with OA risk. When OA risk was compared among breeds, larger breeds had higher risk, consistent with previous findings (Supplementary Figures 2, 3 and Supplementary Table 2).
In an effort to disentangle the effects of body size and overweight to some extent, we examined the interaction between the percentage increase above mature body weight and the risk for OA. This analysis showed that weight gain after maturity, likely representing excess adipose mass, increases the risk of OA. This effect is greater in larger dogs, possibly due to an exacerbation of the already elevated risk posed by larger body size or because the absolute mechanical burden of weight gained after maturity is greater in larger dogs.
Ideally, body condition scores should help to isolate overweight and obesity as risk factors independent of mature body size, so it was expected that BCS would be a strong predictor of OA risk. In this study population, 40.1% of dogs were classed as overweight (BCS 6–7/9) and 4.1% as obese (BCS 8–9/9), and the proportion of overweight and obesity was higher in the dogs that developed OA (overweight = 50.8%, obese = 5.3%) than in those that did not (overweight = 36.7%, obese = 3.8%). These figures are broadly consistent with previous reports, though the estimated prevalence of overweight and obesity varies widely depending on the population, time period, assessment methods, and category definitions (22, 42–44).
While BCS was significantly correlated with OA risk, the size of this effect was very small. This relationship was stronger in smaller dogs, unlike the effect of weight gain after maturity. Overall, BCS was not a powerful predictor of OA diagnosis. A possible explanation for this unexpected result may be the practical challenges in the implementation of BCS scoring. There are several scoring systems in use by veterinarians, and the validation data for these are limited, involving relatively small numbers of dogs, breeds, and clinicians (45–48). Some studies have reported that BCS scoring correlates well with objective measures of body fat (47). However, the strength of this correlation varies significantly between breeds. The consistency and reliability of BCS scoring among veterinarians scoring dogs of many different breeds and conformations in general practice is uncertain.
Various assessment tools for body condition were used at different times in the evaluation of this population, and scores from a 3-point and a 5-point scale were mapped onto a 9-point scale for analysis. In principle, a system with more gradations should allow for a more accurate distinction between degrees of overweight among dogs and help to identify more clearly the relationship between BCS and clinical conditions such as OA. In practice, however, the distribution of BCS scores in this dataset was clearly discontinuous, with the vast majority of dogs scored as 5/9 (45.4%) or 7/9 (35%). This suggests that clinicians tended to lump dogs into broad “normal” and “overweight” categories rather than utilizing BCS scoring as a more finely graded, continuous measure.
Although this was true across all sizes of dogs, there was greater use of the full range of scores, particularly those below 5/9, in smaller dogs (Supplementary Figure 4). This suggests that clinicians may find it easier to distinguish gradations of body condition in smaller breeds. The fact that BCS was a stronger predictor of OA in smaller dogs, among which BCS scores were more granular, supports the possibility that the lumping of many dogs into either “normal” or “overweight” obscured the overall relationship between BCS and OA.
Further evaluation of the reliability of extant BCS scoring systems in heterogenous general practice populations and the development and validation of alternative assessment tools that obviate the limitations of BCS would be useful in clarifying the role of overweight and obesity as risk factors for disease.
Neuter status has been consistently identified as a risk for OA, with intact individuals at lower risk than neutered dogs. There is also evidence that earlier neutering is associated with an increased risk of OA and conditions predisposing to OA (hip and elbow dysplasia and cranial cruciate ligament rupture) (6, 16, 18), though this effect may only be significant in larger dogs (over approximately 43 lbs in one recent analysis) (18).
This study is consistent with previous findings that the risk of OA is increased following desexing in most medium and large breed dogs (over 30 lbs) and in some smaller breeds. While predisposing conditions were not evaluated, the impact of neutering on OA risk overall increased with body size, and neutering was less frequently associated with OA risk in smaller breeds, supporting the apparent greater significance of neutering as a risk factor in larger dogs.
Neutering is also an established risk factor for obesity (16, 22, 23, 38), and it is possible that one way in which neutering increases OA risk is by increasing the propensity for overweight and obesity. Because of challenges in the interpretation of BCS scores, it was difficult to compare the occurrence of overweight and obesity between intact and neutered dogs. The effect of body weight gain after maturity on OA risk was not different between intact and neutered dogs, so it is at least clear that overweight and obesity are important risk factors in all dogs regardless of neuter status. While increasing the incidence of overweight and obesity may be one mediator of the impact of neutering on OA risk, this hypothesis could not be directly evaluated in this study.
One previous report, which directly evaluated this relationship in golden retrievers, found that gonadectomy was a risk factor for overweight and obesity but that body condition only mediated 7% of the association between neutering and orthopedic disease in those neutered dogs at increased risk of OA and cruciate ligament disease (only the dogs in this cohort neutered before 6 months of age showed this increased risk for orthopedic disease) (16).
In this study, age at neutering was inversely associated with OA risk in the total population, and risk decreased progressively with delayed neutering up to 2 years of age. However, categorical and quadratic analyses suggest that the impact of age on OA risk is greatest in dogs neutered earliest and that the effects of age at desexing on OA risk diminish as dogs get older. This is broadly consistent with previous studies that have found the risk for OA and related orthopedic conditions to be greatest in dogs neutered before full maturity (16, 24).
This effect was the same in dogs of different body sizes, which is not consistent with some previous reports suggesting earlier neutering (before 12 months of age) increased the risk of conditions such as hip and elbow dysplasia and cruciate ligament rupture, mostly in larger dogs (18). The cohort evaluated in this study was very large, so the power to detect such a difference in risk associated with size should be higher than in other studies. Therefore, these data suggest the increase in OA risk associated with younger age at neutering may apply to dogs of all sizes, at least in similar, heterogenous primary care populations. Differences between this study and others in the appearance of a size effect for this risk factor may be explained by differences in detection strategies, case definitions, or study populations.
This study confirms the importance of several key risk factors for OA in dogs, including age, body weight, and neutering. Chronological age itself is not, of course, modifiable, but the potential to delay the onset of age-associated health conditions, including OA, has been demonstrated in dogs and numerous other species (28, 29). The most successful strategy for achieving this in research animals, severe caloric restriction, is not practical in companion dogs. Such a strategy requires precisely formulated diets and extensive monitoring to prevent malnutrition, and it can actually reduce lifespan in some animals due to nutrition/gene interactions (49, 50). Feeding is also a critical element of the human–animal bond, and asking dog owners to drastically underfeed their canine companions is unlikely to be accepted by many. However, caloric restriction research has done much to elucidate the general mechanisms of aging, raising the possibility that pragmatic therapies to delay aging-associated diseases may be developed.
Body weight is a function of both breed and body condition. Breed risks for specific health conditions, such as OA, can potentially be modified in populations by selective breeding and in individuals by nutritional interventions and, theoretically, the use of drugs targeting the physiologic characteristics of specific breeds that increase disease risk.
Body condition can be altered by nutrition and exercise. Caloric restriction has been shown to be effective at preventing and treating overweight and obesity in dogs (28, 51, 52). Physical activity may also reduce the development of obesity (16, 53). Despite the unexpectedly small impact of BCS score on OA risk in this study, OA incidence was greater in overweight and obese dogs than in dogs scored as normal weight. Weight gain after maturity, which likely reflects the accumulation of excess adipose, was also a significant risk factor for OA. These findings support the role of obesity as a risk factor for OA and the importance of maintaining optimal body condition in dogs to minimize OA risk. This is particularly important in larger dogs, who are at greatest risk of obesity and most significantly impacted by weight gain and other risk factors, such as neutering.
Neutering is an important modifiable risk factor for OA. Overall, gonadectomy appears to increase the risk of OA, particularly in larger dogs. Earlier neutering adds to this effect in both large and small dogs. The pathogenesis of OA, of course, is complex and incompletely elucidated, and the role of neutering should be understood in this context as one of many interacting risk factors. However, recognizing that an association exists between neutering and OA risk can help clinicians identify individuals at elevated risk and discuss this information with owners to help inform their decisions about neutering. This information can also trigger more proactive surveillance to ensure early diagnosis and appropriate preventative and therapeutic interventions in dogs at likely increased risk.
Any potential increase in OA risk associated with neutering must also be considered in the context of other risks and benefits associated with neutering on both an individual and population level. Changes in neutering practices intended to mitigate one health risk may entail unpredictable increases in other risks not yet identified, so judicious integration of such information with the larger context and the circumstances and needs of individual patients is critical.
Future research in such large, primary care cohorts can further clarify the relationship between putative risk factors and common aging-associated diseases such as OA. This information will support efforts to reduce the burden of these conditions on companion dogs and their caregivers by informing appropriate preventative interventions.
As a retrospective cohort study, this analysis relied on electronic medical records collected across thousands of clinics and veterinarians. While these data provide longitudinal insights into risk factors associated with incident diseases, the data themselves were not collected to test hypotheses surrounding risk factors for incident osteoarthritis. The study population (all dogs seen between 1998 and 2019 and dying in 2019) was large and expected to be representative of the patient population at Banfield during this period, but it may not be representative of the overall U.S. companion dog population.
Electronic health records rely on real-time recording of interactions among clinicians, clients, and patients. Many potentially useful variables cannot be considered because the relevant information is unavailable. One example in this dataset is the lack of detailed information about diet or feeding patterns. Information provided by clients, such as age, breed, and prior medical history, may also be subject to misclassification or recall bias. Similarly, information provided by veterinarians, such as diagnosis of OA and scoring of BCS, may be subject to misclassification bias and vary over time and between clinicians.
For example, a diagnosis of OA could be based on clinical impression, history and physical examination, radiographic interpretations, or other factors, and no standardized diagnostic criteria were in place. While this reflects the reality of OA diagnosis in general practice, the possibility of misclassification is greater when standardized and consistent diagnostic criteria are not used. Some differences in the association between exposures and OA might be seen in a population evaluated according to different or more restricted diagnostic criteria, as is often employed in prospective or university-based studies. Additionally, not all information relevant to the development of OA is captured in this dataset. Feeding practices, including type of diet, quantity, and feeding frequency, are not available for analysis.
Overall incidence rates may differ from true population-level incidence and should be interpreted with caution. Additionally, percentiles were used to summarize adult body weight and BCS across the follow-up window, which fails to capture the duration of time over or underweight. Finally, age at desexing was only known for dogs being desexed at a Banfield Pet Hospital clinic, which does not account for desexing occurring outside Banfield clinics.
The data analyzed in this study is subject to the following licenses/restrictions: Data is proprietary and contains unique patient identifiers so public access would compromise client/patient confidentiality. Requests to access these datasets should be directed to NS, bmF0ZS5zcG9mZm9yZEBlZmZlbS5jb20=.
JG: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. BM: Methodology, Project administration, Writing – original draft, Writing – review & editing. ZK: Data curation, Formal analysis, Writing – review & editing. AN: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. NS: Data curation, Resources, Writing – review & editing. JM: Resources, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank Banfield Pet Hospital for sharing the dataset analyzed in this project and the veterinarians and clients of Banfield Pet Hospital for generating this invaluable information resource.
JG, BM, ZK, and AN were employed by Loyal, a biotechnology company developing medicines to extend lifespan in dogs, during the data analysis and manuscript preparation. NS and JM are employed by Banfield Pet Hospital, the institution where the original clinical data were collected.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1275964/full#supplementary-material
1. O'Neill DG, James H, Brodbelt DC, Church DB, Pegram C. Prevalence of commonly diagnosed disorders in UK dogs under primary veterinary care: results and applications. BMC Vet Res. (2021) 17:1–14. doi: 10.1186/s12917-021-02775-3
2. Robinson NJ, Dean RS, Cobb M, Brennan ML. Investigating common clinical presentations in first opinion small animal consultations using direct observation. Vet Rec. (2015) 176:463–463. doi: 10.1136/vr.102751
3. Wolf S, Selinger J, Ward MP, Santos-Smith P, Awad M, Fawcett A. Incidence of presenting complaints and diagnoses in insured Australian dogs. Aust Vet J. (2020) 98:326–32. doi: 10.1111/avj.12981
4. Anderson KL, O'Neill DG, Brodbelt DC, Church DB, Meeson RL, Sargan D, et al. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci Rep. (2018) 8:1–12. doi: 10.1038/s41598-018-23940-z
5. Johnston SA. Osteoarthritis: Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. (1997) 27:699–723. doi: 10.1016/S0195-5616(97)50076-3
6. Anderson KL, Zulch H, O'Neill DG, Meeson RL, Collins LM. Risk factors for canine osteoarthritis and its predisposing arthropathies: a systematic review. Front Vet Sci. (2020) 7:220–220. doi: 10.3389/fvets.2020.00220
7. Belshaw Z, Dean R, Asher L. “You can be blind because of loving them so much”: The impact on owners in the United Kingdom of living with a dog with osteoarthritis. BMC Vet Res. (2020) 16:1–10. doi: 10.1186/s12917-020-02404-5
8. Pegram C, Gray C, Packer RMA, Richards Y, Church DB, Brodbelt DC, et al. Proportion and risk factors for death by euthanasia in dogs in the UK. Sci Rep. (2021) 11:9145. doi: 10.1038/s41598-021-88342-0
9. O'Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. Vet J Lond Engl. (2013) 198:638–43. doi: 10.1016/j.tvjl.2013.09.020
10. Urfer SR, Kaeberlein M, Promislow DEL, Creevy KE. Lifespan of companion dogs seen in three independent primary care veterinary clinics in the United States. Canine Med Genet. (2020) 7:7. doi: 10.1186/s40575-020-00086-8
11. Bonnett BN, Egenvall A. Age patterns of disease and death in insured Swedish dogs, cats and horses. J Comp Pathol. (2010) 142 (Suppl. 1):S33–38. doi: 10.1016/j.jcpa.2009.10.008
12. Clements DN, Carter SD, Innes JF, Ollier WER. Genetic basis of secondary osteoarthritis in dogs with joint dysplasia. Am J Vet Res. (2006) 67:909–18. doi: 10.2460/ajvr.67.5.909
13. Sanderson S. The epidemic of canine obesity and its role in osteoarthritis. Isr J Vet Med. (2012) 67:195–202.
14. Shahid M, Manchi G, Slunsky P, Naseer O, Fatima A, Leo B, et al. A systemic review of existing serological possibilities to diagnose canine osteoarthritis with a particular focus on extracellular matrix proteoglycans and protein. Pol J Vet Sci. (2017) 20:189–201. doi: 10.1515/pjvs-2017-0024
15. Smith GK, Mayhew PD, Kapatkin AS, McKelvie PJ, Shofer FS, Gregor TP. Evaluation of risk factors for degenerative joint disease associated with hip dysplasia in German Shepherd Dogs, Golden Retrievers, Labrador Retrievers, and Rottweilers. J Am Vet Med Assoc. (2001) 219:1719–24. doi: 10.2460/javma.2001.219.1719
16. Simpson M, Albright S, Wolfe B, Searfoss E, Street K, Diehl K, et al. Age at gonadectomy and risk of overweight/obesity and orthopedic injury in a cohort of Golden Retrievers. PLoS ONE. (2019) 14:e0209131. doi: 10.1371/journal.pone.0209131
17. Torres de la Riva G, Hart BL, Farver TB, Oberbauer AM, Messam LL, Willits N, et al. Neutering dogs: effects on joint disorders and cancers in golden retrievers. PLoS ONE. (2013) 8:e55937. doi: 10.1371/journal.pone.0055937
18. Hart BL, Hart LA, Thigpen AP, Willits NH. Assisting decision-making on age of neutering for 35 breeds of dogs: associated joint disorders, cancers, and urinary incontinence. Front Vet Sci. (2020) 7:388–388. doi: 10.3389/fvets.2020.00388
19. Hart BL, Hart LA, Thigpen AP, Willits NH. Long-term health effects of neutering dogs: comparison of Labrador Retrievers with Golden Retrievers. PLoS ONE. (2014) 9:e102241–e102241. doi: 10.1371/journal.pone.0102241
20. McKenzie B. Evaluating the benefits and risks of neutering dogs and cats. CABI Rev. (2010) 2010:1–18. doi: 10.1079/PAVSNNR20105045
21. Lefebvre SL, Yang M, Wang M, Elliott DA, Buff PR, Lund EM. Effect of age at gonadectomy on the probability of dogs becoming overweight. J Am Vet Med Assoc. (2013) 243:236–43. doi: 10.2460/javma.243.2.236
22. Lund EM, Armstrong PJ, Kirk CA, Klausner JS. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. Int J Appl Res Vet Med. (2006) 4:177–86.
23. Bjørnvad CR, Gloor S, Johansen SS, Sandøe P, Lund TB. Neutering increases the risk of obesity in male dogs but not in bitches — A cross-sectional study of dog- and owner-related risk factors for obesity in Danish companion dogs. Prev Vet Med. (2019) 170:104730–104730. doi: 10.1016/j.prevetmed.2019.104730
24. Spain CV, Scarlett JM, Houpt KA. Long-term risks and benefits of early-age gonadectomy in dogs. J Am Vet Med Assoc. (2004) 224:380–7. doi: 10.2460/javma.2004.224.380
25. Kutzler MA. Possible relationship between long-term adverse health effects of gonad-removing surgical sterilization and luteinizing hormone in dogs. Anim Open Access J MDPI. (2020) 10:599. doi: 10.3390/ani10040599
26. Grandi FC, Bhutani N. Epigenetic therapies for osteoarthritis. Trends Pharmacol Sci. (2020) 41:557–69. doi: 10.1016/j.tips.2020.05.008
27. Shen J, Abu-Amer Y, O'Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. (2016) 58:49–63. doi: 10.1080/03008207.2016.1208655
28. Kealy RD, Lawler DF, Ballam JM, Mantz SL, Biery DN, Greeley EH, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc. (2002) 220:1315–20. doi: 10.2460/javma.2002.220.1315
29. Kealy RD, Lawler DF, Ballam JM, Lust G, Smith GK, Biery DN, et al. Five-year longitudinal study on limited food consumption and development of osteoarthritis in coxofemoral joints of dogs. J Am Vet Med Assoc. (1997) 210:222–5.
30. Huck JL, Biery DN, Lawler DF, Gregor TP, Runge JJ, Evans RH, et al. A longitudinal study of the influence of lifetime food restriction on development of osteoarthritis in the canine elbow. Vet Surg. (2009) 38:192–8. doi: 10.1111/j.1532-950X.2008.00487.x
31. Runge JJ, Biery DN, Lawler DF, Gregor TP, Evans RH, Kealy RD, et al. The effects of lifetime food restriction on the development of osteoarthritis in the canine shoulder. Vet Surg VS. (2008) 37:102–7. doi: 10.1111/j.1532-950X.2007.00354.x
32. Hazewinkel H, Goedegebuure SA, Poulos P, Wolvekamp W. Influences of chronic calcium excess on the skeletal development of growing Great Danes. J Am Anim Hosp Assoc. (1985) 21:377–91.
33. Hedhammar A, Wu FM, Krook L, Schryver HF, De Lahunta A, Whalen JP, et al. Overnutrition and skeletal disease. An experimental study in growing Great Dane dogs. Cornell Vet. (1974) 5:5–160.
34. Manfredi S, Di Ianni F, Di Girolamo N, Canello S, Gnudi G, Guidetti G, et al. Effect of a commercially available fish-based dog food enriched with nutraceuticals on hip and elbow dysplasia in growing Labrador retrievers. Can J Vet Res. (2018) 82:154–8.
35. Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. (1972) 34:187–220. doi: 10.1111/j.2517-6161.1972.tb00899.x
36. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer. (2000).
37. Therneau TM, Grambsch PM. A Package for Survival Analysis in S, Version 2.38. Model Surviv Data Extending Cox Model. (2000). Available online at: http://cran.r-project.org/package=survival (accessed October 24, 2023).
38. Benka VA, Scarlett JM, Sahrmann J, Rieke K, Briggs JR, Ruple A, et al. Age at gonadectomy, sex, and breed size affect risk of canine overweight and obese outcomes: a retrospective cohort study using data from United States primary care veterinary clinics. J Am Vet Med Assoc. (2023) 2023:1–10. doi: 10.2460/javma.22.12.0596
39. Wiles BM, Llewellyn-Zaidi AM, Evans KM, O'Neill DG, Lewis TW. Large-scale survey to estimate the prevalence of disorders for 192 Kennel Club registered breeds. Canine Genet Epidemiol. (2017) 4:8. doi: 10.1186/s40575-017-0047-3
40. Millis D, Tichenor MG, Hecht S, Hunt T. Prevalence of osteoarthritis in dogs undergoing routine dental prophylaxis. In: World Small Animal Veterinary Association World Congress Proceedings. Cape Town (2014).
41. Combelles L, Corbiere F, Calavas D, Bronner A, Hénaux V, Vergne T. Impact of imperfect disease detection on the identification of risk factors in veterinary epidemiology. Front Vet Sci. (2019) 6:66. doi: 10.3389/fvets.2019.00066
42. Association for the Prevention of Pet Obesity. State of U.S. Pet Obesity 2022. Calabash, NC: Association for the Prevention of Pet Obesity (2022).
43. Chiang CF, Villaverde C, Chang WC, Fascetti AJ, Larsen JA. Prevalence, risk factors, and disease associations of overweight and obesity in dogs that visited the veterinary medical teaching hospital at the University of California, Davis from January 2006 to December 2015. Top Companion Anim Med. (2022) 48:100640–100640. doi: 10.1016/j.tcam.2022.100640
44. McGreevy PD, Thomson PC, Pride C, Fawcett A, Grassi T, Jones B. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet Rec. (2005) 156:695–702. doi: 10.1136/vr.156.22.695
45. Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract. (1997) 22:10–5.
46. Burkholder WJ. Use of body condition scores in clinical assessment of the provision of optimal nutrition. J Am Vet Med Assoc. (2000) 217:650–4. doi: 10.2460/javma.2000.217.650
47. Mawby DI, Bartges JW, d'Avignon A, Laflamme DP, Moyers TD, Cottrell T. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc. (2004) 40:109–14. doi: 10.5326/0400109
48. German AJ, Holden SL, Moxham GL, Holmes KL, Hackett RM, Rawlings JM, et al. Simple, reliable tool for owners to assess the body condition of their dog or cat. J Nutr. (2006) 136:2031S−3S. doi: 10.1093/jn/136.7.2031S
49. Lee MB, Hill CM, Bitto A, Kaeberlein M. Antiaging diets: separating fact from fiction. Science. (2021) 374:eabe7365. doi: 10.1126/science.abe7365
50. Nikolai S, Pallauf K, Huebbe P, Rimbach G. Energy restriction and potential energy restriction mimetics. Nutr Res Rev. (2015) 28:100–20. doi: 10.1017/S0954422415000062
51. Robertson ID. The association of exercise, diet and other factors with owner-perceived obesity in privately owned dogs from metropolitan Perth, WA. Prev Vet Med. (2003) 58:75–83. doi: 10.1016/S0167-5877(03)00009-6
52. Frye CW, Shmalberg JW, Wakshlag JJ. Obesity, Exercise and Orthopedic Disease. Vet Clin North Am Small Anim Pract. (2016) 46:831–41. doi: 10.1016/j.cvsm.2016.04.006
Keywords: dogs, osteoarthritis, degenerative joint disease (DJD), risk factors, incidence
Citation: Graves JL, McKenzie BA, Koch Z, Naka A, Spofford N and Morrison J (2023) Body weight, gonadectomy, and other risk factors for diagnosis of osteoarthritis in companion dogs. Front. Vet. Sci. 10:1275964. doi: 10.3389/fvets.2023.1275964
Received: 11 August 2023; Accepted: 02 November 2023;
Published: 28 November 2023.
Edited by:
Alasdair James Charles Cook, University of Surrey, United KingdomReviewed by:
Catarina Lavrador, University of Evora, PortugalCopyright © 2023 Graves, McKenzie, Koch, Naka, Spofford and Morrison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brennen A. McKenzie, YnJlbm5lbkBsb3lhbGZvcmRvZ3MuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.