- Department of Veterinary Medical Sciences, University of Bologna, Bologna, Italy
A 14-year-old female domestic short-haired cat with a diagnosed diabetes mellitus and acromegaly was presented for lethargy and dysorexia. On clinical presentation, the patient showed hyperglycemia, hyperthermia, dull mentation, and dehydration. With the suspicion of an inflammatory or infectious complication of diabetes, she was hospitalized with constant rate infusion of insulin, and empirical ampicillin sulbactam was started. Blood culture revealed positivity for Yersinia pseudotuberculosis and the septic picture was confirmed by blood analysis, with leukocytosis, neutrophilia, and an increased serum amyloid A concentration. The isolated Y. pseudotuberculosis strain showed susceptibility to every antimicrobial tested. During the second day of hospitalization, the onset of hypoglycemia and hypotension was treated with norepinephrine and glucose in fluid therapy. The cat recovered well and was discharged with insulin and amoxicillin-clavulanate. This is the first case of septicemia associated with Y. pseudotuberculosis in a cat, suspected of developing the infection after contact with natural reservoirs such as rodents or birds. This route of transmission should be highlighted especially in relation to the zoonotic potential of the bacteria.
1 Introduction
Yersinia pseudotuberculosis is a gram-negative aerobic or facultative anaerobic rod-shaped bacterium considered as a well-known infectious agent in both humans and animals. It was first described in 1883 (1), and according to the European Center for Disease Prevention and Control (2), is the agent responsible for yersiniosis together with Yersinia enterocolitica, the third most prevalent foodborne disease in Europe. In humans, Y. pseudotuberculosis infections typically occur by the oro-fecal route, after the ingestion of contaminated food, water, or milk (3) or after contact with animals or their environment (4, 5). Birds and rodents are considered the natural reservoirs (6, 7). In animals, it has been described as an infectious agent in a broad spectrum of species, including pets. In cats, it was first reported in 1967 in the UK (8), but since then, only a few cases of Y. pseudotuberculosis infections in cats have been published (9–13). The aim of this case report is to describe for the first time a septicemia associated with Y. pseudotuberculosis in a cat presented to an Italian Veterinary University Hospital.
2 Case description
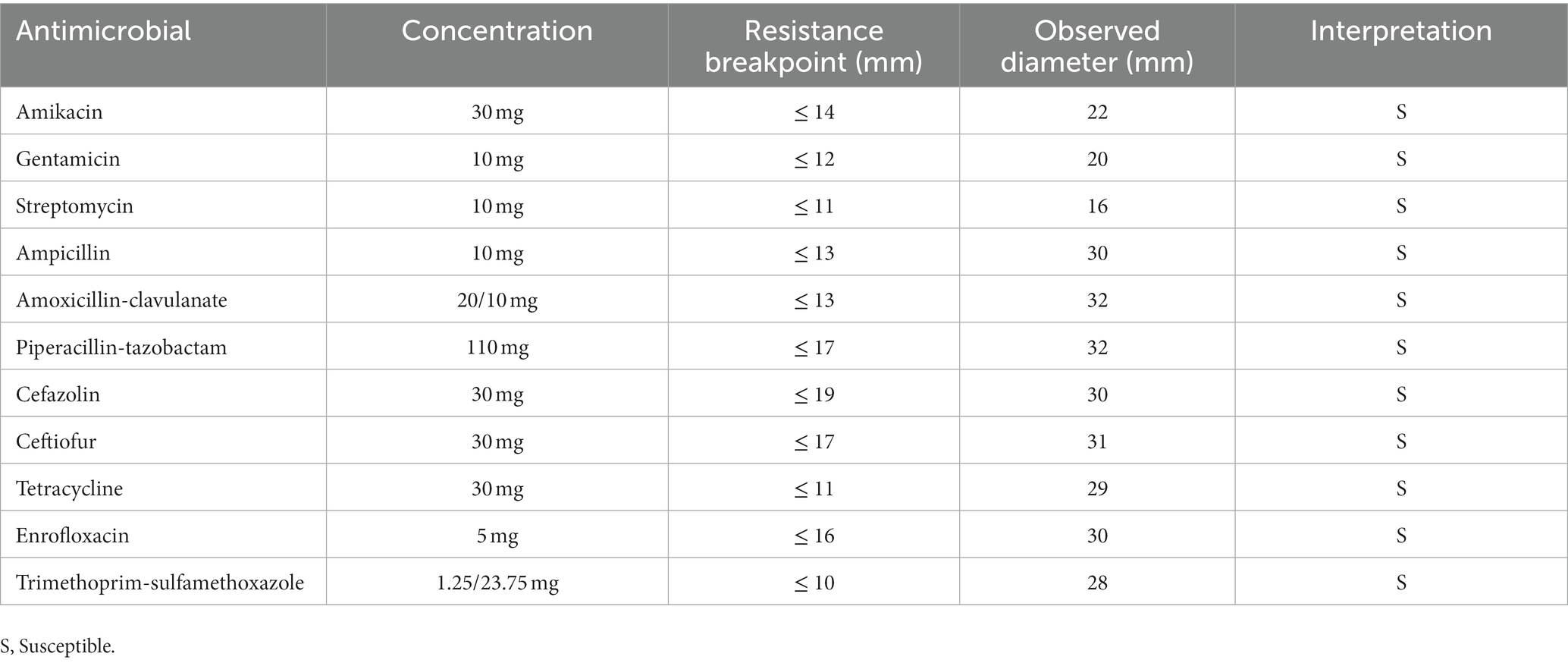
A 14-year-old female spayed domestic short-haired cat was presented at the Veterinary University Hospital at the onset of lethargy and dysorexia in the last few days. The patient was undergoing treatment with insulin (Toujeo 5 UI BID) for a previous diagnosis of acromegaly and diabetes mellitus. Despite the owner continuing to administer insulin, glycemia at home was persistently high (> 500 mg/dL). Before this event, the cat was sporadically seen by the owners hunting wild birds and mice. On physical examination, the cat presented with depression, heart rate of 150 beats/min, blood pressure of 105/76 mmHg, hyperthermia (40.4°C), and clinical signs of dehydration. Hyperglycemia (500 mg/dL) was confirmed in the absence of an increased ketonemia (0.2 mmol/L). Considering liver and kidney function tests, only Alanine Transaminase (ALT) levels were high (280 U/L). According to the primary suspicion of an inflammatory/infectious disease complicating the diabetes mellitus, the patient was hospitalized for proper medical care, and 5 mL of blood, collected by sterile jugular venipuncture, was incubated at 37 ± 1°C in a blood culture bottle (Signal Blood Culture System; Oxoid, Milan, Italy). Replacement fluid therapy was instituted, and once appropriated, a constant rate infusion of regular insulin was administered for glycemic control. Empirical antibacterial treatment with ampicillin-sulbactam (30 mg/kg intravenous TID) was initially prescribed. After 24 h of incubation, the blood culture bottle revealed positivity and was subcultured by streaking 10 microliters in media plates for aerobic (Blood agar with 5% horse blood, MacConkey Agar, Cled Agar), anaerobic (Wilkins-Chalgren Agar, Columbia Agar), and capnophilic (Columbia Agar) bacteria. All the plates were incubated at 37 ± 1°C. At 24 h from subculture, the cultures revealed positivity for isolates in abundant quantity, all in monoculture (Figure 1). One isolate grown from Blood Agar and one from Columbia Agar were subsequently both identified as Yersinia pseudotuberculosis through the matrix-assisted laser desorption-ionization time-of-flight mass spectrometry method (MALDI-TOF MS; Biotyper, Bruker Daltonics, Billerica, MA), following the manufacturer’s instructions (Bruker Daltonik, Bremen, Germany), with a score of 2.47 and 2.35, respectively. To confirm the species-level identification, sequencing of the 16S rRNA portion from the extracted DNA was performed, indicating a 99.91% identity with both Y. pseudotuberculosis and Y.pestis. To rule out Y.pestis, an API 20E test (bioMerieux, France) was performed, confirming the suspicion of Y. pseudotuberculosis (urease-positive). Subsequent Antimicrobic Susceptibility Testing, performed with the disk diffusion method following CLSI standard and breakpoints (14), showed that the strain was susceptible to every tested antimicrobial (see Table 1). Blood profile was consistent with a septic process, according to leukocytosis (17.160 cells/mm3), neutrophilia (14,570/mm3) with basophilia and toxic changes, and an increased serum amyloid A concentration (238 μg/mL). However, a clear septic focus was not identified based on clinical and imaging findings from abdominal ultrasonography. Clinical deterioration was recorded on the morning of the second day of hospitalization with the onset of hypotension and hypoglycemia, consistent with a condition of septic shock, and norepinephrine (0.5 mcg/kg/min intravenous) was administered as a first-line vasopressor and fluids were supplemented with glucose to restore arterial blood pressure and normoglycemia, respectively. The cat recovered soon, and it was possible to stop norepinephrine infusion in the evening. General conditions progressively and significantly improved in the next days and the cat was discharged after 4 days of hospitalization with insulin 5 UI BID and amoxicillin-clavulanate 15 mg/kg SID for 10 days. The follow-up controls revealed good general health conditions and antimicrobial treatment was stopped after 10 days.
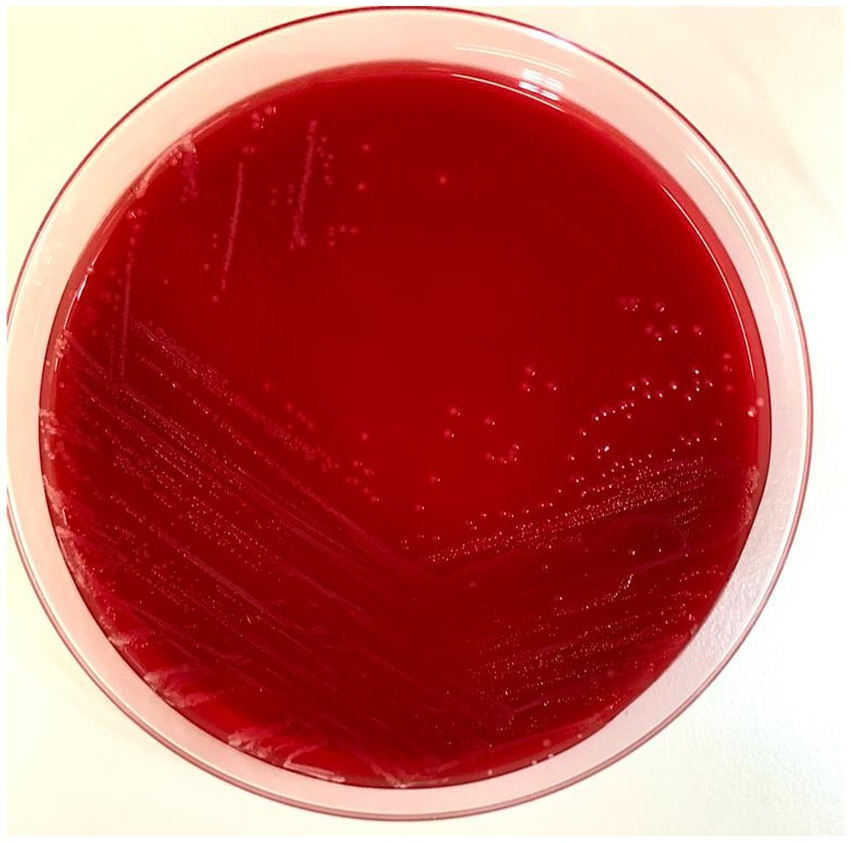
Figure 1. Picture of the Blood Agar with the Y. pseudotuberculosis colonies. Ten microliters of the positive blood culture bottle were streaked with a sterile loop and incubated at 37°C for 24 h.
3 Discussion
From an evolutionary point of view, Y. pseudotuberculosis diverged from a common ancestor with Y.enterocolitica approximately 41–186 million years ago (15). The other human pathogen, Yersinia pestis, is genetically extremely similar to Y. pseudotuberculosis to the point that taxonomically they should be grouped into a single species (16). Furthermore, Y. pseudotuberculosis can be subdivided considering the basis of lipopolysaccharide O-side chain into 15 O-serotypes: in Europe, the most frequent serotypes are O:1-O:3 (16). The recent analysis described other two bacterial populations as a part of the “Y. pseudotuberculosis complex”: Yersinia similis and the “Korean group.” The latter, composed of non-pathogenic strains mostly found in the Korean region (17), has been a matter of debate since Savin et al. (18) proposed to rename it as a novel species, Yersinia wautersii, but Neubauer and Sprague (19) argued that it should continue to be considered as a subgroup of Y.pseudotuberculosis. On the other hand, Y.similis is a non-pathogenic species first identified in 2008 (20), phenotypically indistinguishable from Y. pseudotuberculosis with methods such as MALDI-TOF. In this paper, we used genotypic and phenotypic methods to distinguish between Y. pseudotuberculosis in sensu stricto and the other members of the Y. pseudotuberculosis complex.
In humans, Y. pseudotuberculosis not only causes normally self-limiting gastroenteritis but also pseudoappendicitis, arthritis, pharyngitis, and erythema nodosum (21–23). It can also cause bacteremia and sepsis, with a mortality rate as high as 75% (24). Bacteremia cases more commonly involve patients with immunodeficiency (25, 26), but it has also been reported by Hashimoto et al. (27) as a cause of septic shock in an immunocompetent adult. Its zoonotic potential is well-known (2). Fukushima et al. (22) related the presence of a cat-contaminated environment with the onset of fever and diarrhea in two children with fecal culture positive for Y. pseudotuberculosis that drunk from a puddle near the cat’s litter. In animals, it has been reported as a cause of enteritis (28) and mastitis in cattle (29), ocular disease in goats (30), and septicemia in beavers (31), and it has been related to multiple deaths in zoological collections (32, 33), including mammals like monkeys, meerkats, and paca (34–36), as well as felids such as lions (37). In cats, only a few cases of Y. pseudotuberculosis infections were published (8–13), and there were no reports of isolation from blood. The main symptoms described were lethargy, reduced appetite, and abdominal pain. It has also been described by Thompson (12) and Iannibelli et al. (13) as a cause of hepatitis and enteritis in cats that may become infected by consuming wild animals like rodents and birds. To our knowledge, this is the first report of Y. pseudotuberculosis associated with septicemia in a cat. Although the possibility of contamination of the intravenous catheter cannot be excluded, the onset of clinical symptoms such as hyperthermia, leukocytosis, and the development of septic shock suggested that the positive blood culture reflected a real ongoing infection that was successfully treated. Indeed, the patient’s hunting behaviors described by the owners made it likely to have contact with potential reservoirs such as birds or rodents. Furthermore, diabetes mellitus and acromegaly might have predisposed the cat to infection. In contrast with the case reported by Thompson (12), in this case, no specific neurological signs were observed, suggesting that the infection did not spread to the nervous system. In that case, neurological signs in the absence of hematogenous diffusion suggested they were most likely the result of hepatic encephalopathy, and treatment with a combination of marbofloxacin and amoxicillin-clavulanate was successful. Further investigation about the infection route was complicated to realize, but it can be assumed that pathogen transmission probably occurred from the gastrointestinal tract to the bloodstream through translocation, with possible involvement of the liver through the portal system or by ascending the biliary route.
In conclusion, this case report describes septicemia associated with Y. pseudotuberculosis in a cat, with a positive outcome reached with amoxicillin-clavulanate treatment based on AST results. Cats with external access showing hunting behavior should be monitored for the risk of contracting bacteria such as Y.pseuduberculosis from preys considered to be natural reservoirs, like rodents or birds. This should be considered in relation to the zoonotic potential of the bacteria, especially in situations in which the cat shares the same household with the owners.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because the data used for this study were extrapolated from the clinical data of the patient. Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
RS: Conceptualization, Data curation, Writing – original draft. MG: Writing – original draft, Writing – review & editing. CB: Writing – review & editing. EM: Writing – review & editing. EE: Writing – review & editing. GA: Writing – review & editing. SP: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Malassez, L, and Vignal, W. Sur le micro-organisme de la tuberculose zoogloéique. Arch Physiol Norm Pathol. (1884) 4:81–104.
2. European Centre for Disease Prevention and Control. Yersiniosis. In: ECDC. Annual epidemiological report for 2021. Stockholm: ECDC (2022).
3. Kangas, S, Takkinen, J, Hakkinen, M, Nakari, UM, Johansson, T, Henttonen, H, et al. Yersinia pseudotuberculosis O:1 traced to raw carrots, Finland. Emerg Infect Dis. (2008) 14:1959–61. doi: 10.3201/eid1412.080284
4. Galindo, CL, Rosenzweig, JA, Kirtley, ML, and Chopra, AK. Pathogenesis of Y. Enterocolitica and Y. pseudotuberculosis in human Yersiniosis. J Pathog. (2011) 2011:1–16. doi: 10.4061/2011/182051
5. Kaasch, AJ, Dinter, J, Goeser, T, Plum, G, and Seifert, H. Yersinia pseudotuberculosis bloodstream infection and septic arthritis: case report and review of the literature. Infection. (2012) 40:185–90. doi: 10.1007/s15010-011-0160-2
6. Okwori, AEJ, Martínez, PO, Fredriksson-Ahomaa, M, Agina, SE, and Korkeala, H. Pathogenic Yersinia enterocolitica 2/O:9 and Yersinia pseudotuberculosis 1/O:1 strains isolated from human and non-human sources in the plateau state of Nigeria. Food Microbiol. (2009) 26:872–5. doi: 10.1016/j.fm.2009.06.001
7. Platt-Samoraj, A, Żmudzki, J, Pajdak-Czaus, J, Szczerba-Turek, A, Bancerz-Kisiel, A, Procajło, Z, et al. The prevalence of Yersinia enterocolitica and Yersinia pseudotuberculosis in small wild rodents in Poland. Vector-Borne and Zoonotic Dis. (2020) 20:586–92. doi: 10.1089/vbz.2019.2586
8. Mair, NS, Harbourne, JF, and Greenwood, MT. Pasteurella pseudotuberculosis infection in the cat: two cases. Vet Rec. (1967) 81:461–2.
9. Spearman, JG, Hunt, P, and Nayar, PSG. Yersinia pseudotuberculosis infection in a cat. Can Vet J. (1979) 20:361–4.
10. Obwolo, MJ, and Gruffydd-Jones, TJ. Yersinia pseudotuberculosis in the cat. Vet Rec. (1977) 100:424–5. doi: 10.1136/vr.100.20.424
12. Thompson, D. Successful treatment of Yersinia pseudotuberculosis hepatitis in a cat presenting with neurological abnormalities. J Feline Med Surg Open Rep. (2019) 5:205511691985364. doi: 10.1177/2055116919853644
13. Iannibelli, F, Caruso, A, Castelluccio, A, Castriota, M, d’Agnessa, M, and Chiesa, C. Yersinia pseudotuberculosis in a persian cat. Vet Rec. (1991) 129:103–4. doi: 10.1136/vr.129.5.103
14. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for Bacteria isolated from animals - approved standard. Wayne PA: Clinical and Laboratory Standards Institute (2019).
15. Achtman, M, Zurth, K, Morelli, G, Torrea, G, Guiyoule, A, and Carniel, E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. (1999) 96:14043–8. doi: 10.1073/pnas.96.24.14043
16. Bogdanovich, T, Carniel, E, Fukushima, H, and Skurnik, M. Use of O-antigen gene cluster-specific PCRs for the identification and O-genotyping of Yersinia pseudotuberculosis and Yersinia pestis. J Clin Microbiol. (2003) 41:5103–12. doi: 10.1128/JCM.41.11.5103-5112.2003
17. Laukkanen-Ninios, R, Didelot, X, Jolley, KA, Morelli, G, Sangal, V, Kristo, P, et al. Population structure of the Yersinia pseudotuberculosis complex according to multilocus sequence typing. Environ Microbiol. (2011) 13:3114–27. doi: 10.1111/j.1462-2920.2011.02588.x
18. Savin, C, Martin, L, Bouchier, C, Filali, S, Chenau, J, Zhou, Z, et al. The Yersinia pseudotuberculosis complex: characterization and delineation of a new species, Yersinia wautersii. Int J Med Microbiol. (2014) 304:452–63. doi: 10.1016/j.ijmm.2014.02.002
19. Neubauer, H, and Sprague, LD. Strains of Yersinia wautersii should continue to be classified as the ‘Korean group’ of the Yersinia pseudotuberculosis complex and not as a separate species. Int J Syst Evol Microbiol. (2015) 65:732–3. doi: 10.1099/ijs.0.070383-0
20. Sprague, LD, Scholz, HC, Amann, S, Busse, HJ, and Neubauer, H. Yersinia similis sp. nov. Int J Syst Evol Microbiol. (2008) 58:952–8. doi: 10.1099/ijs.0.65417-0
21. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
22. Fukushima, H, Matsuda, Y, Seki, R, Tsubokura, M, Takeda, N, Shubin, FN, et al. Geographical heterogeneity between far eastern and Western countries in prevalence of the virulence plasmid, the Superantigen Yersinia pseudotuberculosis-derived mitogen, and the high-Pathogenicity Island among Yersinia pseudotuberculosis strains. J Clin Microbiol. (2001) 39:3541–7. doi: 10.1128/JCM.39.10.3541-3547.2001
23. Amphlett, A. Far East scarlet-like fever: a review of the epidemiology, symptomatology, and role of Superantigenic toxin: Yersinia pseudotuberculosis-derived mitogen A. open forum Infect Dis. (2016) 3:ofv202. doi: 10.1093/ofid/ofv202
24. Bennett, J, and Dolin, RM. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 8th ed. Philadelphia, PA: Elsevier (2014).
25. Paglia, MG, D’Arezzo, S, Festa, A, Del Borgo, C, Loiacono, L, Antinori, A, et al. Yersinia pseudotuberculosis septicemia and HIV. Emerg Infect Dis. (2005) 11:112–1130. doi: 10.3201/eid1107.041268
26. Antinori, A, Paglia, MG, Marconi, P, Festa, A, Alba, L, Boumis, E, et al. Short communication: Yersinia pseudotuberculosis septicemia in an HIV-infected patient failed HAART. AIDS Res Hum Retrovir. (2004) 20:709–10. doi: 10.1089/0889222041524599
27. Hashimoto, T, Takenaka, R, Fukuda, H, Hashinaga, K, Nureki, S, Ichi Hayashidani, H, et al. Septic shock due to Yersinia pseudotuberculosis infection in an adult immunocompetent patient: a case report and literature review. BMC Infect Dis. (2021) 21:36. doi: 10.1186/s12879-020-05733-w
28. Slee, K, Brightling, P, and Seiler, R. Enteritis in cattle due to Yersinia pseudotuberculosis infection. Aust Vet J. (1988) 65:271–5. doi: 10.1111/j.1751-0813.1988.tb16141.x
29. Lorusso, A, Addante, L, Capozzi, L, Bianco, A, Del Sambro, L, Gallitelli, ME, et al. Isolation of Yersinia pseudotuberculosis in bovine mastitis: a potential milk-borne hazard. Ital J Food Safety. (2021) 9:8527. doi: 10.4081/ijfs.2020.8527
30. Wessels, ME, Payne, J, Willmington, JA, Bell, SJ, and Davies, IH. Yersinia pseudotuberculosis as a cause of ocular disease in goats. Vet Rec. (2010) 166:699–700. doi: 10.1136/vr.c2801
31. Gaydos, JK, Zabek, E, and Raverty, S. Yersinia pseudotuberculosis septicemia in a beaver from Washington state. J Wildl Dis. (2009) 45:1182–6. doi: 10.7589/0090-3558-45.4.1182
32. Hammerl, JA, vom Ort, N, Barac, A, Jäckel, C, Grund, L, Dreyer, S, et al. Analysis of Yersinia pseudotuberculosis isolates recovered from deceased mammals of a German zoo animal collection. Fenwick B, curatore. J Clin Microbiol. (2021) 59:e03125–04. doi: 10.1128/JCM.03125-20
33. Tsugo, K, Nakamura, SI, Yamanaka, H, and Une, Y. A study on the efficacy of the recombinant Yersinia adhesin a vaccine against yersiniosis in the early phase. J Vet Med Sci. (2017) 79:855–63. doi: 10.1292/jvms.16-0528
34. Iwata, T, Une, Y, Okatani, AT, Kato, Y, Nakadai, A, Lee, K, et al. Virulence characteristics of Yersinia pseudotuberculosis isolated from breeding monkeys in Japan. Vet Microbiol. (2008) 129:404–9. doi: 10.1016/j.vetmic.2007.11.029
35. Nakamura, S, Hayashidani, H, Yonezawa, A, Suzuki, I, and Une, Y. Yersiniosis due to infection by Yersinia pseudotuberculosis in captive meerkats (Suricata suricatta) in Japan. J Vet Diagn Investig. (2015) 27:641–4. doi: 10.1177/1040638715596035
36. Fogelson, SB, Yau, W, and Rissi, DR. Disseminated Yersinia pseudotuberculosis infection in a paca (Cuniculus paca). J Zoo Wildl Med. (2015) 46:130–4. doi: 10.1638/2014-0150R.1
Keywords: Yersinia pseudotuberculosis , bloodstream infection, feline, bacterial, blood, yersiniosis
Citation: Scarpellini R, Giunti M, Bulgarelli C, Mondo E, Esposito E, Assirelli G and Piva S (2024) Case report: First isolation of Yersinia pseudotuberculosis from the blood of a cat. Front. Vet. Sci. 10:1261925. doi: 10.3389/fvets.2023.1261925
Edited by:
Haroon Ahmed, COMSATS University, Islamabad, PakistanReviewed by:
Maria Fredriksson-Ahomaa, University of Helsinki, FinlandSuvendu Kumar Behera, Central Agricultural University, India
Copyright © 2024 Scarpellini, Giunti, Bulgarelli, Mondo, Esposito, Assirelli and Piva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Scarpellini, cmFmZmFlbGUuc2NhcnBlbGxpbmlAdW5pYm8uaXQ=
 Raffaele Scarpellini
Raffaele Scarpellini Massimo Giunti
Massimo Giunti Cecilia Bulgarelli
Cecilia Bulgarelli Elisabetta Mondo
Elisabetta Mondo Silvia Piva
Silvia Piva