- 1IVC Evidensia Small Animal Referral Hospital Arnhem, Neurology, Arnhem, Netherlands
- 2IVC Evidensia Small Animal Referral Hospital Hart van Brabant, Neurology, Waalwijk, Netherlands
- 3Vet Oracle Teleradiology, Norfolk, United Kingdom
A 1.5-year-old female entire French bulldog was referred for neurological evaluation, further diagnostic tests, and treatment 24 h after a road traffic accident. Initial emergency treatment, diagnostic tests, and stabilization had been performed by the referring veterinarian. Neurological examination revealed severe spastic non-ambulatory tetraparesis and was consistent with a C1-5 myelopathy. A magnetic resonance imaging (MRI) study revealed an irregular to elongated ovoid intramedullary lesion centered over the body of C2. The lesion showed marked signal heterogeneity with a central T2W and T2* hyperintense region, surrounded by a hypointense rim on both sequences. The lesion appeared heterogeneously T1W hypointense. The lesion was asymmetric (right-sided), affecting both white and gray matter. The C2-3 intervertebral disk appeared moderately degenerate with a Pfirrmann grade of 3. No evidence of vertebral fracture or luxation was found on radiographs or MRI of the vertebral column. Additional soft tissue abnormalities in the area of the right brachial plexus were suggestive of brachial plexus and muscle injury. A diagnosis of traumatic hemorrhagic myelopathy at the level of C2 and concurrent brachial plexus injury was formed. Conservative treatment was elected and consisted of physiotherapy, bladder care with an indwelling urinary catheter, repeated IV methadone based on pain scoring (0.2 mg/kg), oral meloxicam 0.1 mg/kg q24h, and oral gabapentin 10 mg/kg q8h. The dog was discharged after 4 days, with an indwelling urinary catheter and oral medication as described. The catheter was replaced two times by the referring veterinarian and finally removed after 10 days. Thereafter, voluntary urination was seen. During the 2 months after the road traffic accident, slow recovery of motor function was seen. The right thoracic limb recovery progressed more slowly than the left limb, also showing some lower motor neuron signs during follow-up. This was judged to be consistent with a right-sided brachial plexus injury. The dog was reported ambulatory with mild residual ataxia and residual monoparesis of the right thoracic limb at the last follow-up 3 months post-injury. This case report highlights the MRI-based diagnosis of traumatic hemorrhagic myelopathy in a dog. A fair short-term outcome was achieved with conservative treatment in this case.
Introduction
Hemorrhagic myelopathy, also called hematomyelia, in dogs can be due to various etiologies. These include trauma associated with vertebral fractures/luxation [e.g., due to road traffic accidents (RTA)], trauma associated with congenital anomalies of the craniocervical region (e.g., odontoid process malformation and atlantoaxial instability), iatrogenic trauma (e.g., due to spinal cord puncture during cerebrospinal fluid taps), vascular malformations (e.g., arteriovenous malformation), intervertebral disk disease (e.g., intervertebral disk extrusion), neoplasia (e.g., metastatic hemangiosarcoma or lymphoma), inflammatory disease (e.g., steroid-responsive meningitis arteritis), and hemorrhagic diathesis (e.g., related to Angiostrongylus vasorum infections) (1–9). When no causes are identified, the terms primary hematomyelia or idiopathic hemorrhagic myelopathy may be applicable (9, 10).
In human medical literature, hemorrhages can be found in the spinal cord in cases of spinal cord injury (SCI) without radiographic abnormalities (SCIWORA) (11–15). This is defined as SCI without evidence of vertebral fractures or dislocation based on radiographic studies. Since this is a fairly rare clinical entity, much of its exact pathophysiology remains unknown. Clinically, human patients (often children) are presented with various degrees of neurological dysfunction (12–14). In one systematic review, “complete” SCI defined as a lack of motor and sensory function “below” the level of the lesion in the spinal cord was reported at initial presentation in almost 20% of patients (12). Recent studies have provided evidence for improved outcomes following early surgical intervention (15). No such studies are available regarding clinical canine patients.
In this case report, we describe the magnetic resonance imaging (MRI) based diagnosis of traumatic hemorrhagic cervical myelopathy in a dog without evidence of vertebral fractures or luxation.
Case description
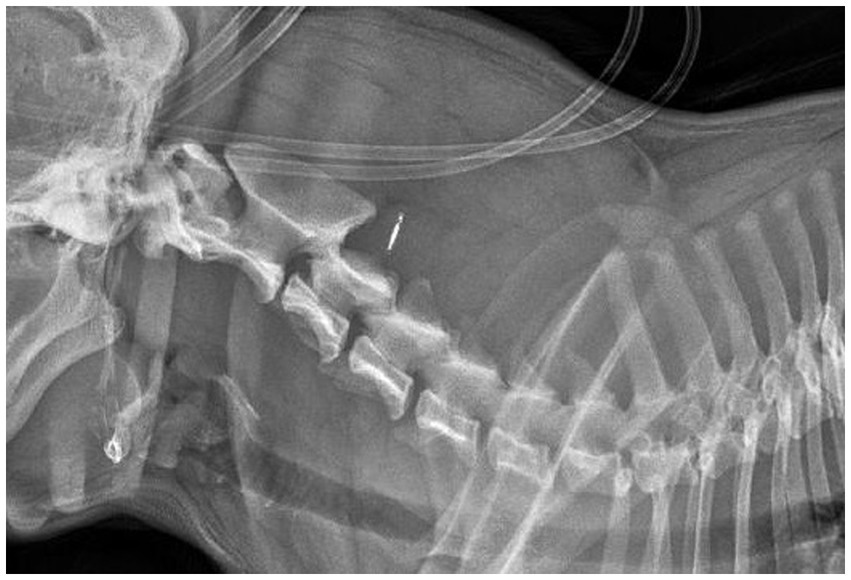
A 1.5-year-old female entire French bulldog was referred for neurological evaluation, further diagnostic tests, and treatment 24 h after a road traffic accident (RTA). The dog was chasing a cat and collided with a moving vehicle head-on, hitting the side of that vehicle. Bystanders reported that the dog immediately collapsed. The dog was rushed to the nearest veterinary practice. The dog was presented there in lateral recumbency with increased extensor tone of all four limbs. Mucous membranes were noted to be slightly blueish. No voluntary movement of limbs was recorded at that time, consistent with spastic tetraplegia. The dog was noted to show reduced responsiveness, and a modified Glasgow coma scale (MGCS) score of 9 was recorded without further details. Initial emergency treatment, diagnostic tests, and stabilization were performed. This included an IV bolus of 15 mL/kg 0.9% sodium chloride, followed by 20 mL/kg/h Ringers solution, and a single IV bolus of 1 g/kg mannitol [based on concerns for increased intracranial pressure (ICP)]. For analgesia, several boluses of methadone (0.2 mg/kg IV) had been administered. Heart rate increased to 70–90 beats/min over the next hour and the dog became more responsive, with a MGCS score of 13, increasing to 18 during the rest of the day on repeat examinations. Pulse oximetry consistently showed a SpO2 of 99–100%. Further diagnostic tests included hematology (no significant abnormalities), biochemistry [hyperglycemia (11.72 mmol/L, reference range 4.11–7.95)] and increased blood lactate (5.26 mmol/L, reference range 0.50–2.50), ultrasound of the thorax and abdomen (possible signs of right-sided lung contusion), laterolateral radiographs of the thorax (signs suggestive of lung contusion), cervical vertebral column (Figure 1), and thoracolumbar vertebral column, and non-invasive blood pressure measurements (80–115 mmHg). Repeat testing of blood lactate showed values within the reference range. When the patient was stable the next day, the owners opted for a referral for further neurological examination, diagnostic testing, and treatment.
During the 2-h drive to the referral hospital, the dog had become hyperthermic (rectal temperature of 40.5 degrees Celsius) and was stabilized by the emergency department. Treatment at that point included active cooling, oxygen supplementation via nasal catheter and flow-by. After achieving normothermia, neurological examination revealed severe spastic non-ambulatory tetraparesis, worse in the thoracic limbs than the pelvic limbs. There was some voluntary movement of the limbs, more so in the pelvic limbs than the thoracic limbs. Spinal reflexes were intact in the pelvic limbs but decreased in the thoracic limbs on both sides. These findings were deemed consistent with a C1-5 myelopathy (likely involving the central cord). After discussion with the owners, an MRI study of the cervical spinal cord was performed, including bilateral brachial plexus regions. Additional imaging studies including an MRI of the brain and computed tomography (CT) of the vertebral column, thorax, and abdomen were declined due to financial restrictions. MRI sequences included T2-weighted (T2W) fast-spin echo (FSE) sagittal plane, T1W FSE sagittal plane, short-tau inversion recovery (STIR) sagittal plane, STIR dorsal plane, 3D fast gradient echo combined with water excitation technique (FFE3D combined with WET), T2W FSE transverse plane, T1W FSE transverse plane, T2*W gradient echo transverse plane, and 3D T1W magnetization prepared—rapid gradient echo (MPRAGE) sagittal plane post-contrast.
The MRI study revealed an irregular to elongated ovoid intramedullary lesion centered over the body of C2 (Figure 2). The lesion showed marked signal heterogeneity with a central T2W and T2* hyperintense region, surrounded by a hypointense rim on both sequences. The lesion appeared heterogeneously T1W hypointense. The lesion was predominantly right-sided and dorsolateral within the spinal cord, affecting both white and gray matter. The C2-3 intervertebral disk appeared moderately degenerate with a Pfirrmann grade of 3. No evidence of vertebral fracture or luxation was found on radiographs of the vertebral column or the MRI study. Additional findings in the area of the right brachial plexus were suggestive of brachial plexus and muscular injury. A diagnosis of traumatic hemorrhagic myelopathy at the level of C2 and concurrent brachial plexus injury was formed.
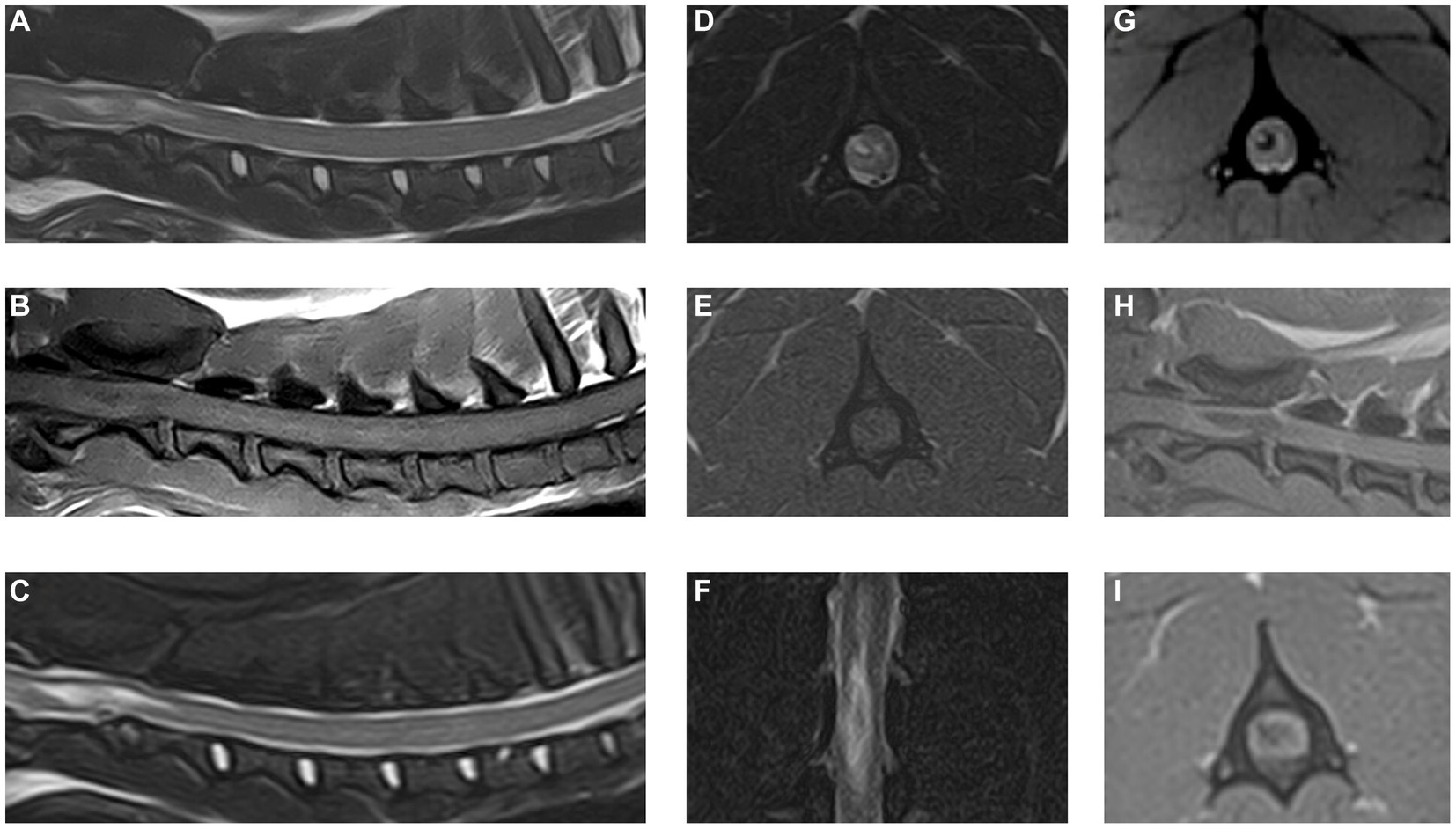
Figure 2. Magnetic resonance images of the cervical spinal cord and surrounding structures. (A) T2W sagittal plane, (B) T1W sagittal plane, (C) STIR sagittal plane, (D) T2W transverse plane at the level of C2 vertebral body, (E) T1W transverse plane at the level of C2 vertebral body, (F) STIR dorsal plane, (G) T2*W transverse plane at the level of C2 vertebral body, (H) 3D T1W MPRAGE sagittal plane, and (I) 3D T1W MPRAGE transverse reconstruction at the level of C2 vertebral body.
Coagulation tests (activated partial-thromboplastin time and prothrombin time) were within the reference range. Repeated clinical examinations, focusing on signs or evidence of hemorrhagic diatheses (e.g., hemorrhages in mucous membranes) did not reveal any abnormalities. Conservative treatment was elected and consisted of fluid therapy, physiotherapy, an indwelling urinary catheter, repeated non-invasive blood pressure measurements, repeated IV methadone boluses based on pain scoring (0.2 mg/kg), oral meloxicam 0.1 mg/kg q24h, and oral gabapentin 10 mg/kg q8h.
Over the next 4 days, progressive signs of recovering motor function were observed in the left thoracic limb, both pelvic limbs, and, to a lesser degree, the right thoracic limb. Withdrawal reflexes were normal in the left thoracic and both pelvic limbs at that point but decreased in the right thoracic limb. Extensor muscle tone in all limbs had decreased, most notably in the right thoracic limb. The dog was discharged after 4 days, with an indwelling urinary catheter and oral medication as described. The catheter was replaced twice by the referring veterinarian and finally removed after 10 days. Thereafter, voluntary urination was seen. Meloxicam was discontinued a week later, and gabapentin was tapered and discontinued 2 weeks later. During the 2 months after the road traffic accident, slow recovery of motor function was seen. The right thoracic limb recovery progressed more slowly than the left limb, also showing some lower motor neuron signs (e.g., flaccid paresis) during follow-up at the referring veterinarian and was also visible on videos sent by the owners for remote evaluation by the neurology department of the referral hospital. The recovery was judged to be consistent with right-sided lateralization of the spinal cord hemorrhage and involvement of the brachial plexus on the right. The dog was reported to be ambulatory with mild residual ataxia and moderate to severe monoparesis of the right thoracic limb at the last follow-up 3 months post-injury. Physiotherapy including hydrotherapy had been commenced by the owners and is being continued long-term with the aid of orthopedic braces for the right thoracic limb. The owners were happy with the outcome at the time of writing and the patient remains under the care and supervision of the referring veterinarian.
Discussion
This case report describes the neurological presentation, MRI findings, conservative management, and short-term outcome of traumatic hemorrhagic cervical myelopathy in a French bulldog. Although the presenting clinical signs were severe directly after the traumatic event (RTA in this case), emergency treatment followed by conservative treatment for the cervical myelopathy and suspected brachial plexus injury resulted in a fair short-term outcome.
For the treatment of traumatic SCI in dogs, it is of vital importance to account for basic support measures and general stabilization before focusing on neurological signs and prognostication (15). In the case reported here, initial stabilization was performed by the referring veterinarian. The dog was transported for further work-up when it was deemed to be stabilized. However, hyperthermia developed during the drive to the referral hospital, and measures needed to be taken to stabilize the patient. Fortunately, there did not seem to have been any neurological deterioration as determined by the results of the neurological examination vs. the descriptions of the referring veterinarian.
As emphasized in several veterinary texts, maintaining adequate tissue (spinal cord) perfusion is a key factor in the treatment of traumatic SCI in dogs (16–18). Spinal cord perfusion pressure (SCPP) is not routinely clinically measured [i.e., calculated from measurements of intradural or “intraspinal” pressure (ISP) and mean arterial blood pressure (MAP)] in canine traumatic SCI cases. Human literature, reporting results in clinical patients, has shown that such measurements can contribute to guiding more efficient and objective treatment measures (19, 20). Even if SCPPs are not performed, maintaining blood pressure (MAP) within the reference range can be and is regarded as a cornerstone of treatment (16, 17). This makes good sense, as low blood pressure will be detrimental to SCPP. High blood pressure is also to be avoided, as the blood-spinal cord barrier (BSCB) and local vascular autoregulation in the injured spinal cord is affected (16–23).
Numerous other possible avenues of treatment are discussed in veterinary as well as human literature (16–18, 23). These include, but are not limited to stem cell therapy, hypothermia, pharmacological treatment (e.g., the dubious role of corticosteroids), and surgery. The role of the latter deserves specific attention based on recent veterinary literature and human literature. As an option for maintaining or increasing SCPP, surgery would provide a way of removing restrictions to the expansion of the spinal cord parenchyma and/or removing compressive lesions. That is to say, surgical opening of the vertebral column and durotomy can provide the spinal cord with space to expand in case of swelling, causing a direct decrease in ISP. Indeed, the role of durotomy in canine spinal cord injury due to intervertebral disk extrusion (IVDE) is the subject of recent studies (24–27). These studies have reported positive effects on outcomes in dogs with severe grades of thoracolumbar spinal cord dysfunction. Durotomy and duraplasty has been studied in humans with SCIWORA as well (15, 20, 28). Myelotomy has also been described in animal models of SCI and is sporadically reported with positive effects in humans (28). Other types of surgery, including stabilization procedures, are reported for the management of SCIWORA in humans as well (29).
With regard to pharmacological treatment, the dog reported here received a variety of intravenous medications and fluids as emergency treatment which included mannitol and methadone. At that point in time, the diagnosis of traumatic hemorrhagic cervical myelopathy had not yet been reached. Importantly, and justly, treatment was focused on stabilizing the trauma patient (16). After the diagnosis of traumatic hemorrhagic cervical myelopathy and concurrent traumatic brachial plexus injury was determined, treatment consisted of fluid therapy, physiotherapy, an indwelling urinary catheter, repeated non-invasive blood pressure measurements, repeated IV methadone boluses based on pain scoring, oral meloxicam, and oral gabapentin. The latter three medications were used with the aim of providing adequate analgesia. Non-steroidal anti-inflammatory drugs (NSAIDs), or cyclooxygenase (COX) inhibitors are particularly preferred for the treatment of SCIWORA in human patients based on animal models and clinical experience (23). However, there are no large prospective, blinded studies assessing the effect of NSAIDs or COX inhibitors in clinical human patients, let alone canine patients with SCIWORA. Nevertheless, the use of these medications would be supported by the importance of inflammatory cascades in SCI pathophysiology as well as the need for analgesia in trauma patients in general (8, 16, 17, 23). Monitoring for side effects, such as gastrointestinal complications, is always advisable. Specifically when corticosteroids have been administered preceding or concurrently. The use of mannitol for SCI is debatable, but as SCPP would benefit from increased blood volume as well as reduction of spinal cord edema, a beneficial effect is not excluded. Future studies may provide the opportunity to develop more evidence-based guidelines. In this trauma patient, mannitol was administered due to concerns of increased ICP. Finally, the use of opioid receptor agonists may be detrimental for SCI patients. Indeed, the use of opioid receptor antagonists (such as naloxone) has shown some positive results in SCI models (16, 23). However, the use of opioid antagonists is not recommended as it would prevent adequate analgesia for a trauma patient such as reported here, and its effectiveness is not adequately supported by evidence in clinical (human or canine) acute trauma patients.
While hemorrhage is a common feature of traumatic SCI in humans in general (30), it is only reported in a minority of cases of cervical SCIWORA in humans (31–33). Up to 67% of human cases of traumatic SCI have signs compatible with spinal cord hemorrhage on MRI (30). In contrast, one study including 59 patients with SCIWORA described MRI findings consistent with hemorrhage in only two cases (31). The finding of an intramedullary hemorrhagic component is considered a robust indicator of irreversible injury and predictor of injury severity (32). Indeed, intramedullary changes including hemorrhage have been shown to negatively affect prognosis in human SCIWORA patients (12). That being said, conservative management of cervical SCIWORA with hematomyelia may still lead to a positive outcome (33). In our case, conservative management resulted in a fair outcome in the short-term, where most of the remaining deficits affected the right thoracic limb. Those deficits were best explained by a concurrent brachial plexus injury, rather than the traumatic cervical hemorrhagic myelopathy in this dog.
In dogs, the use of T2* gradient echo sequences has been reported of value in assessing hemorrhagic lesions of or affecting the spinal cord (34). In the case reported here, this sequence was instrumental for the identification of an intraparenchymal hemorrhage. No signs of linear tracts extending into the spinal cord were found and the lesion epicenter was located over the C2 vertebral body, making an intradural/intramedullary disk extrusion unlikely. There were no signs of vertebral fractures or dislocation on laterolateral radiographs or the MRI study. However, it has been reported that MRI is less sensitive than CT in the identification of vertebral fractures (35). Orthogonal radiographs also have limited sensitivity (36), let alone single-view radiographs which only provide a two-dimensional evaluation of the vertebral column as was available in this case. Thus, a limitation to this case report is the lack of orthogonal radiographs and CT to definitively exclude vertebral fractures. Still, the lack of extraparenchymal hemorrhage and perivertebral muscle abnormalities makes a concurrent vertebral fracture unlikely in this case.
Other limitations to this case report include the lack of further diagnostic tests for the evaluation of the brachial plexus injury (e.g., electromyography, nerve conduction testing) and lack of histopathological confirmation.
In conclusion, we reported the MRI based diagnosis of traumatic hemorrhagic cervical myelopathy in a dog. Conservative management may be considered in such cases, though the role of surgical treatment (including durotomy) deserves attention and consideration in future studies and cases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because the animal was treated in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
KS: Conceptualization, Funding acquisition, Investigation, Visualization, Writing – original draft, Writing – review & editing. IC: Visualization, Writing – review & editing. SP: Conceptualization, Investigation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The publication fee was covered by IVC Evidensia’s fund for publication of peer-reviewed scientific articles.
Acknowledgments
The authors would like to thank all involved veterinary staff members of the referring veterinarian as well as the referral hospital for the contributions made to the treatment and follow-up of this patient.
Conflict of interest
IC and SP were employed by Vet Oracle Teleradiology.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chang, Y, Dennis, R, Platt, SR, and Penderis, J. Magnetic resonance imaging of traumatic intervertebral disc extrusion in dogs. Vet Rec. (2007) 160:795–9. doi: 10.1136/vr.160.23.795
2. Johnson, P, Beltran, E, Dennis, R, and Taeymans, O. Magnetic resonance imaging characteristics of suspected vertebral instability associated with fracture or subluxation in eleven dogs. Vet Radiol Ultrasound. (2012) 53:552–9. doi: 10.1111/j.1740-8261.2012.01959.x
3. Kent, M, Eagleson, JS, Neravanda, D, Schatzberg, SJ, Gruenenfelder, FI, and Platt, SR. Intraaxial spinal cord hemorrhage secondary to atlantoaxial subluxation in a dog. J Am Anim Hosp Assoc. (2010) 46:132–7. doi: 10.5326/0460132
4. Marr, J, Miranda, IC, Miller, AD, and Summers, BA. A review of proliferative vascular disorders of the central nervous system of animals. Vet Pathol. (2021) 58:864–80. doi: 10.1177/0300985820980707
5. Pancotto, TE, Rossmeisl, JH Jr, Zimmerman, K, Robertson, JL, and Werre, SR. Intramedullary spinal cord neoplasia in 53 dogs (1990-2010): distribution, clinicopathologic characteristics, and clinical behavior. J Vet Intern Med. (2013) 27:1500–8. doi: 10.1111/jvim.12182
6. Platt, SR, Dennis, R, Murphy, K, and De Stefani, A. Hematomyelia secondary to lumbar cerebrospinal fluid acquisition in a dog. Vet Radiol Ultrasound. (2005) 46:467–71. doi: 10.1111/j.1740-8261.2005.00085.x
7. Schwab, ML, Ferrarin, DA, Reginatto Wrzesinski, M, Rauber, JDS, Ripplinger, A, Lamego, EC, et al. Clinical and histopathological findings of hemorrhagic progressive Myelomalacia after lumbar tap in 2 dogs: case report. Top Companion Anim Med. (2022) 50:100681. doi: 10.1016/j.tcam.2022.100681
8. Spitzbarth, I, Moore, SA, Stein, VM, Levine, JM, Kühl, B, Gerhauser, I, et al. Canine spinal cord injury consortium (CANSORT-SCI). Current insights into the pathology of canine intervertebral disc extrusion-induced spinal cord injury. Front Vet Sci. (2020) 7:595796. doi: 10.3389/fvets.2020.595796
9. West, N, Butterfield, S, Rusbridge, C, Fernandez, A, Tabanez, J, Rudolf, NJ, et al. Non-traumatic hemorrhagic myelopathy in dogs. J Vet Intern Med. (2023) 37:1129–38. doi: 10.1111/jvim.16694
10. Barker, A, Williams, JM, Chen, A, Bagley, R, and Jeffery, ND. Suspected primary hematomyelia in 3 dogs. Can Vet J. (2015) 56:278–84.
11. Atesok, K, Tanaka, N, O'Brien, A, Robinson, Y, Pang, D, Deinlein, D, et al. Posttraumatic spinal cord injury without radiographic abnormality. Adv Orthop. (2018) 2018:7060654. doi: 10.1155/2018/7060654
12. Boese, CK, and Lechler, P. Spinal cord injury without radiologic abnormalities in adults: a systematic review. J Trauma Acute Care Surg. (2013) 75:320–30. doi: 10.1097/TA.0b013e31829243c9
13. Pang, D, and Wilberger, JE Jr. Spinal cord injury without radiographic abnormalities in children. J Neurosurg. (1982) 57:114–29. doi: 10.3171/jns.1982.57.1.0114
14. Pang, D . Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurgery. (2004) 55:1325–43. doi: 10.1227/01.neu.0000143030.85589.e6
15. Zhu, F, Yao, S, Ren, Z, Telemacque, D, Qu, Y, Chen, K, et al. Early durotomy with duroplasty for severe adult spinal cord injury without radiographic abnormality: a novel concept and method of surgical decompression. Eur Spine J. (2019) 28:2275–82. doi: 10.1007/s00586-019-06091-1
16. Park, EH, White, GA, and Tieber, LM. Mechanisms of injury and emergency care of acute spinal cord injury in dogs and cats. J Vet Emerg Crit Care. (2012) 22:160–78. doi: 10.1111/j.1476-4431.2012.00723.x
17. Olby, N . The pathogenesis and treatment of acute spinal cord injuries in dogs. Vet Clin North Am Small Anim Pract. (2010) 40:791–807. doi: 10.1016/j.cvsm.2010.05.007
18. Saadoun, S, and Jeffery, ND. Acute traumatic spinal cord injury in humans, dogs, and other mammals: the under-appreciated role of the dura. Front Neurol. (2021) 12:629445. doi: 10.3389/fneur.2021.629445
19. Werndle, MC, Saadoun, S, Phang, I, Czosnyka, M, Varsos, GV, Czosnyka, ZH, et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study*. Crit Care Med. (2014) 42:646–55. doi: 10.1097/CCM.0000000000000028
20. Saadoun, S, and Papadopoulos, MC. Acute, severe traumatic spinal cord injury: monitoring from the injury site and expansion Duraplasty. Neurosurg Clin N Am. (2021) 32:365–76. doi: 10.1016/j.nec.2021.03.008
21. Jin, LY, Li, J, Wang, KF, Xia, WW, Zhu, ZQ, Wang, CR, et al. Blood-spinal cord barrier in spinal cord injury: a review. J Neurotrauma. (2021) 38:1203–24. doi: 10.1089/neu.2020.7413
22. Popa, C, Popa, F, Grigorean, VT, Onose, G, Sandu, AM, Popescu, M, et al. Vascular dysfunctions following spinal cord injury. J Med Life. (2010) 3:275–85.
23. Zhang, Y, Al Mamun, A, Yuan, Y, Lu, Q, Xiong, J, Yang, S, et al. Acute spinal cord injury: pathophysiology and pharmacological intervention (review). Mol Med Rep. (2021) 23:417. doi: 10.3892/mmr.2021.12056
24. Hirano, R, Asahina, R, Hirano, T, Hyakkoku, A, Miura, R, Kunihiro, T, et al. Outcomes of extensive hemilaminectomy with durotomy on dogs with presumptive progressive myelomalacia: a retrospective study on 34 cases. BMC Vet Res. (2020) 16:476. doi: 10.1186/s12917-020-02690-z
25. Jeffery, ND, Mankin, JM, Ito, D, Boudreau, CE, Kerwin, SC, Levine, JM, et al. Extended durotomy to treat severe spinal cord injury after acute thoracolumbar disc herniation in dogs. Vet Surg. (2020) 49:884–93. doi: 10.1111/vsu.13423
26. Nakamoto, Y, Uemura, T, Hasegawa, H, Nakamoto, M, and Ozawa, T. Outcomes of dogs with progressive myelomalacia treated with hemilaminectomy or with extensive hemilaminectomy and durotomy. Vet Surg. (2021) 50:81–8. doi: 10.1111/vsu.13514
27. Takahashi, F, Honnami, A, Toki, M, Dosaka, A, Fujita, Y, Hara, Y, et al. Effect of durotomy in dogs with thoracolumbar disc herniation and without deep pain perception in the hind limbs. Vet Surg. (2020) 49:860–9. doi: 10.1111/vsu.13409
28. Telemacque, D, Zhu, FZ, Ren, ZW, Chen, KF, Drepaul, D, Yao, S, et al. Effects of durotomy versus myelotomy in the repair of spinal cord injury. Neural Regen Res. (2020) 15:1814–20. doi: 10.4103/1673-5374.280304
29. Qi, C, Xia, H, Miao, D, Wang, X, and Li, Z. The influence of timing of surgery in the outcome of spinal cord injury without radiographic abnormality (SCIWORA). J Orthop Surg Res. (2020) 15:223. doi: 10.1186/s13018-020-01743-1
30. Leypold, BG, Flanders, AE, and Burns, AS. The early evolution of spinal cord lesions on MR imaging following traumatic spinal cord injury. AJNR Am J Neuroradiol. (2008) 29:1012–6. doi: 10.3174/ajnr.A0962
31. Liu, Q, Liu, Q, Zhao, J, Yu, H, Ma, X, and Wang, L. Early MRI finding in adult spinal cord injury without radiologic abnormalities does not correlate with the neurological outcome: a retrospective study. Spinal Cord. (2015) 53:750–3. doi: 10.1038/sc.2015.45
32. Talbott, JF, Huie, JR, Ferguson, AR, Bresnahan, JC, Beattie, MS, and Dhall, SS. MR imaging for assessing injury severity and prognosis in acute traumatic spinal cord injury. Radiol Clin N Am. (2019) 57:319–39. doi: 10.1016/j.rcl.2018.09.004
33. Pillai, A, Crane, E, Chappell, A, and Buchan, M. Traumatic cervical hematomyelia: report of a rare spinal cord injury without radiographic abnormality. J Trauma. (2008) 65:938–41. doi: 10.1097/01.ta.0000197909.10358.0f
34. Hammond, LJ, and Hecht, S. Susceptibility artifacts on T2*-weighted magnetic resonance imaging of the canine and feline SPINE. Vet Radiol Ultrasound. (2015) 56:398–406. doi: 10.1111/vru.12245
35. Gallastegui, A, Davies, E, Zwingenberger, AL, Nykamp, S, Rishniw, M, and Johnson, PJ. MRI has limited agreement with CT in the evaluation of vertebral fractures of the canine trauma patient. Vet Radiol Ultrasound. (2019) 60:533–42. doi: 10.1111/vru.12785
Keywords: hemorrhage, hematomyelia, spinal cord, recovery, durotomy, short-term outcome
Citation: Santifort KM, Carrera I and Platt S (2023) Case report: Traumatic hemorrhagic cervical myelopathy in a dog. Front. Vet. Sci. 10:1260719. doi: 10.3389/fvets.2023.1260719
Edited by:
Adriano Wang-Leandro, University of Veterinary Medicine Hannover, GermanyReviewed by:
Pia M. Vidal, Catholic University of the Most Holy Conception, ChileSam Long, Veterinary Referral Hospital, Australia
Copyright © 2023 Santifort, Carrera and Platt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koen M. Santifort, a29lbi5zYW50aWZvcnRAZXZpZGVuc2lhLm5s
 Koen M. Santifort
Koen M. Santifort Ines Carrera3
Ines Carrera3