- 1Department of Reproduction and Clinic of Farm Animals, Wrocław University of Environmental and Life Sciences, Wrocław, Poland
- 2Physiology of Reproduction and Behaviors (PRC), INRAE, CNRS, University of Tours, Tours, France
- 3Museum National d’Histoire Naturelle, Réserve Zoologique de la Haute Touche, Obterre, France
Introduction: Due to their capacity to release growth factors and cytokines, co-culture using mesenchymal stem cells has been considered a good alternative to promoting the maturation of the oocytes and the embryo’s development quality in vitro in different mammalian species. In this regard, we investigated the effect of feline Wharton’s jelly MSCs as feeders layer in oocyte maturation—consequently, the development of resulting embryos in co-culture.
Methods: Oocytes with dark cytoplasm and a few layers of cumulus cells were collected and subjected to in vitro maturation and embryo culture using commercial media with and without MSCs addition. The oocytes’ nuclear maturation and the degree of cumulus expansion in different groups were assessed after 24 h; the development of the embryo was evaluated every 12 h until day eight.
Results: Although MSCs increased the proportion of cumulus cells oocytes exhibiting cumulus expansion, there were no significant differences in the percentage of matured oocytes (metaphase II) among the groups (p > 0.05). However, the embryo development differs significantly, with a higher cleavage, morula, and blastocyst percentage in oocytes matured with MSC co-culture conditions than in commercial media alone (p < 0.05). Also, we observed higher morula and blastocyst rates in the embryos co-cultured with MSCs during the in vitro culture (p > 0.05).
Conclusion: Based on our results, the co-culture with MSCs during the oocyte maturation resulted in better embryo development, as well as the MSCs addition during embryo culture returned an increased number of morula and blastocysts. Further research is needed to fully understand and optimize the use of MSCs in oocyte maturation and embryo development.
Introduction
Due to the decreasing number of wild felids, domestic cats (Felis catus) as a model for studying reproduction physiology and developing new assisted reproductive technologies (ART) are gaining more and more importance (1).
ART has been used for several years to preserve genetic material, circumvent problems of subfertility, improve male reproduction, and increase the reproductive results and number of offspring that a single female can obtain. The post-fertilization period is an essential step in culturing embryos in vitro. Therefore, it is crucial to carefully plan the culture conditions during this period to ensure proper embryonic development. Inadequate culture conditions can significantly impact embryonic homeostasis, leading to short-term changes in morphology, cell proliferation, and metabolism and resulting in apoptosis. Such changes can ultimately lead to a reduction in both the number and quality of the formed blastocysts.
After years of investigations, the basic nutritional requirements for oocytes and embryos have been established, guaranteeing successful in vitro development in horses (2), cattle (3), pigs (4), mice and humans (5). However, ART in a feline species is still not as efficient as in other animals. Unfortunately, the culture conditions dedicated for cat oocytes and embryos have not yet been adequately investigated, and on average, in vitro, only around 60% of cats’ oocytes reach the MII phase, and less than a half of the cleaved embryos become blastocyst (6). Furthermore, the low rate of embryo production from feline oocytes reflects the need for a better understanding of the developmental competence of feline oocytes and their specific requirements during in vitro maturation, fertilization and embryo development. Current ART procedures lack knowledge of the interaction of gametes with several components present in the reproductive system during the maturation of oocytes and early stages of embryo development. To mimic the in vivo complex microenvironment in vitro, recent advances used a co-culture of oocytes and embryos with oviduct epithelial cells, mesenchymal stem cells (MSCs), cumulus cells and extracellular vesicles (EVs) in the reproductive environment with the aim to obtain in vitro embryos with developmental levels similar to embryos derived in vivo.
MSCs possess multi-potentiality and properties of immunological and inflammatory regulation. Cell therapy based on their transplant is a promising approach, as these cells can develop into adipocytes, osteoblasts, chondrocytes, smooth muscle cells, and endothelial cells and can express many specific markers depending on the environmental conditions in which they are found (7). The most common sources of MSCs are of adult origins, such as bone marrow or adipose tissue, but their removal requires an invasive clinical procedure. Perinatal sources like an umbilical cord, mainly Wharton’s jelly, offer higher practical accessibility and good quality MSCs with a higher proliferation rate and more potent immunomodulatory properties (8). In vitro, MSCs can thus promote cell viability and angiogenesis by producing growth factors. They also stimulate the recruitment of endogenous stem cells by secreting chemokines and acting locally through cell–cell interactions based on receptor-ligand bonds or through nanotubes that transfer molecules and organelles (9).
MSCs’ properties make them a suitable candidate for improving the performance of in vitro production systems in mammalian species. In fact, many studies have used MSCs or their derived biomaterials in a co-culture system with oocytes and/or embryos, with most studies indicating improved embryo development (10). Furthermore, it has been shown that coculture with MSCs could rescue poor-quality embryos and enhance early embryonic development (11, 12). Additionally, coculture with MSCs has been observed to enhance the cytoplasmic and nuclear oocyte maturation in vitro (13, 14). Based on these findings, we hypothesise that feline Wharton’s Jelly-derived MSCs could improve oocyte maturation and embryo culture in vitro. In this regard, we aimed to evaluate the in vitro effect of fWJ-MSCs added as a feeder layer in the co-culture system during cats’ oocyte maturation and embryo development, in comparison to non-conditioned, commercial maturation and culture media.
Materials and methods
All chemicals and reagents were purchased from Sigma Aldrich Poland unless stated otherwise. Ethical approval was not sought, as it is not required for studies carried out on cells obtained from tissues that were surgical waste (Decision No. 004/2021). Commercial media were used for the oocyte manipulation and maturation: IVF Bioscience, Bickland Industrial Park, Falmouth, United Kingdom.
Mesenchymal stem cells isolation and characterization
MSCs were isolated and characterized, as mentioned in our previous study (8). Umbilical cords were collected from healthy queens (1.5–5 years old) after a normal birth and caesarean sections; the cells were obtained from Wharton’s jelly parts of the umbilical cord (fWJ-MSC—feline Wharton’s jelly mesenchymal stem cells) using collagenase type I at 0.02% in DMEM-LG. The cells were cultured in DMEM-LG containing 10% FBS and 1% PS at 37°C in humidity. Adherents’ cells were grown until reaching 80 to 90% confluence before each passage, and the medium was changed three times a week. Before fWJ-MSCs were used, the cells were identified and characterized based on their expansion rate, tri-lineage differentiation (adipocytes, chondrocytes, and osteoblasts), cell surface markers (CD44, CD90, CD34, and MHC II) and pluripotency genes expression (OCT4, SOX2, NANOG).
Preparation of Wharton’s jelly mesenchymal stem cells
To use fWJ-MSCs as a feeder layer, the cells at passage 2 to 3 were seeded in four well plates at a density of 1 × 104 cells/mL in DMEM-LG containing 10% FBS and 1% PS at 37°C in humidity until reaching 80% to 90% confluence, nonadherent cells were removed by washing twice with PBS. The adherent cells were inactivated with 10 μg/mL mitomycin C for 2 h to avoid nutrients competition. After a series of washes with PBS, the culture was maintained in DMEM-LG for 24 h before the oocytes or embryos were co-cultivated.
Ovaries and oocytes collection
Ovaries were obtained from sexually matured domestic queens subjected to a routine ovariohysterectomy or ovariectomy at the University clinic and local veterinarians in Wroclaw. After surgical removal, ovaries were stored in PBS with 1% of Antibiotic Antimycotic Solution at 4°C for up to 24 h before the recovery of cumulus-oocyte complexes (COCs). COCs were collected by slicing ovaries with a #10 scalpel blade in an OPU medium. Isolated COCs were classified under a dissecting microscope. Only oocytes with evenly pigmented dark ooplasm and some layers of cumulus cells were selected for further procedures.
In vitro maturation of cat oocytes
The selected COCs were placed in a four-well plate in 400 μL of plain bovine maturation medium (BoM) and plain equine maturation medium (EqM) or in the same medium with MSCs co-culture: BoM + MSCs or EqM + MSCs, under mineral oil and matured for 24 h at 38.5°C in 5% CO2 in the air with maximum humidity.
In vitro fertilization
For in vitro fertilization, the oocytes were fertilized with frozen–thawed semen and cryopreserved according to the protocol described by Partyka et al. (15). Semen straw was thawed in a water bath at 37°C then washed in IVF medium followed by centrifugation at 35,000 rpm for 5 min. After 24 h of maturation, cumulus oocytes complex were washed in IVF medium; then incubated with 1 × 106 motile spermatozoa/ml for 18 h in 400 μL of IVF medium under mineral oil at 38.5°C in 5% CO2 in the air with maximum humidity.
Assessment of oocytes maturation
In order to establish the assessment of oocyte maturation after 24 h of IVM, all the cumulus cells were mechanically removed using a glass pipette overheated and pulled to achieve the diameters of approximately 165 μm, slightly larger than the oocyte. Oocytes were aspirated and blown out repeatedly until most cumulus cells were removed. After most of the cumulus cells were removed, the oocytes were washed twice and fixed with 4% formaldehyde for 15 min flowed by washing in PBS and then incubated in DAPI stain solution for 10 min in the dark and mounted on glass slides in drops of Vectashield (Vector Laboratories, Ltd. United Kingdom). The nuclear state of the stained oocytes was assessed under a fluorescence microscope (Olympus IX73) at 360 excitations and 450 nm emission. Oocytes with distinct polar body or two separate and bright chromatin spots were classified as entering the MII stage.
Embryo culture and assessment of the embryo development
After fertilization, presumptive zygotes were washed and transferred to a new plate in a droplet of 50 μL of either BoM or EqM medium or co-culture BoM + MSCs, EqM + MSCs medium (depending on the part of the experiment) covered with mineral oil and incubated at 38.5°C in 5% CO2 in the air with maximum humidity for up to 8 days. To assess embryo development, morphological changes were evaluated and noted every 8 to 12 h. The subsequent developmental stages were noted for each group, and the blastocyst formation was recorded.
Study design
Experiment 1: the effect of the co-culture with MSCs on the oocyte maturation and cumulus cell expansion
This experiment evaluated nuclear maturation and cumulus cell expansion. Oocytes were matured in 400 μL of IVF medium under mineral oil at 38.5°C in 5% CO2 in the air with maximum humidity. In total, 180 oocytes were used in this part of the study, and three independent replicates of 15 oocytes per experimental group were carried out. Study groups were as follows:
• Maturation in BoM (n = 45 oocytes).
• Maturation in EqM (n = 45 oocytes).
• Maturation in BoM + MSCs (n = 45 oocytes).
• Maturation in EqM + MSCs (n = 45 oocytes).
The degree of nuclear maturation was analyzed after 24 h.
Assessment of cumulus cells expansion
The degree of cumulus cells expansion after 24 h of oocyte maturation using two different commercial media and with or without MSC addition was assessed as described by Lee et al. (16). The evaluation system was as follows: no expansion, limited expansion (less than three layers of cumulus cells expended), expended (more than three layers of cumulus cells expanded) and oocytes with no cumulus cells attached were classified as degenerated) (Figure 1).
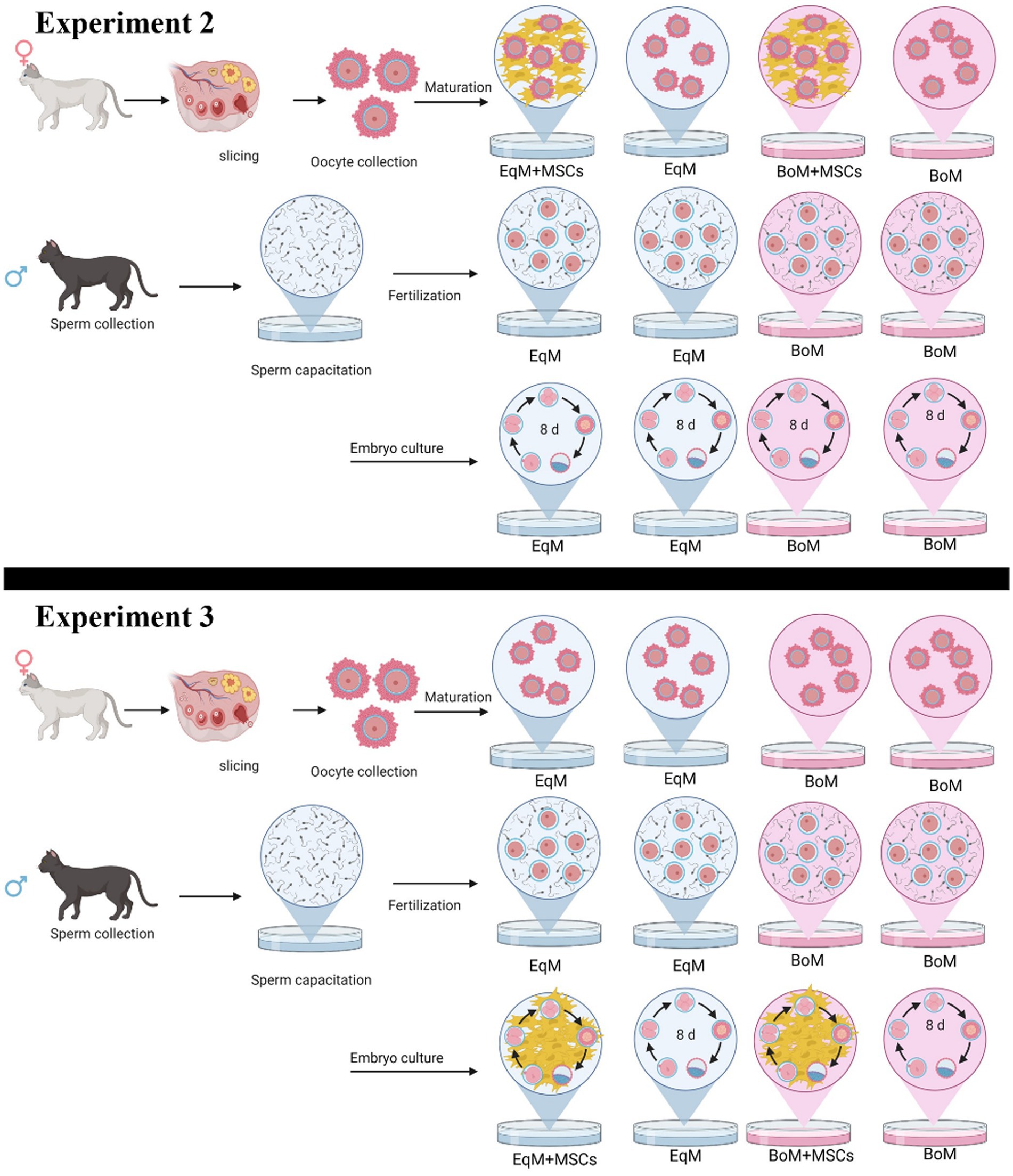
Figure 1. Study design of the experiment 2: the effect of the co-culture with MSC during oocyte maturation on the subsequent embryo development. Oocytes were matured and embryos cultured in four groups: EqM + MSCs/EqM: maturation in EqM + MSCs/embryo culture in EqM (n = 109). EqM/EqM: maturation in EqM/embryo culture in EqM (n = 109). BoM + MSCs/BoM: maturation in BoM + MSCs/embryo culture in BoM (n = 124). BoM/BoM: maturation in BoM/embryo culture in BoM (n = 103) and experiment 3: The effect of MSC addition during embryo development. Oocytes were matured, and embryo culture was carried out in four groups: EqM/EqM + MSCs: maturation in EqM/embryo culture in EqM + MSCs (n = 142). EqM/EqM: maturation in EqM/embryo culture in EqM (n = 109). BoM/BoM + MSCs: maturation in BoM/embryo culture in BOM + MSCs (n = 132). BoM/BoM: maturation in BoM/embryo culture in BOM (n = 103). The figure was prepared with BioRender.
Assessment of nuclear maturation
The nuclear state of the stained oocytes was assessed under the fluorescence microscope (Olympus IX73) at excitation 360 and 450 nm emission. Oocytes with distinct polar bodies or two separate and bright chromatin spots were classified as entering the MII stage (Figure 2).
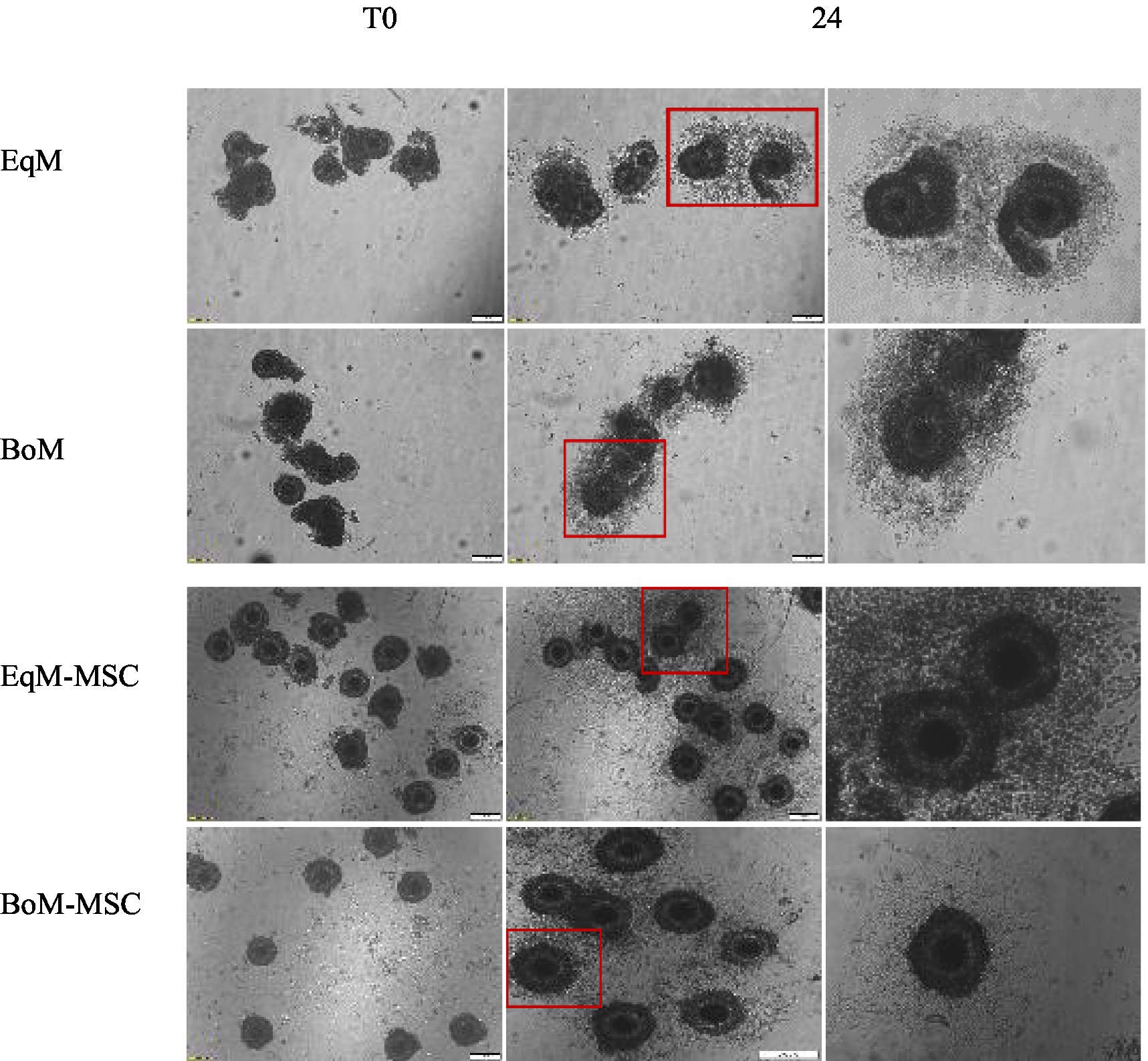
Figure 2. Effect of feline MSCs cells on cumulus expansion of oocytes after IVM. The degree of cumulus expansion at T0 and after 24 h of IVM in four groups EqM + MSC, BoM + MSC, EQM, and BoM. The images were taken with a magnification = 200 um.
Experiment 2: the effect of the co-culture with MSCs during oocyte maturation on embryo development
This part of the study was done to assess the effect of the co-culture system with MSCs during maturation in two commercial media on the subsequent embryo development after in vitro fertilization. In total, 565 oocytes were matured and cultured in four groups,10 replicates per group, as illustrated in Figure 1. Embryonic development (cleavage, morula and blastocysts rate) was compared among all groups.
Experiment 3: the effect of co-culture with MSC during embryo development
At this stage, the oocytes were matured in EqM or in BoM then the MSCs were added during the embryo development to evaluate their effect on the morula and blastocyst formation. In total, 486 oocytes were matured and cultured in four groups, 10 replicates per group, as presented in Figure 1.
Statistical analysis
Data were analysed using one-way ANOVA followed by Tukey’s multiple comparison test using Statistical software (TIBCO, United States). Values are shown as mean ± S.E.M. The significance level was p < 0.05, and at least three independent replicates were performed in all experiments. Nonparametric data, such as differences in the percentage values between groups, were assessed using the chi-square test.
Results
Effect of co-culture on the cumulus cells expansion
The degree of COCs expansion was significantly increased in the EqM + MSCs and BoM + MSCs groups compared to EqM and BoM or groups (p < 0.05) (Figures 2, 3). Furthermore, there was no significant difference between the EqM + MSCs and BoM + MSCs groups. The evaluation of the degree of cumulus cell expansion confirmed that co-culture with MSC showed a considerable increase in the proportion of COCs that showed cumulus expansion (p < 0.05).
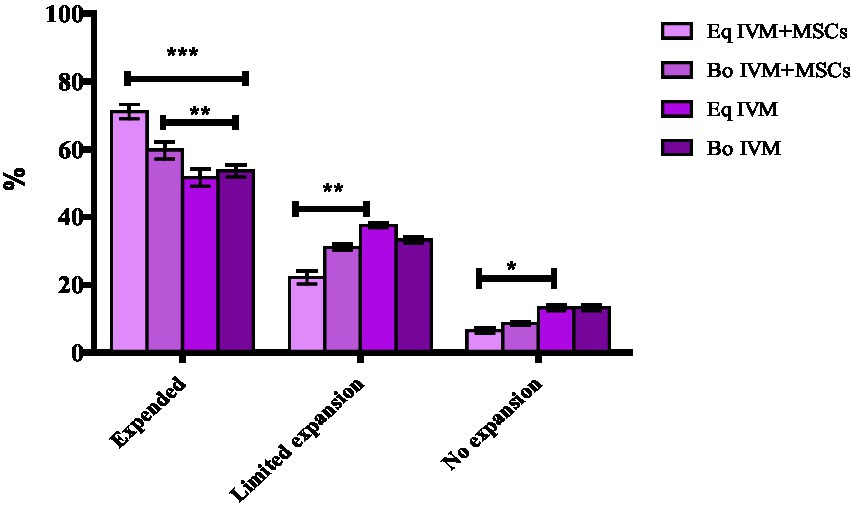
Figure 3. Effect of feline MSCs cells on cumulus expansion of oocytes after in vitro maturation. The cumulus expansion scoring system was as follows: limited expansion, no expansion, degenerated oocytes, denuded and expanded oocytes in four groups EqM + MSCs, BoM + MSCs, EQM, BoM. The Data are shown as the mean ± S.E.M. ***, **, *Within the columns, values are significantly different (p < 0.05).
Effect of different culture conditions on the efficacy of maturation of oocytes
Nuclear maturation was evaluated using DAPI staining and showed a similar percentage of metaphase II (M II) (Figure 4B) in all investigated groups, which ranged from 45% to 55% (p > 0.05). The co-culture system with MSCs did not affect the nuclear maturation of oocytes (Figure 4A).
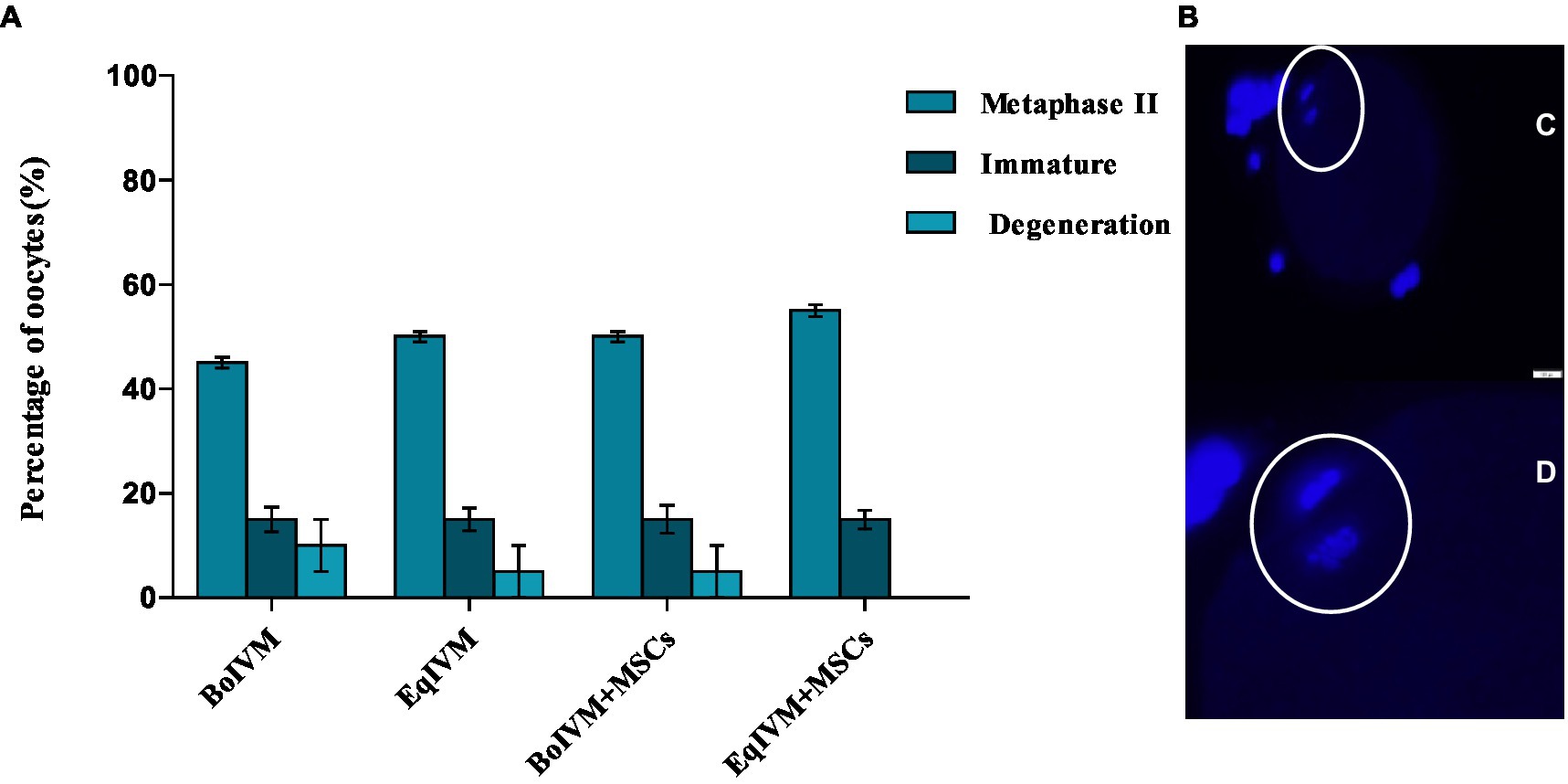
Figure 4. (A) Effect of different maturation conditions on nuclear maturation of feline oocytes. Metaphase II indicated oocyte maturation; the results are shown as the mean ± S.E.M. within the columns (p < 0.05). (B) Representative microscopy images of oocytes in metaphase II illustrating (C, D) polar body extrusion and nucleus stained with DAPI (Bar = 50 μm).
Development of embryos derived from oocytes matured under different maturation conditions
As shown in Table 1, the percentage of the oocytes which cleaved was similar in the co-culture group EqM + MSCs and BoM + MSCs and higher (p < 0.05) than in the EqM and BoM group. The rate of the morula was similar among EqM, BoM and BoM + MSCs groups but higher in the group of oocytes matured in EqM + MSCs. The oocytes matured in EqM + MSCs showed the most promising development and the highest number of blastocysts compared with the BoM + MSCs, BoM and EqM groups. Thus, the use of MSC as a co-culture during oocyte maturation has an effect on the further development of feline embryos (Table 1).
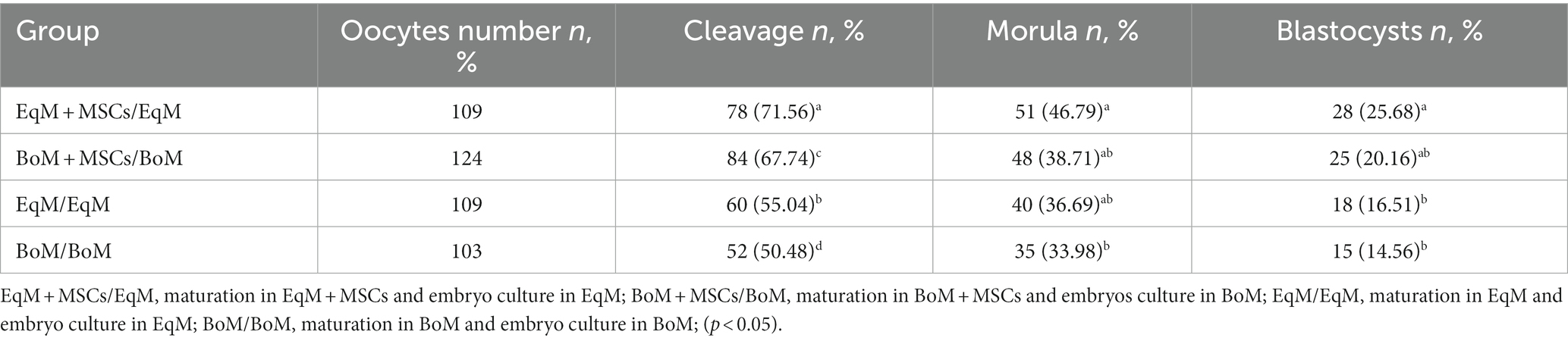
Table 1. Effect of different conditions during oocyte maturation on the subsequent embryo development.
Development of embryos cultured under different conditions
The rate of development of the resulting two-cell embryos was higher in the co-culture group of EqM + MSCs and BoM + MSCs compared with two-cell embryos that were cultured in BoM and EqM (p > 0.05). However, we also noticed that the blastocyst/morula rate was higher in pure EqM media when compared to non-conditioned Bo culture media (p > 0.05).
Discussion
In vivo, oocyte maturation occurs within the ovarian follicle, while fertilization and early embryo development occur in the fallopian tubes. When trying to recreate in vitro these physiological conditions, it is crucial to provide efficient culture systems (17); the first successful in vitro fertilization (IVF) in a domestic cat was achieved 45 years ago using in vivo matured oocytes and in utero-capacitated spermatozoa (18). Despite advances in culture conditions, media and protocols for oocyte maturation and embryo development, in vitro outcomes are still far from desirable compared with embryos produced in vivo (19, 20). In particular, recent studies have shown the beneficial effect of co-culture with MSCs (21), oviduct cells, and cumulus cells (22, 23) on the development of oocytes and embryos in various mammalian species, including cattle (24), horses (25), pigs (26) and canines (27). Therefore, culture conditions are crucial in determining the quality of in vitro-produced embryos. In the present study, we demonstrated for the first time the effect of feline Wharton’s jelly-derived MSCs and different commercial media on the maturation of feline oocytes, cumulus cell expansion and embryo development.
Oocytes with adequate nuclear and cytoplasmic maturation are more competent since many proteins and transcripts stored in their cytoplasm will be required for future embryo development. Therefore, in this study, we investigated whether the co-culture condition with MSCs as a feeder layer can influence oocyte maturation and their ability to develop into embryos. Based on the extrusion of the first polar body (metaphase II) in each experimental group, we did not observe the effect of co-culture with MSCs on the oocytes’ nuclear maturation resulting in comparable percentages ranging between 45 and 55% of MII. Similarly to our results, Ascari et al. (28) showed that murine MSCs or embryonic fibroblasts did not affect the nuclear maturation rate of bovine oocytes. In contrast, the addition of the conditioned medium containing human bone marrow MSCs, as a supplement to enrich the IVM medium used for germinal vesicles in mice polycystic ovary syndrome (PCOS) significantly increased cytoplasmic and nuclear maturation of oocytes (13).
However, when analysing the treatment used during maturation, we observe the morphological difference in cumulus cells expansion after 24 h of maturation; the oocytes cultured in MSCs-conditioned media had significantly increased the cumulus cell expansion compared to oocytes cultured without MSC addition. Similar observations were reported for human adipose-derived stem cells (ASC) added to the medium (ASC-CM) that improved cumulus cell expansion with high transcript abundance of an expansion-related gene in porcine (29). It was also reported by Wang et al. (30) that human Wharton’s jelly MSCs were used to treat mice with induced premature ovarian failure using a daily dose of intraperitoneal CTX injection (50 mg/kg) for 15 consecutive days; the results showed that MSCs reduced cumulus cell apoptosis in investigated mice. Other authors explored the use of human placental MSCs on human ovarian granulosa cells obtained from patients with premature ovarian insufficiency; the reported results showed that MSCs released epidermal growth factor (EGF) that reduced apoptosis and improved proliferation, and restored the oxidative enzyme levels of human granulosa and cumulus cells (31).
After fertilization, we observed differences between the oocytes that matured with and without MSCs. The embryos derived from the oocytes matured with MSCs: EqM + MSCs, BoM + MSCs showed a higher cleavage, morula, and blastocyst rate compared to the oocytes matured in classic BoM and EqM. We observed that the presence of MSCs during the maturation of oocytes did not affect nuclear maturation; it still affected the cleavage rate and blastocyst formation. As reported before, MSCs release several trophic factors, including EGF and cytokines. The trophic effects of these bioactive factors on preantral follicular growth and in vitro maturation of mouse oocytes have been shown (32); it was also reported that the conditioned medium containing human MSCs generated microenvironment that was more appropriate to induce oocyte maturation and increase embryo development of; they also described that high embryonic development rates might be associated with the quality of nuclear and cytoplasmic maturation (33).
The effect of co-culture with MSCs during embryo development was also evaluated in our study. In this part of the experiment, we noticed an improvement in embryo development (Table 2); the morula and blastocysts rate was higher in EqM + MSCs and BoM + MSCs than in BoM and EqM. It is interesting to point out that the embryos co-cultured with MSCs were previously maturated in classic BoM or EqM media. Our current findings are similar to the results of the previous study conducted by Jasmin et al. (34) using mice embryos; they observed that embryos co-cultured with MSCs for 4 days actually formed more blastocysts. Furthermore, our current data are similar to those shown by the same group using murine MSCs and embryonic fibroblast as a co-culture during embryo development (28).
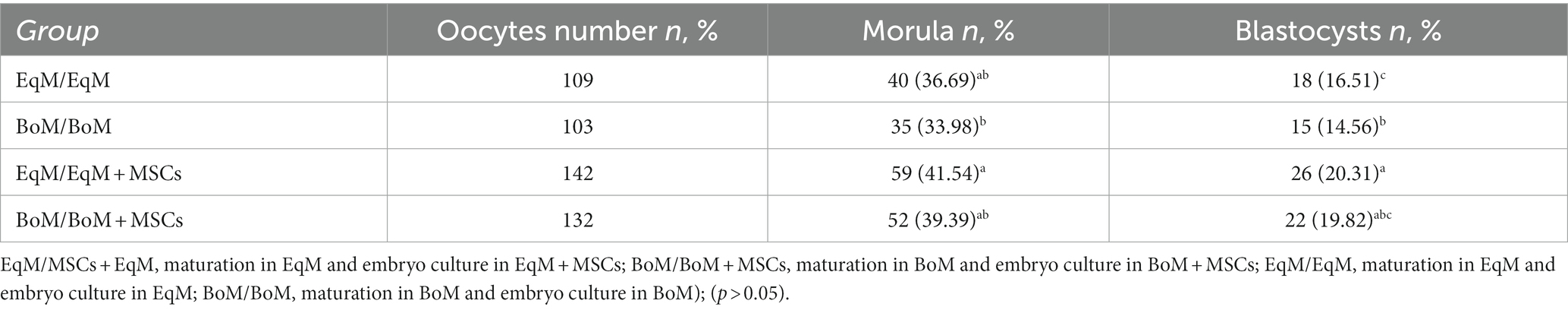
Table 2. Effect of different culture conditions on the development of the embryos from the oocytes matured in BoM or EqM.
In general, the co-culture with somatic cells has shown a positive impact on embryonic development in vitro. Most studies indicate a higher rate of blastocyst formation after culturing embryos with different types of somatic cells (35, 36). However, some studies did not show significant improvement in embryo development (37, 38), and some others indicated a negative effect of the co-culture system on preimplantation embryo development (39). However, the co-culture studies published to date used very different types and concentrations of cells ranging from 1 × 103 to 1 × 106 cells/mL (24, 40), whereas our study used 1 × 104 cells/mL, as a long with the main medium used, time points and oxygen concentrations, so comparisons are very hard and quite limited.
In fact, despite some similarities, each species may have different requirements regarding the substrates in the medium (41), which could explain the minor divergent results observed in our study. Here, we used the same source of mesenchymal cells, but we carried out the culture using two media dedicated to different species (cattle and horses). It is worth noticing that we noted more blastocysts with the use of equine media (EqM) compared to bovine (BoM). The latter could also have some impact on the results obtained during the co-culture experiment.
In summary, we investigated the potential of feline Wharton’s jelly MSC to assist in feline oocyte maturation and embryo growth. With the addition of feline Wharton jelly MSC both to oocyte and embryo culture, we observed an improved embryo development. Furthermore, our results did not show a significant impact on the nuclear maturation process itself, but the addition of MSCs as a feeder layer during the maturation or embryo culture still resulted in a higher rate of embryonic development. In particular, we found that the co-culture with MSCs was most effective during oocyte maturation, as the cleavage and blastocyst rates were higher when MSCs were added during oocytes maturation than during embryos development. These findings suggest that feline Wharton’s jelly MSCs could be a promising tool for improving in vitro feline embryo development in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not sought, as it is not required for studies on cells obtained from surgical waste tissues (Decision No. 004/2021). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MO and MB: conceptualization, data collection, data evaluation, statistical analysis, and manuscript writing. PM: data evaluation and reviewing of the paper. YL: reviewing and editing the paper. WN: consulting and supervising the study and paper reviewing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by UPWr 2.0 project International and Interdisciplinary Development Programme of Wroclaw University of Environmental and Life Sciences, co-financed by the European Social Fund under the Operational Program Knowledge Education Development Program 2014–2020: Axis III Higher Education for the Economy and Development; Action 3.5. Comprehensive University Programs; for Schools of Higher Education (POWR.03.05.00-00-Z062/18).
Acknowledgments
The authors would like to thank the University clinic and Wroclaw local veterinary clinics for their help in collecting samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nowak, A, Kochan, J, Prochowska, S, Partyka, A, Młodawska, W, Witarski, W, et al. The viability of serval (Leptailurus serval) and pallas cat (Felis manul) oocytes after cryopreservation using the rapid-I method. Cryo Letters. (2019) 40:226–30.
2. Landim-Alvarenga, FC, Fernandes, CB, Devito, LG, Derussi, AAP, Blanco, IDP, and Alvarenga, MA. New assisted reproductive technologies applied to the horse industry: successes and limitations. Anim Reprod. (2008) 5:67–82.
3. Ferré, LB, Kjelland, ME, Strøbech, LB, Hyttel, P, Mermillod, P, and Ross, PJ. Review: recent advances in bovine in vitro embryo production: reproductive biotechnology history and methods. Animal. (2020) 14:991–1004. doi: 10.1017/S1751731119002775
4. Romero-Aguirregomezcorta, J, Ramírez, LL, Ortín, A, Ramis, G, Romar, R, and Coy, P. Differences in weight, hierarchy, and incidence of lameness between two groups of adult pigs derived from assisted reproductive technologies. Animals. (2022) 12:3578. doi: 10.3390/ani12243578
5. Legge, M, and Fitzgerald, R. Does the human assisted reproductive technology act 2004 need a review? Policy Q. (2021) 17. doi: 10.26686/pq.v17i1.6734
6. Pope, CE, Gómez, MC, and Dresser, BL. In vitro production and transfer of cat embryos in the 21st century. Theriogenology. (2006) 66:59–71. doi: 10.1016/j.theriogenology.2006.03.014
7. Baouche, M, Ochota, M, Locatelli, Y, Mermillod, P, and Niżański, W. Mesenchymal stem cells: generalities and clinical significance in feline and canine medicine. Animals. (2023) 13:1903. doi: 10.3390/ani13121903
8. Baouche, M, Krawczenko, A, Paprocka, M, Klimczak, A, Mermillod, P, Locatelli, Y, et al. Feline umbilical cord mesenchymal stem cells: isolation and in vitro characterization from distinct parts of the umbilical cord. Theriogenology. (2023) 201:116–25. doi: 10.1016/j.theriogenology.2022.11.049
9. Nie, W-B, Zhang, D, and Wang, L-S. Growth factor gene-modified mesenchymal stem cells in tissue regeneration. Drug Des Devel Ther. (2020) 14:1241–56. doi: 10.2147/DDDT.S243944
10. Vanroose, G, Van Soom, A, and de Kruif, A. From co-culture to defined medium: state of the art and practical considerations. Reprod Domest Anim. (2001) 36:25–8. doi: 10.1046/j.1439-0531.2001.00264.x
11. Heidari, B, Shirazi, A, Naderi, M-M, Akhondi, M-M, Hassanpour, H, Sarvari, A, et al. Effect of various co-culture systems on embryo development in ovine. Czeh J Anim Sci. (2013) 58:443–52. doi: 10.17221/6993-CJAS
12. Kervancioglu, ME, Saridogan, E, Atasu, T, Camlibel, T, Demircan, A, Sarikamis, B, et al. Human fallopian tube epithelial cell co-culture increases fertilization rates in male factor infertility but not in tubal or unexplained infertility. Hum Reprod. (1997) 12:1253–8. doi: 10.1093/humrep/12.6.1253
13. Jafarzadeh, H, Nazarian, H, Ghaffari Novin, M, Shams Mofarahe, Z, Eini, F, and Piryaei, A. Improvement of oocyte in vitro maturation from mice with polycystic ovary syndrome by human mesenchymal stromal cell–conditioned media. J Cell Biochem. (2018) 119:10365–75. doi: 10.1002/jcb.27380
14. Nugraha Setyawan, EM, Oh, HJ, Kim, MJ, Kim, GA, Lee, SH, Bin, CY, et al. Despite the donor’s age, human adipose-derived stem cells enhance the maturation and development rates of porcine oocytes in a co-culture system. Theriogenology. (2018) 115:57–64. doi: 10.1016/j.theriogenology.2017.12.024
15. Partyka, A, Niaski, W, and Ochot, M. “Methods of assessment of cryopreserved semen,” Current Frontiers in Cryobiology. InTech (2012).
16. Lee, SH, Oh, HJ, Kim, MJ, and Lee, BC. Canine oviductal exosomes improve oocyte development via EGFR/MAPK signaling pathway. Reproduction. (2020) 160:613–25. doi: 10.1530/REP-19-0600
17. Luvoni, GC, Colombo, M, and Morselli, MG. The never-ending search of an ideal culture system for domestic cat oocytes and embryos. Reprod Domest Anim. (2018) 53:110–6. doi: 10.1111/rda.13331
18. Hamner, CE, Jennings, LL, and Sojka, NJ. Cat (Felis catus L.) spermatozoa require capacitation. Reproduction. (1970) 23:477–80. doi: 10.1530/jrf.0.0230477
19. Gruber, I, and Klein, M. Embryo culture media for human IVF: which possibilities exist? J Turk Ger Gynecol Assoc. (2011) 12:110–7. doi: 10.5152/jtgga.2011.25
20. Simopoulou, M, Sfakianoudis, K, Rapani, A, Giannelou, P, Anifandis, G, Bolaris, S, et al. Considerations regarding embryo culture conditions: from media to epigenetics. In Vivo. (2018) 32:451–60. doi: 10.21873/invivo.11261
21. Opiela, J, Bülbül, B, and Romanek, J. Varied approach of using MSCs for bovine embryo in vitro culture. Anim Biotechnol. (2019) 30:105–12. doi: 10.1080/10495398.2018.1521820
22. Lee, SH, Oh, HJ, Kim, MJ, Kim, GA, Bin, CY, Jo, YK, et al. Effect of co-culture canine cumulus and oviduct cells with porcine oocytes during maturation and subsequent embryo development of parthenotes in vitro. Theriogenology. (2018) 106:108–16. doi: 10.1016/j.theriogenology.2017.09.015
23. Luciano, AM, Lodde, V, Beretta, MS, Colleoni, S, Lauria, A, and Modina, S. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3?,5?-monophosphate, and glutathione. Mol Reprod Dev. (2005) 71:389–97. doi: 10.1002/mrd.20304
24. Miranda, MS, Nascimento, HS, Costa, MPR, Costa, NN, Brito, KNL, Lopes, CTA, et al. Increasing of blastocyst rate and gene expression in co-culture of bovine embryos with adult adipose tissue-derived mesenchymal stem cells. J Assist Reprod Genet. (2016) 33:1395–403. doi: 10.1007/s10815-016-0779-0
25. Grady, ST, Watts, AE, Thompson, JA, Penedo, MCT, Konganti, K, and Hinrichs, K. Effect of intra-ovarian injection of mesenchymal stem cells in aged mares. J Assist Reprod Genet. (2019) 36:543–56. doi: 10.1007/s10815-018-1371-6
26. Park, A, Oh, HJ, Ji, K, Choi, EM, Kim, D, Kim, E, et al. Effect of passage number of conditioned medium collected from equine amniotic fluid mesenchymal stem cells: porcine oocyte maturation and embryo development. Int J Mol Sci. (2022) 23:6569. doi: 10.3390/ijms23126569
27. No, J, Zhao, M, Lee, S, Ock, SA, Nam, Y, and Hur, T-Y. Enhanced in vitro maturation of canine oocytes by oviduct epithelial cell co-culture. Theriogenology. (2018) 105:66–74. doi: 10.1016/j.theriogenology.2017.09.002
28. Ascari, IJ, Martins, SC, Camargo, LSA, Mendez-Otero, R, and Jasmin,. Development of bovine embryos in vitro in coculture with murine mesenchymal stem cells and embryonic fibroblasts. Mol Biol Rep. (2018) 45:1827–37. doi: 10.1007/s11033-018-4329-y
29. Lee, SH. Human adipose-derived stem cells’ paracrine factors in conditioned medium can enhance porcine oocyte maturation and subsequent embryo development. Int J Mol Sci. (2021) 22:579. doi: 10.3390/ijms22020579
30. Wang, S, Yu, L, Sun, M, Mu, S, Wang, C, Wang, D, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. (2013) 2013:690491. doi: 10.1155/2013/690491
31. Ding, C, Zou, Q, Wu, Y, Lu, J, Qian, C, Li, H, et al. EGF released from human placental mesenchymal stem cells improves premature ovarian insufficiency via NRF2/HO-1 activation. Aging. (2020) 12:2992–3009. doi: 10.18632/aging.102794
32. Ling, B, Feng, DQ, Zhou, Y, Gao, T, Wei, HM, and Tian, ZG. Effect of conditioned medium of mesenchymal stem cells on the in vitro maturation and subsequent development of mouse oocyte. Braz J Med Biol Res. (2008) 41:978–85. doi: 10.1590/S0100-879X2008005000053
33. Akbari, H, Eftekhar Vaghefi, S, Shahedi, A, Habibzadeh, V, Mirshekari, T, Ganjizadegan, A, et al. Mesenchymal stem cell-conditioned medium modulates apoptotic and stress-related gene expression, ameliorates maturation and allows for the development of immature human oocytes after artificial activation. Genes. (2017) 8:371. doi: 10.3390/genes8120371
34. Jasmin,, Peters, VM, Spray, DC, and Mendez-Otero, R. Effect of mesenchymal stem cells and mouse embryonic fibroblasts on the development of preimplantation mouse embryos. In Vitro Cell Dev Biol Anim. (2016) 52:497–506. doi: 10.1007/s11626-015-9997-5
35. Nematollahi-mahani, SN, Pahang, H, Moshkdanian, G, and Nematollahi-mahani, A. Effect of embryonic fibroblast cell co-culture on development of mouse embryos following exposure to visible light. J Assist Reprod Genet. (2009) 26:129–35. doi: 10.1007/s10815-008-9290-6
36. Joo, BS, Kim, MK, Na, YJ, Moon, HS, Lee, KS, and Do, KH. The mechanism of action of coculture on embryo development in the mouse model: direct embryo-to-cell contact and the removal of deleterious components. Fertil Steril. (2001) 75:193–9. doi: 10.1016/S0015-0282(00)01671-X
37. Tucker, MJ, Kort, HI, Toledo, AA, Morton, PC, Wright, G, Ingargiola, PE, et al. Effect of coculture on subsequent survival and implantation of cryopreserved human embryos. J Assist Reprod Genet. (1995) 12:689–92. doi: 10.1007/BF02212894
38. Hu, Y, Maxson, W, Hoffman, D, Ory, S, Eager, S, Dupre, J, et al. Co-culture with assisted hatching of human embryos using Buffalo rat liver cells. Hum Reprod. (1998) 13:165–8. doi: 10.1093/humrep/13.1.165
39. Bernardi, ML, Flechon, J-E, and Delouis, C. Influence of culture system and oxygen tension on the development of ovine zygotes matured and fertilized in vitro. Reproduction. (1996) 106:161–7. doi: 10.1530/jrf.0.1060161
40. Mamidi, MK, Pal, R, Mori, NAB, Arumugam, G, Thrichelvam, ST, Noor, PJ, et al. Co-culture of mesenchymal-like stromal cells derived from human foreskin permits long term propagation and differentiation of human embryonic stem cells. J Cell Biochem. (2011) 112:1353–63. doi: 10.1002/jcb.23052
Keywords: cat, oocytes, embryos, co-culture, mesenchymal stem cells, Wharton’s jelly
Citation: Baouche M, Ochota M, Mermillod P, Locatelli Y and Nizanski W (2023) Feline Wharton’s jelly-derived mesenchymal stem cells as a feeder layer for oocytes maturation and embryos culture in vitro. Front. Vet. Sci. 10:1252484. doi: 10.3389/fvets.2023.1252484
Edited by:
Mehdi Hajian, Royan Institute, IranReviewed by:
Shiva Rouhollahi, Royan Institute, IranDiana Maritza Echeverry Berrio, Universidad San Sebastián, Chile
Copyright © 2023 Baouche, Ochota, Mermillod, Locatelli and Nizanski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Małgorzata Ochota, bWFsZ29yemF0YS5vY2hvdGFAdXB3ci5lZHUucGw=; Wojciech Nizanski, d29qY2llY2gubml6YW5za2lAdXB3ci5lZHUucGw=
 Meriem Baouche
Meriem Baouche Małgorzata Ochota
Małgorzata Ochota Pascal Mermillod2
Pascal Mermillod2 Wojciech Nizanski
Wojciech Nizanski