- 1College of Animal Science and Technology, Sichuan Agricultural University, Chengdu, China
- 2Key Laboratory of Livestock and Poultry Multi-Omics, Ministry of Agriculture and Rural Affairs, College of Animal Science and Technology, Sichuan Agricultural University, Chengdu, China
- 3Farm Animal Genetic Resources Exploration and Innovation Key Laboratory of Sichuan Province, College of Animal Science and Technology, Sichuan Agricultural University, Chengdu, China
Castration of male animals is intended to produce high-enhance quality of animal meat, prevent unpleasant taste, reduce aggressive behavior, and manage overbreeding. Over the years, Tranditional methods of mechanical and surgical castration have been employed over the years, but they fall short of meeting animal welfare requirements due to the associated risk of infection, pain, and stress. Immunocastration, specifically Gonadotropin-releasing hormone (GnRH)-immunocastration, targeting the hypothalamic–pituitary-testis (HPT) axis, has emerged as an animal-friendly alternative to surgical castration, effectively addressing these issues. This review seeks to systematically summarize the principles, development, current applications and challenges of GnRH-immunocastration, offering insights into its role in promoting animal welfare.
1. Introduction
Capon production, an ancient practice dating back over 3,000 years, persists globally (1–3). While capon production constitutes a modest segment of the market, it holds significant growth potential due to its dustinctive sensory attributes cherished by consumers (4–7). Capon are male chickens that undergo surgicalcastration before reaching sexual maturity, a practice also applied to other male livestock like boars and rams. The objectives are to reduce unpleasant odors, increase intramuscular fat deposition, improve carcass composition and meat quality. Castration leads to androgen deficiency, hindering male secondary characteristics, such as the comb and flesh hair, reducing aggressive behaviors and eliminating fighting and snorting (8). The energy consumed by capon in territorial protection, fighting, and courtship behaviors is greatly reduced compared intact rooster, making their feed energy utilization more efficient for growth and fat deposition (9). Consequently, castration enhances fat deposits and intramuscular fat content, elevating meat sensory qualities such as tenderness, juiciness, and flavor (7, 9–11). However, surgical castration also has some limitations, including postoperative complications, increased susceptibility to infections, and animal welfare concerns. Additionally, the procedure needs to be performed at an appropriate age, and the high demands on surgical skills, and other cost-effective resources (12). In contrast, GnRH-immunocastration minimizes animal stress, reduces infection risk and complications associated with surgery, and substantially greatly improves animal welfare. Furthermore, it poses no risk of drug residue, making it easy to apply in production. Consequently, GnRH-immunocastration has the potential to be a safe alternative to surgical castration.
2. The comparison of different castration techniques for male animals
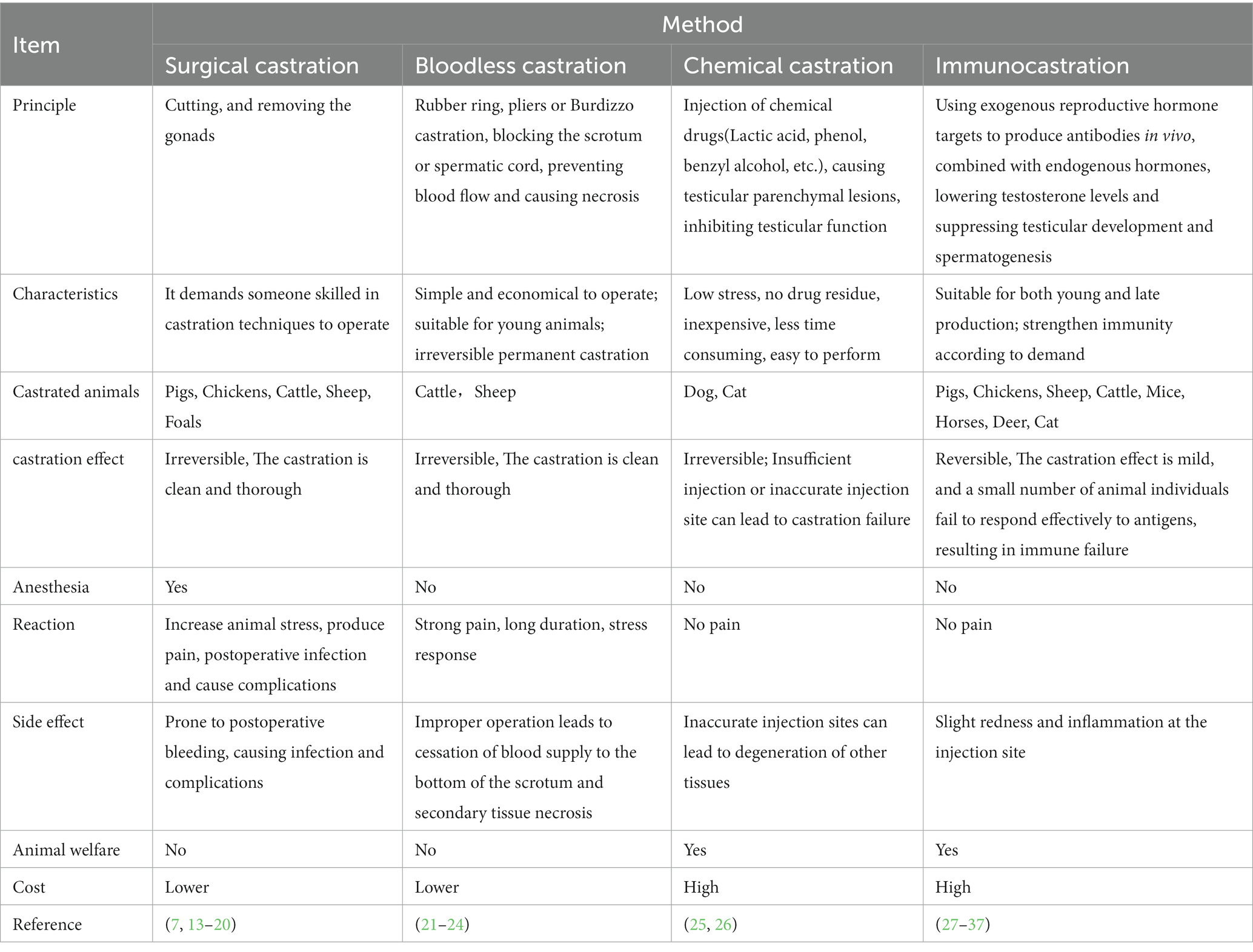
Currently, various techniques for castrating male animals exist, including chemical castration, Bloodless castration, surgical castration, and immunocastration (Table 1). Unlike mammals, rooster’s testicles are located in the abdominal cavity, hanging ventral in the anterior part of the kidney through the mesangium and with the posterior tibial vein and aorta on both sides, which makes avian castration is more challenging than that of mammals. In the poultry industry, traditional surgical castration is performed without anesthesia or analgesic control, resulting in roosters’ suffering and violating animal welfare principles (38). Although geldings are banned in the EU (European Union) due to concerns about animal welfare, they are still used in traditional agricultural systems, representing a derogatory toward age-old practices (39). Surgical castration also incurs mortality rates ranging from 5 to 20%, and sometime even up to 50% (39).
3. Principles of GnRH-immunocastration
Immunocastration primarily targets reproductive hormones within the HPT axis (Figure 1), disrupting reproductive hormone within the HPT axis through immunological means to reduce the concentration of target hormones and achieve castration (29, 40). GnRH is located at the upper end of the HPT axis, plays a pivotal roleinitiating and controlling the physiological functions of the entire reproductive axis (41). Therefore, GnRH-immunization is the most widely used in production compared other targeted hormone immunocastration involves Animals are inoculated with GnRH vaccine, which prompts the production of specific anti-GnRH antibodies in the body, anda lot of anti-GnRH antibodies bind with endogenous GnRH, continuously inactivating endogenous GnRH. Consequently, GnRH-immunization leads to a decrease in luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion. Eventually, this inhibition of animal gonadal functionresults in the achievement of castration (42).
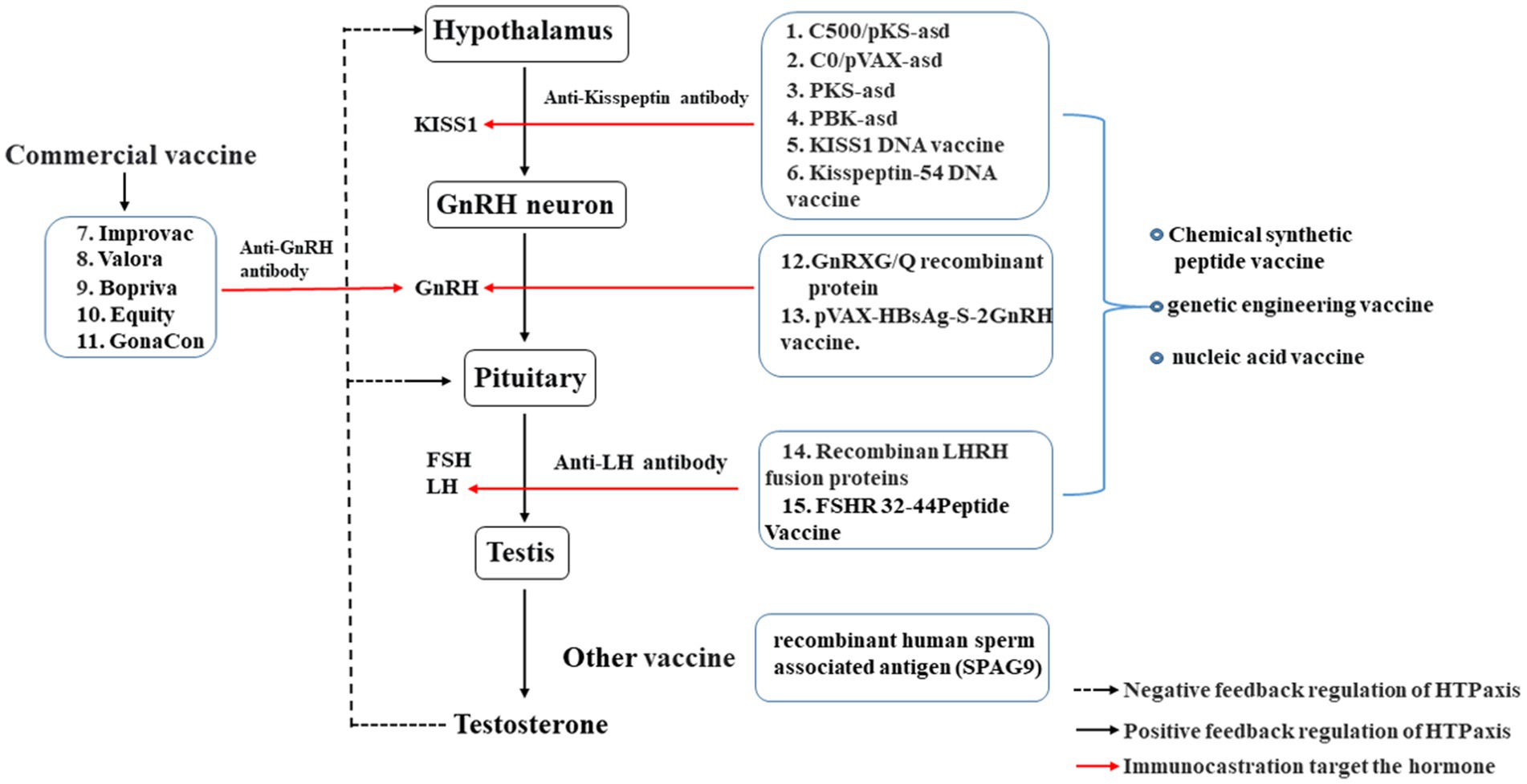
Figure 1. Effect of immunocastration on the hypothalamic–pituitary-testicular axis. The vaccines: 1–6, Immunocastration vaccine targeting Kisspeptin; 7–13, Immunocastration vaccine targeting GnRH; 14–15, Immunocastration vaccine targeting LH.
4. GnRH-immunocastration is a safe castration method in line with animal welfare
Physiological doses of GnRH can significantly increases LH levels and slight increase FSH levels in plasma, reaching the gonads via the pituitary portal circulation, This stimulated the synthesis and secretion of gonadal steroid hormones, promoting gonadal development, gamete production, and the occurrence and maintenance of secondary sexual characteristics. GnRH immunocastration induces a lot of GnRH-antibodies that neutralize endogenous GnRH, and the production of the antibody is a sustained biological effect. As a result, GnRH immunocastration consistently inhibit testicular or ovarian endocrine function, reducing hormone levels and reproductive activity, and associated odors, primarily skatole and androstenone (43–46). Immunization with GnRH leads to a substantial decrease in androstenedione and testosterone in male animals (29, 32, 34, 47, 48). Consequently, European countries are advocating for GnRH-immunocastration as a surgical castration alternative, improving animal welfare. Immunocastration alleviates animal stress, reduces the risk of infection and complications associated with surgical castration, reduces pain and enhances animal welfare. GnRH-immunocastration is considered relatively safe alternative to surgical castration.
5. Current application of GnRH-immunocastration vaccine
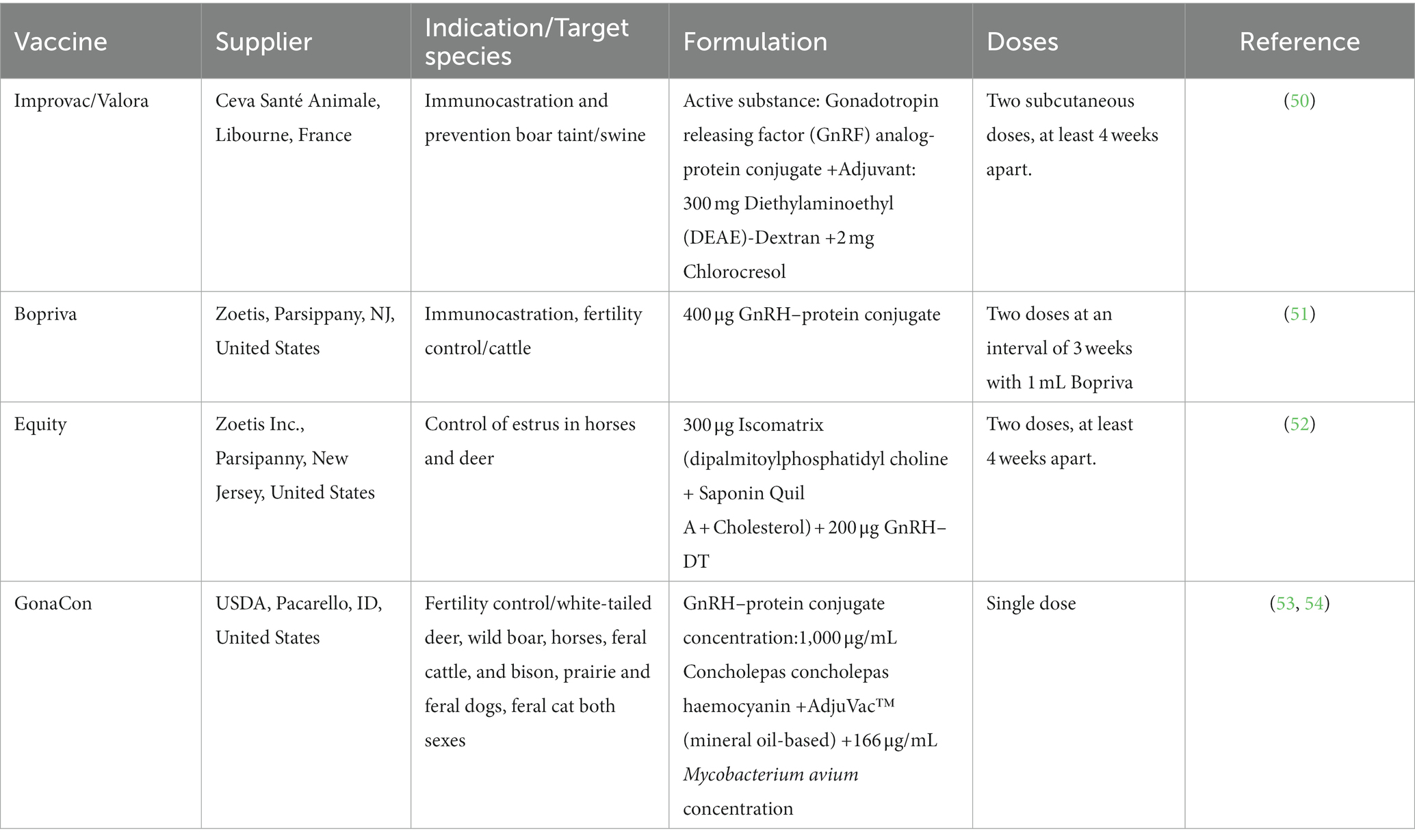
Immunocastration is not limited to pork production but is also employed in other livestock animals as an alternative to surgical castration. Its key advantage lies in eliminating pain, wound infection risks, and potential losses associated with castration (49). Now, several commercial immunocastration products have been applied in animal production (Table 2). However, in Europe, Improvac is the sole product approved for commercial use in pigs., yet its market share is only 2.8% of all male pigs, despite EU approval almost a decade ago. Belgium produces about 15% of the castration vaccine in Europe, while globally, Brazil and Australia hold a market share of more than 50% (55, 56).
6. Current challenges for GnRH-immunocastration in male animals
Immunocastration, often administered using the GnRH vaccine, has undergone extensive investigation in male mammals and birds (Figure 2) (43, 57–61). Outcomes vary based on the animal species, animal age, individual response, and immunization frequency (62). GnRH plays a crucial role in regulating gonadal development and function through the pituitary gland. GnRH-immunocastration significantly decreases reproductive performance of male animals by inhibiting the development testes. Studies have confirmed that immunizing male animals with GnRH can cause infertility, gonadal atrophy, and changes in meat quality by directly or indirectly acting of testosterone (63).
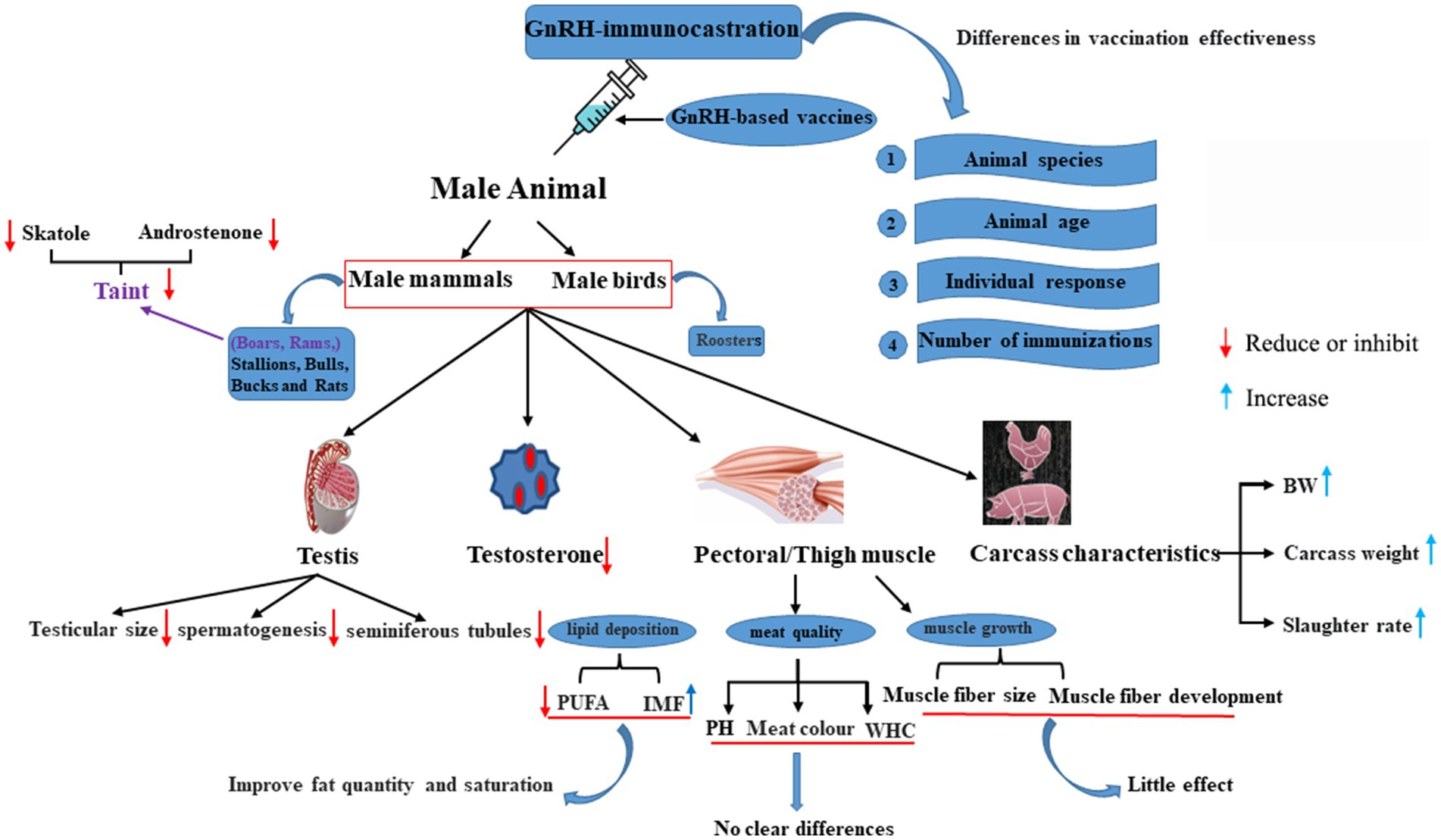
Figure 2. GnRH-immunocastration effects across diverse animals. PUFA, polyunsaturated fatty acids; WHC, water holding capacity; BW, body weight; PH, Pondus Hydrogenii.
6.1. In male mammals
In mammals, the majority of research on GnRH-immunocastration has focused on male animals, and spermatogenesis is inhibited after GnRH immunization. For example, immunizing male animals such as boars, bulls, stallions, rams, bucks, and rats with GnRH vaccine leads to the suppression of testicular, epididymal, and vas deferens development. This results in reduced sperm concentration in the testicles with low viability, constriction of the seminiferous tubules, and inhibition of spermatogonia and spermatoblast production in the deep epithelium (29, 59, 60, 64–68).
At present, GnRH immunocastration is the most widely used in boars. Androstenone is a male hormone that is formed in the cells of the Leydig and has a urine-like odor (69). Skatole is a metabolite of the amino acid tryptophan with a fecal odor that is synthesized by microbial degradation in the colon (44, 70). Immunocastration has been shown to effectively prevent the accumulation of boar taint in adipose tissue by reducing steroid hormone synthesis in the testes (45). However, due to the short duration of the castration effect, the control of boars taint requires multiple doses of GnRH vaccine, and the second vaccination is often carried out 4–6 weeks before slaughter in production, and even the third dose of vaccine is required for slaughter pigs with higher age and weight to control boars odor, which increases the cost.
Meat quality is increasingly valued by consumers, so male livestock are castrated in production to improve meat quality. Currently, a large number of studies have focused on the improvement of meat quality through immunocastration. GnRH immunocastration reduces the accumulation of taint compounds in adipose tissue and improves meat quality and carcass characteristics in male mammals (71). However, the latest study found that the slaughter rate of immunocastrated boars is lower than that of surgical castrated boars and intact boars, as immunocastrated boars have heavier liver and kidneys (72). The abdomen of immune castrated pigs is fatter than that of entire boars, and the lean meat rate is similar to that of surgical castrated pigs, both of which are lower than that of entire boars. Therefore, to some extent, it will affect consumers’ choices. Bellies from immunocastrated pigs are fatter and firmer than those from boars. In addition, although immunocastration increases intramuscular fat content and reduces polyunsaturated fatty acids, the effect of improving intramuscular fat is still not as effective as surgical castration, and boars that undergo surgical castration have lower polyunsaturated fatty acids (73). Similarly, studies have shown that compared to surgical castration, GnRH immunocastration improves cattle weight, but there are no differences in beef pH, color, fat coverage, cooking loss, or tenderness (74).
6.2. In male birds
The utilization and assessment of immunocastration vaccines in pigs has been extensively reported (34, 48, 75). However, there is currently no commercially available vaccine for chickens. Recently, only three studies have investigated the use of the GnRH vaccine for immunizing roosters. Quaresma and colleagues evaluated the effects of Improvac on the body and bone development, meat color, and composition of roosters, and found that the color parameters of Improvac birds, such as brightness, red, and hue angle, were between roosters and capons (5). In addition, i.c. Antunes et al. found that immunocastration had little effect on the fatty acid profile of broilers, but improved overall lipid markers in breast and leg meat to some extent, which could partially enable GnRH immunization (6). Previous studies have shown that both caponization and ovariectomy likely improve the meat quality of the breast muscle based on the objective indices of IMF, appearance (color), texture, and minor change of the fatty acid profile; ovariectomy improves flavor-related indices (76). In our study, we found that roosters inoculated with Improvac had some effect on muscle development, but the effect was not completely satisfactory (77, 78).
7. Conclusions and perspective
Immunocastration currently faces challenges related to immunization failure. These challenges include significant variations in individual responses among immunized animals, insensitivity to antigens in some individuals, failure to elicit an immune response, or a shorter duration of immune effect. This shorter duration leads to an increase in testosterone concentration during the recovery period compared to the previous phase, resulting in a gradual return of sexual behavior. Multiple vaccinations are necessary to counter this effect, which in turn escalates costs. Moreover, there are associated disadvantages for farmers, including increased expenses for purchasing produce and labor management, the risk of accidental self-injection by farm workers, and uncertainty regarding consumer attitudes toward meat from pharmacologically castrated animals. However, it’s important to note that immunocastration offers several advantages, such as reducing animal stress, lowering the risk of infections and complications associated with surgical castration, significantly improving animal welfare, and being relatively straightforward to implement in production settings. Therefore, immunocastration may remain a safe alternative to surgical castration in the future.
In the future development of commercial castration vaccines, particularly GnRH immunocastration vaccines for male animals, there should be an exploration of the construction of immunogens, immune dosages, immune strategies, and timing. Attention should be directed toward enhancing the effectiveness and prolonging the duration of immune response for these vaccines. Currently, research on GnRH vaccines primarily focuses on chemical synthesis of polypeptides, dual conjugate vaccines, DNA vaccines, tandem conjugate vaccines, among others. However, these approaches have their limitations. Considering the existing challenges with GnRH gene vaccines, it’s worth considering research and development of GnRH gene engineering vaccines and GnRH recombinant adenovirus vaccines in the future. In summary, the future focus of immunocastration vaccine development will revolve around creating products with sustained immunogenicity, easy production, and stable effects. These advancements could hold the key to the future of immunocastration vaccines.
Author contributions
CW: drafting the manuscript. YZ and CY: provision of study materials. MZ: conceptualization and supervision. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Key Research and Development Projects of China (2021YFD1600200).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Calik, J . Capon production–breeding stock, rooster castration and rearing methods, and meat quality–a review. Ann Anim Sci. (2014) 14:769–77. doi: 10.2478/aoas-2014-0050
2. Sokołowicz, Z, Krawczyk, J, and Świątkiewicz, S. 4. Quality of poultry meat from native chicken breeds–a review. Ann Anim Sci. (2016) 16:347–68. doi: 10.1515/aoas-2016-0004
3. Symeon, GK, Mantis, F, Bizelis, I, Kominakis, A, and Rogdakis, E. Effects of caponization on growth performance, carcass composition and meat quality of males of a layer line. Animal. (2012) 6:2023–30. doi: 10.1017/S1751731112001024
4. Amorim, A, Rodrigues, S, Pereira, E, and Teixeira, A. Physicochemical composition and sensory quality evaluation of capon and rooster meat. Poult Sci. (2016) 95:1211–9. doi: 10.3382/ps/pev448
5. Quaresma, MAG, Antunes, IC, Ribeiro, MF, Prazeres, S, Bessa, RJB, and Da Costa, PM. Immunocastration as an alternative to caponization: evaluation of its effect on body and bone development and on meat color and composition. Poult Sci. (2017) 96:3608–15. doi: 10.3382/ps/pex191
6. Antunes, IC, Quaresma, MAG, Ribeiro, MF, Alves, SP, Martins da Costa, P, and Bessa, RJB. Effect of immunocastration and caponization on fatty acid composition of male chicken meat. Poult Sci. (2019) 98:2823–9. doi: 10.3382/ps/pez034
7. Calik, J, and Obrzut, J. Physicochemical characteristics of meat from capons derived from the crossing of conserved breed hens and meat roosters. Poult Sci. (2023) 102:102500. doi: 10.1016/j.psj.2023.102500
8. Mast, MG, Jordan, HC, and Macneil, JH. The effect of partial and complete Caponization on growth rate, yield, and selected physical and sensory attributes of Cockerels1. Poult Sci. (1981) 60:1827–33. doi: 10.3382/ps.0601827
9. Cui, X, Cui, H, Liu, L, Zhao, G, Liu, R, Li, Q, et al. Decreased testosterone levels after caponization leads to abdominal fat deposition in chickens. BMC Genomics. (2018) 19:19. doi: 10.1186/s12864-018-4737-3
10. Gesek, M, Murawska, D, Otrocka-Domagała, I, Michalska, K, and Zawacka, M. Effects of caponization and age on the histology, lipid localization, and fiber diameter in muscles from Leghorn cockerels. Poult Sci. (2019) 98:1354–62. doi: 10.3382/ps/pey459
11. Chen, KL, Chi, WT, Chu, C, Chen, RS, and Chiou, PW. Effect of caponization and testosterone implantation on hepatic lipids and lipogenic enzymes in male chickens. Poult Sci. (2007) 86:1754–9. doi: 10.1093/ps/86.8.1754
12. Windsor, PA, Lomax, S, and White, P. Progress in pain management to improve small ruminant farm welfare. Small Rumin Res. (2016) 142:55–7. doi: 10.1016/j.smallrumres.2016.03.024
13. Miller, R, Grott, A, Patzkéwitsch, D, Döring, D, Abendschön, N, Deffner, P, et al. Behavior of piglets in an observation arena before and after surgical castration with local anesthesia. Animals(Basel). (2023) 13:529. doi: 10.3390/ani13030529
14. Linden, JJG . Welfare implications of swine castration a review of the literature by the American Veterinary Medical Association's animal welfare division, covering methods of castration (surgical and immunocastration) of boar pigs, pain control and alternatives to prevent boar taint. Welf Implications Swine Castration. (2023) 11:2.
15. Yoo, S, Beak, S-H, Kang, HJ, Jung, D, Fassah, D, Jeong, I, et al. Effect of knife castration on leukocyte cytokine expression and indicators of stress, pain, and inflammation in Korean cattle bull calves. Anim Biosci. (2023) 36:521–8. doi: 10.5713/ab.22.0368
16. Dustan, B . Performing surgery in goats. Part 2: surgical techniques. Inpractice. (2023) 45:101–6. doi: 10.1002/inpr.294
17. Kongara, K, Corner-Thomas, R, Bruere, S, Lawrence, K, and Gates, M. Practices and opinions of New Zealand sheep farmers towards pain management in lambs during castration and/or tail docking. N Z Vet. (2023) 71:8–17. doi: 10.1080/00480169.2022.2135626
18. Cognie, J, Freret, S, Lansade, L, Parias, C, Barriere, P, Gesbert, A, et al. Early castration in foals: consequences on physical and behavioural development. Equine Vet J. (2023) 55:214–21. doi: 10.1111/evj.13580
19. Sellon, D, Sanz, M, and Kopper, J. Perioperative pain management protocols of veterinarians in the United States for horses undergoing routine orchiectomy (castration). Europe PMC (2023).
20. Ryad, HM, Yadav, SK, Bostami, MB, Sutradhar, BC, and Das, B. Effect of caponization on growth performance and blood parameter in Fayoumi cock. GSC Adv Res Rev. (2022) 13:105–15. doi: 10.30574/gscarr.2022.13.1.0258
21. Ibrahim, A, Ali, MM, Abou-Khalil, NS, and Ali, M. Evaluation of chemical castration with calcium chloride versus surgical castration in donkeys: testosterone as an endpoint marker. BMC Vet Res. (2016) 12:1–9. doi: 10.1186/s12917-016-0670-3
22. Nurmi, H, Laaksonen, S, Häätylä, T, Valros, A, Sauvala, M, and Hänninen, L. The impact of clamp castration on the behaviour and body temperature of reindeer (Rangifer tarandus tarandus)–effects of local anesthesia and non-steroidal anti-inflammatory drug. Appl Anim Behav Sci. (2022) 255:105719. doi: 10.1016/j.applanim.2022.105719
23. Boyle, LA, Conneely, M, Kennedy, E, O’Connell, N, O'Driscoll, K, and Earley, B. Animal welfare research–progress to date and future prospects. Ir J Agric Food Res. (2022) 61:1–12. doi: 10.15212/ijafr-2020-0151
24. Saeed, IH, Alameen, AO, and Abdelatif, A. Physiological responses of adult Nubian goats (Capra hircus) to orchiectomy. Asian Res J Gynaecol Obstet. (2022) 7:28–46. Available at: https://www.sdiarticle5.com/review-history/85037
25. Spruijt, A, Kooistra, H, Oei, C, Vinke, C, Schaefers-Okkens, A, and De Gier, J. The function of the pituitary-testicular axis in dogs prior to and following surgical or chemical castration with the GnRH-agonist deslorelin. Reprod Domes Anim. (2023) 58:97–108. doi: 10.1111/rda.14266
26. Hami, M, Veshkini, A, Jahandideh, A, Rafiee, SM, and Mortazavi, P. Evaluation of testosterone, blood antioxidants, and histopathological changes following chemical castration with calcium chloride in rats. Crescent J Med Biol Sci. (2022) 9:207–12. doi: 10.34172/cjmb.2022.34
27. Needham, T, Musa, AS, Kotrba, R, Ceacero, F, Hoffman, LC, Lebedová, N, et al. Carcass and offal yields of farmed common eland (Taurotragus oryx) males, as affected by age and immunocastration. Animals (Basel). (2022) 12:2893. doi: 10.3390/ani12212893
28. Pérez-Ciria, L, Ripoll, G, Sanz, MÁ, Blanco, M, Miana-Mena, FJ, and Latorre, MAJMS. Impact of gilt immunocastration on weight losses and instrumental and chemical characteristics of Teruel dry-cured ham. Meat Sci. (2023) 199:109125. doi: 10.1016/j.meatsci.2023.109125
29. Ahmed, S, Bo, D, Zhao, J, Liu, G, Ding, Y, Jiang, X, et al. Immunocastration with gene vaccine (KISS1) induces a cell-mediated immune response in ram testis: a transcriptome evaluation. Reprod Domest Anim. (2022) 57:653–64. doi: 10.1111/rda.14106
30. Ny, V, Needham, T, Bartoň, L, Bureš, D, Kotrba, R, Musa, AS, et al. Effects of immunocastration and supplementary feeding level on the performance and blood biochemical markers of farmed yearling fallow deer (Dama dama). J Anim Physiol Anim Nutr (Berl). (2023) 107:1158–66. doi: 10.1111/jpn.13807
31. Kotula-Balak, M, Pawlicki, P, Gałuszka, A, Pardyak, L, Tuz, R, Dubniewicz, K, et al. Effect of immunocastration using Improvac on the regulation of adiponectin and leptin in the testes of landrace boars. Med Weter. (2022) 77:1–6. doi: 10.21521/mw.6685
32. Pan, F, Du, H, Tian, W, Xie, H, Zhang, B, Fu, W, et al. Effect of GnRH immunocastration on immune function in male rats. Front Immunol. (2022) 13:1023104. doi: 10.3389/fimmu.2022.1023104
33. Gautier, C, Kaps, M, Aurich, J, and Aurich, C. Resumption of sexual behaviour and testicular function in immunocastrated Shetland pony stallions after treatment with a GnRH agonist. Anim Reprod Sci. (2022) 247:107127. doi: 10.1016/j.anireprosci.2022.107127
34. Pawlicki, P, Galuszka, A, Pardyak, L, Tuz, R, Płachno, BJ, Malopolska, M, et al. Leydig cells in Immunocastrated polish landrace pig testis: differentiation status and steroid enzyme expression status. Int J Mol Sci. (2022) 23:6120. doi: 10.3390/ijms23116120
35. Chang, A-M, Chen, C-C, Lee, J-W, Hou, D-L, Huang, H-H, and Ke, G. Effects of a novel recombinant gonadotropin-releasing Hormone-1 vaccine on the reproductive function of mixed-breed dogs (Canis familiaris) in Taiwan. Vaccine. (2023) 41:2214–23. doi: 10.1016/j.vaccine.2023.02.061
36. Font-I-Furnols, M, Claret, A, Guerrero, L, and Dalmau, A. Consumers’ expectations about meat from surgical castrated or immunocastrated male and female Iberian pigs. Animals. (2022) 12:468. doi: 10.3390/ani12040468
37. Ochoa, JS, Favre, RN, García, MF, Stornelli, MC, Sangache, WC, Rearte, R, et al. Immunocontraception of male domestic cats using GnRH vaccine Improvac. Theriogenology. (2023) 198:211–6. doi: 10.1016/j.theriogenology.2022.12.020
38. Garcia, A, and McGlone, JJ. Animal welfare and the acknowledgment of cultural differences. Animals(Basel). (2022) 12:474. doi: 10.3390/ani12040474
39. Rikimaru, K, Takahashi, H, and Nichols, MA. An efficient method of early caponization in slow-growing meat-type chickens. Poult Sci. (2011) 90:1852–7. doi: 10.3382/ps.2010-01270
40. Van den Broeke, A, Aluwé, M, Kress, K, Stefanski, V, Škrlep, M, Batorek, N, et al. Effect of dietary energy level in finishing phase on performance, carcass and meat quality in immunocastrates and barrows in comparison with gilts and entire male pigs. Animal. (2022) 16:100437. doi: 10.1016/j.animal.2021.100437
41. Tzoupis, H, Nteli, A, Androutsou, ME, and Tselios, T. Gonadotropin-releasing hormone and GnRH receptor: structure, function and drug development. Curr Med Chem. (2020) 27:6136–58. doi: 10.2174/0929867326666190712165444
42. Kaprara, A, and Huhtaniemi, IT. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism. (2018) 86:3–17. doi: 10.1016/j.metabol.2017.11.018
43. Kowalski, E, Vossen, E, Millet, S, Ampe, B, De Smet, S, and Aluwé, M. Effect of terminal sire line and timing second vaccination on effectiveness of immunocastration, performance, and carcass and meat quality. Meat Sci. (2021) 175:108451. doi: 10.1016/j.meatsci.2021.108451
44. Han, X, Zhou, M, Cao, X, Du, X, Meng, F, Bu, G, et al. Mechanistic insight into the role of immunocastration on eliminating skatole in boars. Theriogenology. (2019) 131:32–40. doi: 10.1016/j.theriogenology.2019.03.017
45. Lin-Schilstra, L, and Fischer, ARH. Paradoxical consumers in four European countries: meat-eating justification and willingness to pay for meat from animals treated by alternatives to surgical castration. Meat Sci. (2022) 188:108777. doi: 10.1016/j.meatsci.2022.108777
46. Lin-Schilstra, L, and Ingenbleek, PTM. A scenario analysis for implementing Immunocastration as a single solution for piglet castration. Animals (Basel). (2022) 12:12. doi: 10.3390/ani12131625
47. Nolan, MB, Bertschinger, HJ, Roth, R, Crampton, M, Martins, IS, Fosgate, GT, et al. Ovarian function following immunocontraceptive vaccination of mares using native porcine and recombinant zona pellucida vaccines formulated with a non-Freund's adjuvant and anti-GnRH vaccines. Theriogenology. (2018) 120:111–6. doi: 10.1016/j.theriogenology.2018.07.044
48. Batorek-Lukač, N, Kress, K, Čandek-Potokar, M, Fazarinc, G, Škrlep, M, Poklukar, K, et al. Immunocastration in adult boars as a model for late-onset hypogonadism. Andrology. (2022) 10:1217–32. doi: 10.1111/andr.13219
49. Borell, EV, Bonneau, M, Holinger, M, Prunier, A, Stefanski, V, Zöls, S, et al. Welfare aspects of raising entire male pigs and Immunocastrates. Animals (Basel). (2020) 10:2140. doi: 10.3390/ani10112140
50. Sjöqvist, E . Effect of vaccination against gonadotrophin-releasing factor of male pigs on meat and fat quality. Food Sci. (2023) 2023:1–29. Available at: https://stud.epsilon.slu.se/18649/1/sj%C3%B6qvist-e-230216.pdf
51. Goto, A, Yoshida, N, Nakada, K, Inoue, Y, Hisaeda, K, Inaba, T, et al. Efficiency of immunocastration with an anti-gonadotropin-releasing hormone vaccine on cryptorchid bulls. J Vet Med Sci. (2023) 85:551–6. doi: 10.1292/jvms.22-0571
52. Schwarzenberger, F, Krawinkel, P, Jeserschek, SM, Schauerte, N, Geiger, C, Balfanz, F, et al. Immunocontraception of male and female giraffes using the GnRH vaccine Improvac®. Zoo Biol. (2022) 41:50–64. doi: 10.1002/zoo.21651
53. Gray, ME, and Cameron, EZ. Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence. Reproduction. (2010) 139:45–55. doi: 10.1530/REP-08-0456
54. Massei, GJA . Fertility control for wildlife: a European perspective. Animals (Basel). (2023) 13:428. doi: 10.3390/ani13030428
55. Mancini, MC, Menozzi, D, and Arfini, F. Immunocastration: economic implications for the pork supply chain and consumer perception. An assessment of existing research. Livest Sci. (2017) 203:10–20. doi: 10.1016/j.livsci.2017.06.012
56. Čandek-Potokar, M, Škrlep, M, and Zamaratskaia, GJT. Immunocastration as alternative to surgical castration in pigs. Theriogenology. (2017) 6:109–26. doi: 10.5772/intechopen.68650
57. Rocha, LF, Souza, RS, Santana, ALA, Macedo, DS, Santana, AMS, Silva, RCD, et al. Reproductive parameters of lambs immunocastrated with anti-GnRH vaccine. Anim Reprod. (2021) 18:e20200237. doi: 10.1590/1984-3143-ar2020-0237
58. Doroteu, EM, Viana, JH, Ferreira Junior, JA, Macedo, JT, Oliveira, RA, and Pedroso, PM. Effect of a single or two doses of an anti-GnRH vaccine on testicle morpho-functional characteristics in Nelore bulls. Trop Anim Health Prod. (2021) 53:1–8. doi: 10.1007/s11250-021-02600-x
59. Birrell, JR, Schulman, ML, Botha, AE, Ganswindt, A, Fosgate, GT, and Bertschinger, HJ. Vaccination against GnRH as a prelude to surgical castration of horses. Equine Vet J. (2021) 53:1141–9. doi: 10.1111/evj.13411
60. Giriboni, J, Martínez-Nevado, E, García, J, Velázquez, R, Toledano-Díaz, A, Ungerfeld, R, et al. Single or repeated immunization against GnRH fails to completely abolish spermatogenesis in dwarf bucks (Capra hircus). Zoo Biol. (2022) 42:364–70. doi: 10.1002/zoo.21743
61. Novak, S, Yakobson, B, Sorek, S, Morgan, L, Tal, S, Nivy, R, et al. Short term safety, immunogenicity, and reproductive effects of combined vaccination with anti-GnRH (Gonacon) and rabies vaccines in female feral cats. Front Vet Sci. (2021) 8:650291. doi: 10.3389/fvets.2021.650291
62. Heyrman, E, Kowalski, E, Millet, S, Tuyttens, FAM, Ampe, B, Janssens, S, et al. Monitoring of behavior, sex hormones and boar taint compounds during the vaccination program for immunocastration in three sire lines. Res Vet Sci. (2019) 124:293–302. doi: 10.1016/j.rvsc.2019.04.010
63. Noya, A, Ripoll, G, Casasús, I, and Sanz, A. Effects of immunocastration performed at two live weights on the growth physiology, temperament and testicular development of feral beef bulls. Anim Sci J. (2020) 91:e13307. doi: 10.1111/asj.13307
64. Mitjana, O, Bonastre, C, Tejedor, MT, Garza, L, Latorre, M, Moreno, B, et al. Immuno-castration of female and male pigs with anti-gonadotrophin releasing hormone vaccine: morphometric, histopathological and functional studies of the reproductive system. Anim Reprod Sci. (2020) 221:106599. doi: 10.1016/j.anireprosci.2020.106599
65. Xu, M, Xu, C, Liu, F, Shen, X, Meng, J, Chen, H, et al. Effects of active immunization with newly modified GnRH peptides on spermatogenesis and production performance of Holstein bulls. Biol Reprod. (2018) 99:461–72. doi: 10.1093/biolre/iox176
66. Yao, Z, Si, W, Tian, W, Ye, J, Zhu, R, Li, X, et al. Effect of active immunization using a novel GnRH vaccine on reproductive function in rats. Theriogenology. (2018) 111:1–8. doi: 10.1016/j.theriogenology.2018.01.013
67. Curtis, AK, Jones, DE, Kleinhenz, M, Montgomery, S, Martin, M, Weeder, M, et al. Delivering an Immunocastration vaccine via a novel subcutaneous implant. Animals (Basel). (2022) 12:2698. doi: 10.3390/ani12192698
68. Rocha, LF, Santana, ALA, Souza, RS, Machado-Neves, M, Oliveira, JC, Dos Santos, ESC, et al. Testicular morphometry as a tool to evaluate the efficiency of immunocastration in lambs. Anim Reprod. (2022) 19:e20210041. doi: 10.1590/1984-3143-ar2021-0041
69. Squires, EJ, Bone, C, and Cameron, J. Pork production with entire males: directions for control of boar taint. Animals (Basel). (2020) 10:1665. doi: 10.3390/ani10091665
70. Aluwé, M, Heyrman, E, Kostyra, E, Żakowska-Biemans, S, Almeida, J, Citek, J, et al. Consumer evaluation of meat quality from barrows, immunocastrates and boars in six countries. Animal. (2022) 16:100455. doi: 10.1016/j.animal.2022.100455
71. Lents, MP, Barbosa, LP, Santana, ALA, Pinheiro, EEG, Mugabe, LC, Biscarde, CEA, et al. Immunocastration of goats using anti-gonadotrophin releasing hormone vaccine. Theriogenology. (2018) 114:7–13. doi: 10.1016/j.theriogenology.2018.03.013
72. Zomeño, C, Gispert, M, Čandek-Potokar, M, Mörlein, D, and Font, IFM. A matter of body weight and sex type: pig carcass chemical composition and pork quality. Meat Sci. (2023) 197:109077. doi: 10.1016/j.meatsci.2022.109077
73. Bee, G, Quiniou, N, Maribo, H, Zamaratskaia, G, and Lawlor, PG. Strategies to meet nutritional requirements and reduce boar taint in meat from entire male pigs and Immunocastrates. Animals (Basel). (2020) 10:1950. doi: 10.3390/ani10111950
74. RH, P, Vidal, S, Larraín, R, and Saénz, L. Effectiveness of a new recombinant antiGnRH vaccine for Immunocastration in bulls. Animals (Basel). (2021) 11:11. doi: 10.3390/ani11051359
75. Argemí-Armengol, I, Villalba, D, Vall, L, Coma, R, Roma, J, and Álvarez-Rodríguez, J. Locally grown crops and Immunocastration in fattening heavy pigs: effects on performance and welfare. Animals (Basel). (2022) 12:1629. doi: 10.3390/ani12131629
76. Cui, X, Liu, R, Cui, H, Zhao, G, Zheng, M, Li, Q, et al. Effects of caponization and ovariectomy on objective indices related to meat quality in chickens. Poult Sci. (2017) 96:770–7. doi: 10.3382/ps/pew346
77. Wang, C, Zeng, YT, Chen, XY, Wu, QY, Yang, LQ, Xu, L, et al. Improvac induces immunocastration by affecting testosterone levels and disrupting spermatogenesis in male broiler chickens. Poult Sci. (2019) 98:6034–45. doi: 10.3382/ps/pez228
Keywords: immunocastration, surgical castration, male animal, animal welfare, GnRH
Citation: Wang C, Yang C, Zeng Y and Zhang M (2023) GnRH-immunocastration: an alternative method for male animal surgical castration. Front. Vet. Sci. 10:1248879. doi: 10.3389/fvets.2023.1248879
Edited by:
Izhar Hyder Qazi, Shaheed Benazir Bhutto University of Veterinary and Animal Sciences, PakistanReviewed by:
Waseem Ali Vistro, Yangzhou University, ChinaCopyright © 2023 Wang, Yang, Zeng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Zhang, emhhbmdtaW5nQHNpY2F1LmVkdS5jbg==
 Chun Wang1
Chun Wang1 Ming Zhang
Ming Zhang