
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Vet. Sci., 21 August 2023
Sec. Veterinary Clinical, Anatomical, and Comparative Pathology
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1238544
This article is part of the Research TopicInsights in Veterinary Experimental and Diagnostic Pathology: 2023View all 7 articles
 Woncheoul Park1*†
Woncheoul Park1*† Han-Ha Chai1†
Han-Ha Chai1† Dajeong Lim1
Dajeong Lim1 Changgwon Dang2
Changgwon Dang2 Jaegu Lee2
Jaegu Lee2 Jongho Kim3
Jongho Kim3 Hogyun Jeong4
Hogyun Jeong4 Taekwon Lee4
Taekwon Lee4 Ki-Chang Lee4
Ki-Chang Lee4 Kyunghyun Lee3*
Kyunghyun Lee3*Schistosomus reflexus (SR) is one of the most common congenital anomalies found in cases of cattle dystocia; this disorder occurs mostly in cattle. Congenital anomalies such as SR are caused by various genetic and environmental factors, but no specific cause has been elucidated for SR. This study reports a case of SR in a Holstein dairy cattle fetus with congenital anomalies in Korea. Grossly, a distinct spine curvature was observed between the thoracic and lumbar vertebrae, accompanied by a consequential malformation from the sacrum to the occipital bone. Furthermore, the thoracic and abdominal organs were exposed. In computed tomography (CT) images, mild and severe kyphoscoliosis was observed in T1~11 and L1~6, respectively. Additionally, vertebral dysplasia was observed in S1~5 and Cd 1~5. To pinpoint the causal genes and mutations, we leveraged a custom 50K Hanwoo SNP-Chip and the Online Mendelian Inheritance in Animals (OMIA) database. As a result, we identified a nonsense mutation in apoptotic protease activating factor 1 (APAF1) within HH1 that was associated with a decrease in conception rate and an increase in abortion in Holstein dairy cattle. The genotype of the SR case was A/A, and most of the 1,142 normal Holstein dairy cattle tested as a control group had the genotype G/G. In addition, the A/A genotype did not exist in the control group. Based on the pathological, genetic, and radiological findings, the congenital abnormalities observed were diagnosed as SR.
Schistosomus reflexus (SR) is the most form of common congenital anomaly found in cases of cattle dystocia; it occurs mainly in cattle, but is also common in other domesticated animals. The prevalence rate of this fatal congenital syndrome ranges from as low as 0.01% to as high as 1.3% of dystocia cases (1, 2). SR occurs mainly in bovines and rarely in other species such as sheep, goats, pigs, dogs, cats, donkeys, and sea turtles (Lepidochelys olivacea) (3–8). The main morphological symptom of SR is a body malformation falling within the category of celosomy. Specifically, SR is characterized by the presence of exposed abdominal and sometimes thoracic viscera (schistosomus), which causes their submersion in amniotic fluid; moreover, it is also characterized by a marked ventral curvature of the thoracic vertebrae (reflexus), which causes the occipital bone to approach the sacrum, lateral curvature of the body and walls of the chest, pelvic deformity, and an abnormally cystic liver shape. These symptoms result in fetal dystocia (1, 9).
Apparent genetic factors that cause SR have not been identified in bovines, such as SNPs, indels, or CNVs. However, there are reports on genes associated with a syndrome similar to SR in other species. First, four genes have been reported in mice: transforming growth factor beta 2 (TGFB2) and beta 3 (TGFB3), paired-like homeodomain transcription factor 2 (PITX2), and activator protein 2 (AP2) (10–12). Second, the thoracoabdominal syndrome (THAS) gene on the X chromosome has been reported in humans (13, 14). Very few studies have been conducted on the causes of SR, and especially few have examined the genetic factors. In addition, most SR case reports have reported on the clinical signs and pathological lesions in some species. In this study, in order to fill this gap, we collected customized Hanwoo 50k SNP-Chip data to identify genetic factors and monitored the clinical signs and pathological lesions occurring in a case of SR in a calf of Holstein dairy cattle in Korea.
A Holstein (pure breed) dam had a stillbirth of twins on the 220th day of pregnancy. One fetus had a crown–rump length of 67 cm, a body weight of 14.5 kg, and no specific problems on gross observation. However, the other fetus showed congenital abnormalities. Gross findings indicated full exposure of the thoracoabdominal organs; this observation was followed by additional computed tomography (CT) scanning. Externally, the body exhibited SR with exposed abdominal organs; the spine was curved and inverted laterally and dorsoventrally, and the anterior surface of the posterior limb was directed posteriorly; and the body weighed 11.6 kg (Figure 1A). The thoracic and visceral organs were exposed. The vertebral column bent to the left, and the sacrum approached the cranium, as observed by noting that the caudal lumbar vertebrae presented with a v-shaped lateral twist of the vertebrae (Figure 1B). The diaphragm was intact, and the thoracic cavity was reduced in size. The lung and heart were deformed in shape and size. The liver was markedly deformed in shape and thickness. The pelvic cavity was reduced in size by compression laterally to the left.
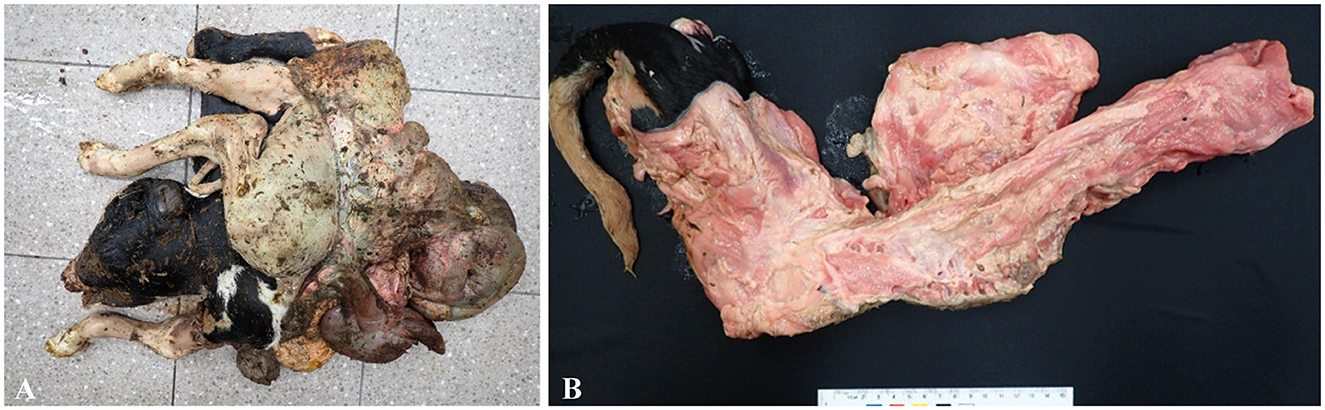
Figure 1. (A) Schistosomus reflexus of the fetus: the body and chest walls bent laterally with exposed thoracic and abdominal viscera, with a gross lesion. (B) V-shaped bent laterally twisted vertebrae were observed, with a gross lesion.
In the CT images, seven cervical vertebrae and the alignment of these vertebrae were relatively normal. Bone density in the cervical vertebral body was generally decreased, and all the endplates were unfused. There was a 1-mm-wide gap longitudinally along the central aspect of the C1 (atlas) vertebral arch (Figure 2A) and also a 1-mm-wide gap longitudinally along the left lateral and right lateral aspects of the vertebral body. These gaps were assessed to be a non-fused vertebral arch and vertebral body. The C2 vertebra (axis) was observed to be divided into two parts, cranial and caudal. The cranial part of C2 was observed to have a round shape with a thickness of approximately 17 mm within the vertebral foramen of C1. The caudal part of C2 was observed without structures such as the vertebral body and the spinous process. There were only 12 thoracic vertebrae, which is one less than the normal number in this species. Abnormal formation of the vertebral curvature was noted along the dorsal aspect of T1-11. The endplates of these vertebrae were all unfused (Figure 2B). The left 1st to 3rd ribs appeared relatively normal. At the T4-8 level (left), approximately five consecutive ribs were fused, giving an abnormal appearance around this region. At the T10-12 level (left), three distinct ribs were observed, with the distal parts of the 11th and 12th ribs fused (Figure 2A). The 1st rib on the right appeared short and thick. At the T1-3 level (right), approximately three consecutive ribs were fused, giving an abnormal appearance around this region. At the T4-9 level (right), approximately seven consecutive ribs were also abnormally fused.

Figure 2. CT image of schistosomus reflexus of the fetus. A developmental defect characterized by a marked curvature of the spine and a deformed pelvis. (A) Kyphoscoliosis was mild in T1~11 and severe in L1~6; left view of the figure. (B) Vertebral dysplasia was observed in S1~5 and Cd 1~5; right view of the figure.
There were six lumbar vertebrae among the lumbar, sacral, and caudal vertebrae. The vertebral curvature along the dorsal aspect of the L1-5 appeared extremely abnormal, with all the endplates unfused and five sacra with irregular margins of the vertebral body for S1-5. Overall bone density was observed to be decreased. The left sacral wing of S1 was articulated with the left iliac wing, while the right sacral wing was separated from the right iliac wing. There were 15 caudal vertebrae. The vertebral bodies of Cd1-5 had irregular margins. In the forelimb and hindlimb, the left humerus was caudodistally displaced and luxated from the left shoulder joint (Figure 2A). The physis of the long bones of the fore- and hindlimbs was unfused. No other significant findings were noted in the fore- and hindlimbs. The curved formation noted in the thoracic vertebrae and lumbar vertebrae is related to kyphoscoliosis, a congenital dysplasia. In addition, the 2nd cervical vertebra was partially formed, with only the vertebral arch present; bilateral fused ribs and the irregular margins of the sacrum and the caudal vertebrae were all assessed to be hypoplastic abnormalities and congenital dysplasias. The separated right sacroiliac joint may have been attributable to sacral dysplasia causing SI joint luxation. The overall low bone density observed may have been due to decay of the bone post-mortem or abnormal growth of the fetus in the form of bone marrow hypoplasia. The gaps and the physis observed in the vertebrae and the limbs are considered normal in an undeveloped fetus.
Our genotyping process was as follows. First, the muscle tissues of the Holstein dairy cattle calf with SR, which had been stored in a deep freezer (−78°C), were thawed, washed, chopped, and then placed in 600 μl of nuclei lysis solution. Second, total genomic DNA was extracted using a Wizard genomic DNA purification kit (Promega, Madison, WI, USA), following the manufacturer's instructions. Subsequently, the DNA concentration and purity were measured using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Finally, the genomic DNA samples were genotyped using a custom 50K Hanwoo SNP-Chip (Illumina, South Korea) involving 58,990 SNPs. In addition, we obtained custom 50K Hanwoo SNP-Chip data from 1,142 Holstein dairy cattle from the Animal Breeding & Genetics Division of the National Institute of Animal Science (NIAS); these data were used to confirm the genotype of normal Holstein dairy cattle as a control group for comparison with the SR case.
We obtained detailed information, including the genotypes for genetic disorders in cattle, from the Online Mendelian Inheritance in Animals (OMIA) database. Subsequently, we summarized the data on 32 genetic disorders that were common between the OMIA database and the custom 50K Hanwoo SNP-Chip data (Table 1). Additionally, we identified the SNP-Chip data for SR in calves of Holstein dairy cattle. As a result, a recessive homozygous genotype (A/A) was identified for abortion due to a haplotype HH1 [Chip ID: ilmnseq_rs448942533-148_B_F_2604252564, apoptosis peptide activating factor 1 (APAF1)], which has been reported as a lethal gene for cattle, and dominant homozygous genotypes were identified for other genetic disorders. Moreover, abortion due to the haplotype HH1 genotype was identified as follows among the 1,142 normal Holstein dairy cattle: 1,108 dominant homozygous (normal, G/G), 30 heterozygous (carriers, G/A), and 4 no genotype information (./.). The G and A allele frequencies were 98.33% and 1.31%, respectively (Figure 3). This result confirmed that the A allele frequency of the haplotype HH1 genotype is being reduced through selection in the Holstein dairy cattle bred at the NIAS, and abortion caused by the haplotype HH1 is also being reduced.
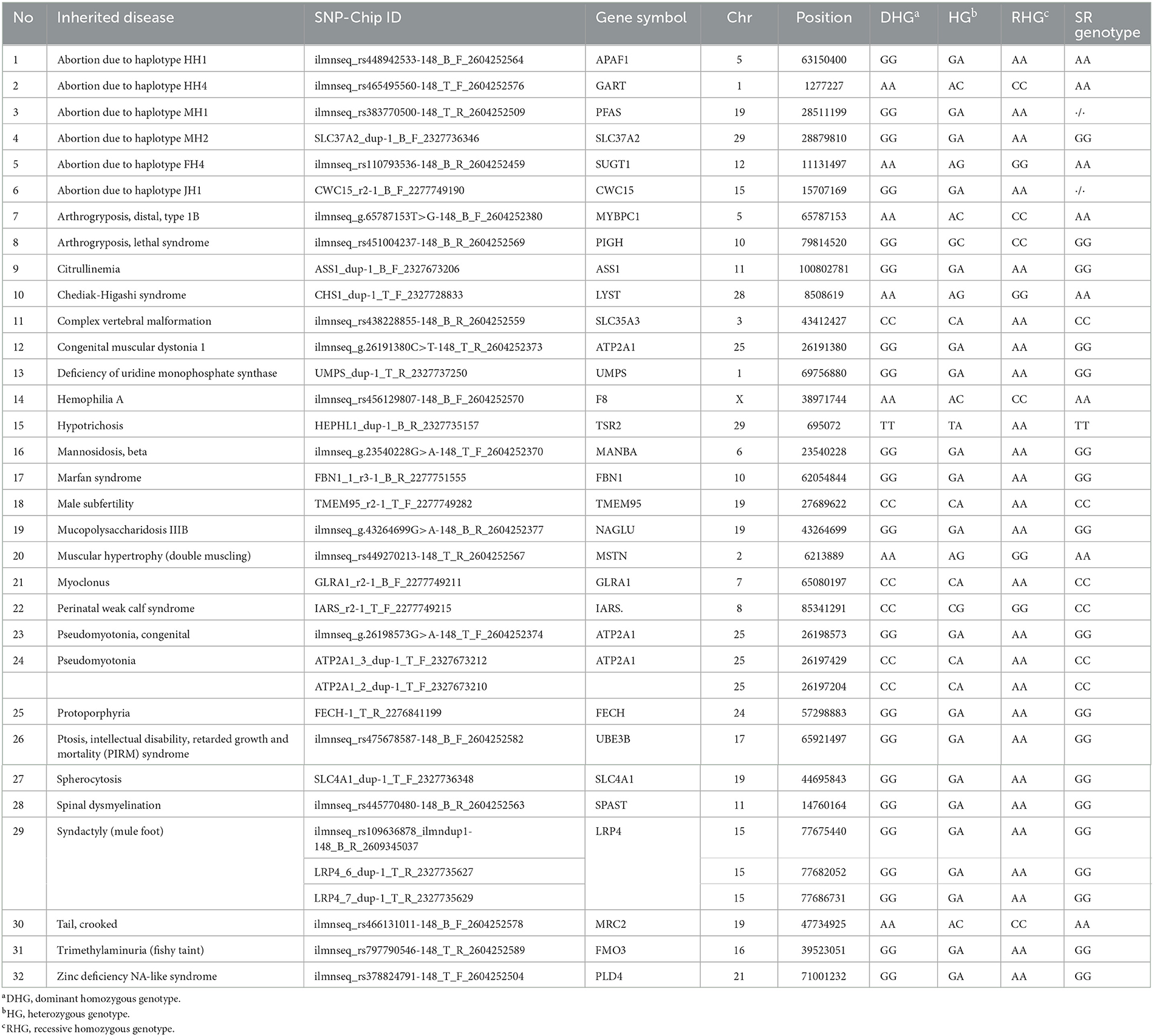
Table 1. Summary of the custom 50 k Hanwoo SNP-Chip genotype of schistosomus reflexus (SR) calf in Holstein dairy cattle using cattle inherited disorders from the OMIA database.
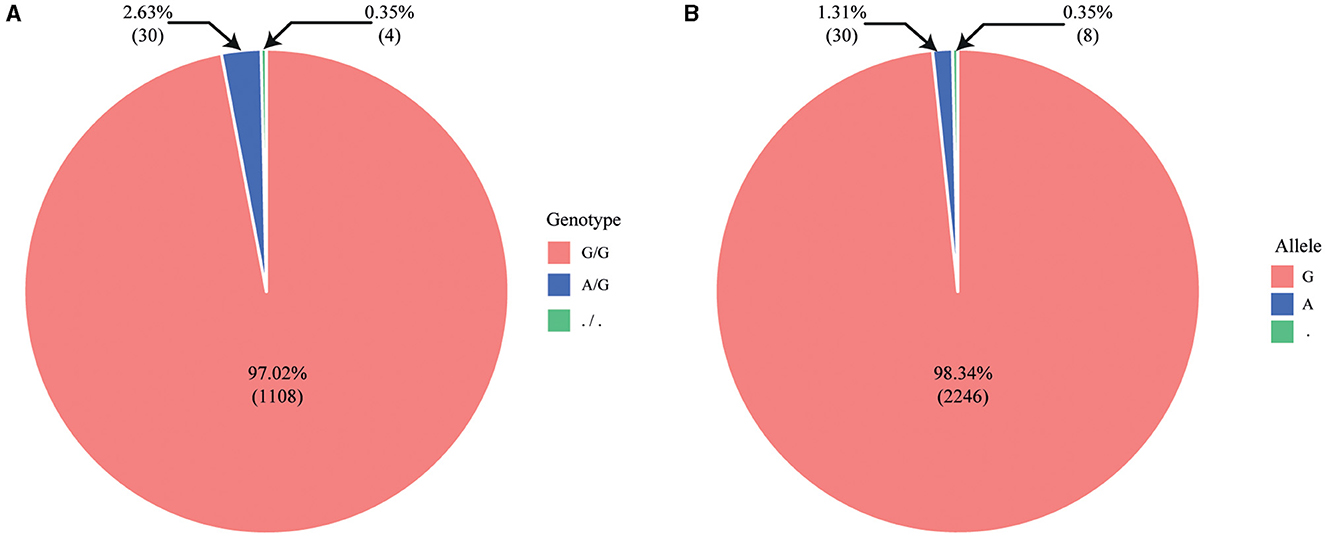
Figure 3. Pie chart illustrating genotype and allele frequency using SNP-Chip data from 1,142 Holstein dairy cattle. (A) Genotype frequency (G/G, A/G,./.); (B) allele frequency (G, A,.).
Most published works have reported that SR, which occurs mainly in cattle, is diagnosed through necropsy. In addition, studies on SR in various livestock species (bovines, sheep, goats, pigs, dogs, cats, donkeys, turtles, etc.) have been reported. However, studies on ways to prevent the disorder of SR and provide opportune treatment to the pregnant dam have yet to be reported. When SR in a calf is diagnosed early, traction, embryotomy, and cesarean section are used to treat the pregnant dam. However, in cases of delayed diagnosis of SR or decayed emphysematous calves, there are instances in which the dam dies due to poor prognosis. Therefore, to prevent the death of dams, methods for early diagnosis of SR are needed.
HH1, a haplotype on chromosome 5, has been reported to decrease conception rates and increase abortion rates in Holstein dairy cattle; this haplotype was identified by VanRaden et al. through high-density SNP genotyping (15). It has also been reported that the HH1 haplotype originated from a single sire born early in advanced animal breeding over 50 years ago (16). Moreover, this HH1 haplotype carried by the sire was identified as a stop-gain (nonsense) mutation in the APAF1 gene. The APAF1 gene is an essential molecule in the cytochrome-c-mediated apoptosis cascade and has been directly implicated in developmental and neurodegenerative disorders. Embryos with these homozygous gene knockouts have been found to die by 16.5 days of development (17).
In this study, we discovered that the HH1 haplotype mutation, which has caused approximately 525,000 spontaneous abortions worldwide over the past 35 years, causing losses of approximately $420 million over the same period (17), is also mutated in the genetic disorder of SR. Previous studies in Holstein dairy cattle have reported diagnoses through pathological methods for calves suspected of having SR. However, based on our results, we recommend diagnosing the genetic disorder of SR in the fetus early on and, at the same time, promptly removing the fetus from the pregnant dam using methods such as traction, embryotomy, and cesarean section. Additionally, more attention should be given to the treatment of the dam. Finally, we believe that, by selecting against the deleterious alleles that cause SR and abortion through systematic breeding schemes, the damage to livestock farms can be reduced by reducing the frequency of carriers in the parental generation.
This study aimed to identify the pathological symptoms and genetic mutations associated with the genetic disorder of SR, which commonly occurs in cattle. To this end, genetic mutations were identified using SNP-Chip data and pathological symptoms of SR cases in Holstein dairy cattle in Korea. As a result, a recessive allele (A/A) was identified in HH1, previously reported in the OMIA database as a lethal gene that causes abortion in Holstein dairy cattle. If additional SR cases are observed in Korea in thefuture, we will identify genetic mutations in the whole genome using re-sequencing and SNP-Chip data.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
This study is only field case not the animal experiments with infection. Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
WP and KL designed and performed the research, analyzed the data, and wrote the manuscript. CD and JL provided and analyzed the SNP-Chip data. JK worked on the pathological examination. HJ and TL worked on the chromatography examination and imaging diagnostics. K-CL provided technical comments on the imaging diagnostics. KL handled technical matters for pathology and imaging. H-HC and DL interpreted the results and finalized the manuscript. All authors read and approved the final manuscript.
This study was carried out with the support of the 2022 RDA Fellowship Program of the NIAS and the Cooperative Research Program for Agriculture Science and Technology Development [Project No. PJ014826, Identification of genetic factors and biomarkers associated with the pregnancy of Korean cattle (Hanwoo)], Rural Development Administration, Republic of Korea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Knight RP. The occurrence of schistosomus reflexus in bovine dystocia. Aust Vet J. (1996) 73:105–7. doi: 10.1111/j.1751-0813.1996.tb09988.x
2. Tierarzt VS, Johnston DE. The causes and treatment of dystocia in beef cattle in Western Victoria: 2 causes, methods of correction and maternal death rates. Austra Vet J. (1967) 43:13–21. doi: 10.1111/j.1751-0813.1967.tb04757.x
3. Dubbin E, Welker F, Veit H, Modransky P, Talley M. Dystocia attributable to a fetal monster resembling schistosomus reflexus in a donkey. J Am Vet Med Assoc. (1990) 197:605–7.
4. Bedford P. Schistosoma Reflexus in a Goat-a Case Report. London: British Veterinary Assoc. (1967) p. 326.
5. Dennis S. Schistosomus reflexus in conjoined twin lambs. Vet Rec. (1972) 90:509–10. doi: 10.1136/vr.90.18.509
6. Cala D, Sánchez H, Jaimes R, Hernández M, Aguinaga JY. Schistosomus reflexus in dogs: case report. Braz J Vet Pathol. (2019) 12:79–82. doi: 10.24070/bjvp.1983-0246.v12i2p79-82
7. Bárcenas-Ibarra A, Rojas-Lleonart I, Lozano-Guzmán R, García-Gasca A. Schistosomus reflexus syndrome in olive ridley sea turtles (Lepidochelys Olivacea). Vet Pathol. (2017) 54:171–7. doi: 10.1177/0300985816651682
8. Kawata K, Tiba T. A rare case of schistosomus reflexus in the cat. Jpn J Vet Res. (1961) 9:179–81.
9. Roberts S. Veterinary Obstetrics and Genital Diseases (Theriogenology). Michigan: Edwards Brothers Inc. (1986).
10. Dünker N, Krieglstein K. Tgfß2–/–Tgfß3–/–double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol. (2002) 206:73–83. doi: 10.1007/s00429-002-0273-6
11. Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, et al. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra-and periocular mesoderm and right pulmonary isomerism. Development. (1999) 126:5749–58. doi: 10.1242/dev.126.24.5749
12. Nottoli T, Hagopian-Donaldson S, Zhang J, Perkins A, Williams T. Ap-2-null cells disrupt morphogenesis of the eye, face, and limbs in chimeric mice. Proc Nat Acad Sci. (1998) 95:13714–9. doi: 10.1073/pnas.95.23.13714
13. Parvari R, Weinstein Y, Ehrlich S, Steinitz M, Carmi R. Linkage localization of the thoraco-abdominal syndrome (Tas) gene to Xq25–26. Am J Med Genet. (1994) 49:431–4. doi: 10.1002/ajmg.1320490416
14. Zucchi I, Jones J, Affer M, Montagna C, Redolfi E, Susani L, et al. Transcription map of Xq27: candidates for several X-linked diseases. Genomics. (1999) 57:209–18. doi: 10.1006/geno.1999.5768
15. VanRaden P, Olson K, Null D, Hutchison J. Harmful recessive effects on fertility detected by absence of homozygous haplotypes. J Dairy Sci. (2011) 94:6153–61. doi: 10.3168/jds.2011-4624
16. VanRaden P, Null D, Olson K, Hutchison J. Reporting of haplotypes with recessive effects on fertility. In: Interbull Bulletin. (2011).
Keywords: schistosomus reflexus, Holstein, genetic mutation, vertebrae dysplasia, fetus, SNP-Chip
Citation: Park W, Chai H-H, Lim D, Dang C, Lee J, Kim J, Jeong H, Lee T, Lee K-C and Lee K (2023) Case report: Investigation of genetic mutations in a case of schistosomus reflexus in a Holstein dairy cattle fetus in Korea. Front. Vet. Sci. 10:1238544. doi: 10.3389/fvets.2023.1238544
Received: 12 June 2023; Accepted: 17 July 2023;
Published: 21 August 2023.
Edited by:
Francisco Javier Salguero, UK Health Security Agency (UKHSA), United KingdomReviewed by:
Alejandra García-Gasca, National Council of Science and Technology (CONACYT), MexicoCopyright © 2023 Park, Chai, Lim, Dang, Lee, Kim, Jeong, Lee, Lee and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woncheoul Park, d2NwYXJrMTk4MkBrb3JlYS5rcg==; Kyunghyun Lee, bXlsb3ZlaHl1bkBrb3JlYS5rcg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.