
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 19 September 2023
Sec. Veterinary Epidemiology and Economics
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1236494
This article is part of the Research Topic Pathogenic and Symbiotic Bacteria in Ruminants: Antimicrobial Resistance and Microbial Homeostasis View all 10 articles
 Ahmed Mohammed Hussen1
Ahmed Mohammed Hussen1 Fikadu Alemu1
Fikadu Alemu1 Ahmed Hasan Hussen1
Ahmed Hasan Hussen1 Abdimalik Hussein Mohamed1
Abdimalik Hussein Mohamed1 Haben Fesseha Gebremeskel2*
Haben Fesseha Gebremeskel2*Introduction: Brucellosis is a zoonosis of major public health and economic importance that is endemic in livestock in Ethiopia with varying levels of seroprevalence.
Methods: A cross-sectional study was carried out to determine the individual and herd-level seroprevalence of brucellosis in small ruminants in the Korahey zone of the Ethiopian Somali Region. A total of 324 sera from 63 herds of small ruminants were collected randomly using a multistage sampling technique and the sera were tested using the Rose Bengal Plate Test, and seropositive reactors were confirmed by the Complement Fixation Test.
Results and discussion: The seroprevalence of brucellosis at the herds and the individual level was 6.35% (95% CI: 0.0–13%) and 1.23% (95% CI: 0.0–2%), respectively; with 1.4% in goats and 0.9% in sheep. Moreover, predicted variables like age group, parity, history of abortion, fetal membranes, herd size, ownership of other livestock species, contact with wild animals in the past year, the introduction of new animals in the past year, and lending of breeding males in the past year were not significantly associated (p > 0.05) with Brucella seropositivity at individual and herd level seroprevalence during multivariable logistic regression analysis. Pastoral community awareness regarding the public health impact of brucellosis and the promotion of an intersectoral One Health approach for the effective control of brucellosis is recommended.
Brucellosis is a bacterial zoonotic disease caused by the genus Brucella that causes reproductive problems such as abortion, retained fetal membranes, and the birth of weak offspring, as well as orchitis and epididymitis in male animals, which is frequently followed by sterility (1). There are 12 known Brucella species that cause brucellosis at this time (2) and six of them, are known to be pathogenic to humans: B. abortus, B. canis, B. inopinata, B. melitensis, B. pinnipedialis, and B. suis (2, 3). This taxonomic classification is primarily based on differences in host preference and pathogenicity that can be attributed to different proteomes, as demonstrated by specific outer-membrane protein markers (4, 5). Around the world, Brucella species that infect both humans and animals are frequently discovered in the interface of the human-animal ecosystem, where strong interactions exist between people, livestock, and wildlife in the same area (6, 7).
Brucella abortus causes abortion, stillbirth, and weak calves in cattle, with abortions typically occurring during the third trimester of pregnancy. B. melitensis is the most common species in developing countries in small ruminants and is linked to clinically visible diseases in humans (8). B. melitensis can cause abortion, retained placenta, orchitis, and epididymitis in goats. Abortions in goats are most common during the fourth month of pregnancy (9). Men’s clinical manifestations of the disease include weakness, fever, excessive sweating, especially at night, weight loss, and generalized body aches (10). Swelling in the testes and burning micturition caused by orchitis and urethritis, respectively, are unusual symptoms of the disease in men (5, 11). The disease has also been reported in wild and marine mammals, as well as birds, in recent years. Another epidemiological concern is the presence of brucellosis in wild animals, with the potential for continuous transmission to domestic animals and from them to humans (12).
Prevalence studies for brucellosis have been carried out in various parts of the country. Brucellosis in animals and humans has been reported in various parts of Ethiopia, most notably in cattle in both intensive and extensive management systems (13–17). The disease has also been reported among small ruminants in pastoral areas of the country. In a study conducted in the Tallalak district of the Afar region, a prevalence of 13.7% in sheep and goats was reported, with the prevalence being higher in goats (15.4%) than in sheep (10.6%), as reported by Wedajo et al. (18), whereas in the Somali region, Mohammed et al. (19) reported a seroprevalence of 1.37% among small ruminants in three woredas of the Jigjiga zone.
Unpasteurized milk products, infected placental material, aborted fetuses, or infected animals, which can shed a variety of bacteria after abortion, are other ways in which this infection in humans can occur in endemic countries (20). Pastoralist communities are more likely to contract brucellosis and other zoonotic diseases than other communities that do not have the same level of association with animals due to their close physical proximity to livestock and their reliance on animal products (19, 21).
The prevalence of brucellosis in pastoralist areas is thought to be influenced by several variables, including grazing patterns, management techniques, and the age and sex composition of herds (22, 23). Additionally, for a variety of reasons, pastoralists do not isolate or get rid of animals that may be infected with Brucellosis, which raises the risk of transmission to healthy animals. Female sheep and goats are kept in the herds for a longer period than males and are sold as soon as they mature. Unlike males, infected females shed the bacteria more frequently and may contribute to the likelihood of brucellosis in pastoral areas. Other factors contribute to the prevalence of brucellosis in pastoralist areas, such as the consumption of unpasteurized milk; unsafe handling and improper disposal of potentially infective materials, such as aborted fetuses, fetal membranes, and bodily fluids, which may contain concentrations of the bacteria; and a lack of awareness about zoonotic risks and methods of transmission (4, 16, 17, 20).
Nonetheless, the extent of the disease and its impact on pastoralist health are understudied, at least in the context of the proposed study area: the Korahey zone. Similarly, no research has been done on the risk factors associated with disease occurrence or the existing knowledge, attitudes, and practices in pastoralist areas that may play a role in zoonotic transmission. Therefore, the objective of this study was to estimate seroprevalence and associated risk factors of brucellosis in small ruminants in selected districts of the Korahey zone, Somali regional state, Ethiopia.
The study was conducted in three purposively selected districts of the Korahey zone, namely, Doboweyn, Kebridahar, and Sheygosh, located 470, 380, and 280 km, respectively, south of Jigjiga, the capital of the Somali Region of Ethiopia, which is situated approximately 630 km to the east of Addis Ababa. The Korahey zone is located in the south of the region and consists of 10 districts and one city administration; the zonal capital is Kebridahar. The zone has semiarid agroecology with a bimodal rainfall pattern. The main rainy season is Gu (April–June), and the second rainy season, Deyr, is received between September and November. The major production system in the zone is pastoralism, with some mixed livestock crop production practiced around Kebridahar. The livestock population of the zone is 3,576,492, consisting of 311,243 cattle, 1,323,491 goats, 1,265,585 sheep and 582,860 camels (24). Kebridahar is located at 6°44′N latitude and 44°16′E longitude and has a total of 799,367 of livestock population. Doboweyn is located at 6°41′N latitude and 43° 69′E longitude and has a total of 626,674 of livestock population. Sheygosh is located at 7° 41’ N latitude and 43° 56′ E longitude and has a total of 520,900 of livestock population (Figure 1).
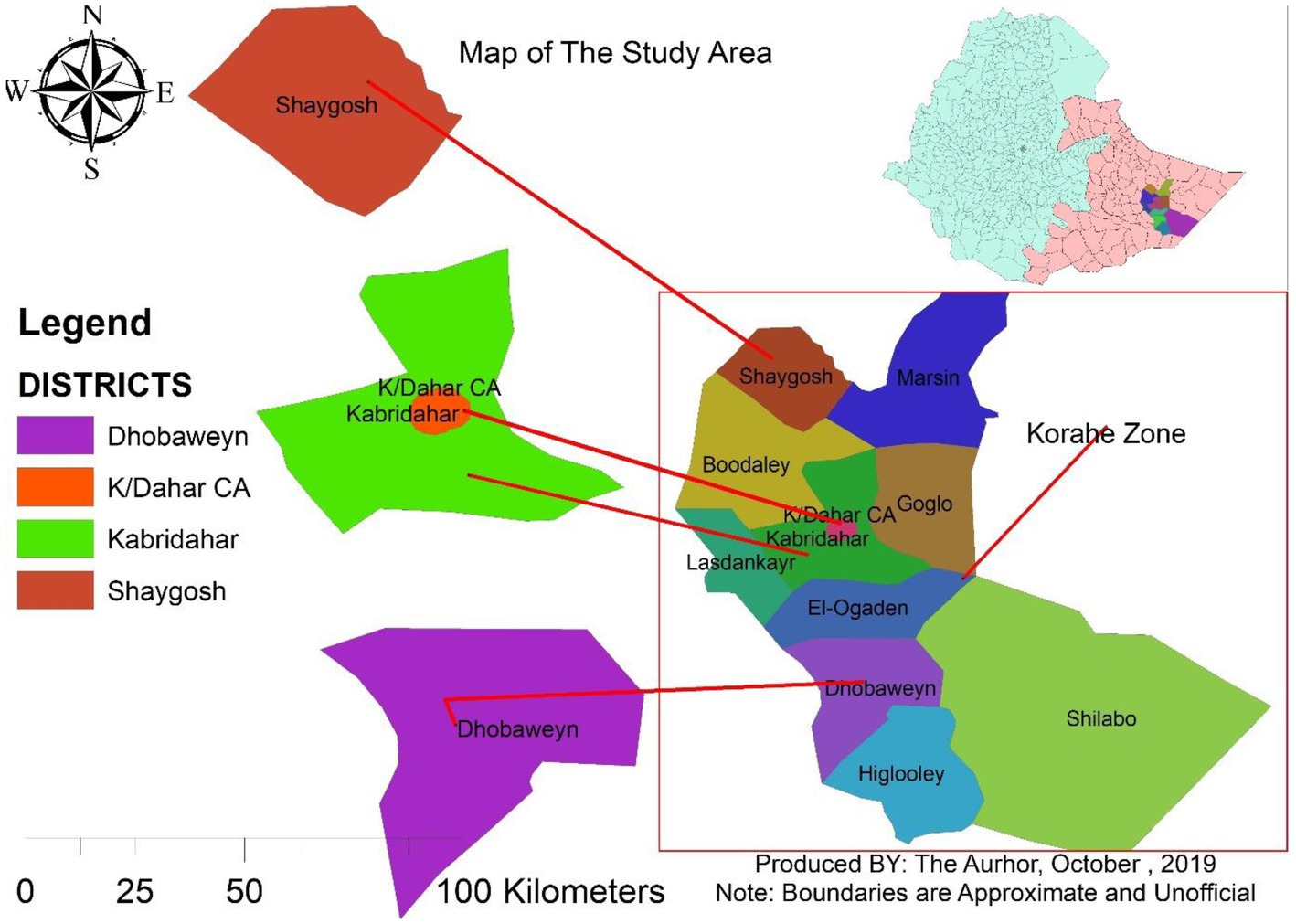
Figure 1. Map of the three study districts of the Korahey zone, Somali regional state, and eastern Ethiopia.
There are approximately 1,323,491 goats and 1,265,585 sheep in the Korahey zone (25). The study population consisted of small ruminants kept under an extensive management system in three purposively selected districts of the Korahey zone. The study animals were indigenous Somali goats and black head Ogaden sheep. In addition, sheep and goats, which were above 6 months of age and had no history of vaccination against brucellosis, were included in the study.
A cross-sectional study design was used to estimate the seroprevalence and associated individual animal-level and herd -level risk factors for small ruminant brucellosis and to evaluate the knowledge, attitudes and practices of pastoralists toward brucellosis in selected districts of the Korahey zone, Somali region of Ethiopia.
A multistage sampling technique was employed. The pastoralist association was the primary sample, pastoralist families/herds the secondary sample and the individual animals the tertiary sample. The primary sample was selected purposively based on livestock population and accessibility, whereas the secondary and tertiary samples, herds and individual animals, were selected using a systematic random sampling technique.
Individual animals were sampled from the herds above using a systematic random sampling method by first putting the animals in a crush. Then, the animals were allowed to leave the fence one animal at a time. The herd’s owner was asked to randomly pick an animal from among the first 5, leaving the fence. Then, every kth animal was selected; the value of k was determined based on the size of the herds being sampled and the number of animals sampled from each herd. Sheep and goats were sampled separately.
The sample size was determined using Thrusfield (26) formula as follows:
where n = required sample size, Pexp = expected prevalence and d = desired absolute precision.
Accordingly, the estimated sample size was 23 for goats and 15 for sheep based on the expected brucellosis prevalence of 1.5% in goats and 1% in sheep in other parts of the Somali region by Mohammed et al. (19) and 0.05 desired absolute precision at the 95% level of confidence. However, to increase precision, the sample size was increased to 213 goats and 111 sheep. In total, 324 small ruminants (sheep and goats) were sampled using systematic random sampling (Table 1).
Animals were sampled from nine purposively selected kebeles that were located in three study districts, i.e., three kebeles (peasant associations) per woreda (districts) based on livestock population and accessibility. The distribution of the herds across the kebeles and woredas was determined based on the estimated population size of the respective kebeles.
Approximately 10 mL of blood sample was collected from the jugular vein of each study animal using plain vacutainer tubes, needle holders, and needles. The blood sample from each animal was labeled and centrifuged at 10,000 rpm for 3 min, and sera were removed by siphoning them into sterile cryovials. The serum samples were then transported to the Jigjiga regional veterinary diagnostic and research laboratory in an ice box, where they were stored and kept at −20°C until serology procedures were performed.
The screening procedure with RBPT was performed at Jigjiga Regional Veterinary Diagnostic and Investigation Laboratory and National Veterinary Institute (NVI) at Bishoftu, whereas CFT was conducted at NVI using test protocols as outlined by OIE (27) and the manufacturer’s specifications for the tests. All the samples that tested positive on the RBPT were tested with the complement fixation test (CFT) (Figure 2).
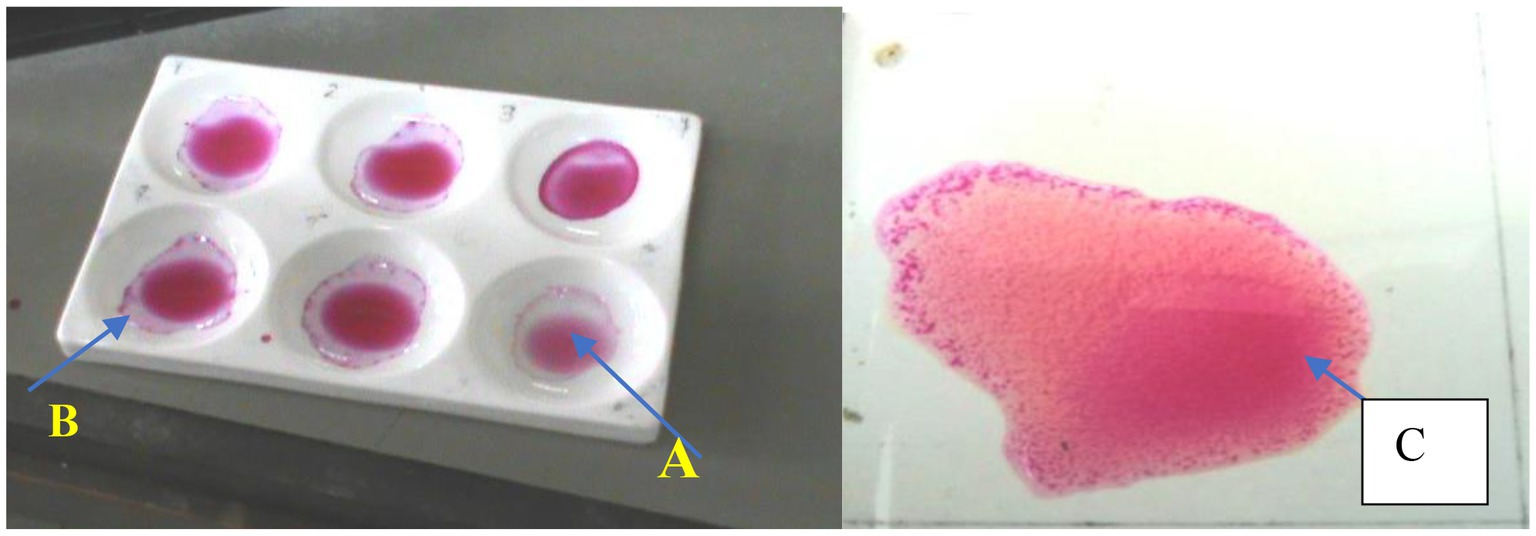
Figure 2. Plate Rose Bengal Plate Test showing positive and negative samples. A, positive sample; B, negative sample; C, positive control.
The CFT and RBPT test antigens (Brucella abortus strain 99), control sera, and other reagents were obtained from Atlas Medical, William James House, Cowley Rd. Cambridge Cb4, 4 WX and sensitized sheep red blood cells (SRBCs) were obtained from the NVI. The serum specimens were tested serially first using RBPT and then CFT for those that tested positive for RBPT. An animal was considered positive if the serum specimen tested positive on both RBPT and CFT, whereas a herd was considered positive if at least a single serum specimen from an animal within the herds tested positive on both RBPT and CFT. For RBPT, the Rose Bengal test antigen was prepared from the killed standard strain of B. abortus strain 99 and stained with Rose Bengal dye in an acidic buffer pH 3.65.
A pretested structured questionnaire was used to collect information about potential factors associated with brucellosis seropositivity at animal and herd levels. The potential factors at the individual animal level included age, parity stage, and previous history of reproductive problems such as abortion, retained fetal membranes, orchitis, and epididymitis. At the herd’s level, the factors assessed were herd size, keeping other animals with sheep and goats, the introduction of new animals in the past year, abortion in the herds in the past year, contact with other small ruminant herds and wild animals, presence of calving/lambing/kidding pens, vaccination history and whether owners sought veterinary service or advice in the past year.
Serological data were entered into a Microsoft Excel Spreadsheet version 2016 (Microsoft Corporation) along with the corresponding data generated with the questionnaire. The statistical software package SPSS version 20 (SPSS Inc., IBM, Chicago, IL, United States) was used for data analysis. Descriptive statistics like frequency and proportion were employed for the description of the seroprevalence of the disease. A herd level and individual animal seroprevalence were calculated by dividing the number of positive test results by the total number of herds and animals sampled, respectively. Univariable analysis using Fisher’s exact test was used for the analysis of the association between individual animal-level and herds Brucella seropositivity and the potential factors. Furthermore, a multivariable logistic regression model was used to analyze risk factors of the disease that was found statistically significant when using univariable analysis and the results were reported by odds ratio using 95% confidence interval to assess the strength of the association. The final multivariable logistic regression model selection was based on p value (p value ≤0.25) and stepwise backward elimination procedure, dropping the least significant independent variable until all the remaining predictor variables were significant. The statistical significance level was set at 95% confidence level and 5% level of precision so that p ≤ 0.05 was considered significant.
A total of 324 animals composed of 213 (65.7%) goats and 111 (34.3%) sheep were sampled. These animals were sampled from 63 herds from 9 kebeles (peasant associations) in Doboweyn, Kebridahar, and Sheygosh districts of the Korahey zone, Somali Region of Ethiopia. The 63 herds had a total of 4,026 animals, of which 2,512 were goats and 1,514 were sheep. The median sheep and goat herds’ size was 56 (range: 20–180 animals). One-third of the herds in the study (33.3%) had more than 60 heads of sheep and goats, and the remaining 66.7% had a herd size of 60 heads or below.
Of the 324 serum samples examined, 1.85% (95% CI: 0.0–3.0%) were positive for Brucella antibodies on RBPT. Of the samples positive for RBPT, 2.3% (95% CI: 0.0–4%) were from goats, and 0.9% (95% CI: 0–3%) were from sheep. Of the samples testing positive on RBPT, 1.23% (95% CI: 0.0–2%) tested positive on CFT, of which 1.4% (95% CI: 0.0–3%) were goats and 0.9% (95% CI: 0–3%) were sheep. In this study, the animal-based seroprevalence of small ruminants brucellosis in three district of the Korahey zone of Somali regional state was 1.85% by RBPT and 1.23% by combined RBPT and CFT. Thus, the overall seroprevalence was 1.23% and were taken for subsequent data analyses (Table 2).
Based on CFT, the highest seroprevalence of 5.9% (95% CI: 2–14%) was recorded in the Dalad kebele of Kebridahar district, followed by 2.9% (95% CI: 3–9%) in the Harano kebele of Doboweyn district and then by 2.6% (95% CI: 3–8%) in the 01 kebele in Sheygosh town of Sheygosh district. In the remaining 6 locations, namely, 01 kebele of Kebridahar town and Bundada kebele of Kebridahar district; 01 kebele of Doboweyn town and Jidhale kebele of Doboweyn district and Harir and Wijiwaji kebeles of Sheygosh district, zero seroprevalences were recorded.
Of the 63 herds sampled, the overall true herd seroprevalence was 6.35% (95% CI: 0.0–13%). None of the herds had more than one animal testing positive for Brucella species. All the herds sampled had a mixture of sheep and goats; hence, comparing herds’ seropositivity levels by species was deemed irrelevant.
In determining the risk factors associated with individual animal Brucella seropositivity, several factors were examined in the bivariate analysis. These include species, sex, age, number of parties, previous history of abortion and/or retained fetal membrane, and previous history of orchitis and epididymitis.
In terms of sex, 276 (85.2%) were females, and 48 (14.8%) were males. All the seropositive animals were females, but there was no statistically significant difference in the brucellosis seroprevalence between the two sexes (p > 0.05).
The majority, 196/324 (60.5%), of the sampled animals were between 3 and 4 years old, and only 18 (5.5%) were older than 4 years. A significant difference (p = 0.001) was observed between the different age categories with regard to Brucella seropositivity, with the highest prevalence being in the >4-year-old age category (16.7%), followed by the category aged 3–4 years (0.5%). None of the animals in the age range of 1–2 years tested positive for Brucella.
Of the female animals in the sample, 258/276 (93.4%) gave birth at least once, with 13 (4.7%) of them being primiparous, giving birth only once. Most of the female animals (84.8%) gave birth between 2 and 4 times. Only 11 females (4%) gave birth more than four times. The highest seroprevalence was observed in the category of female animals that gave birth more than 4 times, with 3 of the 11 female animals in this category (27.3%) showing seropositivity with CFT. This was followed by the females that gave birth between 3 and 4 times, in which a seropositivity prevalence of 0.6% was recorded.
A quarter, 69/276 (25%), of the female animals sampled had a history of abortion at least once in the past, whereas 49/276 (17.8%) of female animals experienced retained fetal membranes. Regarding male animals, 11/48 (23%) had a history of experiencing either orchitis or epididymitis, but this study did not find any seropositive males. All the seropositive animals had a previous history of abortion and retained fetal membranes, and it was found that there was a significant difference (p < 0.05) between animals with a history of previous abortion and those without a previous history of abortion (Table 3).
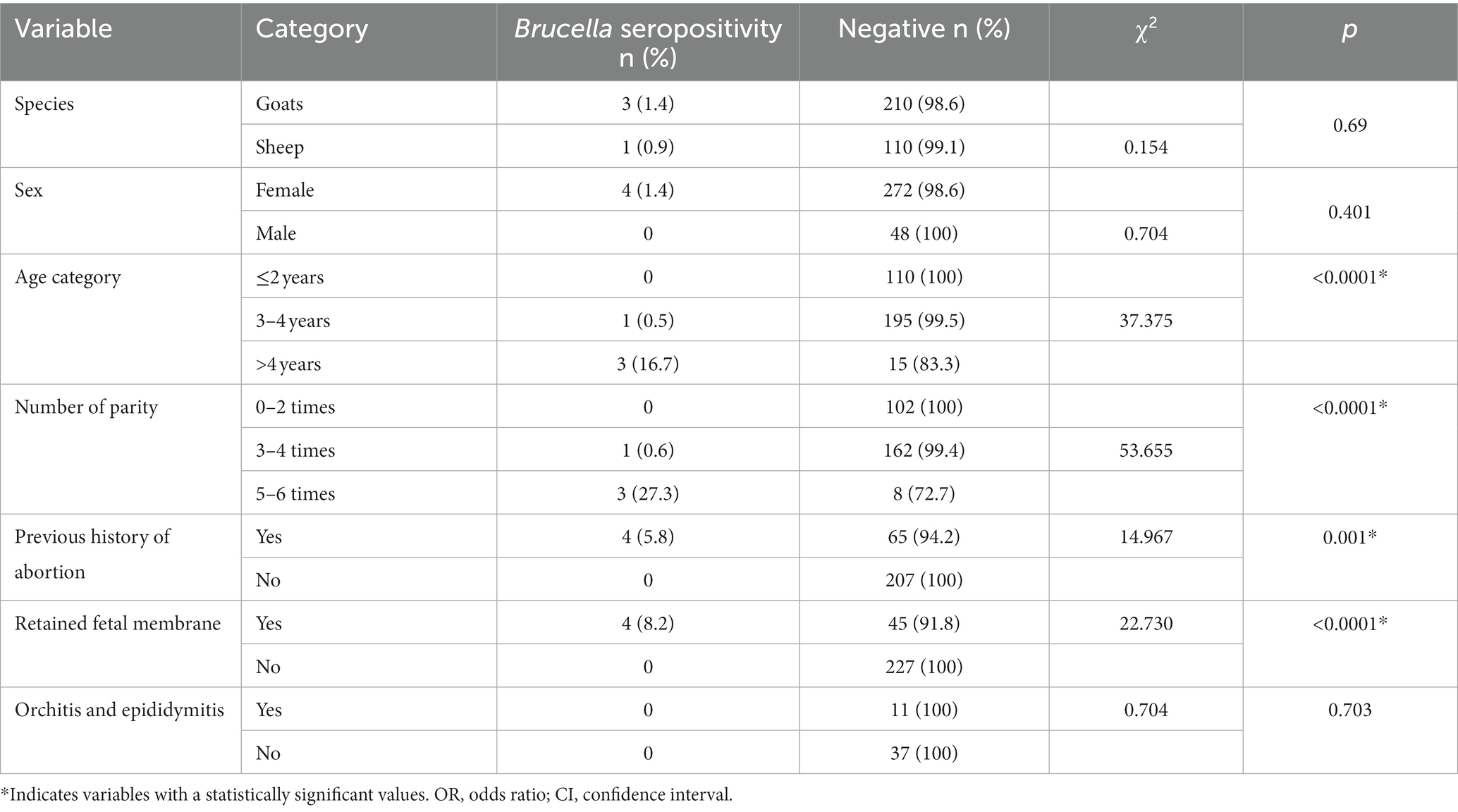
Table 3. Univariate analysis of seropositivity of Brucella with associated risk factors at individual animal level (n = 324).
In determining the risk factors associated with herds Brucella seropositivity, several factors were examined in the bivariate analysis. These included herds’ size, ownership of other livestock species, ownership of calving/kidding/lambing pens, contact with other herds and contact with wildlife, history of abortion in the herds, the introduction of new animals, lending/borrowing of male animals, and veterinary service-seeking behavior of the owner. All but the last factor, i.e., veterinary service-seeking behavior, was considered a risk factor, while the latter variable was treated as a protective factor.
One-third of the herds (21/63, 33.3%) had more than 60 heads of sheep and goats, and the remaining herds had a maximum herd size of 60 sheep and goats. The herds with a size of more than 60 animals had almost 7 times higher odds of having positive reactors for Brucella compared to those herds with smaller herds sizes, but this was not statistically significant (OR = 6.833; 95% CI: 0.665–70.235, p = 0.106).
Of the 63 sampled sheep and goat herds, 25 (39.7%) are kept together with other livestock species, as the owner also keeps other species of livestock apart from goats and sheep, and the other 38 (60.3%) households possess only small ruminants. Almost all of the respondents, 24/25 (96%), who also own other livestock species raise the different herds separately, and only one respondent said that they house the different species of animals together. Most of the other livestock species kept in the area are camels, cattle, and donkeys ordered in terms of population. Three of the herds with positive reactors to Brucella (75%) came from herds whose owners also keep other livestock species, and even though it is not statistically significant, sheep and goat herds belonging to owners who also keep other livestock species have 5 times more odds of getting brucellosis compared to other herds whose owners do not keep other livestock species as well (OR = 5.045; 95% CI: 0.494–51.540, p = 0.172).
Nearly all of the herds 62/63 (98.4%) did not have a calving/lambing/kidding pen where animals delivered offspring, and all the herds with positive reactors to Brucella were from those herds that lacked the calving pen, but this was not statistically significant (p > 0.05). Since the area is predominantly pastoralist with grazing lands shared communally, there is a high chance of contact between different herds. In the present study, 52/63 (82.5%) of the herds came into contact with other herds of sheep and goats in the past year, but the current study did not find a statistically significant association between Brucella herds seropositivity and contact with other herds (OR = 0.612; 95% CI: 0.058–6.505, p = 0.684).
On the other hand, 14/63 (22%) of the herds came into contact with wild animals in the past year. The most commonly encountered wild animals were antelopes such as dik-dik, warthogs, foxes, and hyenas. Although it was not statistically significant, herds that came into contact with wild animals had a higher chance of having positive reactions to Brucella (OR = 3.917; 95% CI: 0.499–30.728, p = 0.194).
Abortion in sheep and goat herds was reported in 32/63 (50.8%) of the herds in the past year. Of the 4 herds that tested seropositive for Brucella, 3 (75%) had experienced abortion in the past year compared to 29 (49%) in seronegative herds. Herds with abortion reports in the past year had an approximately 3 times higher chance of containing seropositive reactors than herds that did not experience abortion in the past year, but this difference was not significant (OR = 3.103; 95% CI: 0.305–31.580, p = 0.339).
New animals were introduced into 14/63 (22.2%) of the herds in the past year mainly through purchases and gifts from relatives. Comparing herds that introduced a new animal into the herds in the past year to those herds that did not, the study found that herds that introduced new animals into the herds had 13 times increased chances of having seropositive reactors for Brucella infection (OR = 13.091; 95% CI: 1.241–138.11, p = 0.032).
Sharing of breeding males is less common, and only 13/63 (20.6%) of the herds have lent their breeding male sheep or goat animals over the last year. Herds that lend male animals for breeding purposes have a more than 14-fold increased chance of having seropositive reactors compared to herds that do not lend their male animals to other herds for breeding. This was statistically significant (OR = 14.7; 95% CI: 1.384–156.179, p = 0.026).
Although 26/63 (41.3%) of the herds were vaccinated in the past year, no vaccination was given against brucellosis, and only 19 (30%) respondents had received/obtained veterinary advice last year, with the majority, 44 (70%), not getting veterinary advice of any kind in the past year. No statistically significant difference (p > 0.05) in herds’ seropositivity was observed between the herds in relation to vaccination and receiving veterinary advice (Table 4).
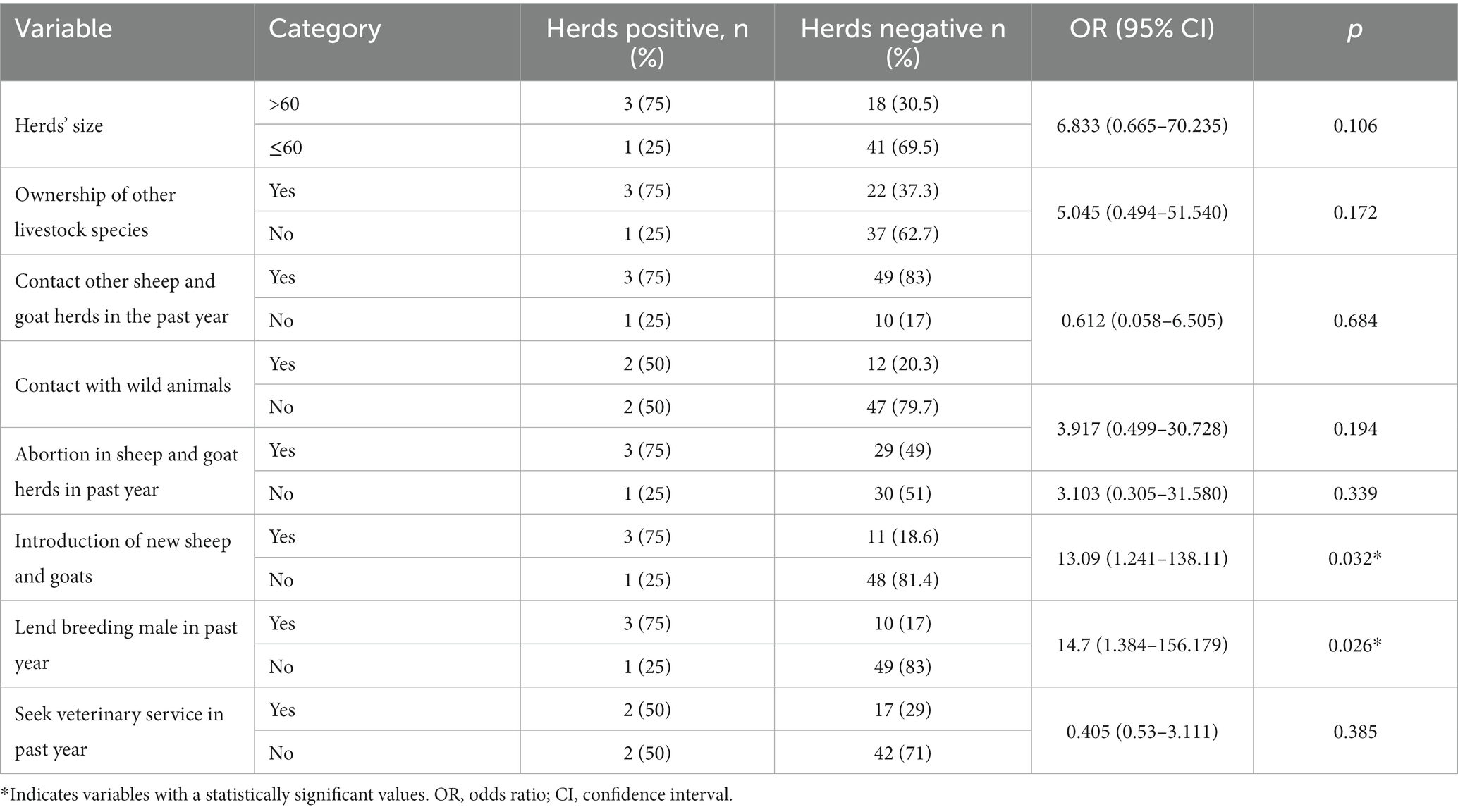
Table 4. Univariate analysis comparison of factors associated with Brucella seropositivity among positive and negative herds.
Based on the entry criteria stated in the methodology (p ≤ 0.25), herds’ size, ownership of other livestock species, contact with wild animals in the past year, the introduction of new animals in the past year, and lending of breeding males in the past year were selected for the multivariable analysis using a binary regression model. Additionally, age category, previous history of abortion, number of parity and retained fetal membrane history were selected for multivariable regression model from individual seropositive animal level factors. Nevertheless, none of the variables showed a significant association (p > 0.05) in the multivariable analysis (Table 5).
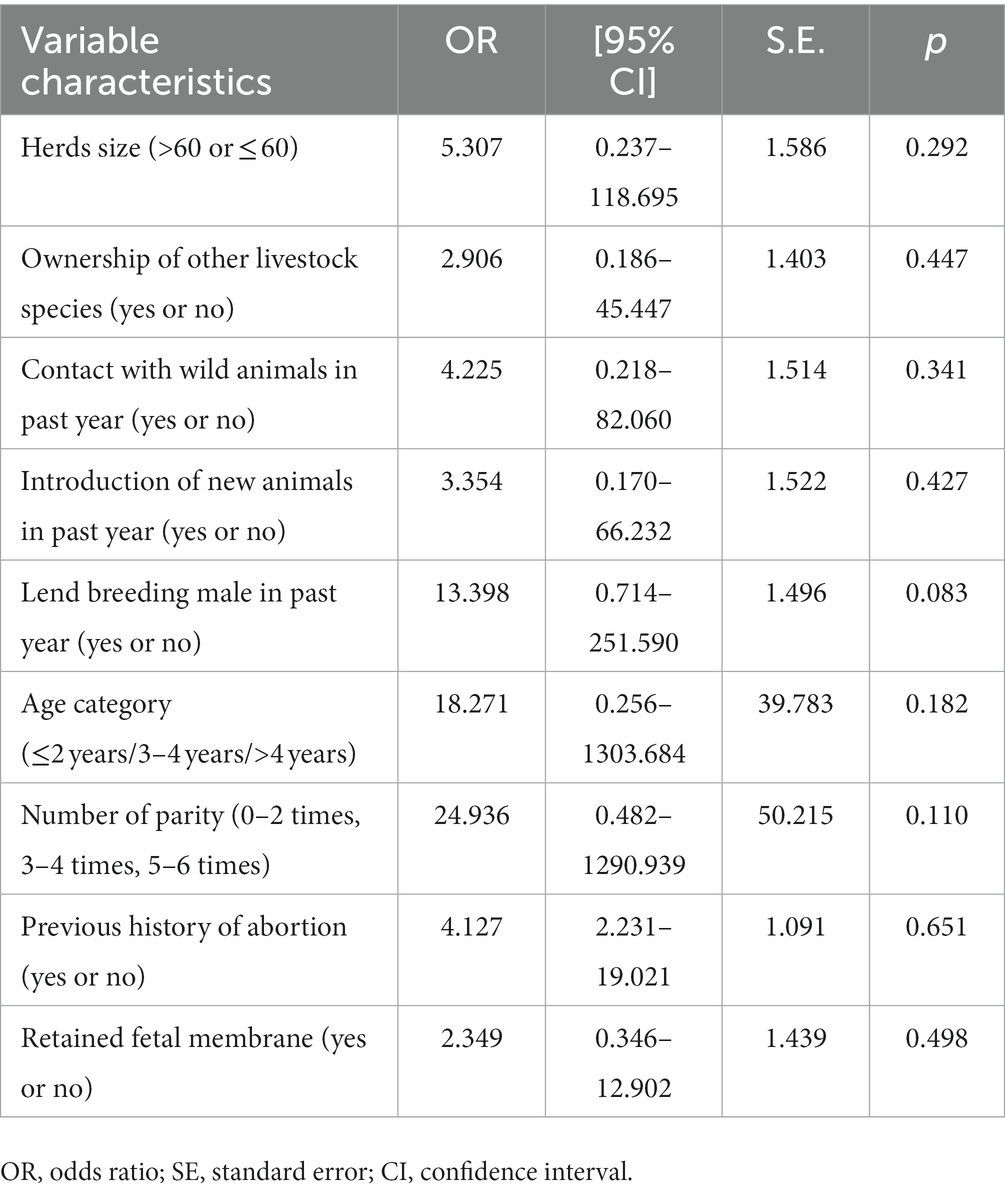
Table 5. Multivariable logistic regression analysis of the variables associated with herds and individual-level seropositivity for Brucella in sheep and goats.
In this study, the animal-based seroprevalence of small ruminant brucellosis in three districts of the Korahey zone of Somali regional state was 1.23% and this was in line with the results reported by Mohammed et al. (19) in the Jigjiga zone of the Somali region, with a prevalence of 1.37%; Tsegay et al. (28) in Debrezeit and Modjo, with a seroprevalence of 1.76%; and Dabassa et al. (29), who found a similar result (1.56%) in a study of small ruminant brucellosis in Yabello. The present finding is lower than results reported by Aloto et al., (30) who reported 4.1% in two zones of southern Ethiopia, Dosa et al. (31) who reported 3.33% in two selected districts of Wolaita Zone southern region, Teshome et al. (32) who reported 17.36% in goats in Borana pastoral area, Deddefo et al. (33) who reported 4.6% in Arsi, Teshale et al. (34) who reported 9.7% in Afar, and Negash et al. (35) who reported 9.11% in the Dire Dawa area. Similarly, Wedajo et al. (18) reported a higher seroprevalence (13.7%) in the Tallalak district of the Afar region.
However, the current result is relatively higher than the previous study by Ferede et al. (36) who reported 0.4% in and around Bahirdar and Tewodros and Dawit (37) who reported 0.7% in and around Kombolcha, Amhara region. This variation might be caused by variations in sample size, agroecological location, and animal management practices.
In the present study, RBPT was used to detect the seroprevalence of Brucella species in small ruminants which was used in this study to screen individual animals, is a low-cost, quick, and highly sensitive test (27). However, due to cross-reactivity with antibodies from closely related gram-negative bacteria such as Yersinia enterocolitica, Escherichia coli, Salmonella spp., and Sternotrophomonas maltophilia as well as antibodies produced by the B. abortus S19 vaccine, its specificity is low (38). In the current study, only samples that gave signals for both RBPT and CFT were considered positive since no single test is appropriate in all epidemiological situations due to problems of sensitivity and or specificity of the tests as recommended by OIE (27) and other reports (39).
The current study revealed that the seroprevalence of brucellosis was 1.57 times higher than sheep’s seroprevalence, although this difference was not statistically significant. This finding is comparable to that of Mohammed et al. (19), who also reported higher seroprevalence in goats in the Somali region. Similar findings were also reported by Wedajo et al. (18), Teshale et al. (34), and Ashenafi et al. (40) in the Afar region; Aloto et al. (30) in two zones of southern, Ethiopia; Mengistu (41) in Konso, southern Ethiopia; Tewodros and Dawit (37) in Kombolcha of the Amhara region; and Negash et al. (35) in Dire Dawa.
However, a study by Bekele and Kasali (42) in the central highlands of Ethiopia and Samaha et al. (43) in Egypt showed a higher prevalence in sheep than in goats, mainly due to differences in husbandry systems and the susceptibility of the sheep and goat breeds in the particular area. The difference may also be due to variations in the species breakdown of the samples examined by the various researchers. Because goats are more susceptible to Brucella infection than sheep and excrete the bacterium for a longer period of time, goats exhibit higher seroprevalence than sheep (21).
The seroprevalence of brucellosis of female sheep and goats were higher than males one, although this difference was not statistically significant (p > 0.05). The result of this study is in agreement with other findings by Tewodros and Dawit (37) and Yesuf et al. (44), who also reported higher seroprevalence in females than in males, although Yesuf et al. (44) found a statistically significant (p < 0.05) difference in Brucella seroprevalence between the two sexes. The lower number of males (n = 48) sampled and tested compared to a higher number of females (n = 276) in the sample may have contributed to the higher female animal seropositivity. It is also possible that male animals are less likely to contract Brucella infection because they do not contain erythritol (45). The fact that there was no statistically significant difference between the two sexes may also have contributed to the very low number of positive results observed in the current study.
The study revealed a significantly (p < 0.05) higher seroprevalence of small ruminant brucellosis in the old age group than in the medium and young age groups. This is consistent with the findings of Tsegaye et al. (28) and Adugna et al. (46), who also reported a higher seroprevalence of brucellosis in small ruminants more than 2 years of age than in the younger age categories. Similarly, Megersa et al. (14), Mohammed et al. (19), and Tigist et al. (47) reported higher seroprevalence in older age groups than in younger animals, even though the difference was not statistically significant. In contrast, Wedajo et al. (18) and Negash et al. (35) reported a higher seroprevalence in younger animals than in adult sheep and goats.
The study also found a statistically significant association between seroprevalence and parity stage, with higher seropositivity in higher parity stage females than in lower parity stage females. The various findings regarding the variation in brucellosis seroprevalence among the various age groups may be related to variations in the relative proportion of the various age groups in the samples examined by the various researchers. The risk of contracting Brucella infection is higher in sexually mature and pregnant animals than in sexually immature animals of either sex. This might be due to the concentration of erythritol and sex hormones, which promote the growth and reproduction of Brucella species organisms, rising with age and sexual maturity (48).
In the present study, the relationship between Brucella seropositivity and the presence of reproductive issues, such as a history of abortion or retained fetal membranes, was investigated. Male animals of both species were excluded from this discussion because no male animals tested positive for Brucella infection by chance. The seroprevalence of brucellosis in small ruminants with a history of abortion or retained fetal membranes was found to be higher (p < 0.05) than in those without these problems. Similar findings were reported by Wedajo et al. (18) and Wubishet et al. (49) in the Afar and Guji zones of the Oromia region, respectively. It is known that abortion in livestock represents the major complaint attributed to Brucella infections (50–52).
The seroprevalence of brucellosis was higher in large (>60 heads) herds’ sizes than in small (≤60 heads), but the difference was not significant. This is in line with the findings reported by Wedajo et al. (18) in the Afar region. Walker (53) shows that herd sizes and animal densities are directly related to disease prevalence and complicate infection control in a population. Similarly, sheep and goat herds kept alongside other livestock species had higher brucellosis seroprevalence than herds kept solely, where the owner did not own any other livestock species besides sheep and goats.
The introduction of new animals from unscreened herds into sheep and goat herds was a major risk factor observed in this study. Pastoralists usually introduce these animals into the herds as replacement stock through purchases, gifts, or donations from relatives. This finding is consistent with the findings of several authors who discovered that the introduction of animals from non-free Brucellosis herds or herds with unknown Brucellosis status was a major factor associated with Brucellosis in sheep, goat, and cattle herds (50, 54–57).
Other research suggests that introducing infected animals can increase individual-level prevalence because the longer they are in contact with the rest of the herds, the greater the risk of spread (1, 58). Animal movement between herds has also been found to be a potentially dangerous practice. One suggested key preventive measure is to avoid the introduction of infected animals by maintaining completely closed herds or by carefully screening purchased animals before introducing them into the herds, a practice that is very uncommon in pastoral communities. There is evidence that one of the main causes of most brucellosis control campaigns’ ineffectiveness is the lack of control over the movement of animals, and this suggestion is supported by available data (12, 50, 56).
Seroprevalence was higher in herds with female animals that had abortions in the previous year compared to herds without abortions, but this difference was not statistically significant. Tigist et al. (47) and Obonyo (59) revealed a statistically significant correlation between a herd’s seropositivity to Brucella and the presence of female animals that had recently given birth. Brucellosis causes late-term abortions, which increases the risk of the disease spreading to other animals in the herds while they graze on the contaminated pasture lands because aborting animals typically shed the bacteria into the environment. Abortion represents the major complaint attributed to Brucella infections in livestock (4, 50–52, 60). It is known that females infected with brucellosis shed considerable amounts of the pathogen in milk, placental membranes, and aborted fetuses. Such females have been reported to shed organisms for several months (4, 48, 60). This causes environmental contamination, which increases the risk of pathogen transmission between animals in the same herds as well as other herds during free mixing in grazing and watering areas.
Lending or sharing male animals with other herds for natural breeding purposes was significantly associated with herd seropositivity. Other authors have reported that lending male animals for breeding is a risk factor for Brucella seropositivity in animals (59, 61). Although the venereal route is not regarded as a key channel for Brucella transmission in small ruminants under natural settings, procedures that entail the movement of animals between herds s are deemed problematic because of the possibility of mechanical transmission (5, 12, 48).
Although not significant, seropositivity was higher in herds that came into contact with other herds in the past year. This could be attributed to the pastoral lifestyle, which is characterized by the frequent mobility of herds. Considering the contagious nature of Brucella spp. sharing shared grazing areas and drinking holes makes it easier for possibly infected cows and clean herds to spread infections such as brucellosis and others (14, 62).
The current study was limited to the seroprevalence of the small ruminant brucellosis only and did not include other such as cattle and camels are susceptible to brucellosis and both species are kept in the study area but they were not part of the current study. The study also did not attempt to assess the prevalence level of the disease in humans to correlate findings in the animals. The present study did not attempt culture of Brucella species and therefore was not able to identify the various species and biovars of Brucella species circulating in sheep and goats in the study area.
The present study revealed that the seroprevalence of brucellosis in sheep and goats was found to be relatively low at both the individual animal and the herd level. However, the multivariable logistic regression analysis revealed that none of the proposed risk factors were not significantly associated with Brucella seropositivity at individual and herd level. In conclusion, awareness campaign among pastoral community of the seriousness of the causes, modes of transmission, symptoms, risk factors, and methods of prevention of the disease should be undertaken as soon as possible. For effective control of brucellosis that may be present in the area, an integrated approach should be promoted that takes into account the relationship between humans, animals, and the environment in the context of “One Health approach.”
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal studies were approved by the Jigjiga University, College of Veterinary Medicine-Research Review Committee (Protocol No. JJU/CVM/clis/022/14). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
AMH, FA, AHH, AM, and HG contributed to data collection, study design, and interpretation, manuscript draft, and writing. AMH, FA, and AHH contributed to the conception of the research idea, study design, data analysis, writing, revising and editing, the design of the study, and data interpretation. AM and HG contributed to the reference search and manuscript writing and editing. All authors approved the submission of the final manuscript.
This study was funded by the Jigjiga University Research and Community Service Vice President Office.
We are grateful to Jigjiga University for funding the research project, and we would like to thank the staff of Jigjiga Regional Veterinary Diagnostic and Investigation Laboratory, who greatly assisted me in laboratory sample collection, storage, and some serological procedures.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BOFED, Bureau of Finance and Economic Development; CAT, Card agglutination test; CFT, Complement fixation test; CFSPH, Center for Food Security and Public Health; ELISA, Enzyme-linked immunosorbent assay; ICSP, International Committee on Systematics of Prokaryotes; LPDB, Livestock and Pastoralist Development Bureau; NVI, National Veterinary Institute; OIE, Office International des Epizooties; RBPT, Rose Bengal Plate Test; SRBC, Sensitized sheep red blood cells; SRS, Somali regional state; WHO, World Health Organization.
2. El-Sayed, A , and Awad, W . Brucellosis: evolution and expected comeback. Int J Vet Sci Med. (2018) 6:S31–5. doi: 10.1016/j.ijvsm.2018.01.008
3. Moreno, E , and Moriyón, I . The genus Brucella. Prokaryotes. (2006) 5:315–456. doi: 10.1007/0-387-30745-1_17
4. Perez, A , and Berhe, M . Brucella, a bacterium with multiple ways of causing infection. Baylor University Medical Center Proceedings Taylor & Francis (2021).
5. Seleem, MN , Boyle, SM , and Sriranganathan, N . Brucellosis: a re-emerging zoonosis. Vet Microbiol. (2010) 140:392–8. doi: 10.1016/j.vetmic.2009.06.021
6. Whatmore, AM , Koylass, MS , Muchowski, J , Edwards-Smallbone, J , Gopaul, KK , and Perrett, LL . Extended multilocus sequence analysis to describe the global population structure of the genus Brucella: phylogeography and relationship to biovars. Front Microbiol. (2016) 7:2049. doi: 10.3389/fmicb.2016.02049
7. Assenga, JA , Matemba, LE , Malakalinga, JJ , Muller, SK , and Kazwala, RR . Quantitative analysis of risk factors associated with brucellosis in livestock in the Katavi-Rukwa ecosystem, Tanzania. Trop Anim Health Prod. (2016) 48:303–9. doi: 10.1007/s11250-015-0951-z
8. Barril, BP . Francisella, Brucella and Pasteurella In: S Baron , editor. Medical Microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch at Galveston (1996)
9. Ragan, VE . Infectious agents: brucellosis. Bovine Reprod. (2021):742–52. doi: 10.1002/9781119602484.ch60
10. Bosilkovski, M . Brucellosis: it is not only Malta! In: Zoonoses-infections affecting humans and animals : 2nd edn, John Wiley & Sons, Inc. Springer Dordrecht (2015). 287–315. doi: 10.1007/978-94-017-9457-2_11
11. Smits, HL , and Kadri, SM . Brucellosis in India: a deceptive infectious disease. Indian J Med Res. (2005) 122:375–84.
12. Cutler, S , Whatmore, A , and Commander, N . Brucellosis–new aspects of an old disease. J Appl Microbiol. (2005) 98:1270–81. doi: 10.1111/j.1365-2672.2005.02622.x
13. Desta, KB . Analyses of dairy cattle breeding practices in selected areas of Ethiopia. Faculty of Agriculture and Horticulture, the Humboldt University of Berlin, Germany Köster (2002).
14. Megersa, B , Biffa, D , Abunna, F , Regassa, A , Godfroid, J , and Skjerve, E . Seroprevalence of brucellosis and its contribution to abortion in cattle, camel, and goat kept under pastoral management in Borana, Ethiopia. Trop Anim Health Prod. (2011) 43:651–6. doi: 10.1007/s11250-010-9748-2
15. Tesfaye, A , Dejene, H , Admassu, B , Kassegn, TA , Asfaw, D , Dagnaw, GG, et al. Seroprevalence of bovine brucellosis in Ethiopia: systematic review and meta-analysis. Vet Med. (2021) 12:1–6. doi: 10.2147/VMRR.S289721
16. Yohannes, M , Degefu, H , Tolosa, T , Belihu, K , Cutler, RR , and Cutler, S . Brucellosis in Ethiopia. Afr J Microbiol Res. (2013) 7:1150–7. doi: 10.5897/AJMR12.738
17. Degefu, H , Mohamud, M , Hailemelekot, M , and Yohannes, M . Seroprevalence of bovine brucellosis in agro pastoral areas of Jijjiga zone of Somali National Regional State, eastern Ethiopia. Ethiop Vet J. (2011) 15: 37–47. doi: 10.4314/evj.v15i1.67683
18. Wedajo, MT , Fekadu, RG , Tefera, YM , Yalew, TA , Alemayehu, LB , and Abadi, AR . Seroprevalence of small ruminant brucellosis and its effect on reproduction at Tellalak District of Afar region, Ethiopia. J Vet Med Anim Health. (2015) 7:111–6. doi: 10.5897/JVMAH2014.0287
19. Mohammed, M , Mindaye, S , Hailemariam, Z , Tamerat, N , and Muktar, Y . Sero-prevalence of small ruminant brucellosis in three selected districts of Somali region, eastern Ethiopia. J Vet Sci Anim Hus. (2017) 5:105. doi: 10.15744/2348-9790.5.105
20. Kansiime, C , Mugisha, A , Makumbi, F , Mugisha, S , Rwego, IB , Sempa, J, et al. Knowledge and perceptions of brucellosis in the pastoral communities adjacent to Lake Mburo National Park, Uganda. BMC Int Health Hum Rights. (2014) 14:1–11. doi: 10.1186/1471-2458-14-242
21. Tsehay, H , Getachew, G , Morka, A , Tadesse, B , and Eyob, H . Seroprevalence of brucellosis in small ruminants in pastoral areas of Oromia and Somali regional states, Ethiopia. J Vet Med Anim Health. (2014) 6:289–94. doi: 10.5897/JVMAH2014.0331
22. Megersa, B , Biffa, D , Niguse, F , Rufael, T , Asmare, K , and Skjerve, E . Cattle brucellosis in traditional livestock husbandry practice in southern and eastern Ethiopia, and its zoonotic implication. Acta Vet Scand. (2011) 53:1–8. doi: 10.1186/1751-0147-53-24
23. Racloz, V , Schelling, E , Chitnis, N , Roth, F , and Zinsstag, J . Persistence of brucellosis in pastoral systems. OIE Rev Sci Tech. (2013) 32:61–70. doi: 10.20506/rst.32.1.2186
25. Central Statistics Agency . Federal Democratic Republic of Ethiopia agricultural sample survey In: Report on livestock and livestock characteristics, statistical bulletin, 585. Addis Ababa: Central Statistics Agency (2019)
26. Thrusfield, M . Veterinary epidemiology 4th Ed. Oxford, United Kingdom:John Wiley & Sons (2018).
27. Office International des Epizooties (OIE) . Terrestrial manual, bovine brucellosis: Manual of diagnostic tests and vaccines for terrestrial animals. Paris: OIE (2009).
28. Tsegay, A , Tuli, G , Kassa, T , and Kebede, N . Seroprevalence and risk factors of brucellosis in small ruminants slaughtered at Debre Ziet and Modjo export abattoirs, Ethiopia. J Infect Dev Countr. (2015) 9:373–80. doi: 10.3855/jidc.4993
29. Dabassa, G , Tefera, M , and Addis, M . Small ruminant brucellosis: serological survey in Yabello District, Ethiopia. Asian J Anim Sci. (2013) 7:14–21. doi: 10.3923/ajas.2013.14.21
30. Aloto, D , Abebe, R , and Megersa, B . Sero-epidemiology of brucellosis in small ruminants, and herder’s knowledge and risky practices in southern Ethiopia. Small Rumin Res. (2022) 216:106785. doi: 10.1016/j.smallrumres.2022.106785
31. Dosa, D , Mohammed, N , and Mathewos, M . Study on small ruminant brucellosis and owners awareness in two selected districts of southern region, Ethiopia. Vet Med Sci. (2023) 9:907–16. doi: 10.1002/vms3.992
32. Teshome, D , Sori, T , Banti, T , Kinfe, G , Wieland, B , and Alemayehu, G . Prevalence and risk factors of Brucella spp. in goats in Borana pastoral area, southern Oromia, Ethiopia. Small Rumin Res. (2022) 206:106594. doi: 10.1016/j.smallrumres.2021.106594
33. Deddefo, A , Sisay, T , and Tuli, G . Seroprevalence and risk factors of small ruminant brucellosis in selected districts of Arsi and east Shoa zones, Oromia region, Ethiopia. AJMR. (2015) 9:1338–44. doi: 10.5897/AJMR2015.7400
34. Teshale, S , Muhie, Y , Dagne, A , and Kidanemariam, A . Seroprevalence of small ruminant brucellosis in selected districts of Afar and Somali pastoral areas of eastern Ethiopia: the impact of husbandry practice. Rev Med Vet. (2006) 157:557.
35. Negash, E , Shimelis, S , and Beyene, D . Seroprevalence of small ruminant brucellosis and its public health awareness in selected sites of Dire Dawa region, eastern Ethiopia. J Vet Med Anim Health. (2012) 4:61–6.
36. Ferede, Y , Mengesha, D , and Mekonen, G . Study on the seroprevalence of small ruminant brucellosis in and around Bahir Dar, north West Ethiopia. Ethiop Vet J. (2011) 15:35–44. doi: 10.4314/evj.v15i2.67692
37. Tewodros, A , and Dawit, A . Sero-prevalence of small ruminant brucellosis in and around Kombolcha, Amhara regional state, north-eastern Ethiopia. J Vet Sci Med Diagn. (2015) 4:1–4.
38. Nielsen, K . Diagnosis of brucellosis by serology. Vet Microbiol. (2002) 90:447–59. doi: 10.1016/S0378-1135(02)00229-8
39. Tumwine, G , Matovu, E , Kabasa, JD , Owiny, DO , and Majalija, S . Human brucellosis: sero-prevalence and associated risk factors in agro-pastoral communities of Kiboga District, Central Uganda. BMC Int Health Hum Rights. (2015) 15:1–8. doi: 10.1186/s12889-015-2242-z
40. Ashenafi, F , Teshale, S , Ejeta, G , Fikru, R , and Laikemariam, Y . Distribution of brucellosis among small ruminants in the pastoral region of Afar, eastern Ethiopia. Rev Sci Tech. (2007) 26:731. doi: 10.20506/rst.26.3.1781
41. Mengistu, M . Sero epidemiology of brucellosis in small ruminants in southern Ethiopia [Master’s thesis]. Debrezeit, Ethiopia: Addis Ababa University (2007).
42. Bekele, T , and Kasali, O . Brucellosis in sheep and goats in Central Ethiopia. Bull Anim Health Prod Afr. (1990) 38:23–5.
43. Samaha, H , Al-Rowaily, M , Khoudair, RM , and Ashour, HM . Multicenter study of brucellosis in Egypt. Emerg Infect Dis. (2008) 14:1916. doi: 10.3201/eid1412.071452
44. Yesuf, M , Alemu, S , Temesgen, W , Mazengiac, H , and Negussie, H . Seroprevalence of ovine brucellosis in south Wollo, north eastern Ethiopia. Am Eurasian J Agric Environ Sci. (2010) 9:288–91.
45. Hirsh, D , and Zee, Y . Veterinary microbiology. 2nd ed. Oxford: Black well Scientific Publications (1999).
46. Adugna, W , Tessema, TS , and Keskes, S . Sero-prevalence of small ruminants brucellosis in four districts of Afar National Regional State, Northeast Ethiopia. J Vet Med Anim Health. (2013) 5:358–64.
47. Tigist, A , Yosefe, D , and Tadele, T . Seroprevalence of caprine brucellosis and associated risk factors in south Omo zone of southern Ethiopia. Afr J Microbiol Res. (2011) 5:1682–5. doi: 10.5897/AJMR11.377
48. Radostits, OM , Gay, CC , Hinchcliff, KW , and Constable, PD . Veterinary medicine E-book: a textbook of the diseases of cattle, horses, sheep, pigs and goats. London, United Kingdom:Elsevier Health Sciences (2007).
49. Wubishet, Z , Golo, D , Chala, F , Shubisa, A , Huqa, L , Godana, H, et al. Sero-prevalence of brucellosis in goats and community awareness in Liban district of Guji zone, Oromia regional state, southern Ethiopia. JOJ Pub Health. (2017) 2:3–6. doi: 10.19080/JOJPH.2017.02.555587
50. Kabagambe, E , Elzer, P , Geaghan, J , Opuda-Asibo, J , Scholl, D , and Miller, J . Risk factors for Brucella seropositivity in goat herds in eastern and western Uganda. Prev Vet Med. (2001) 52:91–108. doi: 10.1016/S0167-5877(01)00251-3
51. Muma, J , Samui, K , Oloya, J , Munyeme, M , and Skjerve, E . Risk factors for brucellosis in indigenous cattle reared in livestock–wildlife interface areas of Zambia. Prev Vet Med. (2007) 80:306–17. doi: 10.1016/j.prevetmed.2007.03.003
52. Musa, M , Eisa, M , El Sanousi, EM , Wahab, MA , and Perrett, L . Brucellosis in camels (Camelus dromedarius) in Darfur, western Sudan. J Comp Pathol. (2008) 138:151–5. doi: 10.1016/j.jcpa.2007.10.005
53. Walker, RL . Brucella In: CH Dwright and ZY Chunge, editors. Veterinary microbiological science. Massachusetts: Blackwell’s (1999). 196–203.
54. Bamaiyi, PH , Hassan, L , Khairani-Bejo, S , ZainalAbidin, M , Ramlan, M , Krishnan, N, et al. Case–control study on risk factors associated with Brucella Melitensis in goat farms in peninsular Malaysia. Trop Anim Health Prod. (2014) 46:739–45. doi: 10.1007/s11250-014-0557-x
55. Coelho, AM , Coelho, AC , Góis, J , de Lurdes, PM , and Rodrigues, J . Multifactorial correspondence analysis of risk factors for sheep and goat brucellosis seroprevalence. Small Rumin Res. (2008) 78:181–5. doi: 10.1016/j.smallrumres.2008.04.007
56. Refai, M . Incidence and control of brucellosis in the near east region. Vet Microbiol. (2002) 90:81–110. doi: 10.1016/S0378-1135(02)00248-1
57. Sharifi, H , Mashayekhi, K , and Tavakoli, MM . Risk facts of small ruminant brucellosis: a cross-sectional study in Southeast Iran 2012. Hum Vet Med. (2015) 7:42–5.
58. Rahman, AA , Saegerman, C , Berkvens, D , Fretin, D , Gani, MO , Ershaduzzaman, M, et al. Bayesian estimation of true prevalence, sensitivity and specificity of indirect ELISA, rose Bengal test and slow agglutination test for the diagnosis of brucellosis in sheep and goats in Bangladesh. Prev Vet Med. (2013) 110:242–52. doi: 10.1016/j.prevetmed.2012.11.029
59. Obonyo, MO . Sero-prevalence and factors associated with brucellosis in goats and sheep and assessment of pastoralists, knowledge attitude and practices towards brucellosis in Garissa county [Master's thesis in applied epidemiology]. Nairobi: Jomo Kenyatta University of Agriculture and Technology (JKUAT) (2018).
60. Osoro, EM , Munyua, P , Omulo, S , Ogola, E , Ade, F , Mbatha, P, et al. Strong association between human and animal Brucella seropositivity in a linked study in Kenya, 2012–2013. Am J Trop Med Hyg. (2015) 93:224. doi: 10.4269/ajtmh.15-0113
61. al-Majali, AM , Majok, AA , Amarin, NM , and al-Rawashdeh, OF . Prevalence of, and risk factors for, brucellosis in Awassi sheep in southern Jordan. Small Rumin Res. (2007) 73:300–3. doi: 10.1016/j.smallrumres.2007.02.002
Keywords: brucellosis, goat, risk factors, seroprevalence, sheep
Citation: Hussen AM, Alemu F, Hasan Hussen A, Mohamed AH and Gebremeskel HF (2023) Herd and animal level seroprevalence and associated risk factors of small ruminant brucellosis in the Korahey zone, Somali regional state, eastern Ethiopia. Front. Vet. Sci. 10:1236494. doi: 10.3389/fvets.2023.1236494
Received: 08 June 2023; Accepted: 21 August 2023;
Published: 19 September 2023.
Edited by:
Roswitha Merle, Freie Universität Berlin, GermanyReviewed by:
Siti Zubaidah Ramanoon, Universiti Putra Malaysia, MalaysiaCopyright © 2023 Hussen, Alemu, Hasan Hussen, Mohamed and Gebremeskel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haben Fesseha Gebremeskel, aGFiZW4uc2VuYmV0dUB3c3UuZWR1LmV0; dHNleW9uLmhAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.