- 1Global Burden of Animal Diseases (GBADs) Programme, University of Liverpool, Liverpool, United Kingdom
- 2Department of Microbiology, Immunology and Veterinary, Public Health, College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Ethiopia
- 3Animal and Human Health Program, International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia
- 4Department of Livestock and One Health, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom
- 5Modelling, Evidence and Policy Group, School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, United Kingdom
- 6Department of Biomedical Sciences, College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Ethiopia
- 7Food and Markets Department, Natural Resources Institute, University of Greenwich, London, United Kingdom
- 8Animal and Human Health Program, International Livestock Research Institute (ILRI), Nairobi, Kenya
This scoping review identifies and describes the methods used to prioritize diseases for resource allocation across disease control, surveillance, and research and the methods used generally in decision-making on animal health policy. Three electronic databases (Medline/PubMed, Embase, and CAB Abstracts) were searched for articles from 2000 to 2021. Searches identified 6, 395 articles after de-duplication, with an additional 64 articles added manually. A total of 6, 460 articles were imported to online document review management software (sysrev.com) for screening. Based on inclusion and exclusion criteria, 532 articles passed the first screening, and after a second round of screening, 336 articles were recommended for full review. A total of 40 articles were removed after data extraction. Another 11 articles were added, having been obtained from cross-citations of already identified articles, providing a total of 307 articles to be considered in the scoping review. The results show that the main methods used for disease prioritization were based on economic analysis, multi-criteria evaluation, risk assessment, simple ranking, spatial risk mapping, and simulation modeling. Disease prioritization was performed to aid in decision-making related to various categories: (1) disease control, prevention, or eradication strategies, (2) general organizational strategy, (3) identification of high-risk areas or populations, (4) assessment of risk of disease introduction or occurrence, (5) disease surveillance, and (6) research priority setting. Of the articles included in data extraction, 50.5% had a national focus, 12.3% were local, 11.9% were regional, 6.5% were sub-national, and 3.9% were global. In 15.2% of the articles, the geographic focus was not specified. The scoping review revealed the lack of comprehensive, integrated, and mutually compatible approaches to disease prioritization and decision support tools for animal health. We recommend that future studies should focus on creating comprehensive and harmonized frameworks describing methods for disease prioritization and decision-making tools in animal health.
Introduction
Livestock production is an economic process in which resources (inputs) are converted into products (outputs) (1). Livestock plays an important economic role across the world, especially in developing countries, as one of the main sources of livelihood (2). However, the livestock sector is constrained by many factors, including disease, which limits livestock production and productivity (3). Diseases negatively influence the conversion of inputs into outputs in the livestock industry, causing direct and indirect costs (4). Direct costs include mortality, reduction in the efficiency of production processes (e.g., reduced feed conversion), and reduced quantity or quality of products. Indirectly, the cost of a disease is associated with additional expenses due to disease management (vaccinate, treat, or control), public health impacts, and suboptimal use of resources, e.g., feed and water, due to infestation by disease vectors in specific localities (5).
Profitable investment in livestock production requires targeted control and prevention strategies, treatment, and surveillance of important diseases (6). Investment in mitigation of livestock disease and improvements in animal health aim to reduce production losses and minimize control costs. However, deciding where best to invest to optimize returns is complex in a livestock system that is often part of more complex systems; decisions for the farm may be made at the household level and across a range of different options at the policy level. Furthermore, there are various sociocultural and non-financial aspects to be considered in both farm- and national-level animal health control activities. A multitude of diseases and pathogens affect animals, including humans, and control efforts must be prioritized, given there are limited available resources (e.g., time, financial resources, etc.), to ensure optimal resource allocation (7, 8). The nature of the impacts of diseases varies with the pathogen and the livestock system affected. In some cases, the indirect costs of livestock diseases, especially zoonotic diseases, through their impacts on other sectors are much greater than the impact on livestock productivity. The costs of zoonoses are mainly due to non-livestock losses (9). The reaction to the presence of non-zoonotic diseases has major effects on the overall burden of disease; for example, the 2001 foot-and-mouth disease (FMD) outbreak in the UK, where there was a significant reduction in income due to shutting down the countryside and loss of tourism (10). Similarly, FMD control measures in Southern Africa had huge impacts on wildlife ecology and marginalized smallholders (11). However, endemic parasitic worm infections impact mostly through direct impacts on productivity and control costs (12), albeit the impacts of these farm-level productivity losses are rarely translated into wider economic impacts that affect downstream actors in the food system and consumers.
The basic principle of prioritizing different diseases within animal health is to maximize net benefits from allocating resources compared with the opportunity costs of alternative resource use (8). This ensures appropriate resource allocation within targeted actions, maximizing the potential benefits to animal health, public health, and the economy. The development and use of prioritization methods within human healthcare started formally several decades ago; infectious disease control and surveillance measures were targeted under the condition that not all diseases should be given equal weights for prevention and control (13). In animal health, various methods have been developed for disease priority setting and resource allocation programs. These include quantitative and qualitative approaches, such as decision tree analysis, expert opinion elicitation, risk-based assessment, semi-quantitative and quantitative scoring frameworks, and multi-criteria decision tools (8, 14–18).
However, despite its importance, the broad topic of disease prioritization for animal health priority setting lacks structure, and it exists as a range of ad hoc disparate tools and projects. This scoping review aims to collect the approaches used in disease prioritization and tools for animal decision-making processes. The concept of this study was developed with a view to designing a comprehensive framework for animal health decision-making and resource allocation.
Methods
A scoping review investigating methodological approaches to animal health priority setting and decision-making tools was carried out according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-SLR) extension for Scoping Reviews (19). In the review, prioritization was considered a process to decide the relative importance of animal health issues (e.g., the importance of diseases and their pathogenic causes, strategies for prevention, surveillance, control or research, drug treatment choices, etc.). In addition, studies that generated evidence to support decision-making, such as economic analysis, risk assessment, and spatial mapping regarding animal diseases, were also included. Therefore, the review included studies that actually or potentially assisted decisions to optimize resource use for animal disease mitigation.
Information sources and search strategy of articles
Major bibliographic databases were systematically searched using a specific syntax for the articles retrieved. Three electronic databases (Medline/PubMed, Embase, and CAB Abstracts) were examined using the search syntax (Supplementary material 1). Keywords for the searches were identified by a detailed review of the contents of selected articles. Keywords were refined by searching for synonyms and formatted using Boolean operators. Initially, language filters were not applied, but English language literature was targeted during data extraction, and other languages were also included where articles had abstracts available in English.
Eligibility criteria (exclusion and inclusion criteria)
Eligibility criteria defining articles to be included or excluded were developed and applied. Studies had to report “real-life” (not theoretical) application of prioritization methodologies for animal diseases, generating evidence for prioritization or decision-making by targeting the strategies identified for disease control, prevention, or surveillance at various levels (single organization or farm, local, sub-national, national, or international). Publications written in English from 1 January 2000 to 13 August 2021 (last date of search) were included. Discussion papers, reviews, and commentaries not addressing a “real-life” prioritization exercise and not implicating prioritization methods directly were not included. Studies focusing on theoretical prioritization issues (e.g., methodology development without presenting a case study on the application of the developed methods) were excluded. Prioritization studies of purely human diseases (including zoonotic diseases originating in animals but currently transmitting from human to human such as HIV/AIDS) were excluded. However, articles presenting a mix of animal and purely human diseases were considered, taking into account the animal aspects as inclusion criteria.
Literature screening based on inclusion/exclusion criteria
Literature screening was based on the article title and abstract and was carried out by two independent reviewers, with disagreements resolved by discussion. If article abstracts were not available, the full text was screened. During the first-round screening of literature, the aforementioned eligibility criteria were used. Additionally, the geographic range covered was recorded (local, sub-national, national, and global).
In the second round of screening, retained articles were categorized based on (1) the prioritization or priority setting methods used (economic-based, simple ranking of diseases, multi-criteria, qualitative risk assessment, quantitative risk analysis, spatial (geographic) risk mapping, mix of different prioritization techniques, and other) and (2) the prioritization purpose—what the prioritization was designed to assess (disease control and prevention strategies, general importance of diseases or animal health issues, risk of disease prioritization, spatial risk mapping or analysis, disease surveillance, and research priority settings).
Article selection process and data extraction process
The information extracted from the articles included the full bibliographic citation of the retrieved article (including authors, titles, year of publication, and journal), types of prioritizations or types of studies for evidence generation (ranging from economic, multi-criteria, spatial, risk assessment, mathematical modeling, or simple ranking), the continent of the article covered (when specified), and the geographic ranges of the focus of the articles (local to global). The different categories and sub-categories of the prioritization techniques were identified during screening and the extraction of information from the initial articles when preparing a template for full data extraction. The outputs in line with the purposes or objectives of the prioritization or evidence generation for decision-making were classified into various groups, which include general disease importance, assessment of risks (new introduction or spatial risk), and research priority setting. Species of animals and diseases or health issues mentioned in each article were also extracted. The categorization was carried out to determine how the outputs will inform users and influence decision-makers in the sense of what the prioritization process or analysis ultimately answers. The diseases, pathogens, or general health issues under consideration in each article have been extracted and presented as priorities in the form of study outputs. Species of animals considered in each article were also extracted when specified as a single species or a group of animals (e.g., ruminants, wildlife, small ruminants, equine, etc.). Species of animals described in the articles were extracted when applicable, if not presented as animals or livestock in general. The study could be focused on general livestock, specific animal species, or multiple species. Some health situations were described without mentioning diseases.
Data extraction was carried out using a form prepared in Kobotoolbox (www.kobotoolbox.org, an online data collection tool). A template for extraction was developed and pre-tested on 2–3 articles in each of the different categories. The online data capturing tool was used instead of a spreadsheet (Excel) due to its convenience for designing the formats using logics (e.g., skip patterns) and adding entries collaboratively. The online tool can accommodate complex formats, including uploading files to the system. Finally, the extracted data were exported into a spreadsheet and managed.
Results
Retrieved and screened articles
The online search identified 6, 726 articles, and upon manual searching, an additional 64 articles were added and 330 were removed due to duplicates. A total of 6, 460 articles were finally imported into online systematic review management software (20) for screening. Based on inclusion and exclusion criteria, 532 articles passed the first screening, and after a second round of screening, 336 were recommended for full review. In the preparation for data extraction, upon downloading the articles, 40 articles were excluded due to lack of full text, and 11 articles were added by snowballing from the reference lists of already identified articles. Thus, the total number of articles for final inclusion and full review was 307 (Figure 1).
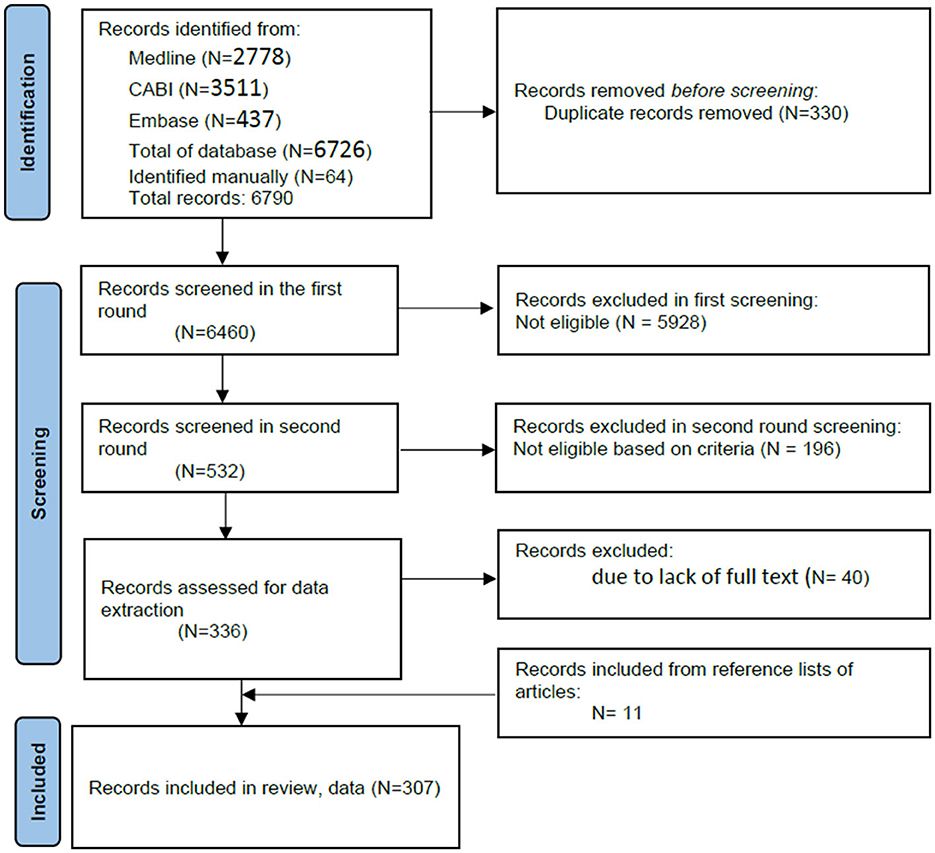
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram depicting the number of articles retrieved and screened sequentially (selected for full-text download, data extraction, and quality check) describing animal disease prioritization methods and processes (n = 307).
Categories of disease or animal health issues prioritization methods, yearly publication, and continental geographic distribution
Of the 307 retained articles that were fully reviewed, the results were presented under the following methods categories: (a) 96 articles on economic analysis (21–116), (b) 59 articles on multi-criteria prioritization (14, 117–173), (c) 57 articles on risk assessment (174–230), (d) 38 articles on spatial analysis (231–268), (e) 27 articles on the simple ranking of disease or animal health issues (269–295), (f) 17 articles on mathematical modeling (296–312), and (g) 13 other articles (313–325) (Table 1).
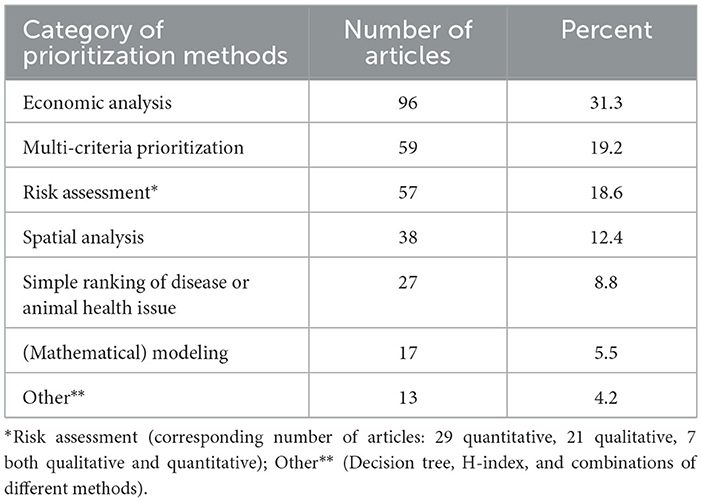
Table 1. Categories of disease or animal health issue prioritization methods in retained papers (n = 307).
The annual number of publications relevant to this review increased over time, with articles using multi-criteria prioritization methods available from 2010 onward and economic analyses more evenly distributed over the duration of the two decades of publication (Figure 2).
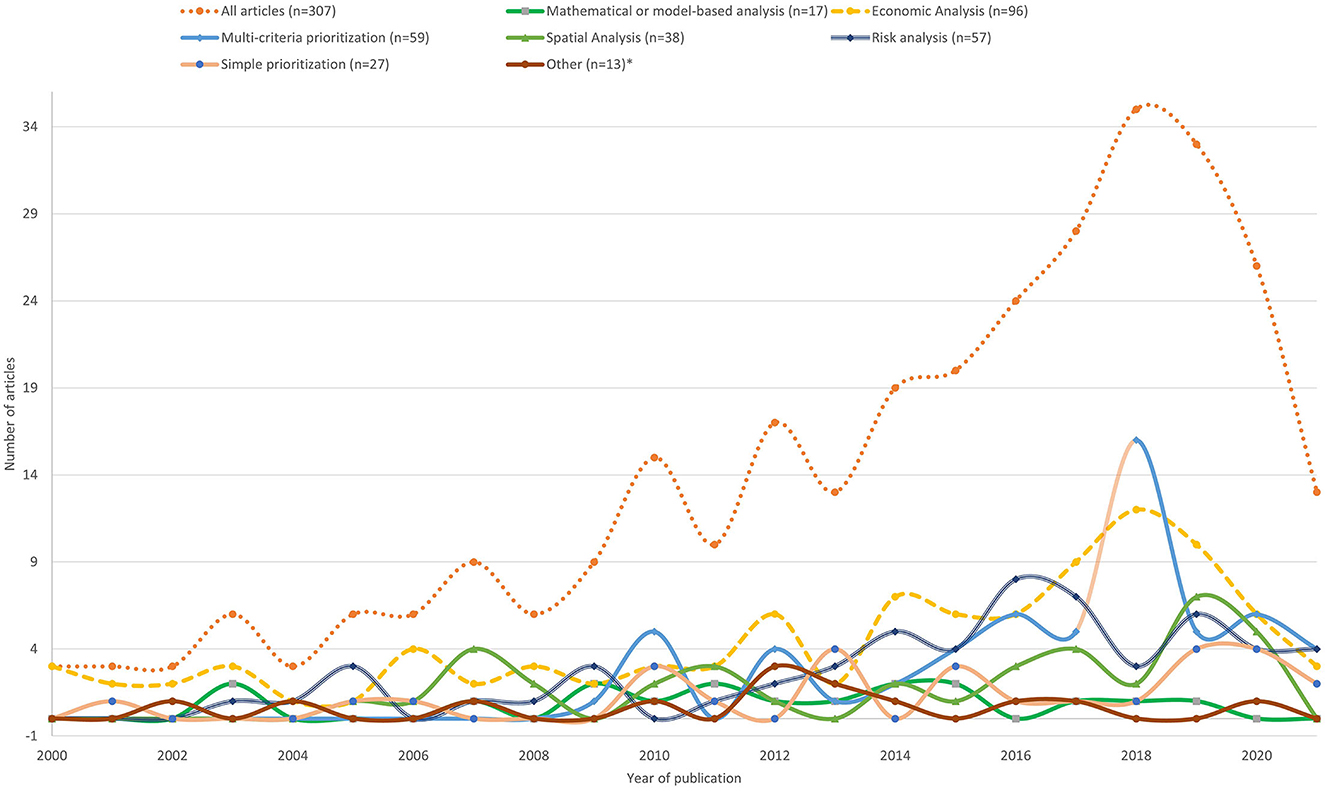
Figure 2. Annual (2000–2021) distribution of the articles included for data extraction disaggregated by animal health (disease) prioritization method (n = 307); *(decision tree, H-index, and combinations of different methods).
The geographic focus of the articles was Europe (35.8%), followed by Africa (18.9%) and then North America (13.0%). European disease prioritization and evidence for decision-making studies focused on risk assessment (52.6%), and African studies mostly (63.0%) used simple ranking of diseases or health problems. Half of the articles (50.8%) focused on national-level prioritization or evidence generation for decision-making, and this figure increased to nearly 60% when multi-criteria methods were used. For articles describing spatial analysis methods, 47.5% of them focused on the national level. The number of articles describing global-level prioritizations was very limited (n = 13) (Table 2).
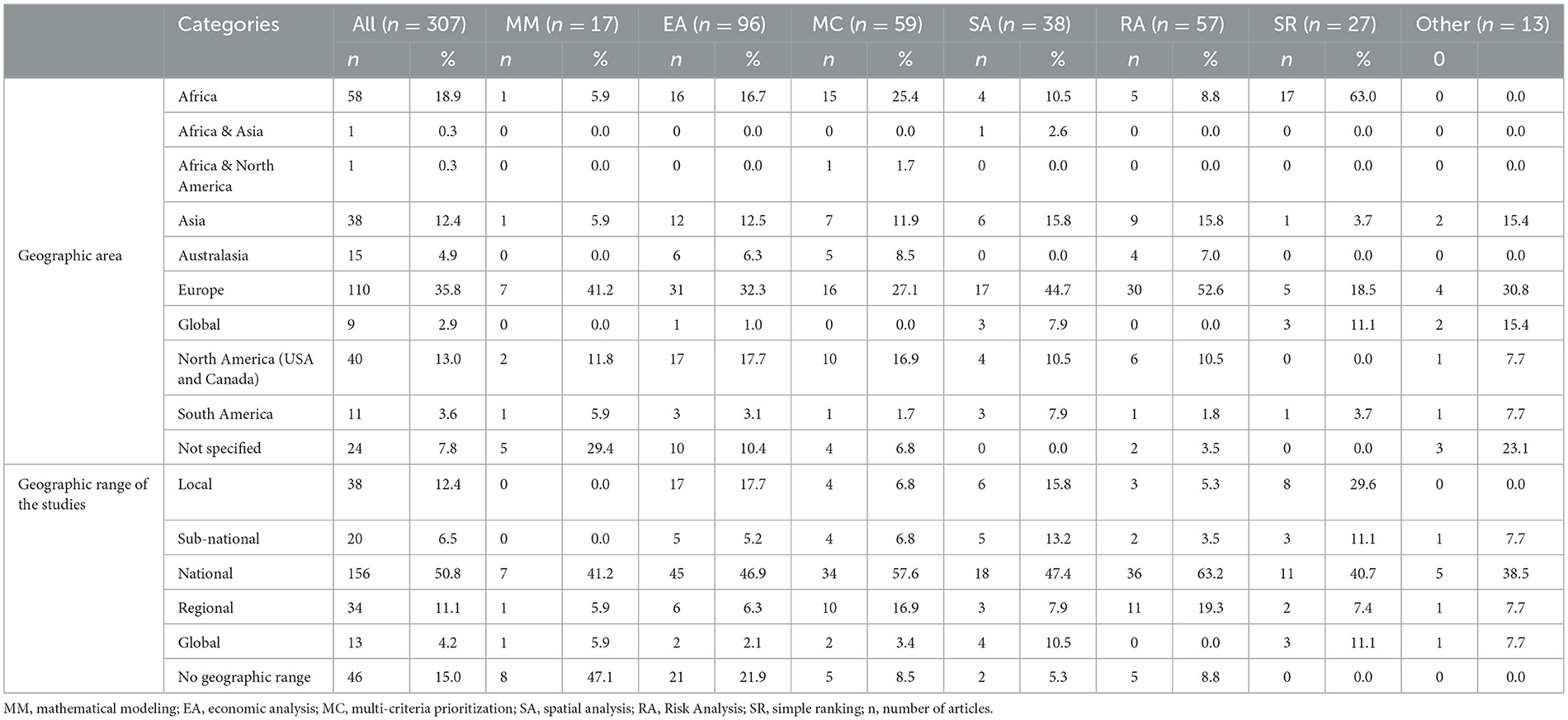
Table 2. Number of articles and methods used for prioritization disaggregated by continent and geographic focus.
Purposes of prioritization
Of the 307 articles reviewed, 39.1% were focused on prioritizations to help target disease control, prevention, or eradication strategies, and 23.0% were to aid in the identification of priority diseases to inform general organizational strategy (Table 3).
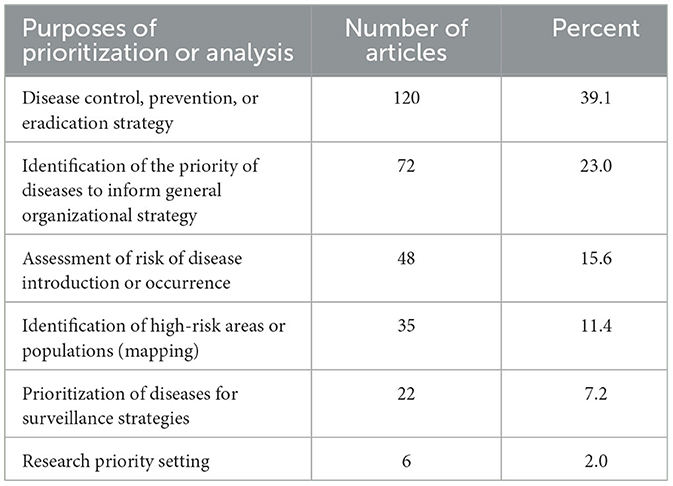
Table 3. The purposes of the prioritizations described in the articles presented in this scoping review (n = 307).
Sub-categories of the methods of multi-criteria prioritization and economic analysis
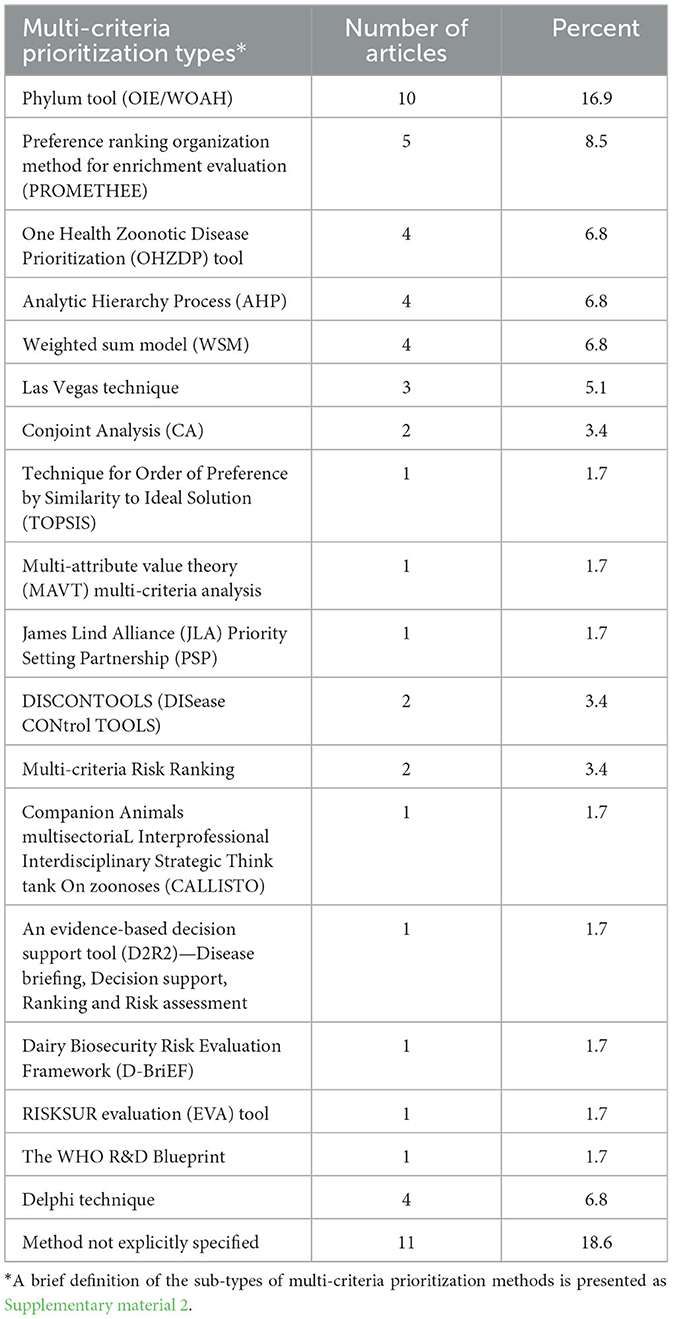
This part of our study describes the sub-categories of prioritization and evidence-generating studies (economic analysis, multi-criteria prioritization, and risk assessment). Different sub-types of multi-criteria prioritization studies largely based on weighting techniques were identified (Table 4). The common technique applied was the Phylum applied in studies in eastern African countries. The brief definitions of the different sub-categories are described in Supplementary material 2.
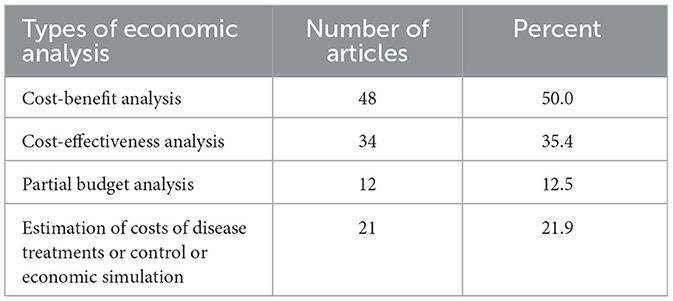
For economic analysis, cost-benefit analysis was the most commonly used economic analysis method for prioritization (48 articles), followed by cost-effectiveness analysis (34 articles). The use of partial budget analysis was reported in 12 articles, respectively (Table 5).
Out of the total articles dealing with risk assessment, 29, 21, and 7 articles used qualitative, quantitative, and both (qualitative and quantitative), respectively.
Prioritized diseases
The importance of different diseases was examined in prioritization exercises involving different methods (Table 6). Paratuberculosis was most commonly examined (10 out of 96 articles) within economic analyses, with rabies, brucellosis, and FMD commonly assessed in multi-criteria prioritizations. African swine fever and FMD were most often assessed in risk assessments. FMD was also the most considered disease using simple ranking methods (Table 6).
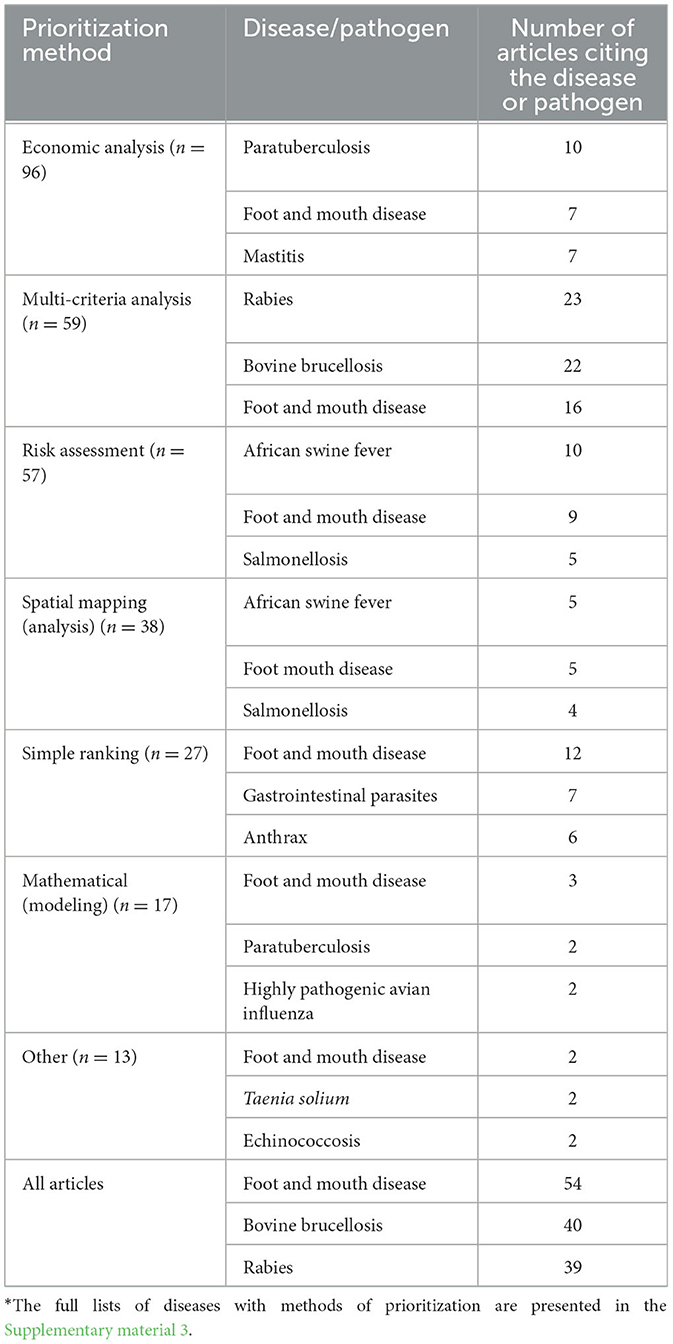
Table 6. The top three diseases or health (with some described by their pathogenic causes) commonly targeted using different disease prioritization methods*.
For all articles combined, FMD (54 articles), bovine brucellosis (40 articles), and rabies (39 articles) were the common diseases considered in the articles. From the total articles, 20 assessed general animal health situations without mentioning specific diseases (Supplementary material 3).
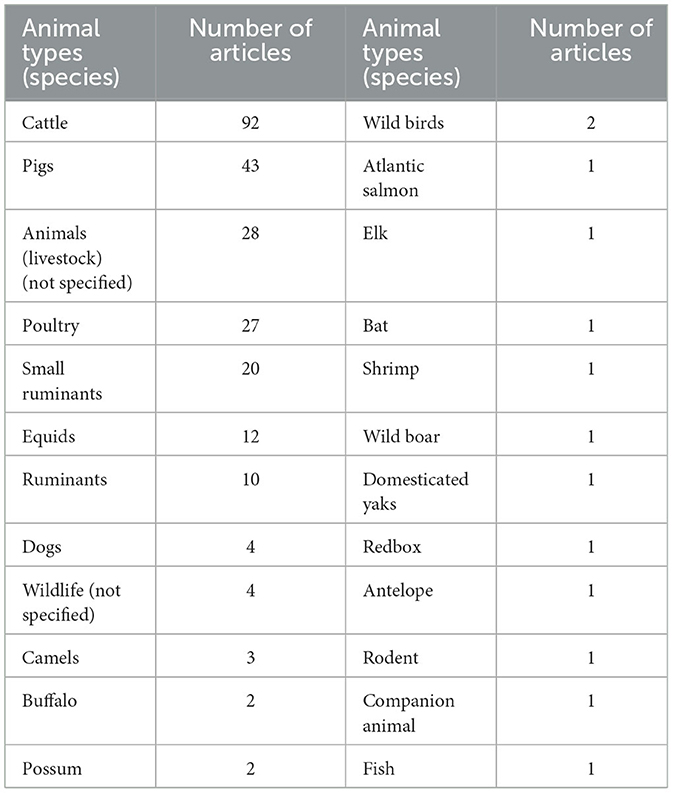
Table 7 depicts the type of animal species or group of species considered in the articles included in the present scoping review. Accordingly, cattle are the most common animals considered by the articles, followed by pigs (Table 7).
Discussion
This scoping review examined published literature describing the prioritization of animal and zoonotic diseases by reviewing a total of 307 published articles. The context of prioritization in the review was taken as studies that either explicitly prioritized the relative importance of diseases, those that prioritized animal health interventions based on one or more criteria, or those generating evidence for wider decision-making. Studies generating evidence used economic analysis, risk assessment (adverse effects of diseases assessed), spatial analysis (risk or disease distribution mapping), or modeling approaches.
In the reviewed articles, those describing economic analysis were dominant, followed by multi-criteria-based prioritizations. Disease prioritization is a complex decision-making process (8), and prioritization based on single approaches such as economic analysis and disease burden estimates may not be rigorous and inclusive enough (326). Health issues are inherently complex, involving an understanding of the economic, social, ethical, and cultural aspects of various stakeholders. These aspects need to be captured in the decision-making process. However, how these different aspects are all considered together, e.g., in multi-criteria prioritization and decision analysis, relies on arbitrary weightings of them; such analytical systems incur costs of time and understanding.
Economic analysis was widely used in prioritization studies by means of a range of approaches. Cost-benefit analysis and cost-effectiveness analysis are most commonly used in animal health, as reported in this and other studies (327). Cost-effectiveness analysis is often used for health interventions that do not have an agreed monetary value, such as human lives or animal welfare. We would expect cost-effectiveness analysis to be used more for zoonoses and diseases with high welfare implications. As an example, cost-effectiveness analysis was not used in any non-zoonotic diseases such as African swine fever, Peste des petits ruminants, and African horse sickness. However, it was used in brucellosis (11.8%), bovine tuberculosis (17.5%), and rabies (17.5%) (Supplementary material 4, raw dataset). Multi-criteria analysis was the second most common approach used for disease prioritization. Developed in the 1960s to aid complex decision-making processes within the environmental, engineering, finance, and management sciences (328, 329), multi-criteria decision analysis combines multiple, often conflicting, alternatives in order to reach a consensus on a given issue (330). The criteria for decision analysis can be measured qualitatively or quantitatively, and the technique has been relatively recently applied within animal health and, particularly, disease control (139). Multi-criteria decision analysis involves multiple steps: (1) identification of pathogens or diseases; (2) selection, weighting, and scoring of criteria; and (3) decision analysis (163). The selection and weighting of criteria according to the views of stakeholders is crucial for outputs to be appropriate (330). Within this study, the weighting methods were Preference Ranking Organization Method Enrichment of Evaluations (PROMETHEE) and Conjoint Analysis (CA) in four articles each; the specific methods used were not explicitly stated for many (34) articles.
Studies dealing with disease prioritization and those generating evidence for decision-making are increasing. This indicates the importance of such approaches in the optimization of resources for animal health and production investment. The finding that most disease prioritization studies are focused disproportionately on developed countries illustrates differences between countries in their capacities to undertake disease prioritization exercises. Several of the disease prioritization methods require expertise and resources in terms of time to complete. Available data to be used as inputs in prioritization methods can also be a reason that most studies come from developed countries. For reliable disease prioritization for resource allocation and decision-making, timely and high-quality data are required. Animal health information systems for developing countries are not well organized, constraining the practice of priority setting in animal health and the overall decision-making process.
The levels of analysis related to geographic or political administrative boundaries of the studies included in the present literature review showed that approximately half of the studies were at the national level, with minor global coverage. There are several highly infectious animal diseases that pose a global threat (transboundary diseases), potentially causing negative socioeconomic and public health consequences beyond national boundaries. This means that the burden and consequences of animal disease go beyond national boundaries; resource allocation for actions should consider such situations. In contrast, endemic diseases are often of local importance, and resources need to be allocated accordingly. This reflects the need for customizable disease prioritization and resource allocation tools that can be used in various scenarios to provide evidence-based decision-making processes for the efficient utilization of animal resources. The present scoping review showed that more complex tools (e.g., risk analysis, modeling, and economic analysis) are commonly used in more economically developed countries (e.g., Europe) compared to simple disease ranking tools being more often used in Africa. This indicates a need to build capacity for the use of complex tools in developing countries.
Various reasons behind the delivery of disease prioritization exercises were mentioned in the reviewed articles. The intention to design control, prevention, or eradication strategies by targeting single or multiple diseases was the most common reason. Overall, the ultimate intent or purpose of disease prioritization is to ensure the allocation of limited resources toward achieving the greatest benefit in improving and maintaining human and animal health (8). Apart from the relatively substantial number of articles identified in the present scoping review, the prioritization tools developed by various organizations and used for practical purposes targeting animal diseases, including zoonoses, were not commonly cited in the peer-reviewed publications identified in this review. Diseases or pathogens targeted in different studies included in the present review were quite diverse. Economic assessment was largely targeted at endemic diseases, of which, for example, paratuberculosis was the most common.
Conclusion
The present scoping review covered various approaches to disease prioritization, including those supporting decision-making in animal health. Some studies focused on explicit disease prioritization, and the remaining studies generated evidence. The scoping review revealed the lack of comprehensive, integrated, and mutually compatible approaches to disease prioritization and decision support tools for animal health; this could lead to sub-optimal resource allocation. It can be concluded that there is neither a dominant tool or sets of data being used nor a comprehensive understanding of how to prioritize actions to control specific diseases. Notably, there was more variation in prioritization analysis than in economic analysis. By far, the most popular approach was cost-benefit analysis (followed by cost-effectiveness analysis) in its true meaning. There is also a complete absence of studies on what investment is needed for the livestock sector in terms of research, education, and coordination. Accordingly, this demands further work to improve disease prioritization through the integration of existing or new approaches that could be related to disease impacts as a result of generic disease risks, spatial distribution, economic impacts, and public health impacts. In the present scoping review, only published articles in English were included, and this can be a limitation of the present study. All disease prioritization outputs may not be found in published formats, and the inclusion of gray literature in possible future studies is recommended.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
KA, KM, and DG conceptualized the study. KA and KM prepared the data retrieval query protocol. TK-J, DG, and JR reviewed the data retrieval protocol. KA and NM extracted data. KA drafted the initial manuscript. All authors made critical contributions in revising the manuscript and approved the final version.
Funding
This study was part of the Global Burden of Animal Diseases Programme (GBADs) funded by the Bill and Melinda Gates Foundation (BMGF) and Foreign, Commonwealth and Development Office (FCDO) UK (Grant number INV 005366).
Acknowledgments
We thank Dr. Vittoria Lutje who helped us to improve the review protocol and search criteria, translated the search strategies for the different electronic databases, ran the searches, and de-duplicated the results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1231711/full#supplementary-material
References
1. Rushton J. The Economics of Animal Health and Production. Wallingford: CAB International (2009), p. 298.
2. Herrero M, Grace D, Njuki J, Johnson N, Enahoro D, Silvestri S, et al. The roles of livestock in developing countries. Animal. (2013) 7:3–18. doi: 10.1017/S1751731112001954
3. Rich KM, Perry BD. The economic and poverty impacts of animal diseases in developing countries: new roles, new demands for economics and epidemiology. Prev Vet Med. (2011) 101:133–47. doi: 10.1016/j.prevetmed.2010.08.002
5. Otte MJ, Chilonda P. Animal Health Economics: An Introduction. Rome: Animal Production and Healthy Division (AGA). (2000).
7. Howe KS. The allocation of resources for animal health. Rev Sci Tech Off Int Epiz. (2017) 36:35–48. doi: 10.20506/rst.36.1.2607
8. Brookes VJ, Del Rio Vilas VJ, Ward MP. Disease prioritization: what is the state of the art? Epidemiol Infect. (2015) 143:2911–22. doi: 10.1017/S0950268815000801
9. Smith KM, Machalaba CC, Seifman R, Feferholtz Y, Karesh WB. Infectious disease and economics: the case for considering multi-sectoral impacts. One Health. (2019) 7:100080. doi: 10.1016/J.ONEHLT.2018.100080
10. Thompson D, Muriel P, Russell D, Osborne P, Bromley A, Rowland M, et al. Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Revue Sci Tech. (2002) 21:675–87. doi: 10.20506/RST.21.3.1353
11. Knight-Jones TJD, McLaws M, Rushton J. Foot-and-mouth disease impact on smallholders - what do we know, what don't we know and how can we find out more? Transbound Emerg Dis. (2017) 64:1079–94. doi: 10.1111/TBED.12507
12. Maqbool I, Wani ZA, Shahardar RA, Allaie IM, Shah MM. Integrated parasite management with special reference to gastro-intestinal nematodes. J Paras Dis Off Org Soc Parasitol. (2017) 41:1. doi: 10.1007/S12639-016-0765-6
13. Carter A. Establishing goals, techniques and priorities for national communicable disease surveillance. The Can J Inf Dis. (1991) 2:37. doi: 10.1155/1991/346135
14. Bianchini J, Humblet MF, Cargnel M, Van der Stede Y, Koenen F, de Clercq K. Prioritization of livestock transboundary diseases in Belgium using a multi criteria decision analysis tool based on drivers of emergence. Trans Emerg Dis. (2020) 67:344–76. doi: 10.1111/tbed.13356
15. Brioudes A, Warner J, Hedlefs R, Gummow B. Diseases of livestock in the Pacific Islands region: setting priorities for food animal biosecurity. Acta Trop. (2015) 143:66–76. doi: 10.1016/j.actatropica.2014.12.012
16. Rist CL, Arriola CS, Rubin C. Prioritizing zoonoses: a proposed one health tool for collaborative decision-making. PLoS ONE. (2014) 9:e109986. doi: 10.1371/JOURNAL.PONE.0109986
17. Eisler MC, Magona JW, Jonsson NN, Revie CW. A low cost decision support tool for the diagnosis of endemic bovine infectious diseases in the mixed crop-livestock production system of sub-Saharan Africa. Epidemiol Infect. (2007) 135:67–75. doi: 10.1017/S0950268806006571
18. Kim E, Carpenter T, Rowanowski S, Cogger N. Criteria and indicators for foot and mouth disease control strategy decision-making in Asia–Oceania countries. Rev Sci Tech'OIE. (2017) 36:867–78. doi: 10.20506/rst.36.3.2720
19. Tricco AC, Lillie E, Zarin W, Colquhoun H, Levac D, Moher D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. ACP J. (2018) 169:467–73. doi: 10.7326/M18-0850
20. Bozada T, Borden J, Workman J, Del Cid M, Malinowski J, Luechtefeld T. Sysrev: A FAIR Platform for Data Curation and Systematic Evidence Review. Front Artif Int. (2021) 4:685298. doi: 10.3389/FRAI.2021.685298
21. van Schaik G, Nielen M, Dijkhuizen AA. An economic model for on-farm decision support of management to prevent infectious disease introduction into dairy farms. Prev Vet Med. (2001) 51:289–305. doi: 10.1016/S0167-5877(01)00224-0
22. Down PM, Bradley AJ, Breen JE, Browne WJ, Kypraios T, Green MJ, et al. Bayesian micro-simulation to evaluate the cost-effectiveness of interventions for mastitis control during the dry period in UK dairy herds. Prev Vet Med. (2016) 133:64–72. doi: 10.1016/j.prevetmed.2016.09.012
23. Garland CB, Leathwick DM. A cost-benefit analysis of pre- and post-lambing anthelmintic treatments to twin-bearing ewes on commercial farms in the southern North Island of New Zealand. N Z Vet J. (2015) 63:220–6. doi: 10.1080/00480169.2015.1012133
24. Goldbach SG, Alban L. A cost-benefit analysis of Salmonella-control strategies in Danish pork production. Prev Vet Med. (2006) 77:1–14. doi: 10.1016/j.prevetmed.2005.10.008
25. Gavin C, Simons RRL, Berriman ADC, Moorhouse D, Snary EL, Smith RP, et al. cost-benefit assessment of Salmonella-control strategies in pigs reared in the United Kingdom. Prev Vet Med. (2018) 160:54–62. doi: 10.1016/j.prevetmed.2018.09.022
26. Gormley AM, Holland EP, Barron MC, Anderson DP, Nugent G. A modelling framework for predicting the optimal balance between control and surveillance effort in the local eradication of tuberculosis in New Zealand wildlife. Prev Vet Med. (2016) 125:10–8. doi: 10.1016/J.PREVETMED.2016.01.007
27. Gummow B, Mapham PH. A stochastic partial-budget analysis of an experimental Pasteurella haemolytica feedlot vaccine trial. Prev Vet Med. (2000) 43:29–42. doi: 10.1016/S0167-5877(99)00071-9
28. Dolecheck KA, Dwyer RM, Overton MW, Bewley JM. A survey of United States dairy hoof care professionals on costs associated with treatment of foot disorders. J Dairy Sci. (2018) 101:8313–26. doi: 10.3168/jds.2018-14718
29. Verteramo Chiu LJ, Tauer LW, Al-Mamun MA, Kaniyamattam K, Smith RL, Grohn YT. An agent-based model evaluation of economic control strategies for paratuberculosis in a dairy herd. J Dairy Sci. (2018) 101:6443–54. doi: 10.3168/jds.2017-13175
30. Rosanowski SM, Carpenter TE, Adamson D, Rogers CW, Pearce P, Burns M, et al. An economic analysis of a contingency model utilising vaccination for the control of equine influenza in a non-endemic country. PLoS ONE. (2019) 14:e0210885. doi: 10.1371/JOURNAL.PONE.0210885
31. Bennett R, Mcclement I, Mcfarlane I. An economic decision support tool for simulating paratuberculosis control strategies in a UK suckler beef herd. Prev Vet Med. (2010) 93:286–93. doi: 10.1016/j.prevetmed.2009.11.006
32. Weng L, Weersink A, Poljak Z, de Lange K, von Massow M. An economic evaluation of intervention strategies for Porcine Epidemic Diarrhea (PED). Prev Vet Med. (2016) 134:58–68. doi: 10.1016/J.PREVETMED.2016.09.018
33. Van der Fels-Klerx HJ, Sorensen JT, Jalvingh AW, Huirne RBM. An economic model to calculate farm-specific losses due to bovine respiratory disease in dairy heifers. Prev Vet Med. (2001) 51:75–94. doi: 10.1016/S0167-5877(01)00208-2
34. Tambi NE, Maina WO, Ndi C. An estimation of the economic impact of contagious bovine pleuropneumonia in Africa. OIE Rev Sci et Technique. (2006) 25:999–1011. doi: 10.20506/rst.25.3.1710
35. Martins SB, Giulio GD, Lynen G, Peters A, Rushton J. Assessing the impact of East Coast Fever immunisation by the infection and treatment method in Tanzanian pastoralist systems. Prev Vet Med. (2010) 97:175–82. doi: 10.1016/j.prevetmed.2010.09.018
36. Isoda N, Asano A, Ichijo M, Ohno H, Sato K, Okamoto H, et al. Assessment of the cost effectiveness of compulsory testing of introduced animals and bulk tank milk testing for bovine viral diarrhea in Japan. J Vet Med Sci. (2019) 81:577–85. doi: 10.1292/jvms.18-0671
37. Trevisan C, Devleesschauwer B, Praet N, Pondja A, Assane YA, Dorny P, et al. Assessment of the societal cost of Taenia solium in Angónia district, Mozambique. BMC Infect Dis. (2018) 18:1–11. doi: 10.1186/S12879-018-3030-Z
38. Archer SC, Mc Coy F, Wapenaar W, Green MJ. Bayesian evaluation of budgets for endemic disease control: an example using management changes to reduce milk somatic cell count early in the first lactation of Irish dairy cows. Prev Vet Med. (2014) 113:80–7. doi: 10.1016/J.PREVETMED.2013.10.011
39. Young JR, Suon S, Rast L, Nampanya S, Windsor PA, Bush RD. Benefit-cost analysis of foot and mouth disease control in large ruminants in Cambodia. Transbound Emerg Dis. (2016) 63:508–22. doi: 10.1111/tbed.12292
40. Bates TW, Carpenter TE, Thurmond MC. Benefit-cost analysis of vaccination and preemptive slaughter as a means of eradicating foot-and-mouth disease. Am J Vet Res. (2003) 64:805–12. doi: 10.2460/AJVR.2003.64.805
41. Halasa T. Bioeconomic modeling of intervention against clinical mastitis caused by contagious pathogens. J Dairy Sci. (2012) 95:5740–9. doi: 10.3168/jds.2012-5470
42. Roberts TW, Peck DE, Ritten JP. Cattle producers' economic incentives for preventing bovine brucellosis under uncertainty. Prev Vet Med. (2012) 107:187–203. doi: 10.1016/J.PREVETMED.2012.06.008
43. Healy JM, Reisen WK, Kramer VL, Fischer M, Lindsey NP, Nasci RS, et al. Comparison of the efficiency and cost of west Nile virus surveillance methods in California. Vector-Borne Zoonotic Dis. (2015) 15:147–55. doi: 10.1089/vbz.2014.1689
44. Athar LA, Khan MN, Sajid MS. Tauseef-Ur-Rehman, Khan IA. Cost benefits analysis of anthelmintic treatment of cattle and buffaloes Pakistan. Vet J. (2011) 31:149–52.
45. Mindekem R, Lechenne MS, Naissengar KS, Oussiguéré A, Kebkiba B, Moto DD, et al. Cost description and comparative cost efficiency of post-exposure prophylaxis and canine mass vaccination against rabies in N'Djamena, Chad. Front Vet Sci. (2017) 4:38. doi: 10.3389/fvets.2017.00038
46. Gormley AM, Anderson DP, Nugent G. Cost-based optimization of the stopping threshold for local disease surveillance during progressive eradication of tuberculosis from New Zealand wildlife. Transbound Emerg Dis. (2018) 65:186–96. doi: 10.1111/tbed.12647
47. Randela R. Cost-benefit analysis of a disease control programme with special reference to ticks and tick-borne diseases in the former Venda region. Dev South Afr. (2005) 22:515–28. doi: 10.1080/03768350500322768
48. Karki S, Lupiani B, Budke CM, Karki NPS, Rushton J, Ivanek R. Cost-benefit analysis of avian influenza control in Nepal. OIE Rev Sci Tech. (2015) 34:813–27. doi: 10.20506/RST.34.3.2397
49. Jemberu WT, Mourits M, Rushton J, Hogeveen H. Cost-benefit analysis of foot and mouth disease control in Ethiopia. Prev Vet Med. (2016) 132:67–82. doi: 10.1016/j.prevetmed.2016.08.008
50. Singh BB, Kostoulas P, Gill JPS, Dhand NK. Cost-benefit analysis of intervention policies for prevention and control of brucellosis in India. PLoS Negl Trop Dis. (2018) 12:6488. doi: 10.1371/journal.pntd.0006488
51. Winter JR, Green LE. Cost-benefit analysis of management practices for ewes lame with footrot. Vet J. (2017) 220:1–6. doi: 10.1016/J.TVJL.2016.11.010
52. Groenendaal H, Zagmutt FJ, Patton EA, Wells SJ. Cost-benefit analysis of vaccination against Mycobacterium avium ssp. paratuberculosis in dairy cattle, given its cross-reactivity with tuberculosis tests. J Dairy Science. (2015) 98:6070–84. doi: 10.3168/jds.2014-8914
53. Roche SM, Von Massow M, Renaud D, Shock DA, Jones-Bitton A, Kelton DF. Cost-benefit of implementing a participatory extension model for improving on-farm adoption of Johne's disease control recommendations. J Dairy Sci. (2020) 103:451–72. doi: 10.3168/jds.2019-16708
54. Barrett DC. Cost-effective antimicrobial drug selection for the management and control of respiratory disease in European cattle. Vet Rec. (2000) 146:545–50. doi: 10.1136/VR.146.19.545
55. Poirier V, Rivière J, Bouveret A, Gardon S, Dufour B. Cost-effectiveness assessment of three components of the bovine tuberculosis surveillance system by intradermal tuberculin testing in French cattle farms by a scenario tree approach. Prev Vet Med. (2019) 166:93–109. doi: 10.1016/j.prevetmed.2019.03.004
56. Rivière J, Le Strat Y, Hendrikx P, Dufour B. Cost-effectiveness evaluation of bovine tuberculosis surveillance in wildlife in France (Sylvatub system) using scenario trees. PLoS ONE. (2017) 12:126. doi: 10.1371/JOURNAL.PONE.0183126
57. Aly SS, Anderson RJ, Whitlock RH, Fyock TL, McAdams SC, Byrem TM, et al. Cost-effectiveness of diagnostic strategies to identify Mycobacterium avium subspecies paratuberculosis super-shedder cows in a large dairy herd using antibody enzyme-linked immunosorbent assays, quantitative real-time polymerase chain reaction, and bac. J Vet Diag Invest. (2012) 24:821–32. doi: 10.1177/1040638712452107
58. Wera E, Mourits MCM, Siko MM, Hogeveen H. Cost-Effectiveness of Mass Dog Vaccination Campaigns against Rabies in Flores Island, Indonesia. Transbound Emerg Dis. (2017) 64:1918–28. doi: 10.1111/tbed.12590
59. Léger A, Grosbois V, Simons R, Stärk KDC, De Nardi M. Cost-effectiveness of surveillance and biosecurity scenarios for preventing CSF in Switzerland. Microbial Risk Anal. (2019) 13:0–1. doi: 10.1016/j.mran.2019.07.001
60. Jensen JD, Christensen T, Olsen JV, Sandøe P. Costs and benefits of alternative strategies to control the spread of livestock-acquired methicillin-resistant staphylococcus aureus from pig production J Int Soc Pharm Outcomes Res. (2020) 23:89–95. doi: 10.1016/J.JVAL.2019.07.006
61. Peeler EJ. Costs and benefits of freedom from shrimp diseases in the European Union. J Invertebr Pathol. (2012) 110:188–95. doi: 10.1016/j.jip.2012.01.014
62. Undurraga EA, Millien MF, Allel K, Etheart MD, Cleaton J, Ross Y, et al. Costs and effectiveness of alternative dog vaccination strategies to improve dog population coverage in rural and urban settings during a rabies outbreak. Vaccine. (2020) 38:6162–73. doi: 10.1016/J.VACCINE.2020.06.006
63. Kangas S, Lyytikäinen T, Peltola J, Ranta J, Maijala R. Costs of two alternative Salmonella control policies in Finnish broiler production. Acta Vet Scand. (2007) 49:1–8. doi: 10.1186/1751-0147-49-35
64. Shaw APM, Rushton J, Roth F, Torgerson PR. DALYs, dollars and dogs: how best to analyse the economics of controlling zoonoses. Rev Sci Tech. (2017) 36:147–61. doi: 10.20506/RST.36.1.2618
65. Hansson H, Lagerkvist CJ. Decision making for animal health and welfare: integrating risk-benefit analysis with prospect theory. Risk Anal. (2014) 34:1149–59. doi: 10.1111/risa.12154
66. Pinzón-Sánchez C, Cabrera VE, Ruegg PL. Decision tree analysis of treatment strategies for mild and moderate cases of clinical mastitis occurring in early lactation. J Dairy Sci. (2011) 94:1873–92. doi: 10.3168/JDS.2010-3930
67. Reichel MP, Hill FI, Voges H. Does control of bovine viral diarrhoea infection make economic sense? N Z Vet J. (2008) 56:60–6. doi: 10.1080/00480169.2008.36809
68. Gethmann J, Probst C, Sauter-Louis C, Conraths FJ. Economic analysis of animal disease outbreaks–BSE and Bluetongue disease as examples. Berl Munch Tierarztl Wochenschr. (2015) 128:478–82. doi: 10.2376/0005-9366-128-478
69. Longworth N, Mourits MCM, Saatkamp HW. Economic Analysis of HPAI Control in the Netherlands I: epidemiological modelling to support economic analysis. Transbound Emerg Dis. (2014) 61:199–216. doi: 10.1111/tbed.12021
70. Henning J, Morton J, Pym R, Hla T, Sunn K, Meers J. Economic analysis of interventions to improve village chicken production in Myanmar. Prev Vet Med. (2013) 110:525–40. doi: 10.1016/j.prevetmed.2013.01.005
71. Cho J, Tauer LW, Schukken YH, Gómez MI, Smith RL, Lu Z, et al. economic analysis of Mycobacterium avium subspecies paratuberculosis vaccines in dairy herds. J Diary Sci. (2012) 95:1855–72. doi: 10.3168/jds.2011-4787
72. Groenendaal H, Galligan DT. Economic consequences of control programs for paratuberculosis in midsize dairy farms in the United States. J Am Vet Med Assoc. (2003) 223:1757–63. doi: 10.2460/javma.2003.223.1757
73. Larson RL, Hardin DK, Pierce VL. Economic considerations for diagnostic and control options for Neospora caninum-induced abortions in endemically infected herds of beef cattle. J Am Vet Med Assoc. (2004) 224:1597–604. doi: 10.2460/javma.2004.224.1597
74. Lhermie G, Sauvage P, Tauer LW, Chiu LV, Kanyiamattam K, Ferchiou A, et al. Grohn YT. Economic effects of policy options restricting antimicrobial use for high risk cattle placed in US feedlots. PLoS ONE. (2020) 15:e0239135. doi: 10.1371/JOURNAL.PONE.0239135
75. Kuczewski A, Hogeveen H, Orsel K, Wolf R, Thompson J, Spackman E, et al. Economic evaluation of 4 bovine leukemia virus control strategies for Alberta dairy farms. J Dairy Sci. (2019) 102:2578–92. doi: 10.3168/jds.2018-15341
76. Pillars RB, Grooms DL, Wolf CA, Kaneene JB. Economic evaluation of Johne's disease control programs implemented on six Michigan dairy farms. Prev Vet Med. (2009) 90:223–32. doi: 10.1016/j.prevetmed.2009.04.009
77. Wolf R, Clement F, Barkema HW, Orsel K. Economic evaluation of participation in a voluntary Johne's disease prevention and control program from a farmer's perspective-The Alberta Johne's Disease Initiative. J Dairy Sci. (2014) 97:2822–34. doi: 10.3168/jds.2013-7454
78. Thomann B, Tschopp A, Magouras I, Meylan M, Schüpbach-Regula G, Häsler B. Economic evaluation of the eradication program for bovine viral diarrhea in the Swiss dairy sector. Prev Vet Med. (2017) 145:1–6. doi: 10.1016/j.prevetmed.2017.05.020
79. Anderson A, Shwiff S, Gebhardt K, Ramírez AJ, Shwiff S, Kohler D, et al. Economic evaluation of vampire bat (Desmodus rotundus) rabies prevention in Mexico. Transbound Emerg Dis. (2014) 61:140–6. doi: 10.1111/tbed.12007
80. Renault V, Hambe HA, Van Vlaenderen G, Timmermans E, Mohamed AM, Ethgen O, et al. Economic impact of contagious caprine pleuropneumonia and cost-benefit analysis of the vaccination programmes based on a one-year continuous monitoring of flocks in the arid and semi-arid lands of Kenya. Transbound Emerg Dis. (2019) 66:2523–36. doi: 10.1111/TBED.13317
81. Awa DN, Njoya A, Tama ACN. Economics of prophylaxis against Peste des Petits ruminants and gastrointestinal helminthosis in small ruminants in North Cameroon. Trop Anim Prod. (2000) 32:391–403. doi: 10.1023/a:1005233703331
82. Cargnel M, Van der Stede Y, Haegeman A, De Leeuw I, De Clercq K, Méroc E, et al. Effectiveness and cost-benefit study to encourage herd owners in a cost sharing vaccination programme against bluetongue serotype-8 in Belgium. Transbound Emerg Dis. (2019) 66:400–11. doi: 10.1111/tbed.13034
83. Kivaria FM, Ruheta MR, Mkonyi PA, Malamsha PC. Epidemiological aspects and economic impact of bovine theileriosis (East Coast fever) and its control: a preliminary assessment with special reference to Kibaha district, Tanzania. Vet J. (2007) 173:384–90. doi: 10.1016/J.TVJL.2005.08.013
84. Hadorn DC, Racloz V, Schwermer H, Stärk KDC. Establishing a cost-effective national surveillance system for Bluetongue using scenario tree modelling. Vet Res. (2009) 40:9040. doi: 10.1051/vetres/2009040
85. Shaw APM, Torr SJ, Waiswa C, Cecchi G, Wint GRW, Mattioli RC, et al. Estimating the costs of tsetse control options: an example for Uganda. Prev Vet Med. (2013) 110:290–303. doi: 10.1016/j.prevetmed.2012.12.014
86. Onono JO, Wieland B, Rushton J. Estimation of impact of contagious bovine pleuropneumonia on pastoralists in Kenya. Prev Vet Med. (2014) 115:122–9. doi: 10.1016/j.prevetmed.2014.03.022
87. Hénaux V, Calavas D. Evaluation of the cost-effectiveness of bovine brucellosis surveillance in a disease-free country using stochastic scenario tree modelling. PLoS ONE. (2017) 12:1–21. doi: 10.1371/journal.pone.0183037
88. Zeng JY, Robertson ID Ji QM, Dawa YL, Bruce M. Evaluation of the economic impact of brucellosis in domestic yaks of Tibet. Transbound Emerg Dis. (2019) 66:476–87. doi: 10.1111/tbed.13049
89. Santman-Berends IMGA, Mars MH, van Duijn L, van Schaik G. Evaluation of the epidemiological and economic consequences of control scenarios for bovine viral diarrhea virus in dairy herds. J Dairy Sci. (2015) 98:7699–716. doi: 10.3168/JDS.2014-9255
90. Hautefeuille C, Azzouguen B, Mouchel S. Peyre M. Evaluation of vaccination strategies to control an avian influenza outbreak in French poultry production networks using EVACS tool. J Prev Med. (2020) 184:105129. doi: 10.1016/j.prevetmed.2020.105129
91. Groenendaal H, Wolf CA. Farm-level economic analysis of the us national Johne's disease demonstration herd project. J Am Vet Med Assoc. (2008) 233:1852–8. doi: 10.2460/JAVMA.233.12.1852
92. van Soest FJS, Mourits MCM, Blanco-Penedo I, Duval J, Fall N, Krieger M, et al. Farm-specific failure costs of production disorders in European organic dairy herds. Prev Vet Med. (2019) 168:19–29. doi: 10.1016/j.prevetmed.2019.03.029
93. Häsler B, Regula G, Stärk KDC, Sager H, Gottstein B, Reist M. Financial analysis of various strategies for the control of Neospora caninum in dairy cattle in Switzerland. Prev Vet Med. (2006) 77:230–53. doi: 10.1016/j.prevetmed.2006.07.006
94. Pham HTT, Antoine-Moussiaux N, Grosbois V, Moula N, Truong BD, Phan TD, et al. Financial Impacts of priority swine diseases to pig farmers in red river and Mekong River Delta, Vietnam. Transbound Emerg Dis. (2017) 64:1168–77. doi: 10.1111/tbed.12482
95. Barratt AS, Rich KM, Eze JI, Porphyre T, Gunn GJ, Stott AW. Framework for estimating indirect costs in animal health using time series analysis. Front Vet Sci. (2019) 6:190. doi: 10.3389/FVETS.2019.00190
96. Lucas P, Horton B. Guidelines for treatment of lice in sheep with long wool based on a model of the development of wool damage. Aust Vet J. (2014) 92:8–14. doi: 10.1111/AVJ.12138
97. Roth F, Zinsstag J, Orkhon D, Hutton G, Cosivi O, Carrin G, Otte J. Human health benefits from livestock vaccination for brucellosis : case study. Bullet WHO. (2003) 81:867–76.
98. Reichel MP, Ellis JT. If control of Neospora caninum infection is technically feasible does it make economic sense? Vet Parasitol. (2006) 142:23–34. doi: 10.1016/j.vetpar.2006.06.027
99. Okello WO, Okello AL, Inthavong P, Tiemann T, Phengsivalouk A, Devleesschauwer B, et al. Improved methods to capture the total societal benefits of zoonotic disease control: Demonstrating the cost-effectiveness of an integrated control programme for Taenia solium, soil transmitted helminths and classical swine fever in northern Lao PDR. PLoS Negl Trop Dis. (2018) 12:1–22. doi: 10.1371/journal.pntd.0006782
100. Charlier J, Rinaldi L, Musella V, Ploeger HW, Chartier C, Vineer HR, et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med. (2020) 182:105103. doi: 10.1016/j.prevetmed.2020.105103
101. Kao SYZ, VanderWaal K, Enns EA, Craft ME, Alvarez J, Picasso C, et al. Modeling cost-effectiveness of risk-based bovine tuberculosis surveillance in Minnesota. Prev Vet Med. (2018) 159:1–11. doi: 10.1016/j.prevetmed.2018.08.011
102. Nathues H, Alarcon P, Rushton J, Jolie R, Fiebig K, Jimenez M, et al. Modelling the economic efficiency of using different strategies to control porcine reproductive and respiratory syndrome at herd level. Prev Vet Med. (2018) 152:89–102. doi: 10.1016/J.PREVETMED.2018.02.005
103. Fasanmi OG, Kehinde OO, Laleye AT, Ekong B, Ahmed SSU, Fasina FO. National surveillance and control costs for highly pathogenic avian influenza H5N1 in poultry: a benefit-cost assessment for a developing economy, Nigeria. Res Vet Sci. (2018) 119:127–33. doi: 10.1016/j.rvsc.2018.06.006
104. Rowe SM, Nydam DV, Godden SM, Gorden PJ, Lago A, Vasquez AK, et al. Partial budget analysis of culture- and algorithm-guided selective dry cow therapy. J Dairy Sci. (2021) 104:5652–64. doi: 10.3168/jds.2020-19366
105. Benavides JA, Rojas Paniagua E, Hampson K, Valderrama W, Streicker DG. Quantifying the burden of vampire bat rabies in Peruvian livestock. PLoS Negl Trop Dis. (2017) 11:e0006105. doi: 10.1371/JOURNAL.PNTD.0006105
106. Welby S, Cargnel M, Saegerman C. Quantitative decision making in animal health surveillance: bovine Tuberculosis Surveillance in Belgium as case study. Transbound Emerg Dis. (2022) 69:e119–29. doi: 10.1111/TBED.14269
107. Gharbi M, Touay A, Khayeche M, Laarif J, Jedidi M, Sassi L, et al. Ranking control options for tropical theileriosis in at-risk dairy cattle in Tunisia, using benefit-cost analysis. Rev Sci et Tech. (2011) 30:763–78. doi: 10.20506/RST.30.3.2074
108. Learmount J, Glover MJ, Taylor MA. Resistance delaying strategies on UK sheep farms: a cost benefit analysis. Vet Parasitol. (2018) 254:64–71. doi: 10.1016/j.vetpar.2018.02.033
109. Cha E, Hertl JA, Bar D, Gröhn YT. The cost of different types of lameness in dairy cows calculated by dynamic programming. Prev Vet Med. (2010) 97:1–8. doi: 10.1016/j.prevetmed.2010.07.011
110. Fasina FO, Ali AM, Yilma JM, Thieme O, Ankers P. The cost-benefit of biosecurity measures on infectious diseases in the Egyptian household poultry. Prev Vet Med. (2012) 103:178–91. doi: 10.1016/j.prevetmed.2011.09.016
111. Jones BA, Rich KM, Mariner JC, Anderson J, Jeggo M, Thevasagayam S, et al. The economic impact of eradicating peste des petits ruminants: a benefit-cost analysis. PLoS ONE. (2016) 11:1–18. doi: 10.1371/journal.pone.0149982
112. Randolph TF, Perry BD, Benigno CC, Santos IJ, Agbayani AL, Coleman P, et al. The economic impact of foot and mouth disease control and eradication in the Philippines. OIE Rev Sci Tech. (2002) 21:645–61. doi: 10.20506/rst.21.3.1355
113. Marschik T, Kopacka I, Stockreiter S, Schmoll F, Hiesel J, Höflechner-Pöltl A, et al. The epidemiological and economic impact of a potential foot-and-mouth disease outbreak in Austria. Front Vet Sci. (2021) 7:1–13. doi: 10.3389/fvets.2020.594753
114. Wang CH, Diderrich V, Kliebenstein J, Patton S, Zimmerman J, Hallam A, et al. Toxoplasma gondii levels in swine operations: Differences due to technology choice and impact on costs of production. Food Control. (2002) 13:103–6. doi: 10.1016/S0956-7135(01)00083-4
115. Stott AW, Gunn GJ. Use of a benefit function to assess the relative investment potential of alternative farm animal disease prevention strategies. Prev Vet Med. (2008) 84:179–93. doi: 10.1016/j.prevetmed.2007.12.001
116. Meyer A, Holt HR, Oumarou F, Chilongo K, Gilbert W, Fauron A, et al. Integrated cost-benefit analysis of tsetse control and herd productivity to inform control programs for animal African trypanosomiasis. Parasites Vectors. (2018) 11:1–14. doi: 10.1186/s13071-018-2679-x
117. Saito EK, Shea S, Jones A, Ramos G, Pitesky M. A cooperative approach to animal disease response activities: analytical hierarchy process (AHP) and vvIBD in California poultry. Prev Vet Med. (2015) 121:123–31. doi: 10.1016/J.PREVETMED.2015.06.001
118. Degeling C, Johnson J, Ward M, Wilson A, Gilbert G. Original contribution a Delphi survey and analysis of expert perspectives on one health in Australia. Ecohealth. (2017) 14:783–92. doi: 10.1007/s10393-017-1264-7
119. Guétin- V, Dufour B, Rivière J. A framework for multicriteria decision-aid analyses in animal health surveillance applied to periodic screening for French bovine tuberculosis. Transb Emerg Dis. (2021) 69:1–13. doi: 10.1111/tbed.14091
120. El Allaki F, Christensen J, Vallières A. A modified TOPSIS (technique for order of preference by similarity to ideal solution) applied to choosing appropriate selection methods in ongoing surveillance for avian influenza in Canada. Prev Vet Med. (2019) 165:36–43. doi: 10.1016/j.prevetmed.2019.02.006
121. Ruzante JM, Davidson VJ, Caswell J, Fazil A, Cranfield JAL, Henson SJ, et al. A multifactorial risk prioritization framework for foodborne pathogens. Risk Anal: Off Pub Risk Anal. (2010) 30:724–42. doi: 10.1111/J.1539-6924.2009.01278.X
122. Ng V, Sargeant JM. A Quantitative and novel approach to the prioritization of zoonotic diseases in North America : a public perspective. PLoS ONE. (2012) 7:e48519. doi: 10.1371/journal.pone.0048519
123. Ng V, Sargeant JM. Prioritizing zoonotic diseases : differences in perspectives between human and animal health professionals in North America. Zoonoses Pu Health. (2016) 63:196–211. doi: 10.1111/zph.12220
124. Pramuwidyatama MG, Hogeveen H. Saatkamp HW. A systematic evaluation of measures against highly pathogenic avian influenza (HPAI) in Indonesia. Front Vet. (2019) 6:33. doi: 10.3389/fvets.2019.00033
125. Bessell PR, Auty HK, Roberts H, McKendrick IJ, Bronsvoort BM, Boden LA. Tool for prioritizing livestock disease threats to Scotland. Front Vet Sci. (2020) 7:223. doi: 10.3389/fvets.2020.00223
126. Wongnak P, Thanapongtharm W, Kusakunniran W, Karnjanapreechakorn S, Sutassananon K, Kalpravidh W, et al. A “what-if” scenario: Nipah virus attacks pig trade chains in Thailand. BMC Vet Res. (2020) 16:1–11. doi: 10.1186/S12917-020-02502-4
127. Brookes VJ, Hernández-Jover M, Cowled B, Holyoake PK, Ward MP. Building a picture: Prioritisation of exotic diseases for the pig industry in Australia using multi-criteria decision analysis. Prev Vet Med. (2014) 113:103–17. doi: 10.1016/j.prevetmed.2013.10.014
128. Renault V, Damiaans B, Sarrazin S, Humblet MF, Lomba M, Ribbens S, et al. Classification of adult cattle infectious diseases: A first step towards prioritization of biosecurity measures. Transbound Emerg Dis. (2018) 65:1991–2005. doi: 10.1111/TBED.12982
129. Muellner P, Hodges D, Ahlstrom C, Newman M, Davidson R, Pfeiffer D, et al. Creating a framework for the prioritization of biosecurity risks to the New Zealand dairy industry. Transbound Emerg Dis. (2018) 65:1067–77. doi: 10.1111/TBED.12848
130. KIM E, CARPENTER T, ROWANOWSKI S, COGGER N. Criteria and indicators for foot and mouth disease control strategy decision-making in Asia-Oceania countries. Rev Sci Tech. (2017) 36:867–78. doi: 10.20506/RST.36.3.2720
131. Hongoh V, Gosselin P, Michel P, Ravel A, Waaub JP, Campagna C, et al. Criteria for the prioritization of public health interventions for climate-sensitive vector-borne diseases in Quebec. PLoS ONE. (2017) 12:49. doi: 10.1371/journal.pone.0190049
132. Gibbens JC, Frost AJ, Houston CW, Lester H, Gauntlett FA. D2R2: an evidence-based decision support tool to aid prioritisation of animal health issues for government funding. Vet Rec. (2016) 179:103684. doi: 10.1136/VR.103684
133. Wentholt MTA, Cardoen S, Imberechts H, Van Huffel X, Ooms BW, Frewer LJ. Defining European preparedness and research needs regarding emerging infectious animal diseases: results from a Delphi expert consultation. Prev Vet Med. (2012) 103:81–92. doi: 10.1016/J.PREVETMED.2011.09.021
134. Clarke AM, More SJ, Maher JW, Byrne AW, Horan M, Barrett D. Development and Application of a Prioritization Tool for Animal Health Surveillance Activities in Ireland. Front Vet Sci. (2020) 7:596867. doi: 10.3389/FVETS.2020.596867
135. O'Brien D, Scudamore J, Charlier J, Delavergne M. DISCONTOOLS: a database to identify research gaps on vaccines, pharmaceuticals and diagnostics for the control of infectious diseases of animals. BMC Vet Res. (2017) 24:1–10. doi: 10.1186/s12917-016-0931-1
136. Tatum RC, McGowan CM, Dean RS, Ireland JL. Equine pituitary pars intermedia dysfunction: Identifying research priorities for diagnosis, treatment and prognosis through a priority setting partnership. PLoS ONE. (2021) 16:e0244784. doi: 10.1371/JOURNAL.PONE.0244784
137. Cardoen S, Van Huffel X, Berkvens D, Quoilin S, Ducoffre G, Saegerman C, et al. Evidence-based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathog Dis. (2009) 6:1083–96. doi: 10.1089/FPD.2009.0291
138. Saegerman C, Bianchini J, Snoeck CJ, Moreno A, Chiapponi C, Zohari S, et al. First expert elicitation of knowledge on drivers of emergence of influenza D in Europe. Transbound Emerg Dis. (2021) 68:3349–59. doi: 10.1111/TBED.13938
139. Mourits MCM, van Asseldonk MAPM, Huirne RBM. Multi Criteria Decision Making to evaluate control strategies of contagious animal diseases. Prev Vet Med. (2010) 96:201–10. doi: 10.1016/J.PREVETMED.2010.06.010
140. Humblet MF, Vandeputte S, Albert A, Gosset C, Kirschvink N, Haubruge E, et al. Multidisciplinary and evidence-based method for prioritizing diseases of food-producing animals and zoonoses. Emerg Infect Dis. (2012) 18:e1. doi: 10.3201/EID1804.111151
141. Hongoh V, Michel P, Gosselin P, Samoura K, Ravel A, Campagna C, et al. Multi-stakeholder decision aid for improved prioritization of the public health impact of climate sensitive infectious diseases. Int J Environ Res Public Health. (2016) 13:419. doi: 10.3390/IJERPH13040419
142. Sekamatte M, Krishnasamy V, Bulage L, Kihembo C, Nantima N, Monje F, et al. Multisectoral prioritization of zoonotic diseases in Uganda, 2017: a One Health perspective. PLoS ONE. (2018) 13:e0196799. doi: 10.1371/JOURNAL.PONE.0196799
143. Yasobant S, Saxena D, Bruchhausen W, Memon FZ, Falkenberg T. Multi-sectoral prioritization of zoonotic diseases: one health perspective from Ahmedabad, India. PLoS ONE. (2019) 14:e0220152. doi: 10.1371/JOURNAL.PONE.0220152
144. Brookes VJ, Barry SC, Hernández-Jover M, Ward MP. Point of truth calibration for disease prioritisation—A case study of prioritisation of exotic diseases for the pig industry in Australia. Prev Vet Med. (2017) 139:20–32. doi: 10.1016/j.prevetmed.2017.01.017
145. Aenishaenslin C, Page D, Gagnier M, Massé A, Fehlner-Gardiner C, Lambert L, et al. Prioritisation of areas for early detection of southward movement of arctic fox rabies based on historical surveillance data in Quebec, Canada. Epidemiol Infect. (2020) 149:3003. doi: 10.1017/S0950268820003003
146. Cito F, Rijks J, Rantsios AT, Cunningham AA, Baneth G. Prioritization of companion animal transmissible diseases for policy intervention in Europe. J Comp Pathol. (2015) 45:1–9. doi: 10.1016/j.jcpa.2015.01.007
147. Mersha TT, Wolde BM, Shumuye NA, Hailu AB, Mohammed AH, Redda YT, et al. Prioritization of neglected tropical zoonotic diseases: a one health perspective from Tigray region, Northern Ethiopia. PLoS ONE. (2021) 16:e0254071. doi: 10.1371/JOURNAL.PONE.0254071
148. Otten A, Fazil A, Chemeris A, Breadner P, Ng V. Prioritization of vector-borne diseases in Canada under current climate and projected climate change. Microbial Risk Anal. (2020) 14:100089. doi: 10.1016/J.MRAN.2019.100089
149. Munyua P, Bitek A, Osoro E, Pieracci EG, Muema J, Mwatondo A, et al. Prioritization of zoonotic diseases in Kenya, 2015. PLoS ONE. (2016) 11:576. doi: 10.1371/JOURNAL.PONE.0161576
150. Trang DT, Siembieda J, Huong NT, Hung P, Ky VD. Original Article Prioritization of zoonotic diseases of public health significance in Vietnam. J Inf Dev. (2010) 9:1315–22. doi: 10.3855/jidc.6582
151. Havelaar AH, Rosse FV, Bucura C, Toetenel MA, Haagsma JA. Prioritizing emerging zoonoses in The Netherlands. PLoS ONE. (2010) 5:e13965. doi: 10.1371/journal.pone.0013965
152. Pieracci EG, Hall AJ, Gharpure R, Haile A, Walelign E, Deressa A, et al. Prioritizing zoonotic diseases in Ethiopia using a one health approach. One Health. (2016) 2:131–5. doi: 10.1016/J.ONEHLT.2016.09.001
153. Zecconi A, Scali F, Bonizzi L, Ferrari N, Ferrero F, Grilli G, et al. Risk prioritization as a tool to guide veterinary public health activities at the regional level in Italy. Vet Ital. (2019) 55:113–21. doi: 10.12834/VETIT.172.518.2
154. More SJ, McKenzie K, O'Flaherty J, Doherty ML, Cromie AR, Magan MJ. Setting priorities for non-regulatory animal health in Ireland: Results from an expert policy Delphi study and a farmer priority identification survey. Prev Vet Med. (2010) 95:198–207. doi: 10.1016/j.prevetmed.2010.04.011
155. de Glanville WA, Vial L, Costard S, Wieland B, Pfeiffer DU. Spatial multi-criteria decision analysis to predict suitability for African swine fever endemicity in Africa. BMC Vet Res. (2014) 10:1–14. doi: 10.1186/1746-6148-10-9
156. Roberts LC. Fosgate T. Stakeholder perceptions of foot-and-mouth disease control in South Africa. J Prev Med. (2018) 156:38–48. doi: 10.1016/j.prevetmed.2018.05.001
157. Kadohira M, Hill G, Yoshizaki R, Ota S. Stakeholder prioritization of zoonoses in Japan with analytic hierarchy process method. (2015) 7:1477–85. doi: 10.1017/S0950268814002246
158. Maino M, Pérez P, Oviedo P, Sotomayor G, Abalos P. The analytic hierarchy process in decision making for caprine health programmes. Rev Sci Tech. (2012) 31:889–98. doi: 10.20506/RST.31.3.2162
159. Peyre M, Hoinville L, Njoroge J, Cameron A, Traon D, Goutard F, et al. The RISKSUR EVA tool (Survtool): a tool for the integrated evaluation of animal health surveillance systems. Prev Vet Med. (2019) 173:777. doi: 10.1016/J.PREVETMED.2019.104777
160. Mehand MS, Al-Shorbaji F, Millett P, Murgue B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res. (2018) 159:63–7. doi: 10.1016/J.ANTIVIRAL.2018.09.009
161. Stebler N, Braam P, Falzon LC. Use of a Modified Delphi Panel to Identify and Weight Criteria for Prioritization of Zoonotic Diseases in Switzerland. Amsterdam: Elsevier BV (2015).
162. Mcfadden AMJ, Muellner P, Baljinnyam Z, Vink D, Wilson N. Use of Multicriteria risk ranking of zoonotic diseases in a developing country: case study of Mongolia. Zoonoses Public Health. (2016) 63:138–51. doi: 10.1111/ZPH.12214
163. Horigan V, De Nardi M, Simons RRL, Bertolini S, Crescio MI, Estrada-Peña A, et al. Using multi-criteria risk ranking methodology to select case studies for a generic risk assessment framework for exotic disease incursion and spread through Europe. Prev Vet Med. (2018) 153:47–55. doi: 10.1016/J.PREVETMED.2018.02.013
164. Cheikh M, Hoch M, Guirreh D, Miguil S, Barkhad M, Djama A, et al. Priority trans-boundary animal diseases and zoonoses and their proposed control strategies for Djibouti. Bullet Anim Health Prod Africa. (2018) 66:231–8.
165. Niyokwishimira A, Nshimirimana Y, Havyarimana J, Moza S, Nsanganiyumwami D, Olaho-Mukani W, et al. Prioritisation of transboundary animal diseases and zoonoses for effective control in Burundi. Bullet Anim Health Prod Africa. (2018) 66:239–47.
166. Uqbazghi K, Teklemariam T, Goitom H, Magona J, Olaho-Mukani W, Muruiki S, et al. Prioritisation and control strategies of transboundry and zoonotic animal diseases in Eritrea. Bullet Anim Health Prod Africa. (2018) 66:249–63.
167. Walelign E, Yilma G, Haile A, Regassa F, Magona J, Olaho-Mukani W, et al. Transboundary animal diseases and zoonoses prioritization and proposed interventions in Ethiopia. Bullet Anim Health Prod Africa. (2018) 66:265–73.
168. Njagi L, Osoro E, Mwololo D, Murekefu W, Thaiya J, Ngeiywa K, et al. Prioritisation of transboundary animal diseases and zoonoses to strengthen control measures in Kenya. Bullet Anim Health Prod Africa. (2018) 66:287–398.
169. Nantima N, Bwire G, Mwebe R, Mugabi K, Ademun A, Kaboyo W, et al. Prioritisation of transboundary animal diseases and zoonoses to strengthen control in Uganda. Bullet Anim Health Prod Africa. (2018) 66:351–61.
170. Adwok D, Yuot M, Jakwot M, Kwai A, Korok J, Magona J, et al. Prioritisation of transboundary animal diseases (TADs) and zoonoses for the development of an effective disease control strategy in South Sudan. Bullet Anim Health Prod Africa. (2018) 66:363–73.
171. Mwenedata J, Kinani J, Kabeja A, Isidore Mapendo I, Kanyandekwe C, Rutagwenda T, et al. Prioritisation and categorisation of transboundary animal diseases and zoonoses for effective surveillance and control in Rwanda. Bullet Anim Health Prod Africa. (2018) 66:387–93.
172. Hanan Yousif M, Faiza Awad A, Abdel Rahman A, Beigi Khidir M, Amel Mahgoub A, Wahba M, et al. Prioritisation of transboundary animal diseases (TADs) and zoonoses for effective control in Sudan. Bullet Anim Health Prod Africa. (2018) 66:395–405.
173. Assenga SP, Sero H, Tinuga DK, Abdu AH, Mtiba PP, Olaho-Mukani W, et al. Transboundary animal diseases and zoonoses: prioritization and interventions in Tanzania. Bullet Anim Health Prod Africa. (2018) 66:415–26.
174. Hernández-Jover M, Schembri N, Holyoake PK, Toribio JALML, Martin PAJ. A comparative assessment of the risks of introduction and spread of foot-and-mouth disease among different pig sectors in Australia. Front Vet Sci. (2016) 3:85. doi: 10.3389/fvets.2016.00085
175. Hwang J, Lee K, Walsh D, Kim SW, Sleeman JM, Lee H. Semi-quantitative assessment of disease risks at the human, livestock, wildlife interface for the Republic of Korea using a nationwide survey of experts: a model for other countries. Transbound Emerg Dis. (2018) 65:e155–64. doi: 10.1111/TBED.12705
176. Chazya R, Muma JB, Mwacalimba KK, Karimuribo E, Mkandawire E, Simuunza M, et al. A qualitative assessment of the risk of introducing Peste des petits ruminants into northern Zambia from Tanzania. Vet Med Int. (2014) 2014:1–14. doi: 10.1155/2014/202618
177. Jori F, Vosloo W, Du Plessis B, Bengis R, Brahmbhatt D, Gummow B, et al. qualitative risk assessment of factors contributing to foot and mouth disease outbreaks in cattle along the western boundary of the Kruger National Park. Rev Sci Tech. (2009) 28:917–31. doi: 10.20506/RST.28.3.1932
178. Kadohira M, Stevenson MA, Høgåsen HR, de Koeijer A. A quantitative risk assessment for bovine spongiform encephalopathy in Japan. Risk Anal. (2012) 32:2198–208. doi: 10.1111/J.1539-6924.2012.01846.X
179. Meester M, Swart A, Deng H, Van Roon A, Trevisan C, Dorny P, et al. A quantitative risk assessment for human Taenia solium exposure from home slaughtered pigs in European countries. Parasites Vectors. (2019) 12:1–18. doi: 10.1186/s13071-019-3320-3
180. Maijala R, Ranta J, Seuna E, Pelkonen S, Johansson T. A quantitative risk assessment of the public health impact of the Finnish Salmonella control program for broilers. Int J Food Microbiol. (2005) 102:21–35. doi: 10.1016/j.ijfoodmicro.2004.11.012
181. Santman-Berends IMGA, Mars MH, Van Duijn L, Van den Broek KWH, Van Schaik G. A quantitative risk-analysis for introduction of Bovine Viral Diarrhoea Virus in the Netherlands through cattle imports. Prev Vet Med. (2017) 146:103–13. doi: 10.1016/J.PREVETMED.2017.08.003
182. Delgado J, Pollard S, Snary E, Black E, Prpich G, Longhurst P, et al. A systems approach to the policy-level risk assessment of exotic animal diseases: network model and application to classical swine fever. Risk Anal. (2013) 33:1454–72. doi: 10.1111/J.1539-6924.2012.01934.X
183. de Vos CJ, Swanenburg M, Tafro N, van Roon A, Stenvers OFJ, Elbers ARW. Animal health risk of legally imported exotic animals into the Netherlands in the period 2013–2014. Microbial Risk Anal. (2017) 6:9–20. doi: 10.1016/J.MRAN.2017.05.002
184. Katsma WEA, De Koeijer AA, Jacobs-Reitsma WF, Mangen MJJ, Wagenaar JA. Assessing interventions to reduce the risk of Campylobacter prevalence in broilers. Risk Anal. (2007) 27:863–76. doi: 10.1111/J.1539-6924.2007.00928.X
185. De la Torre A, Bosch J, Iglesias I, Muñoz MJ, Mur L, Martínez-López B. Assessing the risk of African swine fever introduction into the european union by wild boar. Transbound Emerg Dis. (2015) 62:272–279. doi: 10.1111/tbed.12129
186. Bora M, Bora DP, Manu M, Barman NN, Dutta LJ, Kumar PP, et al. Assessment of risk factors of African swine fever in India: Perspectives on future outbreaks and control strategies. Pathogens. (2020) 9:1–18. doi: 10.3390/pathogens9121044
187. Kyyrö J, Sahlström L, Lyytikäinen T. Assessment of the risk of African swine fever introduction into Finland using NORA-a rapid tool for semiquantitative assessment of the risk. Transbound Emerg Dis. (2017) 64:2113–25. doi: 10.1111/TBED.12633
188. Cargnel M, Maes D, Peeters L, Dispas M. Combining quantitative and qualitative approaches to determine viability of a potential Salmonella Typhimurium vaccination program in pigs in Belgium. Prev Vet Med. (2020) 184:e105132. doi: 10.1016/J.PREVETMED.2020.105132
189. Beaver A, Ruegg PL, Gröhn YT, Schukken YH. Comparative risk assessment for new cow-level Mycobacterium avium ssp. paratuberculosis infections between 3 dairy production types: Organic, conventional, and conventional-grazing systems. J Dairy Science. (2016) 99:9885–99. doi: 10.3168/JDS.2016-11360
190. Benavides B, Casal J, Diéguez JF, Yus E, Moya SJ, Armengol R, et al. Development of a quantitative risk assessment of bovine viral diarrhea virus and bovine herpesvirus-1 introduction in dairy cattle herds to improve biosecurity. J Dairy Sci. (2020) 103:6454–72. doi: 10.3168/JDS.2019-17827
191. Wolf R, Barkema HW, De Buck J, Orsel K. Factors affecting management changes on farms participating in a Johne's disease control program. J Dairy Sci. (2015) 98:7784–96. doi: 10.3168/JDS.2015-9610
192. Wieland B, Batsukh B, Enktuvshin S, Odontsetseg N, Schuppers M. Foot and mouth disease risk assessment in Mongolia-Local expertise to support national policy. Prev Vet Med. (2015) 120:115–23. doi: 10.1016/j.prevetmed.2014.11.017
193. Costard S, Jones BA, Martínez-López B, Mur L, de la Torre A, Martínez M, et al. Introduction of African swine fever into the European union through illegal importation of pork and pork products. PLoS ONE. (2013) 8:e61104. doi: 10.1371/journal.pone.0061104
194. Mul MF, Koenraadt CJM. Preventing introduction and spread of Dermanyssus gallinae in poultry facilities using the HACCP method. Exp Appl Acarol. (2009) 48:167–81. doi: 10.1007/s10493-009-9250-6
195. Miraballes C, Riet-Correa F, Saporiti T, Lara S, Parodi P, Sanchez J. Probability of Rhipicephalus microplus introduction into farms by cattle movement using a Bayesian Belief Network. Ticks Tick Borne Dis. (2019) 10:883–93. doi: 10.1016/j.ttbdis.2019.04.009
196. Gale P, Kelly L, Snary EL. Qualitative assessment of the entry of capripox viruses into Great Britain from the European Union through importation of ruminant hides, skins and wool. Microbial Risk Anal. (2016) 1:13–8. doi: 10.1016/J.MRAN.2015.07.001
197. Kelly L, Kosmider R, Gale P, Snary EL. Qualitative import risk assessment: a proposed method for estimating the aggregated probability of entry of infection. Microbial Risk Anal. (2018) 9:33–7. doi: 10.1016/j.mran.2018.03.001
198. Wieland B, Dhollander S, Salman M, Koenen F. Qualitative risk assessment in a data-scarce environment: a model to assess the impact of control measures on spread of African Swine Fever. Prev Vet Med. (2011) 99:4–14. doi: 10.1016/J.PREVETMED.2011.01.001
199. Fischer EAJ, Martínez López EP, De Vos CJ, Faverjon C. Quantitative analysis of the probability of introducing equine encephalosis virus (EEV) into The Netherlands. Prev Vet Med. (2016) 131:48–59. doi: 10.1016/j.prevetmed.2016.07.005
200. Herrera-Ibatá DM, Martínez-López B, Quijada D, Burton K, Mur L. Quantitative approach for the risk assessment of African swine fever and Classical swine fever introduction into the United States through legal imports of pigs and swine products. PLoS ONE. (2017) 12:e182850. doi: 10.1371/journal.pone.0182850
201. Foddai A, Boklund A, Stockmarr A, Krogh K, Enøe C. Quantitative assessment of the risk of introduction of bovine viral diarrhea virus in Danish dairy herds. Prev Vet Med. (2014) 116:75–88. doi: 10.1016/J.PREVETMED.2014.05.005
202. Sergeant ES, Grewar JD, Weyer CT, Guthrie AJ. Quantitative risk assessment for african horse sickness in live horses exported from South Africa. PLoS ONE. (2016) 11:e0151757. doi: 10.1371/JOURNAL.PONE.0151757
203. Mur L, Martínez-López B, Martínez-Avilés M, Costard S, Wieland B, Pfeiffer DU, et al. Quantitative risk assessment for the introduction of african swine fever virus into the European union by legal import of live pigs. Transbound Emerg Dis. (2012) 59:134–44. doi: 10.1111/j.1865-1682.2011.01253.x
204. Nydam DV, Mohammed HO. Quantitative risk assessment of Cryptosporidium species infection in dairy calves. J Dairy Sci. (2005) 88:3932–43. doi: 10.3168/jds.S0022-0302(05)73079-4
205. Woube YA, Dibaba AB, Tameru B, Fite R, Nganwa D, Robnett V, et al. Quantitative risk assessment of entry of contagious bovine pleuropneumonia through live cattle imported from northwestern Ethiopia. Prev Vet Med. (2015) 122:61–9. doi: 10.1016/j.prevetmed.2015.09.013
206. Martínez-López B, Perez AM. De la Torre A, Rodriguez JMSV. Quantitative risk assessment of foot-and-mouth disease introduction into Spain via importation of live animals. Prev Vet Med. (2008) 86:43–56. doi: 10.1016/j.prevetmed.2008.03.003
207. Cho K, Hyun B, Kim HJ, Kim YJ, Kang HE, Martínez-López B. Quantitative risk assessment of the African swine fever introduction into the Republic of Korea via legal import of live pigs and pig products. Transbound Emerg Dis. (2021) 68:385–96. doi: 10.1111/tbed.13689
208. Beauvais W, Zuther S, Villeneuve C, Kock R, Guitian J. Rapidly assessing the risks of infectious diseases to wildlife species. R Soc Open Sci. (2019) 6:17–23. doi: 10.1098/rsos.181043
209. Kim TY, Kim YS, Kim JK, Shon HJ, Lee YH, Kang CB, et al. Risk analysis of bovine spongiform encephalopathy in Korea. J Vet Med Sci. (2005) 67:743–52. doi: 10.1292/jvms.67.743
210. Mintiens K, Laevens H, Dewulf J, Boelaert F, Verloo D, Koenen F. Risk analysis of the spread of classical swine fever virus through “neighbourhood infections” for different regions in Belgium. Prev Vet Med. (2003) 60:27–36. doi: 10.1016/S0167-5877(03)00080-1
211. Martínez-López B, Carpenter TE, Sánchez-Vizcaíno JM. Risk assessment and cost-effectiveness analysis of Aujeszky's disease virus introduction through breeding and fattening pig movements into Spain. Prev Vet Med. (2009) 90:10–6. doi: 10.1016/j.prevetmed.2009.03.004
212. Knight-Jones TJD, Njeumi F, Elsawalhy A, Wabacha J, Rushton J. Risk assessment and cost-effectiveness of animal health certification methods for livestock export in Somalia. Prev Vet Med. (2014) 113:469–83. doi: 10.1016/J.PREVETMED.2014.01.003
213. Sánchez-García N, Raga JA, Montero FE. Risk assessment for parasites in cultures of Diplodus puntazzo (Sparidae) in the Western Mediterranean: Prospects of cross infection with Sparus aurata. Vet Parasitol. (2014) 204:120–33. doi: 10.1016/j.vetpar.2014.05.013
214. Brookes VJ, Keponge-Yombo A, Thomson D, Ward MP. Risk assessment of the entry of canine-rabies into Papua New Guinea via sea and land routes. Prev Vet Med. (2017) 145:49–66. doi: 10.1016/j.prevetmed.2017.06.011
215. Nathues C, Zimmerli U, Hauser R, Nathues H, Grosse Beilage E, Schüpbach-Regula G. Risk assessment of the introduction of porcine reproductive and respiratory syndrome virus via boar semen into Switzerland as an example of a PRRSV-free country. Transbound Emerg Dis. (2014) 61:546–54. doi: 10.1111/tbed.12059
216. Fanelli A, Buonavoglia D. Risk of Crimean Congo haemorrhagic fever virus (CCHFV) introduction and spread in CCHF-free countries in southern and Western Europe: a semi-quantitative risk assessment. One Health. (2021) 13:100290. doi: 10.1016/j.onehlt.2021.100290
217. Massó Sagüés E, Fernández-Carrión E, Sánchez-Vizcaíno JM. Risk of introduction of infectious animal diseases for Europe based on the health situation of North Africa and the Arabian Peninsula. Front Vet Sci. (2019) 6:293. doi: 10.3389/FVETS.2019.00293
218. Saegerman C, Bertagnoli S, Meyer G, Ganière JP, Caufour P, De Clercq K, et al. Risk of introduction of lumpy skin disease in France by the import of vectors in animal trucks. PLoS ONE. (2018) 13:e0198506. doi: 10.1371/JOURNAL.PONE.0198506
219. Bueno I, Smith KM, Sampedro F, Machalaba CC, Karesh WB, Travis DA. Risk prioritization tool to identify the public health risks of wildlife trade: the case of rodents from Latin America. Zoonoses Public Health. (2016) 63:281–93. doi: 10.1111/zph.12228
220. Ribeiro-Lima J, Schwabenlander S, Oakes M, Thompson B, Wells SJ. Risk profiling of cattle farms as a potential tool in risk-based surveillance for Mycobacterium bovis infection among cattle in tuberculosis-free areas. J Am Vet Med Assoc. (2016) 248:1404–13. doi: 10.2460/javma.248.12.1404
221. Maurella C, Mastrantonio G, Bertolini S, Crescio MI, Ingravalle F, Adkin A, et al. Social network analysis and risk assessment: an example of introducing an exotic animal disease in Italy. Microbial Risk Anal. (2019) 13:100074. doi: 10.1016/J.MRAN.2019.04.001
222. Vaughan-Higgins RJ, Vitali SD, Sims C, Page M, Reiss A. Streamlining disease risk analysis for wildlife using the shark bay bandicoot as a model. Ecohealth. (2021) 18:13–30. doi: 10.1007/s10393-021-01521-3
223. Roelandt S, Van der Stede Y, D'hondt B, Koenen F. The assessment of African swine fever virus risk to Belgium early 2014, using the quick and semi quantitative Pandora screening protocol. Transbound Emerg Dis. (2017) 64:237–249. doi: 10.1111/tbed.12365
224. Hernández-Jover M, Roche S, Ward MP. The human and animal health impacts of introduction and spread of an exotic strain of West Nile virus in Australia. Prev Vet Med. (2013) 109:186–204. doi: 10.1016/J.PREVETMED.2012.09.018
225. Taylor RA, Condoleo R, Simons RRL, Gale P, Kelly LA, Snary EL. The Risk of Infection by African Swine Fever Virus in European Swine Through Boar Movement and Legal Trade of Pigs and Pig Meat. Front Vet Sci. (2020) 6:1–19. doi: 10.3389/fvets.2019.00486
226. Giovannini A, MacDiarmid S, Calistri P, Conte A, Savini L, Nannini D, et al. The use of risk assessment to decide the control strategy for bluetongue in Italian ruminant populations. Risk Anal Off Pub Soc Risk Anal. (2004) 24:1737–53. doi: 10.1111/J.0272-4332.2004.00563.X
227. Delgado J, Pollard S, Pearn K, Snary EL, Black E, Prpich G, et al. Foot and Mouth Disease: A Systemic Risk Assessment of Existing Controls. Risk Anal Off Pub Soc Risk Anal. (2017) 37:1768–82. doi: 10.1111/RISA.12704
228. Squarzoni-Diaw C, Arsevska E, Kalthoum S, Hammami P, Cherni J, Daoudi A, et al. Using a participatory qualitative risk assessment to estimate the risk of introduction and spread of transboundary animal diseases in scarce-data environments: a spatial qualitative risk analysis applied to foot-and-mouth disease in Tunisia 2014-2019. Transbound Emerg Dis. (2021) 68:1966–78. doi: 10.1111/tbed.13920
229. Lapid R, King R, Yakobson B, Shalom U, Moran-Gilad J. Wildlife pathogen surveillance in Israel to inform human and animal infectious disease control: a prioritization exercise. Israel J Vet Med. (2016) 71:33–41.
230. Oliveira ARS, Piaggio J, Cohnstaedt LW, McVey DS, Cernicchiaro N. Introduction of the Japanese encephalitis virus (JEV) in the United States – A qualitative risk assessment. Transbound Emerg Dis. (2019) 66:1558–74. doi: 10.1111/tbed.13181
231. Bosch J, Iglesias I, Muñoz MJ, de la Torre A. A cartographic tool for managing African swine fever in Eurasia: mapping wild boar distribution based on the quality of available habitats. Transbound Emerg Dis. (2017) 64:1720–33. doi: 10.1111/tbed.12559
232. Rubel F, Fuchs K. A decision-support system for real-time risk assessment of airborne spread of the foot-and-mouth disease virus. Methods Inf Med. (2005) 44:590–5. doi: 10.1055/s-0038-1634013
233. Aguilar-Vega C, Fernández-Carrión E, Lucientes J, Sánchez-Vizcaíno JM. A model for the assessment of bluetongue virus serotype 1 persistence in Spain. PLoS ONE. (2020) 15:1–22. doi: 10.1371/journal.pone.0232534
234. Simons RRL, Horigan V, Ip S, Taylor RA, Crescio MI, Maurella C, et al. A spatial risk assessment model framework for incursion of exotic animal disease into the European Union Member States. Microbial Risk Anal. (2019) 13:0–1. doi: 10.1016/j.mran.2019.05.001
235. Clements ACA, Pfeiffer DU, Martin V. Application of knowledge-driven spatial modelling approaches and uncertainty management to a study of Rift Valley fever in Africa. Int J Health Geogr. (2006) 5:1–12. doi: 10.1186/1476-072X-5-57
236. Gerber PJ, Carsjens GJ, Pak-uthai T, Robinson TP. Decision support for spatially targeted livestock policies: diverse examples from Uganda and Thailand. Agric Syst. (2008) 96:37–51. doi: 10.1016/j.agsy.2007.05.004
237. Ippoliti C, Candeloro L, Gilbert M, Goffredo M, Mancini G, Curci G, et al. Defining ecological regions in Italy based on a multivariate clustering approach: a first step towards a targeted vector borne disease surveillance. PLoS ONE. (2019) 14:1–21. doi: 10.1371/journal.pone.0219072
238. Ngari NN, Gamba DO, Olet PA, Zhao W, Paone M, Cecchi G. Developing a national atlas to support the progressive control of tsetse-transmitted animal trypanosomosis in Kenya. Parasites Vectors. (2020) 13:1–12. doi: 10.1186/s13071-020-04156-5
239. Ferrer E, Alfonso P, Ippoliti C, Abeledo M, Calistri P, Blanco P, et al. Development of an active risk-based surveillance strategy for avian influenza in Cuba. Prev Vet Med. (2014) 116:161–7. doi: 10.1016/J.PREVETMED.2014.05.012
240. Volkova VV, Bessell PR, Woolhouse MEJ, Savill NJ. Evaluation of risks of foot-and-mouth disease in Scotland to assist with decision making during the 2007 outbreak in the UK. Vet Record. (2011) 169:124. doi: 10.1136/vr.d2715
241. Hayama Y, Yamamoto T, Kobayashi S, Muroga N, Tsutsui T. Evaluation of the transmission risk of foot-and-mouth disease in Japan. J Vet Med Sci. (2015) 77:1167–70. doi: 10.1292/jvms.14-0461
242. Gierak A, Bocian Ł, Smietanka K. Identification of areas at increased risk of highly pathogenic avian influenza occurrence in commercial poultry in Poland. Avian Dis. (2019) 63:257–62. doi: 10.1637/0005-2086-63.SP1.257
243. Sangrat W, Thanapongtharm W, Poolkhet C. Identification of risk areas for foot and mouth disease in Thailand using a geographic information system-based multi-criteria decision analysis. Prev Vet Med. (2020) 185:105183. doi: 10.1016/j.prevetmed.2020.105183
244. Hardcastle AN, Osborne JCP, Ramshaw RE, Hulland EN, Morgan JD, Miller-Petrie MK, et al. Informing rift valley fever preparedness by mapping seasonally varying environmental suitability. Int J Inf Dis. (2020) 99:362–72. doi: 10.1016/J.IJID.2020.07.043
245. Boender GJ, Nodelijk G, Hagenaars TJ, Elbers ARW, De Jong MCM. Local spread of classical swine fever upon virus introduction into the Netherlands: mapping of areas at high risk. BMC Vet Res. (2008) 4:1–12. doi: 10.1186/1746-6148-4-9
246. Dutra LH, Molento MB, Naumann CRC, Biondo AW, Fortes FS, Savio D, et al. Mapping risk of bovine fasciolosis in the south of Brazil using geographic information systems. Vet Parasitol. (2010) 169:76–81. doi: 10.1016/j.vetpar.2009.12.015
247. Chen WJ, Lai SJ, Yang Y, Liu K, Li XL, Yao HW Li Y, et al. Mapping the distribution of anthrax in mainland China, 2005–2013. PLoS Negl Trop Dis. (2016) 10:2005–13. doi: 10.1371/journal.pntd.0004637
248. Richter V, Kattwinkel E, Firth CL, Marschik T, Dangelmaier M, Trauffler M, et al. Mapping the global prevalence of bovine viral diarrhoea virus infection and its associated mitigation programmes. Vet Record. (2019) 184:711. doi: 10.1136/vr.105354
249. Kracalik IT, Kenu E, Ayamdooh EN, Allegye-Cudjoe E, Polkuu PN, Frimpong JA, et al. Modeling the environmental suitability of anthrax in Ghana and estimating populations at risk: implications for vaccination and control. PLoS Negl Trop Dis. (2017) 11:1–17. doi: 10.1371/journal.pntd.0005885
250. Leta S, Fetene E, Mulatu T, Amenu K, Jaleta MB, Beyene TJ, et al. Modeling the global distribution of Culicoides imicola: an Ensemble approach. Sci Rep. (2019) 9:11487. doi: 10.1038/s41598-019-50765-1
251. Bennema SC, Molento MB, Scholte RG, Carvalho OS, Pritsch I. Modelling the spatial distribution of Fasciola hepatica in bovines using decision tree, logistic regression and GIS query approaches for Brazil. Parasitology. (2017) 144:1677–85. doi: 10.1017/S0031182017000786
252. Rousseau R, McGrath G, McMahon BJ, Vanwambeke SO. Multi-criteria decision analysis to model ixodes ricinus habitat suitability. Ecohealth. (2017) 14:591–602. doi: 10.1007/s10393-017-1247-8
253. Bajardi P, Barrat A, Savini L, Colizza V. Optimizing surveillance for livestock disease spreading through animal movements. J Royal Soc Interface. (2012) 9:2814–25. doi: 10.1098/rsif.2012.0289
254. Epp TY, Waldner C, Berke O. Predictive risk mapping of west nile virus (WNV) infection in Saskatchewan horses. Can J Vet Res. (2011) 75:161–70.
255. Hill A, Gillings S, Berriman A, Brouwer A, Breed AC, Snow L, et al. Quantifying the spatial risk of Avian Influenza introduction into British poultry by wild birds. Sci Rep. (2019) 9:1–8. doi: 10.1038/s41598-019-56165-9
256. Giannakopoulos A, Valiakos G, Papaspyropoulos K, Dougas G, Korou LM, Tasioudi KE, et al. Rabies outbreak in Greece during 2012-2014: Use of geographical information system for analysis, risk assessment and control. Epidemiol Infect. (2016) 144:3068–79. doi: 10.1017/S0950268816001527
257. Leblond A, Sandoz A, Lefebvre G, Zeller H, Bicout DJ. Remote sensing based identification of environmental risk factors associated with West Nile disease in horses in Camargue, France. Prev Vet Med. (2007) 79:20–31. doi: 10.1016/j.prevetmed.2006.11.008
258. Kauffman M, Peck D, Scurlock B, Logan J, Robinson T, Cook W, et al. Risk assessment and management of brucellosis in the southern greater Yellowstone area (I): a citizen-science based risk model for bovine brucellosis transmission from elk to cattle. Prev Vet Med. (2016) 132:88–97. doi: 10.1016/j.prevetmed.2016.08.004
259. Ortiz-Pelaez A, Pfeiffer DU, Tempia S, Otieno FT Aden HH, Costagli R. Risk mapping of Rinderpest sero-prevalence in Central and Southern Somalia based on spatial and network risk factors. BMC Vet Res. (2010) 6:1–14. doi: 10.1186/1746-6148-6-22
260. Boender GJ, Hagenaars TJ, Bouma A, Nodelijk G, Elbers ARW, De Jong MCM, et al. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comput Biol. (2007) 3:e71. doi: 10.1371/JOURNAL.PCBI.0030071
261. Willgert KJE, Schroedle B, Schwermer H. Spatial analysis of bluetongue cases and vaccination of Swiss cattle in 2008 and 2009. Geospat Health. (2011) 5:227–37. doi: 10.4081/gh.2011.175
262. Lim JS, Min KD, Ryu S, Hwang SS, Cho SI. Spatial analysis to assess the relationship between human and bovine brucellosis in South Korea, 2005–2010. Sci Rep. (2019) 9:1–9. doi: 10.1038/s41598-019-43043-7
263. Fonseca O, Moya VM, Montano D de. las N, Centelles Y, Percedo MI, Alfonso P. Spatial modeling of Oestrosis in sheep in Guantánamo province, Cuba. Small Ruminant Res. (2018) 164:32–8. doi: 10.1016/j.smallrumres.2018.05.001
264. De Clercq EM, Leta S, Estrada-Peña A, Madder M, Adehan S, Vanwambeke SO. Species distribution modelling for Rhipicephalus microplus (Acari: Ixodidae) in Benin, West Africa: comparing datasets and modelling algorithms. Prev Vet Med. (2015) 118:8–21. doi: 10.1016/j.prevetmed.2014.10.015
265. Boender GJ, Elbers ARW, Jong MCM de. Spread of avian influenza in The Netherlands: identifying areas at high risk. Vet Ital. (2007) 43:605–9.
266. Pray IW, Wakeland W, Pan W, Lambert WE, Garcia HH, Gonzalez AE, et al. Understanding transmission and control of the pork tapeworm with CystiAgent: a spatially explicit agent-based model. Parasites Vectors. (2020) 13:1–13. doi: 10.1186/S13071-020-04226-8
267. Lian M, Warner RD, Alexander JL, Dixon KR. Using geographic information systems and spatial and space-time scan statistics for a population-based risk analysis of the 2002 equine West Nile epidemic in six contiguous regions of Texas. Int J Health Geogr. (2007) 6:1–10. doi: 10.1186/1476-072X-6-42
268. Downs J, Vaziri M, Jenkins A, Unnasch T. Validation of a risk index model for predicting eastern equine encephalitis virus transmission to horses in Florida. J Med Entomol. (2018) 55:1143–9. doi: 10.1093/jme/tjy067
269. Coffin JL, Monje F, Asiimwe-Karimu G, Amuguni HJ, Odoch T. A One Health, participatory epidemiology assessment of anthrax (Bacillus anthracis) management in Western Uganda. Soc Sci Med. (2015) 129:44–50. doi: 10.1016/j.socscimed.2014.07.037
270. Nthiwa D, Alonso S, Odongo D, Kenya E, Bett B. A participatory epidemiological study of major cattle diseases amongst Maasai pastoralists living in wildlife-livestock interfaces in Maasai Mara, Kenya. Trop Anim Health Prod. (2019) 51:1097–103. doi: 10.1007/s11250-018-01790-1
271. Ghafar A, McGill D, Stevenson MA, Badar M, Kumbher A, Warriach HM, et al. Participatory investigation of bovine health and production issues in Pakistan. Front Vet Sci. (2020) 7:248. doi: 10.3389/FVETS.2020.00248
272. Hlatshwayo M, Mbati PA. A survey of tick control methods used by resource-poor farmers in the Qwa-Qwa area of the eastern Free State Province, South Africa. Onderstepoort J Vet Res. (2005) 72:245–9. doi: 10.4102/ojvr.v72i3.202
273. Knight-Jones TJD, Mylrea GE, Kahn S. Animal production food safety: Priority pathogens for standard setting by the world organisation for animal health. OIE Rev Sci Tech. (2010) 29:523–35. doi: 10.20506/rst.29.3.1994
274. Alemu B, Desta H, Kinati W, Mulema AA, Gizaw S, Wieland B. Application of mixed methods to identify small ruminant disease priorities in Ethiopia. Front Vet Sci. (2019) 6:1–18. doi: 10.3389/fvets.2019.00417
275. Gale P, Brouwer A, Ramnial V, Kelly L, Kosmider R, Fooks AR, et al. Assessing the impact of climate change on vector-borne viruses in the EU through the elicitation of expert opinion. Epidemiol Infect. (2010) 138:214–25. doi: 10.1017/S0950268809990367
276. Motta P, Porphyre T, Handel IG, Hamman SM, Ngwa VN, Tanya VN, et al. Characterizing livestock markets, primary diseases, and key management practices along the livestock supply chain in Cameroon. Front Vet Sci. (2019) 6:101. doi: 10.3389/FVETS.2019.00101
277. Onono JO, Wieland B, Rushton J. Constraints to cattle production in a semiarid pastoral system in Kenya. Trop Anim Health Prod. (2013) 45:1415–22. doi: 10.1007/s11250-013-0379-2
278. Percedo Abreu MI, Guitián J, Herbert-Hackshaw K, Pradel J, Bourriez L, Petit-Sinturel M, et al. Developing a disease prevention strategy in the Caribbean: the importance of assessing animal health-related risks at regional level. OIE Rev Sci Tech. (2011) 30:725–31. doi: 10.20506/rst.30.3.2070
279. Zeleke M, Bekele T. Effect of season on the productivity of camels (Camelus dromedarius and the prevalence of their major parasites in Eastern Ethiopia. Trop Anim Health Prod. (2001) 33:321–9. doi: 10.1023/A:1010540120119
280. Boersema JSC, Noordhuizen JPTM, Lievaart JJ. Hazard perception of Dutch farmers and veterinarians related to dairy young stock rearing. J Dairy Sci. (2013) 96:5027–34. doi: 10.3168/jds.2012-6276
281. Gizaw S, Desta H, Alemu B, Tegegne A, Wieland B. Importance of livestock diseases identified using participatory epidemiology in the highlands of Ethiopia. Trop Anim Health Prod. (2020) 52:1745–57. doi: 10.1007/s11250-019-02187-4
282. Chenais E, Fischer K. Increasing the local relevance of epidemiological research: situated knowledge of cattle disease among Basongora pastoralists in Uganda. Front Vet Sci. (2018) 5:1–12. doi: 10.3389/fvets.2018.00119
283. Stringer AP, Christley RM, Bell CE, Gebreab F, Tefera G, Reed K, et al. Owner reported diseases of working equids in central Ethiopia. Equine Vet J. (2017) 49:501–6. doi: 10.1111/evj.12633
284. Shiferaw TJ, Moses K, Manyahilishal KE. Participatory appraisal of foot and mouth disease in the Afar pastoral area, northeast Ethiopia: implications for understanding disease ecology and control strategy. Trop Anim Health Prod. (2010) 42:193–201. doi: 10.1007/s11250-009-9405-9
285. Chatikobo P, Choga T, Ncube C, Mutambara J. Participatory diagnosis and prioritization of constraints to cattle production in some smallholder farming areas of Zimbabwe. Prev Vet Med. (2013) 109:327–33. doi: 10.1016/j.prevetmed.2012.10.013
286. Chenais E, Wennström P, Kartskhia N, Fischer K, Risatti G, Chaligava T, et al. Perceptions of pastoralist problems: a participatory study on animal management, disease spectrum and animal health priorities of small ruminant pastoralists in Georgia. Prev Vet Med. (2021) 193:105412. doi: 10.1016/j.prevetmed.2021.105412
287. Jaspersen JG, Montibeller G. probability elicitation under severe time pressure: a rank-based method. Risk Anal Off Pub Soc Risk Anal. (2015) 35:1317–35. doi: 10.1111/RISA.12357
288. Upjohn MM, Attwood GA, Lerotholi T, Pfeiffer DU, Verheyen KLP. Quantitative versus qualitative approaches: a comparison of two research methods applied to identification of key health issues for working horses in Lesotho. Prev Vet Med. (2013) 108:313–20. doi: 10.1016/J.PREVETMED.2012.11.008
289. Domenech J, Lubroth J, Eddi C, Martin V, Roger F. Regional and international approaches on prevention and control of animal transboundary and emerging diseases. Ann N Y Acad Sci. (2006) 1081:90–107. doi: 10.1196/annals.1373.010
290. Anzuino K, Knowles TG, Lee MRF, Grogono-Thomas R. Survey of husbandry and health on UK commercial dairy goat farms. Vet Record. (2019) 185:105174. doi: 10.1136/vr.105274
291. Loria GR, Ruocco L, Ciaccio G, Iovino F, Nicholas RAJ, Borrello S. The implications of eu regulation 2016/429 on neglected diseases of small ruminants including contagious Agalactia with particular reference to Italy. Animals. (2020) 10:900. doi: 10.3390/ani10050900
292. Byaruhanga C, Oosthuizen MC, Collins NE, Knobel D. Using participatory epidemiology to investigate management options and relative importance of tick-borne diseases amongst transhumant zebu cattle in Karamoja Region, Uganda. Prev Vet Med. (2015) 122:287–97. doi: 10.1016/j.prevetmed.2015.10.011
293. Mkwanazi MV, Ndlela SZ, Chimonyo M. Utilisation of indigenous knowledge to control ticks in goats: a case of KwaZulu-Natal Province, South Africa. Trop Anim Health Prod. (2020) 52:1375–83. doi: 10.1007/s11250-019-02145-0
294. Campbell Z, Coleman P, Guest A, Kushwaha P, Ramuthivheli T, Osebe T, et al. Prioritizing smallholder animal health needs in East Africa, West Africa, and South Asia using three approaches: Literature review, expert workshops, and practitioner surveys. Prev Vet Med. (2021) 189:105279. doi: 10.1016/J.PREVETMED.2021.105279
295. Ayele B, Tigre W, Deresa B. Investigation of major cattle production constraints in Kembata Tambaro zone of Southern Ethiopia using participatory epidemiology methods. Trop Anim Health Prod. (2016) 48:109–15. doi: 10.1007/s11250-015-0928-y
296. Jewell CP, Kypraios T, Christley RM, Roberts GO. A novel approach to real-time risk prediction for emerging infectious diseases: a case study in Avian Influenza H5N1. Prev Vet Med. (2009) 91:19–28. doi: 10.1016/j.prevetmed.2009.05.019
297. Frössling J, Nusinovici S, Nöremark M, Widgren S, Lindberg A. A novel method to identify herds with an increased probability of disease introduction due to animal trade. Prev Vet Med. (2014) 117:367–74. doi: 10.1016/J.PREVETMED.2014.07.013
298. Smith RL, Sanderson MW, Renter DG, Larson R, White B. A stochastic risk-analysis model for the spread of bovine viral diarrhea virus after introduction to naïve cow-calf herds. Prev Vet Med. (2010) 95:86–98. doi: 10.1016/j.prevetmed.2010.02.009
299. Groenendaal H, Nielen M, Hesselink JW. Development of the Dutch Johne's disease control program supported by a simulation model. Prev Vet Med. (2003) 60:69–90. doi: 10.1016/S0167-5877(03)00083-7
300. Webb CT, Ferrari M, Lindström T, Carpenter T, Dürr S, Garner G, et al. Ensemble modelling and structured decision-making to support emergency disease management. Prev Vet Med. (2017) 138:124–33. doi: 10.1016/j.prevetmed.2017.01.003
301. Meyer A, McAloon CG, Tratalos JA, More SJ, Citer LR, Graham DA, et al. Modeling of alternative testing strategies to demonstrate freedom from Mycobacterium avium ssp. paratuberculosis infection in test-negative dairy herds in the Republic of Ireland. J Dairy Science. (2019) 102:2427–42. doi: 10.3168/JDS.2018-14883
302. Mushayabasa S, Tapedzesa G. Modeling the Effects of Multiple Intervention Strategies on Controlling Foot-and-Mouth Disease. Biomed Res Int. (2015) 2015:234. doi: 10.1155/2015/584234
303. Masry IE, Rijks J, Peyre M, Taylor N, Lubroth J, Jobre Y. Modelling influenza A H5N1 vaccination strategy scenarios in the household poultry sector in Egypt. Trop Anim Health Prod. (2014) 46:57–63. doi: 10.1007/s11250-013-0446-8
304. Bennett RM, McClement I, McFarlane ID, Parker CD. Modelling of control options for an outbreak of Mycoplasma gallisepticum in egg production: a decision support tool. Vet J. (2013) 198:661–5. doi: 10.1016/J.TVJL.2013.09.058
305. Backer JA, Hagenaars TJ, Van Roermund HJW, De Jong MCM. Modelling the effectiveness and risks of vaccination strategies to control classical swine fever epidemics. J the Royal Soc Interface. (2009) 6:849–61. doi: 10.1098/rsif.2008.0408
306. Perez AM, Ward MP, Ritacco V. Modelling the feasibility of bovine tuberculosis eradication in Argentina. OIE Rev Sci Tech. (2011) 30:635–43. doi: 10.20506/rst.30.2.2056
307. Hayama Y, Kobayashi S, Nishida T, Muroga N, Tsutsui T. Network simulation modeling of equine infectious anemia in the non-racehorse population in Japan. Prev Vet Med. (2012) 103:38–48. doi: 10.1016/J.PREVETMED.2011.09.011
308. Vale GA, Hargrove JW, Lehane MJ, Solano P, Torr SJ. Optimal strategies for controlling riverine tsetse flies using targets: a modelling study. PLoS Negl Trop Dis. (2015) 9:e0003615. doi: 10.1371/JOURNAL.PNTD.0003615
309. Probert WJM, Jewell CP, Werkman M, Fonnesbeck CJ, Goto Y, Runge MC, et al. Real-time decision-making during emergency disease outbreaks. PLoS Comput Biol. (2018) 14:1–18. doi: 10.1371/journal.pcbi.1006202
310. Disney WT, Peters MA. Simulation modeling to derive the value-of-information for risky animal disease-import decisions. Prev Vet Med. (2003) 61:171–84. doi: 10.1016/j.prevetmed.2003.07.001
311. Harvey N, Reeves A, Schoenbaum MA, Zagmutt-Vergara FJ, Dubé C, Hill AE, et al. The North American animal disease spread model: a simulation model to assist decision making in evaluating animal disease incursions. Prev Vet Med. (2007) 82:176–97. doi: 10.1016/j.prevetmed.2007.05.019
312. te Beest DE, Hagenaars TJ, Stegeman JA, Koopmans MPG, van Boven M. Risk based culling for highly infectious diseases of livestock. Vet Res. (2011) 42:81. doi: 10.1186/1297-9716-42-81
313. Tomassen FHM, De Koeijer A, Mourits MCM, Dekker A, Bouma A, Huirne RBM, et al. A decision-tree to optimise control measures during the early stage of a foot-and-mouth disease epidemic. Prev Vet Med. (2002) 54:301–24. doi: 10.1016/S0167-5877(02)00053-3
314. Ng V, Sargeant JM. A stakeholder-informed approach to the identification of criteria for the prioritization of zoonoses in Canada. PLoS One. (2012) 7:e29752. doi: 10.1371/journal.pone.0029752
315. Martínez-lópez B, Ivorra B, Fernández-carrión E, Perez AM. A multi-analysis approach for space – time and economic evaluation of risks related with livestock diseases : the example of FMD in Peru. Prev Vet Med. (2014) 114:47–63. doi: 10.1016/j.prevetmed.2014.01.013
316. Del Rio Vilas VJ, Voller F, Montibeller G, Franco LA, Sribhashyam S, Watson E, et al. An integrated process and management tools for ranking multiple emerging threats to animal health. Prev Vet Med. (2013) 108:94–102. doi: 10.1016/J.PREVETMED.2012.08.007
317. Boden LA, Auty H, Reeves A, Rydevik G, Bessell P, McKendrick IJ. Animal health surveillance in Scotland in 2030: using scenario planning to develop strategies in the context of “Brexit.” Front Vet Sci. (2017) 4:1–19. doi: 10.3389/fvets.2017.00201
318. Stevenson MA, Sanson RL, Miranda AO, Lawrence KA, Morris RS. Decision support systems for monitoring and maintaining health in food animal populations. N Z Vet J. (2007) 55:264–72. doi: 10.1080/00480169.2007.36780
319. Berry EA, Hogeveen H, Hillerton JE. Decision tree analysis to evaluate dry cow strategies under UK conditions. J Dairy Res. (2004) 71:409–18. doi: 10.1017/S0022029904000433
320. Van Vaerenbergh B, Koenen F, Pauwels K, Quanten K, Boyen F, Declercq K, et al. Methodology of the biological risk classification of animal pathogens in Belgium. Rev Sci Tech. (2010) 29:513–22. doi: 10.20506/RST.29.3.1995
321. Xiao N, Yao JW, Ding W, Giraudoux P, Craig PS, Ito A. Priorities for research and control of cestode zoonoses in Asia. Inf Dis Poverty. (2013) 2:1–11. doi: 10.1186/2049-9957-2-16
322. Samaan G, Indriani R, Carrasco LR, Lokuge K, Cook AR, Kelly PM, et al. Prioritizing live bird markets at risk of avian influenza H5N1 virus contamination for intervention: a simple tool for low resource settings. Prev Vet Med. (2012) 107:280–5. doi: 10.1016/J.PREVETMED.2012.05.017
323. World Health Organization. Research Priorities for Zoonoses and Marginalized Infections. World Health Organization Technical Report Series. Geneva: WHO (2012).
324. Bertran K, Cortey M, Díaz I. The use of H-index to assess research priorities in poultry diseases. Poult Sci. (2020) 99:6503–12. doi: 10.1016/j.psj.2020.09.017
325. Murray AG, Wardeh M, McIntyre KM. Using the H-index to assess disease priorities for salmon aquaculture. Prev Vet Med. (2016) 126:199–207. doi: 10.1016/j.prevetmed.2016.02.007
326. Baltussen R, Niessen L. Priority setting of health interventions: The need for multi-criteria decision analysis. Cost Effect Res Allocat. (2006) 4:14. doi: 10.1186/1478-7547-4-14
327. Babo Martins S, Rushton J. Cost-effectiveness analysis: adding value to assessment of animal health welfare and production. Rev Sci Tech. (2014) 33:681–9. doi: 10.20506/RST.33.3.2312
328. Huang IB, Keisler J, Linkov I. Multi-criteria decision analysis in environmental sciences: ten years of applications and trends. Sci Total Environ. (2011) 409:3578–94. doi: 10.1016/J.SCITOTENV.2011.06.022
329. Mardani A, Jusoh A, Nor KMD, Khalifah Z, Zakwan N, Valipour A. Multiple criteria decision-making techniques and their applications – a review of the literature from 2000 to 2014. Econ Res-Ekonomska IstraŽivanja. (2015) 28:516–71. doi: 10.1080/1331677X.2015.1075139
Keywords: priority setting, animal health economics, resource allocation, risk assessment, spatial analysis, animal health investment, decision-making
Citation: Amenu K, McIntyre KM, Moje N, Knight-Jones T, Rushton J and Grace D (2023) Approaches for disease prioritization and decision-making in animal health, 2000–2021: a structured scoping review. Front. Vet. Sci. 10:1231711. doi: 10.3389/fvets.2023.1231711
Received: 30 May 2023; Accepted: 06 September 2023;
Published: 06 October 2023.
Edited by:
Paola Scaramozzino, Institute of Experimental Zooprophylactic of the Lazio and Tuscany Regions (IZSLT), ItalyReviewed by:
Sara Savic, Scientific Veterinary Institute Novi Sad, SerbiaBiswajit Bhowmick, The University of Tennessee, Knoxville, United States
Jordi Casal, Autonomous University of Barcelona, Spain
Copyright © 2023 Amenu, McIntyre, Moje, Knight-Jones, Rushton and Grace. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kebede Amenu, ay5hbWVudUBjZ2lhci5vcmc=
 Kebede Amenu
Kebede Amenu K. Marie McIntyre
K. Marie McIntyre Nebyou Moje
Nebyou Moje Theodore Knight-Jones
Theodore Knight-Jones Jonathan Rushton
Jonathan Rushton Delia Grace
Delia Grace