
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 20 September 2023
Sec. Animal Nutrition and Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1230190
Introduction: The objective of this study was to investigate the effects of N-carbamylglutamate (NCG) supplementation on the growth performance, hindgut microbiota composition, and short-chain fatty acid (SCFA) contents in Charollais and Small Tail Han crossbred sheep.
Methods: A total of 16 female crossbred mutton sheep (Charollais × Small Tail Han), aged 4 months, with an initial body weight of 30.03 ± 0.08 kg, were utilized in a 60 days experiment. The sheep were divided into two groups based on their initial body weight. Each group consisted of 8 replicates, with each individual sheep considered as a replicate. The dietary treatments comprised a basal diet supplemented with either 0.00% or 0.12% NCG.
Results and discussion: Our findings indicate that NCG supplementation did not have a significant effect on the growth performance of mutton sheep. However, it did lead to changes in hindgut SCFA contents. Specifically, NCG supplementation increased the content of propanoic acid while decreasing acetic acid and hexanoic acid in the hindgut. Through microbiota analysis using the 16S rRNA technique, we identified Lachnospiraceae_NK3A20_group and Parasutterella as biomarkers for the hindgut microbiota in mutton sheep fed a diet containing NCG. Further analysis of the microbiota composition revealed that NCG supplementation significantly increased the abundance of Lachnospiraceae_NK3A20_group and Parasutterella, while decreasing unclassified_f_Lachnospiraceae and Lachnoclostridium. Correlation analysis between hindgut SCFA contents and microbiota composition revealed that the abundance of Lachnoclostridium was positively correlated with the contents of acetic acid and hexanoic acid, but negatively correlated with propanoic acid. Additionally, the abundance of Lachnospiraceae_NK3A20_group and Parasutterella was positively correlated with the content of propanoic acid, while being negatively correlated with acetic acid and hexanoic acid. Based on these findings, we conclude that dietary supplementation of 0.12% NCG can modulate hindgut SCFA contents in mutton sheep by regulating the composition of the hindgut microbiota.
In recent years, there has been a growing interest among animal nutritionists in studying the intestinal microbiota due to its close relationship with the growth and production performance of animals (1). The intestinal microbiota thrives in the nutrient-rich environment provided by the intestine and plays a pivotal role in modulating various host functions, including energy homeostasis, nutrient processing, and immune system development. Bacteria within the intestine produce proteins and metabolites that are influenced by the diet, and among them, short-chain fatty acids (SCFA) hold particular importance in maintaining intestinal homeostasis. SCFA are generated through the bacterial fermentation of dietary fibers and serve as essential fuels for intestinal epithelial cells. They regulate the functions, proliferation, and differentiation of intestinal epithelial cells, thus significantly influencing intestinal function and host metabolism (2).
Given the significant impact of diet on the composition of the intestinal microbiota, animal nutritionists often explore strategies to modulate the intestinal microbiota by providing animals with different types of exogenous additives (3). The objective is to identify suitable additives that can effectively regulate the production of intestinal SCFA by modulating the intestinal microbiota and subsequently improving the animals’ growth performance. N-carbamylglutamate (NCG) is one such additive that has demonstrated efficacy in enhancing endogenous arginine synthesis (4). Acting as a functional analog of N-acetylglutamate, NCG helps restore or improve urea cycle function (5). By entering the mitochondria and activating the key enzyme carbamoyl phosphate synthase-1 in the urea cycle, NCG accelerates citrulline synthesis and promotes endogenous arginine production (6). In monogastric animals, NCG supplementation has been reported to improve growth and reproductive performance (7–9).
While limited research has investigated the effects of NCG supplementation on hindgut SCFA contents and the intestinal microbiota in ruminants, some studies have provided promising insights. For instance, Buchon et al. (10) found that rabbits fed a diet supplemented with NCG exhibited improved development of the jejunum and ileum. Zhang et al. (11) supplemented NCG in the diet of Tan sheep and observed a significant increase in carcass weight and net meat weight. Additionally, Zhao and Zhong (12) reported that daily supplementation of NCG in cows’ feed improved their antioxidant capacity. These findings suggest that NCG may function as a suitable exogenous additive for enhancing the performance of ruminants. In comparison to other crossbred sheep, the Charollais and Small Tail Han crossbred sheep have demonstrated excellent reproductive performance and disease resistance (13). However, improvements are needed in their growth performance. Given the significance of hindgut SCFA contents and intestinal microbiota for animal health, further investigation is warranted to explore the effects of NCG supplementation on the growth performance, hindgut microbiota composition, and SCFA contents of Charollais × Small Tail Han crossbred sheep. We hypothesize that NCG supplementation will increase hindgut SCFA contents in mutton sheep by modulating the hindgut microbiota composition, potentially resulting in enhanced growth performance.
A total of 16 healthy female crossbred mutton sheep (Charolais × Small Tail Han), aged 4 months, with similar initial body weight (30.03 ± 0.08 kg), were utilized in a 60 days experiment. The sheep were divided into two groups based on their initial body weight, with 8 replicates per group, and each sheep served as a replicate. The dietary treatments consisted of a basal diet supplemented with either 0.00% or 0.12% NCG. The dosage of NCG used in this study was determined based on previous research (14), and the NCG used was obtained from Anhui Pusheng Pharmaceutical, a commercial company.
The sheep were housed in a cleaned and disinfected facility with natural ventilation and programmed lighting schedule. They were fed twice a day at 7 am and 6 pm, with free access to feed and water throughout the experimental period. The dietary ingredients and their chemical composition are presented in Table 1. The methods and procedures used in this study were approved by the Ethics Committee of Jinzhou Medical University.
The individual body weights of the sheep were recorded weekly to calculate the average daily gain (ADG). The feed intake of each pen was measured weekly to calculate the average daily feed intake (ADFI). Feed efficiency was calculated as the ratio of ADG to ADFI (G:F).
On the final day of the experiment, four sheep were randomly selected from each group to obtain fresh stool samples for hindgut SCFA analysis using an Agilent Technologies Inc. 7,890-5,977 GC/MS (Agilent Technologies Inc., CA, United States). Data were manually processed after exportation from the instrument. The SCFA content of each sample was calculated as μg/mg = (sample concentration read by the instrument × final constant volume of the sample × the dilution factor)/sample weight.
Fresh stool samples obtained above were immediately placed in iceboxes and transported to the laboratory. Total DNA was extracted from the microbial community using the E.Z.N.A.® Soil DNA Kit (Omega Bio-Tek, Norcross, GA, United States) following the kit’s instructions. The quality of DNA extraction was assessed through 1% agarose gel electrophoresis, and the DNA density and purity were measured using the NanoDrop 2000 UV–vis spectrophotometer (Thermo Scientific, United States). The V3-V4 variable region of the 16S rRNA gene was amplified by PCR using primers 338F (5′-ACTCCTACGGGAGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Sequencing was performed using the Illumina MiSeq PE300/Novaseq PE250 platform. The original sequencing sequences were subjected to quality control using Fastp software (version 0.20.0). Line joining was performed using FLASH software (version 1.2.7) and UPARSE software (version 7.1). The operational taxonomic units (OTUs) were clustered at a 97% similarity level, and chimeric sequences were removed. The RDP Classifier (version 2.2) was used to annotate the species classification of each sequence, with comparisons made in the SILVA 16S rRNA database (V138) using a comparison threshold of 70%. Alpha-diversity was estimated using the Chao1, Shannon, and Simpson indices, while beta-diversity was assessed using the p-value of Adonis analysis. The LEfSe analysis was performed using LEfSe software, with a linear discriminant analysis score threshold set at 3.5.
Data on growth performance, hindgut SCFA contents, alpha-diversity of hindgut microbiota, and differences in hindgut microbiota at the genus level were analyzed using the student’s t-test procedure in SAS software (SAS Inst. Inc., Cary, NC, United States). The normality of the data was assessed using the Shapiro–Wilk test and QQ plots. Beta-diversity of the hindgut microbiota was analyzed using Adonis analysis. Spearman analysis was conducted to evaluate correlations among hindgut microbiota and hindgut SCFA contents. The results were presented as means ± standard deviation, with a probability value below 0.05 considered statistically significant.
Dietary supplementation of NCG had no significant effects on the growth performance of mutton sheep. The final body weight, ADG, ADFI, and feed efficiency did not differ among groups (Table 2).
The contents of acetic acid (p < 0.05) and hexanoic acid (p < 0.05) in the hindgut of mutton sheep fed with an NCG-containing diet were lower than those fed with a basal diet. However, those fed with an NCG-containing diet presented a higher content of propanoic acid in the hindgut (p < 0.05) (Table 3).
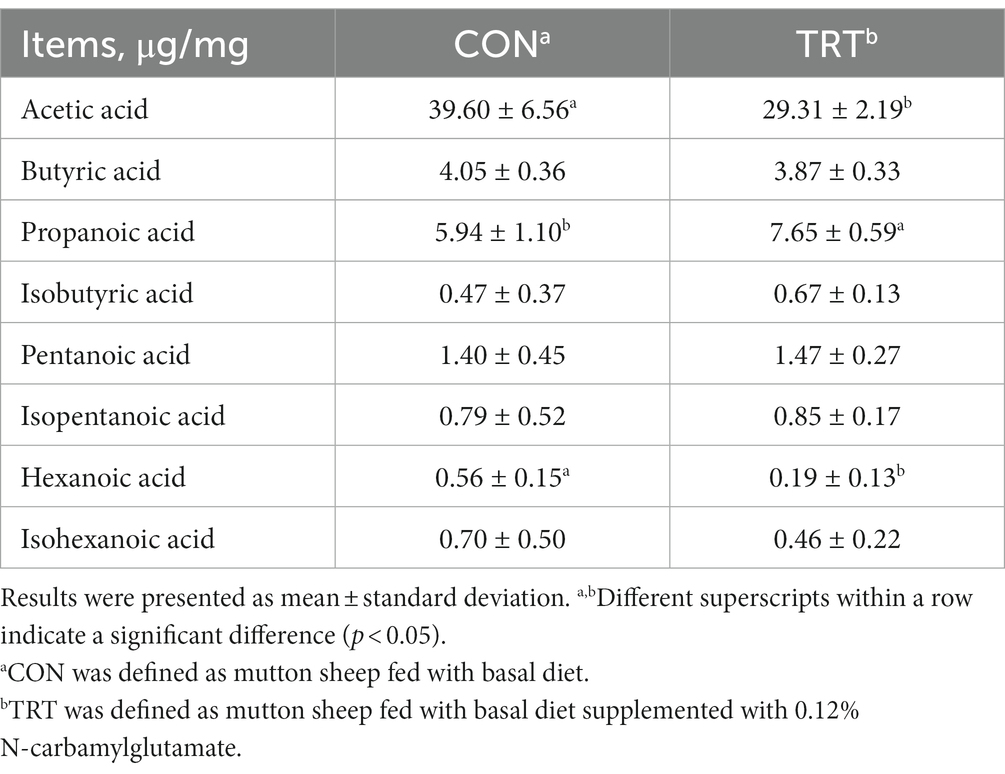
Table 3. Contents of short-chain fatty acids from hindgut as affected by N-carbamylglutamate supplementation.
NCG supplementation did not affect the Shannon, Simpson, Ace, and Chao1 indexes of the hindgut microbiota (Table 4).
Spearman correlation analysis revealed that the abundance of Lachnoclostridium was positively correlated with the contents of acetic acid (p < 0.05) and hexanoic acid (p < 0.05), but negatively correlated with propanoic acid (p < 0.01). Additionally, the abundance of Lachnospiraceae_NK3A20_group and Parasutterella were positively correlated with the content of propanoic acid (p < 0.05), while being negatively correlated with acetic acid (p < 0.05) and hexanoic acid (p < 0.05) (Table 5).
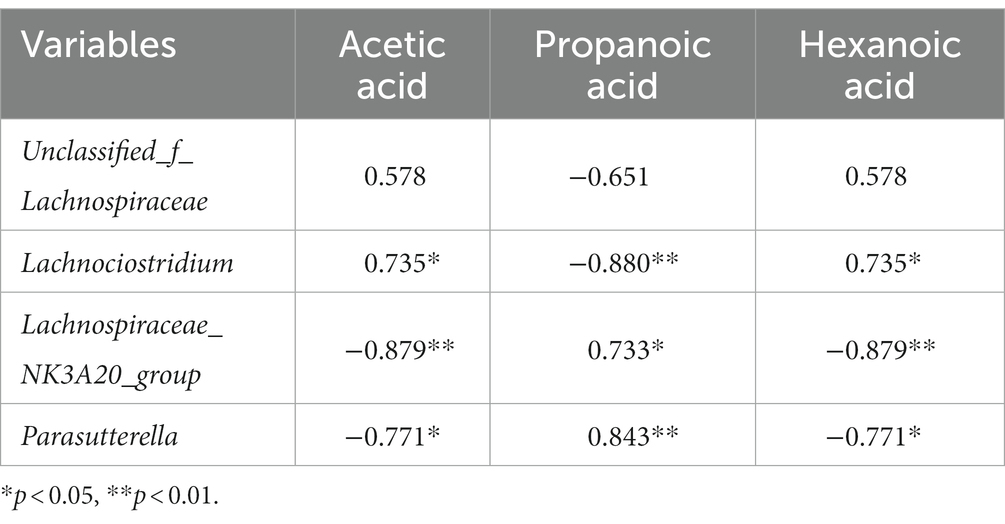
Table 5. Spearman correlation analysis among hindgut microbiota and hindgut short-chain fatty acid contents.
The Venn diagram showed that 559 bacterial OTUs were shared among groups, while 108 bacterial OTUs were unique in the hindgut microbiota of mutton sheep fed with a basal diet; 95 bacterial OTUs were unique in the hindgut microbiota of mutton sheep fed with an NCG-containing diet (Figure 1).
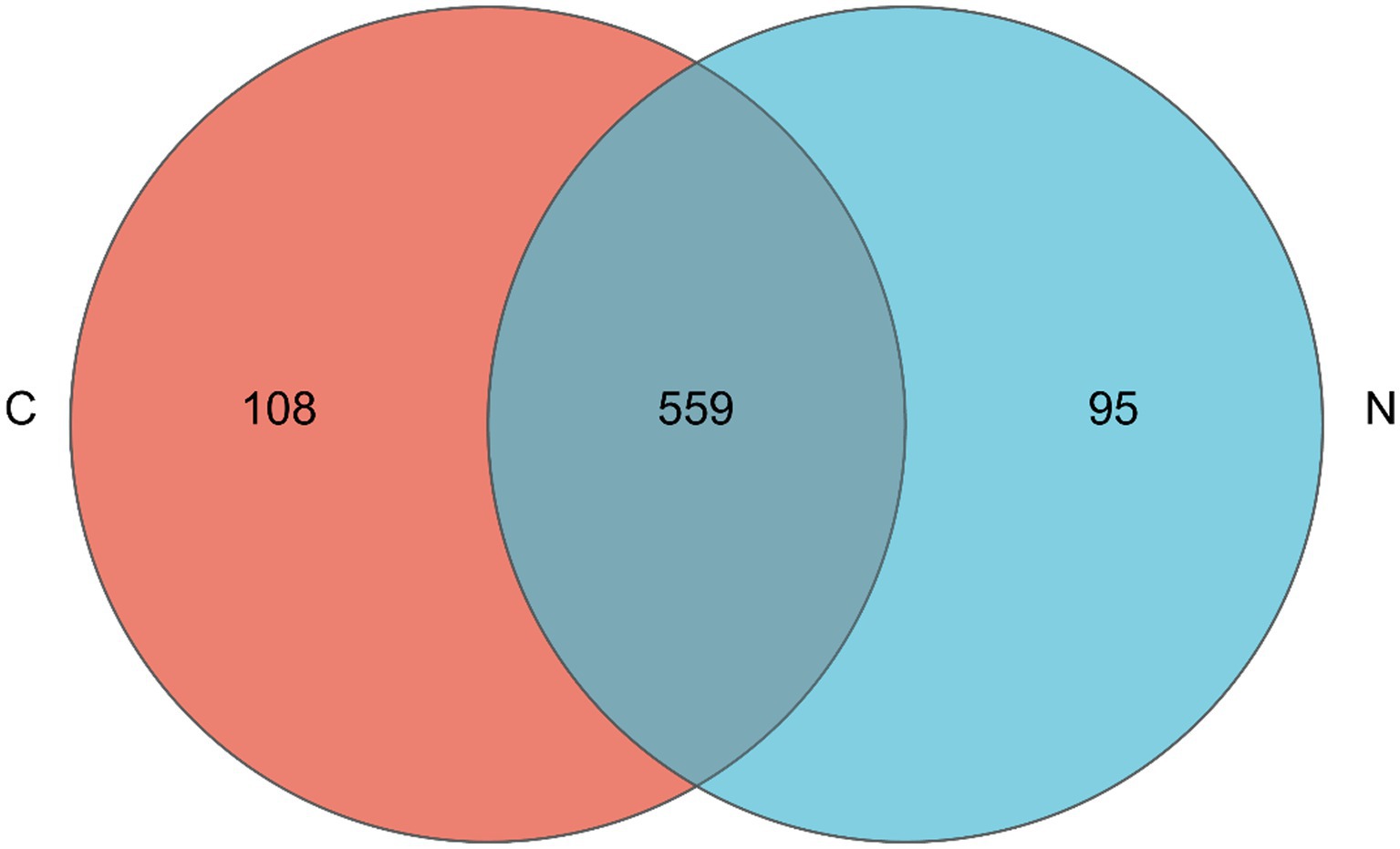
Figure 1. Venn diagram for hindgut microbiota of mutton sheep. Group C was defined as the sample from mutton sheep fed with basal diet. Group N was defined as the sample from mutton sheep fed with basal diet supplemented with 0.12% N-carbamylglutamate.
Beta-diversity, presented in a PCoA plot based on unweighted UniFrac distance, displayed that the samples from all groups did not separate, indicating a similar hindgut microbiota community composition between groups (Figure 2).
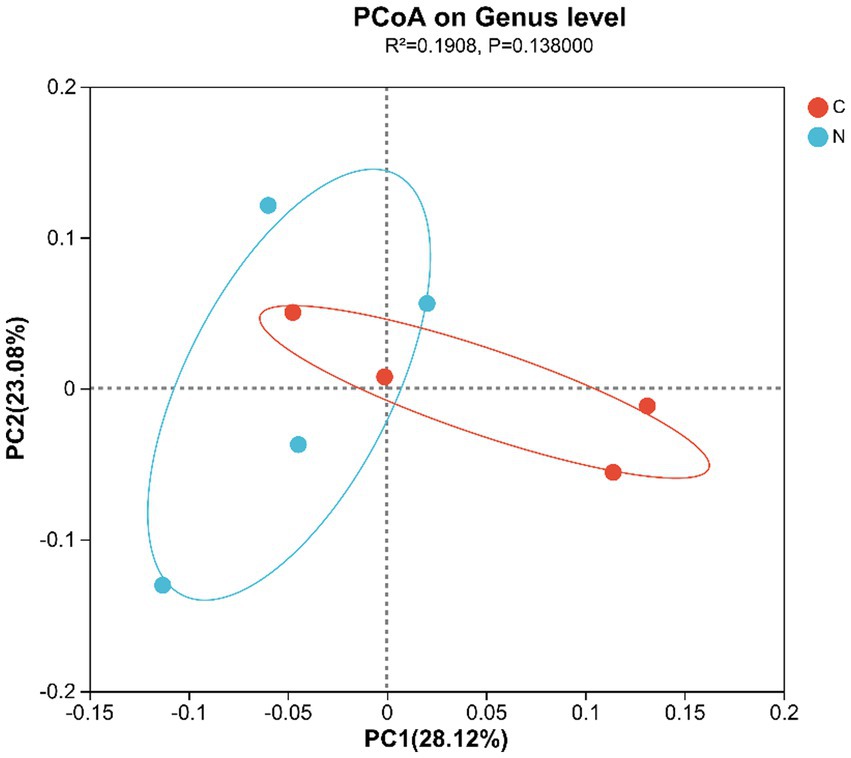
Figure 2. Differential analysis of the hindgut microbiota composition of mutton sheep, PCoA plot based on unweighted UniFrac distance. Group C was defined as the sample from mutton sheep fed with basal diet. Group N was defined as the sample from mutton sheep fed with basal diet supplemented with 0.12% N-carbamylglutamate.
The ten most predominant bacteria in the hindgut were unclassified_f_Lachnospiraceae, UCG-005, Parabacteroides, Treponema, Prevotellaceae_NK3B31_group, Phascolarctobacterium, Succinivibrio, Prevotella, norank_f_Muribaculaceae, and Bacteroides (Figure 3).
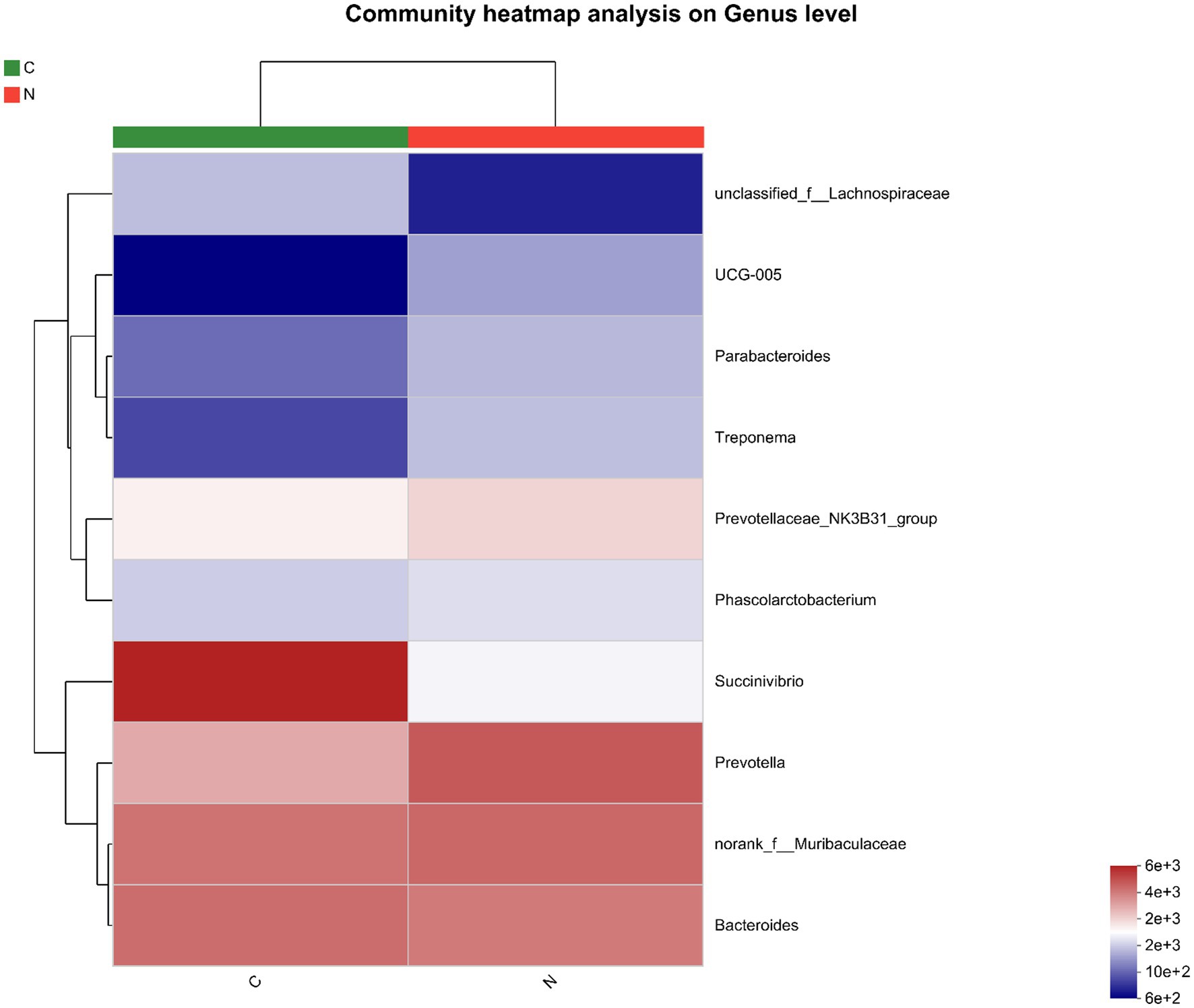
Figure 3. Heatmap of the ten most abundant bacteria of hindgut microbiota of mutton sheep on genus level. Group C was defined as the sample from mutton sheep fed with basal diet. Group N was defined as the sample from mutton sheep fed with basal diet supplemented with 0.12% N-carbamylglutamate.
Four genera (Lachnoclostridium, Escherichia-Shigella, Prevotellaceae_UCG-004, and Eubacterium_siraeum_group) with LDA scores <3.5 were identified as biomarkers for the hindgut of mutton sheep fed with a basal diet at the genus level. Additionally, two genera (Lachnospiraceae_NK3A20_group and Parasutterella) with LDA scores <3.5 were identified as biomarkers for the hindgut of mutton sheep fed with a basal diet supplemented with NCG at the genus level (Figure 4).
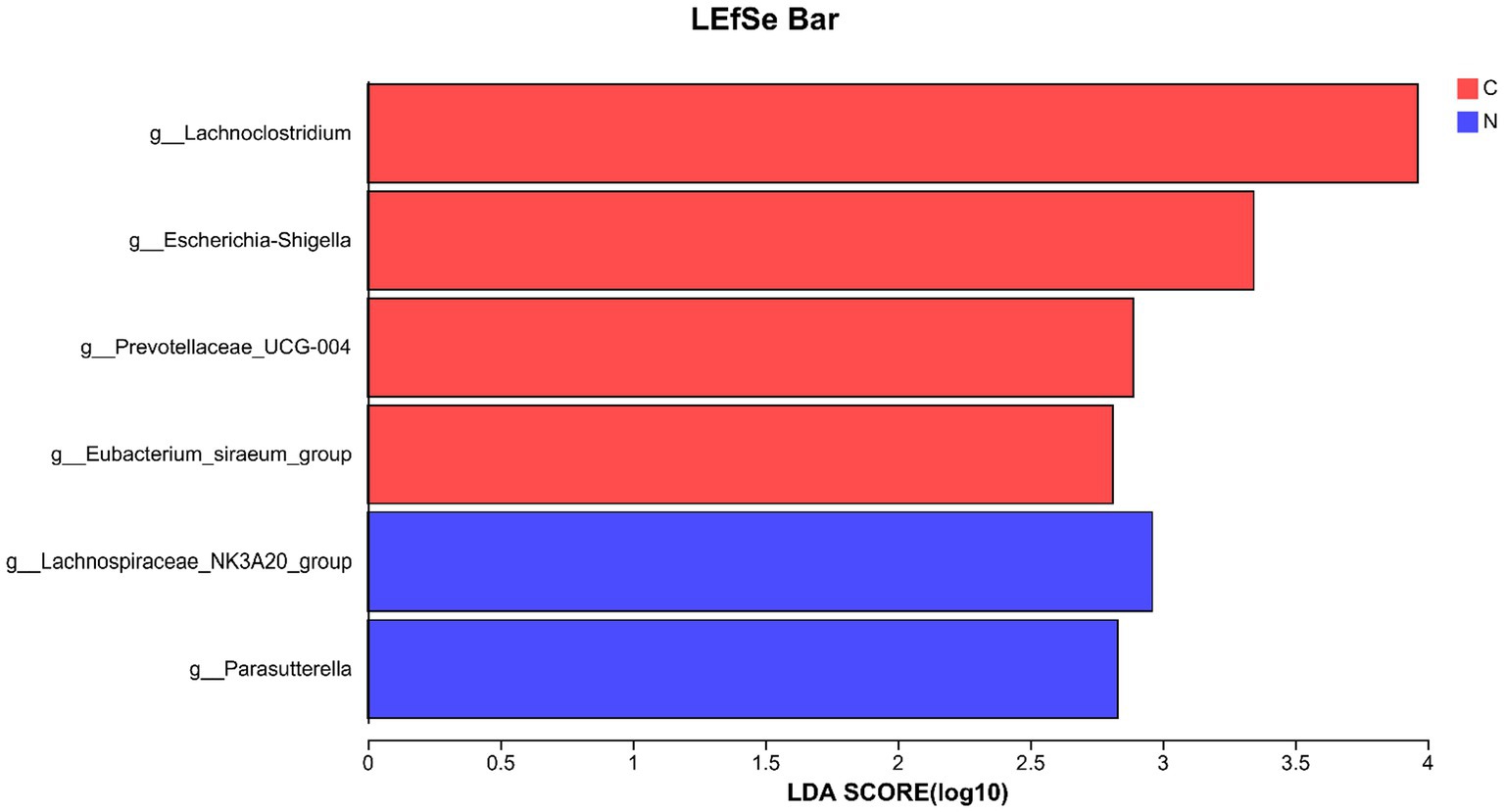
Figure 4. Biomarker of hindgut microbiota on genus level in different groups. The lineages in the values of least discriminant analysis higher than 3.5 are displayed. Group C was defined as the sample from mutton sheep fed with basal diet. Group N was defined as the sample from mutton sheep fed with basal diet supplemented with 0.12% N-carbamylglutamate.
Statistical differences in hindgut microbiota between groups, analyzed by t-test on genus level, revealed that dietary supplementation of NCG significantly increased the abundance of Lachnospiraceae_NK3A20_group (p < 0.05) and Parasutterella (p < 0.05), while decreasing unclassified_f_Lachnospiraceae (p < 0.05) and Lachnoclostridium (p < 0.05) (Figure 5).
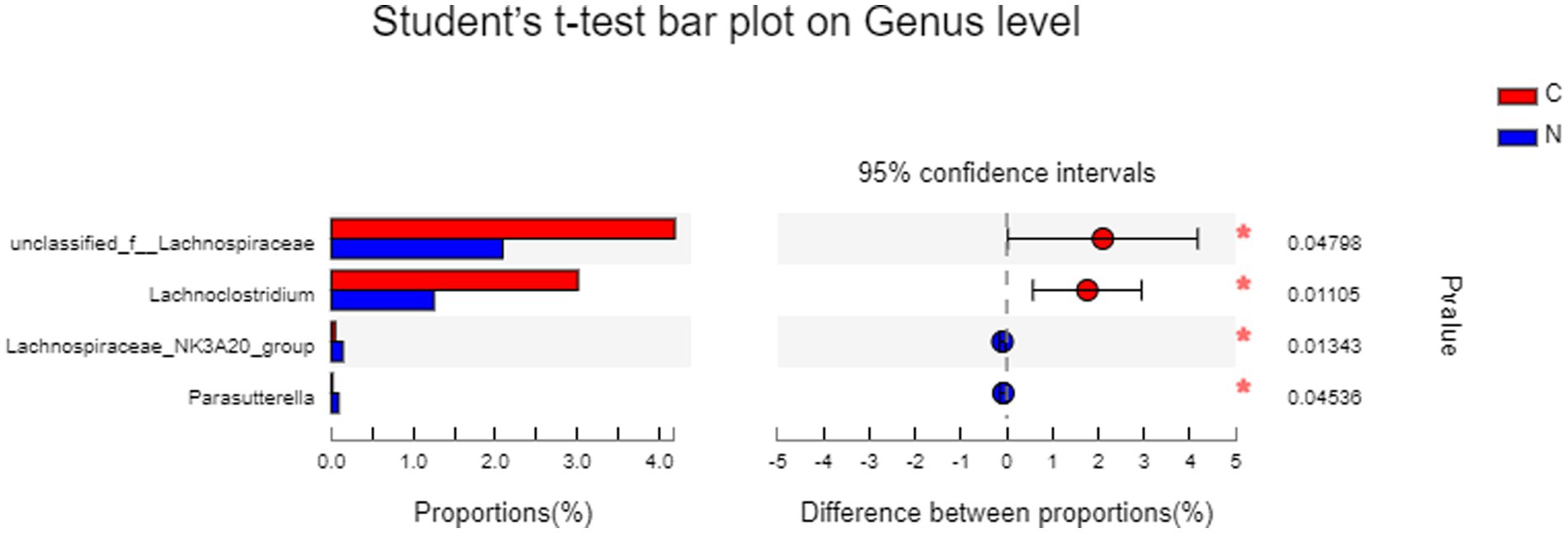
Figure 5. Statistical difference of hindgut microbiota between groups analyzed by t-test on genus level. Group C was defined as the sample from mutton sheep fed with basal diet. Group N was defined as the sample from mutton sheep fed with basal diet supplemented with 0.12% N-carbamylglutamate.
The hindgut microbiota encompasses a diverse array of organism communities that exert profound influences on the host’s development and metabolism. Preceding research has unveiled the modulatory potential of NCG upon the intestinal microbiota (15). Observations by Zhang et al. (16) demonstrated that dietary NCG supplementation ameliorated the diminished abundance of Clostridium, Lactobacillus, and Streptococcus within the colonic mucosa of lambs beset by intrauterine growth restriction. Likewise, Li et al. (17) noted that NCG supplementation invoked an elevation in unidentified_Prevotellaceae coupled with a decrease in Acetitomaculum, Marvinbryantia, and unidentified_Christensenellaceae within the fecal microbiome of heat-stressed lactating dairy cows. In line with this, our investigation revealed a consistent pattern of effects following NCG supplementation. Specifically, there was a noteworthy reduction in the abundance of unclassified_f_Lachnospiraceae and Lachnoclostridium, coupled with an increase in the abundance of Lachnospiraceae_NK3A20_group and Parasutterella within the fecal samples. Of particular significance, our analysis identified Lachnospiraceae_NK3A20_group and Parasutterella as distinctive biomarkers in mutton sheep that were fed the basal diet supplemented with NCG. The significance of these findings is underscored by the role of Lachnospiraceae_NK3A20_group, a recognized probiotic strain that actively participates in the production of SCFA (18). In parallel, the presence of Parasutterella stands as a reliable marker, indicating the presence of a health-promoting core microbiome in fecal compositions (19). Furthermore, the inverse correlation between the abundance of unclassified_f_Lachnospiraceae and the host’s immune status has been previously established (20). Notably, the heightened presence of Lachnoclostridium has been linked to instances of colorectal adenoma and cancer (21). Therefore, founded upon these robust findings, we proffer the notion that NCG supplementation exerts a favorable influence upon the composition of the hindgut microbiota. This influence is distinctly characterized by the augmentation of beneficial bacterial populations and the concomitant reduction of pathogenic strains.
SCFA serves as pivotal metabolites synthesized through the fermentation process carried out by the gut microbiota within the hindgut. These SCFAs substantially contribute to the overall health of the intestinal environment (22). In the context of our study, the administration of an NCG-enriched diet to mutton sheep resulted in elevated propanoic acid levels, concomitant with reduced acetic acid and hexanoic acid levels within the hindgut. Furthermore, the Spearman correlation analysis conducted within this study unveiled the intricate interplay between the abundance of specific bacterial genera and SCFAs content. These findings find support in the work of Rico et al. (23), who explored the interrelationship between the structure of the microbiome and fatty acid production stemming from cellulosic feedstocks in bison rumens. They discerned a negative correlation between the levels of acetic acid and the abundance of Lachnospiraceae_NK3A20_group. Similarly, in another study, Yi et al. (24) scrutinized the effects of the dietary concentrate-to-forage ratio on the bacterial community composition and metabolome within the rumens of yaks. They documented a positive correlation between propionate acid levels and the abundance of Lachnospiraceae_NK3A20_group. Therefore, we speculate that alterations in the SCFAs contents within the hindgut are intricately linked to shifts in the composition of the intestinal microbiota.
However, despite the discernible regulatory effects of dietary NCG supplementation on the composition of the hindgut microbiota and the contents of SCFA, no discernible discrepancies in growth performance were observed among the groups. This finding aligns with the outcomes of a prior study conducted by Sun et al. (25), wherein Holstein bulls were subjected to a daily regimen of NCG-infused diet, yet no notable impacts on body weight were discerned. The growth performance of animals represents an indicator influenced by a multitude of factors. As highlighted by Cai et al. (26), the augmentation of rumen fermentation in heat-stressed goats can be effectively achieved through the supplementation of exogenous probiotics, which serve to improve growth performance. Furthermore, Wang et al. (27) established that the introduction of organic acids can enhance the growth performance of lambs by enhancing their antioxidant ability. Consequently, our speculation rests on the premise that the enhancement of hindgut microbiota composition and SCFA contents, while influential, may not suffice to catalyze improvements in the growth performance of mutton sheep.
To conclude, our findings underscore the prospective utility of NCG as a dietary adjunct capable of modulating hindgut SCFA contents and the composition of the hindgut microbiota in mutton sheep. However, further investigations are warranted to delve into the underlying mechanisms at play and to comprehensively assess the enduring impacts of NCG supplementation on animal health and performance over the long term.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://figshare.com/, https://doi.org/10.6084/m9.figshare.21608295.v1.
The animal study was approved by the methods and procedures used in the present study were approved by the Ethics Committee of Jinzhou Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
WM and MY: conceptualization and methodology. SC: data collection. WM: writing—original draft preparation. CW: investigation, supervision, and writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
This research was funded by Liaoning Provincial Natural Science Foundation Project 2022-M S-387; Jinzhou Medical University College of Animal Husbandry and Veterinary Research Projects 2023rk02 and 2023rk10.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dang, DX, Zou, Q, Xu, Y, Cui, Y, Li, X, Xiao, Y, et al. Feeding broiler chicks with Bacillus subtilis, Clostridium butyricum, and Enterococcus faecalis mixture improves growth performance and regulates cecal microbiota. Probiotics Antimicrob Proteins. (2022). doi: 10.1007/s12602-022-10029-3
2. Martin-Gallausiaux, C, Marinelli, L, Blottière, HM, Larraufie, P, and Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. (2021) 80:37–49. doi: 10.1017/S0029665120006916
3. Dang, DX, Lee, H, Lee, SJ, Song, JH, Mun, S, Lee, KY, et al. Tributyrin and anise mixture supplementation improves growth performance, nutrient digestibility, jejunal villus height, and fecal microbiota in weaned pigs. Front Vet Sci. (2023) 10:1107149. doi: 10.3389/fvets.2023.1107149
4. Gu, FF, Wang, DM, Yang, DT, Liu, JX, and Ren, DX. Effects of dietary N-carbamoylglutamate supplementation on the milk amino acid profile and mozzarella cheese quality in mid-lactating dairy cows. J Dairy Sci. (2020) 103:4935–40. doi: 10.3168/jds.2019-17385
5. Olgac, A, Kasapkara, C, Kilic, M, Derinkuyu, B, Azapagasi, E, Kesici, S, et al. A rare urea cycle disorder in a neonate: N-acetylglutamate synthetase deficiency. Arch Argent Pediatr. (2020) 118:e545–8. doi: 10.5546/aap.2020.eng.e545
6. Lai, YJ, An, YN, Sun, W, Wang, CP, Gao, H, and Jiang, KJ. Research progress in the application of N-carbamyl glutamate in mutton sheep production. Chin J Anim Sci. (2018) 54:26–9.
7. Ma, Y, Yao, J, Zhou, S, Mi, Y, Li, J, and Zhang, C. Improvement of eggshell quality by dietary N-carbamylglutamate supplementation in laying chickens. Poult Sci. (2020) 99:4085–95. doi: 10.1016/j.psj.2020.04.004
8. Ma, W, Dang, DX, Zhang, J, Wang, C, and Li, D. Effects of dietary supplementation of N-carbamylglutamate on the haematology parameters, secondary sexual characteristics and testicular gene expression in roosters. J Anim Physiol Anim Nutr. (2022) 107:621–30. doi: 10.1111/jpn.13714
9. Ma, W, Lu, Y, and Wang, C. Production performance, egg quality, and uterine gene expression for layers as affected by N-Carbamylglutamate supplementation. Front Vet Sci. (2023) 10:88. doi: 10.3389/fvets.2023.1110801
10. Buchon, N, Broderick, NA, and Lemaitre, B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. (2013) 11:615–26. doi: 10.1038/nrmicro3074
11. Zhang, GJ, Kou, QF, He, LR, Niu, WZ, Zhang, LC, Wen, QQ, et al. Effects of rumen protective N-carbamylglutamate (NCG) on performance, slaughter performance and fat distribution of Tan sheep. Chin J Anim Sci. (2015) 51:28–32.
12. Zhao, X, and Zhong, Y. The effect of N-carbamylglutamic acid on the antioxidant performance and hormones of dairy cows. Chin J Vet Med. (2020) 56:16–9.
13. Lu, J., Ding, F., and Zhang, L. (Charlotte sheep × Small Tail Han sheep) reproductive performance test. Chinese livestock and poultry industry (2008) 2:74.
14. Ma, W, Wang, S, Zhang, J, Zhang, Y, Zhong, J, and Wang, C. Effect of N-aminetroyamine on the production of sheep and plasma antioxidant capacity. Feed Res. (2021) 9:16–9.
15. Li, W, Liu, B, Liu, A, Han, M, Yin, Y, Xu, G, et al. Dietary supplementation of N-carbamylglutamate promotes growth performance by modulating the homeostasis of gut microbiota in tilapia (Oreochromis niloticus). Aquac Rep. (2021) 20:100750. doi: 10.1016/j.aqrep.2021.100750
16. Zhang, H, Zheng, Y, Zha, X, Ma, Y, Liu, X, Elsabagh, M, et al. Dietary L-arginine or N-Carbamylglutamate alleviates colonic barrier injury, oxidative stress, and inflammation by modulation of intestinal microbiota in intrauterine growth-retarded suckling lambs. Antioxidants. (2022) 11:2251. doi: 10.3390/antiox11112251
17. Li, Y, Ma, N, Ren, L, Wang, M, Hu, L, Shen, Y, et al. Microbiome-metabolome responses in ruminal content and feces of lactating dairy cows with N-carbamylglutamate supplementation under heat stress. Front Vet Sci. (2022) 9:902001. doi: 10.3389/fvets.2022.1080927
18. Wang, Y, Nan, X, Zhao, Y, Wang, Y, Jiang, L, and Xiong, B. Ruminal degradation of rumen-protected glucose influences the ruminal microbiota and metabolites in early-lactation dairy cows. Appl Environ Microbiol. (2021) 87:e01908–20. doi: 10.1128/AEM.01908-20
19. Ju, T, Kong, JY, Stothard, P, and Willing, BP. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. (2019) 13:1520–34. doi: 10.1038/s41396-019-0364-5
20. Wu, G, Zhang, W, Zheng, N, Zu, X, Tian, S, Zhong, J, et al. Integrated microbiome and metabolome analysis reveals the potential therapeutic mechanism of Qing-Fei-Pai-Du decoction in mice with coronavirus-induced pneumonia. Front Cell Infect Microbiol. (2022) 2022:1208. doi: 10.3389/fcimb.2022.950983
21. Liang, JQ, Li, T, Nakatsu, G, Chen, YX, Yau, TO, Chu, E, et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut. (2020) 69:1248–57. doi: 10.1136/gutjnl-2019-318532
22. Dalile, B, Van Oudenhove, L, Vervliet, B, and Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
23. Rico, JL, Reardon, KF, and De Long, SK. Inoculum microbiome composition impacts fatty acid product profile from cellulosic feedstock. Bioresour Technol. (2021) 323:124532. doi: 10.1016/j.biortech.2020.124532
24. Yi, S, Dai, D, Wu, H, Chai, S, Liu, S, Meng, Q, et al. Dietary concentrate-to-forage ratio affects rumen bacterial community composition and metabolome of yaks. Front Nutr. (2022) 9:927206. doi: 10.3389/fnut.2022.927206
25. Sun, XL, Li, QF, Cao, YF, Yan, JL, Liu, TT, Zhang, YX, et al. Effects of N-carbamyl glutamate on rumen fermentation, rumen fermentation and methane emission in Holstein bulls. Acta Vet Zootech Sin. (2020) 51:1903–12.
26. Cai, L, Hartanto, R, Zhang, J, and Qi, D. Clostridium butyricum improves rumen fermentation and growth performance of heat-stressed goats in vitro and in vivo. Animals. (2021) 11:3261. doi: 10.3390/ani11113261
Keywords: N-carbamylglutamate, mutton sheep, hindgut microbiota, hindgut fermentation, short-chain fatty acid
Citation: Ma W, Yuan M, Chang S and Wang C (2023) N-carbamylglutamate supplementation regulates hindgut microbiota composition and short-chain fatty acid contents in Charollais and Small Tail Han crossbred sheep. Front. Vet. Sci. 10:1230190. doi: 10.3389/fvets.2023.1230190
Received: 28 May 2023; Accepted: 29 August 2023;
Published: 20 September 2023.
Edited by:
Bing Dong, China Agricultural University, ChinaCopyright © 2023 Ma, Yuan, Chang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunqiang Wang, d2Nxd29ybGRAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.