
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Vet. Sci. , 17 October 2023
Sec. Veterinary Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1210089
This article is part of the Research Topic Effective Options Regarding Spay or Neuter of Dogs View all 17 articles
 Navid Ziaei Darounkolaei1*
Navid Ziaei Darounkolaei1* Seyed Mohamad Sadegh Mousavi Kiasary2,3
Seyed Mohamad Sadegh Mousavi Kiasary2,3 Amirhoushang Behzadi4
Amirhoushang Behzadi4 Niki Nabavi Mosavi1
Niki Nabavi Mosavi1 Shima Mahmoodi Ferdowsi4
Shima Mahmoodi Ferdowsi4Objectives: To evaluate a modified ovariohysterectomy (OHE) technique performed by a single person and compare it with the conventional method based on time efficiency, trauma, and postoperative pain.
Methods: In a prospective, randomized, experimental study, 18 healthy, large, deep-chested, mixed-breed intact female dogs were randomly allocated to conventional (n = 9) and instrument shank-assisted (n = 9) groups. On the basis of video recordings, the various surgical step durations were analyzed: total surgery time (TST), pedicle intervention time (PIT), suspensory release time (SRT), shanking time (ShT), clamping time (ClpT), ligating time (LigT), and closure time (CT). The Glasgow composite pain scale short-form (GCMPS-SF), university of Melbourne pain scale (UMPS), and Visual Analogue Scales (VAS) were used to measure pain. C-reactive protein (CRP) fluctuation was also investigated. These evaluations were completed before and 6, 24, 48, and 72 h postoperatively.
Results: Instrument shank-assisted OHE was less time-consuming than conventional OHE (p = 0.005), improved PIT by 30.7% (6.44 min for both pedicles, p = 0.014), and correlated strongly with TST (ρ = 0.862, p = 0.003 and ρ = 0.955, p = 0.000, respectively). The two method’s surgical step durations were also TST = 47.40 ± 9.9 vs. 34.70 ± 6.7 min, PIT = 20.96 ± 5.78 vs. 14.52 ± 3.73 min, SRT = 78.97 ± 69.10 vs. ShT = 20.39 ± 8.18 s (p = 0.035), ClpT = 50.66 ± 45.04 vs. 63.55 ± 37.15 s (p = 0.662), LigT = 12.82 ± 3.37 vs. 8.02 ± 3.11 min (p = 0.005), and CT = 16.40 ± 4.5 vs. 11.60 ± 2.5 min (p = 0.013), respectively. While both techniques inflicted pain on the animals, the novel approach resulted in a reduction of pain at T6 (GCMPS-SF, p = 0.015 and VAS, p = 0.002), T24 (UMPS, p = 0.003), and T48 (GCMPS-SF, p = 0.015 and UMPS, p = 0.050). Both methods exhibited a peak in CRP level after 24 h, which subsequently returned to baseline after 48 h. However, the shank-assisted method demonstrated a significantly lower reduction in CRP level at the 48-h compared to the other group (p = 0.032).
Conclusion: Instrument shank-assisted technique permitted ovarian removal without an assistant, less damage to animals and reducing its time when compared to a conventional technique, and resulting in an alternative that causes less surgical stress and fatigue. Further research with a larger population size is required to determine the serum CRP levels as an alternative pain biomarker.
Elective ovariohysterectomy (OHE) is one of the most common surgeries done on dogs and cats (1). However, there are still problems with this technique, despite how common it is. Prior to the start of the operation, inexperienced graduates are anxious about how to do OHE on their own, and after the procedure is finished, they are concerned with the reliability of ligatures. As they consider how to do this procedure at an acceptable speed, their anxiety will increase. When a surgeon acquires experience, new issues develop, such as how to properly perform a high-risk, quick surgery with several implications, such as hemorrhage (2, 3), surgical trauma, organ manipulation, inflammation (4, 5), surgical stress (6), wound healing, and acute pain. The frequency of ovarian remnant syndrome was increasing mostly among young surgeons because of a concern about vascular rupture while breaking the suspensory ligament and exposing the ovaries inadequately, particularly in patients with obesity or other comorbidities (7–9). According to Berzon’s study, 1% of bitches experienced recurrent estrus following OHE by fourth-year veterinary students (2). However, the overall frequency of complications by final-year veterinary students was found to be as high as 29 (20.6%) out of 141 bitches, with 1 (5%) out of 20 experiencing post-surgical pseudopregnancy (7). As experience grows, less consideration is given to this problem. To achieve the aforementioned results, the surgeon may be skilled in rupturing the suspensory ligament to expose the ovaries and make their pedicle accessible for ligature placement (10). The success of the operation hinges on the surgeon’s willingness to pull, compress, strumming, tear, or sever the suspensory ligament (11), which is accompanied by extensive tissue damage. Drastic tissue damage and the time-consuming nature of its execution have made it the leader in painful surgeries in veterinary medicine, particularly for inexperienced surgeons. The experts, anesthesiologists, surgeons, and experienced researchers have adopted OHE as the acute surgical pain model because of the intensity of the pain caused by an experienced surgeon’s OHE (12–14). Acute postoperative pain has long been a problem for surgeons. Inadequate management of postoperative pain can result in a number of undesirable outcomes, including (1) physiological changes comprising tachycardia, hypertension (due to peripheral vasoconstriction, increased myocardial contractility, and systemic vascular resistance), cardiac arrhythmias, tachypnea, superficial respiratory pattern, pale mucous membranes, mydriasis, sialorrhea, and hyperglycemia (2), behavioral changes comprising vocalization (such as cries, whimpers, and growls), looking and licking the affected area, alteration of the facial expression (submissive attitude), self-mutilation, muscle stiffness or weakness, restlessness and anxiety, apathy and inactivity, aggression, fear, and depression, stereotypes, anorexia or hyporexia, reduction of grooming, prayer posture, sleep disorders, and (3) changes in biochemical parameters by the decrease of PaO2, PaCO2, HCO3, and an increase of H+, cortisol, lactate, and glucose (15–20), prolonging the recovery of patients (17, 21, 22). Despite these hazards, OHE is considered to be a very straightforward procedure, and numerous dog owners visit a veterinary clinic every day to have their pets spayed. Hence, the incidence of problems justifies the adoption of procedures, such as instrument shank-assisted OHE, as well as the many theories pertaining to surgical duration, pain, and trauma.
The length of surgery is a primary factor of the severity of postoperative issues, and it is inversely related to the surgeon’s skills and expertise (13). Skill is considered in two fields: non-technical skills (e.g., knowledge, situational awareness, decision-making, conscientiousness, intraoperative communication, teamwork, and leadership) (23–25) and technical skills (psychomotor actions). The latter would be gained and empowered via an educational program known as Objective Structured Assessment of Technical Skills (OSATS), which has been thoroughly introduced and verified (26, 27). Besides these abilities, some surgical procedures, such as minimally invasive procedures, are time-consuming and instrument-dependent (28, 29), which may not be an option for some animals. Naturally, each operation consists of a succession of procedures with varying durations, since some are simpler than others, such as entering the abdominal cavity through the linea alba, while others, such as the anatomical access to the ovaries, provide challenges. Understanding the elements that determine the duration of surgical steps as well as the total duration makes it simpler and more objective to estimate its sufficiency and leads to a more trustworthy conclusion.
Animal pain is hard to judge because it depends on many things, such as the amount of pain, the type of injury, and the animal’s own characteristics. As a result of the intricate nature of pain perception, several multidimensional questionnaires for qualitative pain assessment and validated behavioral scales have been developed to assess pain intensity in dogs (30, 31). Each of these methodologies assigns a different number of points to certain animal behavioral changes. The sum of the points indicates the observed pain level of the animal. Commonly used pain scales include the Glasgow composite measure pain scale (GCMPS-SF), the University of Melbourne pain scale (UMPS), and the Visual Analogue Scale (VAS), with corresponding ranges of 0–28, 0–24, and 0–10. Each of these solutions seems capable of filling some of the gaps left by the others, since they possess almost separate criteria with little overlap (32).
It is considered that the pain will always correspond to specific parameters that changed when the discomfort began or emerged. Clinical studies may describe a vast array of biomarker variations. Some parameters represent a range of events, but others may be directly triggered by the existing pain. The inflammatory response to surgical trauma or stress (33) activates the hypothalamus, causing it to release corticotropin-releasing hormone and arginine vasopressin, both of which stimulate anterior pituitary adrenocorticotropic hormone production, which in turn stimulates cortisol secretion by the adrenal cortex (19). Cortisol levels vary based on the severity or grade of surgery. The surgical interventions were categorized based on the modified Johns Hopkins surgical criteria, which delineate three levels of invasiveness: grade I, indicating minimally invasive procedures; grade II, indicating moderately invasive procedures; and grade III, indicating highly invasive procedures (34). When comparing grade 2 and grade 3 operations to grade 1, these differences may be identified, but they cannot be separated. Cortisol is a commonly utilized measurement for assessing stress levels and has demonstrated efficacy in evaluating intraoperative noxious stimuli. However, its sensitivity may be inadequate for capturing the variations that arise from repeated intraoperative noxious stimuli in a single animal (35). Cortisol levels seem to fluctuate with age, gender, disease, and the degree of surgical or anesthetic invasiveness. As a result, based on the research conducted so far, it is difficult to determine which is the primary cause of the alterations (36). Glucose is another biochemical parameter that surgery affects, and its clinical monitoring appears straightforward. Growth hormone (somatotrophin) levels increase in response to surgery and trauma; their release from the anterior pituitary is promoted by hypothalamic growth hormone-releasing factor (37, 38), which has an anti-insulin effect by inhibiting glucose uptake and utilization by cells. However, glucose utilization by cells is limited during surgery due to high cortisol levels (39). As a consequence, blood glucose levels rise. Furthermore, cortisol and catecholamines promote glucose production. In addition, a hyperglycemic response may result from a drop in insulin concentration during induction of anesthesia and during surgery, resulting in insulin secretion failure. Ultimately, the surgical invasion causes an increase in blood glucose content (40). Regardless of the causes of elevated glucose levels, the amount of rise in simple operations is negligible (19). Immunological mediators such as cytokines or interleukins (ILs) such as IL-1, IL-6, and tumor necrosis factor-alpha (TNF-α) mediate the rapid activation of the immune system following surgery. The presence of IL-6 depends on the extent of the surgical tissue damage (20). Despite the fact that the plasma level of IL-6 molecules with a short half-life increases within 30–60 min and becomes substantial after 2–4 h with quick returns to baseline, the maximum level may be attained 24 h after major operations, which may be prolonged 48–72 h postoperatively (19, 41). IL-6 stimulates the release of proteins, especially C-reactive protein (CRP), from the liver to commence the “acute phase response,” which comprises a variety of changes (19, 42). Based on a comparable study design (42), the postoperative CRP concentration increased more slowly and reached its peak after 48 h. After that, it went down at a slower rate, with a mean half-life of 62 h compared to 15 h for IL-6 (43). This might make it a valuable and accurate marker for regular diagnostics of systemic inflammation in dogs (44, 45) and a predictor of surgical trauma severity (46).
Therefore, one of the most difficult things for surgeons to do is choose a technique that will cause the least amount of damage, cause the least amount of pain after the surgery, and take the least amount of time. These are a trio of the key challenges for surgeons. The development or modification of minimally invasive techniques has only been able to improve the first two of these aspects, but with significant limitations (47). The aim of this study is to compare a modified OHE procedure that only needs one person to do it with the standard procedure in terms of time, trauma, and pain after the surgery. This will help researchers come up with a way to reduce the length of surgery, the amount of trauma, and immediate postoperative pain.
This research was authorized by the Iranian biomedical research ethics committee [IR.IAU.BABOL.REC.1399.004 (48), IR.IAU.BABOL.REC.1399.015 (49) and IR.IAU.BABOL.REC.1399.093 (50)] and conducted at the Babol branch of Azad University.
In a randomized controlled trial, 18 healthy, large, deep-chested intact female mixed-breed dogs were included. Animals were divided into two equal groups randomly by coin flipping, using a sterile suture sachet (51–53) (NZD) after inducing anesthesia and draping the surgical area. The sample size was evaluated using the software GPower 3.1.9.7. The presence of 9 dogs in each group resulted in a power of 0.9 (Power = 1 − β = 0.9) for TST with effect size d > 1.50 at a significance level of = 0.05.
Shelter dogs with ASA I (the American Society of Anesthesiologists) physical status enrolled in the study (54). The physical exam checked the patient’s heart rate (HR), breathing rate (RR), and rectal temperature (RT). It also checked for internal and external parasites, did a complete blood count, and looked at the Hb, PCV, CRP, and glucose levels in the blood. The study was conducted on bitches in diestrus, based on vaginal smear cytology. Animals with a body condition score between 4 and 6 out of 9 were chosen. Animals under 1 year old, in estrus, pregnant, or lactating, with a weak or no response to painful stimuli (a needlestick in the lower abdomen), with a history of physical or behavioral issues, or with abnormal vaginal secretions were excluded.
Each dog scheduled for surgery on a particular day spent 3 days before and 3 days after the surgery in a separate cage with free access to food and water. Ten days after surgery, the sutures were removed.
The animal’s resting vital parameters (HR, RR, and RT) were recorded before anesthesia. Anesthesia and analgesia were provided by acepromazine (10 mg mL−1, Neurotranq; Alfasan, Woerden, Holland), midazolam (5 mg mL−1, Midazolam; Caspian, Rasht, Iran), pethidine (100 mg 2 mL−1, Petholan; Adeka, İstanbul, Turkey), medetomidine (1,000 mcg mL−1, Dorbene Vet; Syva, León, Spain) and ketamine (50 mg mL−1, Ketamine HCl Inj.; Rotexmedica GmbH, Trittau, Germany), and ketorolac (30 mg mL−1, Ketorolac; Alborz Darou, Tehran, Iran). A 19G catheter was placed aseptically in the cephalic vein for a given lactated Ringer’s solution (250 mL, lactated Ringer’s solution; Shahid Ghazi, Tabriz, Iran) at a rate of 5 mL kg−1 h−1.
The animals were premedicated by acepromazine at 0.02 mg kg−1, midazolam at 0.5 mg kg−1, meperidine at 2 mg kg−1, medetomidine at 20 mcg kg−1, and ketamine at 4 mg kg−1 IM. They received ketorolac at 1 mg kg−1 immediately before surgical asepsis. The maintenance of anesthesia was achieved by administering a consistent anesthetic mixture (ketamine at 4 mg kg−1 and midazolam at 0.27 mg kg−1) at a variable rate of 0.2–0.5 mg kg−1, depending on the ketamine levels present in the mixture. The administration of the anesthetic was monitored through the use of several parameters, including SpO2, ECG, non-invasive blood pressure measured through a blood pressure cuff (size #4) placed proximal to the carpus over the radial artery at five-minute intervals, and respiration. The surgical procedure and manipulation were also taken into account during the administration of anesthesia. The Pm-7000vet, manufactured by Wuhan Zoncare Bio-medical Electronics Co., Ltd., was used to monitor animals. The animals’ cardiorespiratory parameters were monitored until they demonstrated full recovery.
A nociceptive response was defined as a 20% or more rise in heart rate over the base rate, accompanied with an increase in breathing frequency and blood pressure proportionate to a painful surgical procedure (20, 55, 56). Ketamine at 0.5 mg kg−1 was used for rescue analgesia during surgery. The timeline details of measures were provided in Table 1.
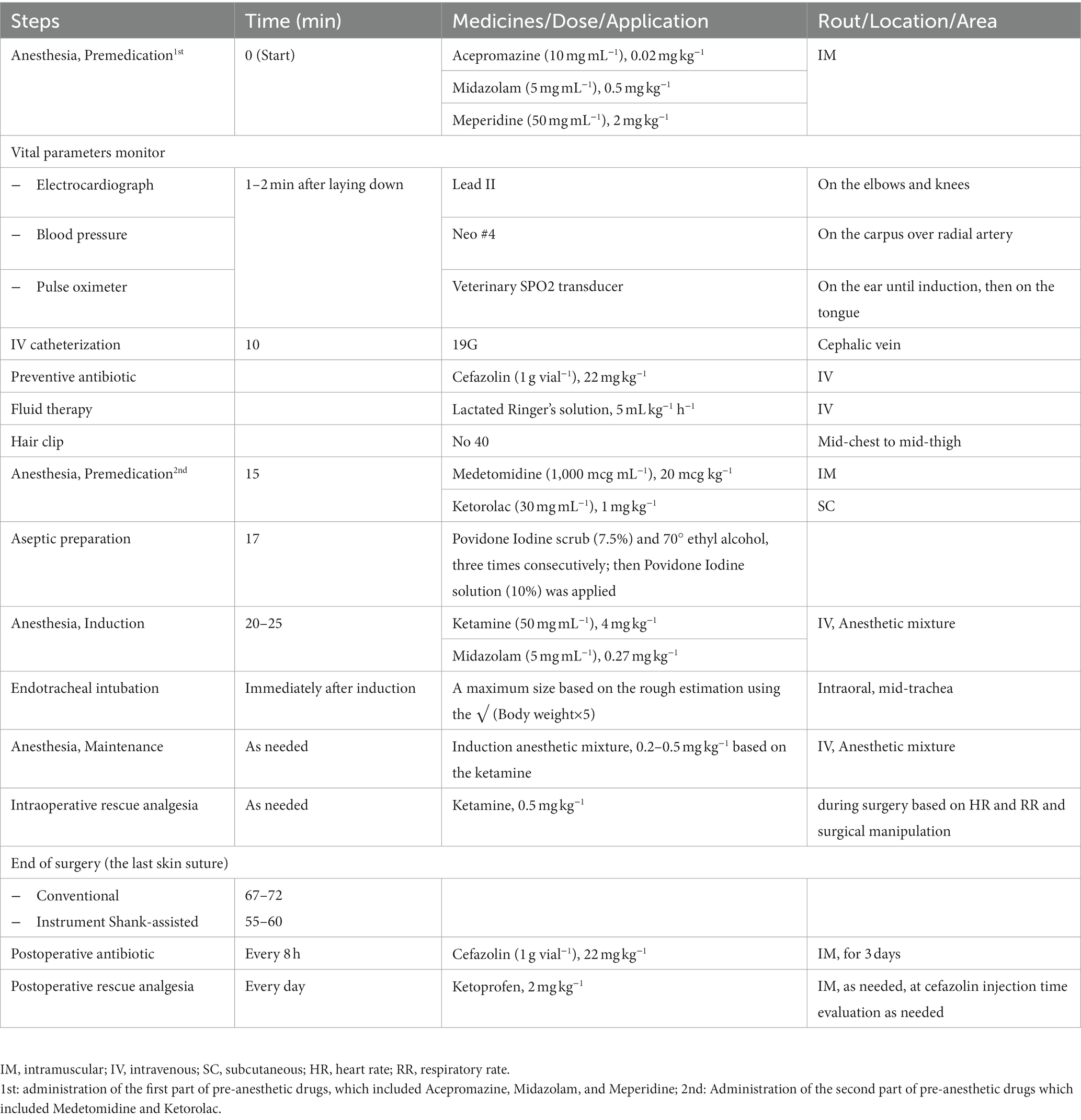
Table 1. The measures carried out during the conventional (n = 9) and Instrument Shank-assisted ovariohysterectomy (n = 9).
Two months after graduation, a female doctor of veterinary medicine (DVM) with minimum experience (according to the veterinary training course) in the conventional approach and no expertise with the new methodology has been selected as the surgeon (NNM). The selected surgeon and surgical team underwent a one-week training course for each surgery 10 days prior to the start of the study. During the training course, one surgery was done on each technique by the advisor, and then the techniques were randomly performed on 12 dogs (6 dogs each technique) by the surgeon conducting the study. The random approach has been a coin toss (51–53); thus, the first-day method was determined by tossing a coin, and the next day the opposite technique must be followed. The study surgeries were performed by the same team under the supervision of the dissertation adviser (NZD).
In dorsal recumbency, the ventral abdomen was aseptically prepped after hair removal from mid-chest to the end of the pelvic symphysis and the inner thigh. During pre-surgical aseptic preparation, the skin was alternately scrubbed three times with 7.5% povidone-iodine and 70% ethyl alcohol. After the final povidone-iodine scrub is complete, a 10% povidone-iodine solution is applied to the surgical field (1).
A ventral midline celiotomy was performed immediately caudal to the umbilicus and extending one-third of the way to the pubic rim (11) for both methods, in order to achieve the same incision length (57, 58).
In the control group, the triple-clamp OHE (1) was done after entrance into the abdominal cavity and control of the uterine horn without a spay hook. During this method, the surgeon’s dominant index finger is used to grab the left horn of the uterus. For organ manipulation, rat-toothed Crile forceps secured to the proper ligament were utilized. The suspensory ligament was strummed and released manually in the caudomedial direction. After creating a mesovarium window, two simple ligatures were placed on top of one another in the first clamp crush near the kidney. One transfixation ligature was then tightened in lieu of the middle clamp near the ovary [Polydioxanone (PDS II), 2–0]. The pedicle was transected and inspected for hemorrhage. The same techniques were then conducted on the contralateral pedicle. Separately, the cervix and uterine arteries were ligated (PDS II 2–0). Linea alba (PDS II 0), subcutaneous tissue (PDS II 2–0), and skin (monofilament Polyamide 0) were routinely closed. A stent bandage was then placed over the suture line.
After accessing the visceral organs, first the left uterine horn and ovary were seized. A hemostat forceps was placed on the proper ligament to manipulate the ovary and its pedicle (Figure 1A). Then a window was created in the broad ligament (Figure 1B), and one of the handles of a Mayo-Hegar needle holder was passed through this window (Figure 1C), then secured (Figure 1D). After securing the ratchets, the needle holder was positioned over the surgical incision on the abdomen (Figure 1D). While pulling the first hemostat (on the proper ligament), the second hemostat was put on the ovarian pedicle, as far away from the ovary as possible (between the ovary and the needle holder’s locked handles) (Figure 1E). While doing this, with the second hemostat, the needle holder shanks were pushed along the suspensory ligament toward the viscera to attain the appropriate distance. After securing the second hemostat, it was placed crosswise on the needle holder’s shanks so that it would not be dragged into the abdominal cavity and would stay visible to the surgeon outside the abdomen at all times (Figure 1F). The third and fourth hemostats were then inserted between the ovary and the second forceps (Figure 1F). These forceps have crushed the tissues in preparation for the installation of the ligature. After crushing the pedicle, the forceps were removed, and circumferential and transfixation ligatures were applied to the pedicle in the formed groove. The ovarian pedicle was sharply transected using a scalpel immediately after the transfixation ligature, while it was protected by a hemostat. Throughout the application of these steps, the second forceps remained firmly on the ovarian pedicle, preventing it from being dragged within. The needle holder was put down after being released (Figure 1G). After gently grasping the corner of the ovarian pedicle with tissue forceps, the second forceps was released. The ovarian pedicle was inspected for hemorrhage and then released. The procedures were repeated for the contralateral ovary. The remainder of the procedure up to the final skin gap suture was routinely performed (same as the triple hemostatic method).
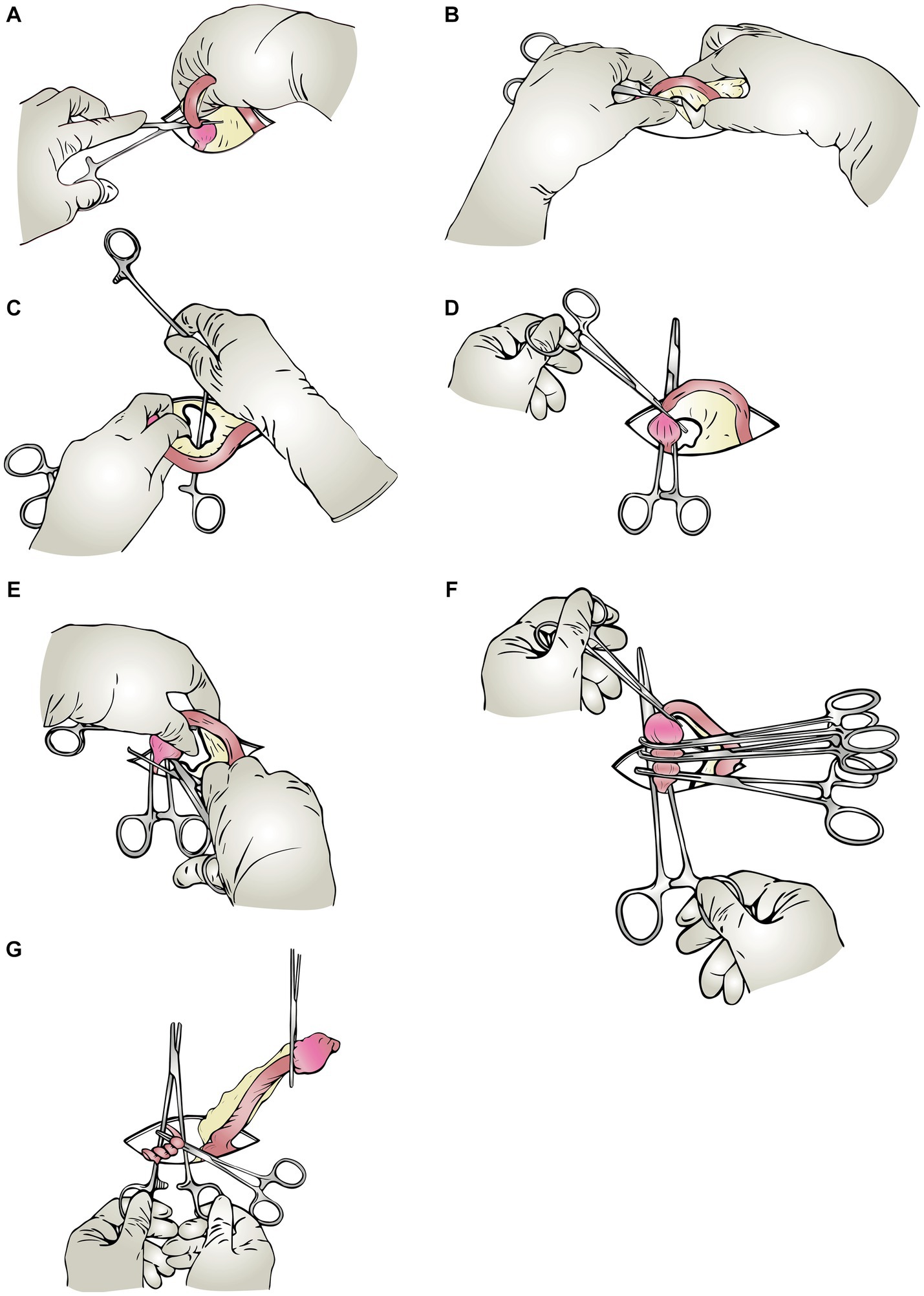
Figure 1. Schematic steps of the modified Instrument shank-assisted ovariohysterectomy. Most steps in this method are similar to the triple-clamp technique. Wherein the pedicle is retained outside the abdomen by a straight Ochsner hemostat placed on the shanks of a needle-holder crossly. (A) Proper ligament clamping: After identifying the uterine horn, the rat-toothed Crile forceps are placed on the proper ligament to manipulate the ovary and its pedicle. (B) Windowing: A window is created in the mesovarium with an index finger. (C) Shanking (needle holder’s shank insertion): The needle holder’s shank is passed through the created window in the previous step, and the ratchets are locked, so the ovarian pedicle locates between the shanks. (D) Shank-IN: The shanks are oriented crossly to the incision on the abdomen. (E) Applying the “Anchoring clamp” (The ovarian pedicle’s 1st clamp): Holding the proper ligament forceps with one hand, push the anchoring clamp (first clamp), which is on the needle holder’s shank, toward the viscera, and lock it on a suitable level of the ovarian pedicle. (F) Triple hemostatic: The “ligating clamps” were secured between the Anchoring clamp and the ovary out of the abdominal cavity to crush the tissue for ligature placement. (G) Ligation and shank-OUT: Apply two simple ligatures and one transfixing ligature close to the ovary in the crushed groove created in the previous step.
All surgeries were video captured, and the time intervals between each step were retrieved. The initiation of the incision and the final abdominal closure suture were considered the beginning and finish of the surgical procedure, respectively. These are the defined time intervals:
Total surgery time (TST): From the initial skin incision to the last skin suture of an abdominal incision.
Pedicle intervention time (PIT): From placing a hemostat on the proper ligament of the left ovary through cutting the right pedicle following the installation of its ligature; incorporating SRT, ShT, ClpT, and LigT for the left and right pedicles.
Suspensory release time (SRT): Digital strumming of the suspensory ligament.
Shanking time (ShT): From the end of windowing (the process of creating a window in the broad ligament) until the start of anchoring clamp placement. An anchoring clamp is a hemostatic clamp placed on the ovarian pedicle as far away from the ovary as possible to prevent dragging into the abdominal cavity.
Clamping time (ClpT): From the placement of the anchoring clamp to the completion of the last ligating clamp, near the ovary. The ligating clamp is a hemostatic forceps that is used to crush the ovarian pedicle and create a groove for the installation of the ligature. After the anchoring clamp, these forceps are secured to the pedicle on the ovary side.
Ligating time (LigT): From the beginning of the first simple ligature until the finish of the trans-fixing ligature of both pedicles.
Other surgical procedures time (OSPT): All surgical procedures, except PIT and CT.
Closure time (CT): Closure time starts from the linea alba to the last skin suture tying.
There are many different kinds of subjective scoring systems, and some of them have been used in veterinary medicine (59). In this study, postoperative pain was measured using the Glasgow composite pain scale short form (GCMPS-SF) (60), the University of Melbourne pain scale (UMPS) (61), and visual analogue scales (VAS) (62). A trained, male examiner (AB) who was single-blinded measured the post-surgical pain (30, 31). He became acquainted with the dogs the day before surgery. Moreover, the similar abdominal closure and the bandage have prevented the procedure from being identified. Multidimensional pain assessments were carried out 1 h before surgery (0) and 6-, 24-, 48-, and 72-h following skin suturing.
Five milliliters of blood were drawn from the lateral saphenous vein after the same pain evaluation times. The samples were kept at the temperature of the operation room for 20–25 min before being transported to the laboratory. The serum separated by centrifuging at 3,000 rpm for 15 min was stored in a microtube at −20°C until the research ended. CRP levels (mg L−1) were determined using the CRP latex agglutination technique using the CRP-LIA kit, Bionik in the laboratory of the faculty (4, 33).
Antimicrobial therapy (63) was started intravenously (IV) during premedication and maintained intramuscularly (IM) every 8 h for 3 days following surgery at 22 mg kg−1 of cefazolin (Exir, Tehran, Iran) in both groups. Ketoprofen (Ketomax; Rooyandarou, Tehran, Iran) at 2 mg kg−1 IM was given to animals having a GCMPS-SF score of 6 or higher out of 24 (45); throughout the examination, the UMPS and VAS ratings were also examined as supplementary criteria for determining a ketoprofen prescription.
The SPSS program (IBM SPSS Statistics for Windows, version 26, IBM Corp., Armonk, NY, United States) was used to look at the data. Along with normality confirmation using the Shapiro–Wilk test (except LigT), the study recruited the suspicious non-normal parametric data (PID, SRT, ShT, ClpT, LigT, and OSPT) after normalization based on the concordance of skewness and kurtosis coupled with stem-and-leaf plots. The duration of surgical steps was analyzed using a T-test. The Pearson correlation coefficient was used to determine the relationship between PIT and TST.
The Friedman test analyzes the progression of pain changes. Using Related-Samples Friedman’s Two-Way ANOVA by Ranks, we compared pain levels between evaluating time intervals throughout each surgical process. Using Kruskal-Wallis H tests on mean, a comparison of pain levels at assessing time intervals between two surgical techniques has been conducted. On the median, Independent-Samples Kruskal-Wallis H followed by Independent-Samples Fisher Exact Sig. (2-sided test, for samples less than 10) has been implemented on the information obtained from three behavioral pain assessment methods.
Variations in CRP were evaluated using repeated measures ANOVA, independent samples t-test, and general linear model-univariate tests.
The correlation between CRP and age, weight, HR, RR, and RT was shown using Pearson’s correlation coefficient. The partial eta squared was used to figure out how CRP and the body condition score (BCS) are related. Spearman’s rho correlation coefficient was used to determine the relation between surgical time intervals and pain (based on GCMPS-SF). The statistical significance level was set at p < 0.05.
All relevant data is contained within the article: The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
There were no significant differences in the distribution of dogs by weight (p = 0.726) or age (p = 0.598) between the two groups. The mean and SD of age, weight, vital parameters (HR, RR, and RT), and BCS are shown in Table 2.
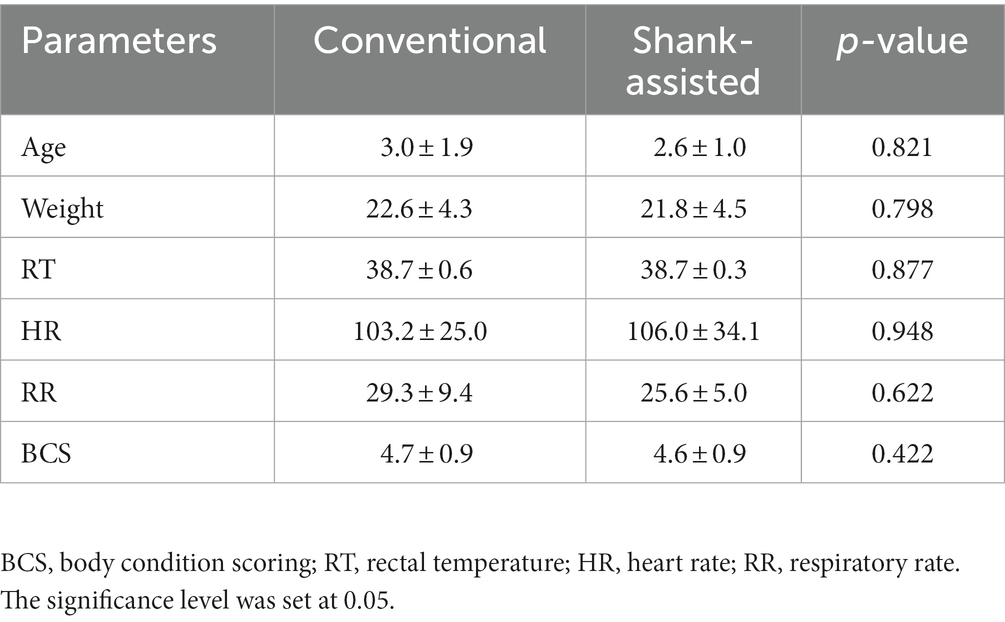
Table 2. Mean ± SD of age, weight, BCS, and preoperative vital parameters before anesthesia for the conventional (n = 9) and Instrument Shank-assisted ovariohysterectomy (n = 9).
Instrument shank-assisted OHE displayed shorter TSTs than the conventional method (34.70 ± 6.7 and 47.40 ± 9.9 min respectively, p = 0.005). Table 3 provides a comparison of the methods’ time intervals. According to the new method, SRT is identical to ShT. ShT required 74% less time than SRT (p = 0.009), and moreover, LigT improved by 37% (p = 0.005) with the novel approach. Additionally, the OSPT and CT got 26 and 29% shorter, respectively.
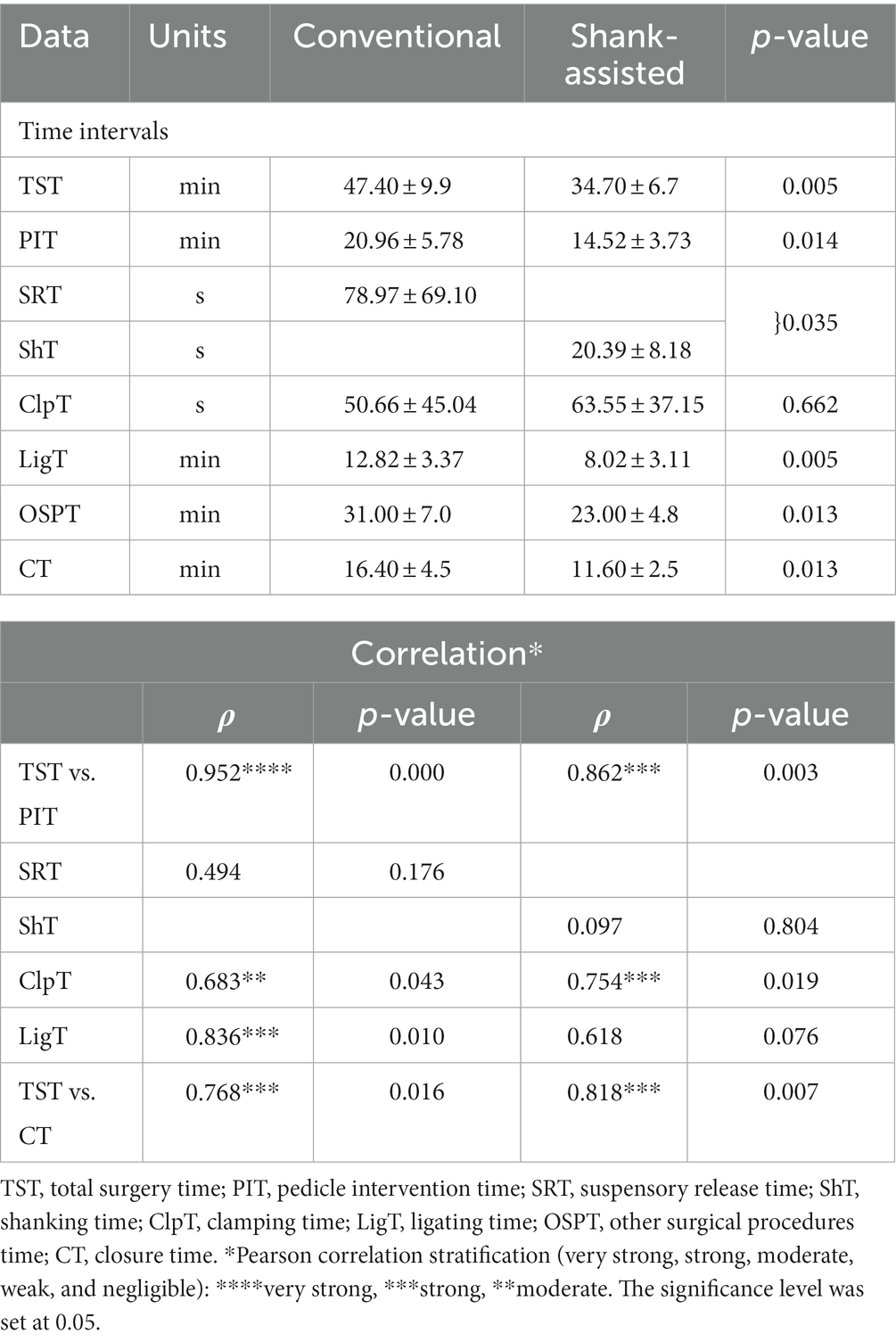
Table 3. Mean ± SD of different surgical steps time intervals recorded in the conventional (n = 9) and Instrument Shank-assisted ovariohysterectomy (n = 9) and correlation of surgical time intervals with total surgery time in deep-chested dogs.
Figure 2 shows how time intervals have changed, and Table 3 gives a statistical analysis of the changes. In each compartment of this figure, the difference favors the Instrument shank-assisted method.
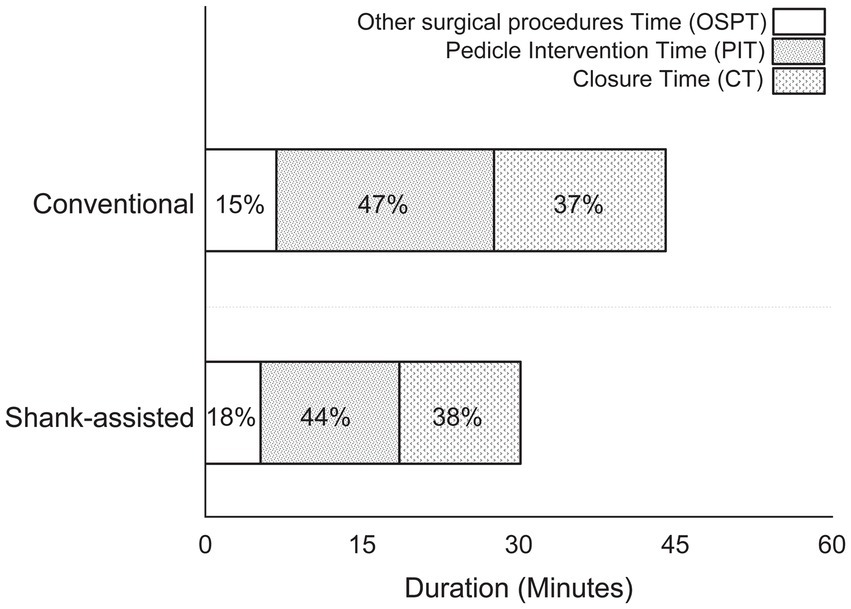
Figure 2. Comparison of the time spent performing different steps in the conventional method (n = 9) and new modified Instrument shank-assisted method of ovariohysterectomy (n = 9) in deep chested-dogs. OSPT, Other surgical procedures time, which consist of all surgical steps, except PIT and CT; PIT, Pedicle intervention time, which is from placing a hemostat on the proper ligament of the left ovary to cutting the right pedicle after placement of its ligatures; CT, Closure time, from the linea alba to the last skin suture tying.
According to the comparable correlation pattern (64) between TST and PIT based on ρ = 0.952, p = 0.0001 and ρ = 0.862, p = 0.003 for conventional and instrument shank-assisted OHE, respectively, PIT plays a crucial role in TST during ovariohysterectomy. The correlation analysis of TST and ShT revealed that, from a temporal perspective, ShT alone did not significantly reduce TST (ρ = 0.097, p = 0.804).
During the conventional OHE, a single intraoperative hemorrhage in the ovarian pedicle was managed. The dog was excluded from the study. None of the dogs in either group had problems after surgery.
Using the Friedman test, pain score changes (Δ Pain in GCMPS-SF, UMPS, and VAS; Table 3) were statistically significant in both groups. The majority of the time, the data showed that the new method was associated with much less pain (Table 4 and Figures 3–5).
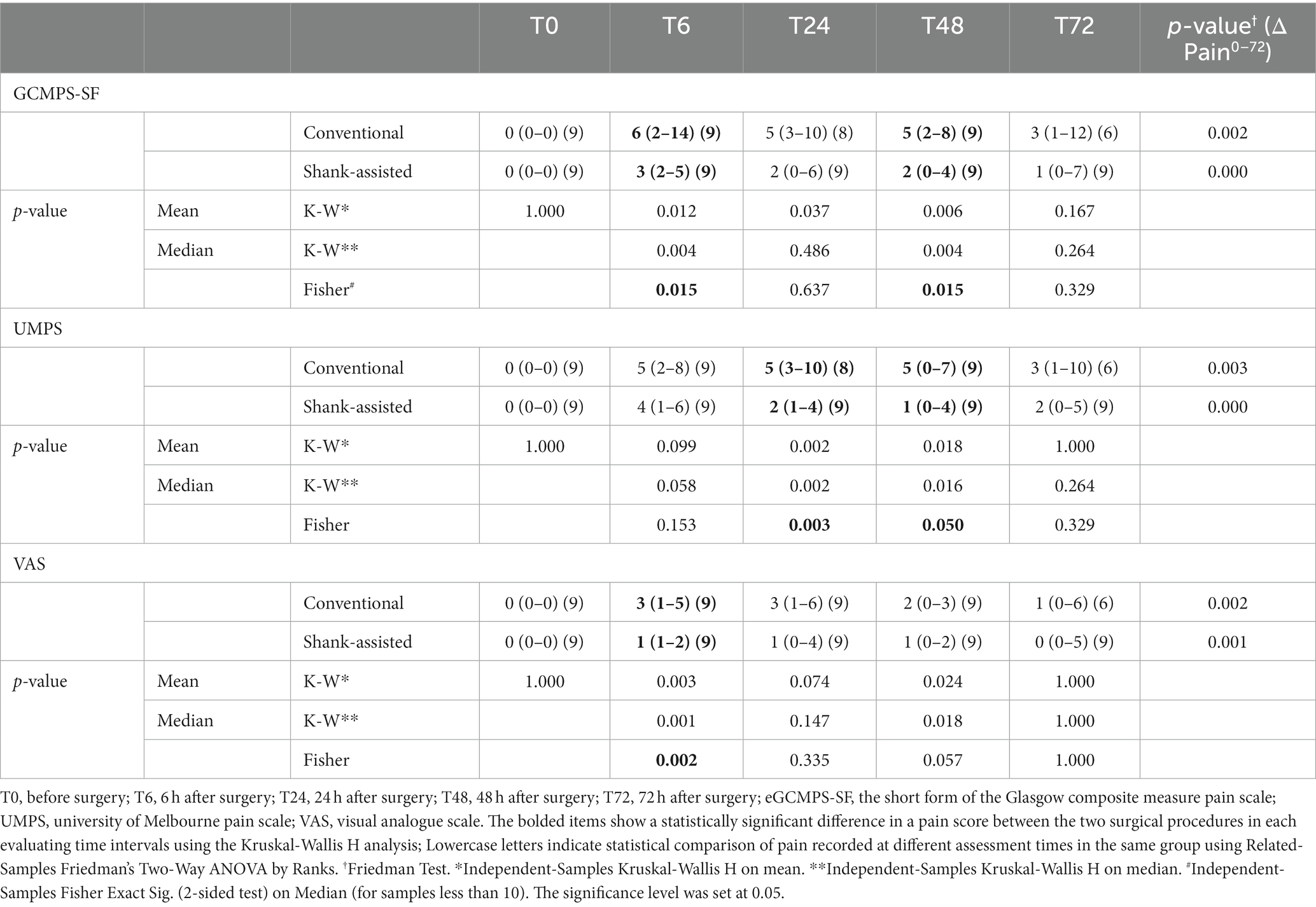
Table 4. Median (Min–Max) of pain scores and Δ Pain0–72 based on Glasgow composite pain scale short-form (GCMPS-SF), university of Melbourne pain scale (UMPS), and visual analogue scales (VAS) scales in the conventional (n = 9) and Instrument Shank-assisted ovariohysterectomy (n = 9) in deep-chested dogs.
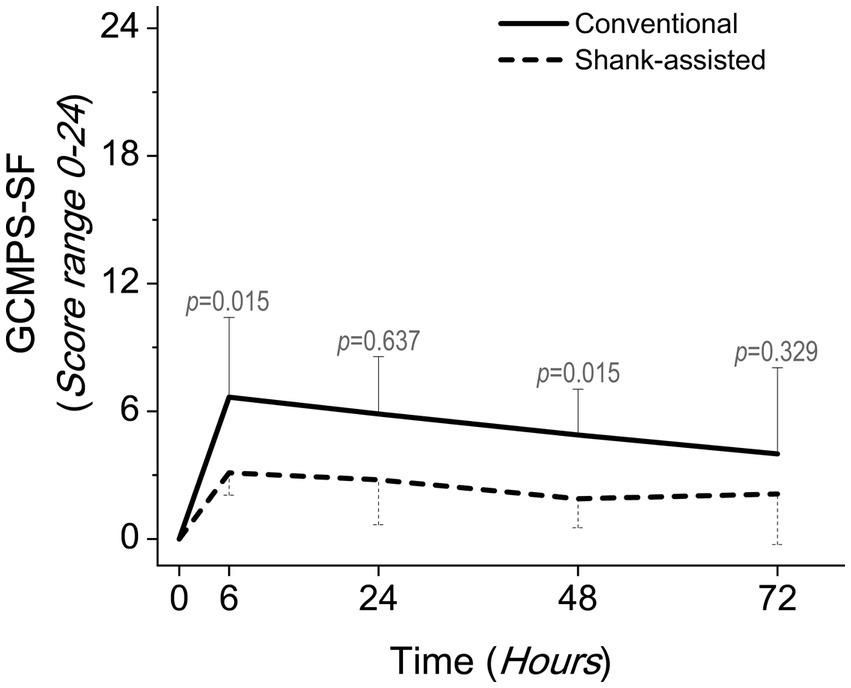
Figure 3. Comparing postoperative pain scores using the Glasgow composite pain scale short-form (GCMPS-SF) in the conventional method (n = 9) and new modified Instrument shank-assisted method (n = 9) of ovariohysterectomy in deep chested-dogs. The p-value was calculated using an Independent-Samples Kruskal-Wallis H on median followed by Independent-Samples Fisher Exact Sig. (2-sided test) on Median shows the statistical difference in sampling times between two groups.
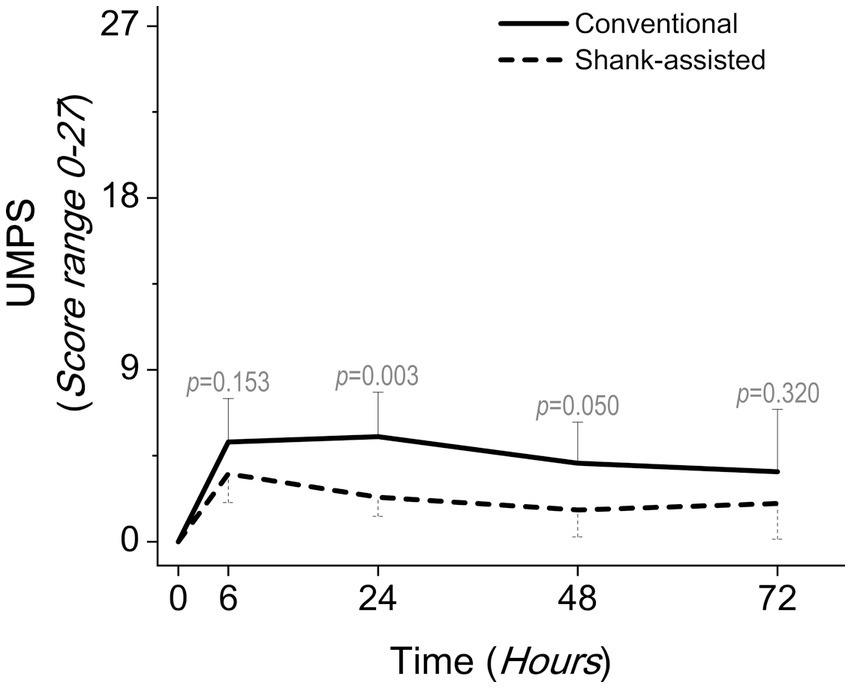
Figure 4. Comparing postoperative pain scores using the university of Melbourne pain scale (UMPS) in the conventional method (n = 9) and new modified Instrument shank-assisted method (n = 9) of ovariohysterectomy in deep chested-dogs. The p-value was calculated using an Independent-Samples Kruskal-Wallis H on median followed by Independent-Samples Fisher Exact Sig. (2-sided test) on Median shows the statistical difference in sampling times between two groups.
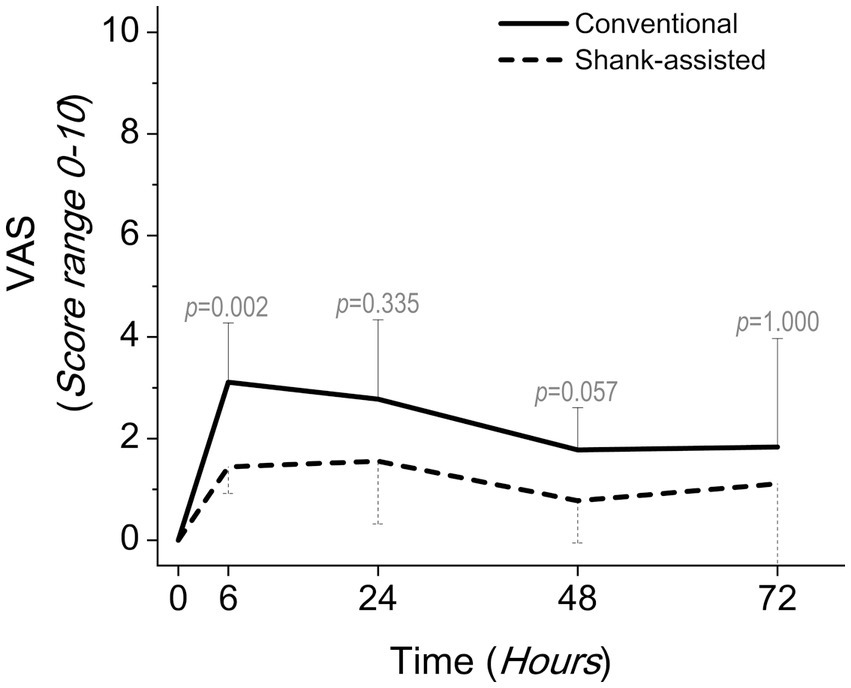
Figure 5. Comparing postoperative pain scores using the visual analogue scales (VAS) in the conventional method (n = 9) and new modified Instrument shank-assisted method (n = 9) of ovariohysterectomy in deep chested-dogs. The p-value was calculated using an Independent-Samples Kruskal-Wallis H on median followed by Independent-Samples Fisher Exact Sig. (2-sided test) on Median shows the statistical difference in sampling times between two groups.
After the surgery, 36 pain assessments have been done in each group, ranging from T6 to T72. These pain assessments have been carried out at 6-, 24-, 48-, and 72-h following surgery on nine animals in each group. The animals were injected with rescue analgesics 13 times (T6: 6, T24: 3, T48: 3, and T72: 1 dog) in the conventional group and twice (T24: 1 and T72: 1, both for one dog) in the novel group. Using the conventional methods, 8 dogs were injected with rescue analgesic, whereas just 1 dog received it using the alternative method.
After 24 h, the highest serum CRP levels were observed in both groups. Figure 6 demonstrates that the conventional group’s rate of rise accelerated more rapidly during the initial 6 h following surgery. The slope of the graph is determined to be y = 24.328x − 18.072 for traditional OHE and y = 4.9556x − 1.5667 for instrument shank-assisted OHE within the first 6 h. The traditional group observed a 4.9-fold increase in CRP acceleration on this basis. After 48 h, the Instrument shank-assisted OHE showed a significant decrease to baseline levels (p = 0.032; see Tables 5, 6). In contrast, although CRP levels decreased in the conventional group, they were not significantly different from their peak levels.
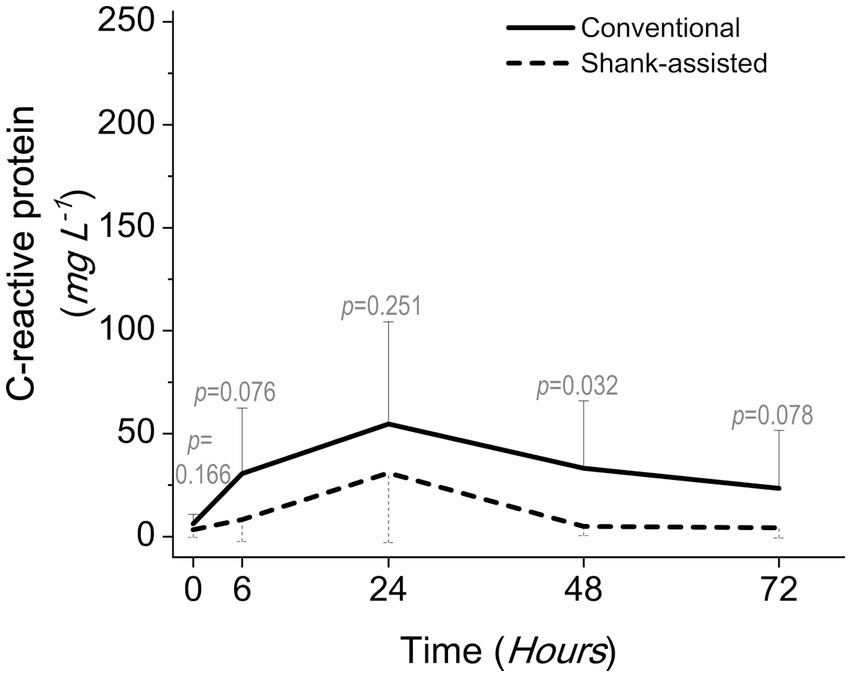
Figure 6. Comparing postoperative serum C-reactive protein levels following the conventional method (n = 9) and new modified Instrument shank-assisted method (n = 9) of ovariohysterectomy in deep chested-dogs. The p-value was calculated using an independent samples T-test shows the statistical difference in sampling times between two groups.
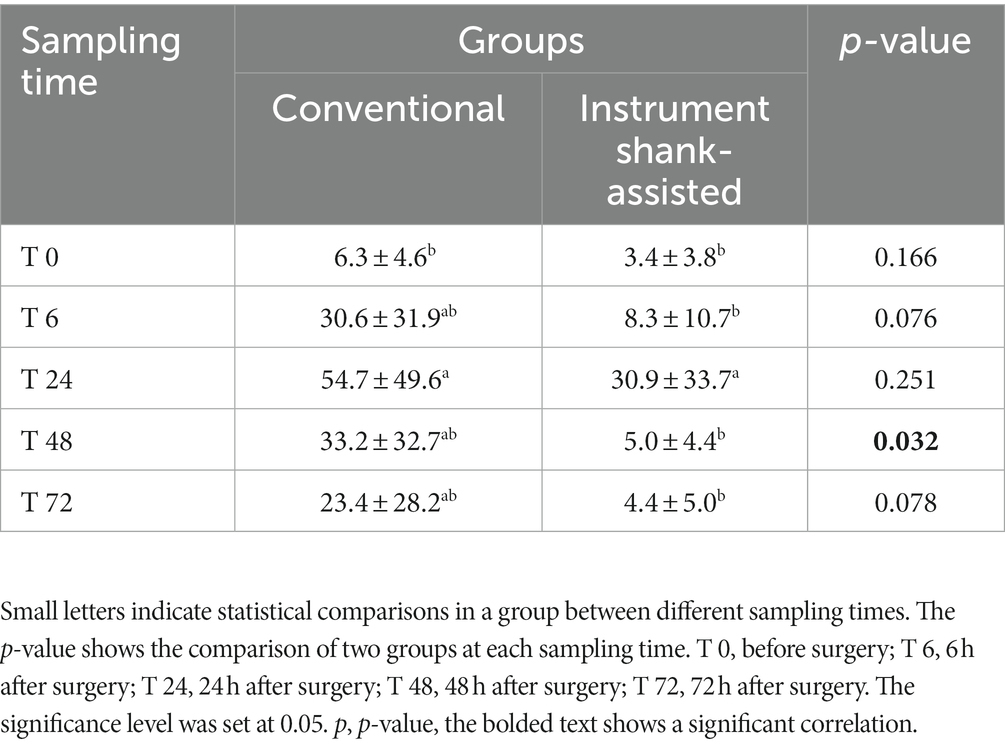
Table 5. Mean ± SD of the serum CRP concentration (mg L−1) following the conventional (n = 9) and Instrument Shank-assisted ovariohysterectomy (n = 9) in deep-chested dogs.
The relationship between pain (as measured by GCMPS-SF, UMPS, and VAS), surgical time parameters, CRP, vital signs, age, weight, and BCS has been studied.
Spearman’s rho correlation coefficient has studied the relationship between the overall surgical duration and the duration of the distinct phases. The analysis is described in full in Table 6, which is stated separately below.

Table 6. The correlation of surgical time intervals with post-surgical pain, which was evaluated using GCMPS-SF, UMPS, and VAS behavioral pain scales, after the conventional (n = 9) and Instrument Shank-assisted ovariohysterectomy (n = 9) in deep-chested mixed-breed dogs.
Only pain in the conventional OHE exhibits a significant positive correlation with LigT at T72, according to GCMPS-SF.
Using the novel method, UMPS found significant negative relationships between pain and TST at T24 and T48. This study indicated that PIT has a vital function in lowering pain in T24.
The VAS has found a greater correlation between specific surgical stages and pain. GCMPS-SF, UMPS, and VAS were able to identify 1, 3, and 7 correlations, respectively, in this regard. In the new method, VAS identified an association between ClpT and less pain at T6 and T24, and between ClpT and OSPT at T48. This assessment method revealed that PIT, LigT, and most notably OSPT have a substantial effect on the incidence of pain on the third day following surgery in the conventional group (see Table 6).
Using Spearman’s rho correlation coefficient, CRP levels and pain were only shown to have significant moderate-to-strong negative relationships in five measurement points of total samples (two groups in total). These associations were detected at T24 (UMPS) and T72 (GCMPS-SF) in the conventional group and at T6 (VAS) and T48 (GCMPS-SF and VAS) in the instrument shank-assisted group, as shown in Table 7.
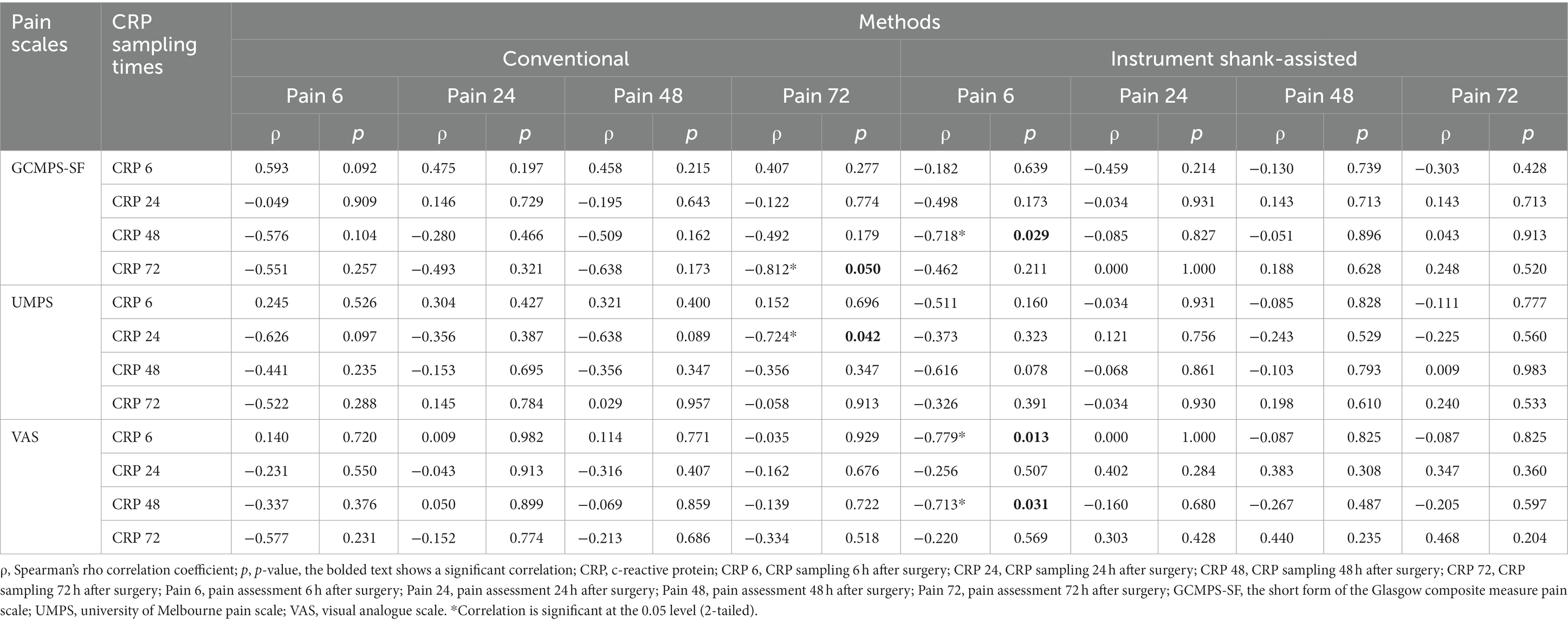
Table 7. The correlation of serum c-reactive protein levels with post-surgical pain, which was evaluated using GCMPS-SF, UMPS, and VAS behavioral pain scales, after the conventional (n = 9) and Instrument Shank-assisted ovariohysterectomy (n = 9) in deep-chested mixed-breed dogs.
Pearson’s correlation coefficient indicated that there was no association between CRP changes and age, weight, HR, RR, and RT in both groups (p > 0.05). Partial eta squared (η P 2) was unable to identify a significant association between CRP and BCS using any of the two techniques.
According to the present results, when implementing OHE with instrumental shank assisted technique, the surgery can be performed by one person with lesser surgical trauma. With the current solutions, digital strumming or sharp tearing of the suspensory ligament in deep-chested dogs has not only been a time-consuming process, but it has also failed to shorten the length of surgery and pain afterward (11). According to current research, a decrease of over 12 min in an alumnus surgeon’s overall surgery length is a positive improvement (57). The reduction in surgical trauma has resulted in a slower increase in CRP levels and a shorter peak. These gains were made using the same equipment and facilities at no additional expense due to a modest modification in the surgical approach.
OHE, like many other surgical procedures, seems to get more challenging as the size and weight of the animal increase. As body mass increases, the chest sinks deeper, and it becomes more difficult to reach and release the suspensory ligament (11). In the present study, the same higher body weight range enhanced the ovarian exposure challenge, allowing the method to be generalized to various sizes of dogs and cats; however, it may be accompanied by fewer problems in smaller animals.
Insufficient exposure during OHE leads to incorrect technique execution and raises the risk of ovarian remnant syndrome (2). The instrument shank-assisted OHE keeps the ovary outside of the abdomen without an assistant while maintaining the suspensory ligament. In obese and deep-chested dogs, as well as for unskilled surgeons who require a longer incision, the surgical assistant is essential (1, 2). So, a small incision without tearing the ligament is another achievement of the modified method, which led to limited surgical complications including incisional swelling, seroma, infection, delayed healing, ventral body wall dehiscence, self-inflicted trauma, pain (65), and hemorrhaging (66).
During a suspensory ligament release (66), an inexperienced surgeon is more likely to break the blood vessel and induce hemorrhage, which prompts the hurried application of surgical sponges. Stress has a negative impact on the non-technical skills of surgeons (67), leading Rodriguez et al. to conclude that intraoperative hemorrhage from an ovarian pedicle probably increased the retention of surgical sponges in veterinary patients (68). Therefore, removing ligament release from the surgical steps would likely reduce the frequency of hemorrhage and sponge retention; however, more research is required before a conclusion can be reached.
Hilgard’s learning theory suggests that experience is a crucial component of the learning process (69). For unbiased comparisons, the study surgeries must be performed by a surgeon with no or equivalent prior experience in both methods. A minimum of surgeon experience in our study to the level of the veterinary training program according to conventional method seems to impose an inevitable minimal bias. However, the presence of an inexperienced surgeon could be a limitation of the present study. Given the possibility that a surgeon’s lack of expertise might exacerbate surgical stress to the point where the impact of the technique is nullified, a week of training for each method prior to the research allowed the current study to reveal the smallest difference between the procedures. TST has been reported to take between 55 and 130 min for inexperienced surgeons (70, 71). Freeman et al. established an optimal duration of 45 min for inexperienced surgeons after six surgeries (70). In this study, an average of almost 40 min TST demonstrated that the surgeon’s skill is enhanced by training before to the start of main operations, and demonstrating our surgeons’ experience (71) made the findings more realistic (11, 13, 70). Alternatively, previous research on pilots has yielded five levels of skill acquisition, including novice, advanced beginner, competent, proficient, and expert (25); If each 10-min improvement for OHE corresponded to one level of improvement for the surgeon’s expert, then our surgeon’s 12.7 min reduction in surgical duration using the instrument shank-assisted technique would theoretically qualify her as “competent.”
Surgical experience makes sick animals healthier (72). Sir Francis Galton thought that talent was completely innate (73), but experience is a learning growth (25). Yet, from a different perspective, the surgeon’s experience may provide no more than a 25% health improvement (72). On the other hand, OHE is still known as a model of acute pain in research studies. So, when animal pain after a technique is still a problem even after experienced surgeons have used it, it is important to look at the technique itself instead of just how it is taught. Consequently, both correct training and the training of correct techniques are emphasized in the surgical training curriculum, and the new method may contribute to the promotion of the latter, as indicated by the high effect sizes reported in the present study.
The type of operation is a stressor for the surgeon (74). Mental (75) and muscle (76) fatigue can delay an operation. Aside from the fact that the stress was not directly evaluated in our study, time as a major component in calculating surgical stress (74, 77) has been meticulously recorded and analyzed. Controversial is the scenario in which the total surgery time is reduced beyond the time spent on the ovarian pedicle. The technical difference between the two methods was SRT and ShT only had a time difference of 58.58 s in favor of the modified method, but TST was improved by 12.7 min. Non-correlation of these variables with TST indicates they did not directly contribute to the difference in TST, while ovarian exposure caused roughly 63% quicker application of ligatures (4.8 min improvement). The remainder of the improved duration was divided into two parts: (1) 3.1 min from the major procedures in OSPT, which include the separation of the broad ligament on both sides, the second ovary access, uterine arteries ligature placement, and uterine body close and cut; and (2) 4.8 min from CT. The procedures conducted in OSPT and CT were comparable among techniques, although the Instrument shank-assisted OHE required significantly less time. When we were nearing the end of the surgery, or, in other words, when the surgeon had reached extreme fatigue, 4.8 min more time was required to close the abdominal wall using the conventional technique. Therefore, significant time reduction in the mentioned two parts could be related to less fatigue and stress, which was paved through the shorter LigT in the new method. Peeters and Kirpensteijn’s unsuccessful attempt to reduce surgical time by utilizing ovariectomy (OVE) instead of OHE (58) is another example of the surgeon’s mental and physical strain at this time, as they did not eliminate digital strumming. Due to the strong correlation between TST and LigT in the present study, it is evident that the modified instrument shank-assisted technique can reduce the impact of the time required to install the ligatures, which was the primary factor in the significantly longer surgical time when the previous technique was used. Additionally, the small standard deviation (78) in these two portions may indicate the surgeon’s optimal state of stability and fewer technical obstacles during instrument shank-assisted OHE, which may require further investigation.
According to research findings, the surgeon’s stress level may be enhanced due to a lack of familiarity with the members of the surgical team (67). While the present study did not measure the stress level of the surgeon, efforts were made to mitigate concerns regarding the presence of new team members. This was achieved through measures such as facilitating familiarity and collaboration among team members during the pre-study training course as well as maintaining a consistent surgical team.
Surgical supervisor changes complicate student-led surgical procedures (57). This study’s experienced academic surgeon continuously supervised the surgical procedures, eliminating the possibility of this error, as observed in Harris’s study due to a change in supervisor.
The time interval proportion found in this study could be used in general, even though an experienced surgeon could cut the total time needed for surgery. Hence, based on Shivley’s yearly savings of 73.3 h (11), a 26.7% decrease in TST in instrument shank-assisted OHE could save 195.7 h (>2.5 times).
In concluding, as ovarian manipulation and pedicle ligatures were identified as the most essential procedures of OHE in a previous study (79), these findings have been meticulously confirmed in the present study. In the instrument shank-assisted OHE, a more accessible ovarian pedicle facilitated all subsequent steps of the surgery.
Due to the surgical discussion’s focus on inexperienced surgeons, it is reasonable to assume that the significance of this technique will change as the surgeon’s experience grows, whereas the pain-related advantages of this surgical technique will be discussed in a separate chapter, considering all surgeons to be subject to the implementation of this technique.
The current study confirmed that, compared to instrument shank-assisted OHE, digital strumming of the ovarian pedicle during conventional OHE makes ligature placement more challenging, possibly due to unwanted visceral interferences or manipulations, prolongs the duration of surgery, and increases pain. Digital strumming is an unpleasant surgical procedure followed by considerable surgical trauma. Thereby, it is anticipated that modifying traditionally invasive procedures would improve patients’ recoveries and well-being.
Subjective pain scales (44, 80) and, controversially, CRP have been used to measure pain since vital signs are not sensitive enough (81) and animals cannot communicate verbally (47). Several multidimensional structured behavior scales have been adapted for use in veterinary medicine (59), and the multidimensional GCMPS-SF for acute pain has been authorized for use in dogs (45, 60) with more sensitive and consistent results (82). The UMPS (which comprises six categories of physiological data) and VAS (with more flexibility) were used to compensate for the insufficiency of the GCMPS-SF and reduce the secrecy of the pain.
The results of pain assessments vary based on the experience and knowledge of the veterinarians, which are affected by age, gender, and time since graduation (31, 83). It was anticipated that using a trained, blinded assessor (30), a wound dressing, and three distinct pain scales would reduce the influence of qualitative variable bias in the current study. On the other hand, demographic data and dog acclimation before surgery suggest that individual pain tolerance, species, age, body condition, and environmental factors that can change or mask pain intensity are not confounding variables.
Since severe pain after surgery is often underestimated (84), it needs to be measured in a new surgical procedure (85). The perception of postoperative pain is dependent not only on surgical duration and technique (86), but also on analgesic type (87), dose, multimodality (88–90), the use of preventative analgesia (91), route of administration, and the pharmacokinetics of medications (92). Pain can result in delayed wound healing and surgical site infection (93), and bandages may not be adequate for preventing suture line contamination (94). So, surgeons prefer to modify surgical procedures to reduce postoperative pain (47). This was one of the most important goals of instrument shank-assisted OHE, which had an effect size d of >1.27, > 1.32, and > 1.77 and a power of 0.811, 0.836, and 0.968 at T6, T24, and T48, respectively, based on provided GCMPS-SF pain measurements.
Sampling time is essential for accurately determining pain on time. In this study, sampling times were adjusted according to four theoretical elements: (1) the clinical duration of action of the analgesic agent (meperidine, medetomidine, and ketorolac), which may provide enough pain relief; (2) the plasma half-life of the anesthetic selected for premedication, induction, and maintenance of anesthesia (acepromazine, midazolam, and ketamine), which may change the responses given for pain evaluation; (3) the minimum estimated duration for pain onset, peak, and subsidence; and (4) the pathophysiology of CRP turnover. By giving dogs nonsteroidal anti-inflammatory drugs (NSAIDs) before surgery to help with pain after surgery (95, 96), it was thought that ketorolac might be enough for the first few hours after surgery. However, the results have only shown that this is true for the new technique. It was anticipated that the pharmacologic effects of the long-half-life (t½) drugs acepromazine (97), ketorolac (98, 99), and ketamine (nor-ketamine) (100) with residual effects of 7.1, 4.5 (or 10 based on relevant reference), and 6.2 h, respectively, would be felt from premedication until endotracheal extubation. Because sedatives, analgesics, and injectable dissociative anesthetics can change responses like facial expression, salivation, mydriasis, and cardiorespiratory parameters, which were evaluated in the GCMPS-SF, UMPS, and VAS, early pain assessment (<6 h) was not considered in this study.
The requirement of rescue analgesia may be regarded as a reliable indicator of the surgical technique’s incompetence. According to a previous report in humans (101), after hip and knee arthroplasty, ketoprofen had the same analgesic effect as extradural morphine. Mathews et al. find that ketoprofen has a comparable impact to meloxicam and conclude that it could be a useful way for controlling postoperative pain (102). Similar to carprofen, meloxicam, and tolfenamic acid, ketoprofen produced excellent postoperative analgesia in cats, but with a lesser effect on tenderness (103). In the meantime, more recent studies indicate the administration of NSAIDs is superior to opioids due to faster recovery of normal functions and greater satisfaction with postoperative well-being (104). Nevertheless, these findings should be taken with care when applied to OHE in veterinary medicine. In the present study, animals that got rescue analgesia were not excluded, and 8 dogs in the conventional group who received rescue analgesia at 13 evaluation times were included for analysis. If getting rescue analgesics improved outcomes, the dogs in the first group were unable to demonstrate superior outcomes despite receiving frequent pain treatment. The number of animals administered rescue analgesics may be a reflection of the severity of surgical trauma and the invasiveness of the conventional technique. Receiving rescue analgesics in 1 dog out of 2 evaluation times can be attributed to greater well-being using the modified method and can be interpreted in two aspects: first, a standard protocol of analgesics is still recommended after surgery, and second, it may be useful in shelter or stray dogs that may not receive proper follow-up treatment, for example.
At 6 h postoperatively, eight out of nine dogs treated with the conventional method received rescue analgesia. This demonstrates at least two important points: (1) ketorolac provides inadequate postoperative analgesia for the conventional OHE performed by an inexperienced surgeon, although it has been used in humans to control moderate to severe post-operative pain, and it may be effective in dogs (105), as effective as flunixin, and more effective than butorphanol or a low dose of oxymorphone (106), by affecting opioid receptors centrally with comparable efficacy to morphine (99), and (2) the current modification has decreased postoperative pain to the point that ketorolac could control it, so that none of the dogs in the second group required T6 rescue analgesia.
Our “competent” surgeon has made the surgery quick and competitive in terms of time (see “Surgical perspective”). Since the new method causes less pain and this surgery is still done to create a model of acute pain for research, it is safe to assume that this surgical method is not limited to a certain group of surgeons, no matter how much experience they have, and that it is better to make it more general.
In addition to the psychological burden experienced by the surgeon, contemporary approaches have been developed to assess the degree of pain and surgical stress imposed on the patient. While certain methods, including pupillometry, surgical pleth index (SPI), skin conductance, cardiovascular, and cardiorespiratory indices, require further advancements in sensor technology and interpretation algorithms to investigate animal responses to anesthesia and surgery, their applicability has not yet been confirmed. In the field of veterinary medicine, additional quantitative techniques have been introduced for animal assessment. These include the parasympathetic tone activity index (PTA index), which analyzes heart rate variability, and the bispectral index (BIS), which analyzes electroencephalography, with the potential for animal interpretation. Despite ongoing debates regarding the universal implementation of their use in all treatment procedures and drug protocols (107), the accessibility of these remedies may not always be assured. It is worth noting that the application of PTA as a means of assessing pain in conscious animals within the field of veterinary medicine is challenging. This is primarily due to the presence of unwanted movements by the animal, which directly impact heart rate variability. Consequently, alternative models capable of detecting these fluctuations should be employed (20, 32, 107, 108). The use of more recent medications, and therefore the recording of the quantity of anesthetics used (55) and the vital parameters throughout the operation, seems to be a large issue that would need review in separate research, other than that this technology was not available for the current study.
A number of different physiological parameters, such as plasma vasopressin, urine noradrenaline, and creatinine concentrations, have been suggested to assess the degree of irritation and pain caused by a surgical method. It appears that documenting additional facts and aiding in the final assessment of the effectiveness of the presented technique may be accomplished by comparing the changes in these parameters during anesthesia between the two methods. The conventional OHE involves applying extremely stretching stress to digitally strumming the suspensory ligament, while the new method involves keeping this ligament under tension all the way through the ligature placement process. Further research is warranted to compare the two methods from this perspective, as acute noxious stimuli during stretching of the pedicles can increase systolic blood pressure, heart rate, plasma vasopressin concentration, and urinary noradrenaline/creatinine ratio (55).
One of the initial observable events following surgery is the elevation in temperature and inflammation of the surgical site, attributed to enhanced blood circulation to the area where surgery was performed. Infrared thermography (IRT) is a proficient technique for assessing thermal variations in problematic areas, as it measures the surface temperature of the skin through thermographic maps. This technique may be used to recognize the localized changes in blood supply and localized increases in temperature that occur in response to stress. The assessment of the efficacy of local anesthesia through the analysis of alterations in surface blood circulation linked to sympathetic activity is among the additional functionalities of IRT (109). However, it has been observed that this technique has not demonstrated sufficient effectiveness in dogs (110). The present study suggests that while the implementation of IRT for evaluating pain and inflammation in the surgical approach area was effective, practical limitations arose due to the bandage covering the surgical site. Conversely, the thermographic assessment of regions where the suspensory ligament has been torn, situated on the roof of the abdominal cavity, may not be deemed reliable in theory. This is due to the fact that the heating of the dermis surface is directly linked to the local dermal microcirculation, which is under the control of the ANS. Nevertheless, the non-invasive nature of this method of evaluation may be an appealing subject for further research.
Pupil shape has been a key indicator for neurological assessment for over a century (111), and automated pupillometers have become increasingly important due to the difficulty of detecting the “reactive pupil” characteristic (112). In addition, assessing pupil reactivity using a pupillometer offers an objective, rather than subjective, evaluation of the neurological examination. Automated pupillometers can distinguish between canine conscious and anesthetic pupillary light reflex (PLR) and continuously assess an animal before, during, and after anesthesia (113). It seems that PLR devices could be useful in research like the current one, provided that the assessments are standardized.
Evaluation of a novel surgical procedure extends beyond surgical parameters and postoperative discomfort. Surgical invasiveness may be assessed separately. Based on past research, the invasiveness of the surgical procedure may be related to postoperative pain. Consequently, the question of surgical trauma severity is a separate topic that will be addressed further in “CRP perspective.”
In this study, the traditional OHE method was changed in a way that reduced the amount of trauma. With the new technique, the amount of surgery-related trauma had a smoother pattern and went back to normal almost 30% faster. These results predicted a shorter period of recovery time subsequent to instrument shank-assisted OHE.
The postoperative acute-phase response develops faster in dogs compared to humans. Tissue damage and pain after invasive surgery are associated with a rise in blood acute-phase proteins, primarily CRP, which may be a valuable diagnostic biological marker of early postoperative complications (114, 115). The CRP, a sensitive biomarker of infection (116), inflammation, and tissue damage (46), is more sensitive than serum cortisol (81) in detecting surgical trauma (13) and can assess various surgical procedures in dogs (33, 117), peaking 24 h postoperatively (42). The short half-life of canine CRP (19 h) makes it a useful marker for identifying the intensity of mild clinical stressors (33, 118, 119) whose effects dissipate more rapidly.
It has been stated earlier that the slope of changes is a reliable predictor variable for the expected peak (13). A five-fold smoother slope in the elevation of CRP concentration generated a milder peak after instrument shank-assisted ovariohysterectomy, so its return level to the base value showed a significant reduction at T48 compared to that of the animals in the opposite group. Thus, it is suggested that future research on the slope of the post-OHE CRP increase would also be planned to reduce study duration and be used for designing and scheduling postoperative analgesia protocols and lengths. As CRP is elevated approximately 6 h after a single stimulation (119), the present data revealed that there is no clinical necessity for sampling before 6 h after surgery. Moreover, because serum concentration peaks between 24 and 48 h in dogs (42, 115, 119), and CRP has a short half-life (33, 118, 119), the last measurement time of 72 h after surgery was appropriate for CRP return.
It has been imagined that OVE can satisfy surgeons’ hopes by reducing the consequences of OHE. Moldal et al. did not find any differences in the levels of CRP, glucose, or iron in the blood between them. Therefore, the greatest trauma in OHE occurs during the surgeon’s manipulation of the ovarian pedicle (86). Thereby, the instrument shank-assisted method’s unique characteristic can be considered an advantage. By reducing manipulation of the ovarian pedicle through eliminating digital strumming, the surgical trauma has also been reduced to a level close to the lowest expected minimum. Experienced surgeons have the advantage of avoiding unnecessary organ manipulation (26, 27), which causes minimal surgical trauma and postoperative serum CRP concentration. Hence, the present modified technique, which entails less organ touch, presents surgical quality closer to that of an experienced surgeon.
It is not without merit to state that CRP concentrations seem to be a decent predictor of how invasive an operation is (120), despite some contradictory reports, such as that there is no correlation between the length of surgery and CRP, despite its rise (121), or that CRP concentrations may not be significant in the diagnosis of a disease (118). In the present study, the return to baseline after a moderate rise in CRP levels utilizing the instrument shank-assisted OHE, as contrasted to the conventional group’s high CRP levels, suggests that the instrument shank-assisted OHE could be concluded as having a minor invasive nature.
The risk of infection is increased when abnormal CRP responses are seen 5 or 7 days following surgery (116). A progressive decrease in serum CRP content in both groups could indicate the absence of infection. It is not unlikely that a procedure requiring fundamentally less organ manipulation would result in improved recovery, but ultimate healing was not the focus of this study.
Finally, the pattern of CRP changes followed the pain charts without correlation, according to the data. So, at least when the surgery is minor, there may not be a statistical correlation between changes in the CRP and pain. This may be because of the wide range of its reported changes. In this instance, the CRP profile may be able to anticipate pain patterns, but it cannot be used to make statistical conclusions.
The CRP changes showed that OVE and other similar procedures cannot be the final solution to animal comfort, and modification of the surgical technique on the more severe parts of the surgery is necessary, whether it is the method proposed in the current study or other solutions that may be introduced later. Although the graphs of CRP and pain changes appear similar, establishing a definitive relationship or statistically significant correlation between them requires further research.
The current study supported the practicability of a single-person ovariohysterectomy in deep-chested adult mixed-breed dogs without tearing the suspensory ligament, along with a reduction in surgical length, CRP, and pain. On the basis of the results, there are still questions regarding the efficiency of using serum CRP concentration as an alternative pain assessment indicator.
Future studies evaluating physiological values, pain, the amounts of analgesics and anesthetics consumed during the operation, hemorrhage, heat loss, instrument handling errors, wound size, and more tissue handling can help determine the surgical technique more precisely. Intraoperative evaluations need an up-to-date drug anesthesia protocol, which was not available in this study because certain nonsteroidal anti-inflammatory drugs were not available in the area and veterinarians were not allowed to use opioids or isoflurane. Further research will be needed to compare the performance of surgeons with varying degrees of expertise and animals of varying ages, making it more difficult to generalize the findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was approved by Iranian biomedical research ethics committee IR.IAU.BABOL.REC.1399.004, IR.IAU.BABOL.REC.1399.015, and IR.IAU.BABOL.REC.1399.093. The study was conducted in accordance with the local legislation and institutional requirements.
NZ: idea, project design, project implementation, statistical analysis, drawing illustrations manually, article writing, article translation, translation editing, and final proofing. SM: project design, project implementation, digitizing Illustrations, article writing, article translation, and translation editing. AB: project design, project implementation, article writing. NN: project design, project implementation, translation editing. SF: project implementation. All authors contributed to manuscript revision, read, and approved the submitted version.
This study conducted as a self-funded project.
This manuscript was based on three approved dissertations at Islamic Azad University, Babol Branch, with registration numbers 1562920629286531398124173 (method development) (48), 1562920629286531399162302942 (postoperative pain) (49), and 1562920629286531398165022 (postoperative serum-CRP) (50), authored by SM, AB, and SF, respectively. We are grateful for Iraj Nowrouzian’s help and insightful suggestions. Mr. Mousavi-Kiasary S.H. is thanked for supplying the resources for these dissertations. The authors would like to thank Farshad Rajabi and Mohammadreza Abdullahzadeh-Delavari, who assisted us in the early planning stage prior to the start of the study. Particular appreciation is extended to the Adib Veterinary Surgical Center (AVSC) for providing equipment and facilities.
The correspondence supplied the idea of instrument shank-assisted OHE. This technique has been registered with the Patent Office-Real Estate Registration Organization of Iran under application number 140050140003000955, date of registration: 2021-04-25, patent number: 107195, date of patent: 2022-06-12, YEKTA identifier: 140150340003001229, verification code: 216204. Thereby, by releasing this article, the authors consider just the rights to intellectual property to be theirs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1210089/full#supplementary-material
1. MacPhail, C, and Fossum, TW. Surgery of the reproductive and genital systems In: TW Fossum, editor. Small animal surgery. Philadelphia, Pennsylvania, USA: Elsevier Health Sciences (2018). 720–87.
2. Berzon, J. Complications of elective ovariohysterectomies in the dog and cat at a teaching institution: clinical review of 853 cases. Vet Surg. (1979) 8:89–91. doi: 10.1111/j.1532-950X.1979.tb00615.x
3. Pearson, H. The complications of ovariohysterectomy in the bitch. J Small Anim Pract. (1973) 14:257–66. doi: 10.1111/j.1748-5827.1973.tb06457.x
4. Freeman, LJ, Rahmani, EY, Sherman, S, Chiorean, MV, Selzer, DJ, Constable, PD, et al. Oophorectomy by natural orifice transluminal endoscopic surgery: feasibility study in dogs. Gastrointest Endosc. (2009) 69:1321–32. doi: 10.1016/j.gie.2008.10.028
5. Höglund, O, Olsson, K, Hagman, R, Öhlund, M, Olsson, U, and Lagerstedt, A-S. Comparison of Haemodynamic changes during two surgical methods for neutering female dogs. Res Vet Sci. (2011) 91:159–63. doi: 10.1016/j.rvsc.2010.08.013
6. Hansen, BD. Assessment of pain in dogs: veterinary clinical studies. ILAR J. (2003) 44:197–205. doi: 10.1093/ilar.44.3.197
7. Burrow, R, Batchelor, D, and Cripps, P. Complications observed during and after ovariohysterectomy of 142 bitches at a veterinary teaching hospital. Vet Rec. (2005) 157:829–33. doi: 10.1136/vr.157.26.829
8. Freeman, LJ, Rahmani, EY, Al-Haddad, M, Sherman, S, Chiorean, MV, Selzer, DJ, et al. Comparison of pain and postoperative stress in dogs undergoing natural orifice transluminal endoscopic surgery, laparoscopic, and open oophorectomy. Gastrointest Endosc. (2010) 72:373–80. doi: 10.1016/j.gie.2010.01.066
9. Fransson, BA, Lagerstedt, AS, Bergstrom, A, Hagman, R, Park, JS, Chew, BP, et al. C-reactive protein, tumor necrosis factor Α, and Interleukin-6 in dogs with Pyometra and sirs. J Vet Emerg Crit Care. (2007) 17:373–81. doi: 10.1111/j.1476-4431.2006.00203.x
10. Bowlt, K, Murray, J, Herbert, G, Delisser, P, Ford-Fennah, V, Murrell, J, et al. Evaluation of the expectations, learning and competencies of surgical skills by undergraduate veterinary students performing canine ovariohysterectomies. J Small Anim Pract. (2011) 52:587–94. doi: 10.1111/j.1748-5827.2011.01120.x
11. Shivley, JM, Richardson, JM, Woodruff, KA, Brookshire, WC, Meyer, RE, and Smith, DR. Sharp transection of the suspensory ligament as an alternative to digital strumming during canine ovariohysterectomy. Vet Surg. (2019) 48:216–21. doi: 10.1111/vsu.13121
12. Devitt, CM, Cox, RE, and Hailey, JJ. Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs. J Am Vet Med Assoc. (2005) 227:921–7. doi: 10.2460/javma.2005.227.921
13. Michelsen, J, Heller, J, Wills, F, and Noble, G. Effect of surgeon experience on postoperative plasma cortisol and C-reactive protein concentrations after ovariohysterectomy in the dog: a randomised trial. Aust Vet J. (2012) 90:474a–4478a. doi: 10.1111/j.1751-0813.2012.01013.x
14. Tsai, TY, Chang, SK, Chou, PY, and Yeh, LS. Comparison of postoperative effects between lidocaine infusion, meloxicam, and their combination in dogs undergoing ovariohysterectomy. Vet Anaesth Analg. (2013) 40:615–22. doi: 10.1111/vaa.12064
15. Campagnol, D, Teixeira-Neto, FJ, Monteiro, ER, Restitutti, F, and Minto, BW. Effect of intraperitoneal or incisional bupivacaine on pain and the analgesic requirement after ovariohysterectomy in dogs. Vet Anaesth Analg. (2012) 39:426–30. doi: 10.1111/j.1467-2995.2012.00728.x
16. Merskey, H, and Watson, G. The lateralisation of pain. Pain. (1979) 7:271–80. doi: 10.1016/0304-3959(79)90084-8
17. Morton, DB, and Griffiths, P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. (1985) 116:431–6. doi: 10.1136/vr.116.16.431
18. Robertson, S, Taylor, P, Lascelles, B, and Dixon, M. Changes in thermal threshold response in eight cats after Administration of Buprenorphine, Butorphanol and morphine. Vet Rec. (2003) 153:462–5. doi: 10.1136/vr.153.15.462
19. Desborough, JP. The stress response to trauma and surgery. Br J Anaesth. (2000) 85:109–17. doi: 10.1093/bja/85.1.109
20. Hernández-Avalos, I, Flores-Gasca, E, Mota-Rojas, D, Casas-Alvarado, A, Miranda-Cortés, AE, and Domínguez-Oliva, A. Neurobiology of anesthetic-surgical stress and induced behavioral changes in dogs and cats: a review. Vet World. (2021) 14:393–404. doi: 10.14202/vetworld.2021.393-404
21. Goldberg, ME, and Shaffran, N. Pain Management for Veterinary Technicians and Nurses. Philadelphia, Pennsylvania, USA: John Wiley & Sons (2014).
22. Grisneaux, E, Pibarot, P, Dupuis, J, and Blais, D. Comparison of Ketoprofen and Carprofen administered prior to orthopedic surgery for control of postoperative pain in dogs. American Veterinary Medical Association (1999).
23. Hoffman, BM, Coons, MJ, and Kuo, PC. Personality differences between surgery residents, nonsurgery residents, and medical students. Surgery. (2010) 148:187–93. doi: 10.1016/j.surg.2010.04.005
24. Pfandler, M, Stefan, P, Mehren, C, Lazarovici, M, and Weigl, M. Technical and nontechnical skills in surgery: a simulated operating room environment study. Spine. (2019) 44:E1396–400. doi: 10.1097/brs.0000000000003154
25. Sadideen, H, Alvand, A, Saadeddin, M, and Kneebone, R. Surgical experts: born or made? Int J Surg. (2013) 11:773–8. doi: 10.1016/j.ijsu.2013.07.001
26. Hatala, R, Cook, DA, Brydges, R, and Hawkins, R. Constructing a validity argument for the objective structured assessment of technical skills (Osats): a systematic review of validity evidence. Adv Health Sci Educ Theory Pract. (2015) 20:1149–75. doi: 10.1007/s10459-015-9593-1
27. Niitsu, H, Hirabayashi, N, Yoshimitsu, M, Mimura, T, Taomoto, J, Sugiyama, Y, et al. Using the objective structured assessment of technical skills (Osats) global rating scale to evaluate the skills of surgical trainees in the operating room. Surg Today. (2013) 43:271–5. doi: 10.1007/s00595-012-0313-7
28. Brun, MV, Silva, MA, Mariano, MB, Motta, AC, Colomé, LM, Feranti, JP, et al. Ovariohysterectomy in a dog by a hybrid notes technique. Can Vet J. (2011) 52:637–40.
29. Pukacz, M, Kienzle, B, and Braun, J. Simple, minimally invasive technique for ovariohysterectomy in the dog. Vet Rec. (2009) 165:688–90. doi: 10.1136/vr.165.23.688
30. Mich, PM, Hellyer, PW, Kogan, L, and Schoenfeld-Tacher, R. Effects of a pilot training program on veterinary Students' pain knowledge, attitude, and assessment skills. J Vet Med Educ. (2010) 37:358–68. doi: 10.3138/jvme.37.4.358
31. Capner, A, Lascelles, B, and Waterman-Pearson, A. Current British veterinary attitudes to perioperative analgesia for dogs. Vet Rec. (1999) 145:95–9. doi: 10.1136/vr.145.4.95
32. Hernandez-Avalos, I, Mota-Rojas, D, Mora-Medina, P, Martínez-Burnes, J, Casas Alvarado, A, Verduzco-Mendoza, A, et al. Review of different methods used for clinical recognition and assessment of pain in dogs and cats. Int J Vet Sci Med. (2019) 7:43–54. doi: 10.1080/23144599.2019.1680044
33. Moldal, ER, Kjelgaard-Hansen, MJ, Peeters, ME, Nødtvedt, A, and Kirpensteijn, J. C-reactive protein, glucose and Iron concentrations are significantly altered in dogs undergoing open ovariohysterectomy or ovariectomy. Acta Vet Scand. (2018) 60:32. doi: 10.1186/s13028-018-0384-6
34. Donati, A, Ruzzi, M, Adrario, E, Pelaia, P, Coluzzi, F, Gabbanelli, V, et al. A new and feasible model for predicting operative risk. Br J Anaesth. (2004) 93:393–9. doi: 10.1093/bja/aeh210
35. Höglund, OV, Hagman, R, and Stridsberg, M. Chromogranin a and cortisol at intraoperative repeated noxious stimuli: surgical stress in a dog model. SAGE Open Med. (2015) 3:2050312115576432. doi: 10.1177/2050312115576432
36. Prete, A, Yan, Q, Al-Tarrah, K, Akturk, HK, Prokop, LJ, Alahdab, F, et al. The cortisol stress response induced by surgery: a systematic review and Meta-analysis. Clin Endocrinol. (2018) 89:554–67. doi: 10.1111/cen.13820
37. Cusack, B, and Buggy, DJ. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. (2020) 20:321–8. doi: 10.1016/j.bjae.2020.04.006
38. Anand, K. The stress response to surgical trauma: from physiological basis to therapeutic implications. Prog Food Nutr Sci. (1986) 10:67–132.
39. Burton, D, Nicholson, G, and Hall, G. Endocrine and metabolic response to surgery. Cont Educ Anaesth Crit Care Pain. (2004) 4:144–7. doi: 10.1093/bjaceaccp/mkh040
40. Radunovic, M, Radunovic, M, Radunovic, M, Lazovic, R, Panic, N, and Bulajiic, M. Biohumoral and endocrine parameters in assessment of surgical trauma in open and laparoscopic cholecystectomy. Vojnosanit Pregl. (2013) 70:555–60. doi: 10.2298/vsp1306555r
41. James, K. Interactions between cytokines and Α2-macroglobulin. Immunol Today. (1990) 11:163–6. doi: 10.1016/0167-5699(90)90067-J
42. Christensen, MB, Eriksen, T, and Kjelgaard-Hansen, M. C-reactive protein: quantitative marker of surgical trauma and post-surgical complications in dogs: a systematic review. Acta Vet Scand. (2015) 57:71. doi: 10.1186/s13028-015-0164-5
43. Wirtz, DC, Heller, KD, Miltner, O, Zilkens, KW, and Wolff, JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. (2000) 24:194–6. doi: 10.1007/s002640000136
44. Reid, J, Scott, M, and Nolan, A. Pain assessment in companion animals: an update. In Pract. (2017) 39:446–51. doi: 10.1136/inp.j4513
45. Reid, J, Nolan, A, Hughes, J, Lascelles, D, Pawson, P, and Scott, E. Development of the short-form Glasgow composite measure pain scale (CMPS-SF) and derivation of an analgesic intervention score. J Appl Anim Welf Sci. (2007) 16:97–104. doi: 10.1017/S096272860003178X
46. Stahl, WM. Acute phase protein response to tissue injury. Crit Care Med. (1987) 15:545–50. doi: 10.1097/00003246-198706000-00001
47. Bradbury, G, and Morton, K. Using Behavioural science to improve pain management. In Pract. (2017) 39:339–41. doi: 10.1136/inp.j3251
48. Mousavi-Kiasary, SMS. Shank-assisted ovariohysterectomy in deep-chested mixed breed dogs [original research]. Babol: Babol Branch, Islamic Azad University (2020).
49. Behzadi, A. Qualitative evaluation of postoperative pain in shank-assisted ovariohysterectomized dogs compared to the conventional method [original research]. Babol: Babol Branch, Islamic Azad University (2021).
50. Mahmoodi-Ferdowsi, S. Evaluation of serum CRP changes in shank-assisted ovariohysterectomy dogs compared to the conventional method [original research]. Babol: Babol Branch, Islamic Azad University (2020).
51. Tomlinson, T. How to be fair, and power research? Select patients by flipping a coin. Am J Bioeth. (2020) 20:29–31. doi: 10.1080/15265161.2020.1795534
52. Schulz, KF, and Grimes, DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. (2002) 359:515–9. doi: 10.1016/S0140-6736(02)07683-3
53. Brewin, CR, and Bradley, C. Patient preferences and randomised clinical trials. BMJ. (1989) 299:313–5. doi: 10.1136/bmj.299.6694.313
54. AHo, D. ASA physical status classification system. American Society of Anesthesiologists. (2018).
55. Höglund, OV, Hagman, R, Olsson, K, Olsson, U, and Lagerstedt, A-S. Intraoperative changes in blood pressure, heart rate, plasma vasopressin, and urinary noradrenalin during elective ovariohysterectomy in dogs: repeatability at removal of the 1st and 2nd ovary. Vet Surg. (2014) 43:852–9. doi: 10.1111/j.1532-950X.2014.12264.x
56. Höglund, OV, Lövebrant, J, Olsson, U, and Höglund, K. Blood pressure and heart rate during ovariohysterectomy in Pyometra and control dogs: a preliminary investigation. Acta Vet Scand. (2016) 58:80. doi: 10.1186/s13028-016-0263-y
57. Harris, KP, Adams, VJ, Fordyce, P, and Ladlow, J. Comparison of surgical duration of canine Ovariectomy and ovariohysterectomy in a veterinary teaching hospital. J Small Anim Pract. (2013) 54:579–83. doi: 10.1111/jsap.12147
58. Peeters, ME, and Kirpensteijn, J. Comparison of surgical variables and short-term postoperative complications in healthy dogs undergoing ovariohysterectomy or ovariectomy. J Am Vet Med Assoc. (2011) 238:189–94. doi: 10.2460/javma.238.2.189
59. Mathews, K, Kronen, PW, Lascelles, D, Nolan, A, Robertson, S, Steagall, PV, et al. Guidelines for recognition, assessment and treatment of pain: WSAVA global pain council members and co-authors of this document. J Small Anim Pract. (2014) 55:E10–68. doi: 10.1111/jsap.12200
60. Holton, L, Pawson, P, Nolan, A, Reid, J, and Scott, E. Development of a behaviour-based scale to measure acute pain in dogs. Vet Rec. (2001) 148:525–31. doi: 10.1136/vr.148.17.525
61. Firth, AM, and Haldane, SL. Development of a scale to evaluate postoperative pain in dogs. J Am Vet Med Assoc. (1999) 214:651–9.
62. Holton, L, Scott, E, Nolan, A, Reid, J, and Welsh, E. Relationship between physiological factors and clinical pain in dogs scored using a numerical rating scale. J Small Anim Pract. (1998) 39:469–74. doi: 10.1111/j.1748-5827.1998.tb03681.x
63. Allegranzi, B, Zayed, B, Bischoff, P, Kubilay, NZ, de Jonge, S, de Vries, F, et al. New who recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. (2016) 16:e288–303. doi: 10.1016/S1473-3099(16)30402-9
64. Schober, P, Boer, C, and Schwarte, LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. (2018) 126:1763–8. doi: 10.1213/ANE.0000000000002864
65. Van Goethem, BS-OA, and Kirpensteijn, J. Making a rational choice between ovariectomy and ovariohysterectomy in the dog: a discussion of the benefits of either technique. Vet Surg. (2006) 35:136–43. doi: 10.1111/j.1532-950X.2006.00124.x
66. Hill, LN, and Smeak, DD. Suspensory ligament rupture technique during ovariohysterectomy in small animals. Compendium. (2010) 32:E1–7.
67. Anton, NE, Athanasiadis, DI, Karipidis, T, Keen, AY, Karim, A, Cha, J, et al. Surgeon stress negatively affects their non-technical skills in the operating room. Am J Surg. (2021) 222:1154–7. doi: 10.1016/j.amjsurg.2021.01.035
68. Rodriguez, FR, Kirby, BM, and Ryan, J. Evaluation of factors associated with retained surgical sponges in veterinary patients: a survey of veterinary practitioners. J Small Anim Pract. (2018) 59:570–7. doi: 10.1111/jsap.12873
69. Braungart, MM, Braungart, RG, and Gramet, P. Applying learning theories to healthcare practice. Jones and Bartlett Learning (2003).
70. Freeman, LJ, Ferguson, N, Fellenstein, C, Johnson, R, and Constable, PD. Evaluation of learning curves for ovariohysterectomy of dogs and cats and castration of dogs. J Am Vet Med Assoc. (2017) 251:322–32. doi: 10.2460/javma.251.3.322
71. Shaver, SL, Larrosa, M, and Hofmeister, EH. Factors affecting the duration of anesthesia and surgery of canine and feline Gonadectomies performed by veterinary students in a year-long preclinical surgery laboratory. Vet Surg. (2019) 48:352–9. doi: 10.1111/vsu.13163
72. Stulberg, JJ, Huang, R, Kreutzer, L, Ban, K, Champagne, BJ, Steele, SR, et al. Association between surgeon technical skills and patient outcomes. JAMA Surg. (2020) 155:960–8. doi: 10.1001/jamasurg.2020.3007
73. Ericsson, KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. (2004) 79:S70–81. doi: 10.1097/00001888-200410001-00022
74. Giannoudis, PV, Dinopoulos, H, Chalidis, B, and Hall, GM. Surgical stress response. Injury. (2006) 37:S3–9. doi: 10.1016/S0020-1383(07)70005-0
75. Engelmann, C, Schneider, M, Kirschbaum, C, Grote, G, Dingemann, J, Schoof, S, et al. Effects of intraoperative breaks on mental and somatic operator fatigue: a randomized clinical trial. Surg Endosc. (2011) 25:1245–50. doi: 10.1007/s00464-010-1350-1
76. Slack, P, Coulson, C, Ma, X, Webster, K, and Proops, D. The effect of operating time on Surgeons' muscular fatigue. Ann R Coll Surg Engl. (2008) 90:651–7. doi: 10.1308/003588408X321710
77. Haga, Y, Ikei, S, and Ogawa, M. Estimation of physiologic ability and surgical stress (E-pass) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. (1999) 29:219–25. doi: 10.1007/BF02483010
78. Gillespie, BM, Chaboyer, W, and Fairweather, N. Factors that influence the expected length of operation: results of a prospective study. BMJ Qual Saf. (2012) 21:3–12. doi: 10.1136/bmjqs-2011-000169
79. Tallant, A, Ambros, B, Freire, C, and Sakals, S. Comparison of intraoperative and postoperative pain during canine ovariohysterectomy and ovariectomy. Can Vet J. (2016) 57:741–6.
80. Reid, J, Scott, M, Nolan, A, and Wiseman-Orr, L. Pain assessment in animals. In Pract. (2013) 35:51–6. doi: 10.1136/inp.f631
81. Hansen, BD, Hardie, EM, and Carroll, GS. Physiological measurements after ovariohysterectomy in dogs: what's normal? Appl Anim Behav Sci. (1997) 51:101–9. doi: 10.1016/S0168-1591(96)01079-9
82. Guillot, M, Rialland, P, Nadeau, MÈ, Del Castillo, J, Gauvin, D, and Troncy, E. Pain induced by a minor medical procedure (bone marrow aspiration) in dogs: comparison of pain scales in a pilot study. J Vet Intern Med. (2011) 25:1050–6. doi: 10.1111/j.1939-1676.2011.00786.x
83. Fajt, VR, Wagner, SA, and Norby, B. Analgesic drug administration and attitudes about analgesia in cattle among bovine practitioners in the United States. J Am Vet Med Assoc. (2011) 238:755–67. doi: 10.2460/javma.238.6.755
84. Gerbershagen, HJ, Aduckathil, S, van Wijck, AJM, Peelen, LM, Kalkman, CJ, and Meissner, W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. (2013) 118:934–44. doi: 10.1097/ALN.0b013e31828866b3
85. Crompton, S. Reviewing pain assessment and scoring models in cats and dogs–part one. Vet Times. (2014) 14:15–8.
86. Horta, RS, Figueiredo, MS, Lavalle, GE, Costa, MP, Cunha, RMC, and Araújo, RB. Surgical stress and postoperative complications related to regional and radical mastectomy in dogs. Acta Vet Scand. (2015) 57:34. doi: 10.1186/s13028-015-0121-3
87. Cohen, FL. Postsurgical pain relief: Patients' status and Nurses' medication choices. Pain. (1980) 9:265–74. doi: 10.1016/0304-3959(80)90013-5
88. Peters, CL, Shirley, B, and Erickson, J. The effect of a new multimodal perioperative anesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after Total joint arthroplasty. J Arthroplast. (2006) 21:132–8. doi: 10.1016/j.arth.2006.04.017
89. Kehlet, H, and Dahl, JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. (1993) 77:1048–56. doi: 10.1213/00000539-199311000-00030
90. Filos, KS, and Lehmann, KA. Current concepts and practice in postoperative pain management: need for a change? Eur Surg Res. (1999) 31:97–107. doi: 10.1159/000008627
91. Ong, CK-S, Lirk, P, Seymour, RA, and Jenkins, BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. (2005) 100:757–73. doi: 10.1213/01.Ane.0000144428.98767.0e
92. Garimella, V, and Cellini, C. Postoperative pain control. Clin Colon Rectal Surg. (2013) 26:191–6. doi: 10.1055/s-0033-1351138
93. Lovich-Sapola, J, Smith, CE, and Brandt, CP. Postoperative pain control. Surg Clin. (2015) 95:301–18. doi: 10.1016/j.suc.2014.10.002
94. Dumville, JC, Gray, TA, Walter, CJ, Sharp, CA, Page, T, Macefield, R, et al. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev. (2016) 12:CD003091. doi: 10.1002/14651858.CD003091.pub4
95. Welsh, EM, Nolan, AM, and Reid, J. Beneficial effects of administering Carprofen before surgery in dogs. Vet Rec. (1997) 141:251–3. doi: 10.1136/vr.141.10.251
96. Lascelles, BD, Cripps, PJ, Jones, A, and Waterman-Pearson, AE. Efficacy and kinetics of Carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet Surg. (1998) 27:568–82. doi: 10.1111/j.1532-950x.1998.tb00533.x
97. Riviere, JE, and Papich, MG. Veterinary pharmacology and therapeutics. Philadelphia, Pennsylvania, USA: John Wiley & Sons (2018).
98. Cagnardi, P, Zonca, A, Gallo, M, Villa, R, Carli, S, Beccaglia, M, et al. Pharmacokinetics and perioperative efficacy of intravenous ketorolac in dogs. J Vet Pharmacol Ther. (2013) 36:603–8. doi: 10.1111/jvp.12044
99. Papich, M. Papich mg Papich handbook of veterinary drugs. Philadelphia, Pennsylvania, USA: WB Saunders (2021).
100. Moaddel, R, Sanghvi, M, Ramamoorthy, A, Jozwiak, K, Singh, N, Green, C, et al. Subchronic administration of (R, S)-ketamine induces ketamine ring hydroxylation in Wistar rats. J Pharm Biomed Anal. (2016) 127:3–8. doi: 10.1016/j.jpba.2016.03.030
101. Hommeril, J-L, Bernard, J-M, Gouin, F, and Pinaud, M. Ketoprofen for pain after hip and knee arthroplasty. Br J Anaesth. (1994) 72:383–7. doi: 10.1093/bja/72.4.383
102. Mathews, KA, Pettifer, G, Foster, R, and McDonell, W. Safety and efficacy of preoperative Administration of Meloxicam, compared with that of Ketoprofen and Butorphanol in dogs undergoing abdominal surgery. Am J Vet Res. (2001) 62:882–8. doi: 10.2460/ajvr.2001.62.882
103. Slingsby, LS, and Waterman-Pearson, A. Postoperative analgesia in the cat after ovariohysterectomy by use of Carprofen, Ketoprofen, meloxicam or Tolfenamic acid. J Small Anim Pract. (2000) 41:447–50. doi: 10.1111/j.1748-5827.2000.tb03139.x
104. Rugytė, D, and Gudaitytė, J. Intravenous paracetamol in adjunct to intravenous Ketoprofen for postoperative pain in children undergoing general surgery: a double-blinded randomized study. Medicina. (2019) 55:86. doi: 10.3390/medicina55040086
105. Mathews, KA, Paley, DM, Foster, RA, Valliant, AE, and Young, SS. A comparison of ketorolac with Flunixin, Butorphanol, and Oxymorphone in controlling postoperative pain in dogs. Can Vet J. (1996) 37:557.
106. Hanson, PD, and Maddison, JE. Nonsteroidal anti-inflammatory drugs and Chondroprotective agents. Small Anim Clin Pharmacol. (2008) 5:287. doi: 10.1016/B978-070202858-8.50015-4
107. Mansour, C, Merlin, T, Bonnet-Garin, J-M, Chaaya, R, Mocci, R, Ruiz, CC, et al. Evaluation of the parasympathetic tone activity (PTA) index to assess the analgesia/nociception balance in anaesthetised dogs. Res Vet Sci. (2017) 115:271–7. doi: 10.1016/j.rvsc.2017.05.009
108. Hernández-Avalos, I, Valverde, A, Ibancovichi-Camarillo, JA, Sánchez-Aparicio, P, Recillas-Morales, S, Rodríguez-Velázquez, D, et al. Clinical use of the parasympathetic tone activity index as a measurement of postoperative analgaesia in dogs undergoing ovariohysterectomy. J Vet Res. (2021) 65:117–23. doi: 10.2478/jvetres-2021-0004
109. Casas-Alvarado, A, Mota-Rojas, D, Hernández-Ávalos, I, Mora-Medina, P, Olmos-Hernández, A, Verduzco-Mendoza, A, et al. Advances in infrared thermography: surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J Therm Biol. (2020) 92:102664. doi: 10.1016/j.jtherbio.2020.102664
110. Küls, N, Blissitt, KJ, Shaw, DJ, Schöffmann, G, and Clutton, RE. Thermography as an early predictive measurement for evaluating epidural and femoral–sciatic block success in dogs. Vet Anaesth Analg. (2017) 44:1198–207. doi: 10.1016/j.vaa.2016.11.009
111. Boev, AN, Fountas, KN, Karampelas, I, Boev, C, Machinis, TG, Feltes, C, et al. Quantitative Pupillometry: normative data in healthy pediatric volunteers. J Neurosurg Pediatr. (2005) 103:496–500. doi: 10.3171/ped.2005.103.6.0496
112. Olson, DM, Stutzman, S, Saju, C, Wilson, M, Zhao, W, and Aiyagari, V. Interrater reliability of pupillary assessments. Neurocrit Care. (2016) 24:251–7. doi: 10.1007/s12028-015-0182-1
113. Kim, J, Heo, J, Ji, D, and Kim, MS. Quantitative assessment of pupillary light reflex in normal and anesthetized dogs: a preliminary study. J Vet Med Sci. (2015) 77:475–8. doi: 10.1292/jvms.14-0387
114. Dąbrowski, R, Wawron, W, and Kostro, K. Changes in CRP, SAA and haptoglobin produced in response to ovariohysterectomy in healthy bitches and those with Pyometra. Theriogenology. (2007) 67:321–7. doi: 10.1016/j.theriogenology.2006.07.019
115. Conner, J, Eckersall, P, Ferguson, J, and Douglas, T. Acute phase response in the dog following surgical trauma. Res Vet Sci. (1988) 45:107–10. doi: 10.1016/S0034-5288(18)30902-0
116. Kang, B-U, Lee, S-H, Ahn, Y, Choi, W-C, and Choi, Y-G. Surgical site infection in spinal surgery: detection and management based on serial C-reactive protein measurements. J Neurosurg Spine. (2010) 13:158–64. doi: 10.3171/2010.3.SPINE09403
117. Kjelgaard-Hansen, M, Strom, H, Mikkelsen, LF, Eriksen, T, Jensen, AL, and Luntang-Jensen, M. Canine serum C-reactive protein as a quantitative marker of the inflammatory stimulus of aseptic elective soft tissue surgery. Vet Clin Pathol. (2013) 42:342–5. doi: 10.1111/vcp.12063
118. Malin, K, and Witkowska-Piłaszewicz, O. C-reactive protein as a diagnostic marker in dogs: a review. Animals. (2022) 12:2888. doi: 10.3390/ani12202888
119. Pepys, MB, and Hirschfield, GM. C-reactive protein: a critical update. J Clin Invest. (2003) 111:1805–12. doi: 10.1172/JCI200318921
120. Neumaier, M, Metak, G, and Scherer, MA. C-reactive protein as a parameter of surgical trauma: CRP response after different types of surgery in 349 hip fractures. Acta Orthop. (2006) 77:788–90. doi: 10.1080/17453670610013006
121. Tylicka, M, Matuszczak, E, Karpińska, M, Hermanowicz, A, Dębek, W, and Ostrowska, H. Proteasome and C-reactive protein inflammatory response in children undergoing shorter and longer lasting laparoscopic cholecystectomy. Scand J Clin Lab Invest. (2017) 77:610–6. doi: 10.1080/00365513.2017.1385839
Keywords: instrument shank-assisted, ovariohysterectomy, pain score, deep-chest, dog, OHE
Citation: Ziaei Darounkolaei N, Mousavi Kiasary SMS, Behzadi A, Nabavi Mosavi N and Ferdowsi SM (2023) Instrument shank-assisted ovariohysterectomy: a randomized clinical trial of surgical and pain alleviation efficiency of a single-person modified technique. Front. Vet. Sci. 10:1210089. doi: 10.3389/fvets.2023.1210089
Received: 21 April 2023; Accepted: 03 October 2023;
Published: 17 October 2023.
Edited by:
Lynette Arnason Hart, University of California, Davis, United StatesReviewed by:
Ismael Hernández Avalos, National Autonomous University of Mexico, MexicoCopyright © 2023 Ziaei Darounkolaei, Mousavi Kiasary, Behzadi, Nabavi Mosavi and Ferdowsi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Navid Ziaei Darounkolaei, emlhZWlAYmFib2xpYXUuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.