- 1Departamento de Fisiología y Farmacologia, Faculta de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México, Ciudad de México, Mexico
- 2Departamento de Química Analítica, Facultad de Química, Universidad Nacional Autónoma de México, Ciudad de México, Mexico
Introduction: The use of florfenicol must follow particular pharmacokinetic/pharmacodynamic (PK/PD) ratios, i.e., it requires achieving serum concentrations at or slightly above the pathogen's minimum inhibitory concentration (MIC) during the dosing interval and that the ratio of area under the concentration vs. time curve (AUC)/MIC should be as high as possible (still undetermined for poultry). As an alternative to the standard soluble florfenicol that is administered to the flock through drinking water, florfenicol premix is often recommended as feed medication in Latin America. However, no particular pharmaceutical design has been proposed.
Methods: This study compared the PK of two preparations of florfenicol in broiler chickens and pondered the possibility of each covering the referred PK-PD ratios as predictors of clinical efficacy. The preparations comprise a pharmaceutical form as FOLA pellets (F = bioavailability; O = optimum; and LA = long-acting) and the premix formulation. The former are small colored pellets with vehicles and absorption enhancers of florfenicol designed for long action, and the latter is the reference premix of the antibiotic. First, these two pharmaceutical forms of florfenicol were administered as oral boluses (30 mg/kg), aided by a probe. In a second trial of the dosing form, both pharmaceutical preparations of florfenicol were administered in feed and ad libitum (110 ppm; ~30 mg/kg).
Results: In both cases, FOLA-florfenicol presented much higher relative bioavailability (3.27 times higher) and mean better residence time than florfenicol premix (two times high when forced as bolus dose). Consequently, FOLA-florfenicol possesses better PK/PD ratios than less sensitive pathogens, i.e., E. coli. It is proposed that if a metaphylactic treatment of a bacterial outbreak in poultry is implemented with florfenicol prepared as FOLA, better PK/PD ratios will be obtained than those of standard florfenicol premix.
Discussion: Clinicians must confirm that feed consumption in the flock has not been affected by the particular disease if FOLA pellets of florfenicol are used.
Introduction
Florfenicol is a wide-spectrum, synthetic antibacterial structurally related to chloramphenicol and thiamphenicol. To date, there is no evidence of toxicity or relevant adverse effects in poultry for this derivative. In contrast, chloramphenicol has been banned in most countries due to its involvement in human toxicity, i.e., aplastic anemia that can be developed even with residual amounts (1). The recommended dose in poultry is 30–40 mg/kg bw/day for 3 days via drinking water, and its pharmacokinetics has been defined for this vehicle (2, 3). In contrast, no published data recommend its use as an in-feed medication in poultry species. Nevertheless, several florfenicol premix preparations are available for poultry in Latin American countries. However, in broiler chickens, the oral bioavailability appears to be lower in fed (55%) than in fasted animals (87–96%) (3, 4). This feature may indicate that administering florfenicol in poultry as an in-feed medication may or may not achieve adequate serum and tissue concentrations and consequently may or may not deliver good clinical efficacy. Florfenicol shows an elimination half-life of at least 106.6 min in poultry when administered through their drinking water (5, 6), and it exhibits a reasonably good apparent volume of distribution at a steady state (3.5 ml/kg) (6). When experimentally administered by an oral gavage at a dose of 40 mg/kg bw, the duration of therapeutic plasma concentrations can be stretched up to 8 h (7). If administered ad libitum through the drinking water (approximate dose of 26 mg/kg bw) in an 18:6 dark:light cycle for 3 days, the estimated mean serum concentration of florfenicol averaged ~ 0.7 μg/ml, and no florfenicol was detected in serum 72 h after the terminal dose. Florfenicol is partially metabolized into florfenicol amine, which is still bioactive (3).
Florfenicol is usually prescribed for treating gastrointestinal and respiratory tract infections in poultry. The presence of a bacterial disease must be established to administer florfenicol as a metaphylaxis treatment (8). Based on the above features of florfenicol, it is postulated that it could be administered in poultry as feed medication and achieve good bioavailability in broiler chickens. According to Murugayan et al. (8), it is necessary to seek alternatives in dosing antibacterial drugs to chicken to optimize pharmacokinetic/pharmacodynamic ratios and reduce the emergence of bacterial resistance caused by the misuse of these drugs. This trial was set to test this hypothesis; that is, a comparative pharmacokinetic study was carried out in broiler chickens, evaluating an experimental long-acting dosage form of florfenicol, prepared as small colored pellets, and named FOLA [Patent No. MX/a/2012/013222 and PCT/MX2013/000137, Universidad Nacional Autónoma de México [UNAM]; FOLA stands for bioavailability (F), optimum (O), and long-acting preparation (LA)]. A standard commercial premix of florfenicol for in-feed administration was taken as a reference preparation.
Materials and methods
The study design and animal handling complied with Mexican regulations for experimental animals, as stated by CICUA No. 0673 (Internal Committee for the Care and Use of Animals, UNAM) and Mexican prescripts in NOM-062-ZOO-1999. This trial was carried out in an experimental chicken house in Mexico City. Overall, 360 15-day-old Ross-308 broiler chickens, weighing approximately 450 g with a daily gain of 60 g, were distributed by simple randomization in four groups. They were allocated within a single chicken house. It was divided by wire mesh into eight smaller areas to contain 45 chicken broilers per group, i.e., a group and its replica as follows: for bolus dose, (FREF − bolus) florfenicol premix from the product NF-180® 8% (PiSA Agropecuaria S.A. de CV, Mexico; https://www.avicultura.mx/producto/nf-180-8) approved in Mexico and other Latin American countries for poultry.
The bolus administration of the florfenicol premix or the florfenicol FOLA was carried out at 6 a.m. Each pharmaceutical preparation was weighed to meet individual broiler chicken weights. Each preparation was suspended in tap water with 2% gelatin and stirred prior to its dosing. The administration was achieved by employing a plastic probe attached to a 10 ml syringe and introduced into the included samples. In both groups, the established dose was 30 mg/kg (15 mg/chicken broiler). Once ensured that no regurgitation occurred, broiler chickens were allowed to feed and water ad libitum. For the ad libitum dosing (FREFad − lib and FFOLAad − lib), florfenicol, either as premix from NF-180® 8% or FOLAs, was incorporated into their powdered feed at a rate of 110 ppm of florfenicol, considering a feed intake of 140 g/chicken and a final dose of ~ 30 mg/kg/day (15 mg/chicken, considering a 10% feed-waste). Medicated feeds were prepared daily. In addition, FOLAs were added to the powdered feed as a dressing at the same dose rate, establishing visually that the distribution of pellets was even. In both ad libitum groups, florfenicol was administered for 3 days, making feeders available from 6:00 a.m. to 12:00 p.m. during these days. Diet composition is presented in Table 1.
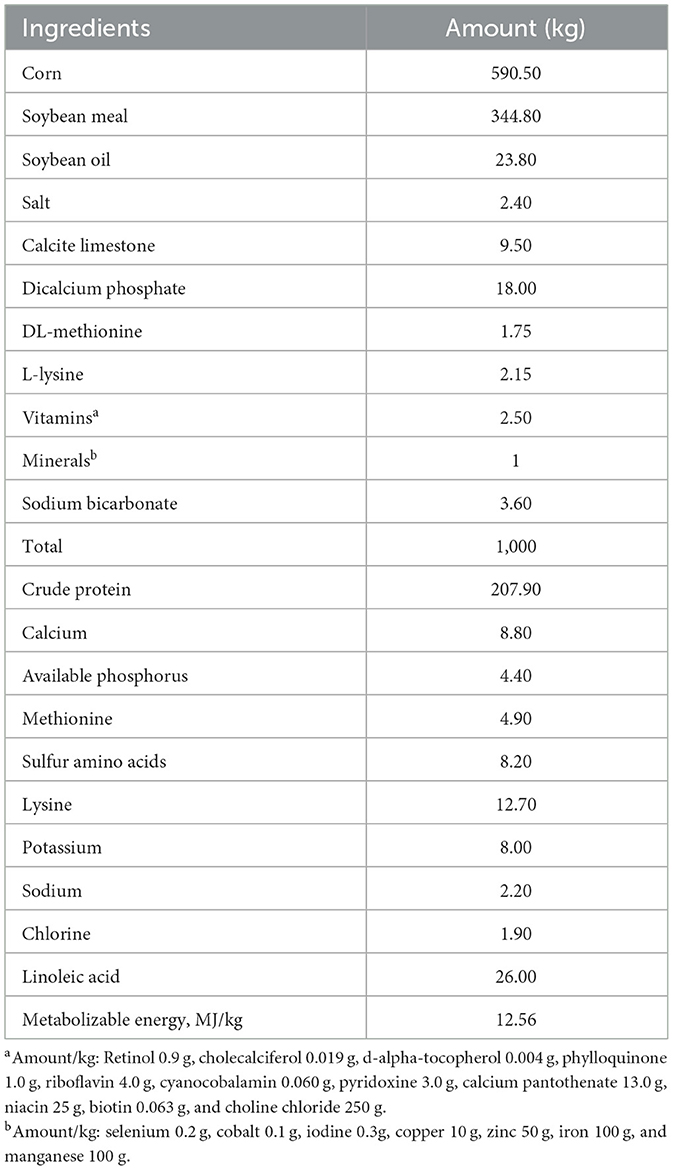
Table 1. Diet composition for this trial with 15-day-old Ross-308 broiler chickens, weighing ~ 450 g.
The pharmacokinetic approach of this study was to obtain PK parameters following a type of naïve pooled sampling since each animal was not sampled more than two times. Hence, for the oral bolus dosing (FREF − bolus and FFOLA − bolus) of this trial aided by technical assistance, ~ 1 ml of blood samples was obtained by jugular or radial wing vein puncture with 3 ml syringes and 25g x 1 in pediatric needles. A total of 10 broiler chickens were bled-sampled each time, i.e., 5 from each group and 5 from each repetition. The set times were as follows: 0.5, 1, 2, 4, 6, 8, 12, and 24 h. No bird was sampled more than twice in a 24-h period.
For the ad libitum dosing, blood samples (1 ml per chicken) were obtained from 5 animals in each group and 5 from its repetition during 3 days of medication at fixed times as follows: 2, 4, 8, 14, and 24 h after dosing, i.e., 8 a.m., 10 a.m., 2 p.m., 8 p.m., and 6 a.m. the next morning and on each day. Paint marking of the sampled broiler chickens allowed for an even sampling in each group. Blood samples were centrifuged at 1,000 × g for 5 min, and serum was harvested and stored at −20°C until analyzed.
The FOLA pellets of florfenicol were manufactured in our laboratory as described in Patent No.MX/a/2012/013222 and CT/MX2013/000137 (UNAM), observing good laboratory practices. In brief, 1% carpool with butylhydroxytoluene as an antioxidant was mixed in a base of 1:1 parts of wheat and corn flour. Then, a yellow-orange vegetable dye was added. Finally, florfenicol was incorporated at a rate of 10%. The mixture was mixed and then extruded at temperatures not higher than 30°C, using ethylalcohol and cotton-seed oil as lubricants. The final concentration of florfenicol in FOLAS was 97.6%, as determined by high-performance liquid chromatography (HPLC) based on the established technique (10).
Concentrations of florfenicol and its active metabolite florfenicol-amine were determined in plasma samples by HPLC using the method described by Kowalski et al. (11), with thiamphenicol as an internal standard. In brief, the extraction procedure was initiated by thawing the plasma samples at 20–25°C laboratory temperature. Then, 0.5 ml of plasma aliquot thiamphenicol was added as internal standard (0.5 μg in 0.2 ml), along with 0.2 ml of 1.0 M sodium hydroxide and 3 ml of ethyl acetate. Each sample was vortex mixed and centrifuged at 5,000 g for 15 min, and the organic layer was carefully transferred to another tube. The supernatant was dissolved in 0.5 ml of the mobile phase. Then, the samples were filtered through a membrane (nylon 0.45 μm) and injected into the HPLC with a 0.6 ml/min flow. Acetonitrile–water (25:75, v/v), adjusted to a pH of 2.7 with 85% orthophosphoric acid, was utilized as the mobile phase. Detection and quantitation were performed at 224 nm for excitation wavelength and 290 nm for emission wavelength. Calibration curves for florfenicol and florfenicol-amine were prepared from 0.05 to 20.48 μg/ml (n = 5).
The apparatus used was a Jasco XLC HPLC system (LC-2000Plus; Jasco Benelux, the Netherlands) with a Symmetry-C18 column (4.6 mm × 100 mm, 3.5 μm; Waters, USA) and equipped with a fluorescence detector. Data were analyzed using Empower-3 software from Waters (Mexico). The chromatographic method was validated, and the analytical procedure was demonstrated as specific. The method produced a linear result from 0.05 to 20.48 μg/ml (r2 = 0.984; y = 500030 x−107 046). Recovery of florfenicol and florfenicol-amine was calculated by applying a linear regression analysis. Precision was demonstrated by the inter-day coefficient of variance (3.0) and the inter-assay error value (< 3.8). The lower quantification limit for florfenicol in plasma was 0.05 μg/ml, with a detection limit of 0.008 μg/ml, and linearity was established from 0.05 to 20.48 μg/ml.
A pharmacokinetic analysis of plasma concentration–time data for florfenicol was carried out using PKAnalyst® (Micromath, Scientific Software, SLM, USA). The pharmacokinetic model (11) was based on choosing the most similar one having the highest r after examining the concentration–time curves (r > 0.98). Then, the number of exponential terms required to describe the plasma concentration–time data for each dosing form was determined by applying Akaike's information criterion (12). The peak concentration in serum (CMAX) and the time to CMAX (TMAX) were estimated by observing data from tabulated plasma concentrations. Relative bioavailability (Fr%) was derived by comparing AUC0 − 24 with florfenicol-FOLA and florfenicol-reference groups.
The serum concentration vs. time data was graphed with Origin Lab-Pro 8C. Areas under the concentration vs. time curve (AUC) on days 1 and 3 were calculated through the trapezoidal method and confirmed with PKAnalyst®. Statistical AUC values were compared with ANOVA and successive Dunnet's test.
Results
Figure 1 shows an example of the chromatograms obtained. The method utilized showed linearity when florfenicol-fortified chicken serum samples were analyzed. Mean ± 1 SD serum concentrations vs. time profiles of florfenicol in broiler chickens after forced oral bolus medication with either florfenicol premix from NF-180® or florfenicol prepared as FOLAs are presented in Figure 2. Figure 3 shows the serum concentrations of florfenicol after its administration ad libitum (110 ppm) for 3 days and their pharmacokinetic variables are shown in Table 2. Pharmacokinetic data obtained for oral bolus administration are shown in Table 3. In addition, two pharmacokinetic/pharmacodynamic (PK/PD) ratios (AUC0 − 24/MIC and %T ≥ MIC) are presented for two pathogenic bacteria whose MIC values were taken from the formal literature: one sensitive (0.25 μg/ml) and the other moderately sensitive (2.0 μg/ml) (13, 14). The relative bioavailability value of FFOLA − bolus was 328%. Comparisons for MRT and Tβ showed that these variables were statistically longer (P < 0.05) in FFOLA − bolus compared with FREF − bolus. The time the serum florfenicol levels remain above or equal to MIC values (T≥MIC) and the AUC0 − 24/MIC ratios for FFOLA − bolus show that the latter had larger ratios than the former. Florfenicol administered as premix remained above MIC values of the theoretically sensitive bacteria (0.25 μg/ml) 31% of the dosing interval (24 h), while florfenicol prepared as FOLA was capable of covering the whole dosing interval. However, the same T ≥ MIC ratio is reduced to 16% and 30% for FREF − bolus and FFOLA − bolus, respectively, if the challenged bacteria are moderately sensitive (2.0 μg/ml). AUC0 − 24/CMI ratios follow the same pattern favoring the FOLA form of florfenicol.
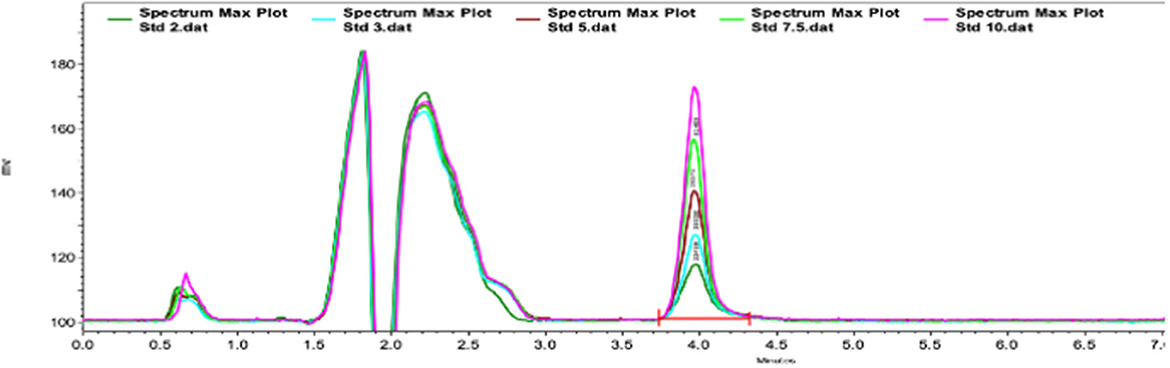
Figure 1. Type chromatogram of florfenicol in chicken serum samples. The retention time for florfenicol was 4 min.
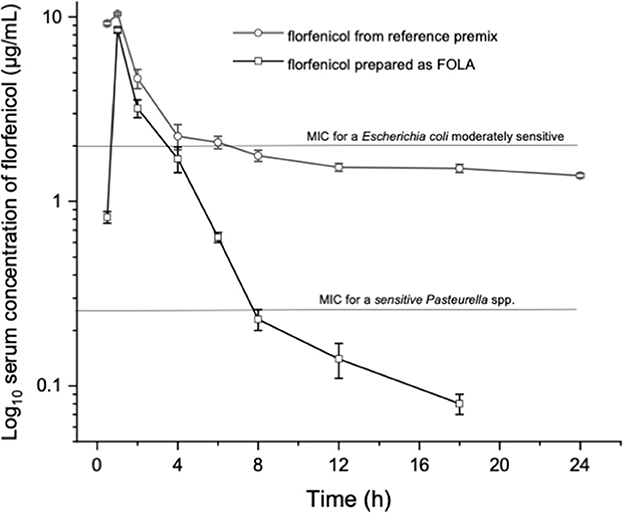
Figure 2. Florfenicol serum concentrations in broiler chickens treated with 30 mg/kg florfenicol either as a reference premix (FREFbolus) or as FOLA pellets (FFOLAbolus), employing a probe and suspending either preparation in water plus 2% gelatin.
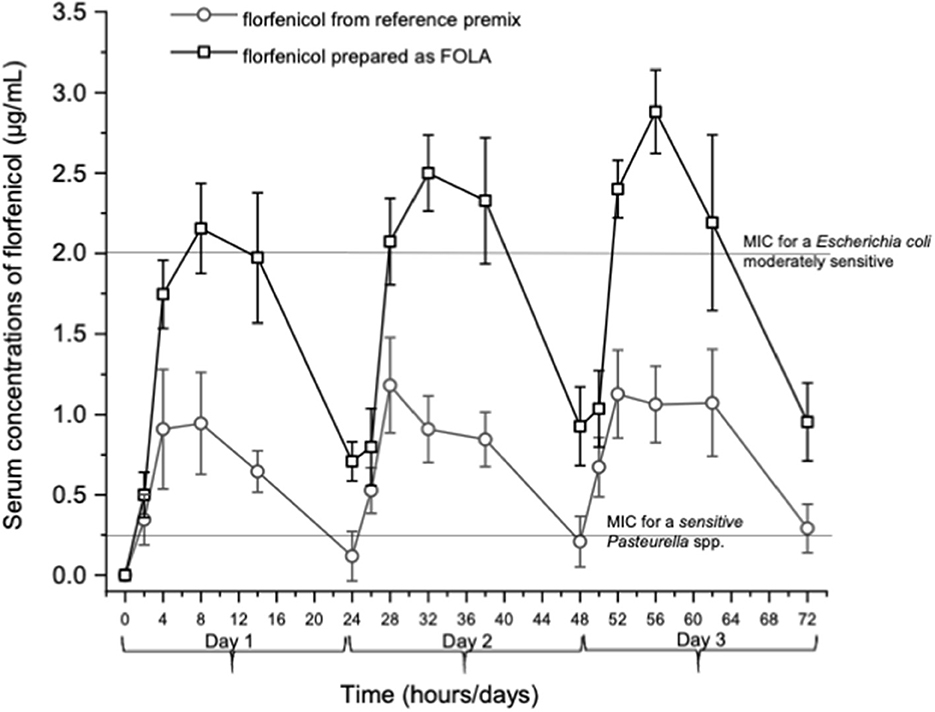
Figure 3. Serum concentrations of florfenicol in broiler chickens receiving two pharmaceutical forms of florfenicol. Feed medication was made available to chickens at 6:00 a.m. using powdered premix (NF-180®, PiSA Agropecuaria S.A. de CV, Mexico) or small colored pellets (FOLA-see text). In both instances, 110 ppm of florfenicol was incorporated into the feed. Given a food intake of 140 g/chicken (weighing 450 g), a dose of ~30 mg/kg was administered.
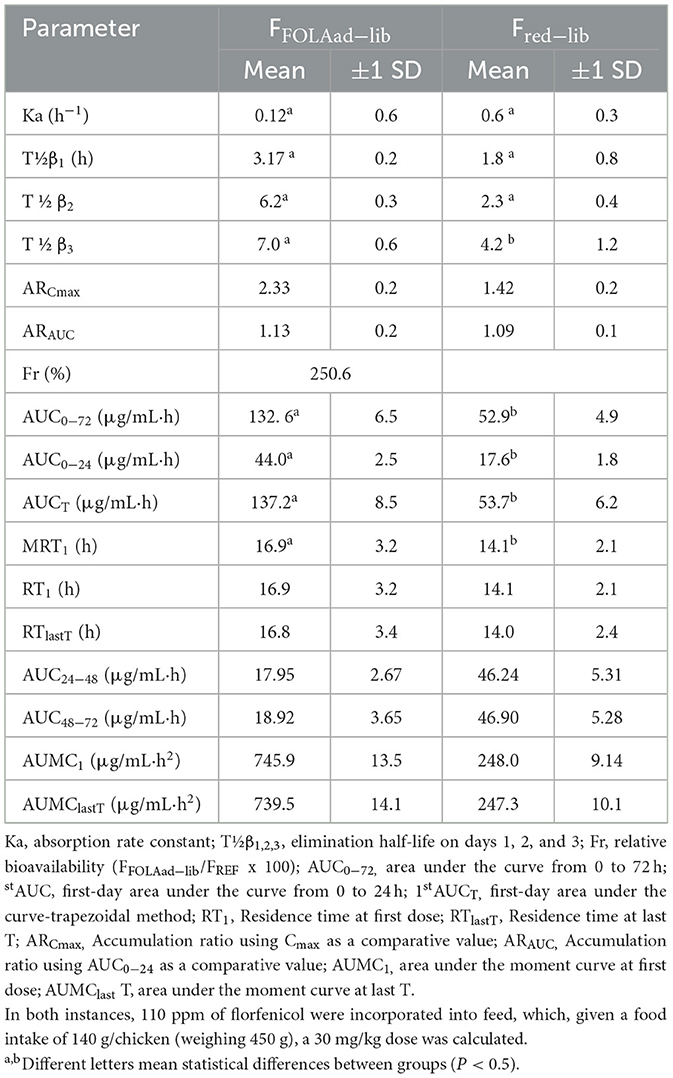
Table 2. Mean ± 1 SD pharmacokinetic parameters of florfenicol in broiler chickens receiving two pharmaceutical forms of florfenicol as in-feed medication and ad libitum: powdered premix (NF-180®, PiSA Agropecuaria S.A. de CV, Mexico) (FREFad−lib), and as FOLA (FFOLAad−lib).
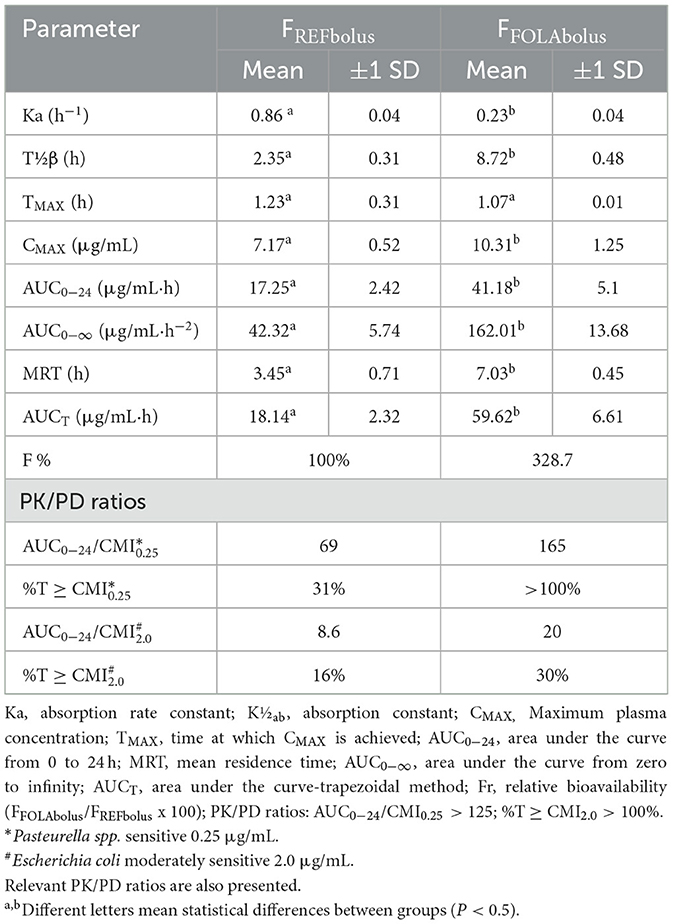
Table 3. Mean ± 1 SD of the oral pharmacokinetic parameters of florfenicol in broiler chickens after a 30 mg/kg administration either as a reference premix (FREFbolus) or as FOLA pellets (FFOLAbolus), utilizing a probe and suspending either preparation in water plus 2% gelatin.
In the ad libitum administration, a decisive difference in the serum profiles of florfenicol for the FOLA form of florfenicol is appreciated. The values of AUC0 − 72 and 1stAUC0 − 24 and 1st MRT and Tβ were statistically higher than those achieved with the reference florfenicol premix (P < 0.05 in all cases). Consequently, this complies well with a lower Ka obtained for FFOLAbolus and FFOLAad − lib.
Discussion
Respiratory diseases are the primary problem in poultry production worldwide, and although much can be done with good husbandry, bacterial disease outbreaks occur and must be treated (15, 16). Antimicrobial drugs are then chosen and are critical to solving the problem. However, their utility has been compromised in recent years by the emergence of resistance to antimicrobial drugs. Given the increasingly limited availability of antimicrobial drugs for poultry production, it is reasonable to think that an immediate operational line of research is to optimize the pharmaceutical design of each active principle (9). The study on the absorption and bioavailability processes of antibacterial drugs in poultry is limited (17). Most pharmaceutical forms available for poultry medicine have been the product of trial and error. They were not designed to optimize their PK/PD ratios when included in food or drinking water (18). Thus, when an outbreak of a respiratory disease occurs, such as complicated chronic respiratory disease, infectious coryza (Haemophilus paragallinarum), or fowl cholera (Pasteurella multocida), soluble florfenicol is often administered as the drug of choice. Early intervention is required (metaphylaxis), and it has been postulated that sick birds tend to reduce feed or water intake, but their consumption patterns before signs of the referred diseases appear have yet to be established; that is, unless a critical part of the flock is affected, water or feed intake variations are rarely detected during the early stages of the disease. This is a task that requires careful research. However, in this context, several preparations of florfenicol in premix became available in Latin America for metaphylaxis treatment.
Florfenicol is rapidly eliminated from the broiler chickens' plasma. This study conceived and tested an attempt to extend the clearance of florfenicol with its inclusion in FOLA pellets and as an in-feed medication. The results show improved florfenicol PK/PD ratios. It is postulated that the gastrointestinal retentive properties of carbopol in FOLA pellets modify absorption into a type of sustained release, and therefore, elimination is extended.
Prudent use of highly potent antimicrobial drugs in veterinary medicine, such as florfenicol, is required (9, 17). To achieve this goal, it is necessary to design pharmaceutical preparations that better comply with each drug's competent PK/PD ratios (19). In turn, this may contribute to maintaining its efficacy in the future (10, 20, 21). It is in this context that florfenicol in FOLAs was conceived. FOLAs can be described as pharmaceutically designed carriers prepared as small color pellets that are readily consumed by poultry, which allow the inclusion of vehicles and help improve the absorption of the active ingredient by modulating the GI-transit time (10, 21).
The pharmacokinetic parameters obtained for the reference florfenicol as premix were very similar to what was achieved in other studies with soluble florfenicol. Minor differences can be attributed to the use of different bloodlines of chicken, different ages, feeding, and housing peculiarities (22, 23). More efficient absorption of florfenicol, when prepared as FOLA pellets, was anticipated as sustained-release formulations tend to achieve better bioavailability values (20, 24), and such behavior has already been obtained with FOLAs for doxycycline and tylosin in poultry (19, 23). Furthermore, the lower Ka obtained for FFOLAbolus and FFOLAad − lib may suggest that a flip-flop phenomenon is occurring. In this study, the FFOLAbolus preparation achieved better values in CMAX and AUC0 − 24 than the premix formulation. In addition, MRT and Tβ were statistically higher than the premix formulation (P < 0.05). Consequently, these features generate notable differences in the bioavailability of the preparations, with a notable advantage for the pharmaceutical form of type FOLA, i.e., Fr from 250 to 328%. Having higher plasma concentrations of the antibacterial drug and a total load of florfenicol achieved (AUC/CMI), as well as slower elimination parameters for florfenicol FOLA compared with reference florfenicol premix (T ≥ CMI), signify a notable improvement in the pharmacokinetic ratios of this drug. Florfenicol is considered a time-dependent antibacterial, and it would seem that there is no advantage in using FOLAs when treating sensitive bacteria (MIC = 0.25 μg/ml). However, it is evident that if a more resistant pathogen is involved in an outbreak, such as E. coli (24), only florfenicol prepared as FOLA will achieve adequate PK/PD ratios. This latter view is even more evident in the ad libitum dosing, where the AUC values, TB, and MRT parameters were substantially higher.
In summary, during the metaphylactic treatment of a bacterial outbreak in poultry, the clinician can safely use florfenicol prepared as FOLA, as better PK/PD ratios than those achieved with florfenicol premix will be obtained. In turn, better clinical results are foreseen. These pharmacokinetic considerations apply if the clinician can confirm that feed consumption in the flock has not been affected by the particular disease. In addition, given the prolonged MRT, ARCmax, and ARAUC found for FOLA preparations, a new withdrawal time may be necessary to avoid unauthorized drug residues. Nevertheless, according to the standards proposed by the European Medicines Agency for the modified release of pharmaceutical preparations, the obtained value for the FOLA pellets (ARAUC < 1.25) can be considered as leading to non-relevant drug accumulation (24).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by CICUA No. 0673 (Internal Committee for the Care and Use of Animals, UNAM) and Mexican prescripts in NOM-062-ZOO-1999.
Author contributions
HS and LG conceived and designed the study and carried out the pharmacokinetic and statistical analyses. AG-F and LO carried out the clinical trial. MM-B and AG-F performed the analytical phase. All authors have read and accepted the manuscript as it is presented to the journal.
Funding
The support from the PAPIIT-UNAM IT200222 project for carrying out this research is acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pooja S, Bansode FW, Singh RK. Chloramphenicol Toxicity: a review. J Med Medic Sci. (2011) 13:1313–6.
2. Shen J, Wu X, Wu D, Jiang H. Pharmacokinetics of florfenicol in healthy and Escherichia coli-infected broiler chickens. Res Vet Sci. (2002) 73:137–40. doi: 10.1016/S0034-5288(02)00033-4
3. Anadón A, Aranzazu M, Martinez M, Rios A, Caballero V, Ares I, Martinez-Larrañaga MR. Plasma and tissue depletion of florfenicol and florfenicol amine in chickens. J Agric Food Che. (2008) 56:11049–56. doi: 10.1021/jf802138y
4. Afifi NA, El-Sooud KA. Tissue concentrations and pharmacokinetics of florfenicol in broiler chickens. British Poultry Sci. (1997) 38:425–8. doi: 10.1080/00071669708418013
5. Shen J, Hu D, Wu X, Coats JR. Bioavailability and pharmacokinetics of florfenicol in broiler chickens. J Vet Pharmacol Therapy. (2003) 26:337–41. doi: 10.1046/j.1365-2885.2003.00495.x
6. Lohani M, Ahmad AH, Singh KP, Verma S. Pharmacokinetics and residual study of florfenicol following multiple-dose oral administration in poultry. J App Anim Res. (2010) 38:9–12. doi: 10.1080/09712119.2010.9707145
7. Chang JL, Davis CN, Cheng RH, Shien MK, Koh C, Chou C. Pharmacokinetics and tissue depletion of florfenicol in leghorn and Taiwan native chickens. J Vet Pharmacol Therap. (2010) 33:471–9. doi: 10.1111/j.1365-2885.2009.01155.x
8. Muryagan J, Kumar PA, Rao GS, Iskandar K, Hawser S, Hays JP, et al. Progress in alternative strategies to combat antimicrobial resistance: focus on antibiotics. Antibiotics. (2022) 11:200. doi: 10.3390/antibiotics11020200
9. Gutierrez L, Marcos X, García-Guzman P, Monroy-Barreto M, Sumano H. Pharmaceutical characterization and pharmacokinetics of florfenicol-loaded alginate dried beads in rabbits. World Rabbit Science. (2022) 30:153–62. doi: 10.4995/wrs.2022.16381
10. Kowalski P, Konieczna L, Chmielewska A, Oledzka I, Plenis A, Bieniecki M, et al. Comparative evaluation between capillary electrophoresis and high-performance liquid chromatography for the analysis of florfenicol in plasma. J Pharm Biomed Anal. (2005) 39:983–9. doi: 10.1016/j.jpba.2005.05.032
11. Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. (1978) 6:165–75. doi: 10.1007/BF01117450
12. Shin SJ, Kang SG, Nabin R, Kang ML, Yoo HS. Evaluation of the antimicrobial activity of florfenicol against bacteria isolated from bovine and porcine respiratory disease. Vet Microbiol. (2005) 106:73–7. doi: 10.1016/j.vetmic.2004.11.015
13. Li XS, Wang GQ, Du XD, Cui BA, Zhang SM, Shen JZ. Antimicrobial susceptibility and molecular detection of chloramphenicol and florfenicol resistance among Escherichia coli isolates from diseased chickens. J Vet Scie. (2007) 8:243–7. doi: 10.4142/jvs.2007.8.3.243
14. Glison JR. Bacterial respiratory diseases of poultry. Poult Sci. (1998) 77:1139–42. doi: 10.1093/ps/77.8.1139
15. Kursa O, Tomczyk G, Adamska K, Chrzanowska J, Sawicka-Durkalec A. The microbial community of the respiratory tract of commercial chickens and turkeys. Microorganisms. (2022) 10:987. doi: 10.3390/microorganisms10050987
16. Hu YS, Prisca XYT, Tao TMN, Hui TC, Shangshe X, A. critical review of the pharmacokinetics, pharmacodynamics, and safety data of antibiotics in avian species. Antibiotics. (2022) 11:741. doi: 10.3390/antibiotics11060741
17. Gray P, Jenner R, Norris J, Page S, Browning G. Australian veterinary association Ltd and animal medicines Australia. Antimicrobial prescribing guidelines for poultry. Aust Vet J. (2021) 99:181–235. doi: 10.1111/avj.13034
18. Rodriguez-Gascon A, Soliní MA, Isla A. The role of PK/PD analysis in the development and evaluation of antimicrobials. Pharmaceutics. (2021) 13:833. doi: 10.3390/pharmaceutics13060833
19. Gutierrez L, Zermeño J, Alcala Y, Sumano H. Higher bioavailability of doxycycline in broiler chickens with novel in-feed pharmaceutical formulations. Poult Sci. (2017) 96:2662–9. doi: 10.3382/ps/pex036
20. Gutierrez L, Tapia G, Gutierrez E, Sumano H. Evaluation of a tasteless enrofloxacin pharmaceutical preparations for cats. Naïve pooled-sample approach to study its pharmacokinetics. Animals. (2021) 11:2312. doi: 10.3390/ani11082312
21. Ismail M, El-Kattan YA. Comparative pharmacokinetics of florfenicol in chicken, pigeon, and quail. British Poult Sci. (2009) 50:144–9. doi: 10.1080/00071660802613286
22. Watteyn A, Russo E, Garmyn A, De Baere S, Pasmans F, Martel A, et al. Clinical efficacy of florfenicol administered in the drinking water against Ornithobacterium rhinotracheale in turkeys housed in different environmental conditions: a pharmacokinetics/pharmacodynamics approach. Avian Pathology. (2013) 42:47448. doi: 10.1080/03079457.2013.823144
23. Gutierrez L, Alcala Y, Bernad MJ, Sumano H. Increased bioavailability of tylosin phosphate as in-feed medication formulated for long action pellets in broiler chickens. J Appl Poult Res. (2018) 27:16–22. doi: 10.3382/japr/pfx035
Keywords: florfenicol-premix, florfenicol-FOLA, broiler-chicken, pharmacokinetics, oral dosage
Citation: Gutierrez L, Guzman-Flores A, Monroy-Barreto M, Ocampo L and Sumano H (2023) Oral pharmacokinetics of a pharmaceutical preparation of florfenicol in broiler chickens. Front. Vet. Sci. 10:1208221. doi: 10.3389/fvets.2023.1208221
Received: 18 April 2023; Accepted: 16 May 2023;
Published: 07 June 2023.
Edited by:
Arturo Anadón, Complutense University of Madrid, SpainCopyright © 2023 Gutierrez, Guzman-Flores, Monroy-Barreto, Ocampo and Sumano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hector Sumano, c3VtYW5vQHVuYW0ubXg=
 Lilia Gutierrez
Lilia Gutierrez Aline Guzman-Flores
Aline Guzman-Flores Minerva Monroy-Barreto
Minerva Monroy-Barreto Luis Ocampo
Luis Ocampo Hector Sumano
Hector Sumano