
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Vet. Sci. , 25 May 2023
Sec. Veterinary Neurology and Neurosurgery
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1201484
This article is part of the Research Topic MRI in Veterinary Neurology View all 14 articles
 Diletta Dell'Apa1*
Diletta Dell'Apa1* Martina Fumeo1
Martina Fumeo1 Antonella Volta1
Antonella Volta1 Marco Bernardini2,3
Marco Bernardini2,3 Francesca Fidanzio1
Francesca Fidanzio1 Valentina Buffagni1
Valentina Buffagni1 Matthias Christen4
Matthias Christen4 Vidhya Jagannathan4
Vidhya Jagannathan4 Tosso Leeb4
Tosso Leeb4 Ezio Bianchi1
Ezio Bianchi1Two boxer dogs from the same litter were presented at 3 months of age for urinary and fecal incontinence. Both dogs had an abnormal tail consisting of a small stump, an atonic anal sphincter, and absent perineal reflex and sensation. Neurological evaluation was indicative of a lesion of the cauda equina or sacral spinal cord. Radiology and CT scan of the spine displayed similar findings in the two dogs that were indicative of sacral agenesis. Indeed, they had 6 lumbar vertebrae followed by a lumbosacral transitional vertebra, lacking a complete spinous process, and a hypoplastic vertebra carrying 2 hypoplastic sacral transverse processes as the only remnant of the sacral bone. Caudal vertebrae were absent in one of the dogs. On MRI, one dog had a dural sac occupying the entire spinal canal and ending in a subfascial fat structure. In the other dog, the dural sac finished in an extracanalar, subfascial, well-defined cystic structure, communicating with the subarachnoid space, and consistent with a meningocele. Sacral agenesis—that is the partial or complete absence of the sacral bones—is a neural tube defect occasionally reported in humans with spina bifida occulta. Sacral agenesis has been described in human and veterinary medicine in association with conditions such as caudal regression syndrome, perosomus elumbis, and Currarino syndrome. These neural tube defects are caused by genetic and/or environmental factors. Despite thorough genetic investigation, no candidate variants in genes with known functional impact on bone development or sacral development could be found in the affected dogs. To the best of the authors' knowledge, this is the first report describing similar sacral agenesis in two related boxer dogs.
Neural tube defects (NTD) are the most common human birth defects. In both humans and animals, these malformations can be caused by genetic or environmental factors. Genetic factors account for approximately 70% of human cases (1). Other non-genetic factors involved in the abnormal closure of the neural tube include drugs (e.g., valproic acid) (2), environmental teratogens (industrial waste and pesticides) (3), and maternal factors like obesity, folic acid deficiency, diabetes mellitus, and hyperthermia during the first trimester of pregnancy (1, 4).
NTDs are usually divided into open and closed defects. The open defects are characterized by external protrusion and/or exposure of neural tissue, while in closed defects the neural tissue is covered by other tissue (4).
Spina bifida (SB) is an NTD caused by incomplete caudal neurulation (4). It results in the failure of fusion of one or more vertebral arches with or without protrusion of the meninges and the spinal cord. This malformation, with hemivertebrae and transitional vertebrae, frequently occurs in dogs with screw tails. Vertebral malformations do not necessarily produce neurological signs and are often seen in radiographs as incidental findings (5).
In humans, sacro-caudal dysgenesis and sacral agenesis (SA) are rarely described NTDs. These terms are used to identify a spectrum of malformations of the sacrum and caudal vertebrae of varying severity. In particular, the term SA identifies the partial or complete absence of the sacral bones. In these syndromes, the most frequent neurological signs are urinary and fecal incontinence, sometimes accompanied by hind limb paresis or paralysis (5).
In small animals, similar malformations have previously been reported in Manx cats and occasionally in dogs (6, 7). In Manx cats, sacro-caudal dysgenesis is well known and can also be associated with myelodysplasia, meningocele, meningomyelocele, tethered spinal cord, hydromyelia, and/or syringomyelia (8, 9). An autosomal dominant inheritance with incomplete penetrance has been demonstrated for this feline disease (7). The tailless phenotype and increased risk for sacro-caudal dysgenesis in Manx and other tailless cats is caused by variants in the TBXT gene encoding the T-box transcription factor T (10). Tailless or short-tailed phenotypes in several dog breeds are also caused by dominant alleles of the TBXT gene (11, 12).
Perosomus elumbis (PE) is a complex syndrome that has been commonly described in farm animals and more rarely in puppies. It is characterized by partial or complete agenesis of the lumbar, sacral, and coccygeal vertebra, and sometimes by other malformations involving the limbs, such as arthrogryposis (13, 14).
In this case report we describe the clinical signs, imaging, and electrodiagnostic findings and outcome of SA in two related boxer dogs. The results of genetic investigation are also reported.
Two 3-month-old intact female boxer dogs from the same litter were presented to the Veterinary Teaching Hospital (VTH) of the University of Parma for the presence of urinary and fecal incontinence since birth. The other four littermates and the parents did not present similar clinical signs.
On presentation, physical examination was unremarkable except for the tail, which appeared as a small stump in both dogs. Neurological examination revealed the presence in both dogs of an atonic anal sphincter and the absence of perineal reflex and nociception. No signs of paraparesis or sciatic nerve involvement were present. Hyperestesia of the lumbosacral spine was not detected. A cauda equina or sacral spinal cord lesion was suspected based on neurological examination. Differential diagnoses included, in particular, anomalous diseases such as SB associated with meningocele or meningomyelocele, sacral dysgenesis/agenesis, and other spine malformations.
A radiographic study of the lumbosacral spine was made in the two puppies in both lateral and ventro-dorsal views. Both patients showed six typically shaped lumbar vertebrae, followed by a lumbosacral transitional vertebra lacking the spinous process and a hypoplastic vertebra carrying two hypoplastic sacral transverse processes as the only remnant of the sacral bone. These findings were considered compatible with SA. Caudal vertebrae were absent in dog 1 (Figure 1A), while in dog 2 several coccygeal vertebrae not articulating with the rest of the spine were present (Figure 2A).
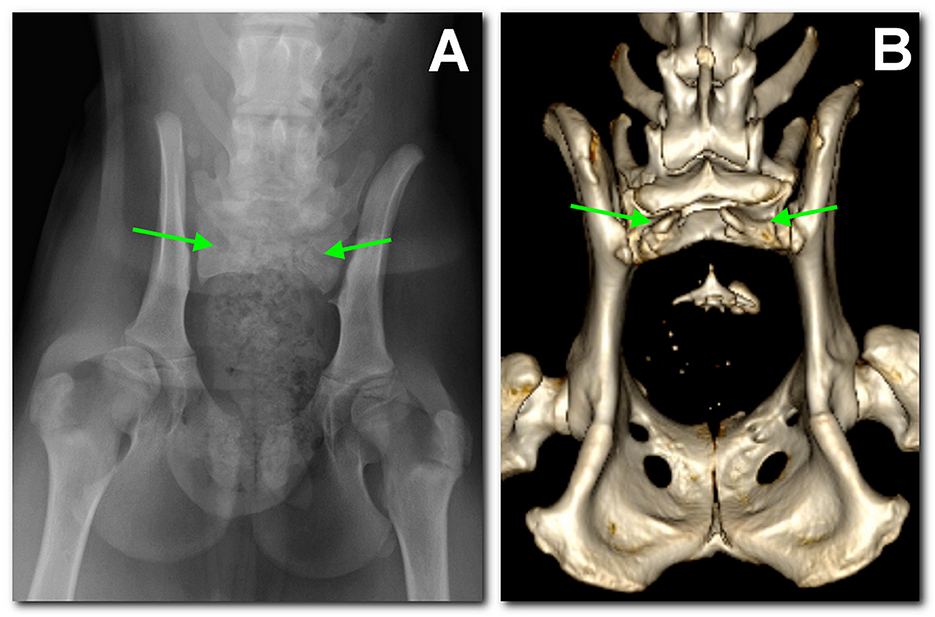
Figure 1. Dog 1, radiographic study with a ventro-dorsal projection of the pelvis performed at 3 months of age (A) in comparison to the tomographic 3D volume rendering reconstruction of the pelvis at 18 months of age (B). There is sacralization of L7. Only S1 is present and hypoplastic; the dorsal part of the arch is not fused and has no spinal process (between arrows). Caudally, there is a single coccygeal vertebra not articulating with the sacrum.
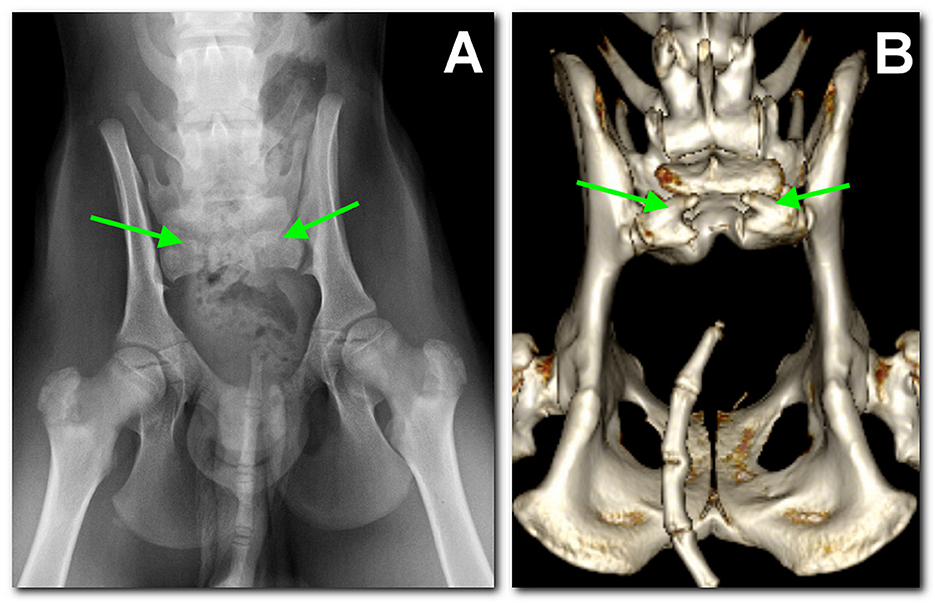
Figure 2. Dog 2, radiographic study with a ventro-dorsal projection of the pelvis performed at 3 months of age (A) in comparison to the tomographic 3D volume rendering reconstruction of the pelvis at 18 months of age (B). Similar findings as in dog 1 are noted: L7 sacralization, S1 with spina bifida (between arrows), and sacral agenesis. Caudally, several coccygeal vertebrae are present, not articulating with S1.
Ultrasound examination of the dorsal sacral region was carried out using a microconvex probe (6–10 MHz). In both patients, the spinal cord was visible, partially protruded caudally, due to the incomplete fusion of the dorsal lamina (Figure 3B). In dog 2, a well-defined anechoic oval 2 x 0.8 cm structure consistent with a meningocele was observed (Figure 4A).
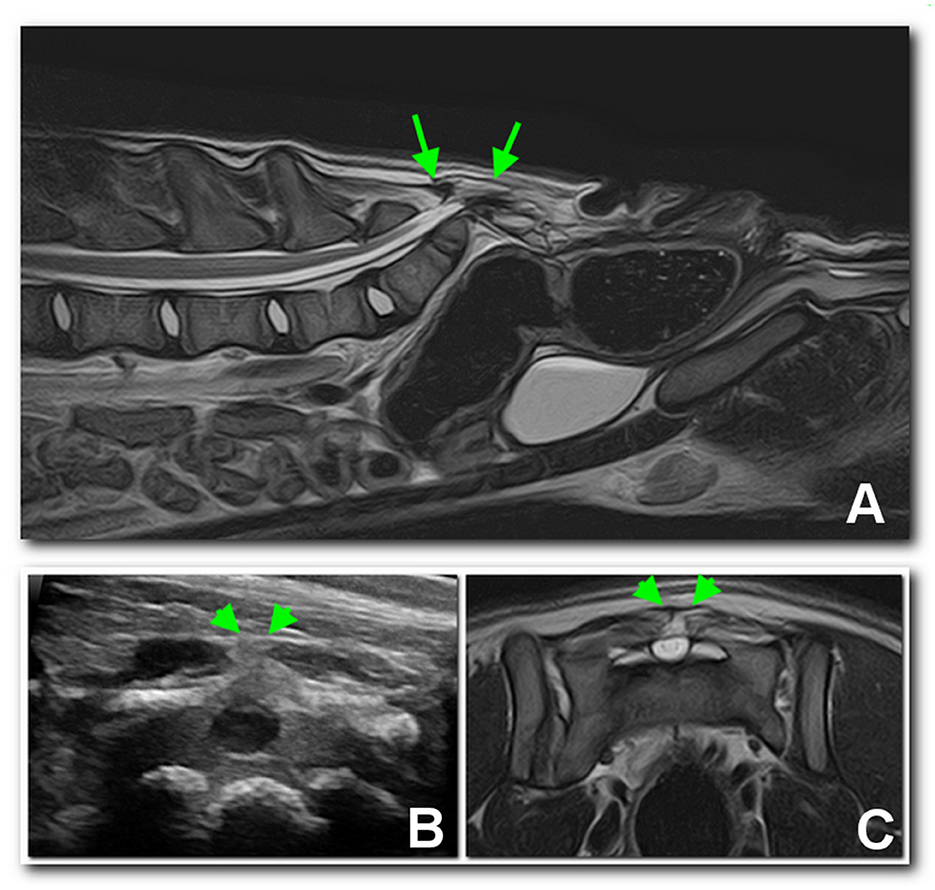
Figure 3. Sagittal (A) and transverse (C) T2 MRI images of the lumbosacral region of dog 1, compared with an ultrasonographic transverse scan (B) of the dorsal sacral aspect. A dural sac occupying the entire spinal canal protruding dorsally (arrows) and ending in a subfascial fat structure may be noted between the arrowheads.
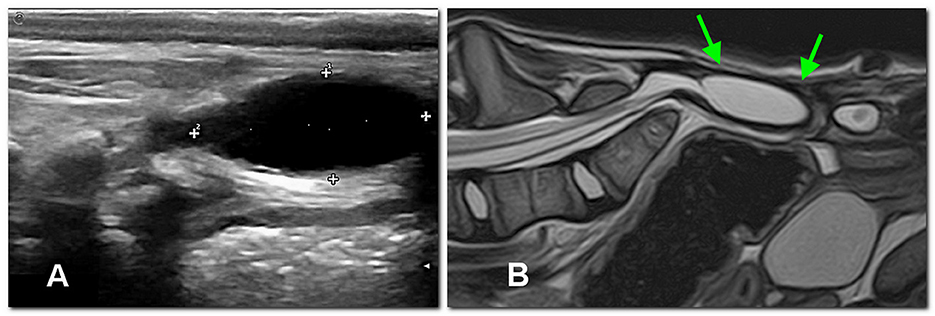
Figure 4. In dog 2, an extracanalar, well-defined subfascial cystic structure caudal to the spine, likely consisting of meningocele, is displayed between calipers by the ultrasonographic sagittal study (A) at 3 months of age and later confirmed by MRI [sagittal T2 image, (B)] at 4 months of age (arrows).
At 4 months of age, complete blood count and blood chemistry values were unremarkable. In view of eventual surgical planning, an MRI (1.5 T, Vantage Elan, Canon Medical Systems Europe B.V., Zoetermeer, The Netherlands) of the lumbosacral spine was performed under general anesthesia with the dogs in dorsal recumbency. The imaging protocol included sagittal and transverse fast spin echo (FSE) T2-weighted (T2W) images [repetition time (ms) (TR)/echo time (ms) (TE), 3886–6353/108]; sagittal and transverse FSE T1-weighted (T1W) images (TR/TE, 480-745/14-15); transverse short tau inversion recovery (STIR) (TR/TE, 7139/48); 3D T1W fat suppressed gradient echo (GE) images (TR/TE, 32.2/5.5). No contrast medium was used. Besides the osteoarticular abnormalities in the radiographs, in dog 1, a dural sac occupying the entire spinal canal and ending abruptly in a subfascial, somewhat organized fat structure was observed (Figures 3A–C). In dog 2, the dural sac occupied the entire spinal canal and ended in a well-defined extracanalar, subfascial cystic structure, caudal to the spine and likely communicating with the subarachnoid space, and probably consisting of a meningocele, thus confirming ultrasound findings (Figure 4B). Surgical treatment of the meningocele was proposed but was declined by the owner. The dogs were discharged without medication, with the indication to monitor urination and defecation.
In the following months, urinary and fecal incontinence persisted without any other major symptoms. On the occasion of ovariohysterectomy, at 18 months of age, a computed tomography (CT) scan was performed as a follow-up. CT examination confirmed and better defined the skeletal malformations previously detected. In particular, both dogs presented L7 sacralization and partial sacral agenesis, consisting of the presence of a vestigial S1, and presenting a severely incomplete body that was not dorsally fused and had no spinous process, likely compatible with a SB (Figures 1B, 2B). The dural sac protruded caudally and dorsally in the subfascial fat, slightly in dog 1, while in dog 2 it formed a 1.3-cm oval hypoattenuating pouch compatible with meningocele. Needle electromyography (EMG) performed following CT scan revealed the presence of spontaneous pathological activity in the perineal muscles of both dogs, represented mainly by complex repetitive discharges.
To identify a potential causative genetic variant, whole genome 2 x 150 bp paired-end-sequencing at 20x coverage of one of the affected dogs was performed. The sequence data were deposited under study accession PRJEB16012 and sample accession SAMEA110415686 in the European Nucleotide Archive. The whole genome sequence data were compared to 924 control genomes (Supplementary Table 1), and variants particular to the boxer with sacral agenesis were identified as described (15). All identified private homozygous and private heterozygous variants are listed in Supplementary Table 2. None of these variants represented a strong plausible candidate for the sacral agenesis.
In this case report we describe an NTD in two boxer dogs from the same litter. To precisely define the spinal anomaly present, the dogs underwent a complete diagnostic work-up that included various imaging modalities. In particular, radiology, ultrasonography, CT, and MRI performed at different stages of the dogs' growth were indicative of the presence of sacral agenesis (SA) associated with meningocele. Further, the incomplete S1 bone, which was not dorsally fused and had no spinous process, was compatible with an SB.
NTDs have a multifactorial etiology, with contributions from genetic, environmental and nutritional factors synergistically affecting normal embryonic development (3, 16).
During neurulation, the ectoderm grows in a longitudinal axis on the notochord and thickens to become a neural plate. Then, a neural groove appears as the edges of the neural plates become raised on each side of a midline depression, and, following the fusion of the edges in a bidirectional way, the neural tube is formed. In dogs, the closure starts in the cervical region and then progresses rostrally to form the future brain, and caudally to form the spinal cord. During this process, the ectoderm separates from the neural tube and fuses dorsally to create the overlying skin (17).
SB is an NTD reported in both humans and animals that results from the incomplete fusion of the dorsal lamina with or without protrusion of meninges (meningocele) or meninges and spinal cord (meningomyelocele). SB can also be classified as closed (occulta) or opened (manifesta/aperta) if there is communication between the neural tissue and the external environment. SA is occasionally reported in humans with SB occulta (5, 18–21).
SA with associated meningocele was initially identified in our patients at 3 months of age with radiographs and ultrasound of the lumbosacral spine. In human medicine, ultrasonography is also commonly used as a first-line screening test for spinal cord abnormalities in infants (9, 22). This rare malformation has previously occasionally been reported in dogs and in Manx cats (6, 7).
A more detailed characterization of SA and SB of the two subjects was possible in the following months with advanced imaging techniques (CT and MRI). Abnormalities similar to those detected in the two dogs have been reported in some rare syndromes described in humans and animals: Caudal regression syndrome (CRS), perosomus elumbis (PE), and Currarino syndrome (CS).
CRS is reported in humans, characterized by different degrees of anomaly of the caudal spinal cord and spine. In CRS there is the partial or total absence of the thoracic, lumbar, sacral, or coccygeal vertebrae associated with visceral organ malformations, but there is also imperforate anus and severe limb deformities. Diagnostic follow-up of suspicion can be made during pregnancy, detecting anomalies in the limbs and vertebral column with prenatal ultrasound. A prevalence of this congenital malformation has been identified in children of diabetic mothers, but other risk factor include cocaine and alcohol consumption. In children, walking disabilities are most commonly detected because of limb deformities (23–25).
SA is considered a part of CRS and can be classified into four types. In type I, there is partial or total unilateral agenesis localized to the sacrum; type II is characterized by partial bilaterally symmetrical agenesis with a firm articulation between the ilia and a normal or hypoplastic first sacral vertebra; in type III there is total sacral agenesis and a variable lumbar vertebra articulating with the ilia; and in type IV the sacrum is absent, while the caudal end plate of the lumbar vertebra is fused with the ilia. These anomalies are associated with a high prevalence of fecal incontinence and urinary symptoms (26, 27). Our patients display similarities with Type II SA.
SA is also a feature of CS, another syndrome reported in human medicine. Two forms of CS have been described: the classic form in which there is partial sacral agenesis, presacral mass (meningocele, lipoma, enteric cyst, teratoma, or a combination of these), and anorectal malformation, and the incomplete form in which only one or two of these malformations are present (28, 29). HLBX9 gene variants have been reported in some CS patients (30–32). In the two boxer dogs included in the present study, anorectal malformation was absent, but presacral mass and SA were detected without other anomalies affecting the hind limbs.
PE is a rare complex congenital malformation that has been described in animals. It has been reported in only one dog, and one cat, and occasionally in cattle, buffalos, sheep, foals, pigs, rhesus macaques (Macaca mulatta), and it has been experimentally induced in mice (13, 33–43). As with CRS, animals with PE display agenesis of the caudal spinal cord and spine. Other signs associated with this syndrome are arthrogryposis and hypoplastic hind limbs, urogenital and intestinal tract defects, craniofacial malformation, synophthalmia, and brachygnatia. If the affected animal survives the first hours of life it usually displays severe difficulty in standing, due to the anomalies affecting the hind limbs and vertebra column. Euthanasia is usually performed, in light of the severity of this condition (34–36, 40).
In children, these congenital malformations require a multidisciplinary approach depending on the identified severity of anomalies. The diagnostic approach to fecal and urinary incontinence in patients with sacral anomalies is complex and involves various tests such as contrast colonography, colonic transit time, anorectal manometry, urodynamics, and EMG (44–46).
Unfortunately, tests for colonic function and urodynamic studies were not performed in these dogs. EMG in both dogs showed the presence of complex repetitive discharges of the perineal muscles as a possible consequence of chronic denervation. The absence of spontaneous pathologic activity at EMG of the muscles of the hind limbs further ruled out the presence of sciatic nerve involvement.
Early surgical treatment of meningocele/meningomyelocele associated with SB has been reported to improve neurological signs in some cases (18). Surgical treatment was declined by the owner, who considered the quality of life of the dogs to be good.
In the long-term follow-up (~3 years from the first examination), the two boxer dogs remained clinically stable and developed no major complications as a result of their urinary and fecal incontinence.
The occurrence of sacral dysgenesis in more than one puppy from a litter with healthy parents and an uneventful pregnancy suggested a genetic disorder with autosomal recessive inheritance. Alternatively, a de novo mutation event in the germline of one of the two parents might have generated a dominant allele causing the SA. Despite extensive genetic investigation, no candidate variants in genes with known functional impact on bone development or sacral development were found. One cautionary note is that short read whole genome sequencing does not have 100% sensitivity in detecting all genetic variants. In particular, large structural variants involving more than ~30 nucleotides are easily missed with the approach. Furthermore, gaps in the genome reference assembly or errors in the gene annotation of the dog genome might also explain why we were not able to identify a candidate for causative variant. The presence of environmental factors (toxic, nutritional, metabolic) as a possible cause of the malformation cannot be excluded.
Moreover, the concomitant presence of both genetic and non-genetic factors acting synergistically is also a possibility in the two boxer siblings. Indeed, in human medicine, the most reliable pathogenetic model for NTDs (multifactorial threshold model) considers either genetic and environmental factors on their own insufficient to affect neural tube closure. The concomitant presence of genetic and non-genetic factors is usually deemed necessary to induce NTDs (4).
To the best of the authors' knowledge, this is the first report of a similar SA in two related boxer dogs. Further studies including subjects with similar NTDs from this and other breeds should be performed in order to improve understanding of the role of genetic and non-genetic factors in these defects of neural tube closure.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/biosample; SAMEA110415686.
Ethical review and approval were not required for the animal study because the case report is a description of a clinical case. Written informed consent was obtained from the owners for the participation of their animals in this study.
DD, EB, MF, AV, and FF contributed to management of the case. MF and AV were in charge of the CT scan, and MB of the MRI. MC, VJ, and TL performed the genetic testing. DD, VB, MF, MC, MB, AV, TL, and EB participated in the review and editing of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1201484/full#supplementary-material
1. Finnell RH, Caiaffa CD, Kim SE, Lei Y, Steele J, Cao X, et al. Gene environment interactions in the etiology of neural tube defects. Front Genet. (2021) 12:659612. doi: 10.3389/fgene.2021.659612
2. Wlodarczyk BJ, Palacios AM, George TM, Finnell RH. Antiepileptic drugs and pregnancy outcomes. Am J Med Genet A. (2012) 158A:2071–90. doi: 10.1002/ajmg.a.35438
3. Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. (2010) 220:217–30. doi: 10.1002/path.2643
4. Avagliano L, Massa V, George TM, Qureshy S, Bulfamante GP, Finnell RH. Overview on neural tube defects: from development to physical characteristics. Birth Defects Res. (2019) 111:1455–67. doi: 10.1002/bdr2.1380
5. Westworth DR, Sturges BK. Congenital spinal malformations in small animals. Vet Clin North Am Small Anim Pract. (2010) 40:951–81. doi: 10.1016/j.cvsm.2010.05.009
6. Choi SY, Lee I, Cho NY, Shin BH, Lee KJ, Choi HJ, et al. Imaging diagnosis of sacrocaudal dysgenesis in a shih-tzu dog. J Vet Clin. (2016) 33:389–91. doi: 10.17555/jvc.2016.12.33.6.389
7. Deforest ME, Basrur PK. Malformations and the Manx syndrome in cats. Can Vet J. (1979) 20:304–14.
8. Leipold HW, Huston K, Blauch B, Guffy MM. Congenital defects on the caudal vertebral column and spinal cord in Manx cats. J Am Vet Med Assoc. (1974) 164:520–3.
9. Plummer SB, Bunch SE, Khoo LH, Spaulding KA, Kornegay JN. Tethered spinal cord and an intradural lipoma associated with a meningocele in a Manx-type cat. J Am Vet Med Assoc. (1993) 203:1159–61.
10. Buckingham KJ, McMillin MJ, Brassil MM, Shively KM, Magnaye KM, Cortes A, et al. Multiple mutant T alleles cause haploinsufficiency of Brachyury and short tails in Manx cats. Mamm Genome. (2013) 24:400–8. doi: 10.1007/s00335-013-9471-1
11. Haworth K, Putt W, Cattanach B, Breen M, Binns M, Lingaas P, et al. Canine homolog of the T-box transcription factor T; failure of the protein to bind to its DNA target leads to a short-tail phenotype. Mamm Genome. (2001) 12:212–8. doi: 10.1007/s003350010253
12. Hytönen MK, Grall A, Hédan B, Dréano S, Seguin SJ, Delattre D, et al. Ancestral T-box mutation is present in many, but not all, short-tailed dog breeds. J Hered. (2009) 100:236–40. doi: 10.1093/jhered/esn085
13. Amaral CB, Romão MA, Ferreira AM. Perosomus elumbis in a puppy. J Comp Pathol. (2012) 147:495–8. doi: 10.1016/j.jcpa.2012.03.003
14. Son JM, Yong HY, Lee DS, Choi HJ, Jeong SM, Lee YW, et al. A case of perosomus elumbis in a Holstein calf. J Vet Med Sc. (2008) 70:521–3. doi: 10.1292/jvms.70.521
15. Jagannathan V, Drögemüller C, Leeb T, Dog Biomedical Variant Database Consortium (DBVDC). A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and eight wolves. Anim. Genet. (2019) 50:695–704. doi: 10.1111/age.12834
16. Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. (2010) 16:6–15. doi: 10.1002/ddrr.93
17. Thomson C, Hahn C(editors). Neuroembriology. In: Veterinary Neuroanatomy: A Clinical Approach. St Louis: Saunders Elsevier Press (2012). p. 11–13.
18. Martín Muñiz L, Del Magno S, Gandini G, Pisoni L, Menchetti M, Foglia A, et al. Surgical outcomes of six bulldogs with spinal lumbosacral meningomyelocele or meningocele. Vet Surg. (2020) 49:200–6. doi: 10.1111/vsu.13342
19. Bertram S, Ter Haar G, De Decker S. Congenital malformations of the lumbosacral vertebral column are common in neurologically normal French bulldogs, English bulldogs, and pugs, with breed-specific differences. Vet Radiol Ultrasound. (2019) 60:400–8. doi: 10.1111/vru.12753
20. Song RB, Glass EN, Kent M. Spina bifida, meningomyelocele, and meningocele. Vet Clin North Am Small Anim Pract. (2016) 46:327–45. doi: 10.1016/j.cvsm.2015.10.007
21. Balioglu MB, Akman YE, Ucpunar H, Albayrak A, Kargin D, Atici Y, et al. Sacral agenesis: evaluation of accompanying pathologies in 38 cases, with analysis of long-term outcomes. Childs Nerv Syst. (2016) 32:1693–702. doi: 10.1007/s00381-016-3022-5
22. Ladino Torres MF, DiPietro MA. Spine ultrasound imaging in the newborn. Semin Ultrasound CT MR. (2014) 35:652–61. doi: 10.1053/j.sult.2014.08.001
23. Kylat RI, Bader M. Caudal regression syndrome. Children. (2020) 7:211. doi: 10.3390/children7110211
24. Singh SK, Singh RD, Sharma A. Caudal regression syndrome–case report and review of literature. Pediatr Surg Int. (2005) 21:578–81. doi: 10.1007/s00383-005-1451-4
25. Akhaddar A. Caudal regression syndrome (spinal thoraco-lumbo-sacro-coccygeal agenesis). World Neurosurg. (2020) 142:301–2. doi: 10.1016/j.wneu.2020.07.055
26. Dewberry L, Peña A, Mirsky D, Ketzer J, Bischoff A. Sacral agenesis and fecal incontinence: how to increase the index of suspicion. Pediatr Surg Int. (2019) 35:239–42. doi: 10.1007/s00383-018-4402-6
27. Macedo M, Martins JL, Freitas Filho LG. Sacral ratio and fecal continence in children with anorectal malformations. BJU Int. (2004) 94:893–4. doi: 10.1111/j.1464-410X.2004.05053.x
28. Garcia-Barceló MM, Chi-Hang Lui V, Miao X, So MT, Yuk-yu Leon T, Yuan ZW, et al. Mutational analysis of SHH and GLI3 in anorectal malformations. Birth Defects Res A Clin Mol Teratol. (2008) 82:644–8. doi: 10.1002/bdra.20482
29. Emans PJ, Kootstra G, Marcelis CL, Beuls EA, van Heurn LW. The Currarino triad: the variable expression. J Pediatr Surg. (2005) 40:1238–42. doi: 10.1016/j.jpedsurg.2005.05.004
30. Bevanda K, MemidŽan I, Boban-RaguŽ A. Caudal regression syndrome (Currarino syndrome) with chromosome mutation 9. Radiology Case Rep. (2020) 15:1184–8. doi: 10.1016/j.radcr.2020.05.023
31. Martucciello G, Torre M, Belloni E, Lerone M, Pini Prato A, Cama A, et al. Currarino syndrome: proposal of a diagnostic and therapeutic protocol. J Pediatr Surg. (2004) 39:1305–11. doi: 10.1016/j.jpedsurg.2004.05.003
32. Dworschak GC, Reutter HM, Ludwig M. Currarino syndrome: a comprehensive genetic review of a rare congenital disorder. Orphanet J Rare Dis. (2021) 16:167. doi: 10.1186/s13023-021-01799-0
33. Hybki GC, Murphy LA, Marchi JP, Patlogar JE, Brisson JO, Nakamura RK. Lumbosacral agenesis in a cat. JFMS Open Rep. (2016) 2:2055116916628555. doi: 10.1177/2055116916628555
34. Patrick T, Gonzalez O, Dick EJ, Jr, Kumar S. Perosomus elumbis in a stillborn rhesus macaque (Macaca mulatta): a case report. J Med Primatol. (2020) 49:110–2. doi: 10.1111/jmp.12459
35. Avedillo LJ, Camón J. Perosomus elumbis in a pig. Vet Rec. (2007) 160:127–9. doi: 10.1136/vr.160.4.127
36. Dennis SM. Perosomus elumbis in sheep. Aust Vet J. (1975) 51:135–6. doi: 10.1111/j.1751-0813.1975.tb09436.x
37. Jones CJ. Perosomus elumbis (vertebral agenesis and arthrogryposis) in a stillborn Holstein calf. Vet Pathol. (1999) 36:64–70. doi: 10.1354/vp.36-1-64
38. Balamurugan B, Pridhavidhar ReddyYV, Jyothi K. Surgical management of dystocia due to Perosomus elumbis in a nondescript buffalo. J Entomol Zool Stud. (2018) 6:2472–4.
39. Agerholm JS, Holm W, Schmidt M, Hyttel P, Fredholm M, McEvoy FJ. Perosomus elumbis in Danish Holstein cattle. BMC Vet Res. (2014) 10:227. doi: 10.1186/s12917-014-0227-2
40. Gerhauser I, Geburek F, Wohlsein P. Perosomus elumbis, cerebral aplasia, and spina bifida in an aborted thoroughbred foal. Res Vet Sci. (2012) 92:266–8. doi: 10.1016/j.rvsc.2010.11.009
41. Piegari G, D'Anza E, Costanza D, Prisco F, Meomartino L, d'Aquino I, et al. Perosomus elumbis in piglets: Pathological, radiological and cytogenetic findings. Animals. (2021) 11:1132. doi: 10.3390/ani11041132
42. Gentile A, Testoni S. Inherited disorders of cattle: a selected review. Slov Vet Res. (2006) 43:17–29.
43. Ehlers K, Stürje H, Merker HJ, Nau H. Valproic acid-induced spina bifida: a mouse model. Teratology. (1992) 45:145–54. doi: 10.1002/tera.1420450208
44. Shen ZY, Zhang J, Bai YZ, Zhang SC. Diagnosis and management of fecal incontinence in children and adolescents. Front Pediatr. (2022) 10:1034240. doi: 10.3389/fped.2022.1034240
45. Sinha S, Shah MA, Babu DM. Symptomatic lower urinary tract dysfunction in sacral agenesis: Potentially high risk? Indian J Urol. (2018) 34:56–61. doi: 10.4103/iju.IJU_184_17
Keywords: sacral agenesis, sacro-caudal dysgenesis, Currarino syndrome, spina bifida, congenital spine malformation, caudal regression syndrome
Citation: Dell'Apa D, Fumeo M, Volta A, Bernardini M, Fidanzio F, Buffagni V, Christen M, Jagannathan V, Leeb T and Bianchi E (2023) Case report: Sacral agenesis in two boxer dogs: clinical presentation, diagnostic investigations, and outcome. Front. Vet. Sci. 10:1201484. doi: 10.3389/fvets.2023.1201484
Received: 06 April 2023; Accepted: 09 May 2023;
Published: 25 May 2023.
Edited by:
Adriano Wang-Leandro, University of Veterinary Medicine Hannover, GermanyReviewed by:
Sam Long, Veterinary Referral Hospital, AustraliaCopyright © 2023 Dell'Apa, Fumeo, Volta, Bernardini, Fidanzio, Buffagni, Christen, Jagannathan, Leeb and Bianchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diletta Dell'Apa, ZGlsZXR0YS5kZWxsYXBhQHVuaXByLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.