- 1Department of Animal Sciences and Technology, Shandong Agricultural University, Tai’an, China
- 2College of Food Science and Engineering, Shandong Agricultural University, Tai’an, China
- 3Laiyang Animal Husbandry and Veterinary Bureau, Yantai, China
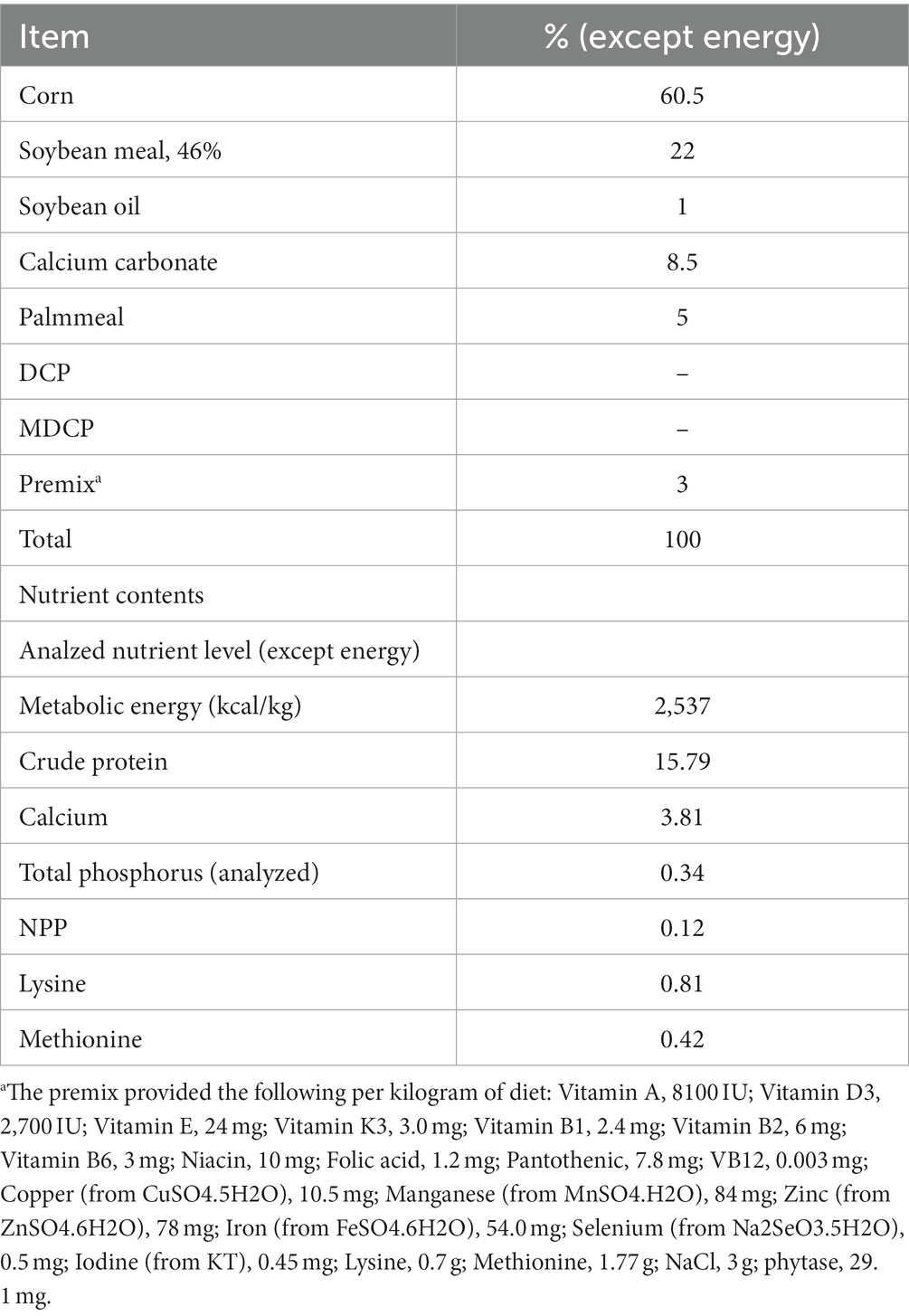
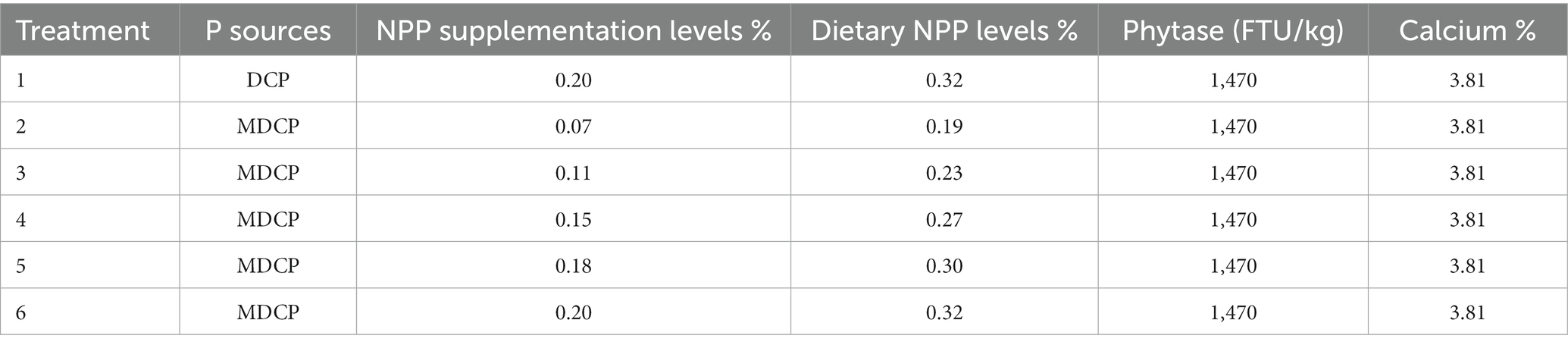
The objective of this study was to evaluate the effect of replacing dicalcium phosphate (DCP) with mono-dicalcium phosphate (MDCP) to formulate low-phosphorus (P) diets on laying performance, egg quality, phosphorus-calcium metabolism, and bone metabolism of 69–78-week-old aged laying hens. Hy-Line Brown laying hens (n = 1,350, 69 weeks old) were randomly assigned to six treatments, each with five replicates of 45 hens. A corn–soybean meal–based diet was formulated to contain 0.12% non-phytate phosphorus (NPP), 3.81% calcium (Ca), and 1,470 FTU/kg phytase. The control group (CON) was supplemented with DCP inorganic phosphorus (Pi) at the NPP level of 0.20% (dietary NPP levels of 0.32%). Test groups (T1–T5) were supplemented with MDCP Pi at NPP levels of 0.07%, 0.11%, 0.15%, 0.18, and 0.20% (dietary NPP levels of 0.19, 0.23, 0.27, 0.30, and 0.32%, respectively). Calcium carbonate levels were adjusted to ensure all experimental diets contained the same Ca levels (3.81%). The feeding trial lasted 10 weeks, with hens increasing in age from 69 to 78 weeks. When supplemented with 1,470 FTU/kg phytase, extra DCP Pi or MDCP Pi did not affect (p > 0.05) laying performance (day laying rate, average egg weight, feed intake, feed-to-egg mass ratio, broken egg rate), egg quality (eggshell strength, albumen height, haugh units), or serum P, Ca, copper (Cu), iron (Fe), zinc (Zn), and manganese (Mn) levels. However, when laying hens were fed MDCP Pi (NPP levels of 0.07 to 0.20%), yolk color improved (p = 0.0148). The tibia breaking strength was significantly higher (p < 0.05) in the 0.18 and 0.20% NPP MDCP Pi groups than in the 0.20% NPP DCP Pi group. The breaking strength, Ca content, and P content of tibia in 0.11% and 0.15% NPP MDCP Pi hens were not significantly (p > 0.05) different from those in 0.20% NPP DCP Pi hens. Hens fed 0.07% NPP MDCP Pi had higher (p < 0.01) serum levels of osteoprotegerin (OPG), type-I collagen c-telopeptide (CTX-I), and tartrate-resistant acid phosphatase 5b (TRACP-5b) than those in all other groups. Serum levels of TRACP-5b and CTX-I in the 0.11% and 0.15% NPP MDCP Pi group were significantly lower than those in 0.18 and 0.20% NPP MDCP Pi groups and the 0.20% NPP DCP Pi group (p < 0.0001). Hens fed 0.07% and 0.11% NPP MDCP Pi had higher (p < 0.05) serum levels of parathyroid hormone (PTH) than those in all other groups. No differences were detected in serum calcitonin (CT), 1,25-dihydroxy-vitamin D3 (1,25-(OH)2D3), bone alkaline phosphatase (BAP), osteocalcin(OCN), and osteopontin (OPN) among all groups (p > 0.05). The expression of P transporters type IIa Na/Pi cotransporter (NaPi-IIa) in 0.11% and 0.15% NPP MDCP Pi hens were higher than those in 0.20% NPP MDCP Pi group and 0.20% NPP DCP Pi group (p < 0.05). The results indicated that both renal P reabsorption and bone resorption were involved in adapting to a low-P diet. In summary, when MDCP was used instead of DCP to supplement P, NPP levels could be reduced to 0.11% (dietary NPP level of 0.23%) without negative effects on laying performance and skeletal health of aged hens. In addition, MDCP was more beneficial than DCP for tibia quality. The results of the current study would provide references for the application of MDCP in low-P diets of aged laying hens.
Introduction
Phosphorus (P) is an essential nutrient for animal growth and development that has a crucial role in skeletal development and bone mineralization (1). However, more than two-thirds of the P in plant-based raw materials is phytate P. Monogastric animals, including poultry, cannot use bound P in phytate efficiently because of insufficient levels of endogenous phytase (2). Therefore, inorganic phosphates (Pi) such as mono-dicalcium phosphate (MDCP) and dicalcium phosphate (DCP) are commonly used in diets to support animal health and performance (3). However, P digestibility by poultry varies with different sources of Pi (3). Sauvant et al. (4) reported actual ileal P digestibility of MDCP and DCP produced from rock phosphate was 69.3 and 60.2%, respectively. According to Bikker et al. (5), actual ileal phosphate digestibility of DCP was 59.0%, whereas that of MDCP reached 70.7%. An et al. (6) reported apparent ileal digestibility of P in MDCP and DCP by poultry was 86.0 and 76.2%, respectively. Previous studies indicate that actual phosphate digestibility was lower for DCP from rock phosphates than for MDCP. Differences in Pi digestibility are attributed to phosphate characteristics as well as animal age, Ca:P ratio, and application of phytase (7, 8). Therefore, in-depth exploration of different mineral sources of P is required to adjust dietary requirements and ensure highly efficient production while reducing pressure on the environment.
In addition, phytase has been widely used in poultry diets to reduce Pi supplementation. Phytase is one of the most popular environmentally friendly feed additives of the 21st century. Supplementing with large amounts of phytase can further improve P utilization (9). Keshavarz (10) reported that supplementation with 300 FTU/kg phytase effectively restores laying performance when dietary NPP content is <0.25% at laying hens from 18 to 51 weeks of age. Wang et al. (11) indicated that laying performance could be maintained ifphytase at 360 FTU/kg was supplemented into a basal diet with supplementation of <0.10% NPP. Taheri et al. (12) and Adeola and Cowieson. (13) reported that dietary supplementation with phytase above 1,000 FTU/kg could reduce the amount of Pi, even without extra supplementation. Ren et al. (14) reported that corn–soybean meal-based diets containing 0.12% NPP and 2000 FTU/kg phytase would meet the requirements for egg production in Hy-Line Brown laying hens from 29 to 40 wk. of age. It is necessary to decrease dietary Pi supplementation because phosphate ore resources are limited globally and fecal P contaminates soils (15). Based on our understanding of the current literature, information is scant regarding the formulation of low-P diets for aged laying hens by replacing DCP with MDCP. The objective of this study was to evaluate the effect of replacing DCP with MDCP to formulate low-P diets on laying performance, egg quality, calcium-phosphorus metabolism, and bone metabolism of 69–78-week-old aged laying hens.
Materials and methods
The Animal Care and Use Committee of Shandong Agriculture University (protocol code SDAUA-2021-019) approved this study.
Animals and diets
A corn–soybean meal-based diet was formulated to contain 0.12% NPP, 3.81% Ca, and 1470 FTU/kg phytase (Table 1). Hy-Line Brown laying hens (n = 1350, 69 weeks old) were randomly assigned to six treatments, each with five replicates of 45 hens. The hens were housed in an environmentally controlled, three-tier, high-rise cage (depth × width × height = 47 × 36 × 105 cm) system. Three hens in the each of cage. The control group (CON) was supplemented with DCP at the NPP levels of 0.20% (dietary NPP levels of 0.32%), which is the current NPP level commonly set by Chinese premix companies when using phytase. Test groups (T1–T5) were supplemented with MDCP at NPP levels of 0.07%, 0.11%, 0.15%, 0.18%, and 0.20% (dietary NPP levels of 0.19%, 0.23%, 0.27%, 0.30%, and 0.32%, respectively). The MDCP was purchased from Yunnan Phosphate Group Co., Ltd (Kunming, China; Total p ≥ 21%), and the DCP was purchased from Sichuan Longmang Group Co., Ltd (Mianzhu, China; Total p ≥ 16.5%). Phytase (≥50,000 FTU/g; Ucommercial name: Habio phytase; 6-phytase produced by Escherichia coli) was purchased from Jinan Bestzyme Bio-engineering (Jinan, China). Calcium carbonate levels were adjusted to ensure all experimental diets contained the same level of Ca (3.81%). The trial was conducted under appropriate management to minimize discomfort to laying hens. The temperature in the laying room was maintained between 20.0°C and 25.0°C. Hens were provided with 16 h of light per day and had free access to feed and water throughout the experiment. The feeding trial was conducted for 10 weeks. The P sources and P levels of experimental diets are shown in Table 2. Laying performance, phosphorus-calcium metabolism, and bone metabolism were evaluated in Hy-Line Brown laying hens from 69 to 78 weeks of age.
Laying performance and egg quality
Feed consumption, number of eggs and total egg weight were recorded daily to calculate feed intake and feed-to-egg mass ratio. After the end of the feeding trial, 10 eggs per replicate (50 eggs per treatment) were randomly collected to determine egg quality. Haugh units, yolk color, and albumen height were measured using an automatic egg quality analyzer (EMT-5200; Robotmation, Co., Ltd., Tokyo, Japan). Eggshell strength was tested using a strength gauge (EFG-0503; Robotmation Co., Ltd.).
Serum biochemistry
At the end of the feeding trial, 10 hens (two hens per replicate) were randomly selected from each group. Fasting blood was collected from the left wing vein using coagulation-promoting tubes at 8:00 and 10:00 AM. Serum samples were extracted by centrifugation at 3,500 rpm for 15 min and then stored at −20°C until analysis (16). Serum Ca and P concentrations were determined at Shandong Agricultural University (Taian, China) using a fully automatic biochemical analyzer (L-7020; Hitachi, Tokyo, Japan). Serum concentrations of copper (Cu), iron (Fe), zinc (Zn), and manganese (Mn) were analyzed using a flame atomic absorption spectrometer (Z-8200, Hitachi) according to the method described in Chen et al. (17).
Tibia quality
After blood collection, hens were euthanized by cervical dislocation for tibia and kidney samples. Right tibia breaking strength was determined using a three-point bending test with a microcomputer-controlled electronic universal mechanical test machine (YAW-5000F; Jinan Pilot Gold Group Co., Jinan, China). The support distance of the instrument was 40 mm, and the testing speed was 2 mm/min. Left tibia samples were defatted in anhydrous ethanol (degreasing time: 8 h) and then dried (drying temperature: 105°C) to determine defatted dry weight. Then, samples were ashed (600°C, 8 h), and Ca (EDTA titration method) and P (ammonium metavanadate colorimetric method) contents were determined as reported in the study by Ren et al. (18). Ash, Ca and P contents of the defatted and oven-dried tibia were calculated.
Determination of serum Ca and P metabolism-related hormones
Ca and P metabolism-related hormones, including CT, PTH, and 1,25-(OH)2D3 were determined with the relevant enzyme-linked immunosorbent assay (ELISA) kits (Jiangsu Meimian Industrial, Yancheng, China) according to previous studies by Ren et al. (19).
Determination of serum bone turnover markers
Serum bone formation markers, including BAP, OCN and OPG, were determined with the relevant ELISA kits (Jiangsu Meimian Industrial) according to previous studies by Ren et al. (19).
Serum bone resorption markers, including CTX-I, OPN, and TRACP-5b, were determined with the relevant ELISA kits (Jiangsu Meimian Industrial) according to previous studies by Ren et al. (19).
Real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Kidney samples were snap-frozen in liquid nitrogen and stored at – 80°C. Primer sequences used for real-time PCR and the procedure to determine relative mRNA expression were according to previous publication (20–22). The β-actin gene was amplified in parallel as the internal control for gene normalization, and primer sequences are shown in Table 3. The 2−∆∆Ct method was used to calculate relative mRNA expression in renal samples.
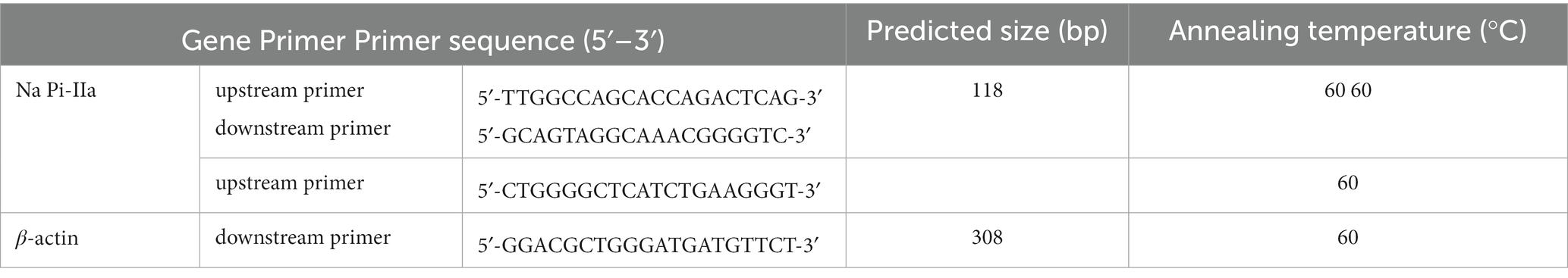
Table 3. Primers for real-time PCR analysis in samples from Hy-Line Brown laying hens (69 to 78 weeks of age).
Statistical analyses
All data were analyzed by using the general linear model (GLM) in SAS 9.4 (SAS Institute Inc., Cary, NC, United States). The experimental unit was the replicate for the analysis of laying performance, each egg for egg quality parameters, and each hens for all other variables. The differences among treatments were compared with Tukey’s multiple range tests. Data are the mean ± standard error. Significance was considered at p < 0.05.
Results
Laying performance and egg quality
Laying performance (day laying rate, average egg weight, feed intake, feed-to-egg mass ratio, broken egg rate) and egg quality (eggshell strength, albumen height, haugh units, yoke color) of hens from different groups are shown in Figure 1. Laying performance and egg quality (eggshell strength, albumen height, haugh units) were not affected by dietary supplementation with MDCP Pi (p > 0.05). The exception was yoke color, which improved in hens fed MDCP Pi compared with those fed DCP Pi (p = 0.0148).
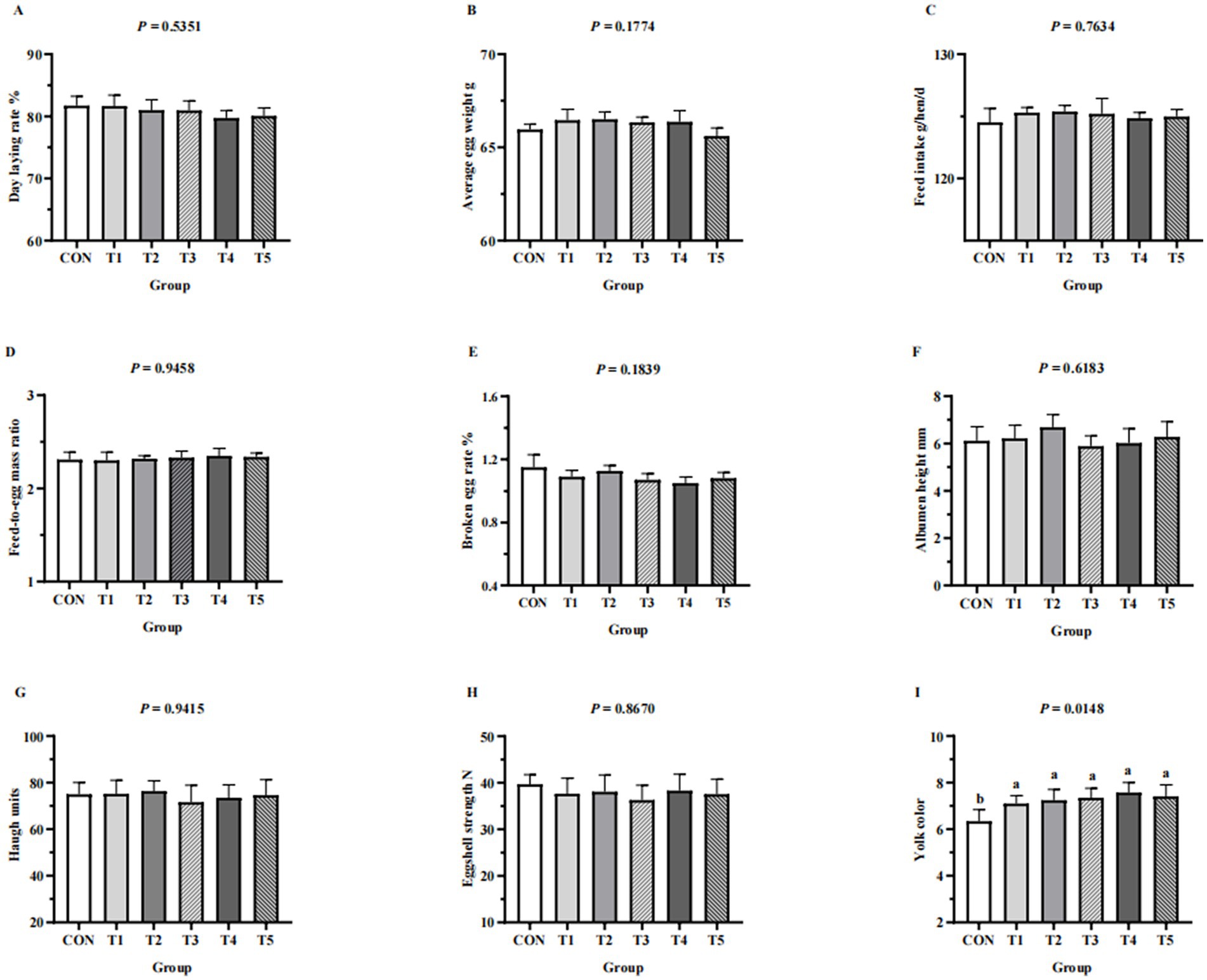
Figure 1. Effects of replacing DCP with MDCP to supplement P on laying performance and egg quality of Hy-Line Brown aged laying hens (69 to 78 weeks of age). (A–E) Laying performance; (F–I) egg quality. CON: DCP, with NPP supplementation at 0.20%. T1–T5: MDCP, with NPP supplementation at 0.07% (T1), 0.11% (T2), 0.15% (T3), 0.18% (T4), and 0.20% (T5). Phytase was used at 1470 FTU/kg diet. The statistical unit was each replicate (n = 5) for laying performance parameters and each egg (n = 50, with 10 eggs per replicate randomly selected) for egg quality parameters. a,bMeans within a row lacking a common superscript differ (p < 0.05).
Serum biochemistry and serum Ca and P metabolism-related hormones
Across groups, no differences were detected in serum levels of Ca (p = 0.1,212), P (p = 0.8354), Cu (p = 0.6545), Fe (p = 0.8221), Zn (p = 0.8166), and Mn (p = 0.9557) (Table 4). Hens fed 0.07% and 0.11% NPP MDCP Pi had higher (p < 0.05) serum levels of PTH than those in all other groups (Figure 2). No differences were detected among groups in serum CT and 1,25-(OH)2D3 (p > 0.05).
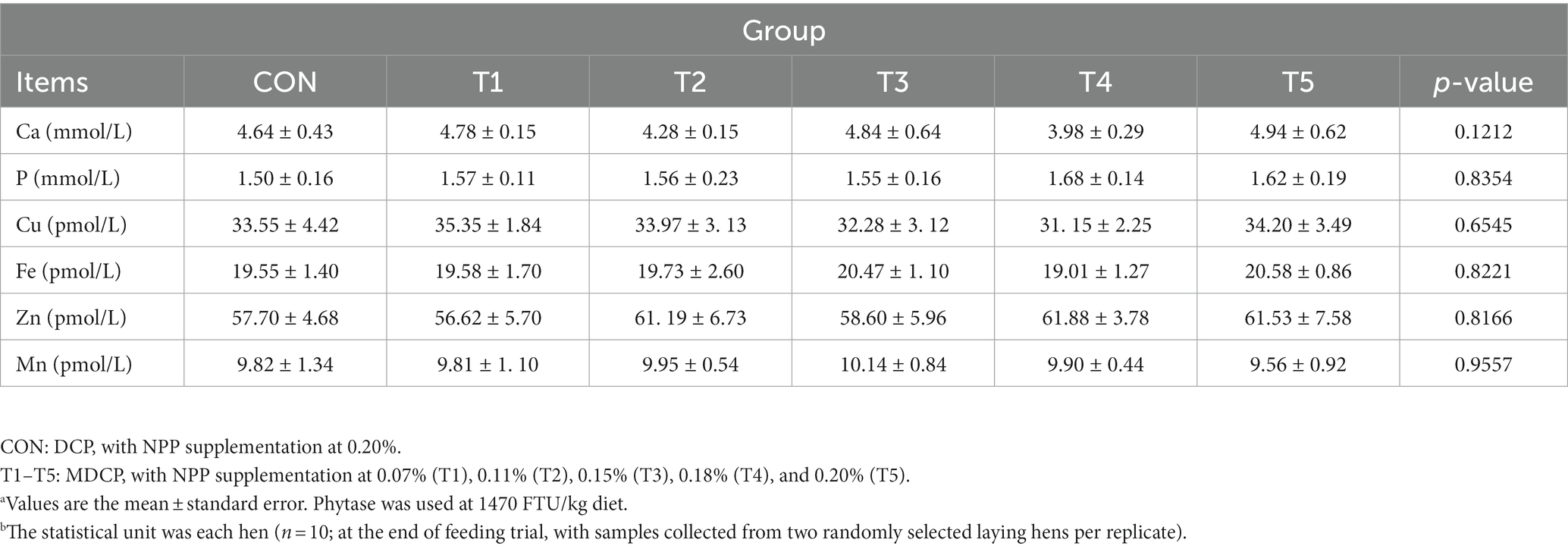
Table 4. Effects of replacing DCP with MDCP to supplement P on serum biochemistry of Hy-Line Brown aged laying hens (69 to 78 weeks of age).a,b
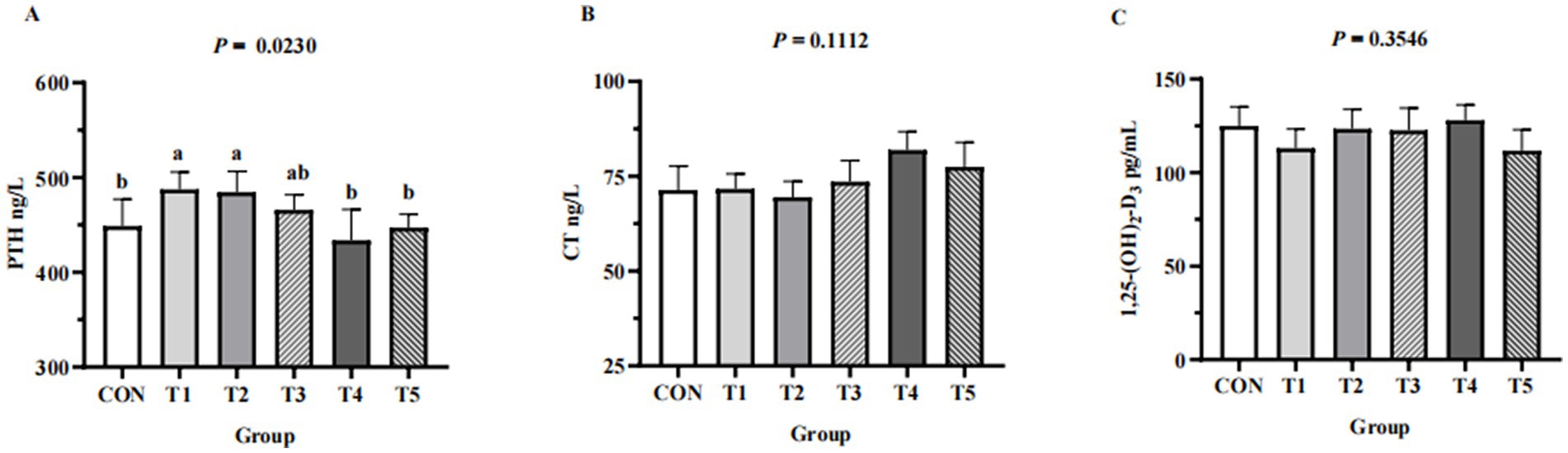
Figure 2. Effects of replacing DCP with MDCP to supplement P on serum Ca and P metabolism-related hormones of Hy-Line Brown aged laying hens (69 to 78 weeks of age). (A) PTH, parathyroid hormone; (B) CT, calcitonin; (C) 1,25-(OH)2D3, 1,25-dihydroxy-vitamin D3. CON: DCP, with NPP supplementation at 0.20%. T1–T5: MDCP, with NPP supplementation at 0.07% (T1), 0.11% (T2), 0.15% (T3), 0.18% (T4), and 0.20% (T5). a,b,c Means within a row lacking a common superscript differ (p < 0.05).
Tibia quality
The tibia breaking strength was significantly higher in the 0.18 and 0.20% NPP MDCP Pi groups than in the 0.20% NPP DCP Pi group (Table 5). The breaking strength, Ca content, and P content of tibia in 0.11% and 0.15% NPP MDCP Pi hens were not significantly (p > 0.05) different from those in 0.20% NPP DCP Pi hens (Table 5). In the 0.07% NPP MDCP group, the Ca and P contents of tibia were significantly lower (p < 0.05) than those in all other groups (Table 5).
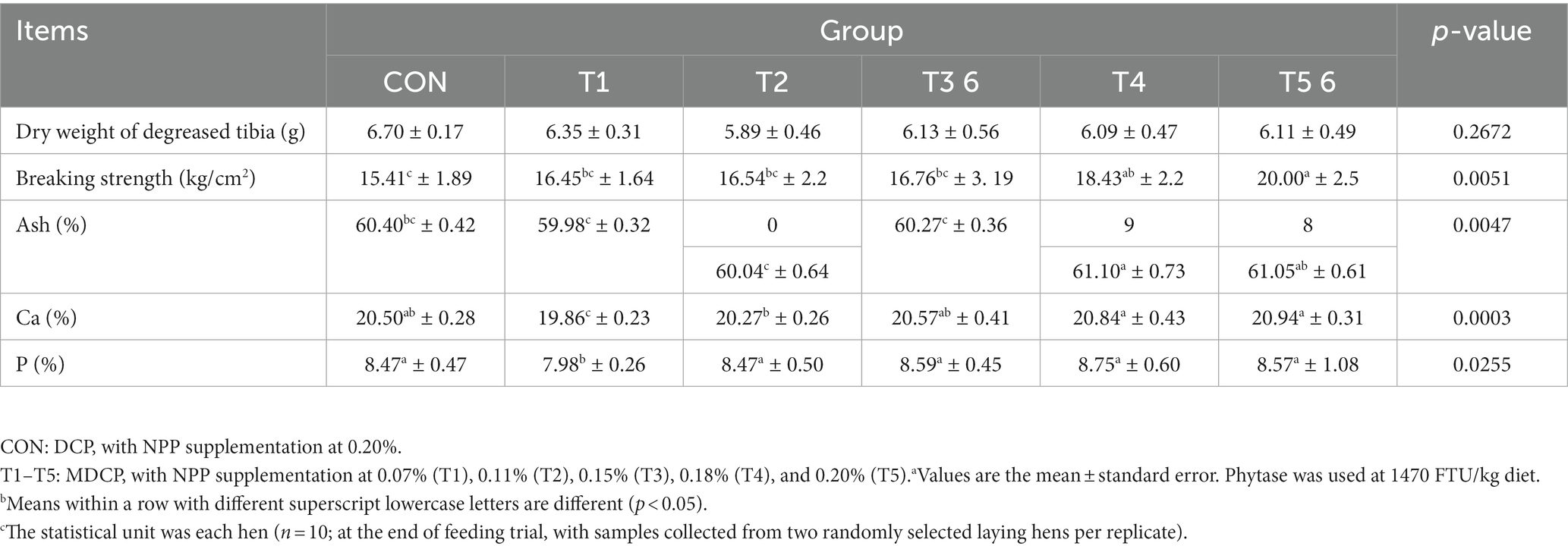
Table 5. Effects of replacing DCP with MDCP to supplement P on tibia quality of Hy-Line Brown aged laying hens (69 to 78 weeks of age).a,b,c
Serum bone turnover markers
Serum bone formation markers (BAP, OCN, OPG) and serum bone resorption markers (CTX-I, OPN, TRACP-5b) of hens from different groups are shown in Figure 3. Serum levels of TRACP-5b and CTX-I in the 0.11% and 0.15% NPP MDCP Pi group were significantly lower than those in 0.15 to 0.20% NPP MDCP Pi groups and the 0.20% NPP DCP Pi group (p < 0.0001). Hens fed 0.07% NPP MDCP Pi had higher (p < 0.01) serum levels of OPG, CTX-I, and TRACP-5b than those in all other groups. Serum levels of OPG in the 0.11% NPP MDCP Pi group were not significantly different from those in 0.15 to 0.20% NPP MDCP Pi groups and the 0.20% NPP DCP Pi group (p > 0.05). No differences were detected in serum BAP, OCN, and OPN among groups (p > 0.05).
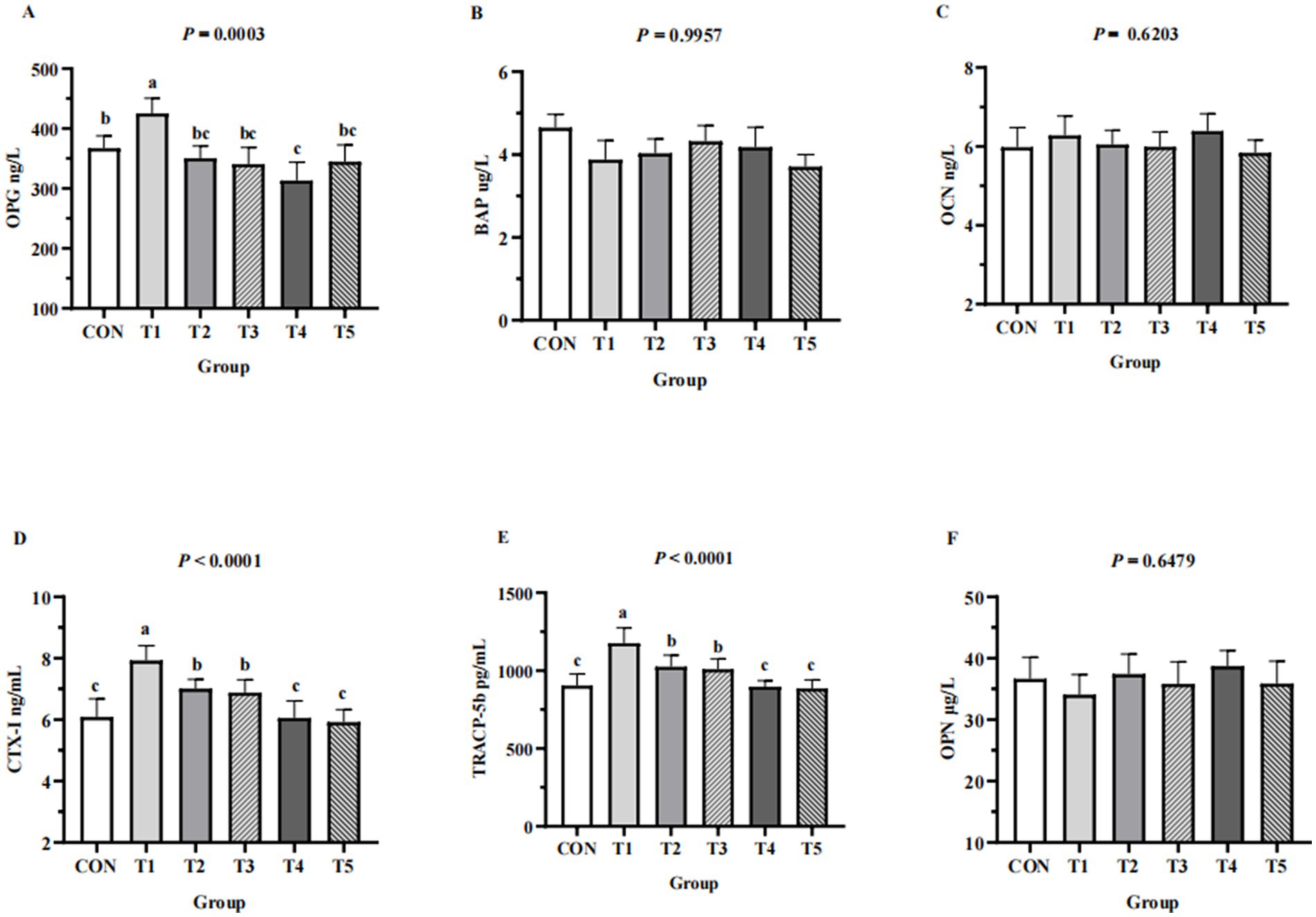
Figure 3. Effects of replacing DCP with MDCP to supplement P on serum bone turnover markers of Hy-Line Brown aged laying hens (69 to 78 weeks of age). CON: DCP, with NPP supplementation at 0.20%. T1–T5: MDCP, with NPP supplementation at 0.07% (T1), 0.11% (T2), 0.15% (T3), 0.18% (T4), and 0.20% (T5). (A–C) Serum bone formation markers; (D–F) Serum bone resorption markers. BAP, bone-alkaline phosphatase; OCN, osteocalcin; OPG, osteoprotegerin; CTX-I, C-telopeptide of I collagen; OPN, osteoponin; TRACP-5b, tartrate resistant acid phosphatase 5b. a,b,cMeans within a row lacking a common superscript differ (p < 0.05).
Renal expression of NaPi-IIa
The expression of NaPi-IIa in the kidneys of hens from different groups are shown in Figure 4. The expression of NaPi-IIa in 0.11% and 0.15% NPP MDCP Pi hens were higher than those in 0.20% NPP MDCP Pi group and 0.20% NPP DCP Pi group (p < 0.05). Hens fed 0.07% MDCP Pi had the highest (p < 0.0001) renal expression of NaPi-IIa than those in all other groups.
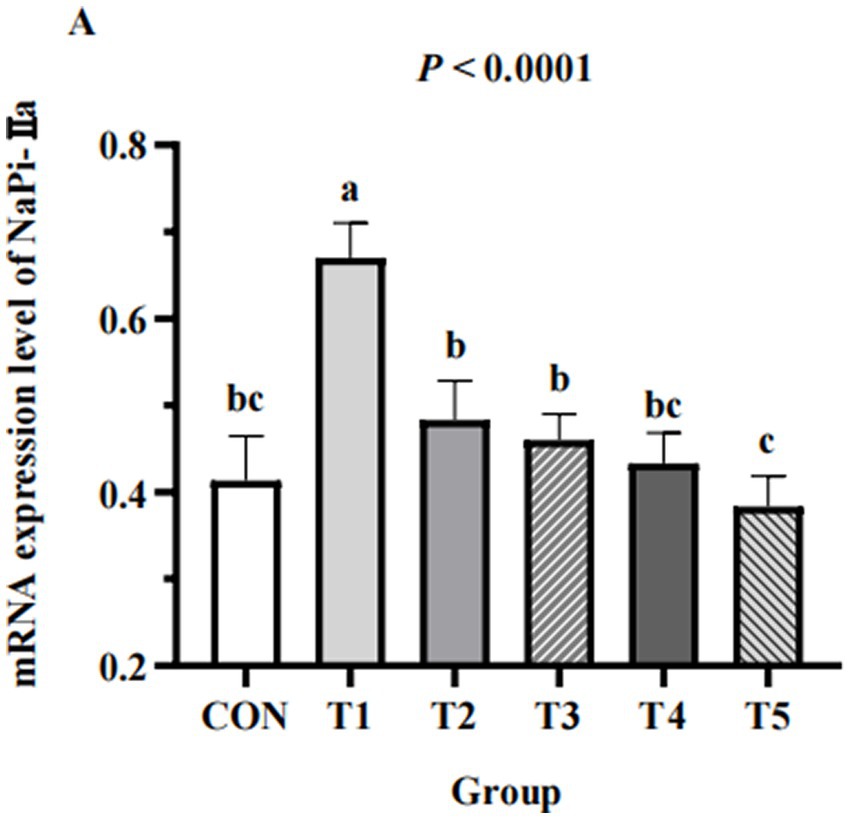
Figure 4. Effects of replacing DCP with MDCP to supplement P on expression of P transporters type IIa Na/Pi cotransporter (NaPi-IIa) in the kidneys of Hy-Line Brown aged laying hens (69 to 78 weeks of age). Relative mRNA expression was calculated for the housekeeping gene β-actin using the 2−ΔΔCt method. a,b,c Means within a row lacking a common superscript differ (p < 0.05).
Discussion
Jing et al. (23) reported that reducing dietary NPP up to 0.15% is adequate to maintain health and performance of laying hens. Cheng et al. (24) reported that laying performance of Hy-Line Brown laying hens(29 to 40 weeks of age) was well maintained by diets supplemented with 2,000 FTU/kg phytase and 0.05%–0.15%NPP MDCP Pi (dietary NPP levels of 0.17–0.27%). Jing et al. (15) indicated that dietary NPP levels could be reduced to 0.10% with the inclusion of 1,000 FTU/kg phytase, without negative effects on production performance and health of the Lohmann hens from 22 to 34 weeks of age. In the current study, laying performance and egg quality were not significantly different among dietary treatments, indicating that 0.07%-0.15% NPP MDCP Pi with 1,470 FTU/kg phytase was sufficient to support the laying performance of Hy-Line Brown 69–78-week-old laying hens compared to 0.20% NPP DCP Pi. Because significant differences were not observed in egg weight and feed-to-egg mass ratio in the current trial, use of MDCP Pi could clearly reduce feed costs and environmental pollution by reducing the amount of Pi. However, the feeding trial was conducted in autumn, and the new corn was low in energy, resulting in an average daily hen intake of more than 120 g, which exceeded the standard of Hy-Line Brown-Conventional Systems in 2021. Given that limitation, long-term feeding studies with large numbers of hens are needed to confirm the findings of this study. There are large changes in P intake due to differences in feed intake.
Tibia quality is a crucial indicator of P nutrition in poultry (25), and inadequate or excess dietary P can cause bone disease (26, 27). Cheng et al. (24) reported that tibia P and Ca storage and mobilization were well maintained when diets were supplemented with 0.05%–0.15% NPP MDCP Pi (dietary NPP levels of 0.17–0.27%). Ren et al. (14) found that 0.20–0.30% DCP Pi (dietary NPP levels of 0.32–0.42%) did not significantly increase tibia breaking strength, ash content, or Ca and P contents in Hy-Line Brown 29–40-week-old laying hens. In the current study, compared with 0.20% NPP DCP Pi, tibia measurements were not affected in hens fed 0.11% and 0.15% NPP MDCP Pi. The supplementation with 0.11% and 0.15% NPP MDCP Pi (dietary NPP levels of 0.23 and 0.27%) is sufficient for the skeleton health of aged laying hens (69 to 78 weeks of age) with the presence of 1,470 FTU/kg phytase. In modern egg production, laying hens face a number of welfare problems related to the increased prevalence of fractures, especially a continuous high egg production in commercial layer lines have led to increased bone fragility and susceptibility to fracture (28, 29). Bone disease are detrimental to egg production, thereby negatively affecting egg quality and production performance (30). Still, minimal information is available on the effects of MDCP use on skeletal-related welfare problems in laying hens. In this study, the tibia breaking strength was significantly higher in the 0.18 and 0.20% NPP MDCP Pi groups than in the 0.20% NPP DCP Pi group, indicating that MDCP was more beneficial than DCP for tibia quality. Therefore, replacing DCP with MDCP to supplement P can be considered a positive welfare attribute.
Ca, P, and related hormones (PTH, CT and 1,25-(OH)2D3) in the serum are crucial indicators for phosphate–calcium metabolism. Jing et al. (23) reported that the concentrations of plasma P were generally reduced in hens on low-P diets. In this study, there were no significant differences in serum Ca and P in hens from different groups. The differences in findings could be related to different testing environments and sources of phytase (31). In addition, serum P are controlled by a variety of factors, and regulation is not limited to Pi intake alone but also to bone resorption and renal reabsorption (32). Meanwhile, the data from the current study showed that hens fed 0.07 and 0.11% NPP MDCP Pi had higher serum levels of PTH than those in all other groups. PTH, secreted from cells of the parathyroid glands, maintains the serum P levels at a normal level by acting directly or indirectly on the intestine, bone, and kidneys (33, 34).
The intake of P plays an important role in bone health, especially during egg laying. The effects of replacing DCP with MDCP and reducing NPP supplementation levels on several serum bone turnover markers indicating bone health were evaluated in this study. A biochemical marker of bone turnover secreted by osteoclasts, TRACP-5b reflects the status of bone resorption, and its blood concentration is used to detect the total amount of bone resorption (25). Obrant et al. (35) reported that serum TRACP-5b is significantly elevated in women with persistent fractures. CTX-I is a special sequence fragments released by osteoclasts from the degradation of type I collagen during bone resorption (36). Therefore, CTX-I reflects the level of bone destruction and is a specific indicator of bone resorption (37). OPG is an irreducible soluble receptor secreted by osteoblasts, which is considered as a protective factor against bone loss (38, 39). OPG could regulate the differentiation of osteocytes and block the formation of osteoclasts (1). Khosla et al. (40) reported a compensatory increase in OPG inhibition of abnormal bone resorption in postnatal rats due to lactation, which disturbed Ca and P metabolism in vivo. The results showed that the serum levels of OPG in the 0.07% NPP MDCP Pi group increased significantly, indicating that the speed of bone resorption was accelerated, which resulted in abnormal bone loss (41). This conclusion is consistent with the lowest tibia Ca and P contents in the 0.07% NPP MDCP group. Aged hens may be more sensitive to P deficiency than young chicks (42). In the current study, the serum levels of TRACP-5b and CTX-I in the 0.11% and 0.15% NPP MDCP Pi hens were significantly lower than those in 0.18 and 0.20% NPP MDCP Pi and 0.20% NPP DCP Pi hens. Low-P intake caused hens to mobilize bone resorption to maintain the body’s calcium-phosphorus metabolism balance.
In addition to the above biochemical indices, the related mRNA expression of a P transporter was also examined. The type II sodium/phosphate cotransporter (NaPi-II) group primarily includes cotransporters NaPi-IIa, NaPi-IIb, and NaPi-IIc, with NaPi-IIb mainly in the small intestine and NaPi-IIa and NaPi-IIc mediating renal P reabsorption (43). The P-transport capacity of the kidney is the most important factor in the maintenance of P homeostasis in hens (44). In a kidney, NaPi-IIa is an electrogenic transporter that preferentially combines with P, whereas NaPi-IIc is an electroneutral transporter that does not easily combine with P. Dietary P deficiency induces upregulation of renal NaPi-IIa mRNA expression in poultry (45). Katsumata et al. (27) reported a significant decrease in renal NaPi-IIa mRNA expression levels after feeding a high-P diet to rats. Li et al. (46) reported that laying hens fed 0.15% available phosphoru (AP) had higher NaPi-IIa mRNA expression levels than those fed 0.41–0.82% AP. In the current study, the renal NaPi-IIa mRNA expression level in the 0.07%–0.15% NPP hens was higher than that in 0.18–0.20% NPP hens, which indicated that the low-P diet increased renal P reabsorption. Because there are no antibodies to NaPi-IIa, protein expression of NaPi-IIa in the kidney was not measured in the current study. Therefore, the effect of a low-P diet on phosphorus balance in laying hens remains to be elucidated.
Conclusion
Renal P reabsorption and bone resorption were both involved in adapting to a low-P diet. In conclusion, NPP levels can be reduced to 0.11% (dietary NPP level of 0.23%) when MDCP is used to supplement NPP in diets instead of DCP without negatively affecting laying performance and skeletal health of aged hens.
The nutritional value of different P sources should be reconsidered in laying hens, especially in the application of phytase. There is great potential to reduce feed costs and decrease phosphorus excretion by reducing the amount of inorganic phosphates without affecting skeletal health and performance of aged hens.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by The Animal Care and Use Committee of Shandong Agriculture University (protocol code SDAUA-2021-019).
Author contributions
WY and SJ received funding. YR, TZ, NJ, and YL conceptualized and designed the study. YR, TZ, LL, ZZ, and KZ conducted animal experiments, chemical analyses, and analyzed the data. YR and TZ wrote the original. NJ, SJ, and YL reviewed and revised the draft. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Major Innovative Projects of Shandong Province (2019JZZY020609). WY and SJ received funding.
Acknowledgments
The authors thank Yu for his excellent experimental assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. van Harn, J , Spek, JW , van Vuure, CA , and van Krimpen, MM . Determination of pre-cecal phosphorus digestibility of inorganic phosphates and bone meal products in broilers. Poult Sci. (2017) 96:1334–40. doi: 10.3382/ps/pew458
2. Selle, PH , and Ravindran, V . Microbial phytase in poultry nutrition. Anim Feed Sci Technol. (2007) 135:1–41. doi: 10.1016/j.anifeedsci.2006.06.010
3. Tassanee, T , Yuwares, R , Ornprapun, S , and Seksom, A . True ileal phosphorus digestibility of monocalcium phosphate, monodicalcium phosphate and dicalcium phosphate for broiler chickens. Anim Feed Sci Technol. (2018) 241:1–7. doi: 10.1016/j.anifeedsci.2018.04.005
4. Sauvant, D , Pere, JM , and Tran, G . Tables of Composition and Nutritional Value of Feed Materials: Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses and Fish. Wageningen: Wageningen Academic Publishers (2004).
5. Bikker, P , Spek, JW , Van Emous, RA , and Van Krimpen, MM . Precaecal phosphorus digestibility of inorganic phosphate sources in male broilers. Br Poult Sci. (2016) 57:810–7. doi: 10.1080/00071668.2016.1222604
6. An, SH , Sung, JY , and Kong, C . Ileal digestibility and Total tract retention of phosphorus in inorganic phosphates fed to broiler chickens using the direct method. Animals (Basel). (2020) 10:2167. doi: 10.3390/ani10112167
7. Rodehutscord, M , Dieckmann, A , Witzig, M , and Shastak, Y . A note on sampling digesta from the ileum of broilers in phosphorus digestibility studies. Poult Sci. (2012) 91:965–71. doi: 10.3382/ps.2011.01943
8. Shastak, Y , Witzig, M , Hartung, K , and Rodehutscord, M . Comparison of retention andprececal digestibility measurements in evaluating mineral phosphorus sources in broilers. Poult Sci. (2012) 91:2201–9. doi: 10.3382/ps.2011.02063
9. Gautier, A , Walk, C , and Dilger, RN . Effects of a high level of phytase on broiler performance, bone ash, phosphorus utilization, and phytate dephosphorylation to inositol. Poult Sci. (2018) 97:211–8. doi: 10.3382/ps/pex291
10. Keshavarz, K . The effect of different levels of nonphytate phosphorus with and without phytase on the performance of four strains of laying hens. Poult Sci. (2003) 82:71–91. doi: 10.1093/ps/82.1.71
11. Wang, S , Tang, CH , Zhang, JM , and Wang, XQ . The effect of dietary supplementation with phytase transgenic maize and different concentrations of non-phytate phosphorus on the performance of laying hens. Br Poult Sci. (2013) 54:466–70. doi: 10.1080/00071668.2013.796339
12. Taheri, HR , Jabbari, Z , Adibnia, S , Shahir, MH , and Hosseini, SA . Effect of high-dose phytase and citric acid, alone or in combination, on growth performance of broilers given diets severely limited in available phosphorus. Br Poult Sci. (2015) 56:708–15. doi: 10.1080/00071668.2015.1093094
13. Adeola, O , and Cowieson, AJ . Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci. (2011) 89:3189–218. doi: 10.2527/jas.2010-3715
14. Ren, Z , Sun, W , Cheng, X , Liu, Y , Han, D , Yan, J, et al. The adaptability of Hy-line Brown laying hens to low-phosphorus diets supplemented with phytase. Poult Sci. (2020) 99:3525–31. doi: 10.1016/j.psj.2020.03.033
15. Jing, M , Zhao, S , Rogiewicz, A , Slominski, BA , and House, JD . Effects of phytase supplementation on production performance, egg and bone quality, plasma biochemistry and mineral excretion of layers fed varying levels of phosphorus. Animal. (2021) 15:100010. doi: 10.1016/j.animal.2020.100010
16. Niu, J , Wang, Q , Jing, C , Liu, Y , Liu, H , Jiao, N, et al. Dietary Galla chinensis tannic acid supplementation in the diets improves growth performance, immune function and liver health status of broiler chicken. Front Vet Sci. (2022) 9:024430. doi: 10.3389/fvets.2022.1024430
17. Chen, X , Ma, XM , Yang, CW , Jiang, SZ , Huang, LB , Li, Y, et al. Low level of dietary organic trace elements improve the eggshell strength, trace element utilization, and intestinal function in late-phase laying hens. Front Vet Sci. (2022) 9:903615. doi: 10.3389/fvets.2022.903615
18. Ren, ZZ , Jiang, SZ , Zeng, QF , Ding, XM , Bai, SP , Wang, JP, et al. Effects of dietary canthaxanthin and 25-hydroxycholecalciferol supplementation on the antioxidant status and tibia quality of duck breeders and newly hatched ducklings. Poult Sci. (2016) 95:2090–6. doi: 10.3382/ps/pew090
19. Ren, Y , Liu, Y , Jiang, K , Li, L , Jiao, N , Zhu, Z, et al. Effects of low-phosphorus diets supplemented with Phytase on the production performance, phosphorus-calcium metabolism, and bone metabolism of aged Hy-line Brown laying hens. Animals (Basel). (2023) 13:1042. doi: 10.3390/ani13061042
20. Wang, Z , Shao, D , Wu, S , Song, Z , and Shi, S . Heat stress-induced intestinal barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. Ecotoxicol Environ Saf. (2022) 244:114053. doi: 10.1016/j.ecoenv.2022.114053
21. Shi, S , Shao, D , Yang, L , Liang, Q , Han, W , Xue, Q, et al. Whole genome analyses reveal novel genes associated with chicken adaptation to tropical and frigid environments. J Adv Res. (2023) 47:13–25. doi: 10.1016/j.jare.2022.07.005
22. Zhang, Q , Zhang, S , Wu, S , Madsen, MH , and Shi, S . Supplementing the early diet of broilers with soy protein concentrate can improve intestinal development and enhance short-chain fatty acid-producing microbes and short-chain fatty acids, especially butyric acid. J Anim Sci Biotechnol. (2022) 13:97. doi: 10.1186/s40104-022-00749-5
23. Jing, M , Zhao, S , Rogiewicz, A , Slominski, BA , and House, JD . Assessment of the minimal available phosphorus needs of laying hens: implications for phosphorus management strategies. Poult Sci. (2018) 97:2400–10. doi: 10.3382/ps/pey057
24. Cheng, X , Yan, JK , Sun, WQ , Chen, ZY , Wu, SR , Ren, ZZ, et al. Effect of inorganic phosphate supplementation on egg production in Hy-line Brown layers fed 2000 FTU/kg phytase. Animal (Basel). (2020) 14:2246–52. doi: 10.1017/S1751731120001597
25. Dhandu, A , and Angel, R . Broiler nonphytin phosphorus requirement in the finisher and withdrawal phases of a commercial four-phase feeding system. Poult Sci. (2003) 82:1257–65. doi: 10.1093/ps/82.8.1257
26. Bai, XY , Miao, DS , Li, JR , Goltzman, D , and Karaplis, AC . Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. (2004) 145:5269–79. doi: 10.1210/en.2004.0233
27. Katsumata, S , Masuyama, R , and Suzuki, MUK . High-phosphorus diet stimulates receptor activator of nuclear factor-kB ligand mRNA expression by increasing parathyroid hormone secretion in rats. Br J Nutr. (2005) 94:666–74. doi: 10.1079/bjn20051552
28. Nannoni, E , Buonaiuto, G , Martelli, G , Lizzi, G , Trevisani, G , Garavini, G, et al. Influence of increased freedom of movement on welfare and egg laying pattern of hens kept in aviaries. Animals (Basel). (2022) 12:2307. doi: 10.3390/ani12182307
29. Sandilands, V , Moinard, C , and Sparks, NHC . Providing laying hens with perches: fulfilling behavioural needs but causing injury? Br Poult Sci. (2009) 50:395–406. doi: 10.1080/00071660903110844
30. Nasr, MA , Murrell, J , and Nicol, CJ . The effect of keel fractures on egg production, feed and water consumption in individual laying hens. Br Poult Sci. (2013) 54:165–70. doi: 10.1080/00071668.2013.767437
31. Hemme, A , Spark, M , Wolf, P , Paschertz, H , and Kamphues, J . Effects of different phosphorus sources in the diet on bone composition and stability (breaking strength) in broilers. J Anim Physiol Anim Nutr (Berl). (2005) 89:129–33. doi: 10.1111/j.1439.0396.2005.00539.x
32. Jacquillet, G , and Unwin, RJ . Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (pi). Pflugers Arch. (2019) 471:83–98. doi: 10.1007/s00424.018.2231.z
33. Crenshaw, TD , Rortvedt, LA , and Hassen, Z . Triennial growth symposium: a novel pathway for vitamin D-mediated phosphate homeostasis: implications for skeleton growth and mineralization. J Anim Sci. (2011) 89:1957–64. doi: 10.2527/jas.2010-3411
34. Raposo, JF , Pires, A , Yokota, H , and Ferreira, HG . A mathematical model of calcium and phosphorus metabolism in two forms of hyperparathyroidism. Endocrine. (2012) 41:309–19. doi: 10.1007/s12020-011-9521-y
35. Obrant, KJ , Ivaska, KK , Gerdhem, P , Alatalo, SL , Pettersson, K , and Väänänen, HK . Biochemical markers of bone turnover are influenced by recently sustained fracture. Bone. (2005) 36:786–92. doi: 10.1016/j.bone.2005.02.009
36. Kučukalić-Selimović, E , Valjevac, A , Hadžović-Džuvo, A , Skopljak-Beganovićm, A , Alimanovic-Alagić, R , and Brković, A . Evaluation of bone remodelling parameters after one year treatment with alendronate in postmenopausal women with osteoporosis. Bosn J Basic Med Sci. (2011) 11:41–5. doi: 10.17305/bjbms.2011.2622
37. Herrmann, M , and Seibel, M . The amino-and carboxyterminal cross-linked telopeptides of collagen by dietary phosphate. Am J Physiol Endocrinol Metab. (2008) 393:57–75. doi: 10.1016/j.cca.2008.03.020
38. Kiesel, L , and Kohl, A . Role of the RANK/RANKL pathway in breast cancer. Maturitas. (2016) 86:10–6. doi: 10.1016/j.maturitas.2016.01.001
39. Amin, N , Boccardi, V , Taghizadeh, M , and Jafarnejad, S . Probiotics and bone disorders: the role of RANKL/RANK/OPG pathway. Aging Clin Exp Res. (2020) 32:363–71. doi: 10.1007/s40520-019-01223-5
40. Khosla, S , Arrighi, HM , Melto, LJ 3rd, Atkinson, EJ , O'Fallon, WM , Dunstan, C, et al. Correlates of osteoprotegerin levels in women and men. Osteoporos Int. (2002) 13:394–9. doi: 10.1007/s001980200045
41. Teng, X , Zhang, W , Xu, D , Liu, Z , Yang, N , Luo, D, et al. Effects of low dietary phosphorus on tibia quality and metabolism in caged laying hens. Prev Vet Med. (2020) 181:105049. doi: 10.1016/j.prevetmed.2020.105049
42. Boling, SD , Douglas, MW , Johnson, MJ , Wang, X , Parsons, CM , Koelkebeck, KW, et al. The effects of dietary available phosphorus levels and phytase on performance of young and older laying hens. Poult Sci. (2000) 79:224–30. doi: 10.1093/ps/79.2.224
43. Biber, J , Hernando, N , Forster, I , and Murer, H . Regulation of phosphate transport in proximal tubules. Pflügers Arch. (2009) 458:39–52. doi: 10.1007/s00424.008.0580.8
44. Huber, K , Zeller, E , and Rodehutscord, M . Modulation of small intestinal phosphate transporter by dietary supplements of mineral phosphorus and phytase in broilers. Poult Sci. (2015) 94:1009–17. doi: 10.3382/ps/pev065
45. Proszkowiec-Weglarz, M , and Angel, R . Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility. J Appl Poult Res. (2013) 22:609–27. doi: 10.3382/japr.2012.00743
Keywords: aged laying hen, egg quality, bone metabolism, phytase, mono-dicalcium phosphate, phosphorus-calcium metabolism
Citation: Ren Y, Zhao T, Zhang K, Zhu Z, Li L, Li Y, Jiang S, Jiao N and Yang W (2023) Effect of replacing dicalcium phosphate with mono-dicalcium phosphate to supplement phosphorus on laying performance, phosphorus-calcium metabolism and bone metabolism of aged laying hens. Front. Vet. Sci. 10:1196334. doi: 10.3389/fvets.2023.1196334
Edited by:
Shourong Shi, Poultry Institute (CAAS), ChinaReviewed by:
Damiano Cavallini, University of Bologna, ItalyTugay Ayasan, Osmaniye Korkut Ata University, Türkiye
Copyright © 2023 Ren, Zhao, Zhang, Zhu, Li, Li, Jiang, Jiao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Jiao, amlhb25pbmdAc2RhdS5lZHUuY24=; Weiren Yang, d3J5YW5nQHNkYXUuZWR1LmNu
†These authors have contributed equally to this work
 Yuechang Ren
Yuechang Ren Tianyu Zhao2†
Tianyu Zhao2† Yang Li
Yang Li Shuzhen Jiang
Shuzhen Jiang Ning Jiao
Ning Jiao