
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 03 May 2023
Sec. Animal Nutrition and Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1190998
This article is part of the Research TopicWaterfowl Production and Management Strategies: Nutrition, Genetics and Breeding, and Diseases PreventionView all 5 articles
Introduction: Gaoyou duck is famous in China and abroad for its good production of double-yolk eggs. However, there has been no systematic research on the egg-laying characteristics of the Gaoyou duck, which limits the development and utilization of breed resource.
Methods: To identify the essential genes related to ovarian development, the transcriptome profiles of the ovaries of Gaoyou ducks at different physiological stages were analyzed. The transcriptome profiles of the ovaries of Gaoyou ducks at 150 d (before laying), 240 d (egg laying) and 500 d (nesting) were constructed, and the differentially expressed genes (DEGs) underwent GO (gene ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses.
Results: The 6 randomly selected DEGs were verified by real-time fluorescent quantitative PCR that their relative expression was consistent with the transcriptional expression profile. Furthermore, KEGG analysis found that 8 candidate signaling pathways were essential for ovarian development, including the MAPK signaling pathway, Progesterone-mediated oocyte maturation, Cell adhesion molecules (CAMs), NOD-like receptor signaling pathway, ECM-receptor interaction, Focal adhesion, TGF-beta signaling path-way and Phagosome. Finally, 5 key DEGs were identified to participate in ovarian development, including TGIF1, TGFBR2, RAF1, PTK2 and FGF10.
Discussion: Our findings reveal the mechanisms under-lying the molecular regulation of related genes in Gaoyou duck ovarian development.
The egg-laying performance of poultry is reportedly closely related to ovarian tissue. The ovary is an important reproductive organ of poultry in which folliculogenesis, selection, maturation, and ovulation occur and represents the endocrine gland that synthesizes and secretes estrogen (1). Its function directly affects the level of egg-laying performance of poultry. Therefore, poultry breeders usually select the ovary to study the egg-laying traits and identify differences related to these traits from the histological and molecular levels to explore the regulatory mechanism of the egg-laying performance (2, 3). In recent years, the ovarian tissues of high- and low-egg-production individuals of poultries, including ducks, geese, and chicken, have been studied through RNA-Seq, which provides a convenient way to explore the candidate genes related to egg-laying performance. Karippadakam et al. (4) compared the high and low egg-laying ovarian transcriptome of Indian domestic ducks and found 38 DEGs potentially related to egg production performance. Kun et al. (5) conducted a comparative study on high and low egg-laying Leizhou black ducks using ovary tissue, revealing two key signaling pathways, steroid biosynthesis and FSH signal pathway, involved in the egg-laying performance of Leizhou black ducks. Sun et al. (6) conducted a comparative study on high and low egg-laying Shan-ma ducks using ovarian tissues, revealing ITGB2, ITGB5, and ITGA8 are candidate genes for the egg-laying performance of Shan-ma duck.
Gaoyou duck, also known as the Gaoyou shelduck, ranks first among the three famous ducks in China. Its origin is Gaoyou City, Jiangsu Province, China (Figure 1). It has been raised for more than 100 years in the Yangtze-Huaihe region of China. It is an excellent local poultry variety for meat and egg production (7, 8). Gaoyou duck has the advantages of roughage resistance, strong foraging ability, suitable for grazing and breeding, fast growth and development, good egg quality, etc. It is famous in China and abroad for its good production of double-yolk eggs, estimated to be up to 3.5% (9). However, until now, there has been no systematic observation and research on the egg-laying characteristics of the Gaoyou duck, which limits the development and utilization of this breed resource.
This study performed a comparative transcriptomic analysis of the Gaoyou duck ovary at different physiological stages (before egg laying, egg laying, and nesting) to identify the differentially expressed genes and signaling pathways involved in regulating ovarian function and follicular development of the Gaoyou duck.
This study was approved by the Institutional Animal Ethics Committee of Jiangsu Agri-animal Husbandry Vocational College, Taizhou, Jiangsu, China. A total of 260 female Gaoyou ducks were raised under the same recommended environmental and nutritional conditions at the National Gene Bank of Waterfowl Resources (Jiangsu, China). At 150 days (before laying, BL), 240 days (egg laying, EL), and 500 days (nesting, NE), three ducks were randomly selected for each day. Ducks were, then, sacrificed at different stages through exsanguination. Samples from the ovarian tissue were collected after removing the egg yolk. All samples above were snap-frozen in liquid nitrogen for follow-up experiments. All animal procedures were performed according to the guidelines provided by the China Council on Animal Care, and the protocols were approved by the Experimental Animal Ethics Committee of Jiangsu Agri-animal Husbandry Vocational College (Ethic approval file No. JAHV-2020-58).
Total RNA was extracted from ovarian tissue using a total RNA Kit (Ambion, Austin, TX, United States) in accordance with the manufacturer's instructions. RNA integrity and concentration were evaluated using NanoDrop (NanoDrop, Thermo Scientific) and Agilent 2100 Bio-analyzer (Agilent Technologies, Palo Alto, CA, United States). All the qualified RNA samples were then transported to OE Biotech. Co. (Shanghai, China) for transcriptome sequencing. The Illumina HiSeq 2500 system sequencing platform was used.
To validate the RNA-Seq results, the expression of six randomly selected DEGs was assessed using qRT-PCR. Total RNA was extracted from ovary samples using TRIzol Kit (Invitrogen). The primers (Supplementary Table 1) were designed using Primer 5.0 software. qPCR was performed on an ABI 7500 Real-time Detection System (Applied Biosystems). The relative gene expression levels of selected DEGs were quantified based on GADPH gene expression by the 2−ΔΔCt method.
Raw reads of each sample were obtained from Illumina sequencing, and clean reads were obtained by Trimmomatic software (10) to make the whole quality control process of each sample. Then, the clean reads were mapped to the duck reference genome (BGI duck 1.0 reference) for the downstream analysis as previously described (6). Genes with an absolute log2|fold-change|≥1.50 and adjusted P ≤ 0.05 were differentially expressed genes (DEGs) in this study. The gene ontology (GO) database was, then, used to identify the functions of the DEGs (5, 11). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were assigned to classify unigenes using the online KEGG Automatic Annotation Server (http://www.genome.jp/kegg/kaas/) (12). The sequencing raw data have been deposited into the Sequence Read Archive in NCBI with accession number PRJNA940302.
All statistical analyses in this study were performed using SPSS version 19.0 (IBM, Armonk, NY, United States). The results were expressed as mean ± standard deviation. Differences were assessed using the independent sample t-test. A P < 0.05 was statistically significant.
A total of nine ovarian tissue transcriptome libraries at different physiological stages (before egg laying, egg laying, and nesting) were constructed. The results are shown in Table 1 and Supplementary Table 2. Both the raw reads and clean reads of each library were more than 49 million. To ensure the quality of the data, quality control and filtering were performed on the data, and high-quality clean reads were obtained by removing the adaptor reads and low-quality reads. The Q30 was >92%, and the GC content was >47%. The clean reads were mapped to the reference genome (Anas platyrhynchos) with a total map ranging from 75.93% to 82.38%. The above sequencing data analysis showed that the data quality was reliable and could be used for further analyses.
Based on the nine ovarian tissue samples at three different physiological stages used for the sequencing, statistical analysis of the expression results was conducted by pairwise comparisons. As shown in Figure 2, a total of 8,157 genes (4,421 upregulated and 3,736 downregulated) were differentially expressed between BL and EL, with only 329 DEGs (141 upregulated and 188 downregulated) differentially expressed between BL and EL, and 7,388 DEGs (3,979 upregulated and 3,409 downregulated) expressed between NE and EL. A comparative analysis of DEGs obtained at three different physiological stages of Gaoyou duck showed that the intersection of DEGS during the pairwise comparisons of BL-vs.-EL, BL-vs.-NE, and NE-vs.-EL yielded 171 co-expressed DEGs (Figure 3), with 1,050, 29, and 332 DEGs expressed in BL-vs.-EL, BL-vs.-NE, and NE-vs.-EL, respectively. To focus on the candidate DEGs for ovarian follicular development of Gaoyou duck at different physiological stages, the top 10 DEGs for each pairwise comparison were screened for further analysis, among which one DEG represented both in BL-vs.-EL and BL-vs.-NE, and data are shown in Table 2.
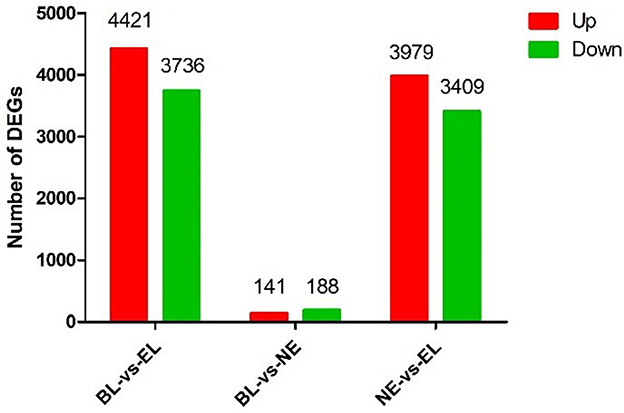
Figure 2. Differentially expressed genes in the ovary of Gaoyou duck at different physiological stages.
To verify the expression levels of DEGs observed in our RNA-seq analysis, six randomly selected DEGs from transcriptomic sequencing were validated by qPCR. The qPCR results showed a similar trend in the expression of these genes, indicating that transcriptomic sequencing data and candidate DEGs from RNA-Seq had high reliability and accuracy (Figure 4).
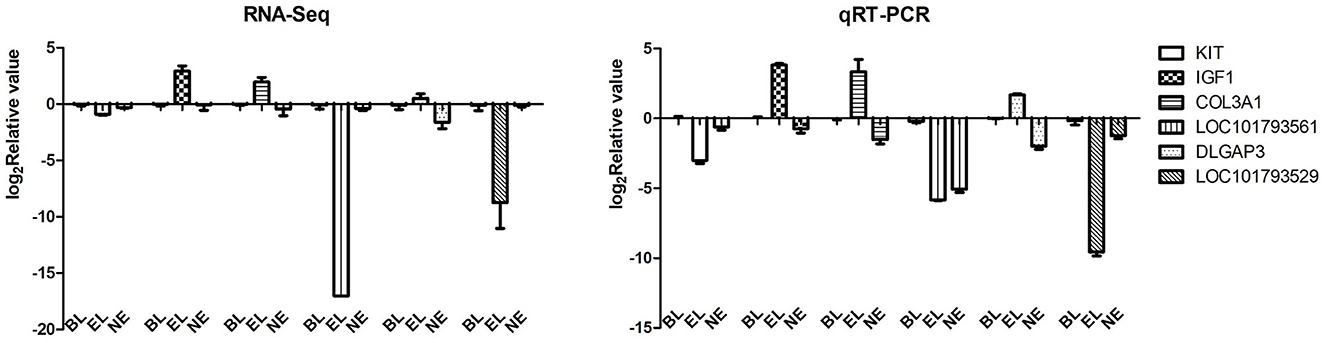
Figure 4. RNA-Seq validation using qRT-PCR. A total of six DEGs were selected randomly to test the accuracy of RNA sequencing.
Gene Ontology (GO) enrichment analysis was performed to further express the functional roles of DEGs in ovarian development. The top 30 most significant GO terms for biological processes (BP), cellular component (CC), and molecular function (MF) in the pairwise three comparisons are shown in Figure 5. The most significant GO terms related to ovarian development in BL-vs.-EL included “steroid hormone-mediated signaling pathway” and “actin crosslink formation” in BF, “tertiary granule membrane” and “ficolin-1-rich granule membrane” in CC, and “BMP receptor activity” and “S100 protein binding” in MF. The most significant GO terms related to ovarian development in BL -vs.-NE included “ovulation cycle” and “cAMP biosynthetic process” in BF, “plasma membrane” and “extracellular region” in CC, and “transforming growth factor beta receptor binding” and “cytokine binding” in MF. The top significantly enriched GO terms related to ovarian development in NE-vs.-EL included “steroid hormone-mediated signaling pathway” and “actin crosslink formation” in BF, “collagen type IV trimer” and “clathrin-coated endocytic vesicle” in CC, and “BMP receptor activity” and “decanoate-CoA ligase activity” in MF. However, there was no common TOP GO term notably enriched among the three comparisons.
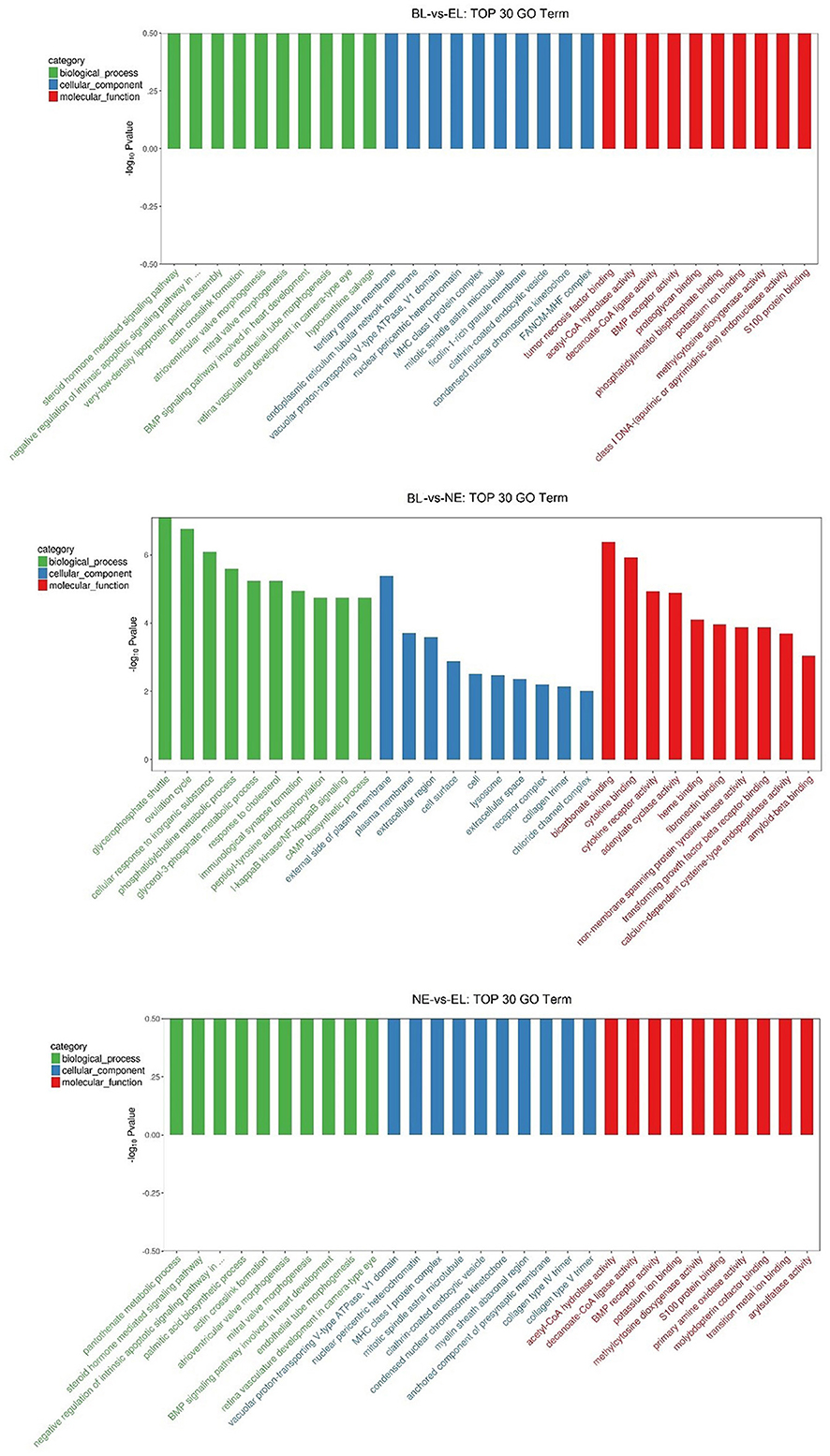
Figure 5. Top 30 GO terms for biological processes, cellular component and molecular function in three comparisons.
To better understand the biological functions and interaction of genes, KEGG pathway analysis was performed for the identified DEGs. The top 20 significantly enriched pathways (P<0.05) by DEGs of each pairwise comparison are shown in Figure 6, including focal adhesion, MAPK signaling pathway, and TGF-beta signaling pathway in BL-vs.-EL; cytokine–cytokine receptor interaction, TGF-beta signaling pathway, and focal adhesion in BL-vs.-NE; focal adhesion, MAPK signaling pathway, and progesterone-mediated oocyte maturation in NE-vs.-EL. Based on the significance levels of KEGG pathways and the literature reviews of the pathway's function, a total of eight pathways were considered as potential pathways related to egg production and ovarian development of Gaoyou duck, including MAPK signaling pathway, progesterone-mediated oocyte maturation, cell adhesion molecules (CAMs), NOD-like receptor signaling pathway, ECM-receptor interaction, focal adhesion, TGF-beta signaling pathway, and phagosome. All the DEGs enriched in these eight pathways were analyzed through Venn software, and 439 candidate DEGs were screened for further analysis (Figure 7).
Furthermore, considering the significance levels of the top 29 DEGs and the 439 DEGs enriched by KEGG pathway results, a total of 468 candidate DEGs were screened from RNA-Seq results of Gaoyou duck ovaries at three different physiological stages. To further narrow candidate genes which harbor great significance, we summarized and clustered the expression patterns of these 468 genes. As shown in Figure 8, among the 16 patterns, we identified three patterns of genes with significant p-values (colored boxes) (numbers 5, 10, and 14), which contained 221, 117, and 47 genes, respectively. Considering ovary development and function at different stages, we focused on profile NO.14. A protein–protein interaction (PPI) network based on the STRING database was generated to visualize the relationship between the 47 potential candidate genes (Figure 9). Based on the PPI network and literature reviews, TGIF1, TGFBR2, RAF1, PTK2, and FGF10 were selected as potential candidate genes for ovarian function and follicular development of Gaoyou duck.
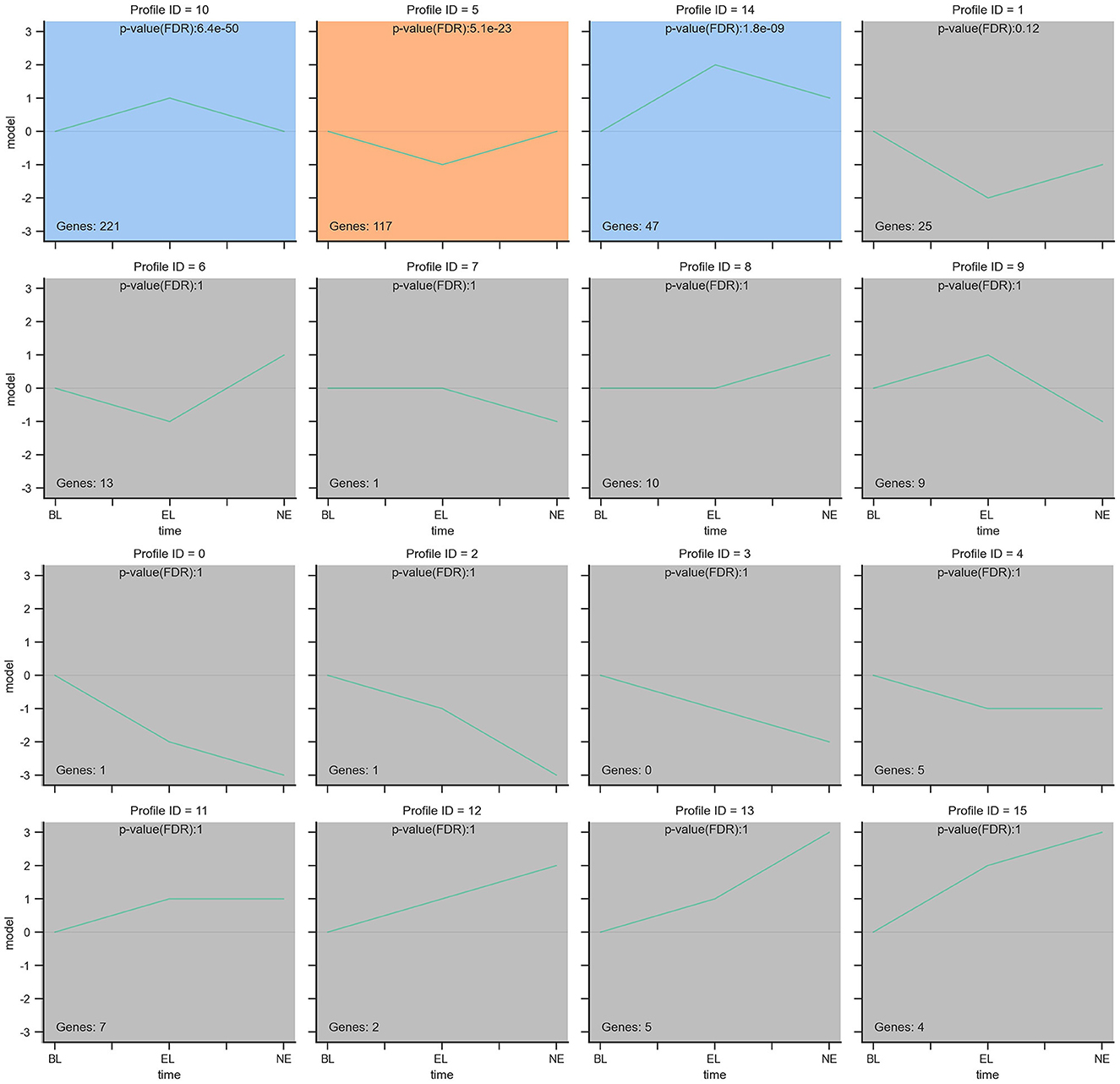
Figure 8. The expression patterns of 468 genes analyzed by model profile. The expression patterns of 468 genes were analyzed and 16 model profiles were used to summarize. Each box represents a model expression profile. 3 expression patterns of genes showed significant P-values (P < 0.05) (colored boxes).

Figure 9. Genes of profile NO.14 of Gaoyou ducks' ovaries at different physiological stages. (A) 47 genes expression profile of NO.14, the horizontal axis represents different physiological stages of Gaoyou duck's ovary, and the vertical axis shows the time series of gene expression levels for the gene after normalized transformation. (B) Heat map showing the differential gene expression of profile NO.14. “blue” represents low relative expression level, and “red” represents high relative expression level. Each column and row represent a sample, and a gene respectively. (C) PPI network of 47 genes based on the STRING database.
The egg-laying process of poultry is a complex developmental biological process, highly affected by the characteristics of the ovarian tissue. The function of the ovary at different development stages directly affects egg production through various genes, which may have different expressions under different physiological conditions (13). In this study, the ovary transcriptomes, which include age at 150 days before laying (BL), 240 days of egg laying (EL), and 500 days of nesting (NE), were analyzed through RNA-Seq to deepen our understanding of Gaoyou duck laying performance.
Based on the transcriptomic studies, 2,782 genes were differentially expressed between the BL and EL groups; 46 and 2,516 DEGs were discovered during the pairwise comparisons of BL-vs.-NE and NE-vs.-EL, respectively. To validate the reliability of the transcriptomic sequencing results, six DEGs were randomly selected for qRT-PCR verification from the original tissue, and the expression levels proved that the six genes were consistent with the RNA-Seq data. The qRT-PCR verification results indicated that the RNA-Seq data and the screened DEGs were highly reliable and could be used for further analysis.
Pathway functional analysis found that focal adhesion, phagosome, cell adhesion molecules (CAMs), MAPK signaling pathway, and regulation of actin cytoskeleton were involved in the whole reproductive process of Gaoyou duck. Focal adhesion is a special structure formed at the contact site between cells and the extracellular matrix, which is used as a combinatorial site for creating different signaling complexes for cells (14). Focal adhesion participates in cell migration, proliferation, and differentiation. Focal adhesion has been identified in previous ovary transcriptome studies, including Jinghai yellow chicken (13), white Muscovy duck (15), and Xinjiang Yili Geese (16). An increasing body of evidence suggests that the MAPK (mitogen-activated protein kinase) signaling pathway participates in cell proliferation, differentiation of apoptosis, and the reproductive process (17). A recent study identified that the MAPK signaling pathway was involved in the egg production and folliculogenesis of Indian domestic ducks (4). The actin cytoskeleton signaling pathway is involved in cellular functions (18). Through actin cytoskeleton reorganization, the proliferation of granulosa cells could be promoted (19). Cheng et al. (20) treated murine ovaries with an actin polymerization-promoting cyclic peptide and discovered that follicle growth was stimulated both in vivo and in vitro. In duck's (6), goose's (21), and goat's (22) ovary transcriptome studies, the actin cytoskeleton has been discovered to be related to ovary function. The phagosome and cell adhesion molecules (CAMs) were consistently reported in a study comparing the ovary of high- and low-laying ducks (23). Overall, these signaling pathways may control ovary development and may be involved in the egg production of Gaoyou duck.
Transforming growth interaction factor 1 (TGIF1) is one of the TALE homolog domain protein family members which participate in many physiological processes including lipid and carbohydrate metabolism (11), inflammatory reaction (24, 25), and inhibition of androgen receptor activity (26). An et al. (27) revealed an insight that TGIF1 could suppress the secretion of E2/P4 in goat's granulosa cells and inhibited the apoptosis of granulosa cells. Martin (28) described that TGIF1 is expressed in chicken cortical and pre-granulosa cells and is required for ovarian development. In this study, we provided preliminary evidence that TGIF1 was deferentially expressed during the development of the Gaoyou duck ovary, suggesting that it may also play a role in ovarian function and follicular development.
Transforming growth factor beta receptor II (TGFBR2) represents a core member of the TGF-beta signaling pathway that plays an important role in ovary function (29). In 1993, Tsuchina et al. (30) found that TGFBR2 mRNA is expressed in the porcine granulosa cells of rat ovaries. Moreover, Roy (31) discovered that TGFBR2 is expressed in human ovarian granulosa cells, corpus luteum cells, and interstitial cells. A study by Li et al. (32) showed that TGFBR2 is highly expressed in the Erhualian pig's ovary. Li (33) compared high- and low-fecundity groups of Hu sheep and found that the expression of EGFBR2 is higher in the ovary than in other tissues and is significantly higher in the high-fecundity group than in the low-fecundity group. Through TGFRB2gc−/− mice, Yang et al. (34) found that TGFRB2 deletion could impair oocyte meiotic arrest, ovulation, and female fertility. In the present study, we found that ovarian TGFBR2 expression was higher during the egg-laying period than the other two periods, suggesting that it might be involved in the egg-laying performance of the Gaoyou duck.
V-RAF-leukemia viral oncogene 1 (RAF1) is the core member of the RAS/RAF/MEK/ERK signaling pathway and has been approved to play an essential role in reproduction and development by regulating steroid hormone synthesis (35, 36). Through in vitro and in vivo studies, Luo et al. (37) found that RAF1 could stimulate E2 synthesis and secretion through the FSH signaling pathway in mouse granulosa cells. Nonetheless, the role of RAF1 in ovarian function and follicular development of poultry has been largely understudied. Based on its function in regulating steroid hormone synthesis, it can be affirmed that RAF1 is essential in Gaoyou duck ovary development.
Protein tyrosine kinase 2 (PTK2) is a member of the PTK family which plays an important role in cell–cell contact by junction formation between cells (38–40). PTK2 has been extensively linked with tumor progression, especially in ovarian cancer (41, 42). Shabbir et al. (43) compared genome-wide transcriptome profiling of 1 month, 3 months, and 8 months Hu sheep's ovaries, in which PTK2 was identified as the target gene of screened essential LncRNAs involved in ovarian and follicular development. By developing an oocyte-specific PTK2-knockout mouse model, PTK2 was indispensable in the communication of oocyte and granulosa cells, which has huge implications for male and female gametogenesis. Due to the importance of PTK2 in gametogenesis, it can be inferred that this gene could participate in the ovarian and follicular development of ducks.
Fibroblast Growth Factor 10 (FGF10) is one of the most important members of the Fibroblast Growth Factor family, which participates in many cellular processes (44). The transcription level of FGF10 has been studied in bovine (45), goat (46), and human ovaries (47), based on which FGF10 is one of the regulators of steroidogenesis and follicular development of the ovary. Oron et al. (48) discovered that FGF10 could be involved in the initial follicular development in humans. Moreover, Chaves et al. (46) cultured goat ovaries with FGF10 in vitro and confirmed that FGF10 could stimulate the growth of follicles. Similarly, Du et al. (49) cultured buffalo oocytes with FGF10 in vitro and found that an appropriate concentration of FGF10 was conducive to oocyte maturation and embryo development. In this study, FGF10 is highly expressed in the egg-laying period, substantiating the importance of FGF10 in the egg production of Gaoyou ducks.
Ovary development is a dynamic process that affects follicle formation and egg-laying performance. This study identified eight important pathways and five candidate genes contributing to ovarian development in Gaoyou ducks. These findings lay the foundation for a better understanding of egg production, especially double-yolk egg production in Gaoyou ducks.
The data presented in the study are deposited in the Sequence Read Archive in NCBI, accession number PRJNA940302.
The animal study was reviewed and approved by Experimental Animal Ethics Committee of Jiangsu Agri-animal Husbandry Vocational College (Ethic approval file No. JAHV-2020-58).
JW: conceptualization. GS and LZ: methodology. GS and JX: software. LZ and XL: validation. LZ: formal analysis, data curation, writing—original draft preparation, supervision, and project administration. LZ and RJ: investigation. LZ and JW: resources and funding acquisition. LZ and JX: writing—reviewing and editing. LZ and XZ: visualization. All authors have read and agreed to the published version of the manuscript.
This research was funded by the National Natural Science Foundation of China (grant number 32002157), the Natural Science Foundation for the Jiangsu Higher Education Institutions of China (grant number 20KJD230001), and the Natural Science Foundation of Jiangsu Agri-animal Husbandry Vocational College (grant number NSFZP202101).
The authors would like to thank all the reviewers who participated in the review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1190998/full#supplementary-material
1. Regan S, Knight PG, Yovich JL, Leung Y, Arfuso F, Dharmarajan A, et al. Involvement of Bone Morphogenetic Proteins (BMP) in the Regulation of Ovarian Function. Vitam Horm. (2018) 107:227–61. doi: 10.1016/bs.vh.2018.01.015
2. Hrabia A. Growth hormone production and role in the reproductive system of female chicken. Gen Comp Endocrinol. (2015) 220:112–8. doi: 10.1016/j.ygcen.2014.12.022
3. Deng Y, Huang H, Rong Y, Hu S, Hu J, Hu B, et al. Molecular characterization, expression profile and transcriptional regulation of the CYP19 gene in goose ovarian follicles. Gene. (2022). 806:145928. doi: 10.1016/j.gene.2021.145928
4. Bhavana K, Foote DJ, Srikanth K, Balakrishnan CN, Prabhu VR, Sankaralingam S, et al. Comparative transcriptome analysis of Indian domestic duck reveals candidate genes associated with egg production. Sci Rep. (2022) 12:0. doi: 10.1038/s41598-022-15099-5
5. Zou K, Asiamah CA, Lu L, Liu Y, Pan Y, Chen T, et al. Ovarian transcriptomic analysis and follicular development of Leizhou black duck. Poult Sci. (2020) 99:6173–87. doi: 10.1016/j.psj.2020.08.008
6. Sun Y, Wu Q, Pan J, Li T, Liu L, Chen D, et al. Identification of differentially expressed genes and signalling pathways in the ovary of higher and lower laying ducks. Br Poult Sci. (2020) 61:609–14. doi: 10.1080/00071668.2020.1792834
7. Zhang L, Zhang XY, Zhang JQ, Zhang Y, Chang GB, Chen GH, et al. Differentiation of ovary structure, reproductive hormones and related genes expression between high and low double-yolk egg laying Gaoyou duck. Jiangsu J Agr Sci. (2018) 34:385–9. doi: 10.3969/j.issn.1000-4440.2018.02.023
8. Tang XW, Zhang L, Zhang JQ, Zhang WY, Zhang XY. Differential expression of lncRNA in ovary of double-yolked egg Gaoyou duck. JGansu Agr Uni. (2021) 56:40–9.
9. Zhang TJ, Li HF, Chen KW, Zhao YG, Chang H, Xue MK, et al. Analysis of fitness predominance for Gaoyou duck's double-yolk egg. J Anim Vet. Adv. (2011) 10:367–71. doi: 10.3923/javaa.2011.367.371
10. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. (2014) 30:2114–20. doi: 10.1093/bioinformatics/btu170
11. Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. (2004) 303:1378–81. doi: 10.1126/science.1089769
12. Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. (2008) 36:D480–D484. doi: 10.1093/nar/gkm882
13. Zhang T, Chen L, Han K, Zhang X, Zhang G, Dai G, et al. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim Reprod Sci. (2019) 208:106114. doi: 10.1016/j.anireprosci.2019.106114
14. Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. (2004) 1692:103–19. doi: 10.1016/j.bbamcr.2004.04.007
15. Bello SF, Xu H, Guo L, Li K, Zheng M, Xu Y, et al. Hypothalamic and ovarian transcriptome profiling reveals potential candidate genes in low and high egg production of white Muscovy ducks (Cairina moschata). Poult Sci. (2021) 100:101310. doi: 10.1016/j.psj.2021.101310
16. Wu Y, Zhao X, Chen L, Wang J, Duan Y, Li H, et al. Transcriptomic analyses of the hypothalamic-pituitary-gonadal axis identify candidate genes related to egg production in Xinjiang Yili geese. Animals. (2020) 10:0. doi: 10.3390/ani10010090
17. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. (2002) 12:9–18. doi: 10.1038/sj.cr.7290105
18. Lunding SA, Andersen AN, Hardardottir L, Olesen HØ, Kristensen SG, Andersen CY, et al. Hippo signaling, actin polymerization, and follicle activation in fragmented human ovarian cortex. Mol Reprod Dev. (2020) 87:711–9. doi: 10.1002/mrd.23353
19. Masayuki S, Tatsuya T, Yoshimi N, Naohito A, Norio I, Koyomi M, et al. Protein tyrosine phosphatase PTP20 induces actin cytoskeleton reorganization by dephosphorylating p190 RhoGAP in rat ovarian granulosa cells stimulated with follicle-stimulating hormone. Mol Endocrinol. (2003) 17:534–49. doi: 10.1210/me.2002-0187
20. Cheng Y, Feng Y, Jansson L, Sato Y, Deguchi M, Kawamura K, et al. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB Journal. (2015) 29:2423–30. doi: 10.1096/fj.14-267856
21. Xu Q, Zhao W, Chen Y, Tong Y, Rong G, Huang Z, et al. Transcriptome profiling of the goose (Anser cygnoides) ovaries identify laying and broodiness phenotypes. PLoS ONE. (2013) 8:e55496. doi: 10.1371/journal.pone.0055496
22. Zi XD, Lu J, Ma Y. Identification L, and comparative analysis of the ovarian microRNAs of prolific and non-prolific goats during the follicular phase using high-throughput sequencing. Sci Rep. (2017) 7:1921. doi: 10.1038/s41598-017-02225-x
23. Sun T, Xiao C, Deng J, Yang Z, Zou L, Du W, et al. Transcriptome analysis reveals key genes and pathways associated with egg production in Nandan-Yao domestic chicken. Comp Biochem Physiol. (2021) 40:100889. doi: 10.1016/j.cbd.2021.100889
24. Melhuish TA, Gallo CM, Wotton D. TGIF2 interacts with histone deacetylase 1 and represses transcription. J Biol Chem. (2001) 276:32109–14. doi: 10.1074/jbc.M103377200
25. Wotton D, Lo RS, Lee S, Massague J. A Smad transcriptional corepressor. Cell. (1999) 97:29–39. doi: 10.1016/S0092-8674(00)80712-6
26. Sharma M, Sun Z. 5'TG3' interacting factor interacts with Sin3A and represses AR-mediated transcription. Mol Endocrinol. (2001) 15:1918–28. doi: 10.1210/me.15.11.1918
27. An X, Cao H, Liu S, Cao B. Effects of TG interaction factor 1 on synthesis of estradiol and progesterone in granulosa cells of goats through SMAD2/3-SP1 signaling pathway. Anim Reprod Sci. (2021) 229:106750. doi: 10.1016/j.anireprosci.2021.106750
28. Estermann MA, Hirst CE, Major AT, Smith CA. The homeobox gene TGIF1 is required for chicken ovarian cortical development and generation of the juxtacortical medulla. Development. (2021) 148:0. doi: 10.1242/dev.199646
29. Rosairo D, Kuyznierewicz I, Findlay J, Drummond A. Transforming growth factor-beta: its role in ovarian follicle development. Reproduction. (2008) 136:799–809. doi: 10.1530/REP-08-0310
30. Tsuchida K, Lewis KA, Mathews LS, Vale WW. Molecular characterization of rat transforming growth factor-beta type II receptor. Biochem Biophys Res Commun. (1993) 191:790–5. doi: 10.1006/bbrc.1993.1286
31. Roy SK, Kole AR. Ovarian transforming growth factor-beta (TGF-beta) receptors: in-vitro effects of follicle stimulating hormone, epidermal growth factor and TGF-beta on receptor expression in human preantral follicles. Mol Hum Reprod. (1998) 4:207–14. doi: 10.1093/molehr/4.3.207
32. Li QQ, Zhang JF, Liu HL, Li QF. Cloning QF, and tissue expression profiles of the TGFBR2 gene in pigs. Ani Hus Vet Med. (2016) 48:44–9.
33. Li EL, Xie XH, XU YF, Z X, LI QF. Relationship between the mRNA expression level of TGF-βReceptor genes in tissues and ovulation rate in hu sheep. Agricult Sci China. (2010) 9:1659–66. doi: 10.1016/S1671-2927(09)60263-7
34. Yang J, Zhang Y, Xu X, Li J, Yuan F, Bo S, et al. Transforming growth factor-beta is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. (2019) 10:558. doi: 10.1038/s41419-019-1797-5
35. Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. (2004) 5:875–85. doi: 10.1038/nrm1498
36. Ghousein A, Mosca N, Cartier F, Charpentier J, Dupuy JW, Raymond AA, et al. miR-4510 blocks hepatocellular carcinoma development through RAF1 targeting and RAS/RAF/MEK/ERK signalling inactivation. Liver Int. (2020) 40:240–51. doi: 10.1111/liv.14276
37. Luo X, Liu H, Guo H, Sun L, Gou K, Cui S, et al. RAF1 mediates the FSH signaling pathway as a downstream molecule to stimulate estradiol synthesis and secretion in mouse ovarian granulosa cells. Ann Transl Med. (2022) 10:314. doi: 10.21037/atm-22-393
38. Kim YJ, Sauer C, Testa K, Wahl JK, Svoboda RA, Johnson KR, et al. Modulating the strength of cadherin adhesion: evidence for a novel adhesion complex. J Cell Sci. (2005) 118:3883–94. doi: 10.1242/jcs.02508
39. Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. (2005) 6:56–68. doi: 10.1038/nrm1549
40. Ogita H, Ikeda W, Takai Y. Roles of cell adhesion molecules nectin and nectin-like molecule-5 in the regulation of cell movement and proliferation. J Microsc. (2008) 231:455–65. doi: 10.1111/j.1365-2818.2008.02058.x
41. Ahmadi HM, Khani D, Sheikhesmaeili F. Nouri, ptk2 B, and mt2a genes expression in gastritis and gastric cancer patients with helicobacter pylori infection. Can J Gastroenterol Hepatol. (2022) 2022:8699408. doi: 10.1155/2022/8699408
42. Fisher L. Retraction: Circular RNA PTK2 modifies the progression and radiosensitivity in gastric cancer via miR-369-3p/ZEB1 axis. RSC Adv. (2021) 11:5643. doi: 10.1039/D1RA90040D
43. Shabbir S, Boruah P, Xie L, Kulyar MF, Nawaz M, Yousuf S, et al. Genome-wide transcriptome profiling uncovers differential miRNAs and lncRNAs in ovaries of Hu sheep at different developmental stages. Sci Rep. (2021) 11:5865. doi: 10.1038/s41598-021-85245-y
44. Mishra SR, Thakur N, Somal A, Parmar MS, Reshma R, Rajesh G, et al. Expression and localization of fibroblast growth factor (FGF) family in buffalo ovarian follicle during different stages of development and modulatory role of FGF2 on steroidogenesis and survival of cultured buffalo granulosa cells. Res. Vet. Sci. (2016). 108:98–111. doi: 10.1016/j.rvsc.2016.08.012
45. Gasperin BG, Ferreira R, Rovani MT, Santos JT, Buratini J, Price CA, et al. FGF10 inhibits dominant follicle growth and estradiol secretion in vivo in cattle. Reproduction. (2012) 143:815–23. doi: 10.1530/REP-11-0483
46. Chaves RN, Lima-Verde IB, Celestino JJ, Duarte AB, Alves AM, Matos MH, et al. Fibroblast growth factor-10 maintains the survival and promotes the growth of cultured goat preantral follicles. Domest Anim Endocrinol. (2010) 39:249–58. doi: 10.1016/j.domaniend.2010.06.006
47. Taniguchi F, Harada T, Iwabe T, Ohama Y, Takenaka Y, Terakawa N, et al. Aberrant expression of keratinocyte growth factor receptor in ovarian surface epithelial cells of endometrioma. Fertil Steril. (2008) 89:478–80. doi: 10.1016/j.fertnstert.2007.02.060
48. Oron G, Fisch B, Zhang XY, Gabbay-Benziv R, Kessler-Icekson G, Krissi H, et al. Fibroblast growth factor 10 in human ovaries. Reprod Biomed Online. (2012) 25:396–401. doi: 10.1016/j.rbmo.2012.07.002
49. Nandi S, Ravindranatha BM, Gupta PS, Raghu HM, Sarma. Developmental competence, and post-thaw survivability of buffalo embryos produced in vitro: effect of growth factors in oocyte maturation medium and of embryo culture system. Theriogenology. (2003) 60:1621–31. doi: 10.1016/S0093-691X(03)00148-1
Keywords: Gaoyou duck, transcriptome, ovarian development, egg production, differentially expressed genes
Citation: Zhang L, Xie J, Sun G, Ji R, Li X, Zhang X and Wang J (2023) Identification of differentially expressed genes and signaling pathways in Gaoyou duck ovary at different physiological stages. Front. Vet. Sci. 10:1190998. doi: 10.3389/fvets.2023.1190998
Received: 21 March 2023; Accepted: 03 April 2023;
Published: 03 May 2023.
Edited by:
Chunhe Wan, Fujian Academy of Agricultural Sciences, ChinaReviewed by:
Mingkun Zhu, Jiangsu University of Science and Technology, ChinaCopyright © 2023 Zhang, Xie, Sun, Ji, Li, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, NTY4NDE4NTgzQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.