- 1Department of Parasitology, University of Agriculture, Faisalabad, Pakistan
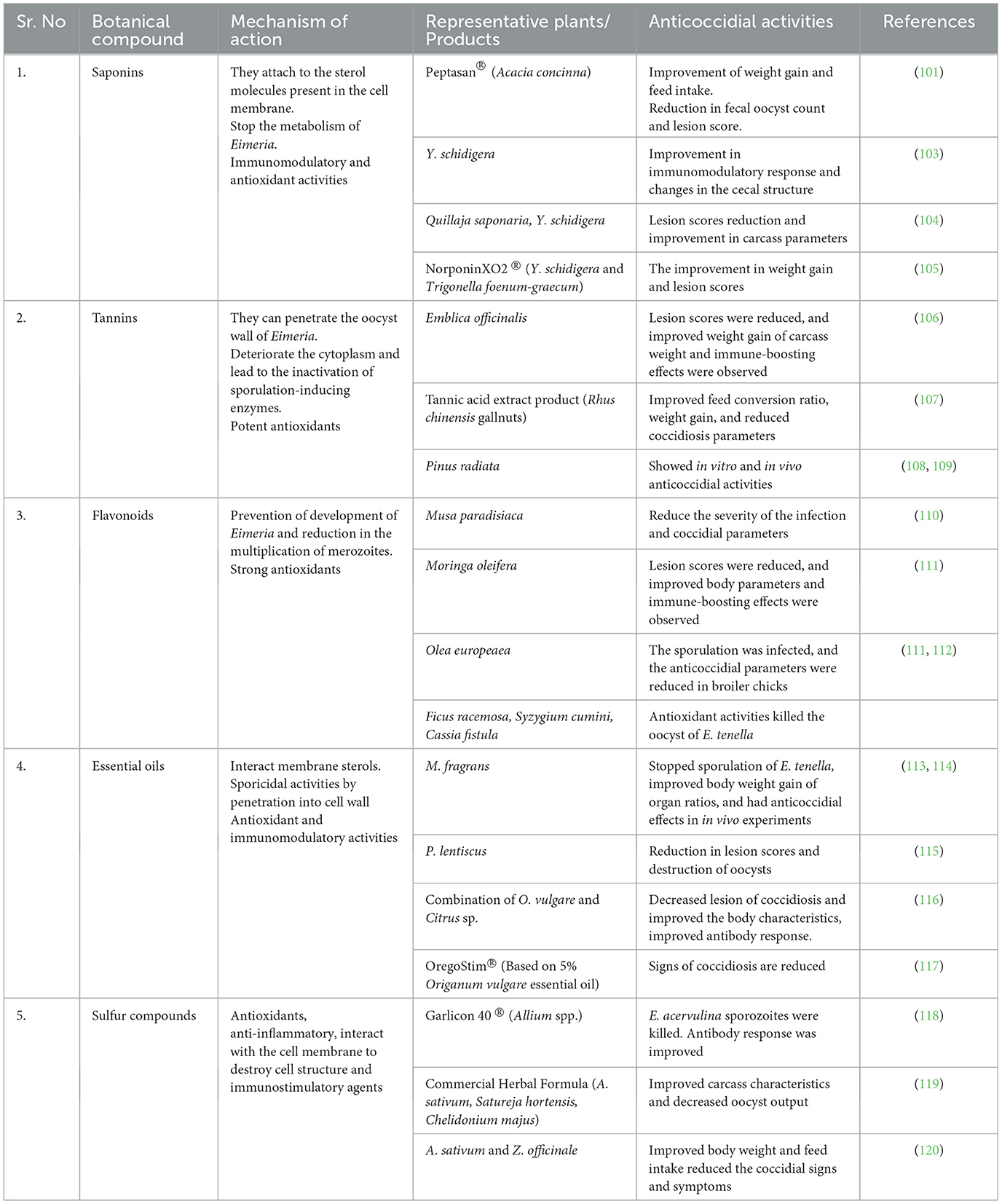
- 2Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia
Avian species have long struggled with the problem of coccidiosis, a disease that affects various parts of the intestine, including the anterior gut, midgut, and hindgut. Among different types of coccidiosis, cecal coccidiosis is particularly dangerous to avian species. Chickens and turkeys are commercial flocks; thus, their parasites have remained critical due to their economic importance. High rates of mortality and morbidity are observed in both chickens and turkeys due to cecal coccidiosis. Coccidiostats and coccidiocidal chemicals have traditionally been added to feed and water to control coccidiosis. However, after the EU banned their use because of issues of resistance and public health, alternative methods are being explored. Vaccines are also being used, but their efficacy and cost-effectiveness remain as challenges. Researchers are attempting to find alternatives, and among the alternatives, botanicals are a promising choice. Botanicals contain multiple active compounds such as phenolics, saponins, terpenes, sulfur compounds, etc., which can kill sporozoites and oocysts and stop the replication of Eimeria. These botanicals are primarily used as anticoccidials due to their antioxidant and immunomodulatory activities. Because of the medicinal properties of botanicals, some commercial products have also been developed. However, further research is needed to confirm their pharmacological effects, mechanisms of action, and methods of concentrated preparation. In this review, an attempt has been made to summarize the plants that have the potential to act as anticoccidials and to explain the mode of action of different compounds found within them.
Introduction
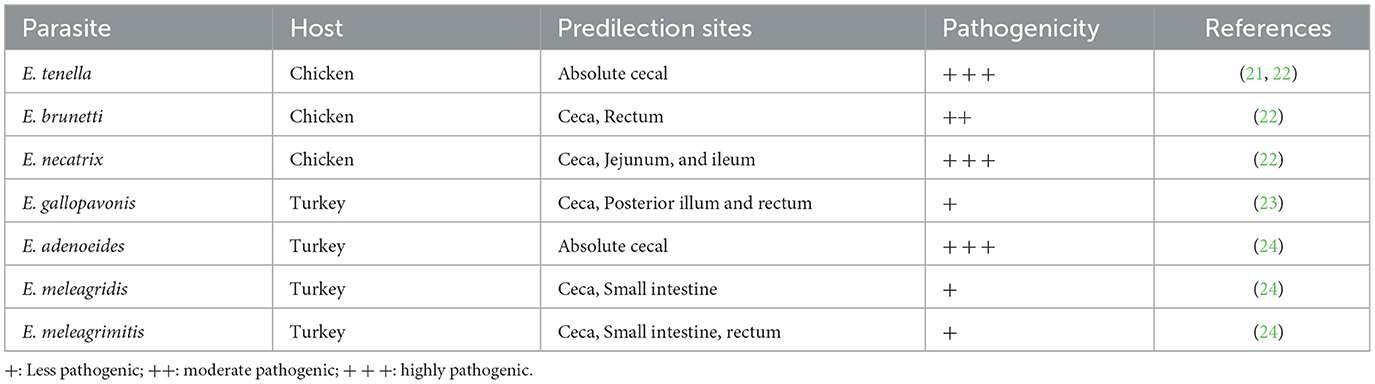
Coccidiosis is a widespread parasitic disease caused by multiple species of protozoan parasites (1, 2). Eimeria spp. are among the most important coccidian parasites, with hundreds of species infecting vertebrates (3, 4). Avian coccidiosis is an absolute intestinal disease characterized by bloody or mucoid diarrhea (5–8). Eimeria species are host- and site-specific, and this specificity is below the organ level, i.e., they infect only the intestine, and within the intestine, they have their reserved regions (9, 10). Various species of Eimeria show symptoms of the disease from the anterior portion of the intestine to the hindgut, depending on their specified predilection sites (11, 12). Cecal coccidiosis is the most dangerous disease among all types of coccidiosis in avian species (13–15). A high and rapid onset of mortality was only observed in cecal coccidiosis (16, 17). The main causative agent for cecal coccidiosis in broiler chickens is Eimeria tenella, which resides in the cecum and causes hemorrhages in it, leading to bloody diarrhea (18–20). Other agents that cause cecal coccidiosis are mentioned in Table 1. This pressing problem highlights the need for scientists to create measures to control coccidiosis, particularly cecal coccidiosis (25, 26).
Currently, multiple drugs are being used in poultry on a daily basis to control coccidiosis (27–29). Due to the acute nature of cecal coccidiosis, preventive measures for controlling this disease are the focus of attention (28–30). Multiple coccidiocidal and coccidiostat drugs are being given in the feed to prevent coccidiosis (31, 32). Anticoccidial drugs target different stages of the Eimeria life cycle, aiming to arrest the parasite at that stage and ultimately control coccidiosis (33).
While chemical anticoccidial drugs have been effective in fighting against coccidiosis, the multiple problems related to this disease have raised doubts about their continued use in the future (34–36).
The primary issue that is being faced with the use of chemical anticoccidials is drug resistance (37–39). Resistance is the ability of the pathogen to escape from the medicine (40, 41). Anticoccidial resistance is a major problem for commercial farmers, as it can result in the wastage of resources and capital on disease control (42, 43). Eimeria are resistant to multiple drugs because of multiple mechanisms (21, 27, 44–47). They have developed genetic modifications (48, 49), altered their metabolism, cell membrane permeability, transport channels, and many other ways to escape the drug interaction, leading to the development of resistance (45, 50, 51). Anticoccidial drugs are becoming less effective in treating coccidiosis due to the emergence of increasingly resistant strains of Eimeria (52–55).
Resistance is not the only issue with anticoccidial drugs. Multiple scientists also have reported public health issues related to anticoccidial drugs (38, 56, 57). The anticoccidial drug metabolites escape the circulatory system and accumulate in various body parts (58–62). These secondary metabolites are transferred to consumers when they consume meat from animals that have been treated with anticoccidial drugs (58, 63). These drug residues cause several problems and may even be carcinogenic or teratogenic (64–66). Anticoccidial drug residues may cause heart, liver, and kidney failure, leading to death and chronic problems in consumers (67–69). Due to these problems, the European Union has banned the routine use of chemical coccidiostats in feed and allowed a limited amount of use only with the veterinarian's prescription (70–72). These issues and the economics of chemical anticoccidial drugs are forcing researchers to investigate alternatives (36, 73).
Commercially, vaccines are one of the most commonly used alternatives for treating cecal coccidiosis (74–77). Anticoccidial vaccines have been developed to prevent various types of coccidiosis (77). Vaccines are being developed to target various stages of the Eimeria and are effective against multiple species of the parasite (56, 78, 79). Anticoccidial vaccines usually use killed parasites or particles of pathogens (80–82). Multiple vaccines, such as Immucox®, Livacox,® Coccivac®, Hatchpack Cocci-III®, Paracox®, etc., are being used in routine farming (17, 56, 83). Anticoccidial vaccines can provide immunity, but some issues limit their use (84–87). Vaccine failure is the primary issue observed with anticoccidial vaccines (30, 85, 88). Vaccine failure results in vaccine-induced coccidiosis (89). Moreover, anticoccidial vaccines provide only temporary protection and require frequent repetition, especially in breeders and layer birds (90, 91). The high cost of anticoccidial vaccines limits their widespread use, making them accessible to only breeding and grandparent flocks of chickens (78). These issues necessitate finding a proper alternative to combat cecal coccidiosis.
Several strategies are being explored for the prevention and control of cecal coccidiosis, including the use of organic acids, amino acids, their derivatives, and so on. Botanical substances are also among these proposed alternatives for controlling coccidiosis, as they are gaining attention from scientists for their antioxidant, anti-inflammatory, immunomodulatory, and anti-infectious properties (92–94). Recently, multiple reviews that summarize botanical products that have been proven to have anticoccidial effects have been published (95–101). These reviews have provided valuable insights into how plants can effectively control various forms of coccidiosis. However, there is a need to further investigate the mechanism of action and pharmacological properties of these botanicals. In this review, we have summarized the effective agents of botanicals and their modes of action, as well as the properties that make them beneficial for use against cecal coccidiosis.
Methodology
This review used Google Scholar (www.scholar.google.com) as the primary search engine. More websites, i.e., ResearchGate (www.researchgate.com) and ScienceDirect (https://www.sciencedirect.com/), and keywords “Cecal coccidiosis;” “Eimeria “tenella” “E. tenella;” “Herbal control of E. tenella;” “Use of essential oils for cecal coccidiosis;” “Use of plant extracts for cecal coccidiosis;” “Botanicals for the control of cecal coccidiosis,” “Plants for the control of cecal coccidiosis” were used. The review articles/secondary data were used as a source for the original articles. The data were not quantified, and the statistical comparison was also not performed (102). Table 2 presents the qualitative effects.
Pathology and methods for controlling cecal coccidiosis
It is necessary to identify the points at which coccidiosis can be effectively controlled before reviewing the plants and plant products used to control cecal coccidiosis. Cecal coccidiosis starts with the ingestion of sporulated oocysts of Eimeria, which then release sporozoites in the stomach (21, 121, 122). Eimeria releases sporozoites. These sporozoites invade the bird's cecum and penetrate the cecal epithelium (17, 123–126). These sporozoites enter epithelial cells and undergo asexual division named merogony/schizogony, resulting in the formation of schizonts inside the cell (127). Cells containing mature schizonts of Eimeria then rupture and release the merozoites (128, 129). These merozoites of Eimeria undergo multiple cycles of schizogony, and then, due to unknown reasons, they mature into male and female gametes (micro- and macrogametes) (130, 131). These gametes fuse to form zygotes that develop into unsporulated oocysts, which are then shed in the bird's feces (132, 133).
The pathogenesis of coccidiosis symptoms arises when the merozoites start infecting the cell and causing the destruction of the epithelium of the cecum (134–136). The epithelium destruction due to schizogony and merozoite invasion induces an immune response (21, 137, 138). This immune response and epithelial destruction result in oxidative stress (139, 140). The reactive oxygen species are produced, and they interact with nearby cells (141–143). These reactive oxygen species extend the damage of coccidiosis to the blood supply to the epithelium, resulting in a blood eruption (144). The repeated cycles of merogony cause extensive damage, leading to bloody diarrhea and death (145). Identifying the different stages of this cycle and their corresponding pathologies provide some points where coccidiosis can be controlled (Figure 1).
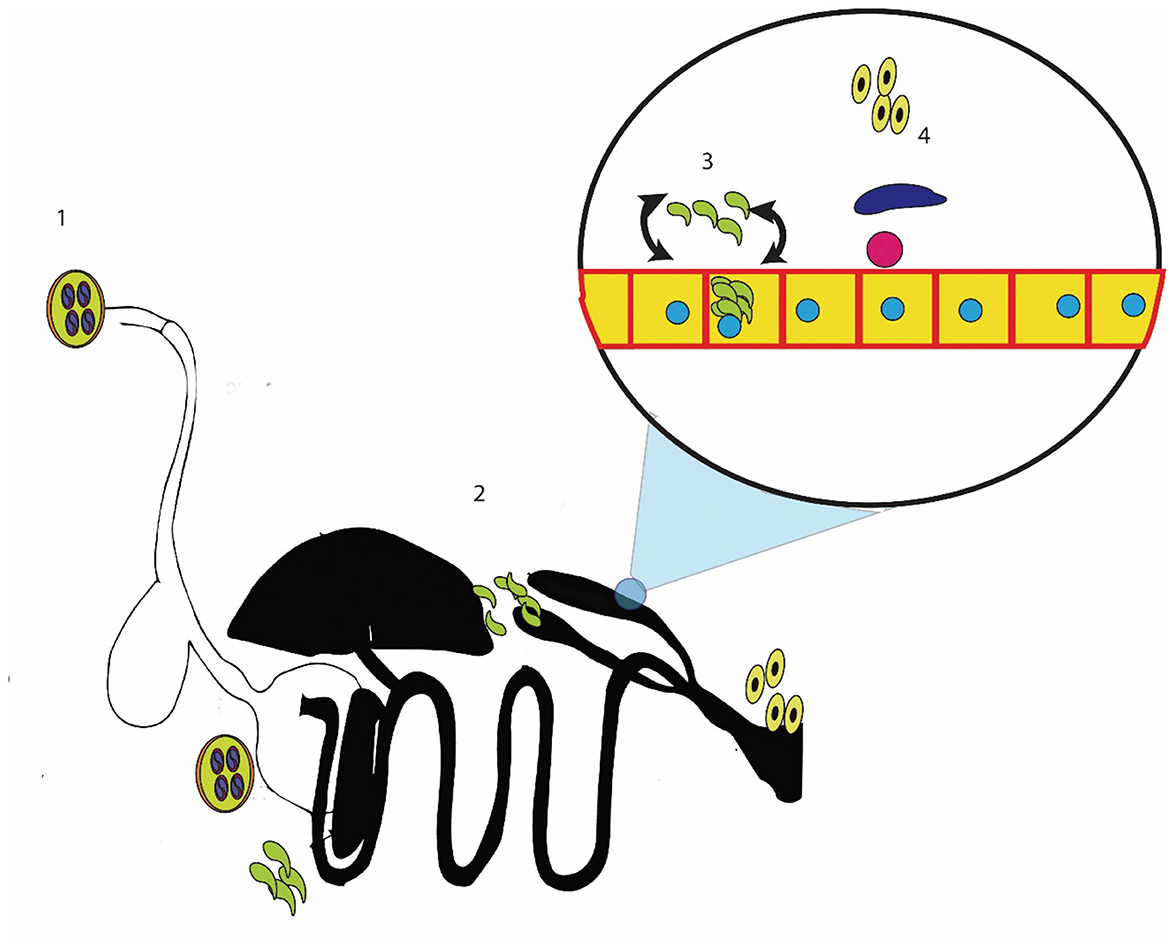
Figure 1. Methods for controlling cecal coccidiosis; 1: killing the sporocyst or reducing the intake of sporocyst; 2: stopping the entry of sporozoites into the cecal epithelium; 3: killing the sporozoites, merozoites, or schizont-containing cells; and 4: controlling the reproductive stage.
First, cecal coccidiosis can be controlled by reducing the intake of sporulated oocysts (21, 146). These sporocysts can be controlled by in vitro sporicidal substances, which may kill the sporocysts or stop the sporulation of oocysts (147). The second method of controlling cecal coccidiosis is by reducing the ingestion of sporozoite/merozoite inside the cell (4, 148). Another important method of controlling cecal coccidiosis is reducing the damage caused by Eimeria. This can be achieved by stopping oxidative stress and inflammation in the cells and supporting the immune system in its efforts to eliminate Eimeria (149, 150). In addition, the number of merogony cycles was reduced and the parasite was forced to enter the sexual phase of the cycle (151, 152). Multiple researchers have explored the use of herbal remedies for controlling cecal coccidiosis, and a few of these remedies have been commercially marketed (119, 153). The active compounds of the plant determine their efficacy in controlling the disease. The active compounds, modes of action, and plants that contain these active ingredients are given in the following sections.
Botanical compounds for controlling cecal coccidiosis
Saponins
Saponins are a group of phytochemicals that are commonly found in many plants (154, 155). They derive their name from their soapy nature, as they can create a foamy texture in aqueous solutions (156–158). Saponins are well known for their antimicrobial, antioxidant, and anticoccidial properties (159–164).
Saponins in botanicals perforate the cell membrane because they interact with cholesterol (158, 165). Cholesterol is the main functional and structural unit of the cell membrane of Eimeria (166, 167). In this way, saponins can kill the sporozoites or merozoites of Eimeria (55, 168–170). Saponins can directly interact with the sporozoite and stop reproduction (171). Saponins have a high antioxidant capacity, i.e., they can control reactive oxygen species and reduce the pathologies associated with oxidative stress, which is highly present in cecal coccidiosis (172–175).
Botanical saponins have an interesting ability to act as an immune booster, thereby making them suitable candidates for use as vaccine adjuvants (176–178). Multiple researchers have reported that plants containing high amounts of saponins can boost the immune system by affecting the immune organ maturation and increasing the antibody levels in the body, providing better defense against cecal coccidiosis (164, 179–182). Plants containing saponins also function as astringents, i.e., they reduce surface tension inside the body and help nutrients enter the cells (183, 184). Due to these activities, multiple saponin-containing plants have been used to control coccidiosis (101, 105, 119, 185–190).
Tannins
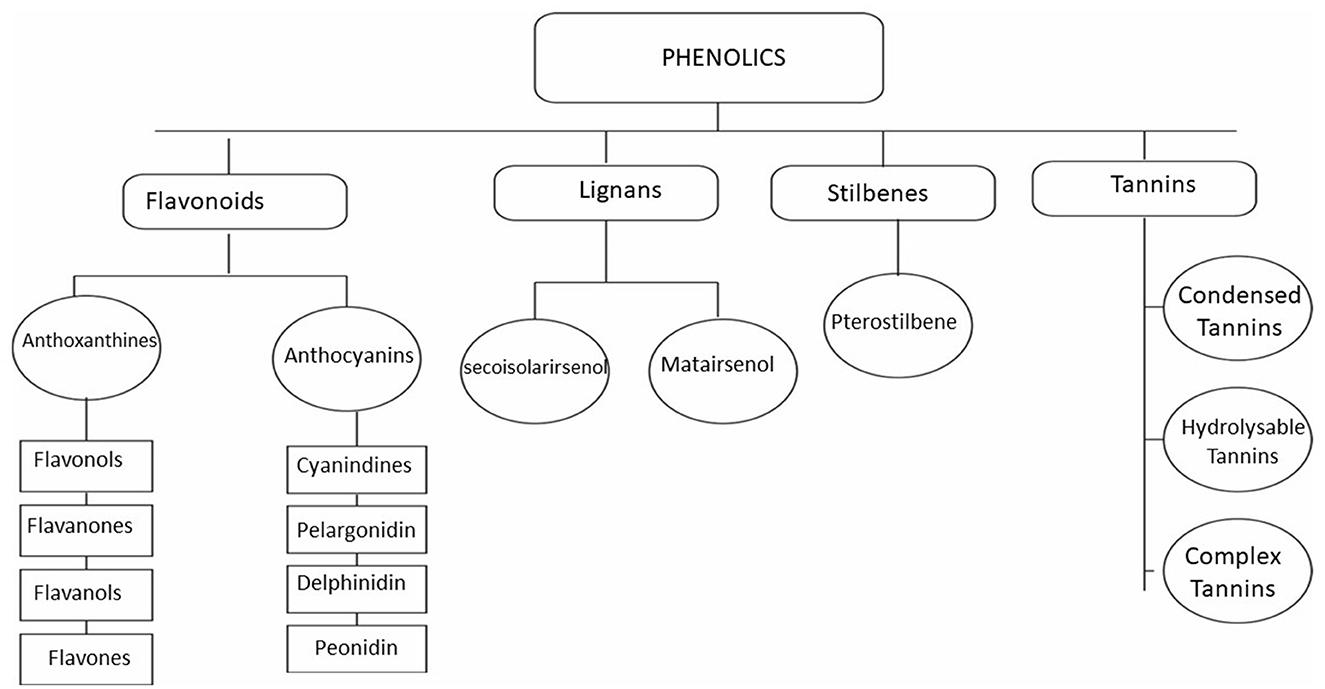
Tannins, or tannic acids, are a ubiquitous class of molecules that occur naturally in plants as defense compounds (191–193). Tannins are classified as phenolic compounds (Figure 2), and they contain various functional groups (194–196). They act as pesticides in plants, protecting them from invading pests and microbes (197, 198). They help regulate plant growth by protecting them from infectious agents (199–203). Tannins can coagulate bacterial cell walls by affecting their peptides (196, 204, 205). Peptide units also make the oocyst wall of Eimeria (206, 207); thus, they perforate the oocyst wall of Eimeria oocysts and denature them (188). Tannins can also protect the epithelium cells from damage and maintain the integrity of nucleic acid during microbial infections (208–210). Tannins also show immunomodulatory activities and potentiate the immune response, helping control infections (211–214). Tannins are also antioxidants and reduce oxidative stress, a condition commonly associated with numerous diseases, especially cecal coccidiosis (21, 215). Numerous tanniferous plants have been used to control coccidiosis (30, 216–222). The efficacy of these plants in controlling cecal coccidiosis can be attributed to their immunomodulatory, antioxidant, and direct anticoccidial activities (187, 222–225).
Flavonoids
Flavonoids are a diverse and broad class of plant phenolic compounds (Figure 2) (226–228) and constitute one of the most prevalent phenolic compounds (229–232). They are further divided into flavonols, flavanols, flavanones, flavones, etc., as described in Figure 2 (233–235). They have multiple modes of action depending on the type and class of flavonoids (236–238). Flavonoids have the potential to control coccidiosis due to their well-known oxidative stress reduction activities (239–241). Flavonoids can also penetrate the cell membranes and cause sporozoite and oocyst death (171, 242). Multiple plants, such as, Moringa oliefera and Syzygium aromaticum, with active flavonoids have anticoccidial activity (170, 188, 240, 243–245).
Essential oils
Essential oils are volatile, short-chain, lipophilic portions of plants that can be extracted using hydro or steam distillation techniques (246–249). They usually contain terpenes, terpene derivatives, aldehydes, and other compounds (250–252). Essential oils are highly active antioxidants and immunomodulators (253–257). These oils can kill the oocyst of Eimeria (15, 258–260) and stop sporulation by penetrating the walls of the Eimeria oocyst (147, 261–263). Essential oils can also help reduce coccidial signs and symptoms because of their direct and indirect anticoccidial activities (264). Essential oils have been widely used to control coccidiosis, especially the cecal kind (15, 116, 117, 259, 262, 265–272).
Vitamins and minerals
Vitamins and minerals are the crucial micronutrients needed by the body to regulate multiple functions and the metabolism of the body (273, 274). Vitamins and minerals are naturally present in plants. They play an important role in many metabolic reactions and help the body maintain growth. Similarly, multiple minerals act as co-factors of the enzymes and have been used to control coccidiosis (259). Vitamins and minerals act as antioxidants and immune stimulators, helping control cecal coccidiosis (275–277). Vitamins and minerals, i.e., vitamin E, selenium (278, 279), vitamin K (280), vitamin A, vitamin D (281, 282), etc., have been shown to be effective in reducing the signs and symptoms of coccidiosis in various multiple experiments.
Sulfur compounds
Sulfur compounds are usually present in the garlic family (Allium spp.) and cannabis (283, 284). Allicin, diallyl disulfide, propyl thiosulfinate oxide, allyl methyl thiosulfate, etc., are commonly found sulfur compounds in plants (285, 286). They have been shown to have high antimicrobial, antioxidant, and anti-aging properties in multiple experiments (287, 288). They have shown multiple bioactivities in many experiments. Sulfur compounds can potentially kill Eimeria by destroying the sporozoites (21). Garlic in multiple forms, i.e., essential oils, extracts, and powders, has been used to treat coccidiosis, and it has shown anticoccidial activity due to these compounds (89, 120, 124, 217, 289, 290).
Pharmacological interactions of botanicals
In a single plant, hundreds of compounds may show multiple activities (291, 292). In multiple experiments, more than one plant was used to control cecal coccidiosis (119, 120, 293). When we added multiple bioactive compounds, there was a possibility of multiple pharmacological interactions occurring among them (294). These interactions may be synergistic, additive, antagonistic, etc. Thus, it is necessary to determine whether these plant products exhibit synergistic or additive effects or if they demonstrate antagonistic properties (295–297). Several experiments have demonstrated that botanical compounds can exhibit additive and synergistic effects against caecal coccidiosis (56, 244, 298). Due to the presence of multiple compounds, plants can employ multiple mechanisms to control Eimeria, thereby reducing the likelihood of drug resistance (27, 299). Although reports suggest that interactions exhibit synergistic or additive effects, further research is needed to explore their potential antagonistic effects.
Conclusion
Studies have shown that plants contain multiple types of botanical compounds that differ in their quantities and ratios. These compounds have demonstrated antioxidant and anticoccidial properties against avian caecal coccidiosis. Phenolics, saponins, essential oils (including terpenes and derivatives), sulfur compounds, etc., have been proven to exhibit anticoccidial effects through diverse mechanisms of action. Moreover, it should be noted that these plant compounds may possess antinutritional properties, which can lead to reduced feed intake, growth inhibition, and adverse effects on body growth. However, studies on their potential toxicity are lacking with respect to coccidiosis. Before considering their therapeutic use, it is imperative to investigate their toxicological profiles. Furthermore, further research is needed to evaluate their pharmacological interactions within the body.
Author contributions
ZS wrote the article and created the illustrations. KA managed the references and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The researchers would like to thank the Deanship of Scientific Research, Qassim University, Saudi Arabia, for funding the publication of this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Matsubayashi M, Takami K, Abe N, Kimata I, Tani H, Sasai K, et al. Molecular characterization of crane coccidia, Eimeria gruis and E. reichenowi, found in feces of migratory cranes. Parasitol Res. (2005) 97:80–3. doi: 10.1007/s00436-005-1404-9
3. Tucker MS, Khan A, Jenkins MC, Dubey JP, Rosenthal BM. Hastening progress in cyclospora requires studying Eimeria surrogates. Microorganisms. (2022) 10:1977. doi: 10.3390/microorganisms10101977
4. Cruz-Bustos T, Feix AS, Ruttkowski B, Joachim A. Sexual development in non-human parasitic apicomplexa: just biology or targets for control? Animals. (2021) 11:2891. doi: 10.3390/ani11102891
5. Imran A, Alsayeqh A. Anticoccidial efficacy of citrus sinensis essential oil in broiler chicken. Pak Vet J. (2022) 42:461–6. doi: 10.29261/pakvetj/2022.082
6. Ahmed AM, AlBakri HS. Phynotypic and genotypic identification of Eimeria species in backyard chicken in Nineveh Governorate, Iraq. Iraqi J Vet Sci. (2021) 35:41–6. doi: 10.33899/ijvs.2021.130487.1834
7. Mohmed RMA. Prevalenceand Risk Factors of Chicken Coccidiosis in Atbara Locality, Sudan (Master's Dissertattion). Sudan University of Science and Technology (2021).
8. Bindari YR, Gerber PF. Centennial review: factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult Sci. (2022) 101:101612. doi: 10.1016/j.psj.2021.101612
9. Kay EE. Host-Parasite Dynamics in the Endangered Yellow-Eyed Penguin (Megadyptes Antipodes): Investigations of Plasmodium and Eimeria in Geographically Distinct Populations. Palmerston North: Massey University (2021).
10. Niu Z, Xue L, Yin X, Shen B. Genetic manipulation toolkits in Apicomplexan parasites. Zoonoses. (2022) 26:27. doi: 10.15212/ZOONOSES-2022-0027
12. López-Osorio S, Chaparro-Gutiérrez JJ, Gómez-Osorio LM. Overview of poultry Eimeria life cycle and host-parasite interactions. Front Vet Sci. (2020) 7:384. doi: 10.3389/fvets.2020.00384
13. Sharaban H, Seadawy M, El-khayat F, El-Gohary AE-G. Evaluation of coccidiosis vaccines in chicken. Kafrelsheikh Vet Med J. (2021) 19:13–9. doi: 10.21608/kvmj.2021.73171.1018
14. Cheng Y, Geng Z, Li Y, Song X, Li L, Wen A, et al. Effects of “Shi Ying Zi” powder and osthole on immune and antioxidant function of E. tenella-infected broilers. Exp Parasitol. (2022) 22:108451. doi: 10.1016/j.exppara.2022.108451
15. Chang L-Y, Di K-Q, Xu J, Chen Y-F, Xi J-Z, Wang D-H, et al. Effect of natural garlic essential oil on chickens with artificially infected E. tenella. Vet Parasitol. (2021) 300:109614. doi: 10.1016/j.vetpar.2021.109614
16. Ismail M. A Case Report on Coccidiosis in Broiler Chicken at Upazila Livestock Office and Veterinary Hospital, Ckakaria, Cox's Bazar. Chattogram: Chattogram Veterinary and Animal Sciences University (2022).
17. McDougald LR, Cervantes HM, Jenkins MC, Hess M, Beckstead R. Protozoal infections. Dis Poultry. (2020) 20:1192–254. doi: 10.1002/9781119371199.ch28
18. Shahid SRA, Shah MA, Riaz A, Malik AM, Hasan MU, Xiangrui L, et al. Identification and molecular characterization of E. tenella based on Etmic5 gene in Pakistan. Pak Vet J. (2020) 40:443–8. doi: 10.29261/pakvetj/2020.063
19. Wang X, Zou W, Yu H, Lin Y, Dai G, Zhang T, et al. RNA sequencing analysis of chicken cecum tissues following E. tenella infection in vivo. Genes. (2019) 10:420. doi: 10.3390/genes10060420
20. Ugwuoke GM, Pewan S. Effect of methanol extract of parkia biglobosa root bark on organ and carcass weight and histopathological changes in E. tenella infected broiler chickens. Anim Res Int. (2020) 17:3587–95.
21. Nahed A, Abd El-Hack ME, Albaqami NM, Khafaga AF, Taha AE, Swelum AA, et al. Phytochemical control of poultry coccidiosis: a review. Poult Sci. (2022) 101:101542. doi: 10.1016/j.psj.2021.101542
22. Yu M, Heo JM. A comprehensive overview of coccidiosis in chicken. Anim Ind Technol. (2021) 8:53–63. doi: 10.5187/ait.2021.8.2.53
23. Hafez HM. Poultry coccidiosis: prevention and control approaches. Archiv Fur Geflugelkunde. (2008) 72:2–7.
24. Chapman HD. Coccidiosis in the Turkey. Avian Pathol. (2008) 37:205–23. doi: 10.1080/03079450802050689
25. Lin X, Mohsin M, Abbas RZ Li L, Chen H, Huang C, et al. Evaluation of immunogenicity and protective efficacy of Eimeria maxima immune mapped protein 1 with eda adjuvant in chicken. Pak Vet J. (2020) 40:209–13. doi: 10.29261/pakvetj/2020.043
26. Craig AD, Khattak F, Hastie P, Bedford MR, Olukosi OA. The similarity of the effect of carbohydrase or prebiotic supplementation in broilers aged 21 days, fed mixed cereal diets and challenged with coccidiosis infection. PLoS ONE. (2020) 15:e0229281. doi: 10.1371/journal.pone.0229281
27. Chapman HD, Rathinam T. Focused review: the role of drug combinations for the control of coccidiosis in commercially reared chickens. Int J Parasitol Drugs Drug Resist. (2022) 18:32–42. doi: 10.1016/j.ijpddr.2022.01.001
28. Attree E, Sanchez-Arsuaga G, Jones M, Xia D, Marugan-Hernandez V, Blake D, et al. Controlling the causative agents of coccidiosis in domestic chickens; an eye on the past and considerations for the future. CABI Agricult Biosci. (2021) 2:1–16. doi: 10.1186/s43170-021-00056-5
29. Jordan B, Albanese G, Tensa L. Coccidiosis in chickens (Gallus Gallus). In: Coccidiosis in Livestock, Poultry, Companion Animals, and Humans. New York, NY: CRC Press (2019). p. 169–73. doi: 10.1201/9780429294105-15
30. Adhikari P, Kiess A, Adhikari R, Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poult Res. (2020) 29:515–34. doi: 10.1016/j.japr.2019.11.005
31. Martins RR, Silva LJG, Pereira AMPT, Esteves A, Duarte SC, Pena A. Coccidiostats and poultry: a comprehensive review and current legislation. Foods. (2022) 11:2738. doi: 10.3390/foods11182738
32. Sharma UNS, Fernando DD, Wijesundara KK, Manawadu A, Pathirana I, Rajapakse RPVJ. Anticoccidial effects of phyllanthus emblica (Indian Gooseberry) extracts: potential for controlling avian coccidiosis. Vet Parasitol Reg Stud Rep. (2021) 25:100592. doi: 10.1016/j.vprsr.2021.100592
33. Adams DS. Novel Methods for Controlling and Diagnosing Avian Coccidiosis (PhD Thesis). North Carolina State University (2021).
34. Mesa-Pineda C, Navarro-Ruíz JL, López-Osorio S, Chaparro-Gutiérrez JJ, Gómez-Osorio LM. Chicken coccidiosis: from the parasite lifecycle to control of the disease. Front Vet Sci. (2021) 8:787653. doi: 10.3389/fvets.2021.787653
35. Blake DP, Worthing K, Jenkins MC. Exploring Eimeria genomes to understand population biology: recent progress and future opportunities. Genes. (2020) 11:1103. doi: 10.3390/genes11091103
36. Selzer PM, Epe C. Antiparasitics in animal health: quo vadis? Trends Parasitol. (2021) 37:77–89. doi: 10.1016/j.pt.2020.09.004
37. Cervantes HM, McDougald LR. The use of anticoccidial sensitivity tests (asts) by the poultry industry. Avian Dis. (2022) 66:1–5. doi: 10.1637/21-00110
38. Owusu-Doubreh B, Appaw WO, Abe-Inge V. Antibiotic residues in poultry eggs and its implications on public health: a review. Sci Afr. (2022) 22:e01456. doi: 10.1016/j.sciaf.2022.e01456
39. Zaheer T, Abbas RZ, Imran M, Abbas A, Butt A, Aslam S, et al. Vaccines against chicken coccidiosis with particular reference to previous decade: progress, challenges, and opportunities. Parasitol Res. (2022) 121:2749–63. doi: 10.1007/s00436-022-07612-6
40. Pham TN, Loupias P, Dassonville-Klimpt A, Sonnet P. Drug delivery systems designed to overcome antimicrobial resistance. Med Res Rev. (2019) 39:2343–96. doi: 10.1002/med.21588
41. Cillóniz C, Dominedò C, Torres A. Multidrug resistant gram-negative bacteria in community-acquired pneumonia. Ann Update Intens Care Emerg Med. (2019) 2019:459–75. doi: 10.1007/978-3-030-06067-1_36
42. Collett SR, Smith JA, Boulianne M, Owen RL, Gingerich E, Singer RS, et al. Principles of disease prevention, diagnosis, and control. Dis Poultry. (2020) 20:1–78. doi: 10.1002/9781119371199.ch1
43. Amare Tadese A. Food Quality and Safety Risks Along the Commercial Broiler Chicken Value Chain in Bishoftu and Addis Ababa, Ethiopia. Addis Ababa: Addis Ababa University (2019).
44. Flores RA, Nguyen BT, Cammayo PLT, Võ TC, Naw H, Kim S, et al. Epidemiological investigation and drug resistance of Eimeria species in Korean chicken farms. BMC Vet Res. (2022) 18:277. doi: 10.1186/s12917-022-03369-3
45. Xie Y, Huang B, Xu L, Zhao Q, Zhu S, Zhao H, et al. Comparative transcriptome analyses of drug-sensitive and drug-resistant strains of E. tenella by RNA-sequencing. J Eukaryotic Microbiol. (2020) 67:406–16. doi: 10.1111/jeu.12790
46. Huang W, Zhu S, Chen T, Zhao Q, Dong H, Huang B, et al. Molecular characterization of glyceraldehyde-3-phosphate dehydrogenase from E. tenella. Parasitol Res. (2022) 121:1749–60. doi: 10.1007/s00436-022-07508-5
47. Vandendoren M. Establishment of an In Vitro Assay for Anticoccidial Drug Efficacy Testing in Eimeria Zuernii. Laramie: University of Wyoming (2020).
48. Tang X, Suo J, Liang L, Duan C, Hu D, Gu X, et al. Genetic modification of the protozoan E. tenella using the crispr/Cas9 system. Vet Res. (2020) 51:1–5. doi: 10.1186/s13567-020-00766-0
49. Mohsin M, Li Y, Zhang X, Wang Y, Huang Z, Yin G, et al. Development of Crispr-Cas9 based RNA drugs against E. tenella infection. Genomics. (2021) 113:4126–35. doi: 10.1016/j.ygeno.2021.10.019
50. Cheng P, Wang C, Zhang L, Fei C, Liu Y, Wang M, et al. Label-free quantitative proteomic analysis of ethanamizuril-resistant vs. -sensitive strains of E. tenella. Parasit Vect. (2022) 15:319. doi: 10.1186/s13071-022-05412-6
51. Nguyen BT, Flores RA, Cammayo PLT, Kim S, Kim WH, Min W. Anticoccidial activity of berberine against Eimeria-infected chickens. Korean J Parasitol. (2021) 59:403. doi: 10.3347/kjp.2021.59.4.403
52. Hsieh M-T, Lai J-M, Su Y-C, Kung W-C, Lee C-H, Weng H-X. Case report: the emergence of anticoccidial-resistant strains of Eimeria in Taiwan. Taiwan Vet J. (2020) 46:45–8. doi: 10.1142/S1682648519720065
53. Madlala T, Okpeku M, Adeleke MA. Understanding the interactions between eimeria infection and gut microbiota, towards the control of chicken coccidiosis: a review. Parasite. (2021) 28:1559. doi: 10.1051/parasite/2021047
54. Yu Y, Dong H, Zhao Q, Zhu S, Liang S, Wang Q, et al. Molecular characterization and analysis of the atpase asna1 homolog gene of E. tenella in a drug sensitive strain and drug resistant strains. Int J Parasitol Drugs Drug Resist. (2021) 15:115–25. doi: 10.1016/j.ijpddr.2021.02.005
55. El-Ghany WAA. Intervention strategies for controlling poultry coccidiosis: current knowledge. J World's Poult Res. (2021) 11:487–505. doi: 10.36380/jwpr.2021.58
56. Khater HF, Ziam H, Abbas A, Abbas RZ, Raza MA, Hussain K, et al. Avian coccidiosis: recent advances in alternative control strategies and vaccine development. Agrobiol Rec. (2020) 1:11–25. doi: 10.47278/journal.abr/2020.003
57. Mohsin M, Abbas RZ, Yin G, Sindhu Z-U-D, Abbas A, Huang Z, et al. Probiotics as therapeutic, antioxidant and immunomodulatory agents against poultry coccidiosis. World's Poult Sci J. (2021) 77:331–45. doi: 10.1080/00439339.2021.1883412
58. Rana MS, Lee SY, Kang HJ, Hur SJ. Reducing veterinary drug residues in animal products: a review. Food Sci Anim Resour. (2019) 39:687. doi: 10.5851/kosfa.2019.e65
59. Feddern V, Scheuermann GN, Coldebella A, Gressler V, Bedendo GC, Caron L, et al. Nicarbazin residue in tissues from broilers reared on reused litter conditions. Animals. (2022) 12:3107. doi: 10.3390/ani12223107
60. Said AA, El-Nabtity SM, El-Aziz AMA, Elassal EI. Residues of anticoccidial drug (Diclazuril) in different broiler tissues by high performance liquid chromatography. Adv Anim Vet Sci. (2019) 7:19–25. doi: 10.17582/journal.aavs/2019/7.s2.19.25
61. Kumar-Sharma H, Joseph M. Effect of deep frying on furazolidone anticoccidial drug residues in liver and muscle tissues of chicken. Afr J Agricult Food Sec. (2019) 7:001–5.
62. Mooney D, Coxon C, Richards KG, Gill LW, Mellander PE, Danaher M, et al. new sensitive method for the simultaneous chromatographic separation and tandem mass spectrometry detection of anticoccidials, including highly polar compounds, in environmental waters. J Chromatogr A. (2020) 1618:460857. doi: 10.1016/j.chroma.2020.460857
63. Kadykalo S, Roberts T, Thompson M, Wilson J, Lang M, Espeisse O. The value of anticoccidials for sustainable global poultry production. Int J Antimicrob Agents. (2018) 51:304–10. doi: 10.1016/j.ijantimicag.2017.09.004
64. Beyene T. Veterinary drug residues in food-animal products: its risk factors and potential effects on public health. J Vet Sci Technol. (2016) 7:1–7.
65. Darwish WS, Eldaly EA, El-Abbasy MT, Ikenaka Y, Nakayama S, Ishizuka M. Antibiotic residues in food: the African scenario. Jpn J Vet Res. (2013) 61 (Supplement):S13–22.
66. Khan AH, Aziz HA, Khan NA, Hasan MA, Ahmed S, Farooqi IH, et al. Impact, disease outbreak and the eco-hazards associated with pharmaceutical residues: a critical review. Int J Environ Sci Technol. (2021) 14:1–12. doi: 10.1007/s13762-021-03158-9
67. Zhang K, Wang X, Wang M, Liu Y, Zhang L, Wang C, et al. Rat 90-day oral toxicity study of a novel coccidiostat–ethanamizuril. Regulat Toxicol Pharmacol. (2020) 111:104550. doi: 10.1016/j.yrtph.2019.104550
68. Ekinci IB, Chłodowska A, Olejnik M. Ionophore toxicity in animals: a review of clinical and molecular aspects. Int J Mol Sci. (2023) 24:1696. doi: 10.3390/ijms24021696
69. KoŽárová I, Juščáková D, Šimková J, Milkovičová M, KoŽár M. Effective screening of antibiotic and coccidiostat residues in food of animal origin by reliable broad-spectrum residue screening tests. Ital J Anim Sci. (2020) 19:487–501. doi: 10.1080/1828051X.2020.1761270
70. Nunan C. Ending routine farm antibiotic use in Europe. In: Achieving Responsible Farm Antibiotic Use through Improving Animal Health and Welfare in Pig and Poultry Production. Belgium: European Public Health Alliance (2022).
71. Rybicki MJ. Coccidiostats in treating coccidiosis. ZYWNOSC Nauka Technologia Jakość. (2020) 27:125. doi: 10.15193/zntj/2020/125/364
72. Dasenaki ME, Thomaidis NS. Multi-residue methodology for the determination of 16 coccidiostats in animal tissues and eggs by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Food Chem. (2019) 275:668–80. doi: 10.1016/j.foodchem.2018.09.138
73. Felici M, Tugnoli B, Piva A, Grilli E. In vitro assessment of anticoccidials: methods and molecules. Animals. (2021) 11:1962. doi: 10.3390/ani11071962
74. Lee Y, Lu M, Lillehoj HS. Coccidiosis: recent progress in host immunity and alternatives to antibiotic strategies. Vaccines. (2022) 10:215. doi: 10.3390/vaccines10020215
75. Soutter F, Werling D, Tomley FM, Blake DP. Poultry coccidiosis: design and interpretation of vaccine studies. Front Vet Sci. (2020) 7:101. doi: 10.3389/fvets.2020.00101
76. Savary RK, Fiss TA, Abbott DA, Nicholds JA, Van Kessel AG, Classen HL. Development of a coccidiosis disease challenge model using a commercially available live oocyst vaccine. Avian Dis. (2021) 65:149–58. doi: 10.1637/aviandiseases-D-20-00105
77. Akanbi OB, Taiwo VO, Ola-Fadunsin SD. Immunisation of chickens with commercial anticoccidial vaccines immucox and livacox showed varied protection against virulent local isolate of E. tenella and houghton strain. Bulgarian J Vet Med. (2021) 10:2021–0045.
78. Venkatas J, Adeleke MA. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol Res. (2019) 118:1701–10. doi: 10.1007/s00436-019-06338-2
79. Zhang S, Tang X, Wang S, Shi F, Duan C, Bi F, et al. Establishment of recombinant Eimeria acervulina expressing multi-copies M2e derived from avian influenza virus H9n2. Vaccines. (2021) 9:791. doi: 10.3390/vaccines9070791
80. Dung HT. Study on the Immune Responses in the Chickens with Eimeria Parasite Infection or Lactic Acid Bacteria Administration: Toward the Application of Lactobacillus Acidophilus L-55 as an Anticoccidial Agent. Okayama: Okayama University (2020).
81. Soutter F, Werling D, Nolan M, Küster T, Attree E, Marugán-Hernández V, et al. A novel whole yeast-based subunit oral vaccine against E. tenella in chickens. Front Immunol. (2022) 13:809711. doi: 10.3389/fimmu.2022.809711
82. Florin-Christensen M, Schnittger L, Bastos RG, Rathinasamy VA, Cooke BM, Alzan HF, et al. Pursuing effective vaccines against cattle diseases caused by apicomplexan protozoa. CABI Rev. (2021) 27:24. doi: 10.1079/PAVSNNR202116024
83. Cai H, Qi N, Li J, Lv M, Lin X, Hu J, et al. Research progress of the avian coccidiosis vaccine. Vet Vacc. (2022) 65:100002. doi: 10.1016/j.vetvac.2022.100002
84. Juárez-Estrada MA, Gayosso-Vázquez A, Tellez-Isaias G, Alonso-Morales RA. Protective immunity induced by an E. tenella whole sporozoite vaccine elicits specific B-cell antigens. Animals. (2021) 11:1344. doi: 10.3390/ani11051344
85. Wang S, Suo X. Still naïve or primed: anticoccidial vaccines call for memory. Exp Parasitol. (2020) 216:107945. doi: 10.1016/j.exppara.2020.107945
86. Nasri T, Sangmaneedet S, Nam NH, Worawong K, Taweenan W, Sukon P. Protective efficacy of new-generation anticoccidial vaccine candidates against Eimeria infection in chickens: a meta-analysis of challenge trials. Vet Parasitol. (2022) 306:109724. doi: 10.1016/j.vetpar.2022.109724
87. Wang X, Peebles ED, Kiess AS, Wamsley KGS, Zhai W. Effects of coccidial vaccination and dietary antimicrobial alternatives on the growth performance, internal organ development, and intestinal morphology of Eimeria-challenged male broilers. Poult Sci. (2019) 98:2054–65. doi: 10.3382/ps/pey552
88. Eckert J, Carrisosa M, Hauck R. Network meta-analysis comparing the effectiveness of anticoccidial drugs and anticoccidial vaccination in broiler chickens. Vet Parasitol. (2021) 291:109387. doi: 10.1016/j.vetpar.2021.109387
89. Jeon Y-S, Kim Y-B, Lee H-G, Park J, Heo Y-J, Chu G-M, et al. Effect of dietary organic and inorganic sulfur on the performance of coccidiosis vaccine challenged broiler chickens. Animals. (2022) 12:1200. doi: 10.3390/ani12091200
90. Rasheed MSA, Matsler PL. Assessment of protection against E. tenella in broiler breeders conferred by a live anticoccidial vaccine and effects of vaccination on early pullet growth. J Appl Poult Res. (2020) 29:447–54. doi: 10.1016/j.japr.2020.02.002
91. Arczewska-Włosek A, Swiatkiewicz S, Ognik K, Józefiak D. Effects of a dietary multi-strain probiotic and vaccination with a live anticoccidial vaccine on growth performance and haematological, biochemical and redox status indicators of broiler chickens. Animals. (2022) 12:3489. doi: 10.3390/ani12243489
92. Hegazy MM, Mostafa RM, El-Sayed YA, Baz MM, Khater HF, Selim A, et al. The efficacy of saussurea costus extracts against hematophagous arthropods of camel and cattle. Pak Vet J. (2022) 42:547–53.
93. Al-Saeed FA, Bamarni SS, Iqbal KJ, ur Rehman T, Zaman A, Faruk SM, et al. In vitro anthelmintic efficacy of haloxylon salicornicum leaves extract using adult heamonchus contortus worms. Pak Vet J. (2023) 15:91.
94. Naseer MU, Iqbal Z, Aslam B. In vitro efficacy of areca catechu against cypermethrin resistant rhipicephalus microplus and its phytochemical analysis. Pak Vet J. (2022) 42:1559.
95. Abbas RZ, Colwell DD, Gilleard J. Botanicals: an alternative approach for the control of avian coccidiosis. World's Poult Sci J. (2012) 68:203–15. doi: 10.1017/S0043933912000268
96. Abbas R, Iqbal Z, Mansoor M, Sindhu Z, Zia MA, Khan JA. Role of natural antioxidants for the control of coccidiosis in poultry. Pak Vet J. (2013) 33:401–7.
97. Rizwan HM, Khan MK, Mughal MAS, Abbas Z, Abbas RZ, Sindhu ZD, et al. A New insight in immunomodulatory impact of botanicals in treating avian coccidiosis. J Parasit Dis. (2022) 46:1164–75. doi: 10.1007/s12639-022-01519-w
98. Kostadinović L, Puvača N, Popović S, Lević J. Botanical supplements as anticoccidial alternatives in poultry nutrition. World's Poult Sci J. (2015) 71:27–36. doi: 10.1017/S0043933915000033
99. Idris M, Abbas RZ, Masood S, Rehman T, Farooq U, Babar W, et al. The potential of antioxidant rich essential oils against avian coccidiosis. World's Poult Sci J. (2017) 73:89–104. doi: 10.1017/S0043933916000787
100. Muthamilselvan T, Kuo T-F, Wu Y-C, Yang W-C. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid Based Compl Alternat Med eCAM. (2016) 2016:16677. doi: 10.1155/2016/2657981
101. Sánchez-Hernández C, Castañeda-Gómez del Campo JA, Trejo-Castro L, Mendoza-Martínez GD, Gloria-Trujillo A. Evaluation of a feed plant additive for coocidiosis control in broilers herbals for coccidiosis control Brazil. J Poult Sci. (2019) 21:19. doi: 10.1590/1806-9061-2018-0846
102. Adjei-Mensah B, Atuahene CC. Avian coccidiosis and anticoccidial potential of garlic (Allium sativum L.) in broiler production: a review. J Appl Poult Res. (2022) 15:100314. doi: 10.1016/j.japr.2022.100314
103. Oelschlager ML, Rasheed MSA, Smith BN, Rincker MJ, Dilger RN. Effects of Yucca Schidigera-Derived Saponin Supplementation During a Mixed Eimeria Challenge in Broilers. Poult Sci. (2019) 98:3212–22. doi: 10.3382/ps/pez051
104. Bafundo KW, Johnson AB, Mathis GF. The effects of a combination of quillaja saponaria and yucca schidigera on Eimeria Spp. in broiler chickens. Avian Dis. (2020) 64:300–4. doi: 10.1637/aviandiseases-D-20-00016
105. El Amine Benarbia M, Gaignon P, Manoli C, Chicoteau P. Saponin-rich plant premixture supplementation is as efficient as ionophore monensin supplementation under experimental Eimeria Spp. challenge in broiler chicken. Front Vet Sci. (2022) 9:371. doi: 10.3389/fvets.2022.946576
106. Kaleem QM, Akhtar M, Awais MM, Saleem M, Zafar M, Iqbal Z, et al. Studies on emblica officinalis derived tannins for their immunostimulatory and protective activities against coccidiosis in industrial broiler chickens. Sci World J. (2014) 2014:58. doi: 10.1155/2014/378473
107. Tonda RM, Rubach JK, Lumpkins BS, Mathis GF, Poss MJ. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poult Sci. (2018) 97:3031–42. doi: 10.3382/ps/pey158
108. Dkhil MA. Anticoccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol Res. (2013) 112:2639–46. doi: 10.1007/s00436-013-3430-3
109. Ahad S, Tanveer S, Malik TA, Nawchoo IA. Anticoccidial activity of fruit peel of P. granatum L. Microb Pathogen. (2018) 116:78–83. doi: 10.1016/j.micpath.2018.01.015
110. Anosa GN, Okoro OJ. Anticoccidial activity of the methanolic extract of musa paradisiaca root in chickens. Trop Anim Health Prod. (2011) 43:245–8. doi: 10.1007/s11250-010-9684-1
111. Mgbojikwe AC, Samuel KV, Olanihan AO, Okeke-Agulu KI, Okpara JO. The evaluation of the anticoccidial properties of aqueous leaf extract of moringa oleifera. Food Environ Saf J. (2022) 20:639. doi: 10.4316/fens.2021.040
112. Debbou-Iouknane N, Nerín C, Amrane-Abider M, Ayad A. In vitro anticoccidial effects of olive leaf (Olea europaea L. Var Chemlal) extract against broiler chickens Eimeria oocysts. Veterinarija ir Zootechnika. (2021) 79:1–8.
113. Sapsuha Y, Suprijatna E, Kismiati S, Sugiharto S. The effect of nutmeg flesh (Myristica fragrans Houtt) extract on growth performance, internal organ and carcass of broiler chickens raised at high stocking density. Livestock Res Rural Dev. (2021) 33:1–7.
114. Thabet A, Alzuheir I, Alnassan AA, Daugschies A, Bangoura B. In vitro activity of selected natural products against E. tenella sporozoites using reproduction inhibition assay. Parasitol Res. (2022) 36:1–10. doi: 10.1007/s00436-021-07360-z
115. Rahmani A, Ahmed Laloui H, Zaak H, Selmania A, Oufroukh K, Chareb N, et al. Effect of Pistacia lentiscus L. vegetable oil on growth performance and coccidiosis in broiler chickens: in vitro and in vivo assessment. Acta Parasitol. (2021) 66:1151–7. doi: 10.1007/s11686-021-00365-9
116. Gordillo Jaramillo FX, Kim D-H, Lee SH, Kwon S-K, Jha R, Lee K-W. Role of oregano and citrus species-based essential oil preparation for the control of coccidiosis in broiler chickens. J Anim Sci Biotechnol. (2021) 12:1–9. doi: 10.1186/s40104-021-00569-z
117. Saini R, Davis S, Dudley-Cash W. Oregano essential oil reduces the expression of coccidiosis in broilers. In: Proceedings of the Fifty-Second Western Poultry Disease Conference. Sacramento, CA: American Association of Avian Pathologists (2003). p. 97
118. Kim DK, Lillehoj HS, Lee SH, Lillehoj EP, Bravo D. Improved resistance to E. acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br J Nutr. (2013) 109:76–88. doi: 10.1017/S0007114512000530
119. Pop LM, Varga E, Coroian M, Nediṣan ME, Mircean V, Dumitrache MO, et al. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasit Vect. (2019) 12:1–9. doi: 10.1186/s13071-019-3595-4
120. Ali M, Chand N, Khan RU, Naz S, Gul S. Anticoccidial effect of garlic (A. sativum) and ginger (Z. officinale) against experimentally induced coccidiosis in broiler chickens. J Appl Anim Res. (2019) 47:79–84. doi: 10.1080/09712119.2019.1573731
121. Kim WH, Chaudhari AA, Lillehoj HS. Involvement of T cell immunity in avian coccidiosis. Front Immunol. (2019) 10:2732. doi: 10.3389/fimmu.2019.02732
122. Gong Y, Liu X, Zhang S, Tang X, Zou J, Suo X. Antibiotic changes host susceptibility to Eimeria falciformis infection associated with alteration of gut microbiota. Infect Immun. (2022) 90:e00229–22. doi: 10.1128/iai.00229-22
123. Sandholt AKS, Wattrang E, Lilja T, Ahola H, Lundén A, Troell K, et al. Dual RNA-Seq transcriptome analysis of caecal tissue during primary E. tenella infection in chickens. BMC Genom. (2021) 22:1–19. doi: 10.1186/s12864-021-07959-7
124. Abd-Elrahman SM, Mohamed SA-A, Mohamed SE, El-Khadragy MF, Dyab AK, Hamad N, et al. Comparative effect of allicin and alcoholic garlic extract on the morphology and infectivity of E. tenella oocysts in chickens. Animals. (2022) 12:3185. doi: 10.3390/ani12223185
125. Ma D, Huang Y, Ma C, Zhang L, Wang J, Wang D, et al. Eimeria tenella: specific etama1-binding peptides inhibit sporozoite entry into host cells. Poult Sci. (2019) 98:4480–91. doi: 10.3382/ps/pez298
126. El-Sherry S, Ogedengbe ME, Hafeez MA, Sayf-Al-Din M, Gad N, Barta JR. Cecal coccidiosis in turkeys: comparative biology of Eimeria species in the lower intestinal tract of turkeys using genetically typed, single oocyst–derived lines. Parasitol Res. (2019) 118:583–98. doi: 10.1007/s00436-018-6147-5
127. Current WL, Upton SJ, Long PL. Taxonomy and life cycles. In: Coccidiosis of Man and Domestic Animals. New York, NY: CRC Press (2019). p. 1–16. doi: 10.1201/9781351070744-1
128. Fayer R, Hammond DM. Development of first-generation schizonts of Eimeria bovis in cultured bovine cells. J Protozool. (1967) 14:764–72.
129. Marugan-Hernandez V, Jeremiah G, Aguiar-Martins K, Burrell A, Vaughan S, Xia D, et al. The growth of E. tenella: characterization and application of quantitative methods to assess sporozoite invasion and endogenous development in cell culture. Front Cell Infect Microbiol. (2020) 10:579833. doi: 10.3389/fcimb.2020.579833
130. Dubey JP, Lindsay DS, Jenkins MC, Bauer C. Biology of intestinal coccidia. In: Coccidiosis in Livestock, Poultry, Companion Animals, and Humans. New York, NY: CRC Press (2019). p. 1–36. doi: 10.1201/9780429294105
131. Ribeiro E. Silva A, Sausset A, Bussière FI, Laurent F, Lacroix-Lamandé S, Silvestre A. Genome-wide expression patterns of rhoptry kinases during the E. tenella life-cycle. Microorganisms. (2021) 9:1621. doi: 10.3390/microorganisms9081621
132. Gondipon R, Malaka R. Overview of coccidiosis in sheep: history of disease incidence in the world and life cycle. Hasanuddin J Anim Sci. (2021) 3:42–51. doi: 10.20956/hajas.v3i1.17958
133. Jeninga MD, Quinn JE, Petter M. Apiap2 transcription factors in apicomplexan parasites. Pathogens. (2019) 8:47. doi: 10.3390/pathogens8020047
134. Gregory MW. Pathology of coccidial infections. In: Coccidiosis of Man and Domestic Animals. New york, NY: CRC Press (2019). p. 235–62. doi: 10.1201/9781351070744-12
135. Klobucher KN, Badger R, Foxall T, Erickson PS. Effect of sodium butyrate, monensin, and butyric acid on the viability of Eimeria bovis sporozoites and their degree of damage to a bovine epithelial cell line. J Anim Sci. (2022) 100:skac360. doi: 10.1093/jas/skac360
136. Winaya IBO, Berata IK, Dwinata IM, Merdana IM. Coccidiosis and hemorrhagic necrotic typhlitis in broilers fed without antibiotic growth promoter. J Adv Vet Res. (2022) 12:509–12.
137. Ryan N, Anderson K, Volpedo G, Varikuti S, Satoskar M, Satoskar S, et al. The Il-33/St2 axis in immune responses against parasitic disease: potential therapeutic applications. Front Cell Infect Microbiol. (2020) 10:153. doi: 10.3389/fcimb.2020.00153
138. Xander P, Cronemberger-Andrade A, Torrecilhas AC. Extracellular vesicles in parasitic disease. In: Exosomes. Amsterdam: Elsevier (2020). p. 179–98. doi: 10.1016/B978-0-12-816053-4.00008-0
139. Mishra B, Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front Vet Sci. (2019) 6:60. doi: 10.3389/fvets.2019.00060
140. Liang D, Zhuo Y, Guo Z, He L, Wang X, He Y, et al. Sirt1/Pgc-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie. (2020) 170:10–20. doi: 10.1016/j.biochi.2019.12.001
141. Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer. (2019) 18:1–10. doi: 10.1186/s12943-019-0961-y
142. Murphy MP, Bayir H, Belousov V, Chang CJ, Davies KJA, Davies MJ, et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metabol. (2022) 4:651–62. doi: 10.1038/s42255-022-00591-z
143. Onyiche TE, Gotep JG, Tanko JT, Ochigbo GO, Ozoani HA, Viyoff VZ, et al. Azadirachta Indica aqueous leaf extracts ameliorates coccidiosis in broiler chickens experimentally infected with Eimeria oocysts. Sci Afr. (2021) 13:e00851. doi: 10.1016/j.sciaf.2021.e00851
144. Mohamed ERA, Elazab MF, El-Habashi N, Elhawary N, Mokhbatly AA. Anticoccidial effect of origanum majoranum aqueous extract on E. tenella-infected chicken. Trop Biomed. (2021) 38:62–72. doi: 10.47665/tb.38.1.011
145. Abdisa T, Hasen R, Tagesu T, Regea G, Tadese G. Poultry coccidiosis and its prevention. Control J Vet Anim Res. (2019) 2:103.
146. El-Ashram SA, Aboelhadid SM, Gadelhaq SM, Arafa WM, Abdel-Razik A-RH, Abohamra S, et al. A oral inoculation of ultraviolet-irradiated Eimeria species oocysts protects chickens against coccidiosis. Parasitol Res. (2019) 118:3173–83. doi: 10.1007/s00436-019-06455-y
147. Kasem SM, Helal IB, Mira NM, Amer S. Evaluating the in vitro efficiency of rosmarinus officinalis extracts formalin and sodium hypochlorite on sporulation of E. tenella oocysts. Jokull J. (2019) 69:36–54.
148. Mequanent A. General veterinary pharmacology and drugs used for treatment of bacteria, virus, fungus and parasites in animals. Life Sci J. (2022) 19:45.
149. Dalgaard TS, Rebel JMJ, Bortoluzzi C, Kogut MH. Factors modulating the avian immune system. Avian Immunol. (2022) 22:419–35. doi: 10.1016/B978-0-12-818708-1.00004-X
150. Khatlab AS, Del Vesco AP, de Oliveira Neto AR, Fernandes RPM, Gasparino E. Dietary supplementation with free methionine or methionine dipeptide mitigates intestinal oxidative stress induced by Eimeria Spp. challenge in broiler chickens. J Anim Sci Biotechnol. (2019) 10:1–17. doi: 10.1186/s40104-019-0353-6
151. Speer CA. The coccidia. In: In Vitro Cultivation of Protozoan Parasites. New York, NY: CRC Press (2019). p. 1–64. doi: 10.1201/9781351073455-1
152. Xie Y, Xiao J, Zhou X, Gu X, He R, Xu J, et al. Global transcriptome landscape of the rabbit protozoan parasite Eimeria stiedae. Parasit Vect. (2021) 14:1–17. doi: 10.1186/s13071-021-04811-5
153. Song X, Li Y, Chen S, Jia R, Huang Y, Zou Y, et al. Anticoccidial effect of herbal powder “Shi Ying Zi” in chickens infected with E. tenella. Animals. (2020) 10:1484. doi: 10.3390/ani10091484
154. Agidew MG. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull Natl Res Centre. (2022) 46:1–22. doi: 10.1186/s42269-022-00770-8
155. Chen C, Zhu H, Kang J, Warusawitharana HK, Chen S, Wang K, et al. Comparative transcriptome and phytochemical analysis provides insight into triterpene saponin biosynthesis in seeds and flowers of the tea plant (Camellia sinensis). Metabolites. (2022) 12:204. doi: 10.3390/metabo12030204
156. Rai S, Acharya-Siwakoti E, Kafle A, Devkota HP, Bhattarai A. Plant-derived saponins: a review of their surfactant properties and applications. Science. (2021) 3:44. doi: 10.3390/sci3040044
157. Sochacki M, Vogt O. Triterpenoid saponins from washnut (Sapindus mukorossi Gaertn)—a source of natural surfactants and other active components. Plants. (2022) 11:2355. doi: 10.3390/plants11182355
158. Liao Y, Li Z, Zhou Q, Sheng M, Qu Q, Shi Y, et al. Saponin surfactants used in drug delivery systems: a new application for natural medicine components. Int J Pharmaceut. (2021) 603:120709. doi: 10.1016/j.ijpharm.2021.120709
159. Youssef IMI, Abdel-Razik AH, Aboelhadid SM, Arafa WM, Shany SA, Abdel-Daim ASA. Comparative effects of dietary saponin and probiotic supplementation on performance, carcass traits and intestinal histomorphology of broilers challenged with E. tenella. Iran J Appl Anim Sci. (2021) 11:147–59.
160. Irivboje OA, Olufayo O, Irivboje YI. Phytogenic compounds: a review of ginger and garlic as an alternative feed additive in poultry nutrition. In: Proceedings of 25th Annual Conference of ASAN 2020, Abuja, Nigeria. Abuja: Animal science association (2020). p. 299–302.
161. Sharma P, Tyagi A, Bhansali P, Pareek S, Singh V, Ilyas A, et al. Saponins: extraction, bio-medicinal properties and way forward to anti-viral representatives. Food Chem Toxicol. (2021) 150:112075. doi: 10.1016/j.fct.2021.112075
162. Góral I, Wojciechowski K. Surface activity and foaming properties of saponin-rich plants extracts. Adv Colloid Interface Sci. (2020) 279:102145. doi: 10.1016/j.cis.2020.102145
163. El Aziz MMA, Ashour AS, Melad ASG. A review on saponins from medicinal plants: chemistry, isolation, and determination. J Nanomed Res. (2019) 8:282–8. doi: 10.15406/jnmr.2019.07.00199
164. Nguyen LT, Farcas AC, Socaci SA, Tofana M, Diaconeasa ZM, Pop OL, et al. An overview of saponins–a bioactive group. Bull UASVM Food Sci Technol. (2020) 77:25–36. doi: 10.15835/buasvmcn-fst:2019.0036
165. Kundishora A, Sithole S, Mukanganyama S. Determination of the cytotoxic effect of different leaf extracts from parinari curatellifolia (Chrysobalanaceae). J Toxicol. (2020) 2020:1487. doi: 10.1155/2020/8831545
166. Liu Y, Wu Q, Wu X, Algharib SA, Gong F, Hu J, et al. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: a review. Int J Biol Macromol. (2021) 173:445–56. doi: 10.1016/j.ijbiomac.2021.01.125
167. Silva LMR, Velásquez ZD, López-Osorio S, Hermosilla C, Taubert A. Novel insights into sterol uptake and intracellular cholesterol trafficking during Eimeria bovis macromeront formation. Front Cell Infect Microbiol. (2022) 2022:70. doi: 10.3389/fcimb.2022.809606
168. Felici M, Tugnoli B, Ghiselli F, Massi P, Tosi G, Fiorentini L, et al. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult Sci. (2020) 99:5350–5. doi: 10.1016/j.psj.2020.07.035
169. Aguinaga-Casañas MA, Mut-Salud N, Falcón-Piñeiro A, Alcaraz-Martínez Á, Guillamón E, Baños A. In vitro antiparasitic activity of propyl-propane-thiosulfinate (Pts) and propyl-propane-thiosulfonate (Ptso) from Allium cepa against E. acervulina sporozoites. Microorganisms. (2022) 10:2040. doi: 10.3390/microorganisms10102040
170. Qureshi NA. In vitro anticoccidial, antioxidant activities and biochemical screening of methanolic and aqueous leaves extracts of selected plants. Pak Vet J. (2021) 41:1946. doi: 10.29261/pakvetj/2020.071
171. Ahmed E, Galal M, Abdelmageed N, Omar MA, Shibat El-hamd DMW, Seddek A-L, et al. An in vitro evaluation of the inhibitory effects of an aqueous extract of acacia nilotica on E. tenella. SVU-Int J Vet Sci. (2022) 5:33–40. doi: 10.21608/svu.2022.147099.1210
172. Lim JG, Park HM, Yoon KS. Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd). Food Sci Nutr. (2020) 8:694–702. doi: 10.1002/fsn3.1358
173. Han Y, Chi J, Zhang M, Zhang R, Fan S, Huang F, et al. Characterization of saponins and phenolic compounds: antioxidant activity and inhibitory effects on α-glucosidase in different varieties of colored quinoa (C. quinoa Willd). Biosci Biotechnol Biochem. (2019) 83:2128–39. doi: 10.1080/09168451.2019.1638756
174. Doost AS, Van Camp J, Dewettinck K, Van der Meeren P. Production of thymol nanoemulsions stabilized using quillaja saponin as a biosurfactant: antioxidant activity enhancement. Food Chem. (2019) 293:134–43. doi: 10.1016/j.foodchem.2019.04.090
175. Mahmood H, Ali Q, Hafeez MM, Malik A. Antioxidant activity of syzygium aromatium and cinnamomum verum seed extracts. Biol Clin Sci Res J. (2021) 2021:84. doi: 10.54112/bcsrj.v2021i1.63
176. Bonam SR, Rénia L, Tadepalli G, Bayry J, Kumar HMS. Plasmodium falciparum malaria vaccines and vaccine adjuvants. Vaccines. (2021) 9:1072. doi: 10.3390/vaccines9101072
177. Pulendran BS, Arunachalam P, O'Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. (2021) 20:454–75. doi: 10.1038/s41573-021-00163-y
178. O'Hagan DT, Lodaya RN, Lofano G. The continued advance of vaccine adjuvants: “We Can Work It Out”. Semin Immunol. (2020) 50:101426. doi: 10.1016/j.smim.2020.101426
179. Lakhani N, Kamra DN, Lakhani P, Alhussien MN. Immune status and haemato-biochemical profile of buffalo calves supplemented with phytogenic feed additives rich in tannins, saponins and essential oils. Trop Anim Health Prod. (2019) 51:565–73. doi: 10.1007/s11250-018-1727-z
180. Shi Z-Y, Zeng J-Z, Wong AST. Chemical structures and pharmacological profiles of ginseng saponins. Molecules. (2019) 24:2443. doi: 10.3390/molecules24132443
181. Ojiako CM, Okoye EI, Oli AN, Ike CJ, Esimone CO, Attama AA. Preliminary studies on the formulation of immune stimulating complexes using saponin from carica papaya leaves. Heliyon. (2019) 5:e01962. doi: 10.1016/j.heliyon.2019.e01962
182. Fleck JD, Betti AH, Da Silva FP, Troian EA, Olivaro C, Ferreira F, et al. Saponins from quillaja saponaria and quillaja brasiliensis: particular chemical characteristics and biological activities. Molecules. (2019) 24:171. doi: 10.3390/molecules24010171
183. Colson E, Savarino P, Js Claereboudt E, Cabrera-Barjas G, Deleu M, Lins L, et al. Enhancing the membranolytic activity of chenopodium quinoa saponins by fast microwave hydrolysis. Molecules. (2020) 25:1731. doi: 10.3390/molecules25071731
184. Yu B, Patterson N, Zaharia LI. Saponin biosynthesis in pulses. Plants. (2022) 11:3505. doi: 10.3390/plants11243505
186. Bafundo KW, Gomez L, Lumpkins B, Mathis GF, McNaughton JL, Duerr I. Concurrent use of saponins and live coccidiosis vaccines: the influence of a quillaja and yucca combination on anticoccidial effects and performance results of coccidia-vaccinated broilers. Poult Sci. (2021) 100:100905. doi: 10.1016/j.psj.2020.12.010
187. Shetshak MA, Jatau ID, Suleiman MM, Ameh MP, Gabriel A. Akefe IO. In vitro anticoccidial activities of the extract and fractions of garcinia Kola (Heckel H.) against E. tenella oocyst. Recent Patents Biotechnol. (2021) 15:76–84. doi: 10.2174/1872208315666210129095213
188. Ishaq AN, Sani D, Abdullhi SA, Jatau ID. In vitro anticoccidial activity of ethanolic leaf extract of citrus Aurantium L. against E. tenella oocysts. Sokoto J Vet Sci. (2022) 20:37–43. doi: 10.4314/sokjvs.v20i5.4
189. Kozłowski K, Vervenne-Zetteler P, Konieczka P, Szymański Ł, van Vilsteren A. Yucca schidigera improves performance and lowers oocyst counts in Eimeria challenged broilers. Animals. (2022) 12:1668. doi: 10.3390/ani12131668
190. Abbas A, Abbas RZ, Khan MK, Raza MA, Mahmood MS, Saleemi MK, et al. Anticoccidial effects of Trachyspermum ammi (Ajwain) in broiler chickens. Pak Vet J. (2019) 39:301–4. doi: 10.29261/pakvetj/2019.056
191. Kumar S, Abedin MM, Singh AK, Das S. Role of phenolic compounds in plant-defensive mechanisms. Plant Phenolics Sustain Agricult. (2020) 1:517–32. doi: 10.1007/978-981-15-4890-1_22
192. Fabbrini M, D'Amico F, Barone M, Conti G, Mengoli M, Brigidi P, et al. Polyphenol and tannin nutraceuticals and their metabolites: how the human gut microbiota influences their properties. Biomolecules. (2022) 12:875. doi: 10.3390/biom12070875
193. Chaudhari SK, Arshad S, Amjad MS, Akhtar MS. Natural compounds extracted from medicinal plants and their applications. Nat Bio-act Comp Prod Appl. (2019) 1:193–207. doi: 10.1007/978-981-13-7154-7_7
194. Hayat J, Akodad M, Moumen A, Baghour M, Skalli A, Ezrari S, et al. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and ftir characterization of marrubium Vulgare L. from 2 different localities of northeast of Morocco. Heliyon. (2020) 6:e05609. doi: 10.1016/j.heliyon.2020.e05609
195. Kaczmarek B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—a minireview. Materials. (2020) 13:3224. doi: 10.3390/ma13143224
196. Bigham A, Rahimkhoei V, Abasian P, Delfi M, Naderi J, Ghomi M, et al. Advances in tannic acid-incorporated biomaterials: infection treatment, regenerative medicine, cancer therapy, and biosensing. Chem Eng J. (2021) 14:134146. doi: 10.1016/j.cej.2021.134146
197. Abubakar Y, Tijjani H, Egbuna C, Adetunji CO, Kala S, Kryeziu TL, et al. Pesticides, history, and classification. In: Natural Remedies for Pest, Disease and Weed Control. Amsterdam: Elsevier (2020). p. 29–42. doi: 10.1016/B978-0-12-819304-4.00003-8
198. Pratyusha S. Phenolic compounds in the plant development and defense: an overview. Plant Stress Physiol Perspect Agricult. (2022) 3:125. doi: 10.5772/intechopen.102873
199. Singh AP, Kumar S. Applications of tannins in industry. Tannins Struct Propert Biol Propert Curr Knowl. (2020) 12:1–13. doi: 10.5772/intechopen.85984
200. Das AK, Islam MN, Faruk MO, Ashaduzzaman M, Dungani R. Review on tannins: extraction processes, applications and possibilities. South Afr J Bot. (2020) 135:58–70. doi: 10.1016/j.sajb.2020.08.008
201. Thi HHP, Nguyen TL. Nutraceutical properties of legume seeds: phytochemical compounds. Legumes Res. (2021) 2:24.
202. Baldwin A, Booth BW. Biomedical applications of tannic acid. J Biomater Appl. (2022) 36:1503–23. doi: 10.1177/08853282211058099
203. Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. (2019) 8:1–28. doi: 10.1186/s13756-019-0559-6
204. Wang N, Tian X, Cheng B, Guang S, Xu H. Calcium alginate/silk fibroin peptide/bletilla striata polysaccharide blended microspheres loaded with tannic acid for rapid wound healing. Int J Biol Macromol. (2022) 220:1329–44. doi: 10.1016/j.ijbiomac.2022.09.123
205. Ahmadian Z, Gheybi H, Adeli M. Efficient wound healing by antibacterial property: advances and trends of hydrogels, hydrogel-metal Np composites and photothermal therapy platforms. J Drug Deliv Sci Technol. (2022) 153:103458. doi: 10.1016/j.jddst.2022.103458
206. Wiedmer S, Kurth T, Buder U, Bleischwitz S, Entzeroth R, Kurth M. Correlative light and electron microscopy of wall formation in Eimeria nieschulzi. Parasitol Res. (2020) 119:2667–78. doi: 10.1007/s00436-020-06765-6
207. Olajide JS, Qu Z, Yang S, Oyelade OJ, Cai J. Eimeria proteins: order amidst disorder. Parasit Vect. (2022) 15:1–16. doi: 10.1186/s13071-022-05159-0
208. Kaewkod T, Tobe R, Tragoolpua Y, Mihara H. Medicinal plant extracts protect epithelial cells from infection and DNA damage caused by colibactin-producing Escherichia coli, and inhibit the growth of bacteria. J Appl Microbiol. (2021) 130:769–85. doi: 10.1111/jam.14817
209. Xu Z, Han S, Gu Z, Wu J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv Healthcare Mater. (2020) 9:1901502. doi: 10.1002/adhm.201901502
210. Chen J, Qiu L, Li Q, Ai J, Liu H, Chen Q. Rapid hemostasis accompanied by antibacterial action of calcium crosslinking tannic acid-coated mesoporous silica/silver janus nanoparticles. Mater Sci Eng C. (2021) 123:111958. doi: 10.1016/j.msec.2021.111958
211. Behl T, Kumar K, Brisc C, Rus M, Nistor-Cseppento DC, Bustea C, et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed Pharmacother. (2021) 133:110959. doi: 10.1016/j.biopha.2020.110959
212. Nassar SA. A Review on natural products as immune system modulators against infections. J Pharmaceut Negat Results. (2022) 51:5307–25.
213. Ali SA, Singh G, Datusalia AK. Potential therapeutic applications of phytoconstituents as immunomodulators: pre-clinical and clinical evidences. Phytother Res. (2021) 35:3702–31. doi: 10.1002/ptr.7068
214. Hoste H, Meza-Ocampos G, Marchand S, Sotiraki S, Sarasti K, Blomstrand BM, et al. Use of agro-industrial by-products containing tannins for the integrated control of gastrointestinal nematodes in ruminants. Parasite. (2022) 29:198. doi: 10.1051/parasite/2022010
215. Kumar K, Sinha RRK, Kumar S, Nirala RK, Kumari S, Sahu SP. Significance of tannins as an alternative to antibiotic growth promoters in poultry production. Pharm J. (2022) 14:257.
216. Rodríguez-Hernández P, Reyes-Palomo C, Sanz-Fernández S, Rufino-Moya PJ, Zafra R, Martínez-Moreno FJ, et al. Antiparasitic tannin-rich plants from the south of Europe for grazing livestock: a review. Animals. (2023) 13:201. doi: 10.3390/ani13020201
217. Ghaniei A, Ghafouri SA, Sadr S, Amiri AA, Tavanaee AET, Charbgoo A, et al. Evaluation of a tannin-based herbal formulation (Artemisia annua, Quercus infectoria, and A. sativum) against coccidiosis in broilers. Res Square. (2022) 14:158. doi: 10.21203/rs.3.rs-1941246/v1
218. Acharya M, Burke JM, Miller JE, Terrill TH, Wood EL, Muir JP. Quebracho tannins aid in the control of Eimeria Spp. and gastrointestinal nematodes in lambs and goat kids. Vet Parasitol. (2020) 288:109295. doi: 10.1016/j.vetpar.2020.109295
219. Dawod A, Fathalla S, El-Seedi HR, Hammad MA, Osman N, Abosheriba N, et al. Efficacy of ficus sycomorus (sycamore fig) extract on intestinal coccidiosis in experimentally infected rabbits. Life. (2022) 12:917. doi: 10.3390/life12060917
220. Choi J, Kim WK. Dietary application of tannins as a potential mitigation strategy for current challenges in poultry production: a review. Animals. (2020) 10:2389. doi: 10.3390/ani10122389
221. Soundararajan C, Nagarajan K, Satish AC, Prakash MA. Use of tamarind seed coat powder for controlling coccidiosis in goats. Indian J Small Ruminants. (2019) 25:247–50. doi: 10.5958/0973-9718.2019.00033.3
222. Perin G, Baldissera MD, Fernandes M, Barreta M, Casagrande RA, Griss LG, et al. Effects of tannin-containing diets on performance, gut disease control and health in broiler chicks. Anim Prod Sci. (2019) 59:1847–57. doi: 10.1071/AN18393
223. Khorrami P, Gholami-Ahangaran M, Moghtadaei-Khorasgani E. The efficacy of pomegranate peel extract on Eimeria shedding and growth indices in experimental coccidiosis in broiler chickens. Vet Med Sci. (2022) 8:635–41. doi: 10.1002/vms3.714
224. Hussain K, Alsayeqh AF, Abbas A, Abbas RZ, Rehman A, Waqar Z, et al. Potential of glycyrrhiza glabra (licorice) extract an alternative biochemical and therapeutic agent against coccidiosis in broiler chickens. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. (2022) 28:264.
225. Fathima S, Selvaraj RK, editors. Application of Nutritional Immunology in the Mitigation of Economic and Production Losses in the Poultry Industry Associated with Food-Borne Pathogens, Coccidiosis, and Necrotic Enteritis Arkansas City, KS: Arkansas Nutrition Conference (2022).
226. Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Fut J Pharmaceut Sci. (2021) 7:1–13. doi: 10.1186/s43094-020-00161-8
227. Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. (2019) 18:241–72. doi: 10.1007/s11101-018-9591-z
228. Delgado AM, Issaoui M, Chammem N. Analysis of main and healthy phenolic compounds in foods. J AOAC Int. (2019) 102:1356–64. doi: 10.5740/jaoacint.19-0128
229. Jahromi SG. Extraction techniques of phenolic compounds from plants. Plant Physiol Aspects Phenolic Comp. (2019) 521:1–18.
230. Tsimogiannis D, Oreopoulou V. Classification of phenolic compounds in plants. In: Polyphenols in Plants. Amsterdam: Elsevier (2019). p. 263–84. doi: 10.1016/B978-0-12-813768-0.00026-8
231. Laura A, Moreno-Escamilla JO, Rodrigo-García J, Alvarez-Parrilla E. Phenolic compounds. In: Postharvest Physiology and Biochemistry of Fruits and Vegetables. Amsterdam: Elsevier (2019). p. 253–71. doi: 10.1016/B978-0-12-813278-4.00012-9
232. Metsämuuronen S, Sirén H. Bioactive phenolic compounds, metabolism and properties: a review on valuable chemical compounds in scots pine and norway spruce. Phytochem Rev. (2019) 18:623–64. doi: 10.1007/s11101-019-09630-2
233. Hu H, Fei X, He B, Luo Y, Qi Y, Wei A. Integrated analysis of metabolome and transcriptome data for uncovering flavonoid components of zanthoxylum bungeanum maxim leaves under drought stress. Front Nutr. (2021) 8:68. doi: 10.3389/fnut.2021.801244
234. Karak P. Biological activities of flavonoids: an overview. Int J Pharm Sci Res. (2019) 10:1567–74.
235. Hazafa A, Rehman K-U, Jahan N, Jabeen Z. The Role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr Cancer. (2020) 72:386–97. doi: 10.1080/01635581.2019.1637006
236. Dias MC, Pinto DCGA, Silva AMS. Plant flavonoids: chemical characteristics and biological activity. Molecules. (2021) 26:5377. doi: 10.3390/molecules26175377
237. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, et al. Important flavonoids and their role as a therapeutic agent. Molecules. (2020) 25:5243. doi: 10.3390/molecules25225243
238. Biharee A, Sharma A, Kumar A, Jaitak V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia. (2020) 146:104720. doi: 10.1016/j.fitote.2020.104720
239. Osinaike A, Adewoga TOS, Fagbemi FT, Uthman TOO, Antia RE. Phytochemical Studies Anticoccidial Potential of the Extract of Carica Papaya (Pawpaw) Leaves in Chicken (2020). Available online at: researchgate.com
240. Biallah MB, Lawal IA, Abdu PA, Okubanjo OO, Kaze PD. Phytochemical profile of the methanolic leaf extract of vernonia Amygdalina del. and in vitro anticoccidial effect of its fractions against E. tenella. Int J Innov Res Dev. (2022) 11:528.
241. Scicutella F, Mannelli F, Daghio M, Viti C, Buccioni A. Polyphenols and organic acids as alternatives to antimicrobials in poultry rearing: a review. Antibiotics. (2021) 10:1010. doi: 10.3390/antibiotics10081010
242. Murshed M, Al-Quraishy S, Qasem MA. In vitro: anti-coccidia activity of calotropis procera leaf extract on eimeria papillata oocysts sporulation and sporozoite. Open Chem. (2022) 20:1057–64. doi: 10.1515/chem-2022-0208
243. López AM, Muñoz MC, Molina JM, Hermosilla C, Taubert A, Zárate R, et al. Anticoccidial efficacy of canary rue (Ruta pinnata) extracts against the caprine apicomplexan eimeria ninakohlyakimovae. J Anim Sci. (2019) 97:101–10. doi: 10.1093/jas/sky389
244. Qaid MM, Mansour L, Al-Garadi MA, Alqhtani AH, Al-abdullatif AA, Qasem MA, et al. Evaluation of the anticoccidial effect of traditional medicinal plants, cinnamomum verum bark and rumex nervosus leaves in experimentally infected broiler chickens with E. tenella. Ital J Anim Sci. (2022) 21:408–21. doi: 10.1080/1828051X.2022.2033139
245. Toah ET, Payne VK, Cedric Y, Nadia NAC, Joël ATR. In vitro oocysticidal sporulation inhibition of E. tenella and antioxidant efficacy of ethanolic and aqueous extracts of conyza aegyptiaca. J Anim Sci Vet Med. (2021) 6:30–40. doi: 10.31248/JASVM2021.249
246. Guzmán E, Lucia A. Essential oils and their individual components in cosmetic products. Cosmetics. (2021) 8:114. doi: 10.3390/cosmetics8040114
247. Thangaleela S, Sivamaruthi BS, Kesika P, Bharathi M, Kunaviktikul W, Klunklin A, et al. Essential oils, phytoncides, aromachology, and aromatherapy—a review. Proc Indian Natn Sci Acad. (2022) 12:4495. doi: 10.3390/app12094495
248. Farahmandfar R, Tirgarian B. Essential oils: in vitro antioxidant activities and their utilizations in storage life increment of foods. J Food Bioprocess Eng. (2020) 3:128–37.
249. de Souza HJB, Dessimoni ALA, Ferreira MLA, Botrel DA, Borges SV, Viana LC, et al. Microparticles obtained by spray-drying technique containing ginger essential oil with the addition of cellulose nanofibrils extracted from the ginger vegetable fiber. Dry Technol. (2021) 39:1912–26. doi: 10.1080/07373937.2020.1851707
250. Stephane FFY, Jules BKJ. Terpenoids as important bioactive constituents of essential oils. In: Essential Oils-Bioactive Compounds, New Perspectives and Applications. London: IntechOpen (2020).
251. Moghaddam M, Mehdizadeh L. Chemistry of essential oils and factors influencing their constituents. In: Soft Chemistry and Food Fermentation. Amsterdam: Elsevier (2017). p. 379–419. doi: 10.1016/B978-0-12-811412-4.00013-8
252. Wani AR, Yadav K, Khursheed A, Rather MA. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb Pathogen. (2021) 152:104620. doi: 10.1016/j.micpath.2020.104620
253. Bhavaniramya S, Vishnupriya S, Al-Aboody MS, Vijayakumar R, Baskaran D. Role of essential oils in food safety: antimicrobial and antioxidant applications. Grain Oil Sci Technol. (2019) 2:49–55. doi: 10.1016/j.gaost.2019.03.001
254. Valdivieso-Ugarte M, Gomez-Llorente C, Plaza-Díaz J, Gil Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: a systematic review. Nutrients. (2019) 11:2786. doi: 10.3390/nu11112786
255. Spisni E, Petrocelli G, Imbesi V, Spigarelli R, Azzinnari D, Donati Sarti M, et al. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: implications in colonic pathophysiology. Int J Mol Sci. (2020) 21:4152. doi: 10.3390/ijms21114152
256. Perez-Roses R, Risco E, Vila R, Penalver P, Canigueral S. Biological and nonbiological antioxidant activity of some essential oils. J Agricult Food Chem. (2016) 64:4716–24. doi: 10.1021/acs.jafc.6b00986
257. Hardin A, Crandall PG, Stankus T. Essential oils and antioxidants derived from citrus by-products in food protection and medicine: an introduction and review of recent literature. J Agricult Food Inform. (2010) 11:99–122. doi: 10.1080/10496501003680680
258. Remmal A, Achahbar S, Bouddine L, Chami N, Chami F. In vitro destruction of eimeria oocysts by essential oils. Vet Parasitol. (2011) 182:121–6. doi: 10.1016/j.vetpar.2011.06.002
259. Upadhaya SD, Cho SH, Chung TK, Kim IH. Anticoccidial effect of essential oil blends and vitamin D on broiler chickens vaccinated with purified mixture of coccidian oocyst from E. tenella and Eimeria maxima. Poult Sci. (2019) 98:2919–26. doi: 10.3382/ps/pez040
260. Abou-Elkhair R, Gaafar KM, Elbahy NM, Helal MA, Mahboub HD, Sameh G. Bioactive effect of dietary supplementation with essential oils blend of oregano, thyme and garlic oils on performance of broilers infected with Eimeria species. Global Vet. (2014) 13:977–85.
261. Isakakroudi N, Talebi A, Allymehr M, Tavassoli M. Effects of essential oils combination on sporulation of Turkey (Meleagris gallopavo) Eimeria Oocysts. Arch Razi Inst. (2018) 73:113–20.
262. Hafeez A, Ullah Z, Khan RU, Ullah Q, Naz S. Effect of diet supplemented with coconut essential oil on performance and villus histomorphology in broiler exposed to avian coccidiosis. Trop Anim Health Product. (2020) 52:2499–504. doi: 10.1007/s11250-020-02279-6
263. Kasem SM, Mira NM, Mahfouz ME, Helal IB. In vitro study to evaluate the efficacy of ultrasonicated ethanolic extract of rosmarinus officinalis and its chitosan-based nanoparticles against E. tenella oocysts of chickens. AAPS PharmSciTech. (2022) 23:295. doi: 10.1208/s12249-022-02445-z
264. Lahlou RA, Bounechada M, Mohammedi A, Silva LR, Alves G. Dietary use of rosmarinus officinalis and thymus vulgaris as anticoccidial alternatives in poultry. Anim Feed Sci Technol. (2021) 273:114826. doi: 10.1016/j.anifeedsci.2021.114826
265. Mohiti-Asli M, Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prevent Vet Med. (2015) 120:195–202. doi: 10.1016/j.prevetmed.2015.03.014
266. Reisinger N, Steiner T, Nitsch S, Schatzmayr G, Applegate TJ. Effects of a blend of essential oils on broiler performance and intestinal morphology during coccidial vaccine exposure. J Appl Poult Res. (2011) 20:272–83. doi: 10.3382/japr.2010-00226
267. Oviedo-Rondón EO, Clemente-Hernández S, Salvador F, Williams P, Losa R, Stephan F. Essential oils on mixed coccidia vaccination and infection in broilers. Int J Poult Sci. (2006) 5:723–30.
268. Langerudi MT, Youssefi MR, Tabari MA. Ameliorative effect of psidium guajava essential oil supplemented feed on chicken experimental coccidiosis. Trop Anim Health Prod. (2022) 54:120. doi: 10.1007/s11250-022-03117-7
269. Dudko P, Junkuszew A, Bojar W, Milerski M, Szczepaniak K, Le Scouarnec J, et al. Effect of dietary supplementation with preparation comprising the blend of essential oil from Origanum vulgare (Lamiaceae) and Citrus Spp. (Citraceae) on coccidia invasion and lamb growth. Ital J Anim Sci. (2018) 17:57–65. doi: 10.1080/1828051X.2017.1346965
270. Lee J-W, Kim D-H, Kim Y-B, Jeong S-B, Oh S-T, Cho S-Y, et al. Dietary encapsulated essential oils improve production performance of coccidiosis-vaccine-challenged broiler chickens. Animals. (2020) 10:481. doi: 10.3390/ani10030481
271. Abdel Haleem M, Abdelnaser A, Ali M. Effect of synbiotics and essential oils in ameliorating the negative drawbacks of necrotic enteritis and coccidiosis in broiler chicks. Benha Vet Med J. (2019) 36:187–98. doi: 10.21608/bvmj.2019.14842.1047
272. Puvaca N, Horvatek Tomić D, Popović S, Ðordević S, Brkić I, Lalić N, et al. Influence of tea tree (Melaleuca alternifolia) essential oil as feed supplement on production traits, blood oxidative status and treatment of coccidiosis in laying hens. Vet Arhiv. (2020) 90:331–40.
273. Godswill AG, Somtochukwu IV, Ikechukwu AO, Kate EC. Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: a systematic review. Int J Food Sci. (2020) 3:1–32. doi: 10.47604/ijf.1024
274. Brancaccio M, Mennitti C, Cesaro A, Fimiani F, Vano M, Gargiulo B, et al. The biological role of vitamins in athletes' muscle, heart and microbiota. Int J Environ Res Public Health. (2022) 19:1249. doi: 10.3390/ijerph19031249
275. Peterson CT, Rodionov DA, Osterman AL, Peterson SN, B. vitamins and their role in immune regulation and cancer. Nutrients. (2020) 12:3380. doi: 10.3390/nu12113380
276. Junaid K, Ejaz H, Abdalla AE, Abosalif KOA, Ullah MI, Yasmeen H, et al. Effective immune functions of micronutrients against Sars-Cov-2. Nutrients. (2020) 12:2992. doi: 10.3390/nu12102992
277. Mitra S, Paul S, Roy S, Sutradhar H, Bin Emran T, Nainu F, et al. Exploring the immune-boosting functions of vitamins and minerals as nutritional food bioactive compounds: a comprehensive review. Molecules. (2022) 27:555. doi: 10.3390/molecules27020555
278. Hafeez MA, Sattar A, Ashraf K, Mehdi M, Rafique A, Mahmood MS, et al. Effect of ibuprofen alone and inconjugation with vitamin E and selenium on experimentally induced coccidiosis in broliers. JAPS. (2020) 30:627–33. doi: 10.36899/JAPS.2020.3.0074
279. Orso C, Cony BL, Silva JP, Furtado JCV, Mann MB, Frazzon J, et al. Effect of live eimeria vaccination or salinomycin on growth and immune status in broiler chickens receiving in-feed inclusion of gelatin and vitamin E. Poult Sci. (2022) 101:102206. doi: 10.1016/j.psj.2022.102206
280. Julien C. Psxiii-21 formulations of vitamin K, organic acids, essential oils and plant hydroalcoholic extracts prevent coccidiosis impact on growth performances in broilers. J Anim Sci. (2022) 100(Supplement_3):319. doi: 10.1093/jas/skac247.582
281. Fatemi SA, Elliott KEC, Macklin KS, Bello A, Peebles ED. Effects of the in ovo injection of vitamin D3 and 25-hydroxyvitamin D3 in ross 708 broilers subsequently challenged with coccidiosis: II immunological and inflammatory responses and small intestine histomorphology. Animals. (2022) 12:1027. doi: 10.3390/ani12081027
282. Oikeh I, Sakkas P, Blake DP, Kyriazakis I. Interactions between dietary calcium and phosphorus level, and vitamin D source on bone mineralization, performance, and intestinal morphology of coccidia-infected broilers. Poult Sci. (2019) 98:5679–90. doi: 10.3382/ps/pez350
283. Iciek M, Kwiecień I, Włodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. (2009) 50:247–65. doi: 10.1002/em.20474
284. Oswald IWH, Ojeda MA, Pobanz RJ, Koby KA, Buchanan AJ, Del Rosso J, et al. Identification of a new family of prenylated volatile sulfur compounds in cannabis revealed by comprehensive two-dimensional gas chromatography. ACS Omega. (2021) 6:31667–76. doi: 10.1021/acsomega.1c04196
285. Hill CR, Shafaei A, Balmer L, Lewis JR, Hodgson JM, Millar AH, et al. Sulfur compounds: from plants to humans and their role in chronic disease prevention. Crit Rev Food Sci Nutr. (2022) 16:1–23. doi: 10.1080/10408398.2022.2057915
286. Ozma MA, Abbasi A, Ahangarzadeh Rezaee M, Hosseini H, Hosseinzadeh N, Sabahi S, et al. A critical review on the nutritional and medicinal profiles of garlic's (A. sativum L.) bioactive compounds. Food Rev Int. (2022) 48:1–38. doi: 10.1080/87559129.2022.2100417
287. Imran M, Umer T, Rehman HU, Saleem M, Farooq H, Bhutta ZA, et al. Anti-inflammatory, immunomodulatory and antioxidant activities of allicin, vitamin C and doxycycline, or their combination against pasteurella multocida infection in rabbits. Continent Vet J. (2022) 2:112–7.
288. Masłowski M, Aleksieiev A, Miedzianowska J, Efenberger-Szmechtyk M, Strzelec K. Antioxidant and anti–aging activity of freeze–dried alcohol–water extracts from common nettle (Urtica dioica L.) and peppermint (Mentha piperita L.) in elastomer vulcanizates. Polymers. (2022) 14:1460. doi: 10.3390/polym14071460
289. Asghar M, Durrani UF, Hussain R, Matloob K, Mahmood AK, Anees M, et al. Comparative efficacy of amprolium, garlic oil (A. sativum) and ginger oil (Zingiber officinale) against coccidiosis in common quail (Coturnix coturnix). J Hellenic Vet Med Soc. (2020) 71:2273–8. doi: 10.12681/jhvms.25072
290. Musa H. The Efficacy of a Commercial Herbal Formulation in the Prevention of Coccidiosis Under Natural Exposure to Eimeria Species. Beijing: Federal University of Technology Minna (2021).
291. Marchiosi R, dos Santos WD, Constantin RP, de Lima RB, Soares AR, Finger-Teixeira A, et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem Rev. (2020) 19:865–906. doi: 10.1007/s11101-020-09689-2
292. Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Microorganisms. (2021) 9:2041. doi: 10.3390/microorganisms9102041
293. Ghafouri SA, Ghaniei A, Tamannaei AET, Sadr S, Charbgoo A, Ghiassi S, et al. Evaluation of therapeutic effects of an herbal mixture (Echinacea purpurea and Glycyrrhiza glabra) for treatment of clinical coccidiosis in broilers. Vet Med Sci. (2022) 17:971.
294. Akuma P, Okagu OD, Udenigwe CC. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front Sustain Food Syst. (2019) 3:23. doi: 10.3389/fsufs.2019.00023
295. Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Natl Prod Rep. (2019) 36:869–88. doi: 10.1039/C9NP00011A
296. Slavova-Kazakova A, Janiak MA, Sulewska K, Kancheva VD, Karamać M. Synergistic, additive, and antagonistic antioxidant effects in the mixtures of curcumin with (–)-Epicatechin and with a green tea fraction containing (–)-Epicatechin. Food Chem. (2021) 360:129994. doi: 10.1016/j.foodchem.2021.129994
297. Chen X, Li H, Zhang B, Deng Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit Rev Food Sci Nutr. (2022) 62:5658–77. doi: 10.1080/10408398.2021.1888693
298. Tsiouris V, Giannenas I, Bonos E, Papadopoulos E, Stylianaki I, Sidiropoulou E, et al. Efficacy of a dietary polyherbal formula on the performance and gut health in broiler chicks after experimental infection with Eimeria Spp. Pathogens. (2021) 10:524. doi: 10.3390/pathogens10050524
Keywords: cecal coccidiosis, botanicals, herbal extracts, Eimeria, E. tenella, poultry, immune response, oxidative stress
Citation: Saeed Z and Alkheraije KA (2023) Botanicals: A promising approach for controlling cecal coccidiosis in poultry. Front. Vet. Sci. 10:1157633. doi: 10.3389/fvets.2023.1157633
Received: 02 February 2023; Accepted: 10 March 2023;
Published: 25 April 2023.
Edited by:
Isa Ozaydin, Kafkas University, TürkiyeReviewed by:
Kun Li, Nanjing Agricultural University, ChinaSara Taha Elazab, Mansoura University, Egypt
Riaz Hussain, Islamia University of Bahawalpur, Pakistan
Copyright © 2023 Saeed and Alkheraije. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khalid A. Alkheraije, ay5hbGtoZXJhaWplQHF1LmVkdS5zYQ==
 Zohaib Saeed
Zohaib Saeed Khalid A. Alkheraije2*
Khalid A. Alkheraije2*