
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 24 August 2023
Sec. Comparative and Clinical Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1155804
This article is part of the Research TopicAdvances in Veterinary Endocrine OncologyView all 6 articles
 Marit F. van den Berg1*
Marit F. van den Berg1* Hans S. Kooistra1
Hans S. Kooistra1 Guy C. M. Grinwis2
Guy C. M. Grinwis2 Stefano Nicoli3
Stefano Nicoli3 Stefania Golinelli4
Stefania Golinelli4 Lisa Stammeleer5
Lisa Stammeleer5 Monique E. van Wolferen1
Monique E. van Wolferen1 Elpetra P. M. Timmermans-Sprang1
Elpetra P. M. Timmermans-Sprang1 Maurice M. J. M. Zandvliet1
Maurice M. J. M. Zandvliet1 Frank G. van Steenbeek1,6†
Frank G. van Steenbeek1,6† Sara Galac1†
Sara Galac1†Pheochromocytomas and paragangliomas (PPGLs) are neuroendocrine tumors arising from the chromaffin cells in the adrenal medulla and extra-adrenal paraganglia, respectively. Local invasion, concurrent disorders, and metastases prevent surgical removal, which is the most effective treatment to date. Given the current lack of effective medical treatment, there is a need for novel therapeutic strategies. To identify druggable pathways driving PPGL development, we performed RNA sequencing on PPGLs (n = 19) and normal adrenal medullas (NAMs; n = 10) of dogs. Principal component analysis (PCA) revealed that PPGLs clearly clustered apart from NAMs. In total, 4,218 genes were differentially expressed between PPGLs and NAMs. Of these, 232 had a log2 fold change of >3 or < −3, of which 149 were upregulated in PPGLs, and 83 were downregulated. Compared with NAMs, PPGLs had increased expression of genes related to the cell cycle, tumor development, progression and metastasis, hypoxia and angiogenesis, and the Wnt signaling pathway, and decreased expression of genes related to adrenal steroidogenesis. Our data revealed several overexpressed genes that could provide targets for novel therapeutics, such as Ret Proto-Oncogene (RET), Dopamine Receptor D2 (DRD2), and Secreted Frizzled Related Protein 2 (SFRP2). Based on the PCA, PPGLs were classified into 2 groups, of which group 1 had significantly higher Ki67 scores (p = 0.035) and shorter survival times (p = 0.04) than group 2. Increased expression of 1 of the differentially expressed genes between group 1 and 2, pleiotrophin (PTN), appeared to correlate with a more aggressive tumor phenotype. This study has shed light on the transcriptomic profile of canine PPGL, yielding new insights into the pathogenesis of these tumors in dogs, and revealed potential novel targets for therapy. In addition, we identified 2 transcriptionally distinct groups of PPGLs that had significantly different survival times.
Pheochromocytomas and paragangliomas (PPGLs) are neuroendocrine tumors arising from the chromaffin cells in the adrenal medulla (pheochromocytomas, PCCs) or extra-adrenal paraganglia (paragangliomas, PGLs) (1, 2). When biochemically functional, they secrete excessive amounts of catecholamines, causing a variety of nonspecific, potentially fatal, clinical signs, such as tachyarrhythmias, hypertension, abdominal pain, and tachypnea. Clinical signs also can be related to the space-occupying or invasive nature of the mass (1, 3, 4).
Vascular invasion is observed in up to 82% of dogs with PPGL, while distant metastasis is reported in up to 24% of cases (4, 5). PCC and PGL are estimated to account for approximately 0.01–0.1 and 0.2%, respectively, of all canine tumors (1). This number is likely underestimated because it is primarily based on autopsy findings rather than the recently available biochemical testing. Currently, surgical resection is the treatment of choice for PPGLs in dogs and humans (1, 2). However, in some patients, surgical removal is prevented by local invasion, concurrent disorders, or distant metastases. In these patients, medical treatment is warranted.
The use of toceranib phosphate, a tyrosine kinase inhibitor (TKI), has been described in a small, retrospective series of 5 dogs with PCCs and 2 retrospective series of 27 and 28 dogs with PGLs, showing clinical benefit to some extent in the majority of dogs (6–8). However, most of these dogs only experienced stable disease as the best response, emphasizing the need for novel medical treatment options for canine PPGL.
The underlying mechanisms driving PPGL formation in humans are diverse, affecting a broad range of biological pathways, which is reflected in The Cancer Genome Atlas (TCGA) classification of PPGLs into 4 transcriptionally defined groups: the pseudohypoxia cluster, kinase signaling cluster, Wnt-altered cluster, and cortical admixture cluster (9). New therapeutic approaches in humans with PPGL are based on targeting these altered signaling pathways, as demonstrated by the use of hypoxia-inducible factor (HIF) inhibitors for tumors from the pseudohypoxia cluster (10–12) and the use of TKIs for metastatic tumors from the pseudohypoxia and kinase signaling cluster (2, 13, 14). In contrast, canine PPGL has not been well characterized on the molecular level, thus hampering the development of molecular-targeted therapies for these tumors in dogs.
Given the current lack of global gene expression data in canine PPGL and the lack of effective medical treatment for these tumors in dogs, we performed RNA sequencing on PPGLs and normal adrenal medullas (NAMs) of dogs, with 2 aims: (1) to unravel their transcriptomic signature, in this way yielding new insight into the pathogenesis of these tumors, and (2) to identify potential novel therapeutic targets.
Tumor tissue was obtained from 21 client-owned dogs with PPGL (20 PCCs, 1 PGL) following surgical removal or immediately after euthanasia. Based on the principal component analysis (PCA) plot, 2 PPGLs were identified as outliers, which were excluded from further analyses, leaving a total of 19 PPGLs (18 PCCs, 1 PGL) in our final analysis cohort. Samples were collected at Utrecht University’s Department of Clinical Sciences, Faculty of Veterinary Medicine, for 8 dogs. In addition, samples were collected from contributing veterinary institutions, including University of Bologna (n = 2), Ghent University (n = 2), University of Ljubljana (n = 1), and veterinary referral centers in Italy (n = 2), Slovenia (n = 1), and the Netherlands (n = 3). Tumor tissues were cut longitudinally, and a representative piece of the tumor was either snap-frozen in liquid nitrogen or first fixed in RNAlater stabilization solution (Invitrogen™, ThermoFisher Scientific, Breda, The Netherlands) and then stored at −70°C until RNA extraction. The remainder of the tissue was formalin-fixed and paraffin-embedded for histopathology and immunohistochemistry (IHC). In all dogs, the diagnosis of PPGL was confirmed by histopathology and IHC using the adrenomedullary markers chromogranin A (CHGA) and synaptophysin (SYP) (1, 15). Normal adrenal tissues were obtained from 10 healthy dogs. Adrenal glands were cut longitudinally, and the medullas were collected and snap-frozen.
Clinicopathological features, such as the presence of excessive catecholamine production, tumor location, tumor size, vascular invasion or the presence of a tumor thrombus, the presence of metastasis, survival time, histopathology results, and IHC results for CHGA, SYP, and Ki67, were recorded for all patients.
Ethical approval was not required because the tissue samples originated from client-owned dogs with naturally occurring pheochromocytoma and paraganglioma, collected after surgical resection for curative purposes, or collected after euthanasia. Permission to use tissues for research purposes was obtained from all dog owners. Normal adrenal tissues were obtained from healthy dogs used in animal research, that were euthanized for reasons unrelated to the present study, which was approved by the Ethical Committee of Utrecht University.
Histopathological examination by a veterinary pathologist (G.G.) and IHC for CHGA and SYP were performed for all canine samples. Slides were deparaffinized and rehydrated according to general histological techniques. For SYP IHC, antigen retrieval was performed with citrate buffer (pH 6) at 97°C for 20 min using PT link (Dako, Agilent Technologies). For CHGA IHC, no antigen retrieval was performed. Slides were incubated with Peroxidase Blocking Solution (Dako, Agilent Technologies) for 5 min to block endogenous peroxidase. Subsequently, slides were blocked with 10% normal goat serum in Tris buffered saline (TBS) for 15 min. Slides were incubated with primary antibodies for 60 min. The mouse monoclonal primary anti-CHGA antibody (ab199014, Abcam, Amsterdam, The Netherlands) was used in a 1:200 concentration, while the mouse monoclonal primary anti-SYP antibody (M731529-2, Agilent Technologies) was used in a 1:100 concentration. Incubation with a secondary antibody (HRP-labelled goat-anti-mouse, Brightvision, ImmunoLogic, WellMed, Duiven, The Netherlands) was performed at room temperature for 30 min. The slides were incubated with chromogen AEC (ab64252, Abcam) for 20 min, and counterstained with hematoxylin using Lab Vision Autostainer 360 (ThermoFisher Scientific). The slides were then rinsed in tap water and mounted with Aquatex (VWR International, Amsterdam, The Netherlands). During intermediate steps, the slides were washed in TBS with Tween-20 (Merck, Darmstadt, Germany) and bovine serum albumin (BSA). Normal canine pancreas with islets of Langerhans or normal canine adrenal medulla were used as positive controls for both CHGA and SYP, although often the histological samples contained pre-existing normal adrenal tissue that could serve as internal positive control (see Supplementary Presentation). Omission of the primary antibody from the immunohistochemical protocol was used as negative control. Cytoplasmatic staining of neoplastic cells for CHGA and SYP was evaluated by 2 observers (G.G. and M.v.d.B.).
Because the Ki67 proliferation index is used in humans with PPGL to assess malignant potential (16), Ki67 IHC is performed as standard of care for all canine PPGLs at our pathology department. Immunohistochemistry for Ki67 was performed as previously described (17), using a mouse monoclonal primary anti-Ki67 antibody (Agilent Cat# M7240, RRID: AB_2142367). Normal canine colon tissue slides were used as positive control, and the primary antibody was omitted for the negative control. The Ki67 score was assessed by 2 observers (M.v.d.B. and M.v.W.) in hot spot areas, in which the percentage of neoplastic cells with nuclear positivity was counted in a minimum of 1,000 cells. ImageJ software was used for this purpose (18). Care was taken to only include PPGL cells, and not areas of necrosis or hemorrhage. The Ki67 score was calculated as the percentage of Ki67 positive nuclei relative to the total number of counted nuclei for each PPGL. The inter-observer agreement was calculated and the average of the 2 Ki67 scores was used for further analyses.
RNA was isolated from PPGLs and NAMs using the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands) following the manufacturer’s instructions. RNA samples were quantified using the Qubit™ RNA high sensitivity Assay Kit (ThermoFisher Scientific, Breda, The Netherlands). The quality of RNA samples was measured with RNA Nano Chips (Agilent Technologies, Middelburg, The Netherlands) on an Agilent Bioanalyzer 2,100 (Agilent Technologies). Samples with an RNA integrity number value <7.5 were excluded from further analysis.
A minimum of 200 ng of RNA from each sample was submitted for library preparation and sequencing at Utrecht Sequencing Facility. Sequencing libraries were made using the TruSeq Stranded mRNA Library Prep Kit (Illumina). Libraries were sequenced using the Nextseq2000 platform (Illumina), producing single-end reads of 50 bp. Reads were aligned to the canine reference genome CanFam3.1 using STAR version 2.4.2a.1 The raw and analyzed files have been uploaded to Gene Expression Omnibus under accession number GSE223526.
The dataset was normalized for composition bias using trimmed mean of M-values (TMM) and fold changes (FCs) were calculated in edgeR (19). Pairwise comparisons for differences in gene expression included NAM against PPGL and, among PPGL, group 1 against group 2, which were defined based on their PCA profile. The Mann–Whitney U-test was used to calculate p-values, which were corrected for multiple comparisons using the false discovery rate (FDR) Benjamini-Hochberg method. For the comparison between NAM and PPGL, genes were considered differentially expressed at an FDR-adjusted p < 0.05 and with a log2 FC of >3 or < −3. For the comparison between PPGL groups 1 and 2, genes were considered differentially expressed with the same cutoffs for the log2 FC, but at an FDR-adjusted p < 0.1. Volcano plots were generated using EnhancedVolcano (R package version 1.12.0) (20). A heatmap of differentially expressed genes (DEGs) was created using gplots.
Pathway enrichment analysis was performed using Gene Set Enrichment Analysis 4.2.3 (21, 22). All unannotated genes were filtered, and the gene list of annotated genes was ranked by multiplying the sign of the log2 FC and − log10 of the FDR-adjusted p value for each gene, following best practices described in the literature (23). The database of pathway gene sets used for pathway enrichment analysis was downloaded from http://baderlab.org/GeneSets (Human_GOBP_AllPathways_no_GO_iea_August_2022_symbol.gmt) and contained pathways from 8 data sources: GO, Reactome, Panther, NetPath, NCI, MSigDB curated gene sets (C2 collection), MSigDB Hallmark (H collection), and HumanCyc (23). To ensure a robust analysis, pathways with an FDR-adjusted p ≤ 0.05 were considered significant.
Further data analysis was performed using the statistical software program IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY, United States). Data were assessed for normality of distribution using the Shapiro–Wilk test. For the comparison of clinicopathological features between PPGL group 1 and 2, differences for continuous and normally distributed data (Ki67 proliferation index, tumor diameter) were assessed using the independent samples t-test, whereas Fisher’s exact test was used for categorical data (tumor location, the presence of excessive catecholamine secretion, vascular invasion, the presence of metastasis). To compare pleiotrophin expression among multiple groups, the non-parametric Kruskal–Wallis test was used. In case of significance, differences between individual groups were assessed by post hoc analysis, and p values were adjusted by the Bonferroni correction method to adjust for multiple comparisons.
The inter-observer agreement score for Ki67 was quantified using the intra-class correlation coefficient for continuous variables. The strength of agreement was interpreted as follows: <0.40, poor; 0.40–0.59, moderate; 0.60–0.79, good; 0.80–1.00, excellent (17).
For the survival analyses, dogs were considered to have had an event when they had died or were euthanized due to PPGL attributable disease signs including metastases. Dogs that had died from a cause that appeared unrelated to PPGL, were lost to follow-up, or were still alive at the end of the study were censored at the timepoint they were last known to be alive. Survival times were recorded as the time between surgical resection of PPGL or initial diagnosis in patients that were not surgically managed, and censoring or death. Survival times were calculated using the Kaplan–Meier product-limit method. Significance between subpopulations was tested using the log-rank test for bivariate (categorical) variables. Values were considered significant at p < 0.05.
The suspicion of PPGL was based on clinical signs, the presence of an adrenal (n = 18) or abdominal mass (n = 1) visualized by abdominal ultrasonography or computed tomography, increased plasma or urinary metanephrine concentrations (n = 10), and cytology following fine needle aspirations of the adrenal mass (n = 1). In 8 dogs, the adrenal mass was an incidental finding. The clinicopathological data of all included dogs can be found in Supplementary Table S1. The inter-observer agreement score for Ki67 was excellent (0.98; p < 0.001).
Gene expression profiles were compared between PPGLs (n = 19) and NAMs (n = 10). In the PCA plot, PPGLs clearly clustered apart from NAMs (Figure 1), illustrating the differences in transcriptional behavior between tumor and normal samples. In total, 4,218 genes were differentially expressed between PPGLs and NAMs with an FDR-adjusted p < 0.05. Of these, 232 had a log2 FC of >3 or < −3, of which 149 were upregulated in PPGLs, and 83 were downregulated (Figure 2; Supplementary Tables S2, S3). Hierarchal clustering of these DEGs revealed distinct clustering of PPGL and NAM tissues (Figure 3). Table 1 shows the top 10 up-and downregulated genes based on their log2 FC.
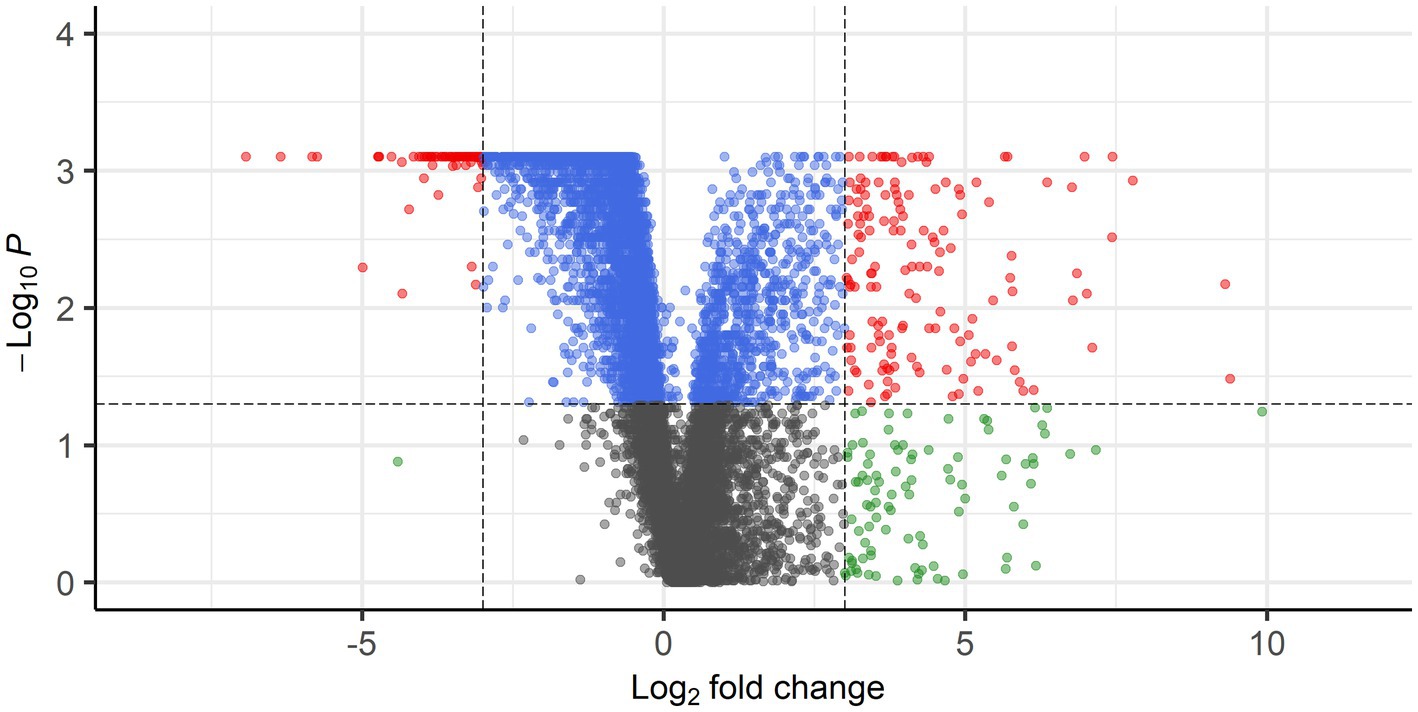
Figure 2. Volcano plot showing DEGs with FDR-corrected p < 0.05 and log2 FC of >3 (149 genes) or < −3 (83 genes) in red. Blue points represent genes with FDR-corrected p <0.05, but log2 FC of <3 and > −3, whereas gray points represent genes with FDR-corrected p > 0.05 and log2 FC of <3 and > −3. Genes with FDR-corrected p > 0.05 and log2 FC of >3 or < −3 are shown in green.

Figure 3. Heatmap of DEGs in PPGLs versus NAM. A total of 232 genes, with a log2 FC of >3 or < −3, are represented. Genes indicated in blue are upregulated, while genes in orange are downregulated.
When researching these 232 up-and downregulated DEGs, data revealed that upregulated genes are associated with biological processes involving pro-tumorigenic factors, the cell cycle, tumor progression and metastasis, hypoxia and angiogenesis, and the Wnt signaling pathway (Table 2). Also, several upregulated genes, that are also highly overexpressed in human PPGL (e.g., Ret Proto-Oncogene [RET] (9), Dopamine Receptor D2 [DRD2] (24)), were identified. Many of the downregulated genes are involved in adrenal steroidogenesis. In addition, several genes that could serve as tumor suppressors are downregulated in PPGLs (Table 2). Table 3 shows a selection of overexpressed genes in canine PPGL that could provide targets for novel therapeutics.
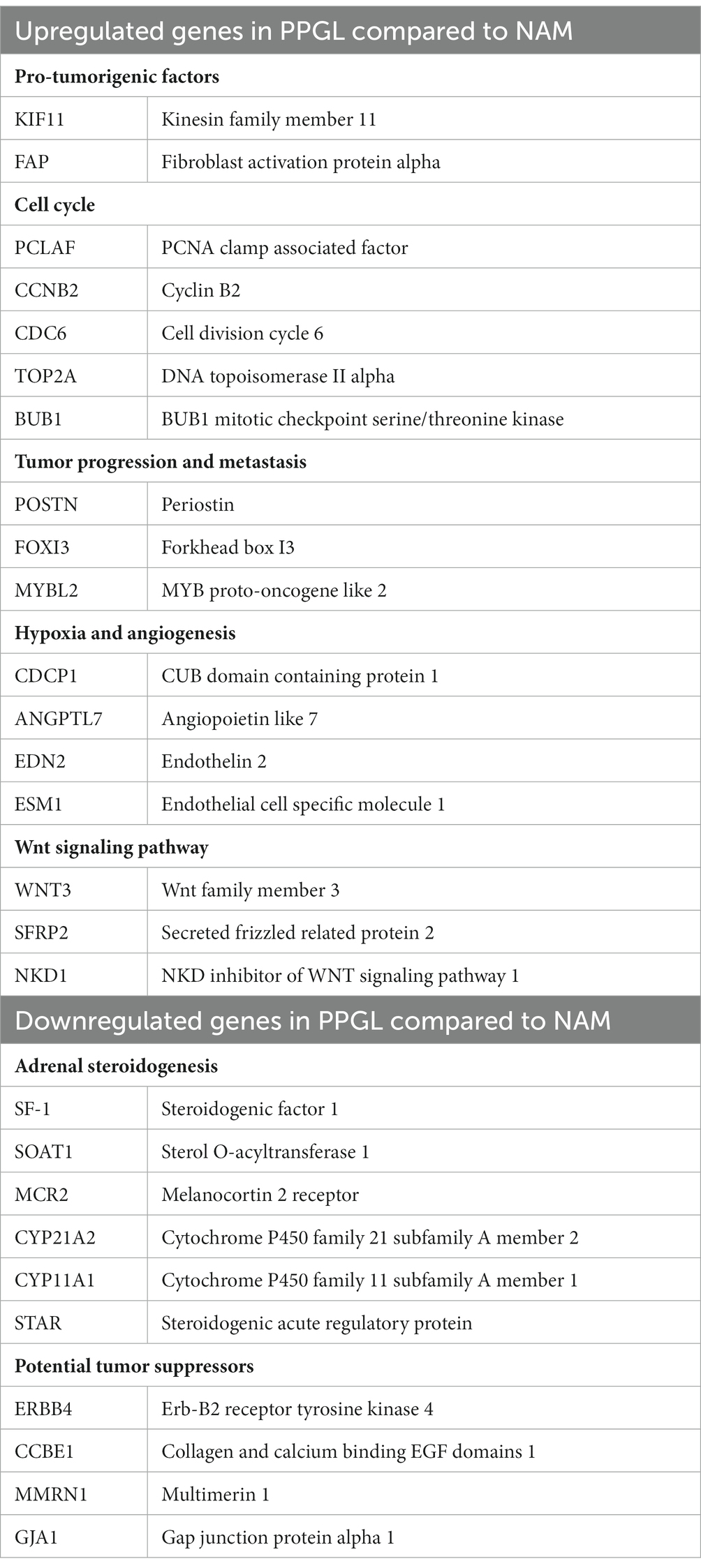
Table 2. Examples of up-and downregulated DEGs between PPGL and NAM that are involved in different biological processes.
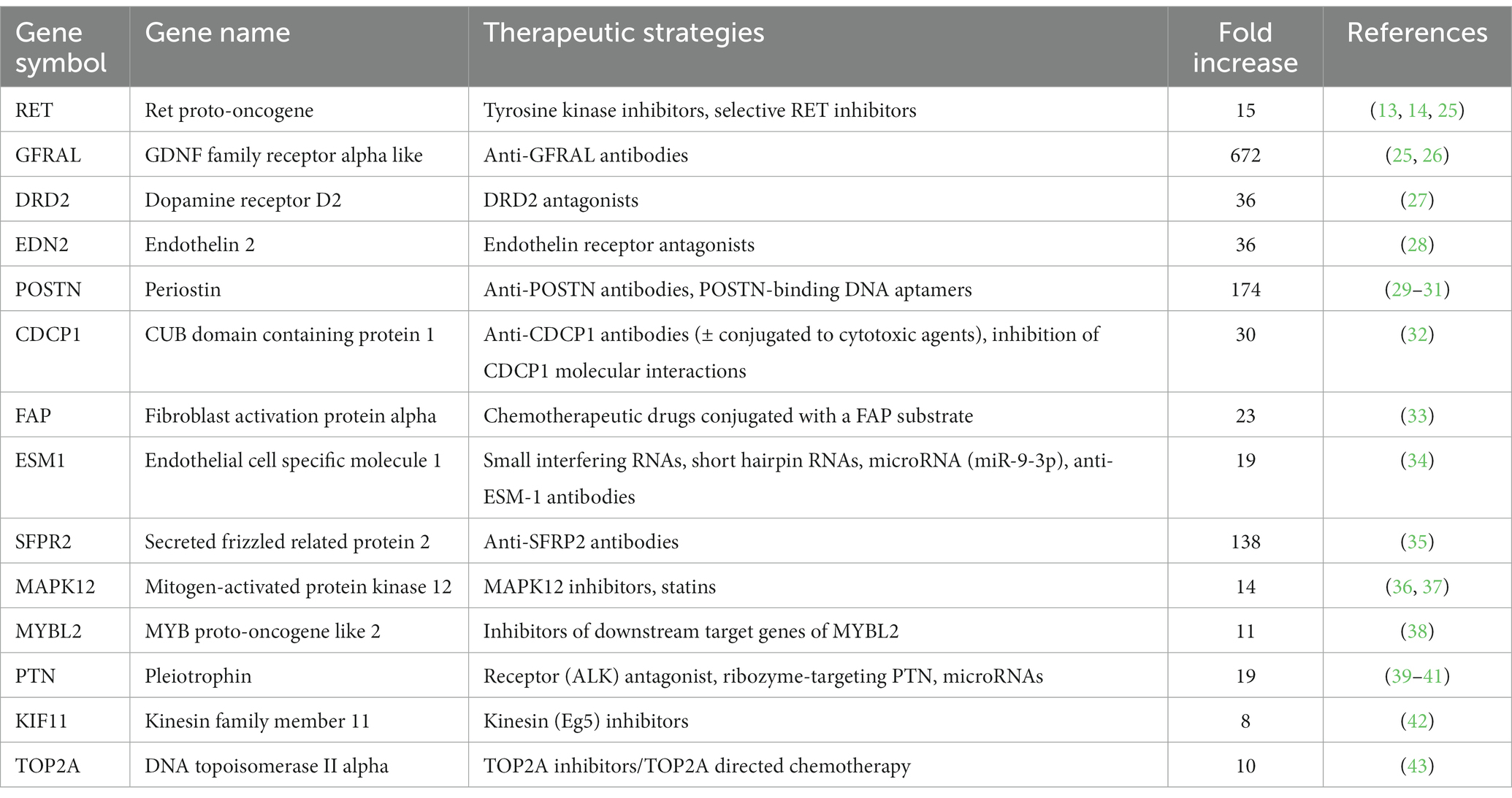
Table 3. Overexpressed DEGs (log2 FC >3, FDR-adjusted p < 0.05) in dogs with PPGL that could provide targets for novel therapeutics.
Using an FDR-adjusted p ≤ 0.05 for enrichment, pathway enrichment analysis revealed 28 overexpressed and 9 underexpressed pathways in PPGLs (Table 4). When comparing PPGL to NAM samples, steroid metabolism and the tricarboxylic acid (TCA) cycle are among the enriched underexpressed pathways, while the most enriched overexpressed pathways are involved in the cell cycle.
Another aim of this study was to evaluate whether subclustering was detectable among dogs with PPGL resulting in different gene expression profiles. We divided dogs with PPGL into 2 groups based on their PCA profile: PPGL group 1 (n = 7) and PPGL group 2 (n = 12) (Figure 4). When comparing these 2 groups based on their clinicopathological parameters, no significant differences between the 2 groups in tumor location (p = 0.78), tumor size (p = 0.16), the presence of excessive catecholamine secretion (p = 0.46), vascular invasion (p = 0.31), or the presence of metastasis (p = 0.34) were found. However, a significantly higher Ki67 score was found in group 1 (p = 0.035; Figure 5). In addition, dogs in group 1 had significantly (p = 0.04) shorter survival times (Figure 6), suggesting a more aggressive phenotype of tumors in group 1. For dogs in group 1, median overall survival time was 45 days (95% confidence interval [CI]: 0–99 days), while the median survival time for dogs in group 2 was not reached. The mean overall survival was 1,789 days (95% CI: 1,125–2,453 days), while mean overall survival was 430 days (95% CI: 0–875 days) and 2,221 days (95% CI: 1,489–2,953 days) for dogs in group 1 and group 2, respectively. Since 4 dogs in group 1 and 1 dog in group 2 did not receive treatment for their tumor, this could have affected the difference in survival times between the two groups. Survival analysis on those dogs that were surgically treated, showed a significantly (p = 0.019) shorter survival time for dogs in group 1 compared to group 2.
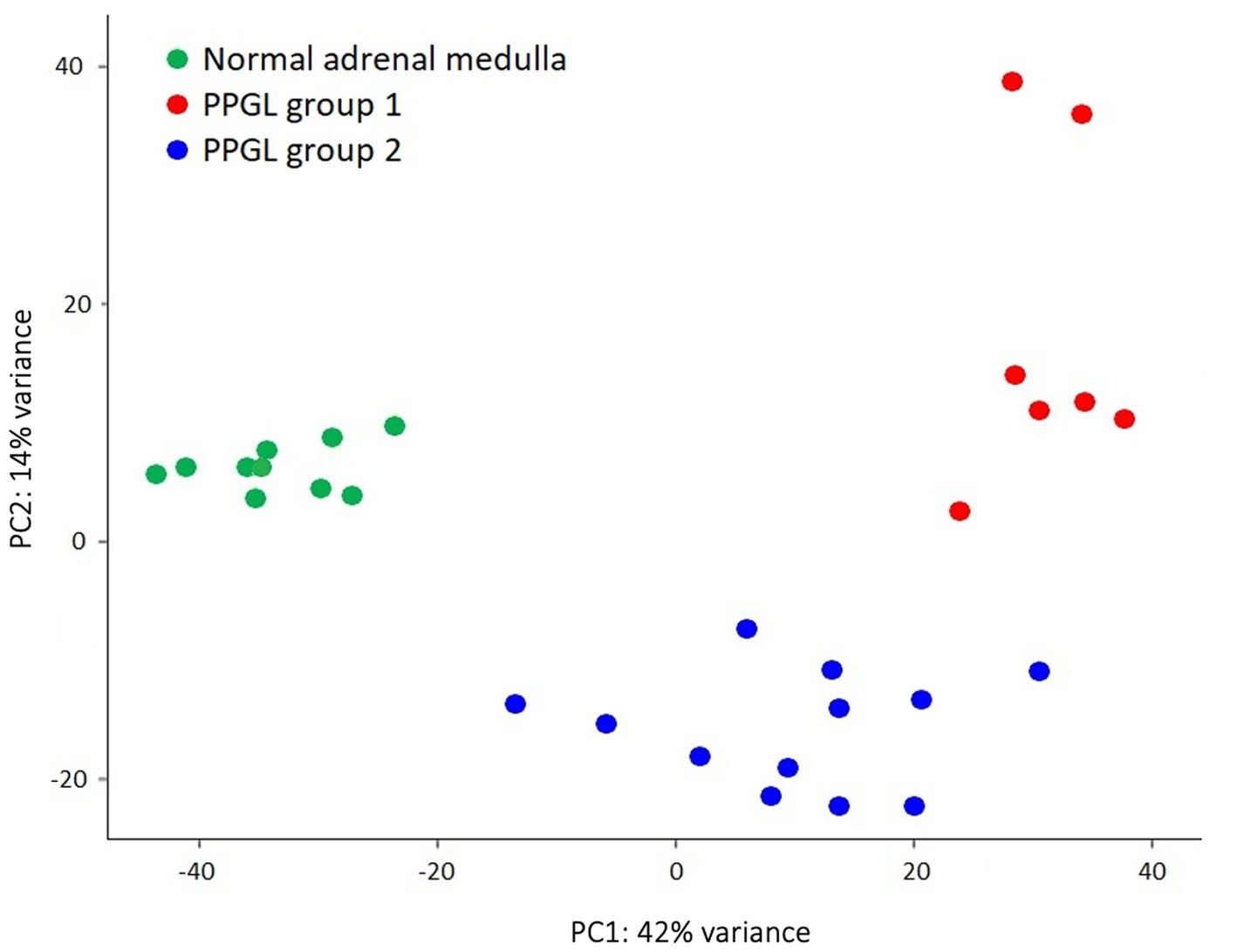
Figure 4. PPGL samples were classified based on their PCA profile as PPGL group 1 (n = 7) and PPGL group 2 (n = 12).
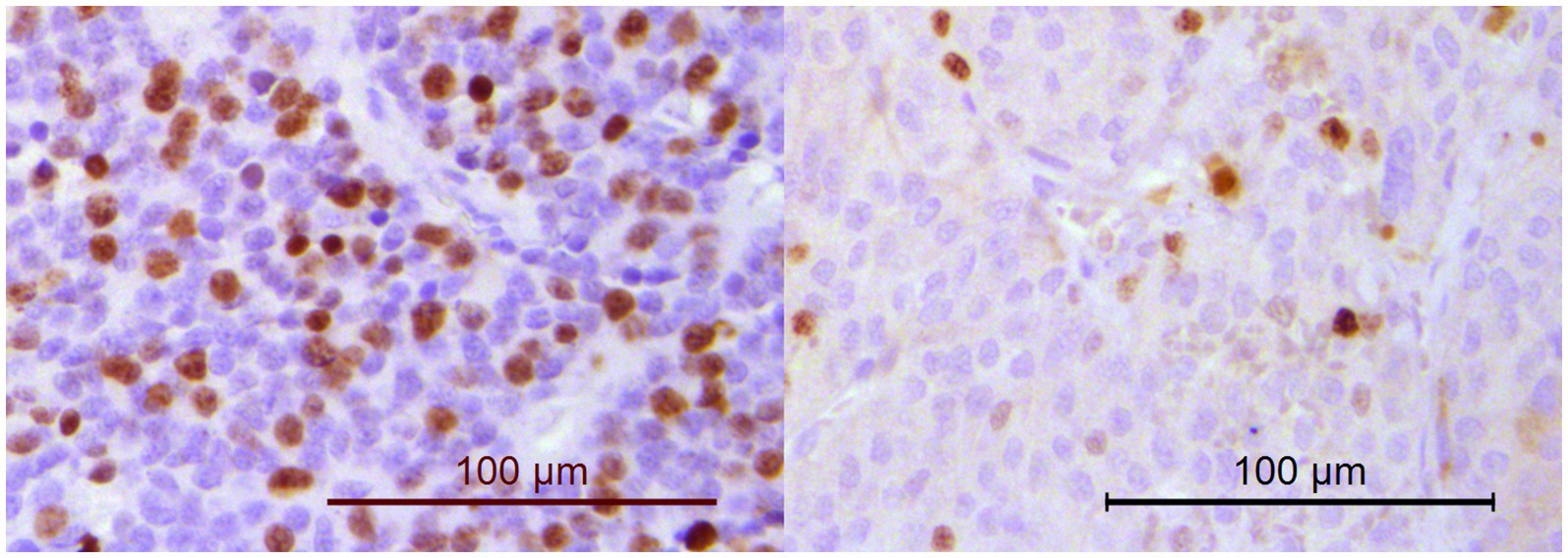
Figure 5. Immunohistochemistry for Ki67. The percentage of neoplastic cells with nuclear positivity was counted in a minimum of 1,000 cells. Dogs in PPGL group 1 (example on the left; Ki67 score of 33.4%) had significantly higher Ki67 scores than dogs in PPGL group 2 (example on the right; Ki67 score of 10.1%).
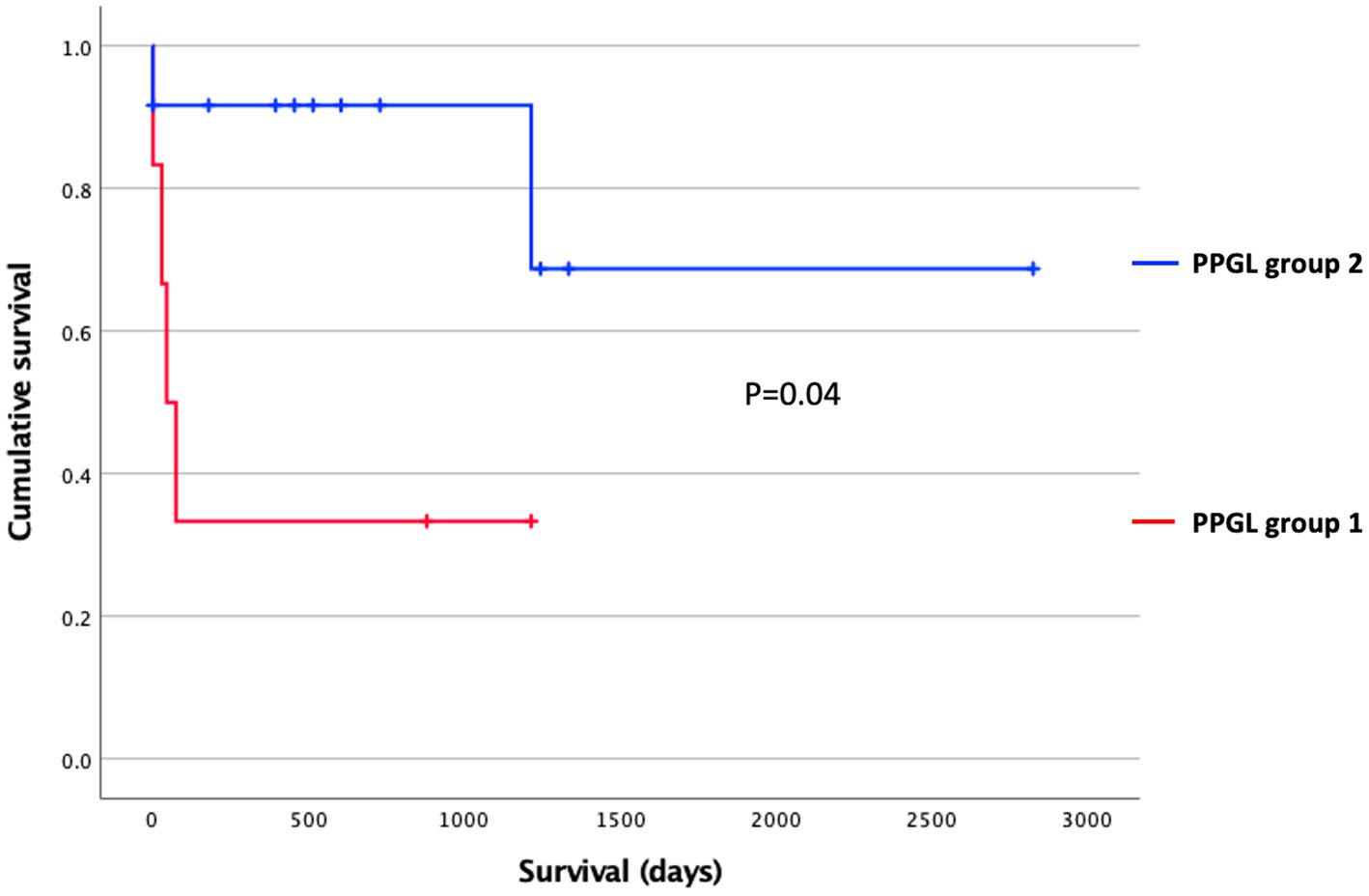
Figure 6. Survival stratified according to the PPGL classification using Kaplan–Meier analysis. Censored dogs are indicated as tick marks. The p value indicates the significance of the difference between PPGL group 1 and 2 as calculated with the log-rank test.
When comparing PPGL group 1 with PPGL group 2, no genes were differentially expressed if an FDR-adjusted p < 0.05 was used. Because of the exploratory nature of this work, we increased the cut-off to an FDR-adjusted p < 0.1, which showed a total of 52 DEGs. Of these, 33 had a log2 FC of >3 (n = 9) or <−3 (n = 24) in PPLG group 1 compared to PPGL group 2 (Figure 7; Supplementary Table S4). Table 5 shows the upregulated and top 10 downregulated genes based on their log2 FC.
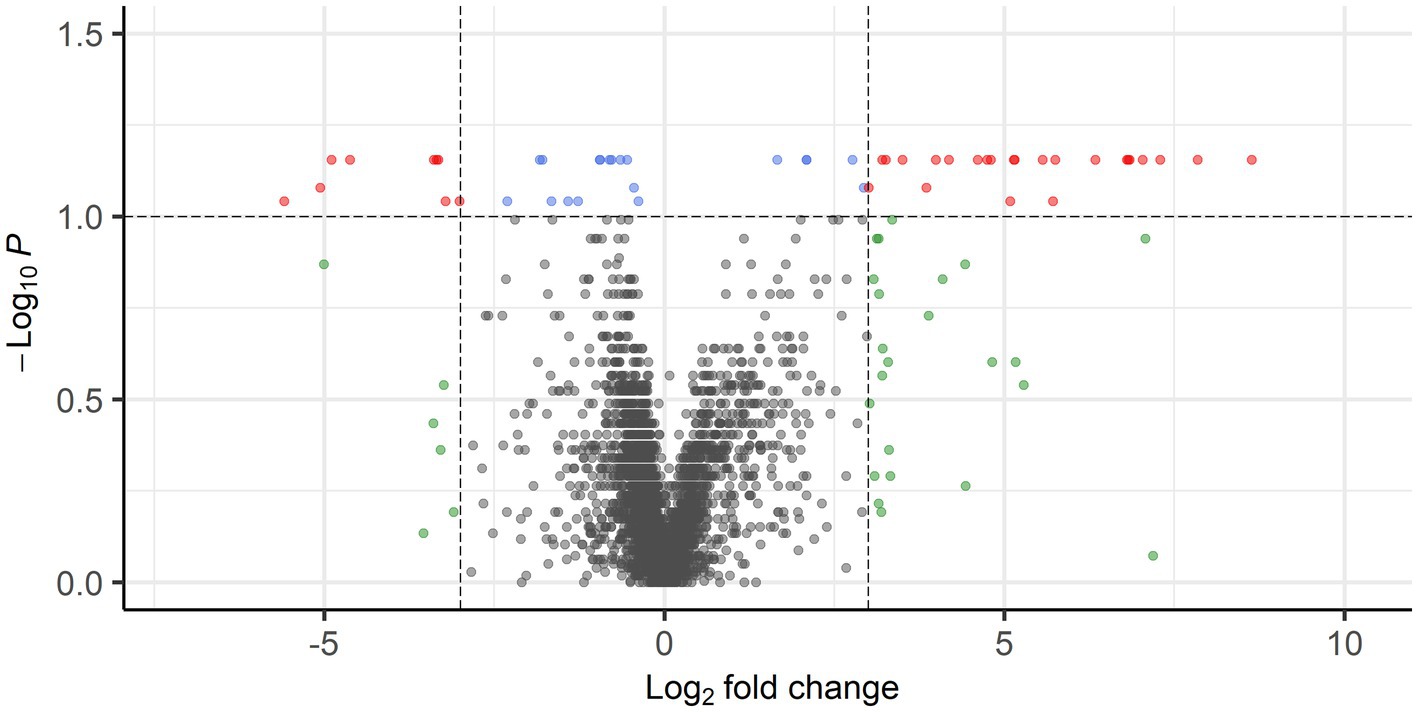
Figure 7. Volcano plot showing DEGs between PPGL group 1 and 2 with FDR-corrected p < 0.1 and log2 FC of >3 (24 genes) or <−3 (9 genes) in red.
When interrogating these up-and downregulated DEGs, data revealed that upregulated genes in group 1 were associated with cancer development and progression (e.g., ISL LIM Homeobox 1 [ISL1], Cartilage Oligomeric Matrix Protein [COMP], pleiotrophin [PTN]), which fits with the more aggressive phenotype of group 1. Many downregulated genes were involved in adrenal steroidogenesis (e.g., Hydroxysteroid 11-beta Dehydrogenase 2 [HSD11B2], Cytochrome P450 Family 11 Subfamily B Member 2 [CYP11B2] and Subfamily A Member 1 [CYP11A1], Cytochrome P450 Family 17 Subfamily A Member 1 [CYP17A1], Cytochrome P450 Family 21 Subfamily A Member 2 [CYP21A2], Steroidogenic Factor 1 [SF-1]). Pathway enrichment analysis revealed 4 underexpressed pathways (Table 6) in PPGL group 1 compared to group 2, but no overexpressed pathways when using an FDR-adjusted p ≤ 0.05 for enrichment.
In search for a therapeutic target that could specifically benefit patients with a poor prognosis (PPGL group 1), we compared DEGs between PPGLs and NAMs, and between PPGL group 1 and 2. When using an FDR-corrected p < 0.05 for the comparison between PPGL and NAMs, and an FDR-corrected p < 0.1 for the comparison between PPGL group 1 and 2, we found 1 gene that was upregulated with a log2 FC >3 and 8 genes that were downregulated with a log2 FC < -3 in both comparisons (Table 7). The expression of PTN was significantly higher in PPGL group 1 compared to NAM (p < 0.001), and in PPGL group 1 compared to group 2 (p = 0.006; Figure 8), which makes it a promising therapeutic target for patients with a poor prognosis.
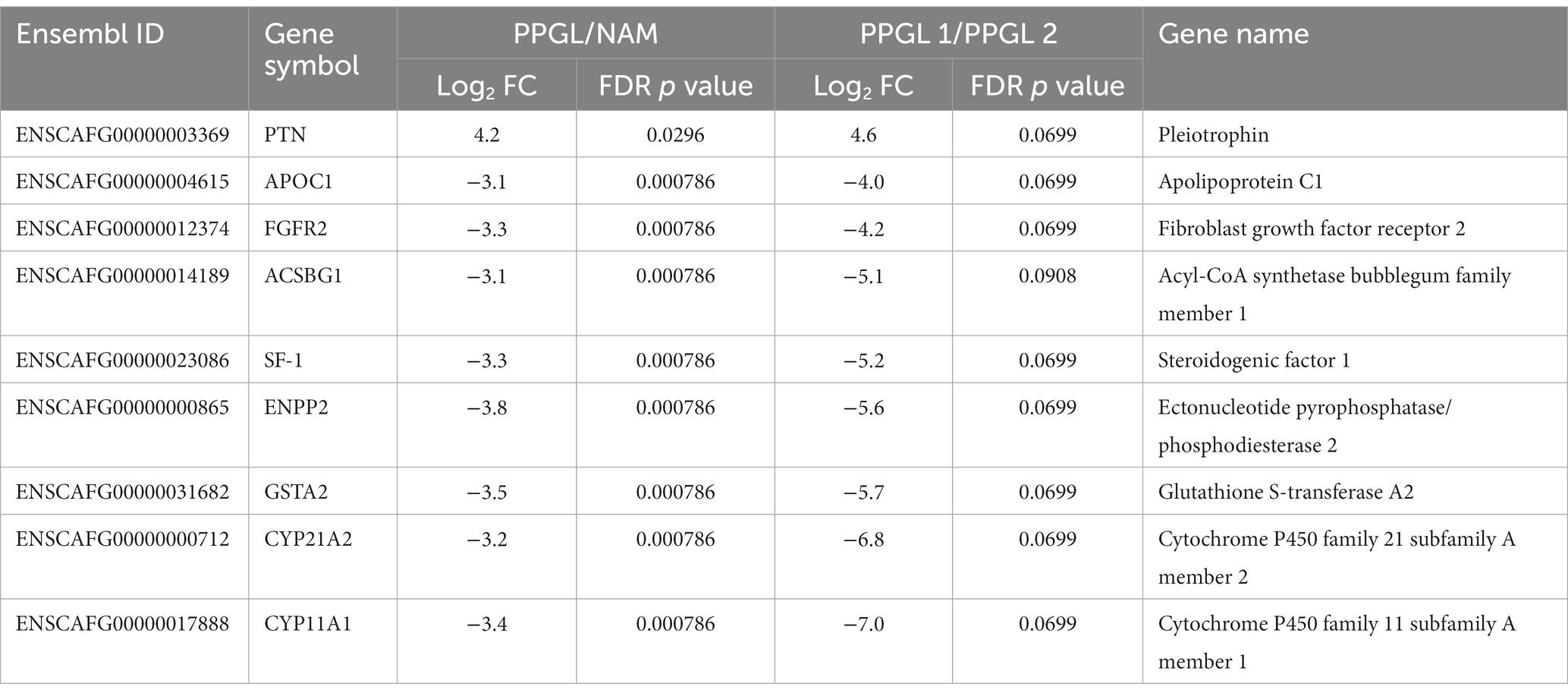
Table 7. Genes that are up-or downregulated in PPGL compared to the NAM, as well as in PPGL group 1 compared to group 2.
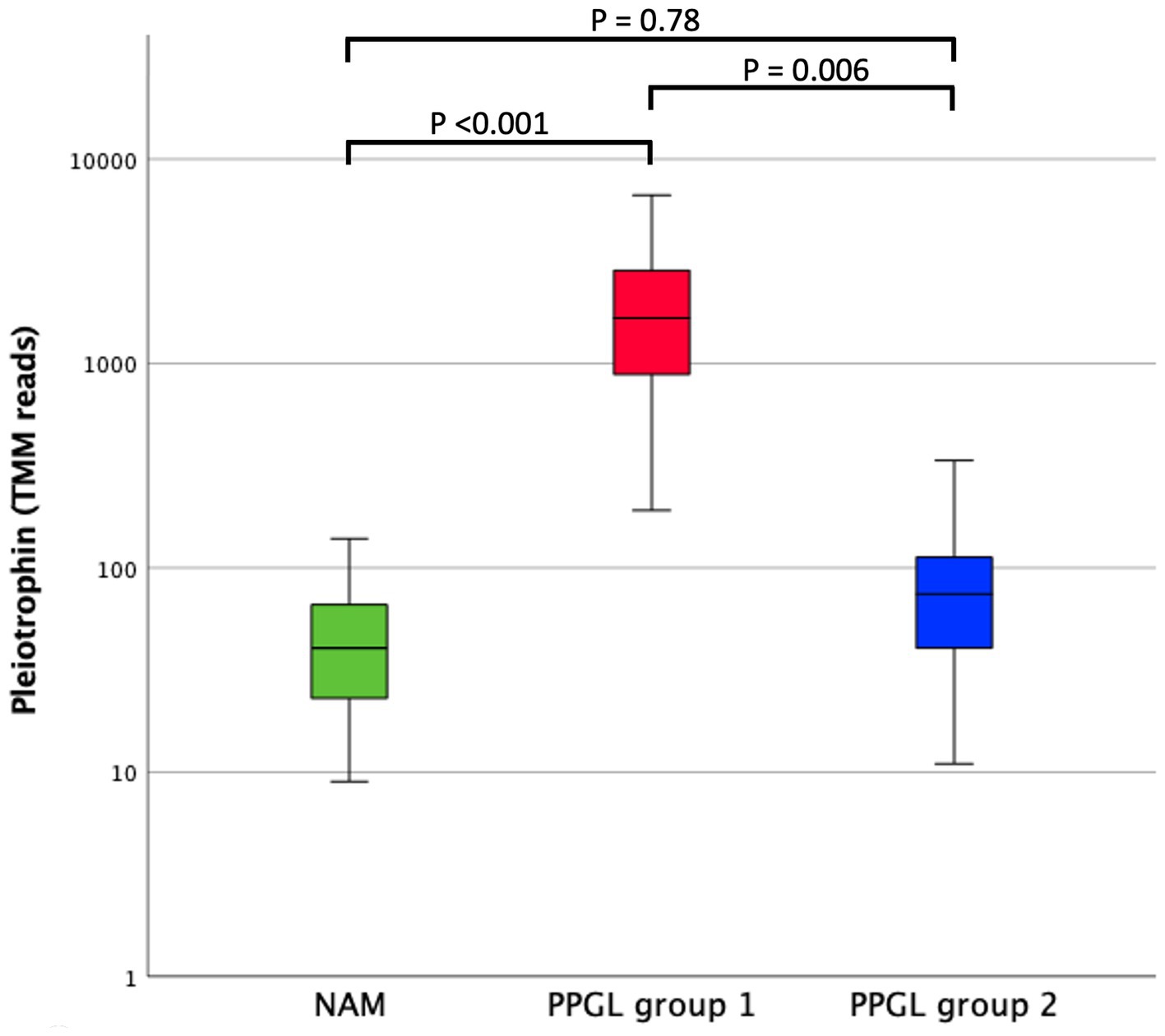
Figure 8. Comparison of pleiotrophin expression (TMM RNA sequencing reads) between NAM, PPGL group 1, and PPGL group 2. The box represents the inter quartile range (i.e., the second and third quartiles). The line within the box represents the median. The whiskers represent the range, extending to a maximum of 1.5-times the interquartile range.
This is the first study in which a transcriptome sequencing approach was applied to identify gene expression characteristics of canine PPGL. We have detected several genes and biological pathways that were dysregulated in PPGLs compared to NAMs, yielding new insights into the pathogenesis of these tumors in dogs and providing potential targets for therapeutic intervention. In addition, we identified 2 transcriptionally distinct groups of PPGLs that had significantly different survival times.
We detected 4,218 DEGs between NAM and PPGL, and hierarchal clustering of these DEGs revealed well-defined clustering of normal and tumor tissues, showing a distinct gene expression profile. Compared with healthy controls, dogs with PPGL had increased expression of genes related to the cell cycle, tumor development, progression and metastasis, hypoxia and angiogenesis, and the Wnt signaling pathway, and decreased expression of genes related to adrenal steroidogenesis.
Aberrant activation of the Wnt pathway is implicated in cancer initiation and progression (44). In humans with PPGL, the Wnt-altered cluster consists of adrenal PCCs with Mastermind Like Transcriptional Coactivator 3 (MAML3) fusion genes and Cold Shock Domain Containing E1 (CSDE1) somatic mutations, resulting in an activation of the Wnt and Hedgehog signaling pathways and overexpression of genes such as Wnt Family Member 4 (WNT4) and Dishevelled Segment Polarity Protein 3 (DVL3) (9). Although these specific genes are not differentially expressed between canine PPGL and NAM, we did find significantly increased expression of Wnt Family Member 3 (WNT3; 21-fold overexpressed), Secreted Frizzled Related Protein 2 (SFRP2; 138-fold overexpressed), and NKD Inhibitor of WNT Signaling Pathway 1 (NKD1; 22-fold overexpressed). Overexpression of WNT3 activates the canonical Wnt pathway, and WNT3 treatment enhanced cell proliferation in rat PCC (PC12) cells (45). SFRP2 can function both as a Wnt antagonist and agonist, whereas NKD1 functions as an inhibitor of the Wnt signaling pathway (35). The role of SFRP2 as enhancer of the Wnt signaling pathway, resulting in the upregulation of WNT3-regulated genes including its antagonist NKD1, has been previously described (46). Additionally, SFRP2 has been shown to play a key role in tumor angiogenesis (35). Thus, the Wnt pathway, and specifically SFRP2, might show great promise as target for anti-cancer therapy in dogs with PPGL.
Besides genes related to the Wnt signaling pathway, our data revealed several other interesting genes that could provide targets for therapeutic intervention (also see Table 2). For example, the transcription factor MYB Proto-Oncogene Like 2 (MYBL2) was significantly overexpressed (11-fold) in PPGLs. MYBL2 is an important physiological regulator of cell cycle progression and cell survival and promotes cancer initiation and/or progression when overexpressed (38). In humans with PPGL, increased MYBL2 expression is significantly correlated with lower 5-years progressive-free interval (47). Although direct MYBL2 inhibitors are not yet available, inhibitors interfering with downstream target genes of MYBL2 may serve as effective novel therapeutics (38). We also noted increased (14-fold) expression of the gene Mitogen-Activated Protein Kinase 12 (MAPK12), which has been shown to play a role in cancer development, invasion, and metastasis (36). In humans with PPGL, the MAPK pathway is mainly associated with tumors from the kinase signaling cluster, but several MAPK-related genes, including MAPK12, are also upregulated in Succinate Dehydrogenase (SDH) B (SDHB)-derived PPGLs (37). It has been shown that treatment of mouse PCC cell lines with statins induced apoptosis by MAPK inhibition (37). Thus, treatment with specific MAPK12 inhibitors or statins might provide a promising treatment option for dogs with PPGL.
In humans with PPGL, several TKIs, including sunitinib, are currently under evaluation in phase II clinical trials2. Sunitinib targets multiple tyrosine kinase receptors, including RET, vascular endothelial growth factor receptors (VEGFR) 1 and 2, platelet-derived growth factor receptor (PDGFR)-β, and c-KIT (13, 14). Tumors from the pseudohypoxia cluster are characterized by activation of pseudohypoxic pathways with resultant activation of HIF target genes implicated in angiogenesis, including VEGF and PDGF (48). Thus, patients from the pseudohypoxia group may derive great benefit from sunitinib treatment (13). In addition, sunitinib may have additional activity in patients with increased RET expression (13). In our canine patients, we did not find increased expression of VEGF, PDGF, and their receptors at the mRNA level. In contrast, RET was significantly overexpressed (15-fold) in dogs with PPGL. Targets of the veterinary available TKI toceranib phosphate include VEGFR, PDGFR, C-KIT, and likely also RET, based on its structural and functional similarity to sunitinib (49). Although these findings should be validated by functional (i.e., protein expression) studies, this suggests that dogs with PPGL might benefit from a new generation of kinase inhibitors with improved selectivity for RET.
In this study, GDNF Family Receptor Alpha Like (GFRAL), which is the receptor for Growth Differentiation Factor 15 (GDF15), was the most upregulated gene in dogs with PPGL (672-fold increased). Of interest, signaling of glial-derived neurotrophic factor (GDNF)-family receptors occurs through the RET receptor tyrosine kinase (25, 50). GDNF-mediated activation of RET is recognized in several cancers and promotes tumor growth, invasion, and metastasis (25). Moreover, GDF15 is considered the main actor of cachexia in cancer signaling through its receptor GFRAL (26). Therefore, strategies targeting the GDF15-GFRAL-RET axis might be of interest to dogs with PPGL, both by their direct anti-tumor effect and by reducing cancer cachexia.
According to TCGA data, human PPGLs have the highest DRD2 mRNA expression of all cancers. A phase II clinical trial of ONC201, a DRD2 antagonist, in human patients with metastatic neuroendocrine tumors including PPGL showed clinical benefit in the majority of patients with PPGL (5 partial responses, 7 stable diseases, 2 progressive diseases) (27). In our canine population, DRD2 was significantly overexpressed (36-fold). Thus, the use of DRD2 antagonist might be beneficial to dogs with PPGL as well.
The decreased expression of adrenal steroidogenesis-related genes and gene pathways in PPGLs compared to NAMs can be explained by contamination of the NAM samples by adjacent cortical cells. PPGLs also showed decreased expression of the TCA cycle pathway. In humans, the pseudohypoxia cluster partially consists of tumors with mutations in TCA cycle-related genes, such as the SDH subunits (SDHA/B/C/D) and fumarate hydratase (2). In dogs with PPGL, mutations in SDHB and SDHD have been described (51, 52), suggestive of common pseudohypoxic pathways. The decreased expression of the TCA cycle pathway in PPGL dogs in our study may reflect this. Future studies on whole-exome or whole-genome sequencing will be needed to fully unravel the genetic background of canine PPGL.
The levels of expression of DEGs were not validated by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) in this study, as the added value of this practice has been questioned recently. It has been shown that the vast majority of ‘non-concordant’ results between RNA sequencing and qRT-PCR analysis (defined as either both approaches leading to differential expression in opposite directions or one of the methods demonstrating differential expression while the other does not) is seen in genes with a log2 fold change of <2 (53), whereas a cutoff of a log2 fold change of >3 or < −3 was used in the present study for genes to be considered differentially expressed.
When comparing gene expression profiles among dogs with PPGL, DEGs only were identified at an FDR-adjusted p < 0.1, suggesting less transcriptomic variance between tumor tissues classified as PPGL group 1 versus group 2 than between PPGL and NAM. However, this also can be related to the small sample size and heterogeneous groups of patients. The less stringent FDR-adjusted p values should be taken into consideration regarding the robustness of the comparisons between PPGL group 1 and 2. Despite this, the classification of PPGL samples based on their position in the PCA plot did allow for the prognostic prediction of dogs with PPGL, showing significantly higher Ki67 scores and worse survival times of tumors in group 1. The survival analyses in this study, however, do have some limitations. In some patients, the cause of death or reason for euthanasia was presumed to be unrelated to PPGL based on the presence of unrelated clinical signs. To definitively exclude this, necropsy would have been required, which was not performed in the majority of dogs. Although a difference in treatment between dogs in group 1 and 2 was excluded as the reason for the shorter survival times of dogs in group 1, survival in patients undergoing surgical resection of PPGL might have been influenced by a combination of factors other than the tumor (such as expertise of the surgeon and anesthetist).
Compared to group 1, PPGL group 2 overexpressed many genes involved in adrenal steroidogenesis. In humans, the cortical admixture cluster is enriched for adrenal cortical markers such as Steroidogenic Acute Regulatory Protein (STAR), CYP11B2, and CYP21A2, which could represent adrenal cortical contamination as an artifact due to impure tumor sampling or could represent a unique distinct tumor biology (9, 54, 55). In our study, most dogs in PPGL group 1 presented with a tumor thrombus (Supplementary Table S1). Sampling of the tumor thrombus, instead of the adrenal mass itself, might have resulted in less contamination with cortical cells in these dogs, possibly explaining these differences between the 2 groups.
Currently, there is no reliable way to predict the metastatic potential of PPGLs in dogs. A previous study evaluated The Pheochromocytoma of the Adrenal Gland Scaled Score (PASS) and several IHC markers in dogs with PCCs, which were shown not to be associated with survival (56). In our study, increased expression of PTN appeared to correlate with a more aggressive tumor phenotype, which might suggest that PTN could have prognostic value in dogs with PPGL. The possibility that PTN might be an interesting prognostic marker needs to be evaluated in a larger cohort of patients with long-term follow-up, and, ideally, by functional studies. In humans, the expression of PTN is increased in several types of cancer, and overexpression is usually associated with a poor prognosis (39, 40, 57). According to the GEPIA database3, PTN is highly expressed in human PPGL. To determine whether PTN could also be a prognostic factor in human PPGL, it would be interesting to consult human RNA sequencing datasets with long-term follow-up. In a study of men with prostate cancer, serum-based PTN was found to be a biomarker for metastatic progression (58). Thus, PTN might provide a promising cancer liquid biopsy marker, which warrants further research to evaluate the utility of PTN as a clinical test in different types of cancer. In addition, PTN may be therapeutically targeted, for example by treatment with microRNAs, anti-PTN antibodies, ribozyme-mediated knockdown, or inhibition of its receptor anaplastic lymphoma kinase (ALK) (39, 59, 60).
In conclusion, this study has shed light on the transcriptomic profile of canine PPGL and revealed novel targets for therapy. Future in vitro and in vivo studies are warranted to determine whether mentioned drugs (such as DRD2 antagonists, MAPK12 inhibitors, RET inhibitors, or drugs targeting PTN) can inhibit tumor growth and improve survival and quality of life in dogs with PPGL.
The raw and analyzed datasets for this study can be found in online repository: NCBI GEO under accession number GSE223526.
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because ethical approval was not required because the tissue samples originated from client-owned dogs with naturally occurring pheochromocytoma and paraganglioma, collected after surgical resection for curative purposes, or collected after euthanasia. Permission to use tissues for research purposes was obtained from all dog owners. Normal adrenal tissues were obtained from healthy dogs used in animal research, that were euthanized for reasons unrelated to the present study, which was approved by the Ethical Committee of Utrecht University. Written informed consent was obtained from the owners for the participation of their animals in this study.
MB, FS, and SGa contributed to conception and design of the study. MB, HK, SN, SGo, LS, MW, ET-S, and SGa contributed to data acquisition. MB, GG, MW, FS, MZ, and SGa contributed to data analysis and interpretation. MB wrote the first draft of the manuscript. FS wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the foundations called ‘Stichting Abri voor Dieren’ and ‘Friends of VetMed’. The open access publication fee is funded by Utrecht University’s Faculty of Veterinary Medicine.
We acknowledge the Utrecht Sequencing Facility (USEQ) for providing sequencing service and data. USEQ is subsidized by the University Medical Center Utrecht and The Netherlands X-omics Initiative (NWO project 184.034.019). We would like to thank Dr. Darja Pavlin from University of Ljubljana, Slovenia and Prof. Dr. Janos Butinar from Animal hospital Postojna, Slovenia, for contributing samples to our dataset. Results were presented as an oral abstract at the 32nd ECVIM-CA 2022 congress and the 6th International Symposium on Pheochromocytoma 2022. The oral abstract received the first oral abstract price, the ESVE-MSD Animal Health award, at the 32nd ECVIM-CA 2022 congress and was, therefore, also presented at the ACVIM Forum 2023.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1155804/full#supplementary-material
1. ^https://github.com/UMCUGenetics/RNASeq
2. ^Clinical Trials. www.ClinicalTrials.gov [accessed January 29, 2023].
3. ^GEPIA 2 (Gene Expression Profiling Interacting Analysis). PTN. http://gepia2.cancer-pku.cn [Accessed January 29, 2023].
1. Galac, S , and Korpershoek, E . Pheochromocytomas and paragangliomas in humans and dogs. Vet Comp Oncol. (2017) 15:1158–70. doi: 10.1111/vco.12291
2. Nölting, S , Ullrich, M , Pietzsch, J , Ziegler, CG , Eisenhofer, G , Grossman, A, et al. Current Management of Pheochromocytoma/Paraganglioma: a guide for the practicing clinician in the era of precision medicine. Cancers (Basel). (2019) 11:1505. doi: 10.3390/cancers11101505
3. Barthez, PY , Marks, SL , Woo, J , Feldman, EC , and Matteucci, M . Pheochromocytoma in dogs: 61 cases (1984-1995). J Vet Intern Med. (1997) 11:272–8. doi: 10.1111/j.1939-1676.1997.tb00464.x
4. Gilson, SD , Withrow, SJ , Wheeler, SL , and Twedt, DC . Pheochromocytoma in 50 dogs. J Vet Intern Med. (1994) 8:228–32. doi: 10.1111/j.1939-1676.1994.tb03222.x
5. Herrera, MA , Mehl, ML , Kass, PH , Pascoe, PJ , Feldman, EC , and Nelson, RW . Predictive factors and the effect of phenoxybenzamine on outcome in dogs undergoing adrenalectomy for pheochromocytoma. J Vet Intern Med. (2008) 22:1333–9. doi: 10.1111/j.1939-1676.2008.0182.x
6. Musser, ML , Taikowski, KL , Johannes, CM , and Bergman, PJ . Retrospective evaluation of toceranib phosphate (Palladia®) use in the treatment of inoperable, metastatic, or recurrent canine pheochromocytomas: 5 dogs (2014-2017). BMC Vet Res. (2018) 14:272. doi: 10.1186/s12917-018-1597-7
7. Coto, GM , Musser, ML , Tropf, MA , Ward, JL , Seo, YJ , Mochel, JP, et al. A multi-institutional retrospective analysis of Toceranib phosphate for presumed or confirmed canine aortic body Chemodectomas. Front Vet Sci. (2021) 8. doi: 10.3389/fvets.2021.635057
8. Lew, FH , McQuown, B , Borrego, J , Cunningham, S , and Burgess, KE . Retrospective evaluation of canine heart base tumours treated with toceranib phosphate (Palladia): 2011-2018. Vet Comp Oncol. (2019) 17:465–71. doi: 10.1111/vco.12491
9. Fishbein, L , Leshchiner, I , Walter, V , Danilova, L , Robertson, AG , Johnson, AR, et al. Comprehensive molecular characterization of Pheochromocytoma and Paraganglioma. Cancer Cell. (2017) 31:181–93. doi: 10.1016/j.ccell.2017.01.001
10. Toledo, RA . New HIF2α inhibitors: potential implications as therapeutics for advanced pheochromocytomas and paragangliomas. Endocr Relat Cancer. (2017) 24:C9–C19. doi: 10.1530/ERC-16-0479
11. Bechmann, N , and Eisenhofer, G . Hypoxia-inducible factor 2α: a key player in tumorigenesis and metastasis of Pheochromocytoma and Paraganglioma? Exp Clin Endocrinol Diabetes. (2022) 130:282–9. doi: 10.1055/a-1526-5263
12. Jochmanová, I , Yang, C , Zhuang, Z , and Pacak, K . Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. (2013) 105:1270–83. doi: 10.1093/jnci/djt201
13. O’Kane, GM , Ezzat, S , Joshua, AM , Bourdeau, I , Leibowitz-Amit, R , Olney, HJ, et al. A phase 2 trial of sunitinib in patients with progressive paraganglioma or pheochromocytoma: the SNIPP trial. Br J Cancer. (2019) 120:1113–9. doi: 10.1038/s41416-019-0474-x
14. Jimenez, C , and Toledo, R . Recent advances in the management of malignant pheochromocytoma and paraganglioma: focus on tyrosine kinase and hypoxia-inducible factor inhibitors. F1000Res. (2018) 7:F1000 Faculty Rev-1148. doi: 10.12688/f1000research.13995.1
15. Reusch, CE . Pheochromocytoma and multiple endocrine neoplasia In: E Feldman, R Nelson, C Reusch, J Scott-Moncrieff, and E Behrend, editors. Canine and Feline Endocrinology. St. Louis, Missouri: Elsevier (2015). 521–54.
16. Kimura, N , Takayanagi, R , Takizawa, N , Itagaki, E , Katabami, T , Kakoi, N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. (2014) 21:405–14. doi: 10.1530/ERC-13-0494
17. Sanders, K , Cirkel, K , Grinwis, GCM , Teske, E , van Nimwegen, SA , Mol, JA, et al. The Utrecht score: a novel histopathological scoring system to assess the prognosis of dogs with cortisol-secreting adrenocortical tumours. Vet Comp Oncol. (2019) 17:329–37. doi: 10.1111/vco.12474
18. Schneider, CA , Rasband, WS , and Eliceiri, KW . NIH image to image J: 25 years of image analysis. Nat Methods. (2012) 9:671–5. doi: 10.1038/nmeth.2089
19. Robinson, MD , McCarthy, DJ , and Smyth, GK . edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. (2009) 26:139–40. doi: 10.1093/bioinformatics/btp616
20. Blighe, K , Rana, S , and Lewis, M . Enhanced Volcano: publication-ready volcano plots with enhanced colouring and labeling. (2022). https://github.com/kevinblighe/EnhancedVolcano.
21. Subramanian, A , Tamayo, P , Mootha, VK , Mukherjee, S , Ebert, BL , Gillette, MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. (2005) 102:15545–50. doi: 10.1073/pnas.0506580102
22. Mootha, VK , Lindgren, CM , Eriksson, K-F , Subramanian, A , Sihag, S , Lehar, J, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. (2003) 34:267–73. doi: 10.1038/ng1180
23. Reimand, J , Isserlin, R , Voisin, V , Kucera, M , Tannus-Lopes, C , Rostamianfar, A, et al. Pathway enrichment analysis and visualization of omics data using g: profiler, GSEA. Cytoscape and EnrichmentMap Nat Protoc. (2019) 14:482–517. doi: 10.1038/s41596-018-0103-9
24. Saveanu, A , Muresan, M , de Micco, C , Taieb, D , Germanetti, AL , Sebag, F, et al. Expression of somatostatin receptors, dopamine D 2 receptors, noradrenaline transporters, and vesicular monoamine transporters in 52 pheochromocytomas and paragangliomas. Endocr Relat Cancer. (2011) 18:287–300. doi: 10.1530/ERC-10-0175
25. Mulligan, LM . GDNF and the RET receptor in cancer: new insights and therapeutic potential. Front Physiol. (2019) 9:1873. doi: 10.3389/fphys.2018.01873
26. Ahmed, DS , Isnard, S , Lin, J , Routy, B , and Routy, JP . GDF15/GFRAL pathway as a metabolic signature for cachexia in patients with cancer. J Cancer. (2021) 12:1125–32. doi: 10.7150/jca.50376
27. Anderson, PM , Trucco, MM , Tarapore, RS , Zahler, S , Thomas, S , Gortz, J, et al. Phase II study of ONC201 in neuroendocrine tumors including Pheochromocytoma-Paraganglioma and desmoplastic small round cell tumor. Clin Cancer Res. (2022) 28:1773–82. doi: 10.1158/1078-0432.CCR-21-4030
28. Ling, L , Maguire, JJ , and Davenport, AP . Endothelin-2, the forgotten isoform: emerging role in the cardiovascular system, ovarian development, immunology and cancer. Br J Pharmacol. (2013). 168:283–95. doi: 10.1111/j.1476-5381.2011.01786.x
29. González-González, L , and Alonso, J . Periostin: a matricellular protein with multiple functions in cancer development and progression. Front Oncol. (2018) 8:225. doi: 10.3389/fonc.2018.00225
30. Lee, YJ , Kim, IS , Park, SA , Kim, Y , Lee, JE , Noh, DY, et al. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther. (2013) 21:1004–13. doi: 10.1038/mt.2013.30
31. Dorafshan, S , Razmi, M , Safaei, S , Gentilin, E , Madjd, Z , and Ghods, R . Periostin: biology and function in cancer. Cancer Cell Int. (2022) 22:315. doi: 10.1186/s12935-022-02714-8
32. Khan, T , Kryza, T , Lyons, NJ , He, Y , and Hooper, JD . The CDCP1 signaling hub: a target for cancer detection and therapeutic intervention. Cancer Res. (2021) 81:2259–69. doi: 10.1158/0008-5472.CAN-20-2978
33. Xin, L , Gao, J , Zheng, Z , Chen, Y , Lv, S , Zhao, Z, et al. Fibroblast activation protein-α as a target in the bench-to-bedside diagnosis and treatment of tumors: a narrative review. Front Oncol. (2021) 11:648187. doi: 10.3389/fonc.2021.648187
34. Zhang, H , Shen, YW , Zhang, LJ , Chen, JJ , Bian, HT , Gu, WJ, et al. Targeting endothelial cell-specific molecule 1 protein in Cancer: a promising therapeutic approach. Front Oncol. (2021) 11:687120. doi: 10.3389/fonc.2021.687120
35. van Loon, K , Huijbers, EJM , and Griffioen, AW . Secreted frizzled-related protein 2: a key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metastasis Rev. (2021) 40:191–203. doi: 10.1007/s10555-020-09941-3
36. Xu, W , Liu, R , Dai, Y , Hong, S , Dong, H , and Wang, H . The role of p38γ in Cancer: from review to outlook. Int J Biol Sci. (2021) 17:4036–46. doi: 10.7150/ijbs.63537
37. Fliedner, SMJ , Engel, T , Lendvai, NK , Shankavaram, U , Nölting, S , Wesley, R, et al. Anti-cancer potential of MAPK pathway inhibition in paragangliomas-effect of different statins on mouse pheochromocytoma cells. PLoS One. (2014) 9:e97712. doi: 10.1371/journal.pone.0097712
38. Musa, J , Aynaud, MM , Mirabeau, O , Delattre, O , and Grünewald, TGP . MYBL2 (B-Myb): a central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis. (2017) 8:e2895. doi: 10.1038/CDDIS.2017.244
39. Wang, X . Pleiotrophin: activity and mechanism. Adv Clin Chem. (2020) 98:51–89. doi: 10.1016/bs.acc.2020.02.003
40. Papadimitriou, E , Pantazaka, E , Castana, P , Tsalios, T , Polyzos, A , and Beis, D . Pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta as regulators of angiogenesis and cancer. Biochim Biophys Acta Rev Cancer. (2016) 1866:252–65. doi: 10.1016/j.bbcan.2016.09.007
41. Zhou, J , Yang, Y , Zhang, Y , Liu, H , and Dou, Q . A meta-analysis on the role of pleiotrophin (PTN) as a prognostic factor in cancer. PLoS One. (2018) 13:e0207473. doi: 10.1371/journal.pone.0207473
42. Liu, X , Gong, H , and Huang, K . Oncogenic role of kinesin proteins and targeting kinesin therapy. Cancer Sci. (2013) 104:651–6. doi: 10.1111/cas.12138
43. Chen, T , Sun, Y , Ji, P , Kopetz, S , and Zhang, W . Topoisomerase IIα in chromosome instability and personalized cancer therapy. Oncogene. (2015) 34:4019–31. doi: 10.1038/onc.2014.332
44. Barker, N , and Clevers, H . Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. (2006) 5:997–1014. doi: 10.1038/nrd2154
45. Morimoto, E , Inagaki, K , Komatsubara, M , Terasaka, T , Itoh, Y , Fujisawa, S, et al. Effects of Wnt-β-catenin signaling and Sclerostin on the phenotypes of rat Pheochromocytoma PC12 cells. J Endocr Soc. (2022) 6. doi: 10.1210/jendso/bvac121
46. von Marschall, Z , and Fisher, LW . Secreted frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling. Biochem Biophys Res Commun. (2010) 400:299–304. doi: 10.1016/j.bbrc.2010.08.043
47. Xin, Z , Li, Y , Meng, DL , Ren, J , and Men, J . Elevated expression of the MYB proto-oncogene like 2 (MYBL2) encoding gene as a prognostic and predictive biomarker in human cancers. Math Biosci Eng. (2022) 19:1825–42. doi: 10.3934/mbe.2022085
48. Papathomas, TG , Suurd, DPD , Pacak, K , Tischler, AS , Vriens, MR , Lam, AK, et al. What have we learned from molecular biology of Paragangliomas and Pheochromocytomas? Endocr Pathol. (2021) 32:134–53. doi: 10.1007/s12022-020-09658-7
49. London, C , Mathie, T , Stingle, N , Clifford, C , Haney, S , Klein, MK, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Vet Comp Oncol. (2012) 10:194–205. doi: 10.1111/j.1476-5829.2011.00275.x
50. Rochette, L , Zeller, M , Cottin, Y , and Vergely, C . Insights into mechanisms of GDF15 and receptor GFRAL: therapeutic targets. Trends Endocrinol Metab. (2020) 31:939–51. doi: 10.1016/j.tem.2020.10.004
51. Holt, DE , Henthorn, P , Howell, VM , Robinson, BG , and Benn, DE . Succinate dehydrogenase subunit D and succinate dehydrogenase subunit B mutation analysis in canine phaeochromocytoma and paraganglioma. J Comp Pathol. (2014) 151:25–34. doi: 10.1016/j.jcpa.2014.03.007
52. Korpershoek, E , Dieduksman, DAER , Grinwis, GCM , Day, MJ , Reusch, CE , Hilbe, M, et al. Molecular alterations in dog pheochromocytomas and paragangliomas. Cancers (Basel). (2019) 11:607. doi: 10.3390/cancers11050607
53. Coenye, T . Do results obtained with RNA-sequencing require independent verification? Biofilms. (2021) 3:100043. doi: 10.1016/j.bioflm.2021.100043
54. Fishbein, L , and Wilkerson, MD . Chromaffin cell biology: inferences from the Cancer genome atlas. Cell Tissue Res. (2018) 372:339–46. doi: 10.1007/s00441-018-2795-0
55. Crona, J , Backman, S , Welin, S , Taïeb, D , Hellman, P , Stålberg, P, et al. RNA-sequencing analysis of adrenocortical carcinoma, pheochromocytoma and paraganglioma from a pan-cancer perspective. Cancers (Basel). (2018) 10:518. doi: 10.3390/cancers10120518
56. Zini, E , Nolli, S , Ferri, F , Massari, F , Gerardi, G , Nicoli, S, et al. Pheochromocytoma in dogs undergoing adrenalectomy. Vet Pathol. (2019) 56:358–68. doi: 10.1177/0300985818819174
57. Perez-Pinera, P , Chang, Y , and Deuel, TF . Pleiotrophin, a multifunctional tumor promoter through induction of tumor angiogenesis, remodeling of the tumor microenvironment and activation of stromal fibroblasts. Cell Cycle. (2007) 6:2877–83. doi: 10.4161/cc.6.23.5090
58. Liu, S , Shen, M , Hsu, EC , Zhang, CA , Garcia-Marques, F , Nolley, R, et al. Discovery of PTN as a serum-based biomarker of pro-metastatic prostate cancer. Br J Cancer. (2021) 124:896–900. doi: 10.1038/s41416-020-01200-0
59. Grzelinski, M , Steinberg, F , Martens, T , Czubayko, F , Lamszus, K , and Aigner, A . Enhanced anjtitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK. Neoplasia. (2009) 11:145–56. doi: 10.1593/neo.81040
60. Li, R , and Morris, SW . Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev. (2008) 28:372–412. doi: 10.1002/med.20109
Keywords: pheochromocytoma, paraganglioma, adrenal, dog, transcriptome, RNA sequencing, therapeutic target, prognostic marker
Citation: van den Berg MF, Kooistra HS, Grinwis GCM, Nicoli S, Golinelli S, Stammeleer L, van Wolferen ME, Timmermans-Sprang EPM, Zandvliet MMJM, van Steenbeek FG and Galac S (2023) Whole transcriptome analysis of canine pheochromocytoma and paraganglioma. Front. Vet. Sci. 10:1155804. doi: 10.3389/fvets.2023.1155804
Received: 31 January 2023; Accepted: 08 August 2023;
Published: 24 August 2023.
Edited by:
Pablo Martín-Vasallo, University of La Laguna, SpainReviewed by:
Priscila Emiko Kobayashi, São Paulo State University, BrazilCopyright © 2023 van den Berg, Kooistra, Grinwis, Nicoli, Golinelli, Stammeleer, van Wolferen, Timmermans-Sprang, Zandvliet, van Steenbeek and Galac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marit F. van den Berg, bS5mLnZhbmRlbmJlcmdAdXUubmw=
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.