- 1School of Public Health and Management, Guang University of Chinese Medical, Nanning, Guangxi, China
- 2Guangxi Key Laboratory of Veterinary Biotechology, Guangxi Veterinary Research Institute, Nanning, Guangxi, China
- 3Department of Employment, Nanning Normal University, Nanning, Guangxi, China
Hepatitis E is a disease of public health significance caused by the cross-species transmission of zoonotic hepatitis E virus (HEV) infection. There are no specific drugs. In this study, network pharmacology was used to reveal the mechanism of treatment of the active constituents of the Abrus cantoniensis Hance on hepatitis E. Based on the previously published representative components of A. cantoniensis Hance, we were screened the active components with OB ≥ 20% and DL ≥ 0.1 in A. cantoniensis Hance based on the TCMSP, predicted the target online through Swiss target prediction, and integrated the hepatitis E target in the GeneCards and DisGenet databases. Then, the core target was screened and the GO and KEGG enrichment and the network of the drug-active-ingredient-disease-pathway-target analysis were performed by the Cytoscape software. There were 11,046 hepatitis E targets, including PI3K-AKt, SRC, MAPK, PTPN11, EGFR, STAT1 and so on. The core ingredients include Oleanolic acid, Butin, β-sitosterol, Soyasapogenol E, 5,7-dihydroxy-2-methyl-8-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one, Stigmasterol, Emodin, Physcion, and Enoxolone. A total of 1,410 GO enrichment results of core targets, including 1,246 biological process, 51 cell composition and 113 molecular function results. KEGG pathway was enriched in 150 related pathways, suggesting that A. cantoniensis Hance acts on cancer signaling pathway, endocrine resistance pathway, PI3K-AKt signaling pathway, MAPK, TNF and other signaling pathway. Through key components such as Oleanolic acid, Butin, β-sitosterol, Stigmasterol, and Enoxolone and other components interferes with AKT1, IL-6 and TNF, and regulates pathway in cancer, PI3K-AKt signaling pathway and MAPK pathway to play a therapeutic role in hepatitis E.
Introduction
Hepatitis is one of the major national and global health challenges (1). Hepatitis E caused by fecal-oral transmission of zoonotic hepatitis E virus (HEV) is one of the major public health hazards (2). Genotypes 1 and 2 HEV are restricted to humans and often associated with large outbreaks and epidemics in developing countries with poor sanitation conditions, whereas genotypes 3 and 4 HEV infect humans, pigs and other animal species and are responsible for sporadic cases of hepatitis E (2–4). HEV not only has a wide range of animal hosts, but also has the characteristics of the cross-species transmission (4, 5). According to data released by the World Health Organization (WHO), about 20 million people worldwide are infected with hepatitis E every year, more than 3 million acute cases of hepatitis E, and nearly 70,000 deaths related to hepatitis E. Especially in developing countries or regions, the prevalence of hepatitis E is high. But in recent years, with the expansion of the transmission range, HEV has also become increasingly serious in developed countries. It is considered to be a new global public health problem with serious threats, which can particularly cause significant mortality in pregnant women and patients with cirrhosis (6), and there is no effective treatment so far. Recently, it has been found that immunocompromised patients (such as organ transplant recipients) are more likely to develop chronic hepatitis E (7). In pregnant women and patients with underlying chronic liver disease, acute hepatitis virus infection can develop into severe hepatitis and liver failure, directly causing the mortality rate of pregnant women and underlying chronic liver disease patients as high as 25 and 75%, yet there is no effective treatment. Currently, the Chinese-developed HEV vaccine, HEV-239, has been used in China for many years (8), but has not been licensed for use outside China. Drug therapy is the most widely used method for hepatitis E treatment at present. Although there are reports that some drugs such as ribavirin, interferon-alpha and Qingkailing injection have some therapeutic effects, none of them are characteristic drugs of HEV (9). Moreover, ribavirin is not a panacea, and the administration of ribavirin for pregnant women is highly debatable. In order to significantly improve the effective rate of treatment, shorten the treatment time, reduce the incidence of adverse reactions, reduce the cost of treatment, and reduce the burden on patients, it is urgent to develop efficient, drug-resistant and specific hepatitis E treatment drugs to help realize the goal of eliminating viral hepatitis as a major public health threat by 2030 proposed by the WHO (10).
Abrus cantoniensis Hance is the whole dried plant of leguminous plant A. cantoniensis Hance, which is a genuine regional drug in Guangxi, China. It tastes sweet and slightly bitter, and has the effects of dampness and get rid of jaundice, clearing heat and detoxifying, soothing liver and relieving pain. It is used for dampness-heat jaundice, side and rib discomfort, abdominal distension pain, and mammarectomy pain (11). It is often used in the treatment of chronic hepatitis B and other liver diseases. In the treatment of chronic hepatitis B, compound composed of chicken bone herb as the main drug combined with Entecavir can not only enhance the inhibition of HBV DNA replication, but also improve liver function indicators and delay the progression of liver fibrosis (10). In particular, through key components such as abrine, abrisapogenol, soyasapogenol and other components regulates hepatitis B signaling pathway to play a therapeutic role in hepatitis B (10). In addition, there have been studies on the combined treatment of 50 cases of toxic hepatitis in 8 weeks with traditional Chinese medicines such as A. cantoniensis Hance, Yinchen and gardenia, and the total effective rate is high (9, 10). So that A. cantoniensis Hance has significant effects on liver protection, anti-inflammatory, antiviral and immune regulation (10). However, its therapeutic effect on hepatitis E and its inhibitory effect on HEV have not been reported.
Network pharmacology is widely used in the field of drug research and development. Based on the core idea of “multi-component, multi-target and multi-pathway” of systems biology, it selects potential targets and pathways by calculating the network parameters of drug component targets, interprets the potential biological mechanism of Traditional Chinese medicine treatment of diseases, and discovers the basis of potential pharmacodynamic substances (12). In this study, network pharmacology was used to explore the mechanism of action of A. cantoniensis Hance in the treatment of hepatitis E, which provided theoretical support for subsequent drug development and mechanism verification.
Materials and methods
Predict active ingredients of A. cantoniensis Hance
The effective ingredients were obtained using “A. cantoniensis” as the keyword, searching through Traditional Chinese Medicine Systematic Pharmacology technology platform (TCMSP, https://tcmspw.com/tcmsp.php) (12), oral bioavailability (OB) ≥ 20%, and drug-likeness (DL) ≥ 0.1. Also, the main effective active ingredients of A. cantoniensis Hance were screened during this process.
Acquire and collect action targets of A. cantonensis Hance
Based on previously published references (12), the TCMSP database and Swiss Target Prediction database were used to predict the target of related active ingredients first, and the target of active ingredients of Abrus cantonensis Hance was obtained afterward by importing it into the Uniprot online protein database (https://www.uniprot.org/).
Acquire hepatitis E-related targets and map with A. cantoniensis Hance prediction targets
The hepatitis E-related targets were obtained using “hepatitis E” as a keyword and searched through the GeneCards database (https://www.genecards.org/) and OMIM database (https://omim.org/). Following, the duplicate target genes were deleted. Finally, both genes of epilepsy and A. cantoniensis Hance were mapped together to get a venny map.
Protein–protein interaction network analysis and the core targets screen
The potential target of A. cantonensis Hance for hepatitis E treatment was introduced into the String database (https://string-db.org/), and the species “Homo sapiens” was selected to obtain a PPI network. The interaction score is set to ≥0.9 confidence level. The data was then imported into the Cytoscape 3.9.1 software.
GO analysis and KEGG pathway enrichment analysis
A total of 62 core gene targets were imported into the Metascape database (https://metascape.org/gp/index) for Gene Ontology (GO) analysis and Kyoto Genomics and Genomics Encyclopedia (KEGG) analysis. Then, import KEGG path relationship file into the Cytoscape 3.9.1 software to predict the degree value, which was used to adjust the node size afterward. Finally, the “target -KEGG path” relationship network diagram was generated.
Construct and analyze “drugs-active ingredients-diseases-pathway-targets” network
The obtained potential targets, KEGG pathways, diseases, and A. cantoniensis Hance for hepatitis E treatment were sent to the Cytoscape software to construct a “drug-active ingredients-diseases-pathway-targets” regulatory network diagram. Then, analyse the potential relationship between genes and components of A. cantoniensis Hance by a network diagram.
Results
Screen active ingredients of A. cantoniensis
We set OB ≥ 20% and DL ≥ 0.1 to obtain 9 main active ingredients of A. cantoniensis Hance, including Oleanolic acid, Butin, β-sitosterol, Soyasapogenol E, 5,7-dihydroxy-2-methyl-8-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] oxychromen-4-one, Stigmasterol, Emodin, Physcion, and Enoxolone (Supplementary Table S1), based on the TCMSP database.
Predict potential targets of A. cantoniensis Hance components in hepatitis E
After repeated targets were removed, 285 targets of A. cantoniensis Hance active ingredients were obtained based on the Swiss target prediction (Supplementary Figure S1).
All 11,046 hepatitis E targets were obtained based on the GeneCards database and PharmGKB database platform. Intersecting the target related to A. cantoniensis Hance with the disease target, we obtained the Wayne diagram of A. cantoniensis Hance target for hepatitis E treatment (Supplementary Figure S1). 258 common targets were obtained, and the three effective components of A. cantoniensis Hance were mapped with hepatitis E genes (Supplementary Figure S1). It was found that there were multiple common targets of 9 components and hepatitis E and could play its role through multiple targets (Supplementary Figure S2).
Screening of core targets
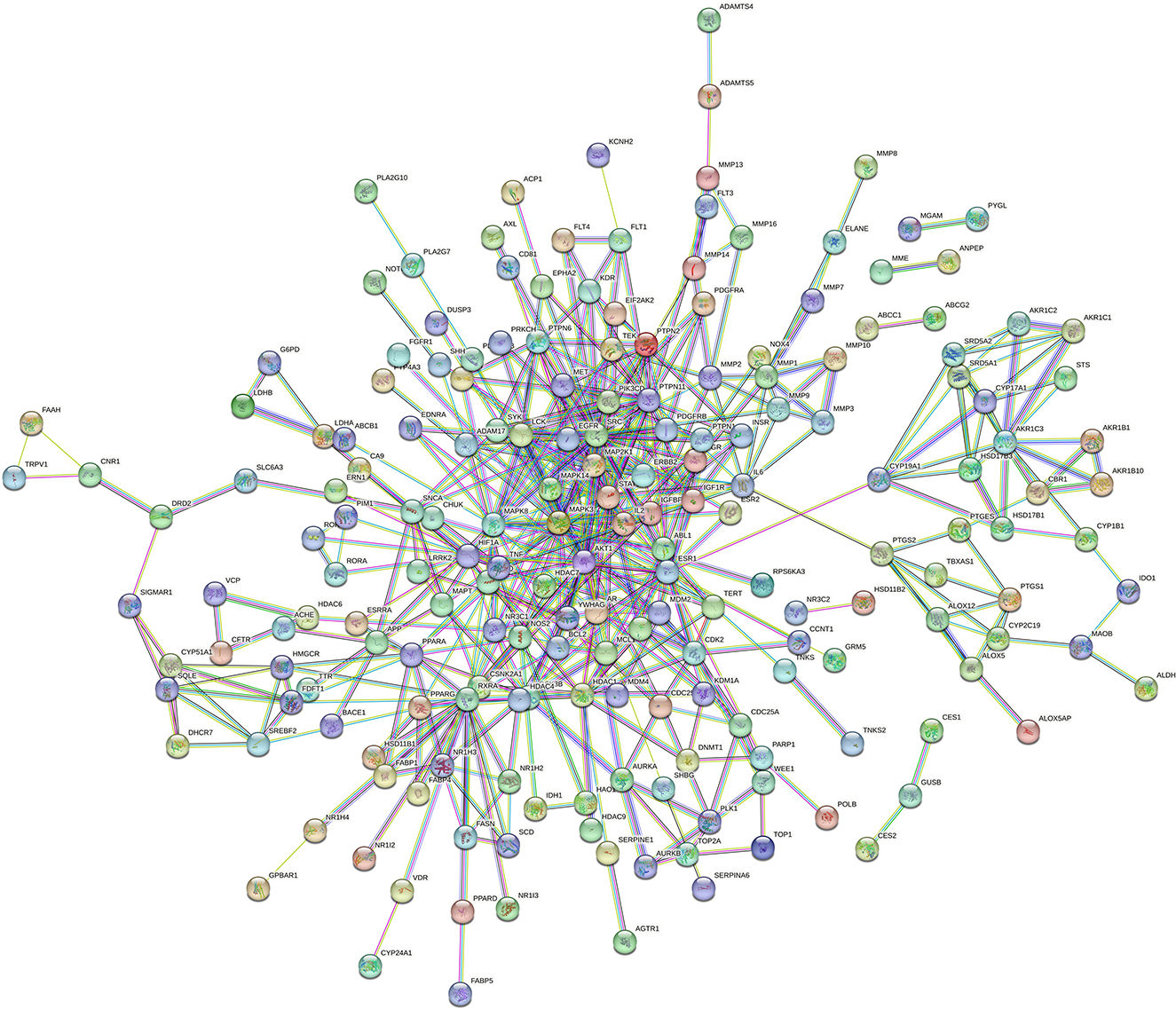
As shown in Figure 1, the PPI network consists of 258 nodes and 501 edges.
Based on the results of PPI, Cytoscape 3.9.1 software was utilized to sequence and visualize the intersection targets according to degree (maximum degree of inner circle), and to draw bar graph/target network graph. We obtained 62 core gene targets (Figure 2A). The top 10 targets with the highest correlation were SRC (70), MAPK3 (64), AKT1 (52), RXRA (50), PTPN11 (50), ESR1 (46), EGFR (38), MAPK8 (38), STAT1 (36), and MAPK14 (34) (Figure 2B). These results suggest that these genes may be potential targets for the treatment of hepatitis E.
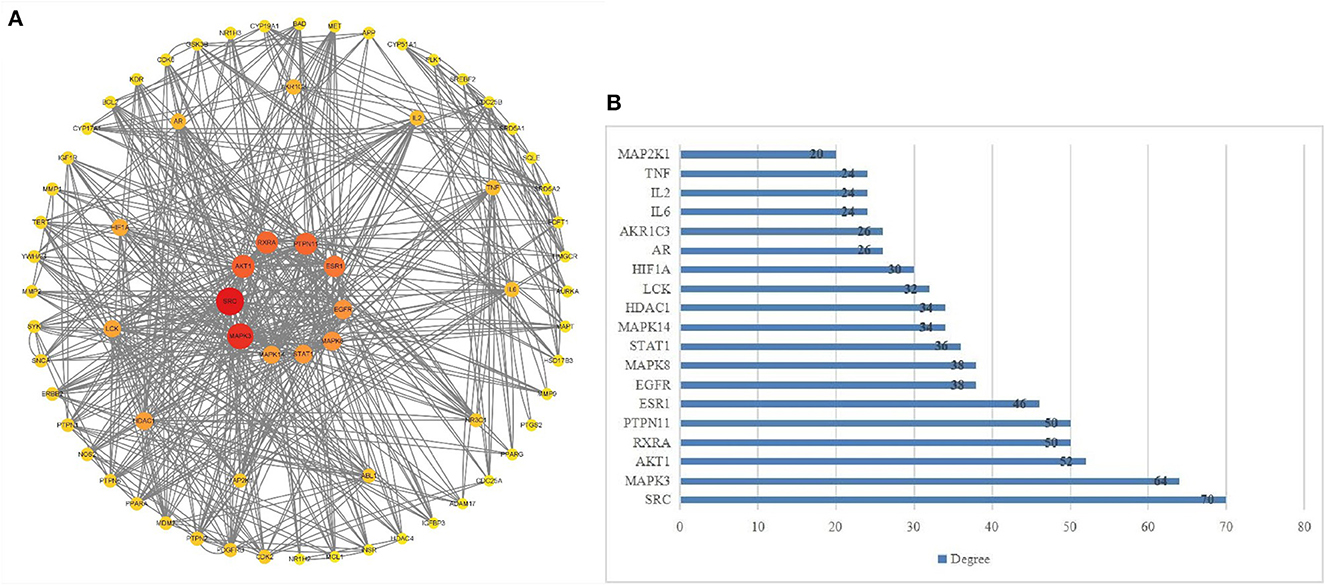
Figure 2. (A, B) Core target network diagram. The target near the center of the circle is the core target.
Biological function and pathway analysis
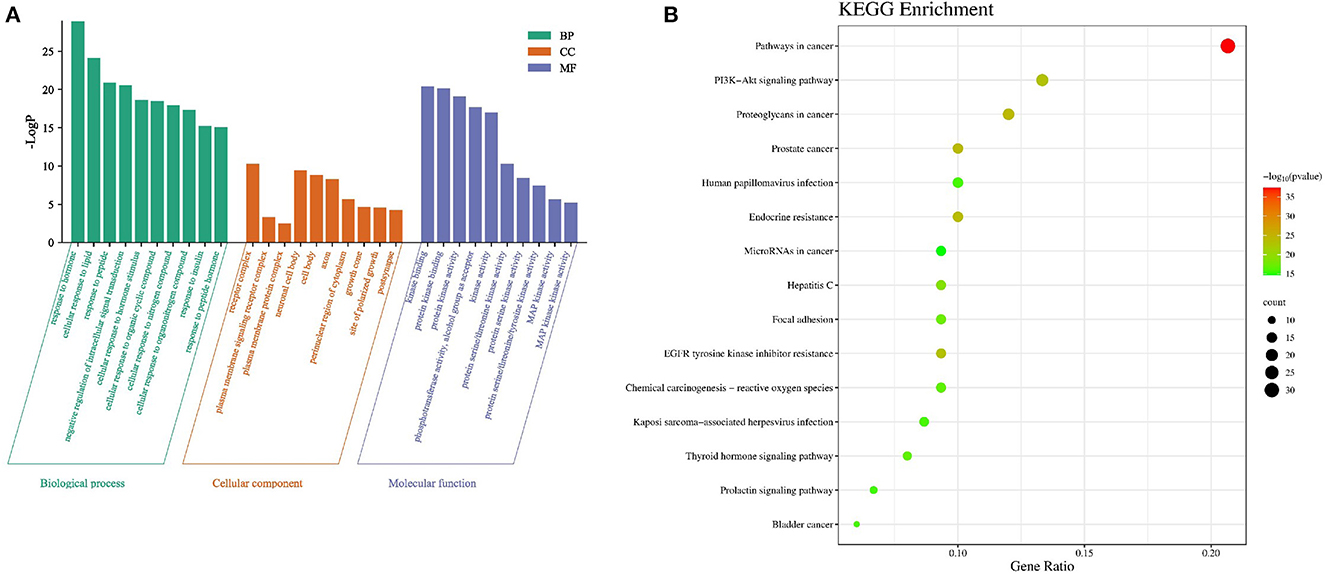
There were 1,410 GO enrichment results of core targets, including 1,246 biological process (BP), 51 cell composition (CC) and 113 molecular function (MF) results (Figure 3A). The results of GO annotation showed that the biological functions of A. cantoniensis Hance, the key target of hepatitis E treatment, were mainly involved in response to hormone, negative regulation of intracellular signal transduction, neuronal cell body, cell body, protein kinase activity, and other biological processes responsiveness. It showed that the effective components of A. cantoniensis Hance played a role in treating hepatitis E by regulating various biological pathways.
There were 150 KEGG enrichment-related pathways (Figure 3B). The enrichment results of KEGG pathway show that the key target genes of A. cantoniensis Hance in treating hepatitis E were significantly enriched in the several signal pathways, including pathways in cancer, PI3K-AKt signaling pathway, EGFR tyrosine kinase inhibitor resistance, Hepatitis C pathway, Thyroid hormone signaling pathway, and etc., besides, the MAPK signaling pathway, p53 signaling pathway, B cell receptor signaling pathway, Hepatitis B, T cell receptor signaling pathway, TNF signaling pathway and so on, were also included (p < 0.05). It is speculated that these pathways may also be in Abrus cantonensis Hance.
Construct a drug-active ingredient-disease-pathway-target network
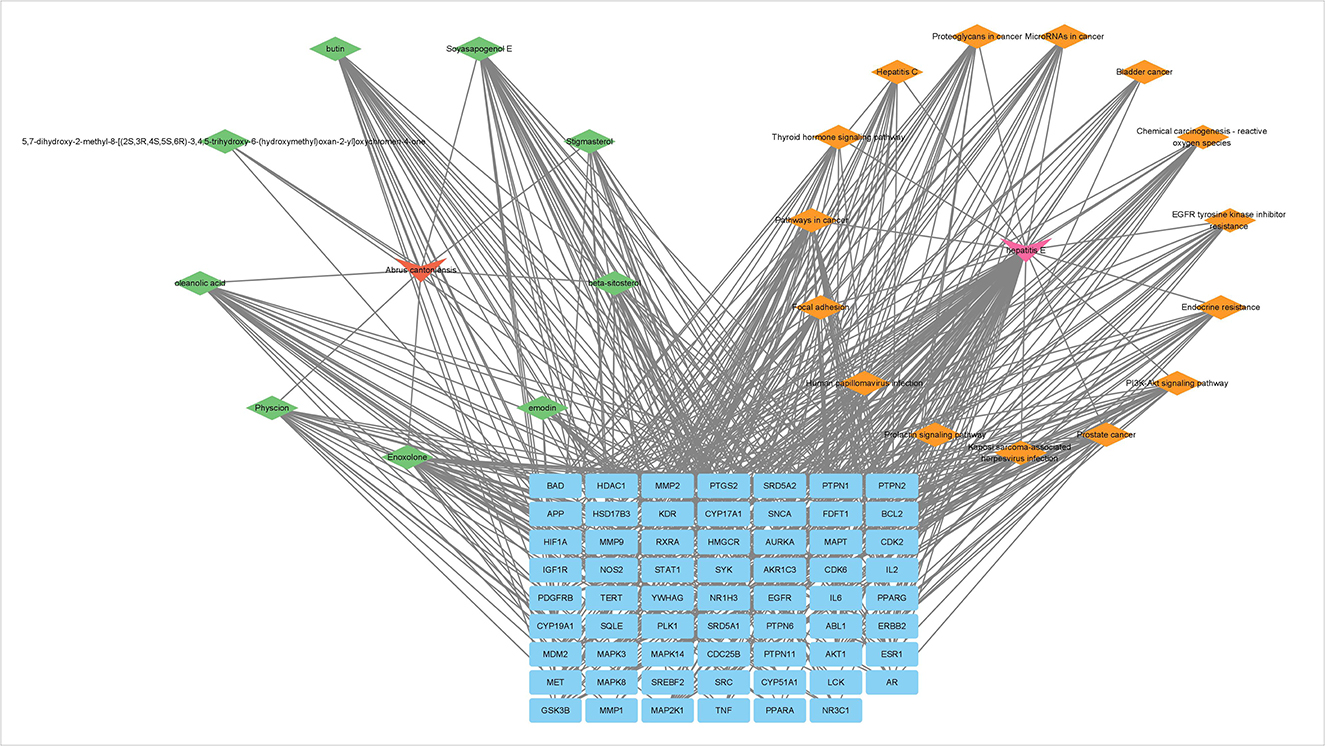
Visualization of KEGG concentration analysis correlated with hepatitis E development, 9 key active components acting 15 key pathways associated with hepatitis E, including PI3K-AKt signaling pathway, MAPK signaling pathway, IL-2/IL-6 signaling pathway and TNF signaling pathway (Figure 4). It is indicated that Abrus cantonensis Hance exerts potential therapeutic effect for hepatitis E through “multiple component, multitarget and multiple pathway.”
Discussions
The results of this study showed that the active constituents of A. cantoniensis Hance acted on multiple targets and pathways. Among them, Oleanolic acid, Butin, 5,7-dihydroxy-2-methyl-8-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one, β-sitosterol, Soyasapogenol E, Stigmasterol, Emodin, Physcion, and Enoxolone are the core chemical components in the treatment of hepatitis E by A. cantoniensis Hance. It has been shown that hepatitis E can be treated by compound glycyrrhizin (SNMC), which is a compound consisting of enoxolone and glucuronic acid (13). In addition, glycyrrhizic acid can inhibit the inflammatory response of liver, reduce the pathological injury of liver, and restore the normal function of damaged liver cells (14). Therefore, this study is the first to confirm that enoxolone, the active ingredient of A. cantoniensis Hance, can play a role in the treatment of hepatitis E. In addition, Butin, β-sitosterol and stigmasterol have been shown to have therapeutic effects on Hepatitis B (10). This study also confirmed that it has the same therapeutic effect on hepatitis E, possibly because these ingredients can inhibit the inflammatory response and play the same role. In addition, 5,7-dihydroxy-2-methyl-8-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one, Soyasapogenol E, Emodin and Physcion were the first reported active ingredient of A. cantoniensis Hance in the treatment of hepatitis E.
The target organ of HEV is the liver, so at the beginning of infection, Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) in the liver are significantly elevated. Suggesting that these enzymes play a role in liver damage. After the action of A. cantoniensis Hance, the binding force of some protein kinases and the activity of some protein kinases are also enhanced. It may be that the effective ingredients of A. cantoniensis Hance regulate the enzyme activity in the body, so as to play the role of liver protection. In addition, nervous system complication is the most common symptom of HEV infection, and also the most concerned point. Shi et al. (15) found that HEV can infect and replicate in the brain and spinal cord tissues of gerbils, and the expression of tight junction protein Zonula occlidens-1 is decreased in the infected gerbils, while the number of astrocytes is significantly increased. This result confirms that HEV can destroy the blood brain barrier and is directly related to the damage of the nervous system. In the GO enrichment study, neuronal cell body was found to be highly enriched in this study, indicating that the active components of A. cantoniensis Hance can effectively improve neuronal cell body, so as to effectively improve the neurological complications caused by HEV.
The results of this study show that The active components of the A. cantoniensis Hance can affect cancer signaling pathway, endocrine resistance, PI3K-AKt signaling pathway, MAPK signaling pathway, IL-2/IL-6/IL-17 signaling pathway and TNF signaling pathway. These signaling pathways play an important role in the occurrence and development of hepatitis and liver cancer. Studies have shown that IL-6 can be expressed in hepatocytes, and the main activators of IL-6 expression are IL-1b and tumor necrosis factor (TNF-α) (16). Activation of the Phosphoinositol 3 kinase (PI3K)-protein kinase activates the B(PKB)/Akt pathway, in which JAK phosphorylates and activates PI3K and then phosphorylates some phosphatidylinositol to phosphatidylinositol-4, 5-diphosphate (PIP2) and phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3, in turn, phosphorylates and activates PKB/Akt, which is recruited into the plasma membrane. This PI3K/Akt pathway contributes to the activation of NF-κB (16). In this study, IL-6 is the key gene in the treatment of hepatitis E, and the regulation of PI3K-Akt signaling pathway is the potential mechanism of the treatment of hepatitis E by the A. cantoniensis Hance. Tumor necrosis factor (TNF) signaling pathway is a classic inflammatory signaling pathway, which can promote the generation of many diseases, including liver diseases. TNF signaling pathway can be regulated upstream and downstream with other inflammatory proteins and cytokines, and then fluctuate other inflammatory pathways or regulate each other, jointly promoting the development of diseases. Recent evidence suggests that cathepsin C interacts with TNF-α/p38 mitogen activated protein kinase (MAPK) signaling pathways to promote hepatocellular carcinoma proliferation and metastasis (17). TNF and IL-17 have the potential to promote liver inflammation and fibrosis induced by activation of Nod-like receptor heat protein domain associated Protein 3 (NLRP3) inflammasome (18). NLRP3 is also considered as a potential pathogenic mechanism involved in HEV infection (19). Therefore, the active constituents of A. cantoniensis Hance can also play a role in the treatment of hepatitis E by regulating TNF signaling pathway. In addition, the MAPK signaling pathway can also mediate the development of liver diseases (20), and the active ingredients of the A. cantoniensis Hance can regulate the above inflammatory signaling pathway, thus playing a potential therapeutic role.
In conclusion, this study reveals the potential mechanism of A. cantoniensis Hance in the treatment of hepatitis E through network pharmacology. We found that A. cantoniensis Hance may enhance the activity of protein kinases, promote the growth of neuronal cells body, and regulate hepatitis related signaling pathways (PI3K-AKt, MAPK, IL-2/IL-6/IL-17 signaling pathway and so on), which can serve as a reference in hepatitis E treatment. However, network pharmacology analysis only provides a theoretical prediction, which needs to be verified by further experiments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZX, CW, and ZL completed data analysis and the draft and contributed to the manuscript. DZ assisted in this manuscript. BD provided the funding of research, reviewed, and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the 2022 Doctoral Research Foundation of Guangxi University of Chinese Medicine and the High-level Talent Cultivation and Innovation Team funding by Guangxi University of Chinese Medicine (2022A009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1155677/full#supplementary-material
Supplementary Table S1. Chemical composition of A. cantoniensis Hance.
Supplementary Figure S1. Intersection target of A. cantoniensis Hance and hepatitis E.
Supplementary Figure S2. Map of core target.
References
1. Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, et al. Hepatitis E. Lancet. (2012) 379:2477–88. doi: 10.1016/S0140-6736(11)61849-7
2. Larrue H, Abravanel F, Péron JM. Hepatitis E, what's the real issue? Liver Int. (2020) 1:43–7. doi: 10.1111/liv.14351
3. Aslan AT, Balaban HY. Hepatitis E virus: epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. (2020) 26:5543–60. doi: 10.3748/wjg.v26.i37.5543
4. Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. (2010) 140:256–65. doi: 10.1016/j.vetmic.2009.03.017
5. Sanford BJ, Emerson SU, Purcell RH, Engle RE, Dryman BA, Cecere TE, et al. Serological evidence for a hepatitis e virus-related agent in goats in the United States. Transbound Emerg Dis. (2013) 60:538–45. doi: 10.1111/tbed.12001
6. Meng XJ. Expanding host range and cross-species infection of hepatitis E virus. PLoS Pathog. (2016) 12:e1005695. doi: 10.1371/journal.ppat.1005695
7. Kamar N, Rostaing L, Izopet J. Hepatitis E virus infection in immunosuppressed patients: natural history and therapy. Semin Liver Dis. (2013) 33:62–70. doi: 10.1055/s-0033-1338115
8. European Association for the Study of the Liver. Electronic address: ZWFzbG9mZmljZUBlYXNsb2ZmaWNlLmV1; European association for the study of the liver. EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. (2018) 68:1256–71. doi: 10.1016/j.jhep.2018.03.005
9. Ma XL, Wu H, Yin DM, Zheng XY, Yang XL, Yao JY. Advances in drug therapy of hepatitis E. J Qiqihar Med Univ. (2022) 43:1175–9. doi: 10.3969/j.issn.1002-1256.2022.12.019
10. Li TP, Wu FF, Sun H, Yan GL, He YM, Tan ZE, et al. Exploring the mechanism of the A. cantoniensis in the treatment of hepatitis B based on network pharmacology and molecular docking. Drugs Clin. (2022) 37:33–41. doi: 10.7501/j.issn.1674-5515.2022.01.005
11. Li TS, Huang SY. Chemical components and clinical application of Abri Herba. Chin J Exp Tradit Med Formul. (2019) 25:226–34. doi: 10.13422/j.cnki.Syfjx.20191038 (in Chinese)
12. Wang Y, Li X, Dou P, Qiao T, Chang Y. Antiepileptic therapy of A. cantoniensis: evidence from network pharmacology. Evid Based Compl Alternat Med. (2022) 2022:7748787. doi: 10.1155/2022/7748787
13. Park HY, Park SH, Yoon HK, Han MJ, Kim DH. Anti-allergic activity of 18beta-glycyrrhetinic acid-3-O-beta-D-glucuronide. Arch Pharm Res. (2004) 27:57–60. doi: 10.1007/BF02980047
14. Zhao YK, Li L, Liu X, Cheng SP, Guo QQ, Fan DP, et al. Explore pharmacological mechanism of glycyrrhizin based on systems pharmacology. China J Chin Mater Med. (2016) 41:1916–20. doi: 10.4268/cjcmm20161026
15. Shi R, Soomro MH, She R, Yang Y, Wang T, Wu Q, et al. Evidence of hepatitis E virus breaking through the blood-brain barrier and replicating in the central nervous system. J Viral Hepat. (2016) 23:930–9. doi: 10.1111/jvh.12557
16. Kaur S, Bansal Y, Kumar R, Bansal G, A. panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg Med Chem. (2020) 28:115327. doi: 10.1016/j.bmc.2020.115327
17. Zhang GP, Yue X, Li SQ. Cathepsin C interacts with TNF-α/p38 MAPK signaling pathway to promote proliferation and metastasis in hepatocellular carcinoma. Cancer Res Treat. (2020) 52:10–23. doi: 10.4143/crt.2019.145
18. Wree A, McGeough MD, Inzaugarat ME, Eguchi A, Schuster S, Johnson CD, et al. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology. (2018) 67:736–49. doi: 10.1002/hep.29523
19. Li Y, Yu P, Kessler AL, Shu J, Liu X, Liang Z, et al. Hepatitis E virus infection activates NOD-like receptor family pyrin domain-containing 3 inflammasome antagonizing interferon response but therapeutically targetable. Hepatology. (2022) 75:196–212. doi: 10.1002/hep.32114
20. Feng PC, Ke XF, Kuang HL, Pan LL, Ye Q, Wu JB. Retraction note: BMP2 secretion from hepatocellular carcinoma cell HepG2 enhances angiogenesis and tumor growth in endothelial cells via activation of the MAPK/p38 signaling pathway. Stem Cell Res Ther. (2022) 13:154. doi: 10.1186/s13287-022-02841-z
Keywords: Abrus cantoniensis Hance, hepatitis E, network pharmacology, PI3K-Akt signaling pathway, β-sitosterol, Soyasapogenol E, Stigmasterol, Enoxolone
Citation: Xu Z, Wang C, Luan Z, Zhang D and Dong B (2023) Exploring the potential targets of the Abrus cantoniensis Hance in the treatment of hepatitis E based on network pharmacology. Front. Vet. Sci. 10:1155677. doi: 10.3389/fvets.2023.1155677
Received: 31 January 2023; Accepted: 06 March 2023;
Published: 23 March 2023.
Edited by:
Wei Wang, Wenzhou University, ChinaReviewed by:
Mixia Cao, Anhui Science and Technology University, ChinaLi Li, Jinzhou Medical University, China
Copyright © 2023 Xu, Wang, Luan, Zhang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baiqing Dong, NzY0OTM3MzE4QHFxLmNvbQ==
†These authors have contributed equally to this work
 Ziheng Xu
Ziheng Xu Can Wang2†
Can Wang2†