- 1Department of Zoonoses, Faculty of Veterinary Medicine, Assiut University, Asyut, Egypt
- 2Poultry Diseases Department, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
- 3Reproductive Diseases Department, Animal Reproduction Research Institute, Giza, Egypt
- 4Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Sohag, Egypt
- 5Department of Biology, College of Education (Majmaah), Majmaah University, Al Majma’ah, Saudi Arabia
- 6Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 7Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 8Zoonoses Department, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
Live bird markets increase the risk of transmission of zoonotic diseases. Few studies have investigated the potential zoonotic transmission of Campylobacter in Egypt. Therefore, our study was carried out to investigate the presence of Campylobacter species, mainly Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli), in pigeons and turkeys sold at poultry shops. Furthermore, the study aimed to explore the potential occupational risk of Campylobacter infection, mainly among workers at poultry shops. Six hundred (n = 600) samples from various organs were obtained from pigeons and turkeys from live bird shops in the Giza and Asyut provinces in Egypt. Additionally, 100 stool samples were collected from persons working at poultry shops. Circulation of thermophilic Campylobacter in pigeons, turkeys, and humans was investigated based on culture and molecular methods. The rate of detection of Campylobacter species from the samples was significant when the culture method was used alone in comparison to when it was used in combination with mPCR. The prevalence rates of Campylobacter species detected by mPCR were 36% (C. jejuni 20%; C. coli 16%), 28% (C. jejuni 12%; C. coli16%), and 29% (C. jejuni 15%; C. coli 14%) in pigeons, turkeys, and workers, respectively. In pigeons, significant variations in the C. jejuni and C. coli occurrence rates were reported in terms of the intestinal content (15, 4%), liver (4, 13%), and skin (9, 7%), respectively. In turkeys, Campylobacter species were mostly detected in liver samples with a percentage of 19%, followed by the skin (12%), and the intestinal content (8%). In conclusion, Campylobacter species are circulating in poultry farms in Egypt and could represent a hazard for humans. It is recommended that biosecurity measures should be applied to mitigate the occurrence of Campylobacter in poultry farms. Moreover, there is an urgent need to transform live bird markets into chilled poultry markets.
1. Introduction
Foodborne gastroenteritis, caused by Campylobacter is a bacterial diarrheal disease that is found worldwide (1). In developing countries, the Campylobacter infection rate varies from 5 to 20% (2). Animals and poultry are implicated in zoonotic transmission to humans (3). The bird intestine is considered the best habitat for the multiplication of Campylobacter species (4). Cross-contamination of poultry meat with Campylobacter usually occurs during evisceration (5). The handling of raw poultry and the consumption of undercooked poultry meat are the main sources of Campylobacter infection in humans (6). In relation to its clinical picture, Campylobacter infection in humans is usually mild and reported as sporadic cases (7–9). However, community outbreaks of Campylobacter have also been reported, and some patients may develop severe illness (10). In addition, C. jejuni infection is associated with Guillain–Barre syndrome and reactive arthritis (11).
Several studies reported the presence of Campylobacter in the intestine of asymptomatic persons in developing countries (12, 13). Among others, C. jejuni is the major cause of Campylobacter gastroenteritis in humans, followed by C. coli, and to a lesser extent, Campylobacter lari (14). In Egypt, the current incidence of Campylobacter enteritis in humans is still unclear due to a lack of national surveillance programs, so the majority of reported human cases have come from research studies. Several investigators have reported variable incidence rates of 2.3 and 9.6% for Campylobacter (15, 16). It is noteworthy to mention that 90% of the chicken meat in Egypt is produced by commercial poultry farms. However, 10% of the meat is provided by small breeders. Additionally, ducks, geese, pigeons, and turkeys are produced in the backyards of villagers which are produced either mainly for self-consumption or sell (17). Moreover, the live poultry trade is considered the principal strategy for the retail of poultry and covers about 60–80% of the overall poultry commercial production. Live birds are sold commercially in poultry shops distributed all over the country (17). Live bird markets (LBMs) increase the risk for the transmission of zoonotic diseases to humans. To our knowledge, very limited information is available on the occurrence of thermophilic Campylobacter in pigeons, turkeys, and humans at live bird markets in Egypt. Therefore, our study was designed to investigate the presence of Campylobacter in pigeons and turkeys sold in poultry shops. In addition, we investigated persons working at poultry shops to elucidate the occupational risk of Campylobacter infection.
2. Materials and methods
2.1. Ethical statement
The present study was approved by Assiut University, Egypt under the approval number 1717300906.
2.2. Study area and sample collection
Six hundred samples were collected from pigeons and turkeys (100 each) from live poultry markets in the Giza and Asyut provinces in Egypt in the period from August 2014 to December 2019. Three samples (intestinal content, liver, and skin) were obtained from each bird. In addition, 100 stool samples were examined from healthy persons working at poultry shops. The workers were aged 18–50 years.
2.3. Isolation and biochemical identification of Campylobacter
In this step, Campylobacter species were isolated in accordance with ISO 10272-2 (18). Samples from the skin or liver, or 10 g of the intestinal content or stools were homogenized in Bolton broth. Samples were then enriched in Bolton broth (Oxoid) and incubated at 37°C for 4–6 h. Then, they were kept at 41.5°C in a microaerophilic atmosphere for 48 h (Campygen; Oxoid). A loopful of the enrichment broth was plated in modified charcoal cefoperazone Campylobacter desoxycholate agar (mCCDA) (Oxoid) and incubated at 41.5°C under microaerobic conditions for 48 h. Colonies were identified according to the procedure mentioned in ISO10272-2 (18).
2.4. Molecular identification of Campylobacter jejuni and Campylobacter coli
2.4.1. Extraction of DNA
The DNA of each strain was extracted using the Genomic DNA Purification Kit (Thermo Scientific Gene Jet Purification Kit#K0721, #K0722) in accordance with the manufacturer’s instructions. Briefly, a purified colony was inoculated into tryptic soya broth and incubated at 37°C for 48 h. Bacterial cells (2 × 109) were harvested in a 1.5 ml microcentrifuge tube by centrifugation at 5000 × g for 10 min. The pellet was suspended in a solution composed of 180 μl of digestion solution and 20 μl of proteinase K, which was then mixed thoroughly at 56°C. RNase A (20 μl) was added. The solution was mixed and then incubated at room temperature for 10 min. Lysis solution (200 μl) was added and the solution was mixed thoroughly by vortexing for about 15 s. Then, 400 μl of ethanol (50%) was added. The solution was mixed and then transferred to a purification column with a collection tube and centrifuged for 1 min at 6000 × g. Collection tubes were then discarded and replaced by 2 ml collection tubes. Washing buffer I (500 μl) was then added, and the solution was centrifuged for 1 min at 8000 × g. Wash buffer II (500 μl) was added, and the solution was centrifuged for 3 min at maximum speed (≥12,000 × g). The elution buffer (200 μl) was added to the center of the purification column membrane, and the solution was incubated for 2 min at room temperature and centrifuged for 1 min at 8000 × g.
2.4.2. Polymerase chain reaction
This step involved the multiplex polymerase chain reaction (mPCR). Three primers targeting the Campylobacter 23S rRNA gene, the hip O gene for C. jejuni, and the glyA gene for C. coli were used to amplify 650, 323, and 126 bp (Supplementary Figure S1), respectively, as described previously (19). PCR was carried out in a volume of 50 μl with 25 μl of the master mix, 10 μl of the DNA template (50 ng), 9 μl of grade water, and 1 μl of each primer (20 pmol). The PCR conditions were as described previously (19), as follows: there was an initial denaturation step of 94°C for 6 min, followed by 35 cycles, each consisting of 30 s at 95°C, 30 s at 59°C, 30 s at 72°C, and a final extension step at 72°C for 7 min. The electrophoresis of PCR products was performed in 1.5% agarose at 80 V in Tris base–boric acid–EDTA buffer for 120 min. The UV trans-illuminator (Biometra) was used for the visualization of amplicons. Then, they were photographed with the Gel Documentation System using BioDocAnalyze software. A negative control and a positive control were also included.
2.5. Statistical analysis
SAS version 9.4 was used to analyze the data. The Chi-square test was used identify the level of significance, and p-values of <0.05 were considered significant.
3. Results
3.1. Campylobacter species in pigeons, turkeys, and humans
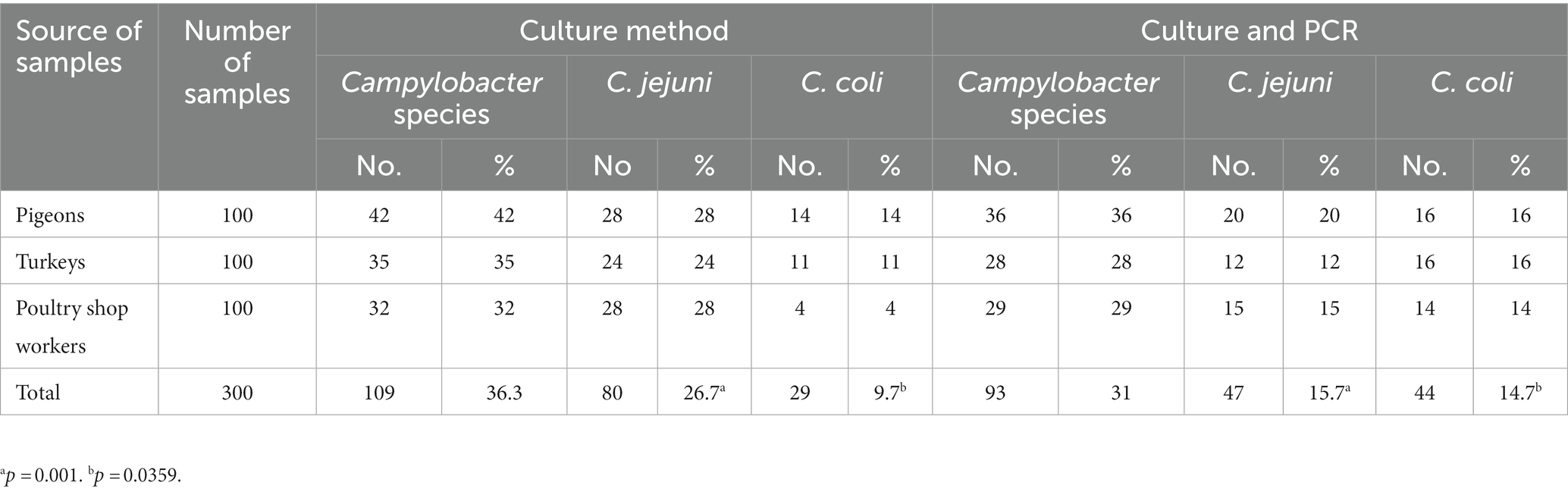
Table 1 shows that the overall prevalence of Campylobacter species determined by the culture method alone and in combination with mPCR was 36.3% (109/300) and 31% (93/300), respectively. The prevalence of C. jejuni determined by the culture method alone and in combination with mPCR was 26.7% (80/300) and 15.7% (47/300), respectively, and the difference was statistically significant (p = 0.001). In addition, as depicted in Table 1, the prevalence of C. coli determined by the culture method alone and in combination with mPCR was 9.7% (29/300) and 14.7% (44/300), respectively, and the difference was statistically significant (p = 0.0359). The prevalence rates of Campylobacter species detected by mPCR were 36% (C. jejuni 20%; C. coli 16%), 28% (C. jejuni 12%; C. coli 16%), and 29% (C. jejuni 15%; C. coli 14%) in pigeons, turkeys, and humans, respectively, and the differences were not statistically significant.
3.2. Distribution pattern of Campylobacter species isolates in pigeons
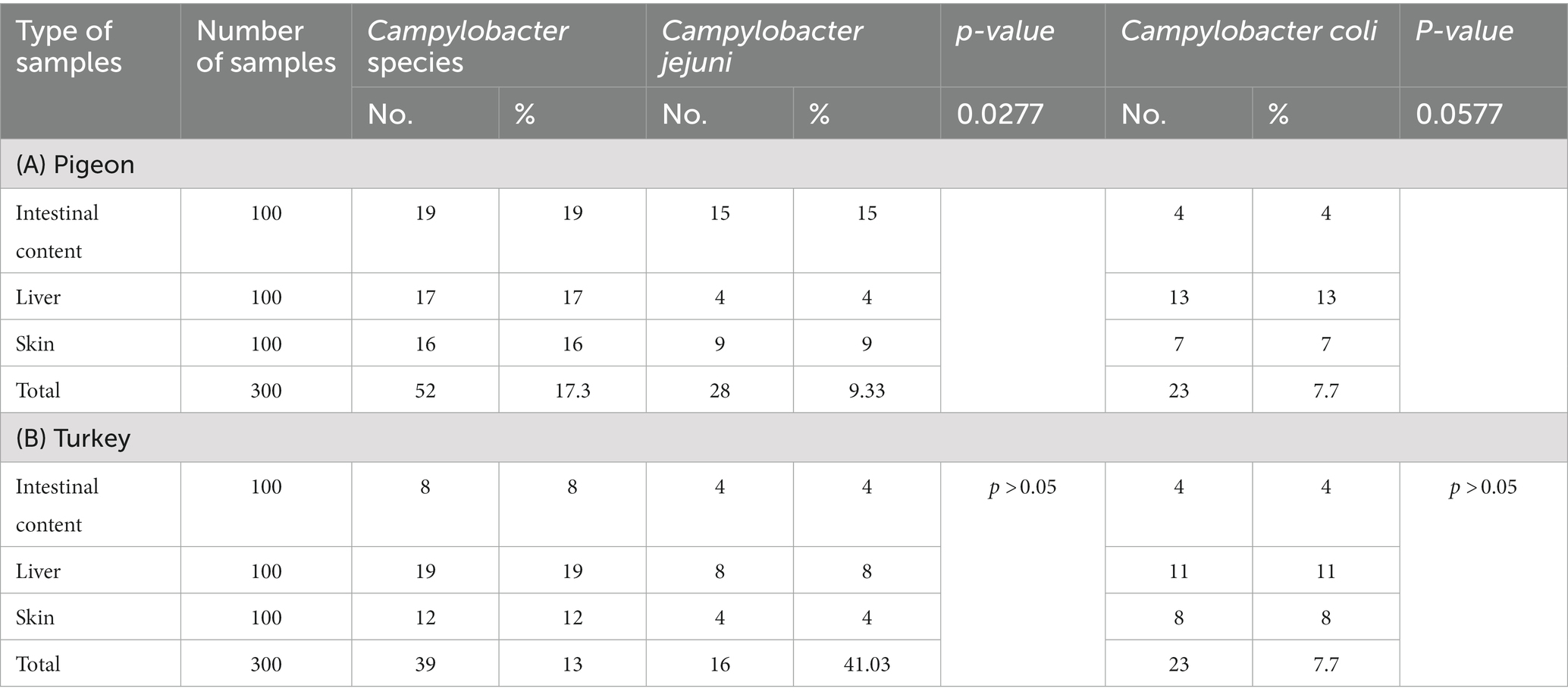
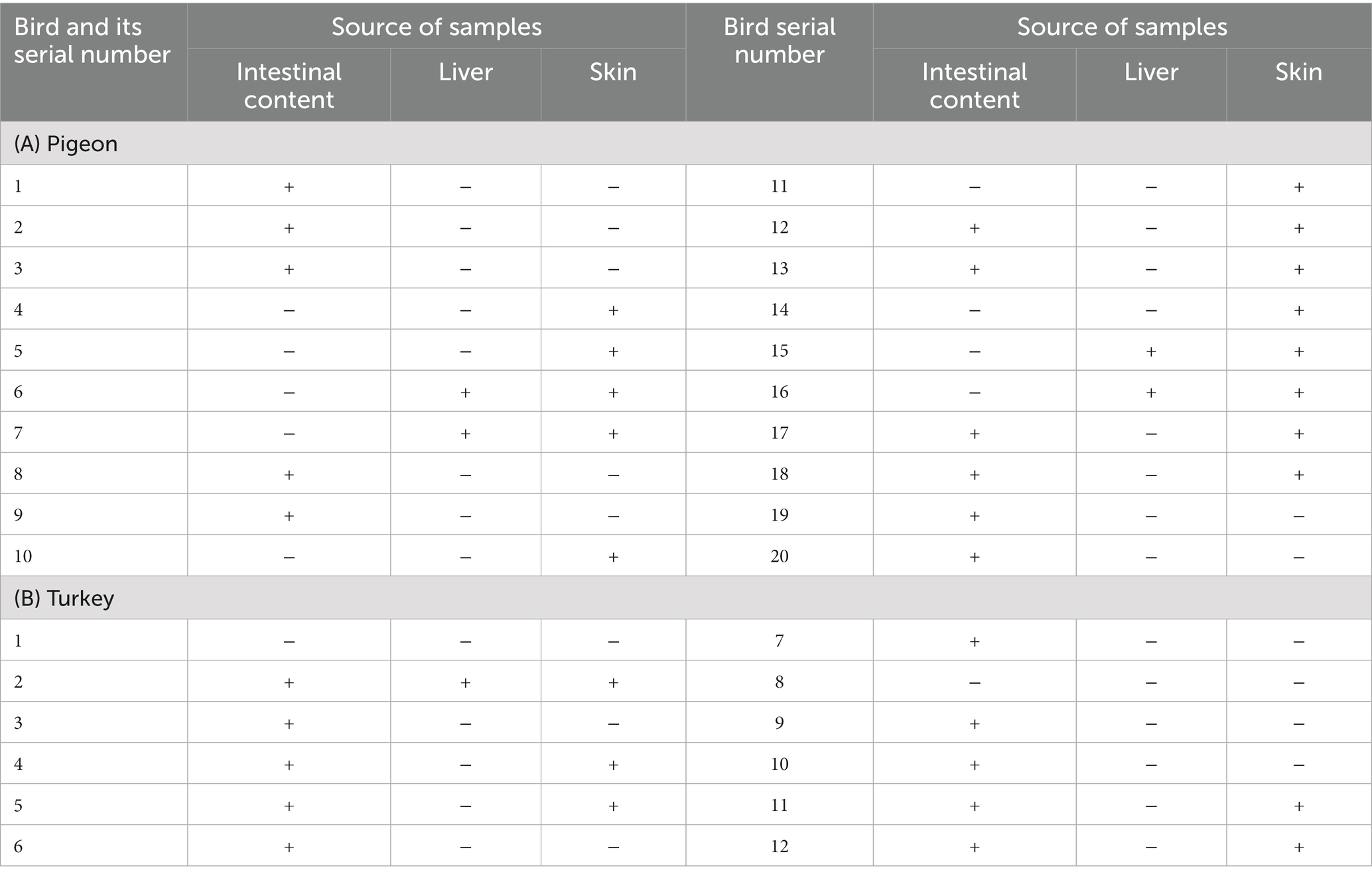
As shown in Table 1, Campylobacter species were detected in 17.3% (52/300) of the examined pigeons, of which 19, 17, and 16% of the positive samples were from the liver, intestinal content, and skin, respectively. C. jejuni was detected in the intestinal content (15%), liver (4%), and skin (9%) samples, and the differences between sample types were statistically significant (p = 0.0277). C. coli was identified in the intestinal content (4%), liver (13%), and skin (7%) samples, and the differences between sample types were statistically significant (p = 0.0577) (Table 2). Twenty-eight isolates of C. jejuni were isolated from 20 pigeons (Tables 1, 2), and different patterns were shown. Two isolates of C. jejuni were retrieved from both the liver and the skin samples of four pigeons (Nos. 6, 7, 15, 16), as shown in Table 3. However, separate isolates of C. jejuni were isolated from the intestine or skin samples of the remaining pigeons (Table 3). Furthermore, twenty-three isolates of C. coli were isolated from 16 pigeons (Tables 1, 2), and different patterns were shown. C. coli was isolated from the intestinal content, liver, and skin samples of the same pigeon (No.1), and C. coli was isolated from both the intestinal content and the skin samples of six pigeons (Nos. 5, 10, 11, 12, 13, and 14). Separate isolates were isolated from the intestine and skin samples of the remaining pigeons (Table 4).
3.3. Distribution pattern of Campylobacter species isolates in Turkey
Campylobacter species were identified in 13% (39/300) of the turkeys, of which 8, 19 and 12% of the positive samples were from the intestinal content, liver, and skin, respectively. C. jejuni was detected in the intestinal content (4%), liver (8%), and skin (4%) samples, and the differences between sample types were not statistically significant. Furthermore, C. coli was identified in intestinal content (4%), liver (11%), and skin (8%) samples, and the differences between sample types were not statistically significant (Table 2). Sixteen isolates of C. jejuni were isolated from 12 turkeys (Tables 1, 2), and different patterns were shown. Three isolates of C. jejuni were isolated from the intestinal content, liver, and skin samples of the same turkey (No.2). C. jejuni was isolated from both the liver and skin samples from four turkeys (Nos. 4, 5, 11, 12), and separate isolates were isolated from the intestinal content and liver samples of the rest of turkeys (Table 3). Twenty-three isolates of C. coli were isolated from 14 turkeys (Tables 1, 2), and different patterns were shown. C. coli was isolated from both the liver and skin samples of six turkeys (Nos.1, 2, 3, 4, 5, and 7), C. coli was isolated from the intestinal content, liver, and skin samples of the same turkey (No.11) C. coli was isolated from the intestinal content and liver samples from turkey No. 14, and separate isolates were isolated from the liver or skin samples of the rest of the turkeys (Table 4).
3.4. Occurrence of Campylobacter species in poultry shop workers
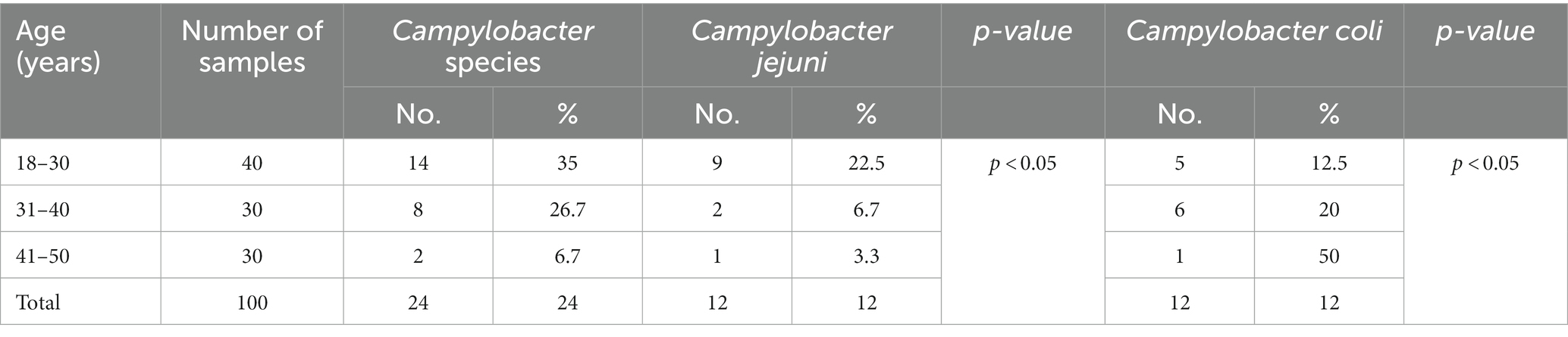
In humans, Campylobacter species were identified in 29% (C. jejuni 15%; C. coli 14%) of the examined workers using mPCR (Table 1). Campylobacter species were detected among workers in percentages of 40, 33.3, and 10% in the 18–30, 31–40, and 41–50 year age groups, respectively, and the differences were significant (p = 0.179) (Table 5). C. jejuni and C. coli were recovered from workers from the 18–30, 31–40, and 41–50 year age groups in percentages of (25%; 15%), (10%; 23.3%), and (6.7%; 3.3), respectively (Table 5).
4. Discussion
Poultry is an important reservoir of food-poisoning microorganisms. In Egypt, the occurrence of Campylobacter in poultry farms continues to be a major problem facing poultry production, especially because the poultry industry mainly includes small-scale producers on farms that do not apply biosecurity measures. In the current study, the identification of Campylobacter species from the examined samples (n = 300) by using the culture method alone or in combination with mPCR was statistically significant. The prevalence rates of C. jejuni (26.7%; 31%) and C. coli (9.7%; 14.7%) determined by the two methods were statistically significant (Table 1). In contrast, no significant difference was reported between the results obtained with the culture and PCR methods for the detection of Campylobacter species in another study (20). The biochemical identification of C. jejuni isolated by the culture method mainly depends on the results of the hippurate hydrolysis test, which reacts positively for C. jejuni and is negative for other species of Campylobacter (21, 22). However, some strains of C. jejuni react negatively to the hippurate hydrolysis test as a result of a failure in the transcription of the hipO gene (23). On the other hand, sometimes, C. coli reacts positively to hippurate hydrolysis as a result of the occurrence of amino acids in the media (24). Hence, the culture method and biochemical reaction are not sufficient to identify Campylobacter species, and confirmation by molecular identification is preferred. In contrast to the prevalence of C. jejuni recovered from the pigeons in this study (Table 1), lower percentages of C. jejuni (11.1 and 8.1%) were obtained in previous studies conducted in California and Croatia, respectively (25, 26). On the other hand, higher percentages (69.1 and 28%) of C. jejuni have been reported in other studies conducted in Spain and Italy, respectively (27, 28). Concerning the prevalence of C. coli in pigeons, a lower percentage (1.1%) was detected in another study (28).
Compared with previous studies carried out in the United States that have reported the prevalence of Campylobacter species (1.6 and 17%) in turkeys (29, 30), our study reported the highest prevalence rate (Table 1). On the other hand, several studies have reported higher percentages (46, and 31.4%) of Campylobacter species in Canada and the UK (20, 31), respectively. In this study, a higher prevalence rate of C. coli (16%) compared to that of C. jejuni (12%) was found in turkeys (Table 1). This result is inconsistent with the results of several studies carried out in Denmark and Hannover (32, 33). In contrast, Noormohamed and Fakhr (29) noted a higher frequency of detection for C. jejuni compared with C. coli.
In pigeons, significant variations in the occurrence rates of C. jejuni and C. coli were reported for the intestinal content (15, 4%), liver (4, 13%), and skin (9, 7%) samples (Table 2). In contrast, a previous study showed a higher percentage of positive C. jejuni (21.7%) samples recovered from the intestinal content (34). Meanwhile, lower percentages of C. jejuni (5.26%) were identified in skin samples in another study (35). In turkey (Table 2), Campylobacter species were detected in the liver (19%), skin (12%), and intestinal content (8%). A similar result was reported in another study conducted in Delta Governorates, Egypt (36). However, the occurrence rates of Campylobacter species in the liver varied between 9.7 and 30% in previous studies conducted in Germany and Egypt (36, 37). Conversely, higher percentages (55, 26.7%) of Campylobacter species were recovered from the skin in previous studies (36, 38). Compared to our findings for the intestinal content (Table 2), higher percentages of Campylobacter (16.7%) were reported elsewhere (36) The variation in the occurrence rates of Campylobacter species noted in different studies might be attributed to variations in the level of cross-contamination that may occur during the slaughter and evisceration of birds. It is clearly evident that the livers of pigeons and turkeys are potential sources of Campylobacter infection, especially when consumed undercooked.
In relation to the rate of Campylobacter infections in human population in Egypt, several previous studies documented that Campylobacterios is an important cause of diarrhea in children in the country (39–41). In this respect, up to 85% of children in Egypt were found infected with Campylobacter sp. in their first year with annual incidence of 1.2 episodes per year (42–45). Regarding the isolation rate of Campylobacter species in Egypt, several studies reported variable percentages (27.55, 5.33, and 18.3%) in Assiut and Aswan Governorates (46–48), respectively. Other studies at various Egyptian governorates reported an isolated rate of 8.5 and 38.09% for C. jejuni isolated from occupational workers (42, 49). Moreover, at species level, C. jejuni and C. coli could be identified in Aswan Governorate at rate of 50% for each (46), while the isolation rate Assiut Governorate (48) was 11.7 and 6.7% for the same species, respectively. Taken into account, in Egypt, farming practices often lack sufficient biosecurity and control which are considering predisposing factors for higher incidence of the pathogen. Among others, it is evident that persons working at poultry shops and dealing with live birds are at high risk of acquiring various zoonotic pathogens, especially during the handling, slaughtering, and evisceration of birds. Therefore, we investigated workers at poultry shops (n = 100) to explore the occurrence of Campylobacter species among them. Interestingly, the overall occurrence of Campylobacter species among the examined workers (Table 5) was 29%. Conversely, several previous studies conducted in in Tanzania, Bangladesh, and Egypt (50–52) have reported lower percentages (9.3, 11.5, and 5.3%) of Campylobacter38–40. C. jejuni was identified in 15% of the examined workers. Conversely, lower percentages of C. jejuni (5.8 and 1.5%) were obtained in other studies conducted in France and India (53, 54). On the other hand, higher percentages of C. jejuni (21.4, 63.6%) were obtained in other studies (32, 55). In this study, C. coli was recovered from 14% of the workers. Lower percentages of C. coli (2.5 and 1.5%) were obtained in other studies (52–54). Higher percentages of C. coli (78.5 and 31.8%) were obtained in previous studies (32, 55). The role of asymptomatic persons in the epidemiology of Campylobacter is still unclear and further investigation is needed to study the role of carriers in the epidemiology of Campylobacter, how long they remain as carriers, and whether nonclinical cases can develop clinical disease. A significant rate of Campylobacter infection in relation to the age of the workers was reported in this study. A similar result was obtained in another study conducted in Tanzania (12).
5. Conclusion
Based on the results obtained in this study, it is apparent that Campylobacter species are circulating in poultry farms in Egypt which might be a risk hazard for humans. Therefore, it is critically important to apply hazard analysis and critical control points at all stages of the production chain until the products reach the consumers. Moreover, the transformation of LBM into chilled poultry markets is recommended. The relatively high occurrence of Campylobacter among workers might reflect the poor hygiene practices applied at live poultry shops. Thus, the awareness of poultry shop workers about safe handling practices in the workplace needs to be increased to decrease the possibility of cross-contamination and to prevent the zoonotic transmission of Campylobacter infection. Further research, at large scale, is highly recommended for exploring the antimicrobial resistance and genotyping of the circulating strains of Campylobacter species from different reservoirs in the Egyptian environment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Assiut University, Egypt with an ethical approval number of 1717300906. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the Institutional Review Board of the Assiut University (Local ethical approval), Assiut University, Egypt with approval number is 17300906. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
AS, AI, MS, EE, and AY designed the idea of the conception, performed the methodology, formal analysis, data curation and supervision besides revision of the manuscript. NA and KA participated in designing of the idea of the conception and drafting of the manuscript. AS, AI, MS, AY, ME-k, and EE drafted the manuscript, prepared the manuscript for publication and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R23), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1150077/full#supplementary-material
References
1. World Health Organization. World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/campylobacter (2020).
2. Oberhelman, RA. Campylobacter infection in developing countries In: I Nachamkin and MJ Blaser, editors. Campylobacter. 2nd ed. Washington: American Society for Microbiology (2000). 139–53.
3. Plishka, M, Sargeant, JM, Greer, AL, Hookey, S, and Winder, C. The prevalence of Campylobacter in live cattle, Turkey, chicken, and swine in the United States and Canada: a systematic review and meta-analysis. Foodborne Pathog Dis. (2021) 18:230–42. doi: 10.1089/fpd.2020.2834
4. Lee, MD, and Newell, DG. Campylobacter in poultry: filling an ecological niche. Avian Dis. (2006) 50:1–9. doi: 10.1637/7474-111605R.1
5. Rahimi, E, and Tajbakhsh, E. Prevalence of Campylobacter species in poultry meat in the Esfahan city. Iran Bulg J Vet Med. (2008) 11:257–62.
6. Evans, MR, Ribeiro, CD, and Salmon, RL. Hazards of healthy living: bottled water and salad vegetables as risk factors for Campylobacter infection. Emerg Infect Dis. (2003) 9:1219–25. doi: 10.3201/eid0910.020823
7. Kim, S, Vela, A, Clohisey, SM, Athanasiadou, S, Kaiser, P, Stevens, MP, et al. Host-specific differences in the response of cultured macrophages to Campylobacter jejuni capsule and O-methyl phosphoramidate mutants. Vet Res. (2018) 49:1–10. doi: 10.1186/s13567-017-0501-y
8. Kuusi, M, Nuorti, J, Hänninen, M-L, Koskela, M, Jussila, V, Kela, E, et al. A large outbreak of campylobacteriosis associated with a municipal water supply in Finland. Epidemiol Infect. (2005) 133:593–601. doi: 10.1017/S0950268805003808
9. Peterson, MC. Campylobacter jejuni Enteristis associated with consumption of raw Milk. J Environ Health. (2003) 65:20–21, 24, 26.
10. Same, RG, and Tamma, PD. Campylobacter infections in children. Pediatr Rev. (2018) 39:533–41. doi: 10.1542/pir.2017-0285
11. Rajendran, P, Babji, S, George, A, Rajan, D, Kang, G, and Ajjampur, S. Detection and species identification of Campylobacter in stool samples of children and animals from Vellore, South India. Indian J Med Microbiol. (2012) 30:85–8. doi: 10.4103/0255-0857.93049
12. Komba, EV, Mdegela, RH, Msoffe, P, Nielsen, LN, and Ingmer, H. Prevalence, antimicrobial resistance and risk factors for thermophilic Campylobacter infections in symptomatic and asymptomatic humans in Tanzania. Zoonoses Public Health. (2015) 62:557–68. doi: 10.1111/zph.12185
13. Megraud, F, Boudraa, G, Bessaoud, K, Bensid, S, Dabis, F, Soltana, R, et al. Incidence of Campylobacter infection in infants in western Algeria and the possible protective role of breast feeding. Epidemiol Infect. (1990) 105:73–8. doi: 10.1017/S095026880004766X
14. Skirrow, MB. Clinical aspects of Campylobacter infection In: I Nachamkin and MJ Blaser, editors. Campylobacter. (2nd) ed. Washington, DC: American Society for Microbiology (2000). 69–88.
15. Wasfy, MO, Oyofo, BA, David, JC, Ismail, TF, El-Gendy, AM, Mohran, ZS, et al. Isolation and antibiotic susceptibility of Salmonella, Shigella, and Campylobacter from acute enteric infections in Egypt. J Health Popul Nutr. (2000) 18:33–8.
16. Abd El-Baky, R, Sakhy, M, and Gad, G. Antibiotic susceptibility pattern and genotyping of Campylobacter species isolated from children suffering from gastroenteritis. Indian J Med Microbiol. (2014) 32:240–6. doi: 10.4103/0255-0857.136550
17. Shatokhin, Y, El Gammal, M, and Prikhodko, D. Broiler poultry industry: investment challenges and opportunities. Rome, Italy: Arab Republic of Egypt Food and Agriculture Organization of the United Nations (2017).
18. ISO. Microbiology of food and animal feeding stuffs – Horizontal method for detection and enumeration of Campylobacter spp. Part 1: Detection method 10272–2 International Organization for Standardization. Geneva, Switzerland: (2006).
19. Wang, G, Clark, CG, Taylor, TM, Pucknell, C, Barton, C, Price, L, et al. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J Clin Microbiol. (2002) 40:4744–7. doi: 10.1128/JCM.40.12.4744-4747.2002
20. Perko-Mäkelä, P, Isohanni, P, Katzav, M, Lund, M, Hänninen, M-L, and Lyhs, U. A longitudinal study of Campylobacter distribution in a Turkey production chain. Acta Vet Scand. (2009) 51:1–10. doi: 10.1186/1751-0147-51-18
21. Burnett, TA, Hornitzky, MA, Kuhnert, P, and Djordjevic, SP. Speciating Campylobacter jejuni and Campylobacter coli isolates from poultry and humans using six PCR-based assays. FEMS Microbiol Lett. (2002) 216:201–9. doi: 10.1111/j.1574-6968.2002.tb11436.x
22. Kulkarni, S, Lever, S, Logan, J, Lawson, A, Stanley, J, and Shafi, M. Detection of Campylobacter species: a comparison of culture and polymerase chain reaction based methods. J Clin Pathol. (2002) 55:749–53. doi: 10.1136/jcp.55.10.749
23. Hani, EK, and Chan, VL. Expression and characterization of Campylobacter jejuni benzoylglycine amidohydrolase (hippuricase) gene in Escherichia coli. J Bacteriol. (1995) 177:2396–402. doi: 10.1128/jb.177.9.2396-2402.1995
24. Denis, M, Soumet, C, Rivoal, K, Ermel, G, Blivet, D, Salvat, G, et al. Development of am-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett Appl Microbiol. (1999) 29:406–10. doi: 10.1046/j.1472-765X.1999.00658.x
25. Jeffrey, J, Atwill, ER, and Hunter, A. Farm and management variables linked to fecal shedding of Campylobacter and Salmonella in commercial squab production. Poult Sci. (2001) 80:66–70. doi: 10.1093/ps/80.1.66
26. Vučemilo, M, Vlahović, K, Dovč, A, MuŽinić, J, Pavlak, M, Jerčić, J, et al. Prevalence of Campylobacter jejuni, Salmonella typhimurium, and avian Paramyxovirus type 1 (PMV-1) in pigeons from different regions in Croatia. Z Jagdwiss. (2003) 49:303–13. doi: 10.1007/BF02189638
27. Bellio, A, Traversa, A, Adriano, D, Bianchi, DM, Colzani, A, Gili, S, et al. Occurrence of thermotolerant Campylobacter in raw poultry meat, environmental and pigeon stools collected in open-air markets. Italian J Food Safety. (2014) 3:157–159. doi: 10.4081/ijfs.2014.1706
28. Vázquez, B, Esperón, F, Neves, E, López, J, Ballesteros, C, and Muñoz, MJ. Screening for several potential pathogens in feral pigeons (Columba livia) in Madrid. Acta Vet Scand. (2010) 52:1–6. doi: 10.1186/1751-0147-52-45
29. Noormohamed, A, and Fakhr, MK. Prevalence and antimicrobial susceptibility of Campylobacter spp. in Oklahoma conventional and organic retail poultry. Open Microbiol J. (2014) 8:130–7. doi: 10.2174/1874285801408010130
30. Zhao, S, Young, S, Tong, E, Abbott, J, Womack, N, Friedman, S, et al. Antimicrobial resistance of Campylobacter isolates from retail meat in the United States between 2002 and 2007. Appl Environ Microbiol. (2010) 76:7949–56. doi: 10.1128/AEM.01297-10
31. Arsenault, J, Letellier, A, Quessy, S, Normand, V, and Boulianne, M. Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and Turkey flocks slaughtered in Quebec, Canada. Prev Vet Med. (2007) 81:250–64. doi: 10.1016/j.prevetmed.2007.04.016
32. Siemer, B, Nielsen, E, and On, S. Identification and molecular epidemiology of Campylobacter coli isolates from human gastroenteritis, food, and animal sources by amplified fragment length polymorphism analysis and penner serotyping. Appl Environ Microbiol. (2005) 71:1953–8. doi: 10.1128/AEM.71.4.1953-1958.2005
33. Weber, RAM, Jung, A, and Glünder, G. Campylobacter infections in four poultry species in respect of frequency, onset of infection and seasonality. Berl. Munch. Tierarztl. Wochenschr. (2014) 127:257–66.
34. Bianchini, V, Borella, L, Benedetti, V, Parisi, A, Miccolupo, A, Santoro, E, et al. Prevalence in bulk tank milk and epidemiology of Campylobacter jejuni in dairy herds in northern Italy. Appl Environ Microbiol. (2014) 80:1832–7. doi: 10.1128/AEM.03784-13
35. Soncini, G, Valnegri, L, Vercellotti, L, Colombo, F, Valle, D, Franzoni, M, et al. Investigation of Campylobacter in reared game birds. J Food Prot. (2006) 69:3021–4. doi: 10.4315/0362-028X-69.12.3021
36. Khalil, M, Moawad, A, Kafafy, M, Fahmy, H, and Sobhy, M. Molecular characterization of Campylobacter species from turkeys flocks in delta governments. Assiut Vet Med J. (2020) 66:111–7. doi: 10.21608/avmj.2020.167255
37. Atanassova, V, Reich, F, Beckmann, L, and Klein, G. Prevalence of Campylobacter spp. in Turkey meat from a slaughterhouse and in Turkey meat retail products. FEMS Immunol Med Microbiol. (2007) 49:141–5. doi: 10.1111/j.1574-695X.2006.00180.x
38. Bouhamed, R, Bouayad, L, Messad, S, Zenia, S, Naïm, M, and Hamdi, T-M. Sources of contamination, prevalence, and antimicrobial resistance of thermophilic Campylobacter isolated from turkeys. Veterinary World. (2018) 11:1074–81. doi: 10.14202/vetworld.2018.1074-1081
39. Abd El-Ghany, WA. One health approach of campylobacteriosis in Egypt: an emerging zoonotic disease. J Infect Develop Countries. (2019) 13:956–60. doi: 10.3855/jidc.11860
40. Saif, NA, Cobo-Díaz, JF, Elserafy, M, El-Shiekh, I, Álvarez-Ordóñez, A, Mouftah, SF, et al. A pilot study revealing host-associated genetic signatures for source attribution of sporadic Campylobacter jejuni infection in Egypt. Transbound Emerg Dis. (2022) 69:1847–61. doi: 10.1111/tbed.14165
41. Kaakoush, NO, Castaño-Rodríguez, N, Mitchell, HM, and Man, SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev. (2015) 28:687–720. doi: 10.1128/CMR.00006-15
42. Omara, ST, El Fadaly, H, and Barakat, A. Public health hazard of zoonotic Campylobacter jejuni reference to Egyptian regional and seasonal variations. Res J Microbiol. (2015) 10:343–54. doi: 10.3923/jm.2015.343.354
43. Sainato, R, ElGendy, A, Poly, F, Kuroiwa, J, Guerry, P, Riddle, MS, et al. Epidemiology of Campylobacter infections among children in Egypt. Am J Trop Med Hygiene. (2018) 98:581–5. doi: 10.4269/ajtmh.17-0469
44. Rao, MR, Naficy, AB, Savarino, SJ, Abu-Elyazeed, R, Wierzba, TF, Peruski, LF, et al. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol. (2001) 154:166–73. doi: 10.1093/aje/154.2.166
45. Liu, L, Johnson, HL, Cousens, S, Perin, J, Scott, S, Lawn, JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. (2012) 379:2151–61. doi: 10.1016/S0140-6736(12)60560-1
46. Karmi, M, Prevalence of Campylobacter spp. And its pathogenic genes in poultry meat, human and environment in Aswan, upper Egypt. Assiut Vet Med J. (2019) 65:151–8. doi: 10.21608/avmj.2019.168777
47. Abushahba, M, Ahmed, S, Ibrahim, A, and Mosa, H. Prevalence of zoonotic species of Campylobacter in broiler chicken and humans in Assiut governorate. Egypt Approaches Poult Dairy Vet Sci. (2018) 3:260–8. doi: 10.31031/APDV.2018.03.000568
48. Abbas, SGE, Karmi, M, Mubarak, AG, and Youseef, AG. Prevalence and virulence genes profile of zoonotic Campylobacter species in chickens and human in Aswan governorate. SVU Int J Veterinary Sci. (2022) 5:15–32. doi: 10.21608/svu.2022.143454.1204
49. Barakat, AM, Abd El-Razik, KA, Elfadaly, HA, Rabie, NS, Sadek, SA, and Almuzaini, AM. Prevalence, molecular detection, and virulence gene profiles of Campylobacter species in humans and foods of animal origin. Veterinary World. (2020) 13:1430–8. doi: 10.14202/vetworld.2020.1430-1438
50. El-Tawab, A, Ashraf, A, El Hofy, FI, Ammar, AM, Ahmed, HA, and Hefny, AA. Bacteriological and molecular identification of Campylobacter species in chickens and humans, at Zagazig city. Egypt Benha Veterinary Med J. (2015) 28:17–26. doi: 10.21608/bvmj.2015.32523
51. Mdegela, R, Nonga, H, Ngowi, H, and Kazwala, R. Prevalence of thermophilic Campylobacter infections in humans, chickens and crows in Morogoro, Tanzania. J Veterinary Med Ser B. (2006) 53:116–21. doi: 10.1111/j.1439-0450.2006.00926.x
52. Sarkar, S, Ray, N, Hossain, M, Paul, S, Sarkar, S, and Kobayashi, N. Multiplex polymerase chain reaction for detection of Campylobacter from stool specimen. Mymensingh Med J. (2014) 23:449–55.
53. Bessède, E, Delcamp, A, Sifré, E, Buissonnière, A, and Mégraud, F. New methods for detection of campylobacters in stool samples in comparison to culture. J Clin Microbiol. (2011) 49:941–4. doi: 10.1128/JCM.01489-10
54. Rajagunalan, S, Bisht, G, Pant, S, Singh, SP, Singh, R, and Dhama, K. Prevalence and molecular heterogeneity analysis of Campylobacter jejuni and Campylobacter coli isolated from human, poultry, and cattle. Vet Arhiv. (2014) 84:493–504.
Keywords: Campylobacter, pigeon, Turkey, human, live bird market, Egypt
Citation: Sayed ASM, Ibrahim AI, Sobhy MM, Elmahallawy EK, Alsowayeh N, Alarjani KM, El-khadragy MF and Youseef AG (2023) Circulation of thermophilic Campylobacter in pigeons, turkeys, and humans at live bird markets in Egypt. Front. Vet. Sci. 10:1150077. doi: 10.3389/fvets.2023.1150077
Edited by:
Fabrizio Bertelloni, University of Pisa, ItalyReviewed by:
Heriberto Fernandez, Austral University of Chile, ChileBarbara Turchi, University of Pisa, Italy
Copyright © 2023 Sayed, Ibrahim, Sobhy, Elmahallawy, Alsowayeh, Alarjani, El-khadragy and Youseef. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amal S. M. Sayed, YW1hbHNheWVkNzNAYXVuLmVkdS5lZw==; Ehab Kotb Elmahallawy, ZWVoYWFAdW5pbGVvbi5lcw==
 Amal S. M. Sayed
Amal S. M. Sayed Ahmed I. Ibrahim2
Ahmed I. Ibrahim2 Mona M. Sobhy
Mona M. Sobhy Ehab Kotb Elmahallawy
Ehab Kotb Elmahallawy Noorah Alsowayeh
Noorah Alsowayeh Khaloud Mohammed Alarjani
Khaloud Mohammed Alarjani Manal F. El-khadragy
Manal F. El-khadragy