- Department of Veterinary Medicine and Animal Productions, University of Naples Federico II, Naples, Italy
1. Introduction
In humans, head and neck squamous cell carcinoma (HNSCC) is among the most common cancers worldwide. This severe disease affects the aerodigestive tract, including oral cavity and oropharynx (1). Tobacco smoking, alcohol abuse and poor oral hygiene are believed to be the main risk factors. However, it is estimated that a subgroup of oral squamous cell carcinoma (OSCC), accounting for 25% of all HNSCCs, is associated with alpha high-risk human papillomavirus (HR-HPV) infection, with the HPV-16/-18 being the major responsible for cancer development. This particular type of tumor arises mainly at oropharyngeal sites, where persistent infection plays a key role toward neoplastic transformation. Viral oncogenes E6 and E7 drive carcinogenesis in infected cells by impairing the molecular pathways of two key tumor suppressors such as p53 and pRb (1). In veterinary oncology, association of PVs with SCC of the upper digestive tract has been ascertained in bovine and equine species (2–5). In cattle, bovine PV type-4 (BPV-4) is considered a main player in early steps of tumorigenesis leading to development of SCC of the esophagus, mouth, and oropharynx, along with environmental co-factors (2, 6). Furthermore, there is increasing evidence that Equus caballus PV-2, likely to be a co-causative agent of genital SCC in horses, is also involved in development of a subset of equine HNSCC, thus being considered an equine equivalent of HPV-16 (3–5). Studies in dogs have suggested a possible role of canine PVs in oral carcinogenesis, particularly in the transformation of oral papillomas into SCC (7–9). Similarly, a rising number of published work indicates that Felis catus papillomaviruses (FcaPVs) exhibit mucosal tropism, being consistently detectable in a subset of OSCC of cat and playing a co-causative role in the development of these tumors (10–16).
2. Association of FOSCC with FcaPVs infection
The first hints of a biologically significant association of feline OSCC (FOSCC) with FcaPVs came from the pioneering studies aimed at characterizing transcriptional activity of FcaPV type 2 (FcaPV-2) in vivo and its biological properties in feline living cells (10, 17). Here, viral DNA and gene expression were reported in one FOSCC case and FcaPV-2 E6 and E7 oncogenes appeared to exert transforming properties comparable to those of HR-HPVs associated with human HNSCC (10, 17). Additional clues were pointed out when FcaPV-2 was shown to be detectable and transcriptionally active in cell lines derived from cat gingival and tongue SCC (18). Moreover, these cell lines showed a molecular scenario compatible with a FcaPV-2 E6-dependent p53 degradation with similarities to that reported for HPV-16 E6 (18). Stronger evidence came from two later independent studies that reported detection of FcaPV-2 in a relevant subset of FOSCC from different geographical areas, with a prevalence of 31% (10/32) in Italy and ~58% (11/19) in Japan (12, 19). Importantly, the study conducted in Italy pointed out expression of FcaPV-2 oncogenes E6E7 in tumors and higher viral load compared to non-neoplastic oral mucosa harboring viral DNA, suggesting active infection and oncogenic functions (19). A later work from Germany confirmed expression of PV antigen in ~21% (5/21) of FOSCC and the presence of PV-induced cellular changes (koilocytes and inclusion bodies) in a subset of samples (15). Furthermore, a recent multicentric study demonstrated the presence of at least one FcaPV type (among these FcaPV-1/-2/-3/-4/-5) in ~21% (22/103) of a pool of FOSCC samples from Italy and Austria (11). Consistent data were presented in two independent published works conducted in USA and New Zealand, denoting the presence and high viral load of FcaPV-3 in 5% (1/20) of FOSCC but not in normal mucosa, and typical PV-induced cellular changes in a FcaPV-3 positive tumor sample (13, 14). Taking these studies together, the association rate of FcaPVs with FOSCC seems to fluctuate between 5 and 58%, however a conference paper from USA dated in 2015 reports that it can even reach 100% (12/12) (20).
In summary, the evidence of a co-causative role of FcaPVs in the development of FOSCC is as follows:
1) Different FcaPV types (-1/-2/-3/-4/-5) exhibit mucosal tropism (16, 21).
2) A subset of FOSCC samples is associated with FcaPVs DNA (11–14, 19, 20).
3) Detection of FcaPVs DNA in a subset of FOSCC is a common finding in different geographical areas, as per independent studies by different research groups (11–14, 19, 20).
4) There is histological, molecular and immunohistochemical evidence of PV active infection in a subset of FOSCC by different research groups (13, 15, 19).
5) FcaPV-2 displays high viral load and expression of E6E7 oncogenes in FOSCC samples (10, 19).
6) FcaPV-2 DNA is detectable and viral oncogenes are expressed in FOSCC-derived cell lines (18).
7) FcaPV-2 E6 and E7 oncoproteins exert transforming properties by impairing p53 and pRb pathways in feline living cells (10, 18, 22).
8) FcaPV-3 induces cellular changes compatible with PV-induced cancer and displays high viral load in FOSCC (13, 14).
Finally, as brilliantly summarized in a recent, excellent critical review of the literature, FcaPVs infection clearly emerges as a risk factor for a subset of FOSCC (~16%). Interestingly, the authors even warn that the number of PV positive cases might be underestimated, due to: (I) DNA fragmentation occurring in formalin fixed-paraffin embedded samples causing false negative PCR results. (II) The use of consensus primers, which exert lower sensitivity than type-specific primers. (III) Infection by genotypes not detectable by the primers employed in elder studies. (IV) The possible occurrence of the “hit and run” mechanism by which the virus may initially induce cellular transformation, to then disappear and go no longer detectable (23).
3. Discussion
Among the numerous diseases classified as HNSCC (SCC of oral cavity, oropharynx, nasopharynx, larynx and upper esophagus), HPV-positive SCC shows different biological features, genetic background and molecular markers compared to HPV-negative counterpart and is now considered as a distinct clinical entity (1, 24). Indeed, due to a less aggressive behavior combined with an improved response to therapies, the 3-year overall survival of patients bearing HPV-positive cancer is 82 vs. 57% of those affected by HPV-negative tumors (1, 24). Recent studies carried out in the context of global scale meta-analyses conducted by the International Agency for Research on Cancer (IARC) confirm improved survival in HPV positive patients, with oropharyngeal tumors driving this trend (25). These data further strengthen the rationale of numerous past, ongoing, and future studies aiming at de-intensificating therapeutic protocols against HPV-related OSCC (25, 26). The focus of these studies is to maintain high cure efficiency, reduce treatment-related toxicity, and preserve the quality of life at the same time (25, 26).
Therefore, HPV testing is a crucial step working as a link between the pathologist and the clinical oncologist toward a complete diagnostic, therapeutic and prognostic evaluation in the practice of cases of human OSCC. Immunohistochemistry (IHC) for p16 is considered a reliable test that serves as a surrogate for HPV detection, since it is a downstream effector of impaired pRb (24, 27). In doubtful cases, IHC may be further integrated by molecular tools such as in situ hybridization (ISH) for viral DNA and E6/E7 gene expression analysis (27).
Oral tumors are frequent in cats, among these SCC is the most common malignancy (28). Surgery, chemotherapy and radiation therapy are available as treatment options, however the prognosis is poor in most of the cases, leading to death or euthanasia (28). We still do not know whether FcaPV-related FOSCC may actually constitute a distinct oncological entity in terms of biological behavior as in human counterpart. There is a need to collect additional data; therefore, we ask ourselves and the broadest community of veterinary oncologists whether it is time to set-up large-scale strategies toward this goal by coordinating scientific efforts worldwide. This means to design multicentric follow up studies with the aim of monitoring the biological behavior and the evolution of these tumors over time, in order to understand whether they represent or not a different clinical subject compared to PV-negative disease (Figure 1). Evaluating the incidence of PV-related cancer in different oral sites would be of great interest as well, to see whether it preferentially develops at oropharynx also in felines. If so, this FOSCC subtype will further confirm its reliability as animal model of HPV-driven HNSCC and vice versa (29). Standard treatment for human HNSCC is surgery followed by high-dose cisplatin with adjuvant radiotherapy and it is widely ascertained that this protocol works particularly well for HPV positive tumors. Importantly, in IARC studies, surgery has been proven to be the most impactful treatment and further indications have emerged that chemo-radiotherapy might be de-escalated (25). Will a similar scenario be confirmed in feline species, it will be even more important to devise possible de-escalation strategies for FcaPV-positive patients (Figure 1). This would be achieved by setting up studies where modulating different combinations of surgery, chemo-, radio- and biological therapy to then compare primary (e.g., overall survival, disease-free survival) and secondary outcomes (e.g., loco-regional control) in differently treated experimental groups. Among the de-intensification strategies eligible for the treatment of human HNSCC, biological therapy based on the use of monoclonal antibody Cetuximab as adjuvant has been object of great attention (30). Hence, it is worth mentioning that our recent work in pre-clinical models of FOSCC has provided promising results, thus encouraging future studies in the feline counterpart (31). We imagine a future perspective where de-escalated therapies may provide advantages for cats, with a more favorable balance between therapeutic efficacy and welfare, and owners, in handling and economic terms. In such a context, viral testing would emerge as a necessary, preliminary step prior to the clinical management, as determined by the diagnostic algorithm in human practice. In this regard, a proportion of FcaPV-2 positive FOSCCs display p16 immunostaining, and this appears as a positive prognostic parameter in cats too. However, the role of p16 as marker of PV infection in this species is controversial (19, 23, 32), thus molecular tests (PCR and RT-PCR) would be recommendable (10, 19). Moreover, ISH would find possible application, considering that it is successfully employed for detection of FcaPVs DNA and mRNA in feline cutaneous SCC (33–35).
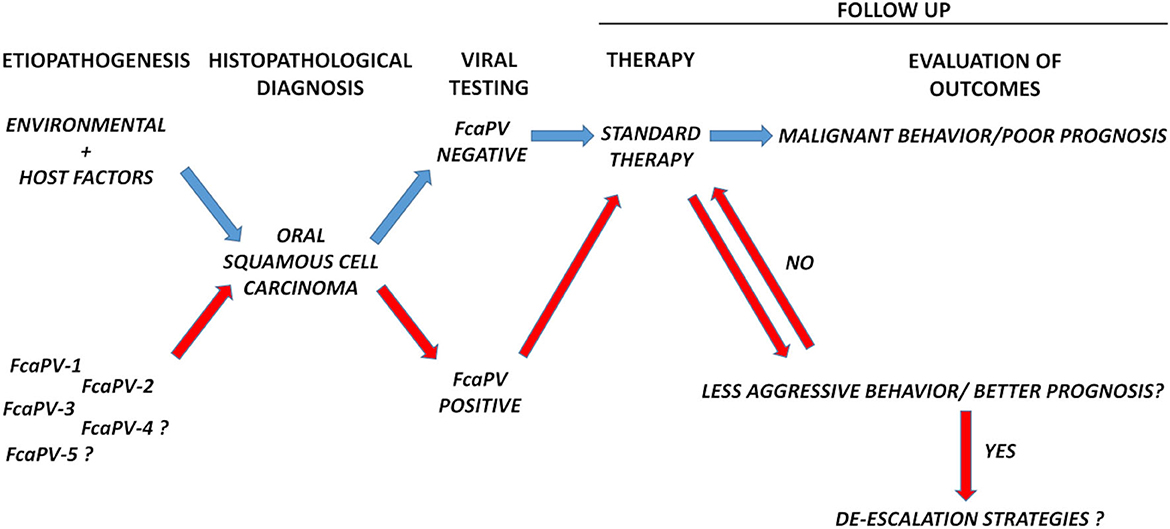
Figure 1. Schematic diagram showing the diagnostic workflow proposed in this article. Follow up of biological behavior and response to therapies of FcaPV-positive vs. FcaPV-negative tumors would help to understand whether, as in humans, the former constitutes a different oncological entity compared to the latter and possibly pave the way to de-escalation strategies. Viral testing would be a necessary, preliminary step before clinical handling.
In conclusion, pending definitive data in humans, we believe it is time to begin studies in domestic feline, with the aim of studying the biology, clinical behavior and response to therapies of FcaPV-related FOSCC. In a future perspective, this would help to ameliorate the approach of the veterinary oncologists in terms of diagnosis, therapeutic and prognostic evaluation toward feline patients.
We are used to think of comparative oncology as the discipline in which studies on animal models pave new ways in human medicine. However, this would be the case were the “human model” traces the path for feline oncology, although we do not yet know what lies at the end of the road.
Author contributions
GA and GB drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by a grant from the Board of Directors, University of Naples Federico II.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tanaka TI, Alawi F. Human papillomavirus and oropharyngeal cancer. Dent Clin North Am. (2018) 62:111–20. doi: 10.1016/j.cden.2017.08.008
2. Borzacchiello G, Roperto F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet Res. (2008) 39:45. doi: 10.1051/vetres:2008022
3. Sykora S, Jindra C, Hofer M, Steinborn R, Brandt S. Equine papillomavirus type 2: an equine equivalent to human papillomavirus 16? Vet J. (2017) 225:3–8. doi: 10.1016/j.tvjl.2017.04.014
4. Knight CG, Dunowska M, Munday JS, Peters-Kennedy J, Rosa BV. Comparison of the levels of equus caballus papillomavirus type 2 (Ecpv-2) DNA in equine squamous cell carcinomas and non-cancerous tissues using quantitative pcr. Vet Microbiol. (2013) 166:257–62. doi: 10.1016/j.vetmic.2013.06.004
5. Strohmayer C, Klang A, Kummer S, Walter I, Jindra C, Weissenbacher-Lang C, et al. Tumor cell plasticity in equine papillomavirus-positive versus-negative squamous cell carcinoma of the head and neck. Pathogens. (2022) 11:2. doi: 10.3390/pathogens11020266
6. Campo MS, Moar MH, Sartirana ML, Kennedy IM, Jarrett WF. The presence of bovine papillomavirus type 4 DNA is not required for the progression to, or the maintenance of, the malignant state in cancers of the alimentary canal in cattle. EMBO J. (1985) 4:1819–25. doi: 10.1002/j.1460-2075.1985.tb03856.x
7. Regalado Ibarra AM, Legendre L, Munday JS. Malignant transformation of a canine papillomavirus Type 1-induced persistent oral papilloma in a 3-year-old dog. J Vet Dent. (2018) 35:79–95. doi: 10.1177/0898756418774575
8. Thaiwong T, Sledge DG, Wise AG, Olstad K, Maes RK, Kiupel M. Malignant transformation of canine oral papillomavirus (Cpv1)-associated papillomas in dogs: an emerging concern? Papillomavirus Res. (2018) 6:83–9. doi: 10.1016/j.pvr.2018.10.007
9. Munday JS, Dunowska M, Laurie RE, Hills S. Genomic characterisation of canine papillomavirus type 17, a possible rare cause of canine oral squamous cell carcinoma. Vet Microbiol. (2016) 182:135–40. doi: 10.1016/j.vetmic.2015.11.015
10. Altamura G, Corteggio A, Pacini L, Conte A, Pierantoni GM, Tommasino M, et al. Transforming properties of felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology. (2016) 496:1–8. doi: 10.1016/j.virol.2016.05.017
11. Altamura G, Cuccaro B, Eleni C, Strohmayer C, Brandt S, Borzacchiello G. Investigation of multiple felis catus papillomavirus types (-1/-2/-3/-4/-5/-6) Dnas in feline oral squamous cell carcinoma: a multicentric study. J Vet Med Sci. (2022) 22:60. doi: 10.1292/jvms.22-0060
12. Yamashita-Kawanishi N, Chang CY, Chambers JK, Uchida K, Sugiura K, Kukimoto I, et al. Comparison of prevalence of felis catus papillomavirus type 2 in squamous cell carcinomas in cats between Taiwan and Japan. J Vet Med Sci. (2021) 83:1229–33. doi: 10.1292/jvms.21-0153
13. Munday JS, Hardcastle M, Dally N. In situ squamous cell carcinoma of the gingiva and nictitating membrane associated with felis catus papillomavirus type 3 in a cat. Vet Pathol. (2022) 59:463–6. doi: 10.1177/03009858221079667
14. Chu S, Wylie TN, Wylie KM, Johnson GC, Skidmore ZL, Fleer M, et al. A virome sequencing approach to feline oral squamous cell carcinoma to evaluate viral causative factors. Vet Microbiol. (2020) 240:108491. doi: 10.1016/j.vetmic.2019.108491
15. Ozturk-Gurgen H, Almilli O, Sennazli G, Majzoub-Altweck M. Histopathological investigation of feline oral squamous cell carcinoma and the possible role of papillomavirus infection. Pak Vet J. (2022) 42:95–101. doi: 10.29261/pakvetj/2021.077
16. Altamura G, Tommasino M, Borzacchiello G. Cutaneous Vs. mucosal tropism: the papillomavirus paradigm comes to an “and”. Front. Microbiol. (2020) 11:588663. doi: 10.3389/fmicb.2020.588663
17. Altamura G, Corteggio A, Borzacchiello G. Felis catus papillomavirus Type 2 E6 oncogene enhances mitogen-activated protein kinases and akt activation but not egfr expression in an in vitro feline model of viral pathogenesis. Vet Microbiol. (2016) 195:96–100. doi: 10.1016/j.vetmic.2016.09.013
18. Altamura G, Power K, Martano M, Degli Uberti B, Galiero G, De Luca G, et al. Felis catus papillomavirus type-2 E6 binds to E6ap, promotes E6ap/P53 binding and enhances P53 proteasomal degradation. Sci Rep. (2018) 8:17529. doi: 10.1038/s41598-018-35723-7
19. Altamura G, Cardeti G, Cersini A, Eleni C, Cocumelli C, Bartolome Del Pino LE, et al. Detection of felis catus papillomavirus type-2 DNA and viral gene expression suggest active infection in feline oral squamous cell carcinoma. Vet Comp Oncol. (2020) 18:494–501. doi: 10.1111/vco.12569
20. Skor O. Presence of papillomavirus DNA in feline squamous cell carcinoma and injection site-sarcoma. In: Veterinary Cancer Society Conference, Fairfax County, Virginia (2015).
21. Munday JS, Knight CG, Luff JA. Papillomaviral skin diseases of humans, dogs, cats and horses: a comparative review. Part 2: Pre-Neoplastic Neoplastic Dis Vet J. (2022) 288:105898. doi: 10.1016/j.tvjl.2022.105898
22. Altamura G, Martano M, Licenziato L, Maiolino P, Borzacchiello G. Telomerase reverse transcriptase (Tert) expression, telomerase activity, and expression of matrix metalloproteinases (Mmp)-1/-2/-9 in feline oral squamous cell carcinoma cell lines associated with felis catus papillomavirus type-2 infection. Front Vet Sci. (2020) 7:148. doi: 10.3389/fvets.2020.00148
23. Sequeira I, Pires MDA, Leitao J, Henriques J, Viegas C, Requicha J. Feline oral squamous cell carcinoma: a critical review of etiologic factors. Vet Sci. (2022) 9:558. doi: 10.3390/vetsci9100558
24. Guo T, Goldenberg D, Fakhry C. Ahns series: do you know your guidelines? management of head and neck cancer in the era of human papillomavirus: educating our patients on human papillomavirus. Head Neck. (2017) 39:833–9. doi: 10.1002/hed.24693
25. Sharkey Ochoa I, O'Regan E, Toner M, Kay E, Faul P, O'Keane C, et al. The role of hpv in determining treatment, survival, and prognosis of head and neck squamous cell carcinoma. Cancers (Basel). (2022) 14:17. doi: 10.3390/cancers14174321
26. Windon MJ, D'Souza G, Fakhry C. Treatment preferences in human papillomavirus-associated oropharyngeal cancer. Future Oncol. (2018) 14:2521–30. doi: 10.2217/fon-2018-0063
27. McMullen C, Chung CH, Hernandez-Prera JC. Evolving role of human papillomavirus as a clinically significant biomarker in head and neck squamous cell carcinoma. Expert Rev Mol Diagn. (2019) 19:63–70. doi: 10.1080/14737159.2019.1559056
28. Supsavhad W, Dirksen WP, Martin CK, Rosol TJ. Animal models of head and neck squamous cell carcinoma. Vet J. (2016) 210:7–16. doi: 10.1016/j.tvjl.2015.11.006
29. Altamura G, Borzacchiello G. Hpv related head and neck squamous cell carcinoma: new evidences for an emerging spontaneous animal model. Oral Oncol. (2019) 88:84. doi: 10.1016/j.oraloncology.2018.11.027
30. Zakeri K, Dunn L, Lee N. Hpv-associated oropharyngeal cancer de-escalation strategies and trials: past failures and future promise. J Surg Oncol. (2021) 124:962–6. doi: 10.1002/jso.26696
31. Altamura G, Borzacchiello G. Anti-Egfr monoclonal antibody cetuximab displays potential anti-cancer activities in feline oral squamous cell carcinoma cell lines. Front Vet Sci. (2022) 9:1040552. doi: 10.3389/fvets.2022.1040552
32. Munday JS, He Y, Aberdein D, Klobukowska HJ. Increased P16(Cdkn2a), but not p53, immunostaining is predictive of longer survival time in cats with oral squamous cell carcinomas. Vet J. (2019) 248:64–70. doi: 10.1016/j.tvjl.2019.04.007
33. Hoggard N, Munday JS, Luff J. Localization of felis catus papillomavirus type 2 E6 and E7 Rna in feline cutaneous squamous cell carcinoma. Vet Pathol. (2018) 55:409–16. doi: 10.1177/0300985817750456
34. Demos LE, Munday JS, Lange CE, Bennett MD. Use of fluorescence in situ hybridization to detect felis catus papillomavirus type 2 in feline bowenoid in situ carcinomas. J Feline Med Surg. (2019) 21:575–80. doi: 10.1177/1098612X18795919
Keywords: papillomavirus, Felis catus, oral squamous cell carcinoma, head and neck cancer, comparative oncology
Citation: Altamura G and Borzacchiello G (2023) Feline oral squamous cell carcinoma and Felis catus papillomavirus: is it time to walk the path of human oncology? Front. Vet. Sci. 10:1148673. doi: 10.3389/fvets.2023.1148673
Received: 20 January 2023; Accepted: 02 May 2023;
Published: 17 May 2023.
Edited by:
Joshua R. Herr, University of Nebraska-Lincoln, United StatesReviewed by:
Sirikachorn Tangkawattana, Khon Kaen University, ThailandCopyright © 2023 Altamura and Borzacchiello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Borzacchiello, Ym9yemFjY2hAdW5pbmEuaXQ=
 Gennaro Altamura
Gennaro Altamura Giuseppe Borzacchiello
Giuseppe Borzacchiello