- 1Department of Biological Safety, Veterinary Faculty, Kazakh National Agrarian Research University, Almaty, Kazakhstan
- 2Department of Parasitology and Invasive Diseases, Faculty of Veterinary Medicine, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland
- 3Department of Microbiology, Virology and Immunology, Veterinary Faculty, Kazakh National Agrarian Research University, Almaty, Kazakhstan
- 4Department of Veterinary Medicine, Agrarian Faculty, Mukhtar Auezov South Kazakhstan University, Shymkent, Kazakhstan
- 5Department of Biology, Faculty of Sciences, Kyrgyz-Turkish Manas University, Bishkek, Kyrgyzstan
Aspergillosis is a severe fungal disease that affects all species and ages of poultry and leads to significant economic losses within the poultry industry. The economic significance of aspergillosis is associated with direct losses due to poultry mortality, a decline in the production of meat and eggs, feed conversion, and poor growth of recovering poultry. Although a decrease in the production of poultry meat and eggs in Kazakhstan due to this fungal disease has been widely reported, studies on the consequent financial losses on affected farms (households) have not been carried out. This study aimed to estimate the financial losses and epidemiological parameters of avian aspergillosis among households affected by the disease in the Almaty region. To achieve the objectives of the research, a survey was conducted involving affected households from February 2018 to July 2019. The affected poultry were diagnosed based on clinical, macroscopical, and microscopical procedures, and once the infection was confirmed, household owners were interviewed. Data were collected from 183 household owners. The median incidence risk and fatality rates were 39 and 26% in chickens, 42 and 22% in turkeys, and 37 and 33% in geese, respectively, with young poultry having a higher incidence risk and fatality rate than adults. Approximately 92.4% of the household owners treated the affected poultry using natural folk methods and 7.6% of household owners used antifungal drugs and antibiotics, spending a median of US $35.20 (min US $0; max US $400) per household throughout the course of the infection. Egg production was reduced by a median of 58.3% when households were affected. The price of poultry fell by a median of 48.6% immediately after recovery due to weight loss. The median of the overall financial losses of households was US $198.50 (min US $11; max US $1,269). The majority of household owners (65%) did not replace their poultry, 9.8% of household owners replaced all their poultry, and the remaining 25.1% replaced only a proportion of the poultry lost at the time of the study. Newly acquired poultry were purchased from neighbors (10.9%), fellow villagers (50%), and state poultry farms (39.1%). This study demonstrates that aspergillosis has an immediate impact on subsistence household owners' livelihoods in the Almaty region of Kazakhstan.
Introduction
The Republic of Kazakhstan (RK) is a country in the center of Eurasia with a territory of 2,724,902 km2. The population is 19,082,467 (1). The World Bank classifies the RK as a country with an upper middle-income economy (World Bank, 1 July 2021). Although agriculture remains a relatively unimportant sector (represents just 10% of GDP) of the Kazakh economy, it is considered the main source of income for the rural population which makes up 40.08% of the total population in the Republic. Currently, 45% of the total gross agricultural output involves livestock (1).
Poultry farming is one of the key sectors of the livestock industry in the RK, which ensures the country's food security. According to official data (1, 2), 65 poultry enterprises in the republic specialize in the production of eggs and poultry meat. Although the RK fully meets the demand of the domestic market for eggs (3), the domestic production of poultry meat covers only 58% of the needs of the domestic market.
Although poultry is predominantly produced by large poultry enterprises and farms, more than 25% of production is provided by households located in rural and urban areas (1) as an additional source of income and food. The market price of household poultry and associated products is higher than that of industrial producers. Unfortunately, infectious diseases with various geneses cause great economic damage to the poultry industry of the RK. An example is the recent outbreaks of the avian flu that swept across the country in 2020 (4, 5), where ~2 million chickens, geese, and ducks died.
Recently, affected poultry with clinical signs compatible with aspergillosis are often admitted to the Department of Pathology of the Kazakh National Agrarian Research University (KNARU) for postmortem examination and microscopic analysis. Due to the lack of clear clinical signs of avian aspergillosis, antemortem diagnosis is a challenge and unreliable (6).
Mycoses caused by fungi of the genus Aspergillus are common in humans (7–9), a wide range of animals (pet, farm, and wild) (10–16), and plants (17). Although there are several hundred species in the genus, subdivided into 22 distinct sections (18, 19), only 14 well-known species are infectious agents (6). Of these, a small number of opportunistic pathogens of the Aspergillus genus cover a wide range of diseases, ranging from localized infections to fatal disseminated diseases, as well as allergic responses to inhaled conidia (6, 7, 10, 20, 21). Moreover, some species belonging to the Aspergillus genus produce numerous mycotoxins (aflatoxins, gliotoxin, and ochratoxin A), which are released into the environment. Mycotoxins provide chemical protection and increase the virulence of the fungus (22–24). Feedstuff contaminated with Aspergillus mycotoxins is a cause of mortality in poultry. In terms of Aspergillosis cases, the most commonly found are caused by A. fumigatus (6, 20) and only a few by other species such as A. flavus, A. niger, and A. terreus (25–27).
In particular, A. fumigatus is the most common airborne fungal infection of the respiratory system of all avian species (10, 28–30). This is facilitated by the peculiarities of the anatomy and physiology of the avian lung-air sac system (31, 32). The relatively small spores of A. fumigatus (33) bypass initial physical barriers and penetrate deeply into the respiratory system to the air sacs where they are deposited (10). Furthermore, the epithelial surface of air sacs is almost devoid of mucociliary transport mechanisms (34). Consequently, poultry placed in a contaminated environment with aerosolized conidia may show significant pathology after only short exposure (6).
Aspergillosis occupies the first place in the structure of the mycotic pathologies of birds. This is due to the ubiquitous distribution of the Aspergillus species, the possibility of Aspergillus surviving on various biological substrates, as well as the morphological features of the fungi and their impact on the bird's organism (22, 35). The economic damage in this pathology is great since the mortality of young birds can range up to 90% (36, 37). Subsequent to data reported by Owings and Dykstra et al., the economic impact related to turkey mortality losses could be worth US $11 million annually. However, there have been no systematic studies on the prevalence of aspergillosis in chickens that would allow a substantiated estimate of the economic impact (38).
Keeping poultry benefit households through both income generation and as a direct source of quality food products for home consumption. Furthermore, households annually provide more than 10% of the market demand for eggs in the RK (1). Aspergillosis reduces meat and egg production, potentially having a negative impact on households' additional sources of income and food products. Nevertheless, the financial impact of avian aspergillosis in households in rural and urban areas of the RK has not been quantified, and disease control and prevention strategies used by the population are unknown.
The main purpose of this study is to assess the financial losses and infection parameters of avian aspergillosis in households in the Almaty region in terms of the affected poultry. Such a quantitative assessment in the endemic areas is critical for the development of disease control programs and for improving preparedness in other regions of the RK.
Materials and methods
Study area and households
The study was conducted among households situated in 13 districts and two cities of the Almaty region from February 2018 to July 2019. The Almaty region is located in the southeast of the RK (Figure 1A). It is divided into 17 districts (Figure 1B) and contains three cities of regional subordination. Agriculture in the Almaty region is the largest sector in terms of employment (27.1% of total employment). There are more than 54,000 agricultural producers and 345,000 households make up the population. In terms of gross output, crop production is responsible for 50.4% and livestock, 49.2%. The region ranks first in the republic in terms of the number of cattle, horses, and poultry and leads in the production of meat (19%), milk (13%), wool (22%), and eggs (23%) (40).
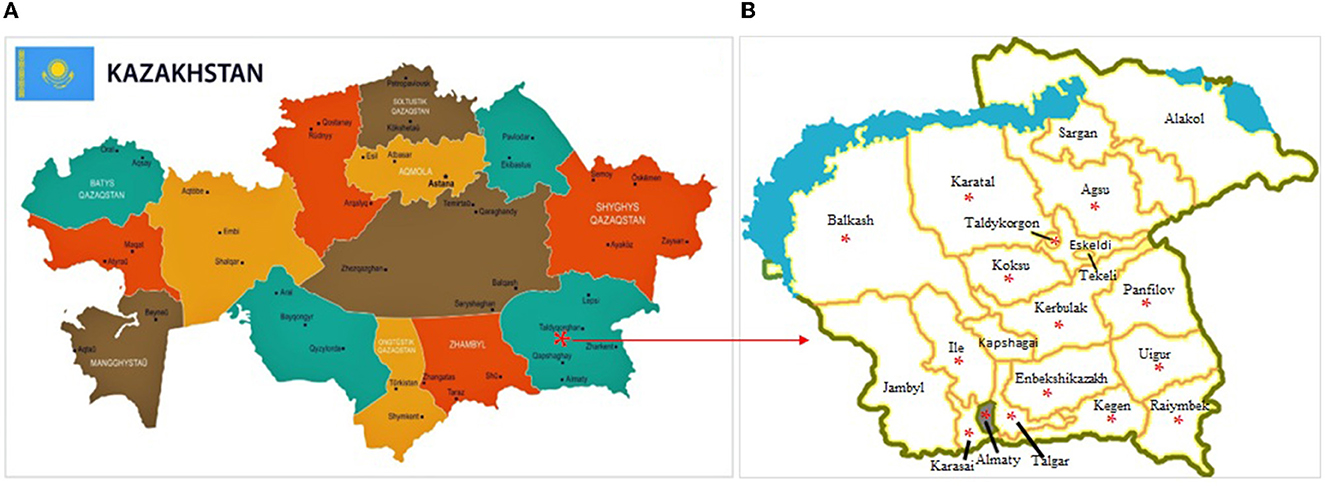
Figure 1. Administrative map of the Republic of Kazakhstan (A) (https://www.orangesmile.com/destinations/kazakhstan/country-maps-provinces.htm) and Almaty region (B) [(39), Almaty Oblast Aksu.png]. Areas where avian aspergillosis were reported to have been noted (*).
The bulk of the rural and urban population, with the exception of officially registered agricultural producers, has an economy consisting mainly of livestock as their main or additional source of income. They have sheep, goats, cattle, horses, camels, and poultry in varying numbers. In general, with regard to poultry, they raise chickens, turkeys, ducks, geese, and others. According to official data (1), a significant number of livestock are maintained in rural households (52.4% cattle, 50.6% sheep, 56% pigs, 67.7% goats, and 25.2% poultry). In these households, there is a mix of all types of livestock, and there are practically no households keeping only one species of domestic animal. The urban population, given the limited conditions, keeps only poultry. In 2022, in all categories of farms in the RK, the poultry population amounted to 45.2 million heads. In total, 71.8% of them were concentrated in large poultry farms, 33.1% in households, and 1.4% in small farms.
Study design and data collection
This study was based on a survey of 183 households, in which poultry were affected by avian aspergillosis. The affected poultry were identified following the household owners' report of an outbreak (or of sporadic cases) to a veterinarian. In addition, students from the KNARU have identified additional cases in their villages. All affected poultry were examined after the infection was reported and diagnosed by a qualified veterinarian. Avian aspergillosis was not registered among poultry raised in large enterprises and farms.
The affected poultry (chickens, turkeys, geese, and ducks), which demonstrated respiratory signs, dyspnea, rales, and weight loss, all died. Their age ranged from 6 days to 28 months. At postmortem examination, samples of organs affected with distinguishable aspergillosis lesions (e.g., granulomas, hemorrhages, and greenish-yellow cottony textures) were fixed in 4% formaldehyde and sent to KNARU in Almaty for the confirmation of the microscopical diagnosis. Paraffin wax-embedded specimens were sectioned for pathohistological examination at 5 μm and stained with hematoxylin and eosin for a general view. Caseous nodules and massive granulomas with necrotic cores surrounded by cells of lymphoid tissue (macrophages and lymphocytes), large foreign-body giant cells, and outer fibrous capsules were detected on the affected poultry organs. The results in terms of the above-described gross and microscopic lesions of avian aspergillosis were published in articles in local scientific journals. In total, 87% of the affected poultry were confirmed as displaying avian aspergillosis, and most of these birds (93%) were admitted from different areas of the Almaty region. Without exception, these were from households.
A total of 183 semi-structured interviews with affected household owners were conducted once the outbreak had concluded. All these owners have kept different species of poultry in their households for many years. The semi-structured interviews were conducted in a free and open manner. Before the interview, the goal of the study was explained to the respondents and verbal consent to participate was obtained. The duration of each interview was ~1.5 h. Details of the interviews were noted in notebooks and were recorded using Sony Dictaphones (ICD-BX140, China) or by using the dictaphone recorder function on the researchers' mobiles.
The basis of each interview was questions relating to aspects such as poultry breeding in the household, species of poultry, the total number of poultry, the purpose of keeping poultry, income from poultry products, poultry diseases, vaccination, treatment methods, and poultry fungal diseases (especially avian aspergillosis). In addition, information was obtained from the owners of the affected poultry with regard to morbidity and mortality, changes in production parameters, prices for healthy poultry (Supplementary Table 1), actions taken with regard to the affected poultry, and costs incurred. Interviews were conducted in Kazakh and Russian. A total of 183 household owners kindly agreed to participate in our interview. Data collection was carried out by the researchers, local veterinarians, and KNARU students.
Data analysis
Data were entered into an Excel spreadsheet and inconsistencies across the data were cross-checked. Descriptive statistics were generated and stratified by household owner, species (chickens, turkeys, ducks, and geese), and age categories (young poultry <180 days and adults ≥180 days). Parameters estimated include incidence risk, fatality rate, treatment cost, the difference in price between healthy and affected (recovered) poultry, and reduction in egg production. Incidence risk and fatality rate were estimated as follows:
and
The percentage reduction in poultry price and egg yield was calculated by comparing the estimated values in affected (recovered) and clinically healthy poultry. Price changes were assumed to be related to aspergillosis only.
Estimation of total financial losses
Financial losses resulting from avian aspergillosis were calculated individually for each household. The main factors were the value of the poultry before the aspergillosis outbreak, the value of the poultry after the aspergillosis outbreak, treatment cost, income loss due to reduced egg production, and total financial losses. The estimation of financial losses was carried out according to the scheme presented in Figure 2.
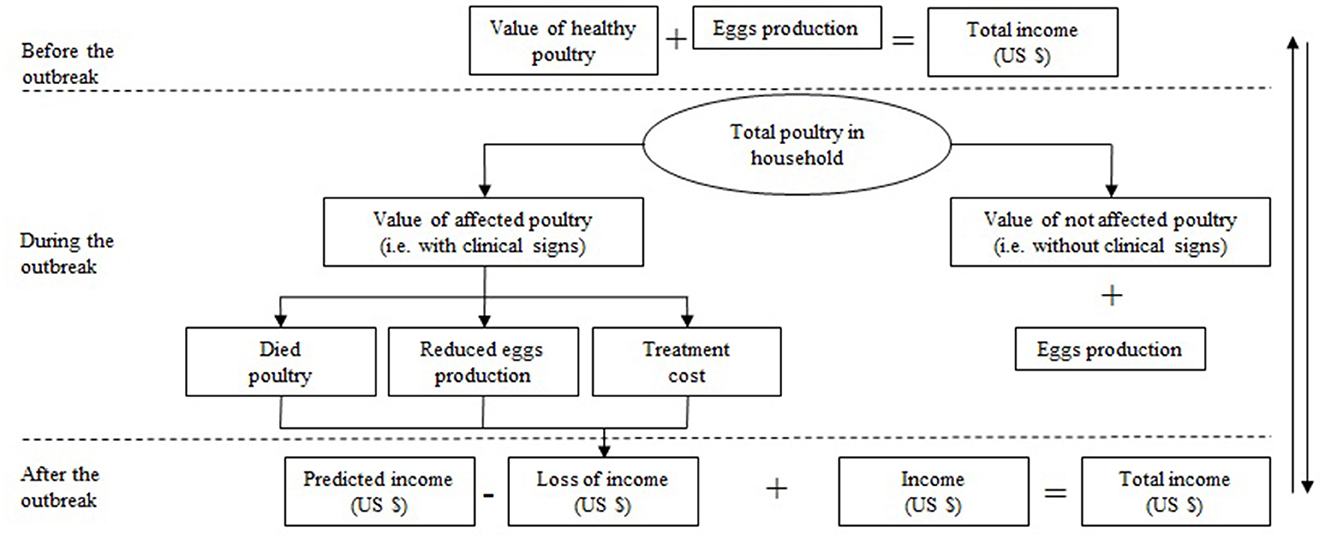
Figure 2. General scheme is used to estimate the financial losses caused by avian aspergillosis in households in the Almaty region of the RK.
First, the value of total poultry (+ eggs) before infection (VPBI) was estimated. The poultry number in the household before the infection equates to the sum of those poultry that presented with clinical signs, plus those that did not present any clinical signs. Owners of households reported the market price of each healthy poultry (eggs) at different ages. The total value of the poultry in a household was then estimated by multiplying the value of one bird by the number of birds stratified by species.
where P – price, T – total number, c – chicken, t – turkey, g - goose, and d – duck.
To estimate the value of the affected poultry after the infection, we assumed that poultry were both affected (with clinical signs) and unaffected (without clinical signs). Affected poultry had two outcomes: they died or remained in the household. In the first case, the value of the poultry became zero due to mortality, and in the second case, the poultry recovered. However, the value of the poultry was lower than it would be if it had not been affected by the disease. To estimate the value of the poultry without clinical disease, we used the value that household owners reported they would be paid if they sold them as healthy.
where VNAPAI is the value of the not affected poultry kept after the infection, N is the number of poultry that did not present clinical signs during the infection;
where VAPKAI is the value of the affected poultry kept after the infection, P' is the price affected poultry kept after the infection, N' is the number of the affected poultry kept after the infection;
Egg production losses due to clinical aspergillosis were estimated only for those household owners who reported selling eggs. The market price of eggs was also reported by the household owners.
where ILREP is the income loss due to reduced eggs production, EYBI is the daily egg yield before the infection, EYDI is the daily egg yield during the infection; ID is the infection duration; EP is the average egg price per piece in the study area.
The total value of the poultry after the infection was then estimated as the sum of the value of unaffected poultry in the household plus the value of affected poultry kept in the household until recovery.
where VPAI - is the total value of the poultry after the infection.
Treatment cost during the infection was equal to the money that household owners spent treating affected poultry with antifungal drugs and antibiotics or using folk remedies. Other expenses, such as time spent treating and looking after affected poultry, were not taken into consideration. Thus, the total financial losses per household owner were estimated as the difference between the value of the poultry before and after the infection, plus the treatment cost and income loss due to reduced egg production.
where TFL is the total financial losses, and M is the money spent on the treatment of poultry.
Statistical analysis
Pearson's chi-squared test (or Fisher's Exact test where appropriate) was used to determine the strength of association between the binary outcomes of the two groups. For continuous variables, parametric (t-test or ANOVA) or non-parametric equivalent, if appropriate (Mann–Whitney U-test or Kruskal–Wallis tests), were used to compare the outcomes of different groups. A value of p < 0.005 was considered as being statistically significant. The analysis was performed using SPSS (23.0).
Results
Characteristics of households included in the study
A total of 201 affected household owners were identified and invited to take part in the study. Data were collected from 183 household owners who agreed to participate in the study. Data were obtained from 168 (91.8%) household owners from rural areas and 15 (8.2%) household owners from urban areas. A majority of the household owners were from the Enbekshikazakh district (n = 28; 15.3%), followed by Panfilov (n = 21; 11.5%), Raiymbek (n = 19; 10.4%), Agsu (n = 17; 9.3%), Karasai (n = 14; 7.6%), Talgar (n = 12; 6.5%), Kerbulak (n = 11; 6%), Karatal (n = 9; 4.9%), Uigur (n = 9; 4.9%), Kegen (n = 8; 4.4%), Ile (n = 7; 3.8%), Koksu (n = 7; 3.8%), Balkash (n = 6; 3.3%) districts, plus Almaty (n = 9; 4.9%) and Taldykorgon (n = 6; 3.3%) in the case of cities (Table 1). All household owners had other main jobs and kept domestic animals as an additional source of income. They have sheep, goats, cattle, horses, donkeys, chickens, turkeys, geese, and ducks in varying numbers. Each of the studied households simultaneously kept different species of livestock and poultry.
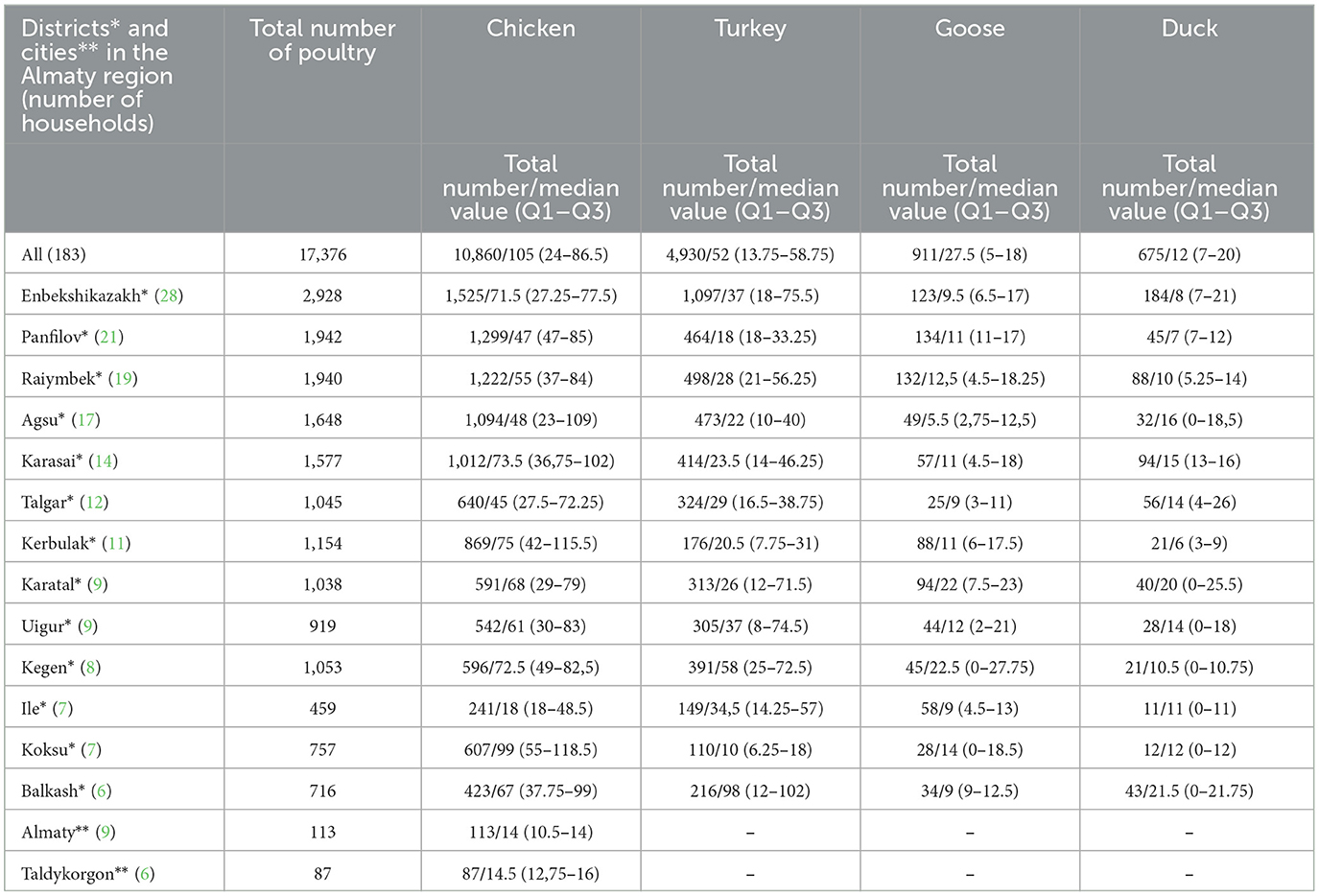
Table 1. The number of poultry in the households included in the study stratified by poultry species and districts (cities) in the Almaty region, RK.
Interviewed owners' reports
According to the reports, the majority of interviewed household owners (n = 168; 91.8%) kept two or more poultry species. A total of 15 (8.2%) household owners, due to limited opportunities, kept only a small number of chickens (from 7 to 17 heads). The total number of poultry in all households (n = 183; 100%) was 17,376, and the number of poultry in each household ranged from 7 to 162 head. In total, 13,504 heads of poultry were classified as adults (≥180 days) and 3,872 (<180 days) as young (Supplementary Tables 2–6). The poultry in the households were of different breeds.
The dominant type of poultry in the households were chickens (10,860, 62.5%) (Supplementary Table 3) and all owners (n = 183) kept them, followed by turkeys (n = 126; 4,930, 28.3%) (Supplementary Table 4), geese (n = 71; 911, 5.2%) (Supplementary Table 5), and ducks (n = 42; 675, 4%) (Supplementary Table 6). The most common reason for keeping poultry was its consumption (meat and eggs) at home (n = 66; 36.1%), commercialization of their meat, or its consumption at home (n = 48; 26.2%), sale of their eggs or their consumption at home (n = 26; 14.2%), selling them alive to earn income according to needs (n = 24; 13.1%), selling eggs (n = 16; 8.7%), and their commercialization regularly (n = 3; 1.6%). According to the report data, the average income from poultry (+eggs) annually was 14% of total income (min 1%; max 38%) (Supplementary Table 2).
In total, 61% (n = 112) of the interviewed household owners were aware of only two infections (Newcastle disease and avian flu diseases), while the rest (39%; n = 71) were unaware of any of the avian infectious diseases. Ectoparasites were known to all of the interviewed owners, and ascariasis was known to 44% (n = 81). Moreover, only one household owner (0.5%) was aware of avian aspergillosis because the owner had previously dealt with this pathology. Three (1.6%) of the interviewed household owners vaccinated chickens against Newcastle disease, while the rest (n = 180; 98.4%) did not. A total of 14 (7.6%) of the interviewed owners used antibiotics and antifungal drugs against different types of avian ailments. The dominant mass of household owners (n = 169; 92.4%) resorted to natural folk methods of treatment and prevention of illness in poultry. Most of them (n = 95; 56.2%) used crushed onion (Allium cepa L.; bulbs and leaves) and garlic (Allium sativum L.; bulbs and peduncles), mixed with feed. In total, 14.8% (n = 25) of the interviewed owners used only onion, 12.4% (n = 21) only garlic, 8.3% (n = 14) red peppers (Capsicum annuum L.), 5.9% (n = 10) root of harmala (Peganum harmala L.) (as an infusion or decoction), and 2.4% (n = 4) a small dose of childrens' urine (only in the case of turkeys) (Supplementary Table 2).
Disease impact
The median infection duration was 11 days (min. 5; max. 26 days). In total, 48.1% (n = 88) of the household owners reported that only chickens were affected, and 8.2% reported that (n = 15) only turkeys were affected. While 30% (n = 55) had chicken and turkey affected, and 7.1% (n = 13) had chickens and geese affected. A total of 12 owners (6.5%) indicated that three poultry species (chicken, turkey, and goose) concurrently showed clinical signs and died. There were no reports of ducks affected by aspergillosis. The median infection duration was longer in households where more than one species of poultry was affected [22 days (min. 16; max. 26)] compared to those in which only one species was affected [9 days (min. 5; max. 12)]. The time between the end of the infection and the inspector's visit averaged 12 days (min 6; max 23).
Considering only infections reported between February 2018 and February 2019 (when materials and data on infections were collected throughout the year), infections were reported year-round with an increase between March and May and a second peak in the period from September to November (Supplementary Figure 1).
The median incidence risk and fatality rate were 39% (min 14; max 100) and 26% (min 5; max 100), respectively, in chicken, 42% (min 5; max 82) and 22% (min 6; max 85), respectively, in turkey, and 37% (min 21; max 80) and 33% (min 10; max 75), respectively, in geese. In young poultry (<180 days), the incidence risk [45 (min 1; max 100)] and fatality rate [58 (min 30; max 100)] were higher than in adult poultry (≥180 days) (32 (min 5; max 89) and 20 (min 4; max 78), respectively) (Table 2). The median yield in egg production per day before the infection was 74 (min 11; max 127) while after infection it was 42 (min 2; max 102), and the median drop in egg production during infection was 30 (min 6; max 57) in chicken, and 23.5 (min 12; max 106), 11 (min 7; max 100), and 10 (min 5; max 39) in turkey, respectively (Table 3, Supplementary Table 7).
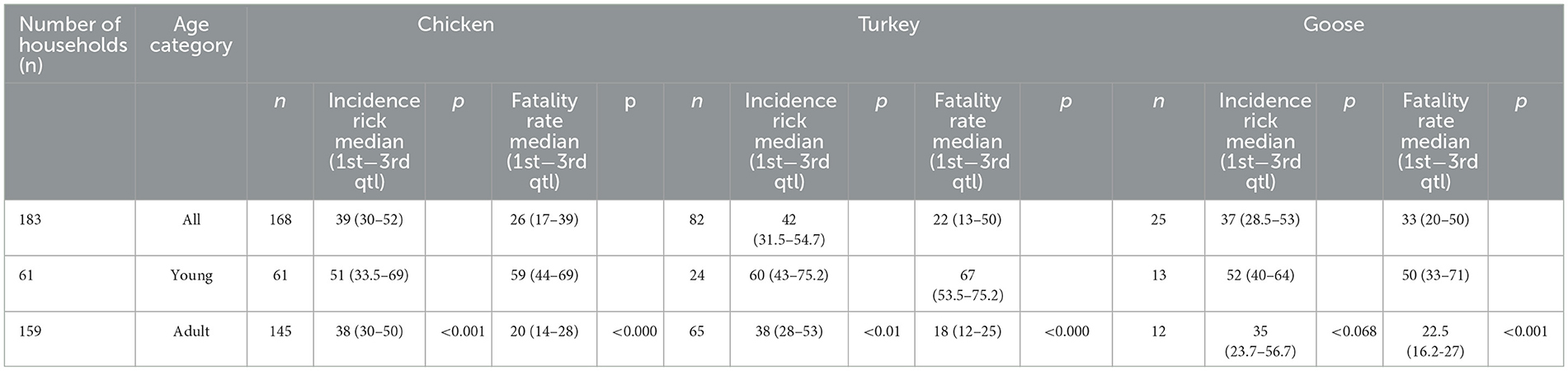
Table 2. Median aspergillosis incidence risk and fatality rate in affected households in the Almaty region, RK.
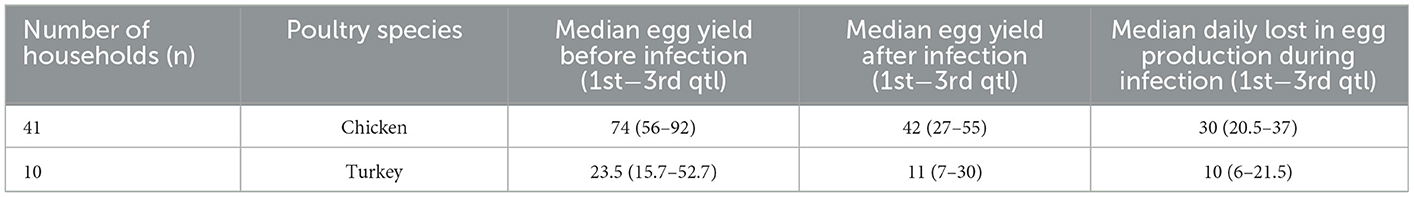
Table 3. Median egg yield before and after infection and daily drop in egg production during the aspergillosis reported by household owners in the Almaty region, RK.
Management and coping strategies
The majority of household owners (n = 155; 84.7%) treated affected poultry with folk methods of treatment, spending a median of 1,760 (US$3.52) (min 32; max 8,000) per household per day (for purchases of garlic, onions, and hot chili peppers) and treated individual poultry for a median of 10 days (min 5; max 25 days). A total of 14 (7.6%) of the household owners did not spend any money as they used decoction or infusion of harmala (n = 10; 5.4%) and urine therapy (n = 4; 2.2%) for the treatment of the affected poultry. The remaining 14 (7.6%) household owners treated affected poultry with appropriate antifungal drugs and antibiotics prescribed by a veterinarian, spending a median of 10,980 (US $21.96) (min 10,980; max 11,310). No information was collected regarding the time when treatment started in relation to the infection onset. The poultry displaying clinical signs were not sold or slaughtered for the sale of their meat.
The majority of household owners (n = 119; 65%) had not replaced poultry at the time of the interview. There were two main reasons for not replacing lost poultry. The first was the reluctance of owners to buy poultry from livestock markets because there was no guarantee that newly purchased poultry were healthy. The second reason was the lack of additional resources. A total of 18 household owners replaced all their poultry (9.8%), while the rest (n = 46; 25.1%) replaced only part of the lost poultry. Out of 64 household owners who provided an answer on the place where the last poultry had been purchased, 39 (60.9%) reported that it had been purchased from neighbors (n = 7; 10.9%) and fellow villagers (n = 32; 50%) who have a lot of poultry and good experience in keeping them, followed by state poultry farms (n = 25; 39.1%). Some of the household owners purchased poultry from more than one source. The median prices reported by household owners for the purchased poultry were 3,800 (US$7.6) for chicken, 10,600 (US$21.2) for turkey, and 8,300 (US$16.6) for geese.
Total financial losses during the infection
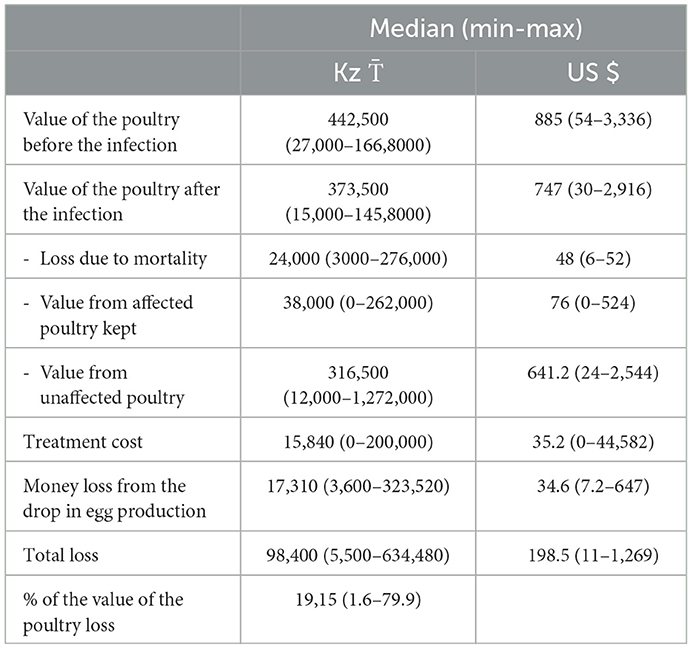
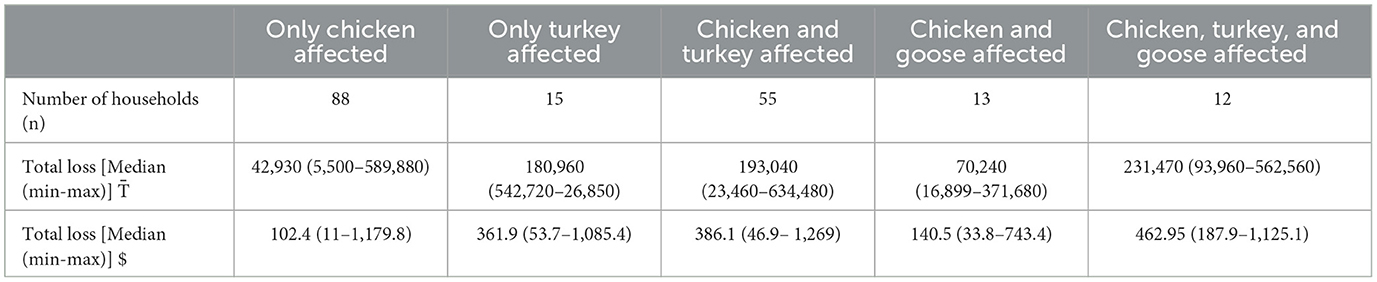
Financial losses during the infection are presented in Tables 4, 5. The median overall losses were 98,400 (US$198.5), ranging from 5,500 (US$11) to 634,480 (US$1,269) (Table 4). The median losses were higher [231,470 (min 93,960; max 562,560)] when three poultry species (chicken, turkey, and goose) were affected, followed by chicken and turkey affected [ 193,040 (min 23,460; max 634,480)], only turkey affected [ 180,960 (min 542,720; max 26,850)], chicken and geese affected [ 70,240 (min 16,899; max 371,680)], and only chicken affected [ 42,930 (min 5,500; max 589,880)] (Table 5).
The median percentage loss in terms of the value of the poultry in households was 19.1% (min 1.6%; max 79.9%). The median percentage loss was higher for chicken and geese affected (median 27.3; min 6.5%, max 50%), followed by chicken and turkey affected (median 21.2; min 4%, max 48.3%), only turkey affected (median 20; min 6.1%; max 31.4%), chicken, turkey and geese affected (median 19; min 8.1%; max 32.5%), and only chicken affected (median 16.5; min 1.6%; max 79.9%) (Supplementary Table 8).
Discussion
In this article, data are provided on financial losses and epidemiological parameters of avian aspergillosis among affected 183 households located in various villages in 13 districts and two cities of the Almaty region in Kazakhstan. The results of this research indicate that avian aspergillosis has an immediate impact on household owners' income. As mentioned above, 33.1% of all poultry in the republic is concentrated in households (1). Furthermore, poultry products produced in households are an organic source of poultry meat and eggs, as well as an additional source of income for the rural population.
Financial losses due to avian aspergillosis are associated with high mortality in the case of infected poultry, the disposal (non-sale) of carcasses of adult poultry, a decrease in egg production, and the purchase of medicines or raw materials for folk remedies.
The results of this study showed that the direct losses associated with the mortality (p < 0.05; p = 0.004) of poultry due to aspergillosis have an obvious immediate negative financial impact on the household owners and on the production of an organic source of poultry meat and eggs (p < 0.05; p = 0.00). The majority of household owners treated affected poultry with folk remedies, spending a median of US $31.5 (minimum $0.5; maximum $400.50). Some of the household owners treated the affected poultry with appropriate antifungals and antibiotics prescribed by a veterinarian, spending a median of US $65.90 (minimum $11.50; maximum $66.50). Thus, the treatment of affected poultry with modern medicines is confirmed statistically (p < 0.05; p = 0.01) as being two times more expensive than treatment with folk remedies. Median losses were higher (p < 0.05; p = 0.00) when three poultry species (chicken, turkey, and geese) were affected. The median percentage loss of poultry value in households was 19.1% (minimum 1.6%; maximum 79.9%). During the outbreak, the affected poultry and its meat were not sold, leading to a statistically significant reduction in the income (p < 0.05; p = 0.03) of the households, many of which, as indicated in Supplementary Table 2, build their daily income from the production of meat and eggs. In total, 65% (n = 119) of household owners reported that they had not replaced poultry that died as a consequence of the outbreak, citing a lack of assurance that healthy poultry could be purchased from livestock markets and a lack of additional resources. Interviewed owners reported that selling affected poultry or slaughtering for any ailments, including aspergillosis, was not carried out. This was because no one will buy diseased poultry, even at a very low price, and the carcasses of dead poultry were thrown in the trash or outer toilet, buried or burned, or given to dogs. Indeed, poultry carcasses resulting from airsacculitis are condemned at inspection following slaughter (41, 42).
Due to a lack of awareness, the household owners dump or give the poultry carcasses to their dogs, which increases the risk of spreading aspergillosis and increases the risk of infection among the owners themselves. As previously mentioned (43, 44), environmental contamination with Aspergillus conidia in poultry farms represents a significant risk for farm workers. Although the official RK veterinary authorities have developed rules for the prevention and elimination of avian aspergillosis (45), household owners were not aware of these rules. Poor awareness on the part of the household owners was confirmed by the results of this survey, where more than 61% of the owners had a superficial knowledge of only two avian infections, only one owner knew about avian aspergillosis, and only 1.6% of the owners had vaccinated chickens against Newcastle disease (Supplementary Table 2). This indicates that the veterinary service and local government authorities do not pay sufficient attention with regard to appropriate awareness-raising activities regarding the conditions required for poultry keeping and the general knowledge about the most common poultry diseases. To overcome these shortcomings, it is necessary to develop and distribute short and easily understandable brochures for ordinary people, organize special television programs, show videos on local channels, and hold local seminars.
According to the survey results, several (7.6%) household owners used antifungal drugs and additional antibiotics for the treatment of avian aspergillosis. Poultry farms have not treated birds with this pathology, although several strategies have been proposed for keeping birds in captivity. Furthermore, there were no appropriate vaccines (6, 46). In the current study, the majority of the interviewed owners (92.4%) prefer to use natural folk methods of treatment (Supplementary Table 2). They used freshly crushed onion (bulb and leaves) and garlic (bulb and peduncles) and red peppers mixed with feed, as well as harmala root as infusion or decoction and a small dose of fresh baby urine. Similar information on the treatment of avian aspergillosis was not found among other ethnoveterinary studies. In addition, more research is needed to specifically determine the effectiveness or otherwise of these remedies.
It is worth noting that all the above-mentioned ethnoveterinary methods of treatment are used among different ethnic groups for the treatment of other animal disorders. Onion has a wide array of uses in ethnoveterinary practices (47–49), ranging from gastrointestinal ailments, the treatment of tympany, indigestion, and bloating to proven insecticidal antiparasitic, repellant, antioxidant, anti-inflammatory, and antimicrobial actions (50, 51). Garlic also has effective pharmacological and medicinal properties (52) and is often used in veterinary practice for the treatment of various animal diseases (48, 49). The harmala is the most commonly used medicinal plant in ethnoveterinary practice (53). Harmala seed and root extracts have been reported to show antimicrobial, antiparasitic, antiviral, and antifungal activities (54). In addition, the results of many studies have proven that red pepper has many pharmacological properties (55). Interestingly, the internal and external application of one's own urine is an ancient Eastern tradition that is gaining popularity in the West (56). Human (56, 57) and animal (58) urine have multiple healing properties and has been noted for their antibacterial, antifungal, and antiviral properties (59). Thus, all of the above-mentioned plants and human urine, despite the lack of direct indications in studies on the treatment of avian aspergillosis, have antifungal properties.
In the current study, the correlation of the ethnoveterinary treatments with natural remedies, and the impact of avian aspergillosis, showed the following results. In the treatment of aspergillosis with onion + garlic, the mean mortality among adult poultry was 5.44 ± 5.22, among young poultry it was 15.85 ± 11.84, and the mean infection duration was 14.62 ± 6.86; with onion, it was 4.76 ± 3.64, 16.16 ± 5.67, and 15.84 ± 5.8, respectively; with garlic, it was 5.94 ± 4.94, 12.11 ± 6.8, and 14.76 ± 6.03, respectively; with red pepper, it was 4.53 ± 4.62, 13.25 ± 4.19, and 14.35 ± 6.23, respectively; with harmala decoction or infusion, it was 5 ± 3.6, 12.5 ± 2.12, and 15 ± 6.68, respectively; with urine, it was 3.66 ± 4.61, 16 ± 0, and 9 ± 0.81, respectively. In the treatment with the above-mentioned natural remedies between adult poultry and young poultry and infection duration, statistically, there were no significant differences (p > 0.05; p = 0.882, p = 0.658, and p = 0.561, respectively). However, there is a statistically significant difference between adult and young poultry (p > 0.05; p = 0.00).
In terms of the epidemiological parameters in the current study, the incidence risk and fatality rate were slightly different in chickens, turkeys, and geese (39–36%, 42–22%, and 37–33%, respectively). However, there is no statistically significant difference between the three groups of poultry (p > 0.05; p = 0.918 and p = 0.283, respectively). Incidence risk and fatality rates were higher in young poultry (45–58%) than in adults (20–32%). This difference was confirmed statistically (p < 0.05; both p = 0.00). Mortality ranged between 4.5 and 90%, while spontaneous avian aspergillosis in birds aged from 3 days to 20 weeks was previously reported (36, 37). This is due to the immaturity of the immune system in young individuals. The median yield in egg production during infection reduced by 59.5% and after infection by 41.7% in chickens, by 57.5 and 53.2% in turkeys, respectively, which indicates the marked and protracted negative impact of avian aspergillosis. The economic significance of aspergillosis was reported only in turkeys (60, 61), and it is especially important to note that it primarily affects expensive breeder toms (62).
In this pathology, the diagnosis was made using a combined method, since the antemortem diagnosis of aspergillosis is considered difficult and unreliable (6, 63, 64). This refers to clinical signs, postmortem, and microscopic features with the mandatory detection of conidia and fungal culture. However, the lack of molecular methods for diagnosing avian aspergillosis and the shortage of local pathologists in Kazakhstan cause great problems when it comes to a correct diagnosis.
It is clear from the results presented here that avian aspergillosis has a negative financial impact on household owners' livelihoods. Furthermore, coping, treatment and prevention strategies, and control measures are not well organized by the official veterinary authorities, which is likely to have consequences for spreading Aspergillus in the study area. It is necessary to raise household owners' awareness of the necessary conditions of poultry keeping and feeding and feed storage and to provide general information with regard to poultry diseases, including avian aspergillosis.
To effectively control this disease, it is recommended that there is a need to develop a structural strategic plan that will include informational materials and training sessions for household owners and poultry farmers. For the timely and correct diagnosis of avian aspergillosis, it is necessary to provide local veterinary laboratories with modern culture, histology, serology, imaging, and molecular techniques and the hiring of competent specialists. It is also important to take into consideration the above-mentioned folk methods of treatment used by the household owners; further study of non-traditional methods of treatment could provide a significant breakthrough in the veterinary treatment of aspergillosis.
Conclusion
This study demonstrates that aspergillosis has an immediate impact on subsistence household owners' livelihoods in the Almaty region of the Republic of Kazakhstan. We have quantified the effect of aspergillosis on production parameters that have not been quantified before in chickens, turkeys, and geese and have assessed the impact of the diseases on subsistence producers from different angles. We have also identified potential transmission routes and areas where appropriate control measures should be directed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Ethics Committee of the Kazakh National Agrarian Research University.
Author contributions
DK and NA conceptualized the study and developed the questionnaire with input from AM, PS, MM, GK, AD, and NN. AM and DK diagnosed affected poultry, according to the results of necropsy and microscopic examination. DK coordinated the data collection (with input from AM) and performed the data entry. NA verified data entry and conducted data analysis with input from DK. DK secured funding. AM, DK, and NA drafted the manuscript. All authors reviewed the manuscript.
Funding
DK acknowledges funding from the Kazakh National Agrarian Research University funded fellowship.
Acknowledgments
The authors gratefully thank the local public practice veterinarians and students for their assistance in data collection, household owners for their invaluable support, and Dr. G Muhametjanova for performing the statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1141456/full#supplementary-material
References
1. Bnasr RK. Bureau of National Statistics of the Agency for Strategic Planning and Reform of the Republic of Kazakhstan. Official government website of the RK (2022).
2. EFKO Kazakhstan LLP. Poultry farming in Kazakhstan: achievements and prospects. J AGRO-BOSS. (2020).
3. Sigarev MI, Nurkuzhaev ZhM, Alshembaeva LT. Poultry farming in Kazakhstan: state and development problems. Kazakhstan agricultural newspaper of “Agrarian of Kazakhstan”. (2019). Available online at: http://abkaz.kz/pticevodstvo-kazaxstana-sostoyanie-i-problemy-razvitiya/ (accessed november 19, 2022).
4. Lewis NS, Banyard AC, Whittard E, Karibayev T, Kafagi TA, Chvala I, et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg Microbes Infect. (2020) 10:148–51. doi: 10.1080/22221751.2021.1872355
5. Amirgazin A, Shevtsov A, Karibayev T, Berdikulov M, Kozhakhmetova T, Syzdykova L, RamankulovY, Shustov AV. Highly pathogenic avian influenza virus of the A/H5N8 subtype, clade2.3.4.4b, caused outbreaks in Kazakhstan in 2020. PeerJ. (2022) 10:e13038. doi: 10.7717/peerj.13038
6. Seyedmousavi S, Guillot J, Arne' P, Sybren de Hoog G, Mouton JW, Melchers WJG, Verweij PE. Aspergillus and aspergilloses in wild and domestic animals: a global health concern with parallels to human disease. J Med Mycol. (2015) 53:765–97. doi: 10.1093/mmy/myv067
7. Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. (1999) 12:310–50. doi: 10.1128/CMR.12.2.310
8. Greub G, Bille J. Aspergillus species isolated from clinical specimens: suggested clinical and microbiological criteria to determine significance. Clin Microbiol Infect. (1998) 4:710–6. doi: 10.1111/j.1469-0691.1998.tb00656.x
9. Clancy CJ, Nguyen MH. Acute community-acquired pneumonia due to Aspergillus in presumably immunocompetent hosts: clues for recognition of a rare but fatal disease. Chest. (1998) 114:629–34. doi: 10.1378/chest.114.2.629
10. Arné P, Thierry S, Wang D, Deville M, Loc'h L, Desoutter A, et al. Aspergillus fumigatus in poultry. Int J Microbiol. (2011) 2011:746356. doi: 10.1155/2011/746356
11. Puntenney SB, Wang Y, Forsberg NE. “Mycotic infections in livestock: Recent insights and studies on etiology, diagnostics and prevention of Hemorrhagic Bowel Syndrome,” In: Southwest Nutrition & Management Conference, Pheonix, Tuscon: University of Arizona, Department of Animal Science (2003), p. 49–63.
12. Sarfati J, Jensen HE, Latge JP. Route of infections in bovine aspergillosis. J Med Vet Mycol. (1996) 34:379–83. doi: 10.1080/02681219680000681
13. Dobesova O, Schwarz B, Velde K, Jahn P, Zert Z, Bezdekova B. Guttural pouch mycosis in horses: a retrospective study of 28 cases. Vet Rec. (2012) 171:561. doi: 10.1136/vr.100700
14. Pérez V, Corpa JM, Marín JFG, Adúriz JJ, Jensen HE. Generalized aspergillosis in dairy sheep. Zentralbl Veterinarmed B. (1999) 46:613–21. doi: 10.1046/j.1439-0450.1999.00290.x
15. Sharman MJ, Mansfield CS. Sinonasal aspergillosis in dogs: a review. J Small Anim Pract. (2012) 53:434–44. doi: 10.1111/j.1748-5827.2012.01245.x
16. Abdo W, Kawachi T, Sakai H, Fukushi H, Kano R, Shibahara T, et al. Disseminated mycosis in a killer whale (Orcinus orca). J Vet Diagn Invest. (2012) 24:211–8. doi: 10.1177/1040638711416969
17. Duarte EAA, Damasceno CL, de Oliveira TAS, Barbosa LD, Martins FM, Silva JR, et al. Putting the mess in order: aspergillus welwitschiae (and Not A. niger) is the etiological agent of sisal bole rot disease in Brazil. Front in Microbiol. (2018) 9:1–21. doi: 10.3389/fmicb.2018.01227
18. Peterson SW, Varga J, Frisvad JC. “Phylogeny and subgeneric taxonomy of Aspergillus,” In:Varga J, Samson RA, , eds., Wageningen: Wageningen Academic Publishers (2008), p. 33–56.
19. Balajee SA. Aspergillus terreus complex. Med Mycol. (2009) 47:S42–6. doi: 10.1080/13693780802562092
21. Tell LA. Aspergillosis in mammals and birds: impact on veterinary medicine. Med Mycol. (2005) 43:S71–3. doi: 10.1080/13693780400020089
22. Pitt JI. The current role of Aspergillus and Penicillium in human and animal health. J Med Vet Mycol. (1994) 32:17–32. doi: 10.1080/02681219480000701
23. Keller NP, Turner G, Bennett JW. Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microbiol. (2005) 3:937–47. doi: 10.1038/nrmicro1286
24. Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. (2003) 16:497–516. doi: 10.1128/CMR.16.3.497-516.2003
25. Charlton B, Chin RP, Barnes HJ. “Aspergillosis,” In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, eds Diseases of Poultry, 12th edn. Iowa State Press, Ames, Iowa (2008), p. 989–1001.
26. Olias P, Gruber AD, Winfried B, Hafez HM, Lierz M. Fungal pneumonia as major cause of mortality in white stork (Ciconia ciconia) chicks. Avian Dis. (2010) 54:94–8. doi: 10.1637/9088-092509-Reg.1
27. Olias P, Hauck R, Windhaus H, van der Grinten E, Gruber AD, Hafez HM. Articular aspergillosis of hip joints in Turkeys. Avian Dis. (2010) 54:1098–110. doi: 10.1637/9232-011110-Case.1
28. Beernaert LA, Pasmans F, Van Waeyenberghe L, Haesebrouck F, Martel A, et al. Aspergillus infections in birds: a review. Avian Pathol. (2010) 39:325–31. doi: 10.1080/03079457.2010.506210
29. Richard JL. Diseases of Poultry, 9th edn. In: Calnek BW, Barnes HJ, Beard CW, Reid WM, Yoder HW, eds Iowa State University Press, Ames, Iowa (1991), p. 326–34.
30. Hoerr FJ. Diseases of Poultry, 9th edn. In: Calnek BW, Barnes HJ, Beard CW, Reid WM, Yoder HW, eds Iowa State University Press, Ames, Iowa (1991), p. 884–915.
31. Fedde MR. Relationship of structure and function of the avian respiratory system to disease susceptibility. Poult Sci. (1998) 77:1130–8. doi: 10.1093/ps/77.8.1130
32. Brown RE, Brain JD, Wang N. The avian respiratory system: a unique model for studies of respiratory toxicosis and for monitoring air quality. Environ Health Perspect. (1997) 105:188–200. doi: 10.1289/ehp.97105188
33. Richard JL, Thurston JR. Rapid hematogenous dissemination of Aspergillus fumigatus and A. flavus spores in turkey poults following aerosol exposure. Avian Dis. (1983) 27:1025–33. doi: 10.2307/1590203
34. Maina JN. The Lung-Air Sac System of Birds: Development, Structure, and Function. Springer: Berlin (2005), p. 159–74.
35. Heitman J. Microbial pathogens in the fungal kingdom. Fungal Biol Rev. (2011) 25:48–60. doi: 10.1016/j.fbr.2011.01.003
36. Akan M, Haziroglu R, Ilhan Z, Sareyyüpoglu B, Tunca R, et al. A case of aspergillosis in a broiler breeder flock. Avian Dis. (2002) 46:497–501. doi: 10.1637/0005-2086(2002)0460497:ACOAIA2.0.CO
37. Steinlage SJT, Sander JE, Brown TP, Lobsinger CM, Thayer SG, Martinez A. Disseminated mycosis in layer cockerels and pullets. Avian Dis. (2003) 47:229–33. doi: 10.1637/0005-2086(2003)0470229:DMILCA2.0.CO
38. Hauck R, Cray C, França M. Spotlight on avian pathology: aspergillosis. Avian Pathology. (2020) 49:115–8. doi: 10.1080/03079457.2019.1696946
39. Safronov. The Administrative Map of the Almaty Region (Aksu District on the Map of Almaty Region). (2008). Available online at: https://commons.wikimedia.org/wiki/File:Almaty_Oblast_Aksu.png
40. Abdykalyk SE. Development of the agro-industrial complex of the Almaty region of the Republic of Kazakhstan. Questions Sci Edu. (2019) 7:44–7.
41. Moretti LD, Dias RA, Telles EO, Balian Sd. Time series evaluation of traumatic lesions and airsacculitis at one poultry abattoir in the state of São Paulo, Brazil (1996–2005). Prev Vet Med. (2010) 94:231–239. doi: 10.1016/j.prevetmed.2010.02.013
42. Lupo C, Bouquin SL, Allain V, Balaine L, Michel V, Petetin I, et al. Risk and indicators of condemnation of male turkey broilers in western France, February–July 2006. Prev Vet Med. (2010) 94:240–50. doi: 10.1016/j.prevetmed.2010.01.011
43. Cafarchia C, Camarda A, Iatta R, Danesi P, Favuzzi V, Di Paola G, et al. Environmental contamination by Aspergillus spp. in laying hen farms and associated health risks for farm workers. J Med Microbiol. (2014) 63:464–70. doi: 10.1099/jmm.0.065946-0
44. Fulleringer SL, Seguin D, Warin S, Bezille A, Desterque C, Arné P, et al. Evolution of the environmental contamination by thermophilic fungi in a turkey confinement house in France. Poult Sci. (2006) 85:1875–80. doi: 10.1093/ps/85.11.1875
45. On approval of the Veterinary Rules for the and Prevention Elimination of Infectious Diseases of Poultry. Veterinary rules for the prevention and elimination of avian aspergillosis. Web portal Information and legal system of normative legal acts of the Republic of Kazakhstan. Available online at: https://adilet.zan.kz/rus/docs/V050003448 (accessed November 19, 2022).
46. Xavier MO, Soares MP, Meinerz AR, Nobre MO, Osório LG, Silva Filho RP, et al. Aspergillosis: a limiting factor during recovery of captive magellanic penguins. Brazilian J Microbiol. (2007) 38:480–4. doi: 10.1590/S1517-83822007000300018
47. Khan K. Rahman IUr, Calixto ES, Ali N, Ijaz F. Ethnoveterinary therapeutic practices and conservation status of the medicinal flora of chamla Valley, Khyber Pakhtunkhwa, Pakistan. Front Vet Sci. (2019) 6:122. doi: 10.3389/fvets.2019.00122
48. Viegi L, Pieroni A, Guarrera PM, Vangelisti R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J Ethnopharmacol. (2003) 89:221–44. doi: 10.1016/j.jep.2003.08.003
49. Volpato G, Saleh SML, Nardo AD. Ethnoveterinary of Sahrawi pastoralists of Western Sahara: camel diseases and remedies. J Ethnobiol Ethnomed. (2015) 11:54. doi: 10.1186/s13002-015-0040-4
50. Marefati N, Ghorani V, Shakeri F, Boskabady M, Kianian F, Rezaee R, et al. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm Biol. (2021) 59:285–300. doi: 10.1080/13880209.2021.1874028
51. Zhao X-X, Lin F-J, Li H, Li H-B, Wu D-T, Geng F, et al. Recent advances in bioactive compounds. Health functions, and safety concerns of onion (Allium cepa L) Front Nutr. (2021) 8:669805. doi: 10.3389/fnut.2021.669805
52. Tudu CK, Dutta T, Ghorai M, Biswas P, Samanta D, Oleksak P, et al. Traditional uses, phytochemistry, pharmacology and toxicology of garlic (Allium sativum), a storehouse of diverse phytochemicals: a review of research from the last decade focusing on health and nutritional implications. Front Nutr. (2022) 9:949554. doi: 10.3389/fnut.2022.929554
53. Aldayarov N, Tulobaev A, Salykov R, Jumabekova J, Kydyralieva B, Omurzakova N, et al. An ethnoveterinary study of wild medicinal plants used by the Kyrgyz farmers. J Ethnopharmacol. (2022)285:114842. doi: 10.1016/j.jep.2021.114842
54. Zhu Z, Zhao S, Wang C. Antibacterial, antifungal, antiviral, and antiparasitic activities of Peganum harmala and its ingredients: a review. Molecules. (2022) 27:4161. doi: 10.3390/molecules27134161
55. Khan FA, Mahmood T, Ali M, Saeed A, Maalik A. Pharmacological importance of an ethnobotanical plant: Capsicum annuum L. Nat Prod Res. (2014) 28:1267–74. doi: 10.1080/14786419.2014.895723
56. Coen van der Kroon. The Golden Fountain. The Complete Guide to Urine Therapy. Book code BK-5170, ISBN 81-7021-742-3 (1921), p. 126.
57. Majesty D, Kenneth N. Assessment of urine on body weight, CD4 cells and testosterone hormone. Canad J Comput Math Nat Sci Eng Med. (2013)4:56–60.
58. Oyedeji-Amusa MO, Ojuromi OT, Ashafa AO. Ethnoveterinary survey of tradomedical importance of Bos taurus L urine, bile and dung in Nigeria and South Africa Tropical. J Pharmaceutical Res August. (2016) 15:1807–13. doi: 10.4314/tjpr.v15i8.30
59. Martha MC. Your own perfect Medicine: The Incredible Proven Natural Miracle Cure that Medical Sciences has Never Revealed. Wishland Inc. (1994), p. 15–202.
60. Owings WJ. Turkey Health Problems: A Summary of 12 Years of IOWA Grower Surveys. Iowa State University Extension Publication (1995), p. PS−257.
61. Dykstra MJ, Charlton BR, Chin RP, Barnes HJ. “Fungal infections,” In:Swayne, DE, Glisson, JR, McDougald, LR, Nolan, LK, Suarez, DL, Nair, V, , eds. Diseases of Poultry, 13th ed. Ames: Iowa State Press (2013), p. 1077–1096. doi: 10.1002/9781119421481.ch25
62. Kunkle RA, Sacco RE. Susceptibility of convalescent turkeys to pulmonary aspergillosis. Avian Dis. (1998) 42:787–90. doi: 10.2307/1592716
63. Aguilar RF, Redig PT. “Diagnosis and treatment of avian aspergillosis,” In Bonagura, JD and Kirk, RW, eds Current veterinary therapy XII. WB Saunders Co., Philadelphia, PA (1995), p. 1294–99.
Keywords: epidemiological characteristics, financial losses, households, poultry, aspergillosis, Kazakhstan
Citation: Kalkayeva D, Maulanov A, Sobiech P, Michalski M, Kuzembekova G, Dzhangabulova A, Nurkhojayev N and Aldayarov N (2023) Epidemiological characteristics and financial losses due to avian aspergillosis in households in the Almaty region, Republic of Kazakhstan. Front. Vet. Sci. 10:1141456. doi: 10.3389/fvets.2023.1141456
Received: 10 January 2023; Accepted: 15 March 2023;
Published: 17 April 2023.
Edited by:
Berhanu Mekibib, Hawassa University, EthiopiaReviewed by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyShamik Polley, West Bengal University of Animal and Fishery Sciences, India
Copyright © 2023 Kalkayeva, Maulanov, Sobiech, Michalski, Kuzembekova, Dzhangabulova, Nurkhojayev and Aldayarov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dinara Kalkayeva, ZGluYXJha2Fsa2F5ZXZhQGdtYWlsLmNvbQ==; Nurbek Aldayarov, bnVyYmVrLmFsZGF5YXJvdkBtYW5hcy5lZHUua2c=
 Dinara Kalkayeva1*
Dinara Kalkayeva1* Przemysław Sobiech
Przemysław Sobiech Nurbek Aldayarov
Nurbek Aldayarov