- 1Environmental and Aquatic Research Laboratory, Department of Pathobiology, College of Veterinary Medicine, Tuskegee University, Tuskegee, AL, United States
- 2Microbiology Diagnostic Laboratory, Department of Pathobiology, College of Veterinary Medicine, Tuskegee University, Tuskegee, AL, United States
- 3Clinical Pathology Diagnostic Laboratory, Department of Pathobiology, College of Veterinary Medicine, Tuskegee University, Tuskegee, AL, United States
Streptococcus pseudoporcinus (S. pseudoporcinus) is a β-hemolytic, Gram-positive novel bacterium first identified in 2006. It is a catalase-negative, non-motile coccus arranged in short chains. Furthermore, it has a broad beta-hemolytic reaction on sheep blood agar and cross-reacts with Lancefield group B antigen agglutination reagent. In this study, we report a case of S. pseudoporcinus infection of a surgical wound on the left metatarsus of a dog. The patient is a 9-year-old spayed female Great Dane dog with a brief history of multiple cutaneous masses being removed. Post-surgery, the post-surgical site on the left metatarsus became infected and discharged purulent material with a fetid odor. Upon preliminary diagnostic testing, we detected catalase-negative Gram-positive cocci exhibiting beta-hemolytic growth on sheep blood agar. A VITEK® 2 Compact machine from bioMérieux identified the bacterium as S. pseudoporcinus. Furthermore, antibiotic testing revealed multidrug resistance. Therefore, we document a multidrug-resistant S. pseudoporcinus isolate as a cause of canine post-surgical wound infection. Furthermore, it was the only isolate detected from the sample; hence, it is the cause of the infection. To our knowledge, this case is the first report of S. pseudoporcinus in a dog.
1. Introduction
Streptococcus pseudoporcinus was first described in 2006 after isolates were recovered from the genitourinary tract of women (1). Although phenotypically identical to Streptococcus porcinus, 16S ribosomal ribonucleic acid (16S rRNA) gene sequencing differentiated these two bacteria (1, 2). On blood agar plates, colonies were small, round, and circular with β-hemolysis. This characteristic is similar to biochemical profiles shown by Streptococcus agalactiae (group B Streptococcus, GBS) (3). Consequently, this cross-reaction with standard GBS test kits raises concerns about the misdiagnosis of this bacterium in suspected GBS cultures. The Centers for Disease Control and Prevention (CDC) has added this bacterium to their Streptococcus Laboratory collections, making it easier for diagnosticians to diagnose it from GBS (4). In addition, this bacterium has often been recovered from pregnant women, infants, and other immunocompromised patients (5–7). While re-evaluating 97 S. porcinus isolates from animal, human, and dairy sources in the CDC Streptococcus strain collection using 16S rRNA and rpoB gene sequencing, none identified as S. pseudoporcinus were from animals. To our knowledge, we report the first case of S. pseudoporcinus from an infected surgical wound in a dog.
2. Case description
A 9-year-old spayed female Great Dane dog was presented to the Tuskegee University Small Animal Veterinary Medical Teaching Hospital for evaluation of multiple cutaneous masses, one of which was on the left metatarsus. The histopathology result indicated a benign tumor in this location. Dexmedetomidine was used for premedication; cerenia, hydromorphone, and ketamine were also administered; and propofol was administered for induction/maintenance during the mass removal surgery. Some masses were surgically removed, including the one on the left metatarsus. The patient was discharged the same day with instructions for post-surgical care while at home, and the owner was prescribed the antibiotic enrofloxacin and anti-inflammatory carprofen for post-operative care. However, 10 days after the operation, the patient returned to the hospital with an inflamed surgical wound on the left metatarsus that oozed purulent material with a fetid odor.
A wound swab was submitted to the Microbiology Laboratory Service at Tuskegee University College of Veterinary Medicine (TUCVM) for aerobic and anaerobic culture and susceptibility testing. After 24 h of incubation, there was no growth on MacConkey agar, but there was growth on trypticase soy agar (TSA). Pinpoint, round, creamy colonies grew on the aerobic culture plate and candle jar culture plate, with the latter revealing more colony growth. Gram stain revealed short chains of Gram-positive cocci, while catalase testing showed no formation of air bubbles. The isolate was subcultured on Brain Heart Infusion blood agar (5% sheep blood) and incubated in a candle jar at 37°C overnight. After 20 h of incubation, a wide beta-hemolysis zone was observed around bacterial colonies. The isolate was re-cultured on TSA and incubated overnight at 37°C. The Gram-positive VITEK® 2 microplate from bioMérieux was inoculated with fresh TSA culture and set in the machine according to the manufacturer's protocol. After 5 h, the machine identified the isolate as S. pseudoporcinus with excellent identification.
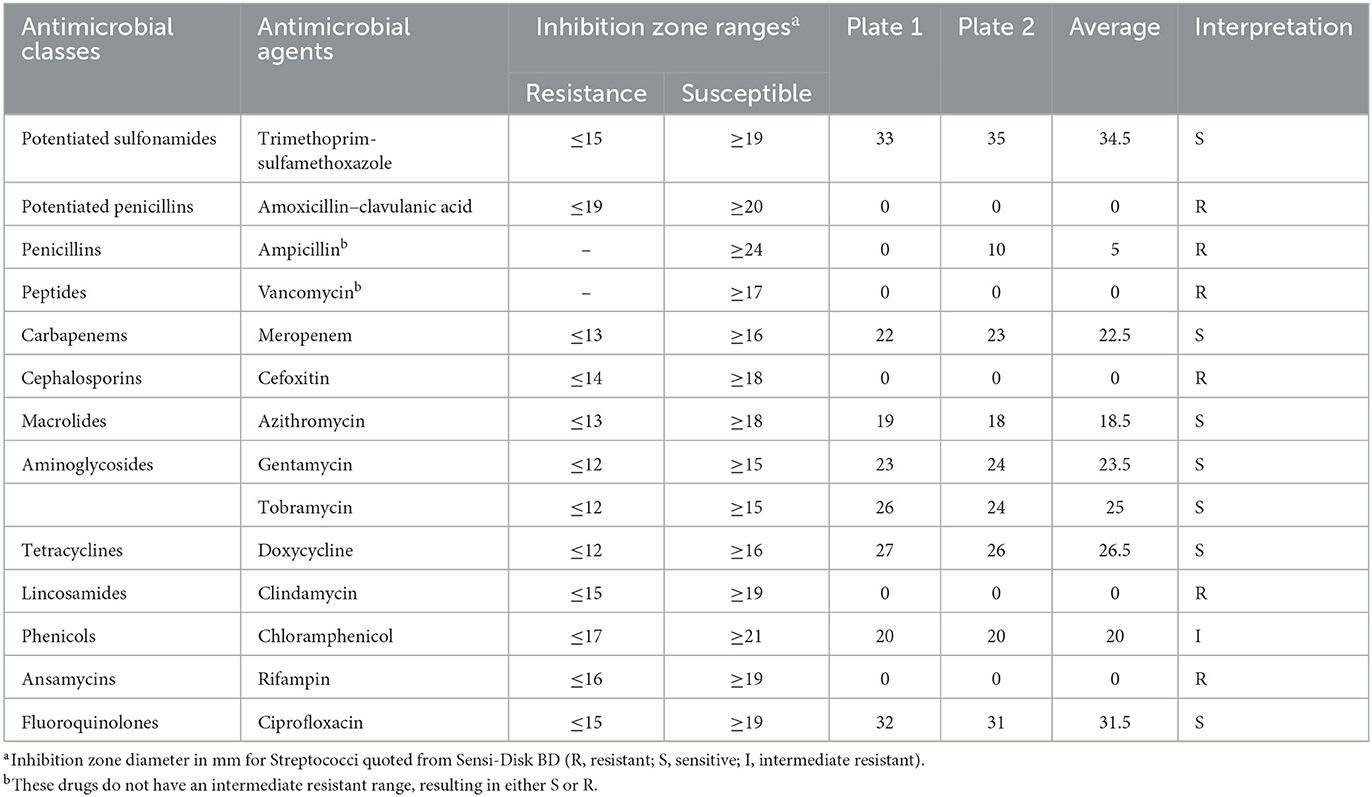
Antibiotic susceptibility testing was carried out using an agar disk diffusion test and a Beckman Coulter Gram-positive microplate (Brea, CA). According to Clinical and Laboratory Standards Institute (CLSI) (M100-S25) guidelines, two plates of Muller-Hinton agar were seeded from 0.5 McFarland bacterial suspension, and the antibiotic disks were placed on the plates. The plates were incubated in a candle jar at 37°C for 20 h. Similarly, following the manufacturer's instructions, the Beckman Coulter microplate was prepared using the Renok rehydration system to determine the drug breakpoint concentrations using CLSI guidelines for Gram-positive bacteria. The rehydrated plate was incubated in a candle jar at 37°C for 20 h. The results of the diameter for the zone of inhibition following agar disk diffusion are shown in Table 1. Similarly, the interpretive results of the isolates tested against various concentrations of antibiotics using the Beckman Coulter microplate are shown in Table 2.
3. Discussion
We report, to our knowledge, a novel case of S. pseudoporcinus in a dog that presented oozing purulent material with a fetid odor 10 days post-surgery. Microbiological testing on the wound swab revealed the causative agent as S. pseudoporcinus. This bacterium is a usual colonizer of healthy female genitourinary tracts and has been implicated in liver cirrhosis, leg cellulitis, endocarditis, thumb infection, and fetal demise (3, 5–9) and has also been associated with bacteremia in a patient co-infected with Syphilis–HIV (5, 10). In the present study, we present for the first time the isolation of S. pseudoporcinus from an infected wound in a dog. With S. pseudoporcinus described as a human strain of Streptococcus spp. and differentiated from S. porcinus by 16S rRNA (1), this case may be considered a reverse zoonosis even though the mechanism of infection in this dog is unclear.
Multidrug resistance (MDR) is non-susceptibility to at least one agent in three or more antimicrobial classes (11). Based on this definition, this study defined MDR based on disk diffusion assay and microplating results; S. pseudoporcinus exhibited resistance to six out of 14 antimicrobial agents and 12 out of 21 antimicrobial agents after agar disk diffusion and Beckman Coulter microplating, respectively. Streptococcus pseudoporcinus showed resistance to ansamycins, peptides, lincosamides, penicillins, and first- and second-generation cephalosporins. On the contrary, S. pseudoporcinus was susceptible to carbapenems, aminoglycosides, fluoroquinolones, phenicols, tetracyclines, third- and fifth-generation cephalosporins, potentiated sulfonamides, and fusidic acid. Our finding is similar to studies reporting the susceptibility of S. pseudoporcinus to fluoroquinolones, tetracyclines (8), and Trimethoprim–sulfamethoxazole (12). Macrolides (including erythromycin and azithromycin) were resistant based on the breakpoint results from microplating but differed in the agar disk diffusion results. Determining the antimicrobial resistance genes present was not achieved in this study as this study focused on the diagnostic and antibiotic susceptibility profiles of the bacterial isolate confirmed to suggest the best treatment course for the dog. Therefore, since this phenotypic-based MDR was observed in S. pseudoporcinus recovered from a dog for the first time in the literature, this study reports this as a novel case of an MDR S. pseudoporcinus in a dog.
In conclusion, we described the first report of S. pseudoporcinus in a dog. Because S. pseudoporcinus is an emerging multidrug-resistant pathogen in humans, its isolation in dogs is a worrying concern, as this could lead to a potential public health menace.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YS wrote the second and preceding drafts of the manuscript and contributed to the conception and design of the study. AE organized and included the database from the clinic. AM diagnosed the case, contributed to the conception and design of the study, and wrote the first draft of the manuscript. All authors contributed to the manuscript revision and read and approved the submitted version.
Funding
This study was supported by grants from USDA/NIFA-AHDRXXXG022.
Acknowledgments
We thank the College of Veterinary Medicine, Tuskegee University's clinical and laboratory team for handling this case and Dr. Anthony Pokoo-Aikins of USDA-ARS for reviewing this study. We thank Dean Ruby Perry of the College of Veterinary Medicine, Tuskegee University, Tuskegee, AE for her continuous support. We also thank the clinical and laboratory team of Tuskegee University's Small Animal Veterinary Medical Teaching Hospital who assisted with this study. Additionally, we thank Dr. Anthony Pokoo-Aikins of USDA-ARS, Athens, GA for his inputs during the initial reviewing of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bekal S, Gaudreau C, Laurence RA, Simoneau E, Raynal L. Streptococcus pseudoporcinus Sp. Nov, a novel species isolated from the genitourinary tract of women. J Clin Microbiol. (2006) 44:2584–86. doi: 10.1128/JCM.02707-05
2. Grundy M, Suwantarat N, Rubin M, Harris R, Hanlon A, Tekle T, et al. Differentiating Streptococcus pseudoporcinus from GBS: could this have implications in pregnancy? Am J Obstet Gynecol. (2019) 220:490.e1–7. doi: 10.1016/j.ajog.2019.01.219
3. Liatsos GD, Tsiriga A, Dourakis SP. Fatal Streptococcus pseudoporcinus disseminated infection in decompensated liver cirrhosis: a case report. J Med Case Rep. (2021) 15:4–9. doi: 10.1186/s13256-021-02832-3
4. Shewmaker PL, Steigerwalt AG, Whitney AM, Morey RE, Graziano JC, Facklam RR, et al. Evaluation of methods for identification and determination of the taxonomic status of strains belonging to the Streptococcus porcinus-Streptococcus pseudoporcinus complex isolated from animal, human, and dairy sources. J Clin Microbiol. (2012) 50:3591–97. doi: 10.1128/JCM.01481-12
5. Gupta K, Mohanty M, Rath S. Bacteremia because of Streptococcus pseudoporcinus in a syphilis-HIV co-infected patient: a case report. J Fam Med Prim Care. (2020) 9:2119–20. doi: 10.4103/jfmpc.jfmpc_663_19
6. Khan S, Wong TT, Prasad N, Lee B, Urban C, Segal-Maurer S, et al. Streptococcus pseudoporcinus : case reports and review of the literature. Case Rep Infect Dis. (2020) (2020):1–6. doi: 10.1155/2020/4135246
7. Pierce SL, Shibib DR, Robison D, Edwards RKA. case of maternal sepsis and fetal demise associated with Streptococcus pseudoporcinus. Case Rep Obstet Gynecol. (2019) 2019:1–3. doi: 10.1155/2019/4309191
8. Mahlen SD, Clarridge JE. Thumb infection caused by Streptococcus pseudoporcinus. J Clin Microbiol. (2009) 47:3041–42. doi: 10.1128/JCM.00802-09
9. Sawamura S, Niimori D, Ihn HA. case of leg cellulitis caused by multidrug-resistant Streptococcus pseudoporcinus. Intractable Rare Dis Res. (2018) 7:280–82. doi: 10.5582/irdr.2018.01110
10. Stoner KA, Rabe LK, Austin MN, Meyn LA, Hillier SL. Incidence and epidemiology of streptococcus pseudoporcinus in the genital tract. J Clin Microbiol. (2011) 49:883–86. doi: 10.1128/JCM.01965-10
11. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
12. Gaudreau C, Simoneau E, Labrecque O, Laurence RA, Laferrière C, Miller M, et al. Epidemiological, biochemical and antimicrobial susceptibility characteristics of Streptococcus pseudoporcinus isolated in Quebec, Canada, from 1997 to 2006. J Med Microbiol. (2007) 56:1620–24. doi: 10.1099/jmm.0.47295-0
Keywords: drug resistance, bacteria, infection, microbiology, case report, purulent discharge
Citation: Soku YK, Etzioni AL and Mohamed A (2023) Case report: Multidrug-resistant Streptococcus pseudoporcinus isolated from an infected surgical wound of a 9-year-old spayed female Great Dane dog. Front. Vet. Sci. 10:1139381. doi: 10.3389/fvets.2023.1139381
Received: 06 January 2023; Accepted: 07 February 2023;
Published: 03 March 2023.
Edited by:
Muhammad Saqib, University of Agriculture, Faisalabad, PakistanReviewed by:
Mashkoor Mohsin, University of Agriculture, Faisalabad, PakistanAbu Baker Siddique, Government College University, Faisalabad, Pakistan
Copyright © 2023 Soku, Etzioni and Mohamed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdelrahman Mohamed, amohamed@tuskegee.edu
 Yesutor K. Soku
Yesutor K. Soku Athema L. Etzioni
Athema L. Etzioni Abdelrahman Mohamed
Abdelrahman Mohamed