
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 30 March 2023
Sec. Comparative and Clinical Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1137774
Background: Reporting of clinical trials conducted in client- and shelter-owned dog and cat populations is not optimal, which inhibits the ability to assess the reliability and validity of trial findings and precludes the ability to include some trials in evidence synthesis.
Objective: To develop a reporting guideline for parallel group and crossover trials that addresses the unique features and reporting requirements for trials conducted in client- and shelter-owned dog and cat populations.
Design: Consensus statement.
Setting: Virtual.
Participants: Fifty-six experts from North America, the United Kingdom, Europe, and Australia working in academia, government (research and regulatory agencies), industry, and clinical veterinary practice.
Methods: A steering committee created a draft checklist for reporting criteria based upon the CONSORT statement and the CONSORT extensions for reporting of abstracts and crossover trials. Each item was presented to the expert participants and was modified and presented again until >85% of participants were in agreement about the inclusion and wording of each item in the checklist.
Results: The final PetSORT checklist consists of 25 main items with several sub-items. Most items were modifications of items contained in the CONSORT 2010 checklist or the CONSORT extension for crossover trials, but 1 sub-item pertaining to euthanasia was created de novo.
Conclusion: The methods and processes used to develop this guideline represent a novel departure from those used to create other reporting guidelines, by using a virtual format. The use of the PetSORT statement should improve reporting of trials conducted in client- and shelter-owned dogs and cats and published in the veterinary research literature.
Veterinarians, regulators, and other veterinary health professionals increasingly are expected to make evidence-based decisions, where the evidence comes from research (1). When evaluating the efficacy of interventions where it is ethical and feasible to allocate study subjects to intervention groups, randomized controlled trials (RCTs) provide the highest level of evidence of the primary research designs (2, 3). However, evaluating the potential for bias and interpreting the results of an RCT requires comprehensive reporting of the trial design and conduct. Earlier studies in human healthcare illustrated inadequacies in reporting of RCTs (4–9). This led to the creation of the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting of two-group parallel design RCTs, first published in 1996 (10), revised in 2001 (11) and again in 2010 (12). The CONSORT statement was developed by expert consensus and consists of a checklist of 25 items that should be reported in all RCT reports. An accompanying elaboration document provided details on the rationale for including each item, as well as examples from the literature illustrating comprehensive reporting for the items (13). Meta-research studies have shown that reporting of RCTs in human healthcare has improved following the publication of the CONSORT statement (14, 15).
In the years since the CONSORT statement was first published, a number of extensions have been published to address variations in trial design or different types of interventions. These include CONSORT extensions for crossover trials (16), multi-arm parallel group randomized trials (17), non-inferiority and equivalence trials (18), and reporting of RCT abstracts in journals or conference proceedings (19).
Formal reporting guidelines developed by expert consensus have been published for trials conducted in animal publications. The REFLECT statement (20) provides guidelines for reporting clinical trials conducted in livestock populations. In addition to providing guidance for reporting some features of livestock trials that differ from human trials, the elaboration document (21) also provides relevant examples from the livestock trial literature. There is evidence that reporting of swine intervention trials (22) and bovine respiratory disease trials (23) has improved since the publication of the REFLECT statement.
The ARRIVE statement for reporting of in vivo experiments in animals originally was published in 2010 (24), with a revised ARRIVE 2.0 published in 2020 (25, 26). An updated document with explanations and examples of the items in ARRIVE 2.0 also was published in 2020 (27). The focus of ARRIVE 2.0 is on comparative studies; thus, studies using dogs and cats would be within the scope of these guidelines. However, trials including dogs were only used in two of the examples in the explanation document, and in both instances the experiments used dogs as animal models of human illness (27). None of the examples described experiments conducted in cats.
Early evaluations of the quality of reporting of clinical trials in dogs and cats have found substantive deficiencies in the reporting of small animal trials (28, 29). In the 2010 evaluation, in addition to documenting inadequate reporting, the authors reported an association between inadequate reporting and trial results; an increased proportion of positive treatment effects within a trial was associated with not reporting key features such as the method used to generate the random allocation sequence, the use of double blinding, and the eligibility criteria for animals. In an updated evaluation of trials published after 2015 in populations of dogs or cats, ~1/5 of published trials used a crossover design (257 / 1190) (30). When evaluating the quality of reporting in 200 trials published during 2019, the authors noted that some trial features, such as method of allocation to intervention group, were well reported. However, despite the availability of relevant reporting guidelines such as CONSORT and ARRIVE, there still were substantive deficiencies in the reporting of trials in dogs and cats (30). The reason for continued inadequacies in reporting is unknown. However, it is possible that individuals conducting trials in dogs and cats are not aware of the existence of relevant guidelines such as CONSORT, the CONSORT extension for cross-over trials, ARRIVE, or REFLECT, or that the explanations and examples in those guidelines are not sufficiently relevant to the trial conditions that they experience.
Thus, there is a need for reporting guidelines for owned dogs and cats, both to modify reporting items to address nuances between trials in livestock, humans, or for biomedical purposes and to provide relevant examples of good reporting for researchers conducting trials in owned dogs and cats. Given the prevalence of cross over designs, there may be value in combining the reporting of parallel and crossover trials into a single guideline to facilitate access for researchers.
Therefore, the objective of this work is to describe the methods used to develop reporting guidelines for parallel group and crossover trials conducted in client- and shelter-owned dog and cat populations (PetSORT). A separate companion paper, the PetSORT explanation and elaboration document (31), provides the methodologic background for the items contained in the PetSORT statement as well as illustrative examples of appropriate reporting. We strongly recommend that the PetSORT checklist be used in conjunction with the explanation and elaboration document for reporting of all trials conducted in dog and cat populations.
The process used for developing reporting guideline statements have been documented and published previously (32–34). Typically, this process has included an in-person consensus meeting occurring over a period of several consecutive days during which members of the working group discuss and reach agreement on items to be included in the guidelines. For this project, however, travel restrictions and lockdowns associated with the COVID-19 pandemic precluded using this approach.
A steering committee of four members (co-authors AR, JS, LS, and AO'C) was formed with the express purpose of developing a reporting guideline for trials that involve client- and shelter-owned dog and cat populations. Two of the steering committee members had previously led initiatives to develop reporting guidelines in veterinary medicine (JS, AO'C). The steering group first met in June 2020, and ultimately were responsible for development of the initial checklist, which was based on the items included in the 2010 CONSORT statement (12), as well as items from the CONSORT extension for crossover trials (16). The steering committee intentionally combined items pertinent to both parallel and crossover trials into one consolidated checklist in order to improve ease of use for investigators. This committee then identified and invited potential participants, coordinated the collection of participants' opinions on each item included in the guidelines, revised and recirculated modifications to participants, and were responsible for all subsequent steps involved in preparation, revisions, and publication of the manuscripts associated with this work.
The aim of the steering committee was to include experts with experience in a wide variety of areas in the consensus group, but all having familiarity with design, analysis, and publication of trials. For this guideline, trials were defined as a controlled experiment where there were at least two groups, the investigator controlled allocation to intervention groups, and disease or outcome occurrence was natural rather than induced and conducted in client- and shelter-owned dogs and cats. Diversity was sought in terms of specific areas of content expertise (e.g., veterinary specialists were included from the specialty areas of oncology, nutrition, internal medicine, ophthalmology, emergency medicine, pharmacology, surgery, and public health) as well as areas of employment (e.g., academia, regulatory agencies, public and private research companies, and clinical practices). Representation from multiple countries was intentionally prioritized and an effort was made to include participants with relevant editorial experience.
Previously published work that included the use of a consensus group reported imposing size limitations on the total number of experts to include in the panel based upon funding and the need to allow for active participation in conversation (34). Due to the virtual nature of this project, the steering committee decided not to cap the total number of participants included in the expert panel. Instead, an initial group of 32 individuals with qualifying expertise were identified by the steering committee and invited to participate in this work via email. The initial group was selected based upon several criteria including their area of expertise, history of publication of controlled trials, previous participation in reporting guidelines consensus groups, geographic location, and the perceived impact reporting guidelines would have on their work. Only two of the experts initially invited had previously published with any of the steering committee members. All invitees, whether they agreed to participate or not, were asked to nominate other experts to participate in this work in order to ensure participation from a robust consensus group.
The email invitation sent to experts requested that individuals who wished to participate complete a modified Delphi survey indicating their thoughts on the initial PetSORT checklist items anonymously. For each item participants were first asked whether they agreed the item should be included. If the participant thought the item should be included, they were further queried if the item was worded appropriately or if they had suggestions about how to modify the item. Experts were also invited to offer additional comments on each item and could include any feedback they thought necessary to communicate to the steering committee. Surveys results were collected using Qualtrics, a web-based survey platform.
The steering committee decided that consensus would be reached when 87.5% of experts agreed upon the exclusion or inclusion of an item as it was currently worded i.e., at least 49 of the 56 experts agreed. Items that the experts agreed should be included, but did not reach consensus in terms of the wording of the item, were modified by the steering committee to address concerns and comments from the expert panel and recirculated for another round of voting that included the contextual reasons and comments provided by experts in the previous survey responses. This was repeated until consensus was reached about the wording for each item. The identities of the experts involved in this process were not revealed until after consensus was reached.
The steering committee compiled the proposed modifications to the initial checklist developed by the steering committee and collated the comments and suggested revisions and used these to develop the final reporting guidelines for use in reporting trials conducted in dog and cat populations. A draft of the explanation and elaboration document was then prepared by the steering committee and circulated among all participants for input. Feedback from all participants was incorporated into the final version of the manuscript by the members of the steering committee.
Seventy-five experts were invited to participate in the consensus group and 52 accepted the invitation and completed all tasks (Figure 1). All four of the steering committee members participated for a total of 56 members in the consensus group. The methodological expertise of the participants included trial design, epidemiology, statistics, regulatory medicine, clinical practice, systematic review and meta-analysis. The majority of experts (n = 43, 76.8%) were employed in the United States, 10 (17.9%) were employed in the United Kingdom, and 3 (5.4%) were employed in Canada, Germany, and Australia. Academicians accounted for the majority of the consensus group (n = 46, 82.1%), 6 (10.7%) members of the group worked for a government agency, and 4 (7.1%) worked in industry or private practice.
The steering committee proposed an initial set of guidelines that included 25 main items which resulted in 38 individual items when sub-items are counted. There was consensus among the experts to include all 38 items in the final checklist for PetSORT but only 18 of the initially proposed items were accepted by the consensus group as they were worded by the steering committee. These 18 items were not further modified by the expert group. Consensus about the wording proposed by the steering committee was not reached for 20 of the items initially presented to the consensus group and revisions and modifications were made in an iterative process to reflect the consensus of the group.
Compared to the original CONSORT 2010 guidelines for parallel trials, only 2 of the items (2b and 7b) included in PetSORT required no modifications by the steering committee (Table 1). Eighteen of the items included in PetSORT (1a, 2a, 6a, 6b, 7a, 8a, 8b, 10, 11b, 12a, 14a, 14b, 15, 17a, 17b, 18, 20, and 21) had only minor modifications made from the CONSORT 2010 statement for parallel trials in order to clarify or add more details specific to the veterinary community. Seventeen PetSORT items include more substantial changes from the CONSORT 2010 statement for parallel trials (Table 2). One item, 6c, was developed specifically to address the need for inclusion of discussion in reporting of trials involving dogs and cats regarding consultation about and performance of euthanasia and the possible impact of losses due to euthanasia on the outcome of trials conducted in pets.
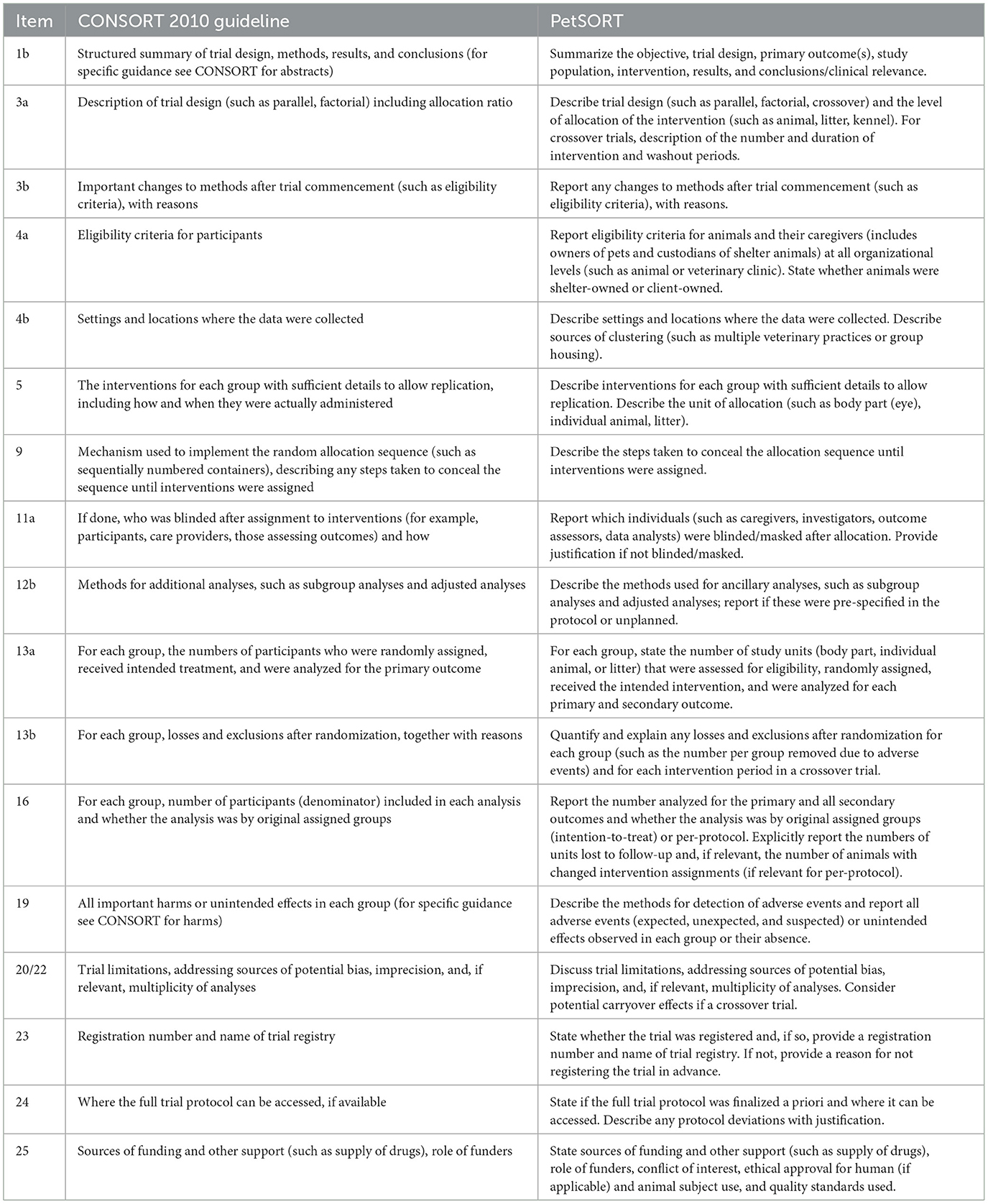
Table 2. PetSORT items paired with the CONSORT 2010 statement for parallel trials item from which the content was substantial changed.
This work describes the development of reporting guidelines for use when reporting on trials conducted in client- and shelter-owned cat and dog populations. This work was based upon both the CONSORT 2010 statement for reporting parallel group randomized trials (12) and the extension to randomized crossover trials (16). The guidelines represent the consensus of a large group of individuals considered to be experts in trials conducted in dog and cat populations. The results of this work, therefore, represent consensus of expert opinion.
In concordance with the CONSORT statement, the intention of these guidelines is to provide guidance for authors when describing the design and results of trials. However, these guidelines are also useful for editors and peer reviewers assessing the comprehensiveness of reporting when considering suitability of trials for publication, researchers conducting systematic reviews, and readers attempting to assess internal and external validity of the trials being reported.
Like the CONSORT statement, the PetSORT guidelines are not intended to be prescriptive regarding the order of reporting. The items were generally ordered to correspond to the CONSORT statement, which follows the typical order of sections within a scientific manuscript. Thus, while it is important that all of the relevant items on the checklist are addressed in sufficient detail within a manuscript, that content does not necessarily need to correspond to the section in which the item number is located on the checklist. It is also important to note that the PetSORT statement is not intended to be used as a tool to assess the quality of the research design or execution of the trial.
It is of note that the majority of the participants in the consensus group were employed in the United States and this may be considered a selection bias. Similarly, most of the consensus group was employed in an academic setting. The effect of the bias, if present, cannot be determined due to the survey responses having been collected anonymously. It might also be considered a limitation that people contacted to participate in this work were asked to nominate other experts to the group. This may have resulted in invitations being extended based upon personal or professional connections which could have resulted in inclusion of experts who think similarly about these concepts. However, conducting this work virtually rather than face-to-face allowed for a larger and potentially more diverse group of individuals to participate in the consensus process and we feel the effect of personal connections with the original group of invitees was mitigated due to the large size of the expert panel.
It was agreed a priori that the exact number of experts in agreement on each item would not be published which is in keeping with standards of guideline development (35–37). The steering committee felt that publication of the specific figures would detract from the purpose of publication of a consensus statement, which is to define the general agreement of the group. However, anonymized aggregated data including individual responses and approval rates can be requested from the corresponding author.
When used with the PetSORT explanation and elaboration document, we expect these guidelines will lead to improved reporting of trials conducted in client- and shelter-owned dog and cat populations.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
AR, JS, LS, and AO'C contributed to the conception and design of this work. AR was primarily responsible for communication between the steering committee and the members of the consensus group. AR and JS drafted the manuscript. LS and AO'C revised the document critically. All authors contributed to the article and approved the submitted version.
The authors would like to acknowledge the efforts made by the consensus group members for their contributions to this project. These members are (in alphabetical order) Karin Allenspach, David J. Argyle, Cynthia Bashore, Erika Berger, Philip J. Bergman, Adrian Boswood, Benjamin M. Brainard, Marnie L. Brennan, Dave Brodbelt, Jeffrey N. Bryan, Steven Budsberg, Jenna H. Burton, Daniel L. Chan, Michael G. Conzemius, William S. Dernell, Nicola Di Girolamo, Richard Evans, Aiden P. Foster, Lisa M. Freeman, Alexander J. German, Michelle A. Giuffrida, Wanda J. Gordon-Evans, Nicolas Granger, Laura L. Hungerford, Nick Jeffery, Unity Jeffery, Chad M. Johannes, Aarti Kathrani, Susan Lana, Amy K. LeBlanc, Sandra L. Lefebvre, Dana N. LeVine, Christopher Loss, Caroline Mansfield, Philipp Mayhew, Sarah A. Moore, Ralf Mueller, Allison L. O'Kell, Dan O'Neill, Natasha Olby, Thierry Olivry, Rodney L. Page, Jessica M. Quimby, Robert B. Rebhun, Carolina H. Ricco Pereira, Courtney Shaw, Douglas H. Thamm, David M. Vail, Kristen M. Weishaar, Constance N. White, Alexandra L. Winter, and Luke A. Wittenberg. In addition, we would like to acknowledge Sarah Totton for her assistance in preparing the checklist.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Holmes M, Cockcroft P. Evidence-based veterinary medicine 1. Why is it important and what skills are needed? In Pract. (2004) 26:28–33. doi: 10.1136/inpract.26.1.28
2. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. (2011) 128:305–10. doi: 10.1097/PRS.0b013e318219c171
3. Sargeant JM, Kelton DF, O'Connor AM. Study designs and systematic reviews of interventions: building evidence across study designs. Zoonoses Public Health. (2014) 61:10–7. doi: 10.1111/zph.12127
4. Ah-See KW, Molony NC, A. qualitative assessment of randomized controlled trials in otolaryngology. J Laryngol Otol. (1998) 112:460–3. doi: 10.1017/S0022215100140770
5. DerSimonian R, Charette LJ, McPeek B, Mosteller F. Reporting on methods in clinical trials. N Engl J Med. (1982) 306:1332–7. doi: 10.1056/NEJM198206033062204
6. Gøtzsche PC. Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Control Clin Trials. (1989) 10:31–56. doi: 10.1016/0197-2456(89)90017-2
7. Pocock SJ, Hughes MD, Lee RJ. Statistical problems in the reporting of clinical trials. N Engl J Med. (1987) 317:426–32. doi: 10.1056/NEJM198708133170706
8. Sonis J, Joines J. The quality of clinical trials published in the journal of family practice, 1974-1991. J Fam Pract. (1994) 39:225–35.
9. Schulz KF, Chalmers I, Grimes DA, Altman DG. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA. (1994) 272:125–8. doi: 10.1001/jama.272.2.125
10. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT Statement. JAMA. (1996) 276:637–9. doi: 10.1001/jama.276.8.637
11. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. (2001) 357:1191–4. doi: 10.1016/S0140-6736(00)04337-3
12. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. (2010) 11:32. doi: 10.1186/1745-6215-11-32
13. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. (2010) 63:e1–37. doi: 10.1016/j.jclinepi.2010.03.004
14. Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev. (2012) 11:MR000030. doi: 10.1002/14651858.MR000030.pub2
15. Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane review. Syst Rev. (2012) 1:60. doi: 10.1186/2046-4053-1-60
16. Dwan K, Li T, Altman DG, Elbourne D. 2010 statement: extension to randomised crossover trials. BMJ. (2019) 366:l4378. doi: 10.1136/bmj.l4378
17. Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. (2019) 321:1610–20. doi: 10.1001/jama.2019.3087
18. Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. (2012) 308:2594–604. doi: 10.1001/jama.2012.87802
19. Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. (2008) 5:e20. doi: 10.1371/journal.pmed.0050020
20. O'Connor AM, Sargeant JM, Gardner IA, Dickson JS, Torrence ME, Dewey CE, et al. The REFLECT statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety. J Vet Intern Med. (2010) 24:57–64. doi: 10.1111/j.1939-1676.2009.0441.x
21. Sargeant JM, O'Connor AM, Gardner IA, Dickson JS, Torrence ME. Consensus meeting participants. the reflect statement: reporting guidelines for randomized controlled trials in livestock and food safety: explanation and elaboration zoonoses. Public Health. (2010) 57:105–36. doi: 10.1111/j.1863-2378.2009.01312.x
22. Moura CAA, Totton S, Sargeant JM. Evidence of improved reporting of swine vaccination trials in the post-REFLECT statement publication period. J Swine. (2019) 27:265–77.
23. Totton SC, Cullen JN, Sargeant JM, O'Connor AM. The reporting characteristics of bovine respiratory disease clinical intervention trials published prior to and following publication of the REFLECT statement. Prev Vet Med. (2018) 150:117–25. doi: 10.1016/j.prevetmed.2017.12.015
24. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. (2010) 8:e1000412. doi: 10.1371/journal.pbio.1000412
25. du Sert NP, Hurst V, Ahluwalia A, Alam S, Altman DG, Avey MT, et al. Revision of the ARRIVE guidelines: rationale and scope. BMJ Open Sci. (2018) 2:e000002. doi: 10.1136/bmjos-2018-000002
26. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci. (2020) 4:e100115.
27. Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. (2020) 18:e3000411. doi: 10.1371/journal.pbio.3000411
28. Lund EM, James KM, Neaton JD. Veterinary randomized clinical trial reporting: a review of the small animal literature. J Vet Intern Med. (1998) 12:57–60. doi: 10.1111/j.1939-1676.1998.tb02095.x
29. Sargeant JM, Thompson A, Valcour J, Elgie R, Saint-Onge J, Marcynuk P, et al. Quality of reporting of clinical trials of dogs and cats and associations with treatment effects. J Vet Intern Med. (2010) 24:44–50. doi: 10.1111/j.1939-1676.2009.0386.x
30. Sargeant JM, Plishka M, Ruple A, Selmic LE, Totton SC, Vriezen ER. Quality of reporting of clinical trials in dogs and cats: an update. J Vet Intern Med. (2021) 35:1957–71. doi: 10.1111/jvim.16204
31. Sargeant JM, Ruple A, Selmic LE, O'Connor AE. The standards of reporting trials in pets (PetSORT): explanation and elaboration. Front Vet Sci. (2023) 10:1137781. doi: 10.3389/fvets.2023.1137781
32. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med. (2008) 148:W60–6. doi: 10.7326/0003-4819-148-4-200802190-00008-w1
33. Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Med. (2010) 7:e1000217. doi: 10.1371/journal.pmed.1000217
34. Sargeant JM, O'Connor AM, Dohoo IR, Erb HN, Cevallos M, Egger M, et al. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology - veterinary (STROBE-Vet) Statement. J Vet Intern Med. (2016) 30:1887–95. doi: 10.1111/jvim.14574
35. Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P, et al. Guidelines international network: toward international standards for clinical practice guidelines. Ann Intern Med. (2012) 156:525–31. doi: 10.7326/0003-4819-156-7-201204030-00009
36. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. (2010) 182:E839–42. doi: 10.1503/cmaj.090449
Keywords: animal reporting guideline, animal health, randomized trials, small animal clinical trials, companion animals
Citation: Ruple A, Sargeant JM, Selmic LE and O'Connor AM (2023) The standards of reporting randomized trials in pets (PetSORT): Methods and development processes. Front. Vet. Sci. 10:1137774. doi: 10.3389/fvets.2023.1137774
Received: 04 January 2023; Accepted: 13 February 2023;
Published: 30 March 2023.
Edited by:
Claire Rebecca Sharp, Murdoch University, AustraliaCopyright © 2023 Ruple, Sargeant, Selmic and O'Connor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audrey Ruple, YXJ1cGxlQHZ0LmVkdQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.