- 1Department of Veterinary Medical Imaging, College of Veterinary Medicine, Jeonbuk National University, Iksan, Republic of Korea
- 2Department of Veterinary Medical Imaging, VIP Animal Medical Centre, Seoul, Republic of Korea
- 3Department of Veterinary Medical Imaging, Bundang Leaders Animal Medical Centre, Seongnam, Republic of Korea
- 4Department of Veterinary Medical Imaging, Nel Animal Medical Center, Anyang-si, Gyeonggi-do, Republic of Korea
Introduction: Common bile duct (CBD) measurements are important for the evaluation of biliary systemic disorders. However, in veterinary medicine, reference ranges for specific body weights (BW) and correlation between CBD diameter and BW have not been studied. This study aimed to establish normal reference ranges of CBD diameter for different BW groups and to analyse correlation between CBD diameter and BW in dogs without hepatobiliary disease. Additionally, normal reference ranges of CBD to aorta ratio (CBD: Ao ratio) were established which is not affected by BW.
Methods: CBD diameter was measured at three different sites: porta hepatis (PH), duodenal papilla (DP) level and mid-portion (Mid) between these points using computed tomography (CT) in 283 dogs without hepatobiliary disease.
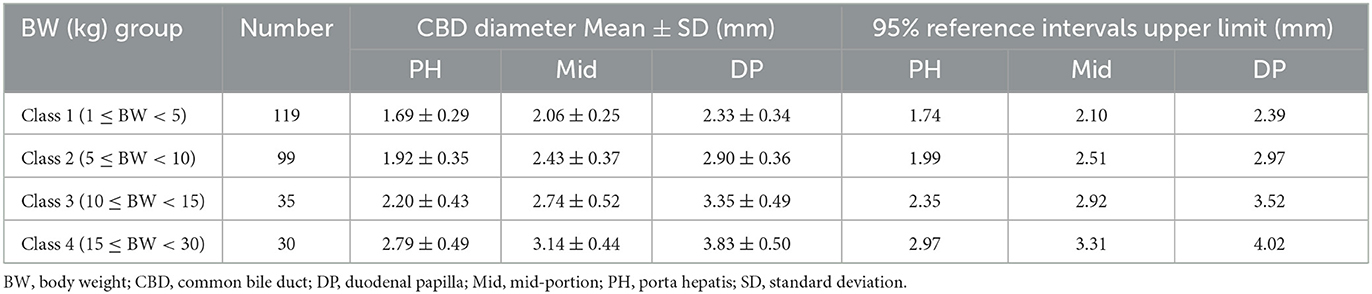
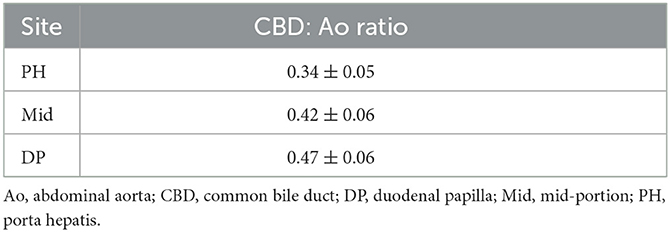
Results: The reference range of CBD diameter at PH level: 1.69 ± 0.29 mm (Class 1; 1 kg ≤ BW < 5 kg), 1.92 ± 0.35 mm (Class 2; 5 kg ≤ BW < 10 kg), 2.20 ± 0.43 mm (Class 3; 10 kg ≤ BW < 15 kg), 2.79 ± 0.49 mm (Class 4; 15 kg ≤ BW < 30 kg); Mid-level: 2.06 ± 0.25 mm (Class 1), 2.43 ± 0.37 mm (Class 2), 2.74 ± 0.52 mm (Class 3), 3.14 ± 0.44 mm (Class 4); DP level: 2.33 ± 0.34 mm (Class 1), 2.90 ± 0.36 mm (Class 2), 3.35 ± 0.49 mm (Class 3), and 3.83 ± 0.50 mm (Class 4). There was a significant difference in CBD diameter at each level among all BW groups. Furthermore, BW and CBD diameter showed positive linear correlation at each level. We devised CBD: Ao ratio at each level that showed no significant difference between the different BW groups; PH level: 0.34 ± 0.05; Mid-level: 0.42 ± 0.06; DP level: 0.47 ± 0.06.
Conclusion: In conclusion, since the CBD diameter for each BW is significantly different, different normal reference ranges of CBD diameter should be applied for each BW, and the CBD: Ao ratio can be used regardless of the BW.
1. Introduction
Extrahepatic bile duct measurement is necessary to evaluate patients affected by cholestasis since this condition is associated with clinical complications such as injury of the liver and gallbladder (GB) and disruption of hepatic circulation (1–6). Common bile duct (CBD) dilation can be caused by obstructive or non-obstructive causes, but most are associated with obstructive causes (7). Extrahepatic biliary tract obstruction (EHBO) has been attributed to a variety of causes including pancreatitis, GB mucocele, pancreatobiliary malignancy, extrinsic luminal compression, obstruction due to intraluminal components (bile duct stones, foreign body, etc.), obstruction and stenosis of the duodenal papilla (DP), and fibrosing cholangitis (6, 8–10). In particular, chronic pancreatitis and cholangiohepatitis with choledocholithiasis are the most common causes of partial or complete EHBO (1, 8, 9).
In veterinary medicine, there are several studies that evaluate bile duct diameter in dogs (10, 11). One previous study evaluated CBD diameter using ultrasound (US) and the upper limit of the normal CBD diameter was defined as 3 mm (10). US has the advantage of being faster and cheaper and can be performed without anesthesia when evaluating the biliary tract. Therefore, US is often used as the first diagnostic tool for evaluation of the biliary system and frequent follow-up monitoring is also possible (8). However, US examination in small animals is more difficult than in humans because the bile duct is often within the rib cage and obscured by gas in the stomach or intestine (8). Therefore, visualization of the CBD may not always be possible and may be inconsistent. In addition, US may not be able to evaluate the entire CBD and the specificity and sensitivity can be greatly affected by the skill and experience of the sonographer (12). In contrast, evaluation of the extrahepatic bile duct using computed tomography (CT) can better visualize the anatomy and location of the extrahepatic bile duct than ultrasound (6, 13). Moreover, CT can be very useful in evaluating periductal mass lesions and the entire CBD length and diameter, in which evaluation of the entire CBD is important to diagnose partial obstruction (8, 13). Although there was a study on the CBD diameter of normal dogs using CT in veterinary medicine (11), to the best of the author's knowledge, there has been no study suggesting reference ranges for specific body weight (BW) groups and a correlation between BW, sex, age and CBD diameter in dogs. Also, weight-independent parameters have not been not identified.
Therefore, the aims of this study were as follows: (1) Establish a reference range for CBD diameter in dogs without hepatobiliary disease and identify the differences of CBD diameter between different BW groups; (2) identify the correlation between BW and CBD diameter; (3) evaluate CBD diameter to abdominal aorta (Ao) diameter ratio (CBD: Ao ratio) as a weight-independent parameter; and (4) analyse the significance of differences in CBD diameter in relation to age and sex.
2. Materials and methods
2.1. Animals
In this multicenter, retrospective, observational study, medical charts including complete blood counts, serum biochemistry, and CT images of 635 dogs were collected from four veterinary clinics (Jeonbuk National University Animal Medical Center, Bundang Leaders Animal Medical Center, VIP Animal Medical Center, and Nel Animal Medical Center) between 2 August 2021 and 20 December 2022. The inclusion criteria were normal serum chemistry results [white blood cell count, alkaline phosphatase (ALP) level, alanine aminotransferase (ALT) level, gamma glutamyl transferase (GGT) level, total bilirubin concentration, albumin concentration, and cholesterol level], no abnormalities on CT images (liver, biliary system, and pancreas), and no clinical signs related to liver and pancreatic disease (anorexia, vomiting, diarrhea, and abdominal pain). Dogs weighing over 30 kg were excluded from this study. In total, 283 dogs were included in the analysis and were classified into four groups based on their BW (Class 1: 1 kg ≤ BW < 5 kg, Class 2: 5 kg ≤ BW < 10 kg, Class 3: 10 kg ≤ BW < 15 kg, Class 4: 15 kg ≤ BW < 30 kg).
This study was approved by the Institutional Animal Care and Use committee of Jeonbuk National University (Approval No. JBNU 2022-037).
2.2. Measurement methods
CT images were acquired using three scanners (Revolution ACT or Brivo CT 385; GE Hangwei Medical System Co., Ltd., Beijing, China/Alexion 16; Toshiba Medical Systems Co Ltd., Otowara, Japan) with the following scan parameters: 120 kVp, 80–200 mAs, 0.625–1.25-mm slice thickness, 0.75–1.0-s rotation time, and a 0.938–1.375 collimation beam pitch. The scan field included at least the cranial margin of the diaphragm to the caudal endplate of the third lumbar vertebra, and scans were performed under breath-holding to minimize artifacts caused by movement due to breathing. All images were reviewed using an abdominal window (window level = 40 Hounsfield units [HU], window width = 400 HU) and a Radiant Dicom viewer (Medixant; Poznan, Poland). Portal venous and delayed phase images were used in all measurements for the best visualization of the liver, CBD, and other surrounding organs. Iohexol (Omnipaque, 750 mgI/kg; GE Healthcare, Ireland) was used for contrast medium and administered via cephalic vein.
CBD diameter was measured at three different sites: at the porta hepatis (PH) level, at the duodenal papilla (DP) level, and mid-portion (Mid) between these points (Figure 1A). At the PH level, the hepatic artery, portal vein, and CBD were located in parallel, and the CBD diameter at the DP level was measured at the location where the CBD inserted into the duodenum. All measurements were performed with only axial plane using electronic caliper. The CBD diameter was measured from the leading edge to the trailing edge, including the ductal wall, and the widest diameter was measured perpendicular to its long axis (Figures 1B–D). CBD:Ao ratio was evaluated at PH, Mid, and DP levels. Ao diameter was measured on the axial plane and at the location where each CBD diameter was measured: PH, Mid, and DP level. For the Ao measurement, minor axis diameter of the vessel was measured (Figure 1E).
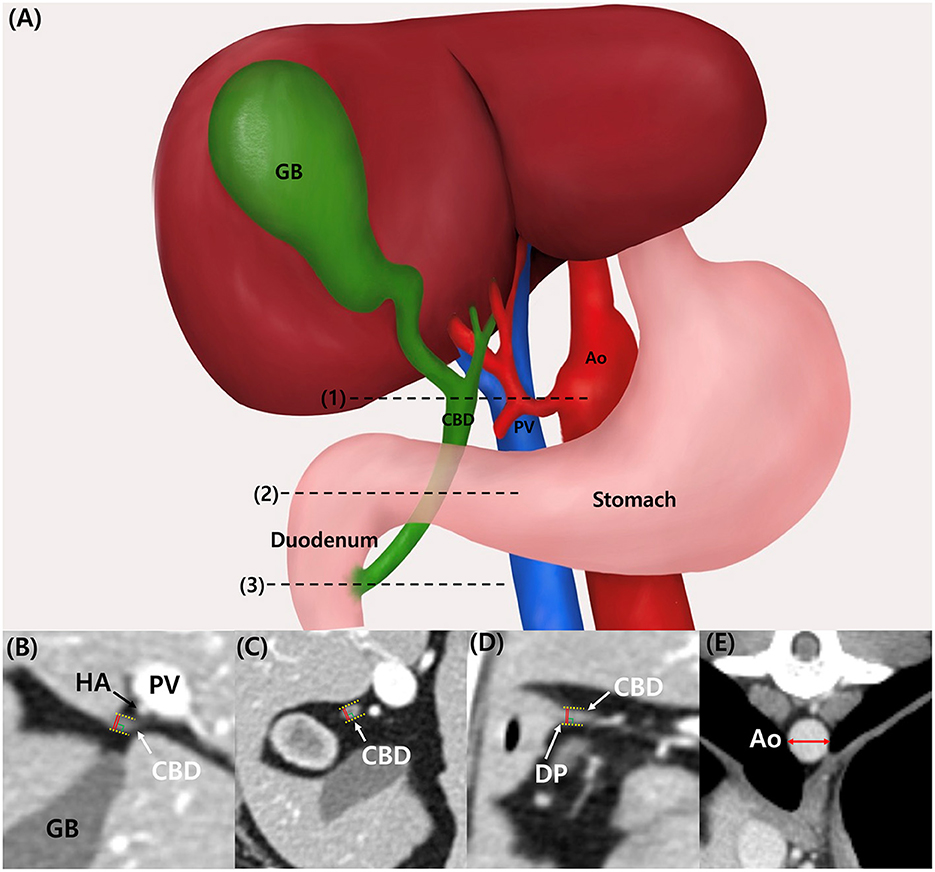
Figure 1. Illustration of common bile duct in dogs. (A) The location of measurement of common bile duct diameter (CBD) were as following: porta hepatis (PH) level (1), duodenal papilla (DP) level (3), and mid-portion (Mid) between these points (2). CT images of CBD at PH level (B), Mid-level (C), and DP level (D). The measurement of CBD is made including the ductal wall (yellow dotted line), and the widest diameter was measured (red line) perpendicular to its long axis. CT images of aortic diameter (Ao) measurement (E). For the Ao measurement, minor axis diameter (red double arrow) was measured. Ao, aorta; CBD, common bile duct; CT, computed tomography; DP, duodenal papilla; GB, gallbladder; HA, hepatic artery, Mid, mid-portion; PH, porta hepatis; PV, portal vein.
For intraobserver, reliability analysis, the CBD diameters at each level were measured twice by observer A. For interobserver reliability analysis, the CBD diameters at each level were measured by three different clinicians, observer A-C (Residents in the Veterinary Medical Imaging Department of the Teaching of Jeonbuk National University).
2.3. Statistics
All values were presented as the mean and standard deviation (SD). Since this study only included dogs without hepatobiliary disease, we established the upper limit of the normal CBD diameter instead of normal cutoff value. The mean + 1.96 SD value was used for the upper limit of the 95% reference range of the CBD diameter. One-way analysis of variance (ANOVA) was used to analyse the differences in CBD diameter, CBD: Ao ratio between BW groups and to analyse difference of CBD diameters between three different sites. Games-Howell and Scheffe's tests were used for post hoc analysis when the ANOVAs were significant (p < 0.05*). Simple linear regression analysis was applied to analyse the correlation between “BW and CBD diameter” and “age and CBD diameter.” The t-test was used to compare whether there was a difference of CBD diameter between sexes. Intraobserver and interobserver reliability for CBD diameter was assessed using the absolute agreement-type intraclass correlation coefficient (ICC) with a 95% CI. IBM Statistical Package for Social Sciences Statistics (SPSS; version 27.0, IBB Corp., Armonk, NY, USA) was used for all statistical analyses, and all experimental values were considered significant at p < 0.05* or p < 0.001**.
3. Results
3.1. Animal
The dogs were of 32 different breeds, including Maltese (n = 59), mixed (n = 39), Poodle (n = 37), Yorkshire terrier (n = 16), Shih tzu (n = 16), Pomeranian (n = 15), Schnauzer (n = 13), Retriever (n = 11), Chihuahua (n = 9), Spitz (n = 9), Dachshund (n = 8), Beagle (n = 8), Cocker spaniel (n = 7), Bichon frise (n = 6), Pekingese (n = 5), French bulldog (n = 3), Samoyed (n = 3), Miniature pinscher (n = 2), Italian greyhound (n = 2), Shetland sheepdog (n = 2), Chow chow (n = 2), Cavalier King Charles spaniel (n = 1), Bull terrier (n = 1), Great Pyrenees (n = 1), Husky (n = 1), Laika (n = 1), Scottish terrier (n = 1), Pug (n = 1), Welsh corgi (n = 1), Shiba inu (n = 1), Australian shepherd (n = 1), and Boston terrier (n = 1); Maltese dogs were the most common. The mean BW was 7.85 kg (range: 1.55–29.5 kg). The average age of all dogs was 8.8 years (range: 0.5–17 years). The study included 144 male and 139 female dogs. The number of dogs in each BW group was as follows: Class 1, n = 119; Class 2, n = 99; Class 3, n = 35; and Class 4, n = 30. The body condition scores (BCS) of the included dogs were 4 or 5/9.
3.2. Reference range and differences in the CBD diameters for different BW groups
Table 1 provides the normal reference range and upper limit of the CBD diameters at each level for each BW group. The CBD diameter at each level differed significantly (p < 0.05* or p < 0.001**) among all BW groups (Figures 2A–C).
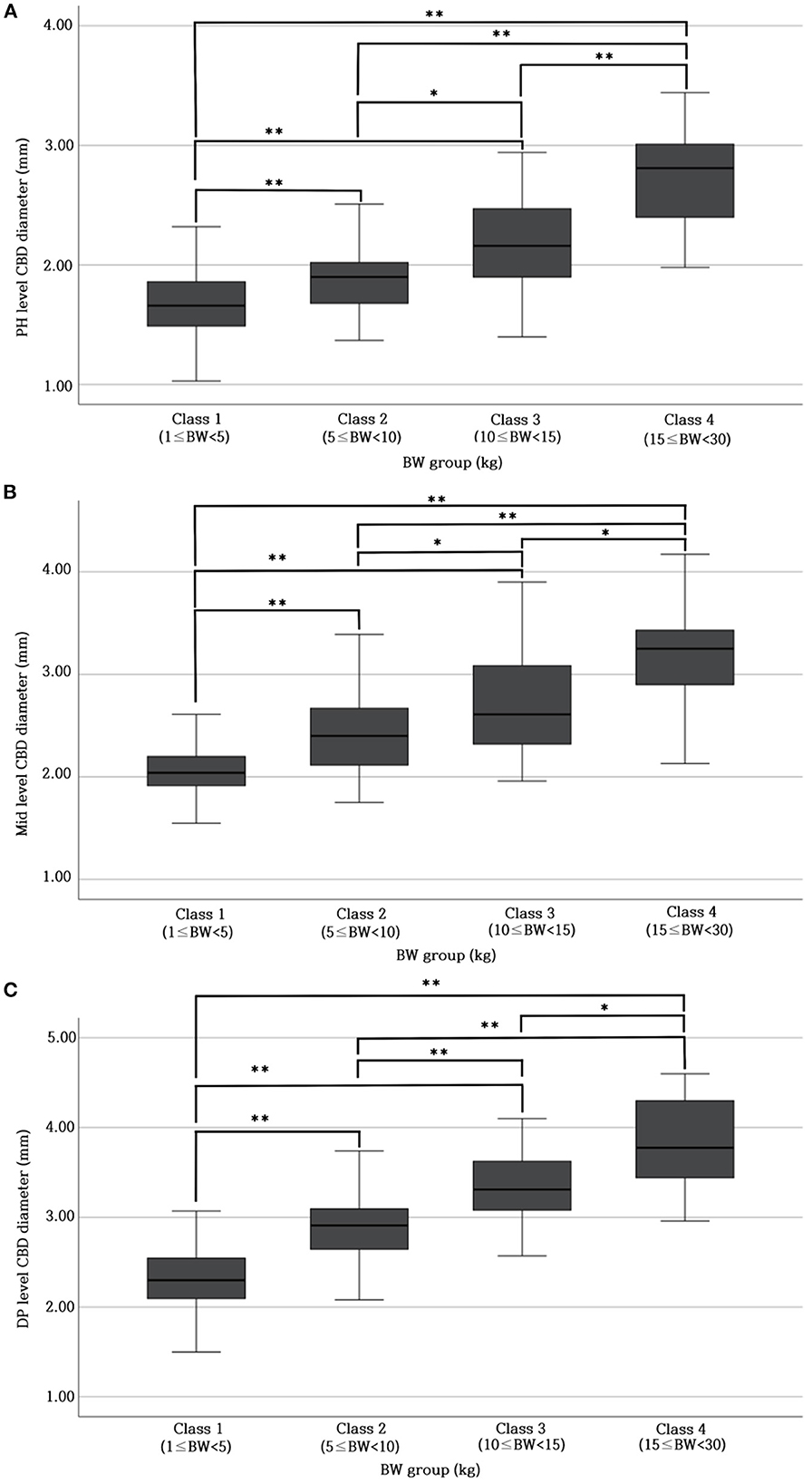
Figure 2. Differences in CBD diameter at PH level (A), Mid-level (B), and DP level (C) between different BW groups. There were significant differences (p < 0.05* or p < 0.001**) in CBD diameter at each level (PH, Mid, and DP level) among all BW groups. BW, body weight; CBD, common bile duct; DP, duodenal papilla; Mid, mid-portion; PH, porta hepatis.
3.3. Correlations between the CBD diameters and BW
The BW and CBD diameter at the PH level showed a moderate positive linear correlation (R2 = 0.535; β = 0.057; p < 0.001**) (Figure 3A). The BW and CBD diameter at the Mid-level showed a moderate positive linear correlation (R2 = 0.511; β = 0.057; p < 0.001**) (Figure 3B). The BW and CBD diameter at the DP level showed a moderate positive linear correlation (R2 = 0.629; β = 0.079; p < 0.001**) (Figure 3C).
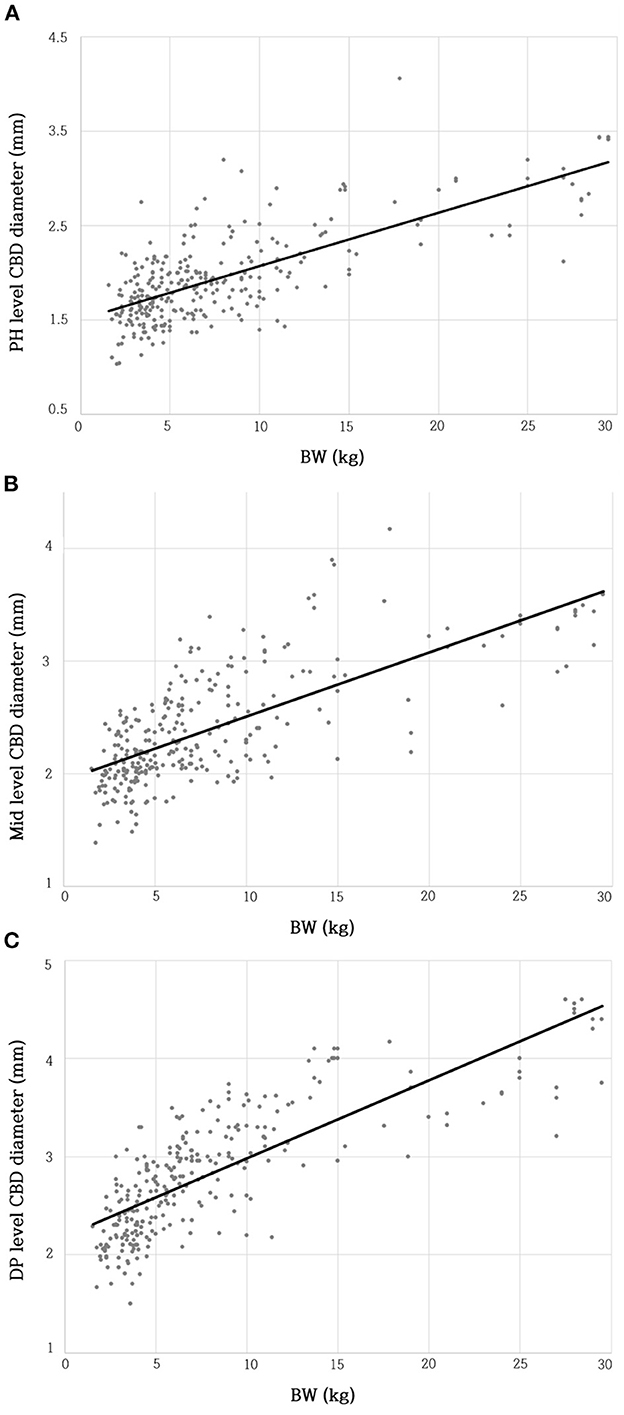
Figure 3. Relationship between BW and CBD diameter at PH level (A), Mid-level (B), and DP level (C). There were moderate linear positive correlations between BW and CBD diameter at each level. BW, body weight; CBD, common bile duct; DP, duodenal papilla; Mid, mid-portion; PH, porta hepatis.
3.4. CBD: Ao ratio
Table 2 provides the reference range of CBD: Ao ratio at three different sites. To verify whether this ratio was not affected by BW, the CBD: Ao ratio was compared between different BW groups. The ratio showed no significant difference at each site (p > 0.05) (Figure 4).
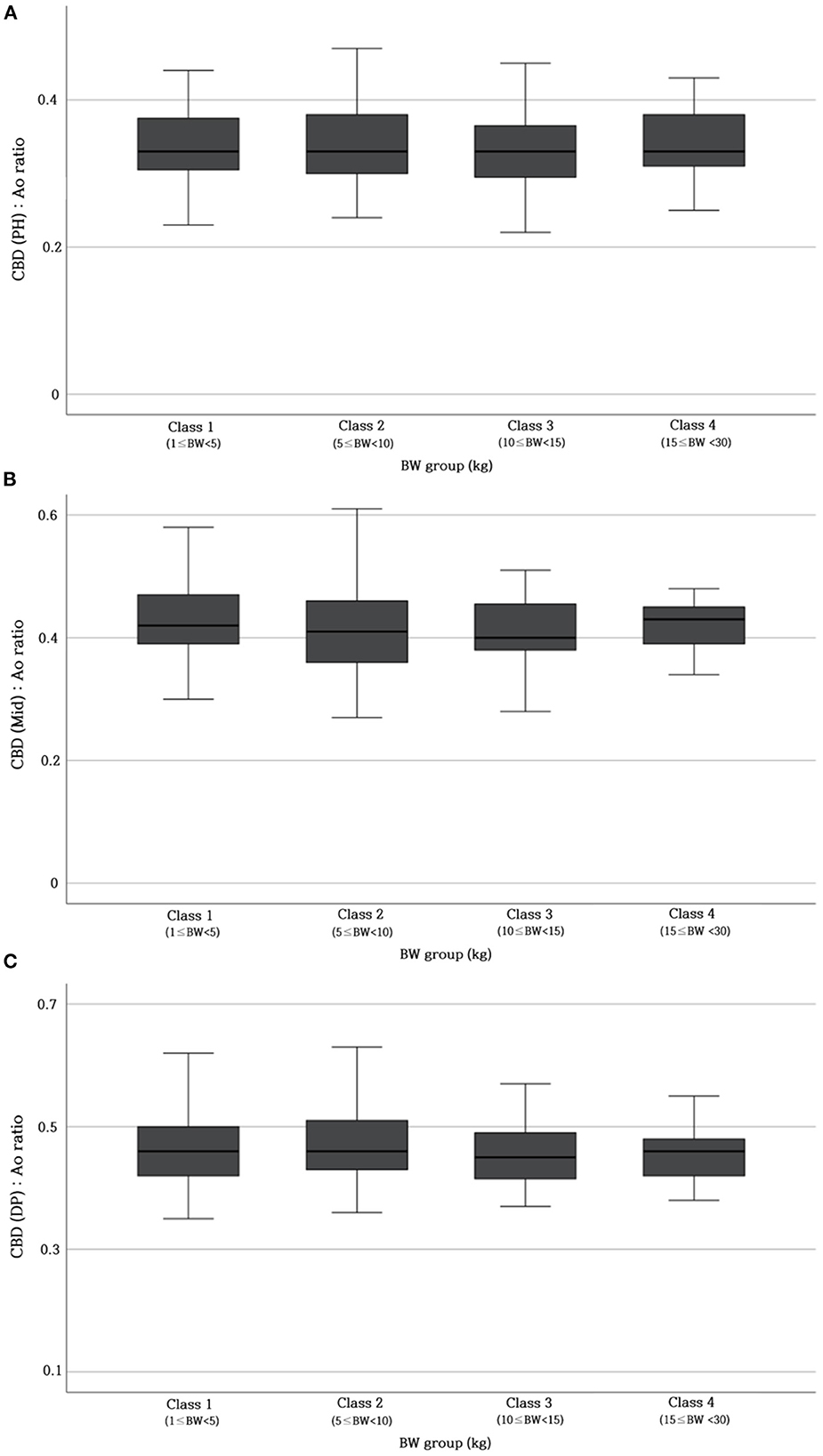
Figure 4. Differences in CBD: Ao ratio at PH level (A), Mid-level (B), and DP level (C) between different BW groups. No significant differences (p > 0.05) are found in the CBD: Ao ratio between the BW groups. Ao, abdominal aorta; BW, body weight; CBD, common bile duct; DP, duodenal papilla; Mid, mid-portion; PH, porta hepatis.
3.5. Differences in the CBD diameters at the three different levels (PH, Mid, and DP level)
There was a significant difference in the mean CBD diameters between the three different levels (mean CBD diameter at PH; 1.95 ± 0.49 mm, Mid; 2.39 ± 0.50 mm, DP; 2.81 ± 0.63 mm; p < 0.001**).
3.6. Correlation between CBD diameter and age
Age showed no correlation with CBD diameter at the PH level (R2 = 0.000; β = −0.002; p = 0.841), Mid-level (R2 = 0.004; β = −0.008; p = 0.320), and DP level (R2 = 0.000; β = −0.003; p = 0.728).
3.7. Difference between the CBD diameters and sex
No significant differences in CBD diameters were observed in relation to sex at PH level (t-value = 0.762, p = 0.447), Mid-level (t-value = 0.613, p = 0.540) and DP level (t-value = 0.497, p = 0.620).
3.8. Intraobserver and interobsever reliability
Table 3 provides the first and the second measured CBD diameters at each level. The intraobserver reliability measured by ICC was 0.962 at the PH level, 0.997 at the Mid-level, and 0.981 at the DP level. The measured values of CBD diameter at each level showed almost perfect reliability for the two measurements. Table 3 also provides the CBD diameters measured by three clinicians (observer A-C) at each level. Interobserver reliability measured by ICC was 0.975 at the PH level, 0.958 at the Mid-level, and 0.956 at the DP level which were consistent with almost perfect reliability.
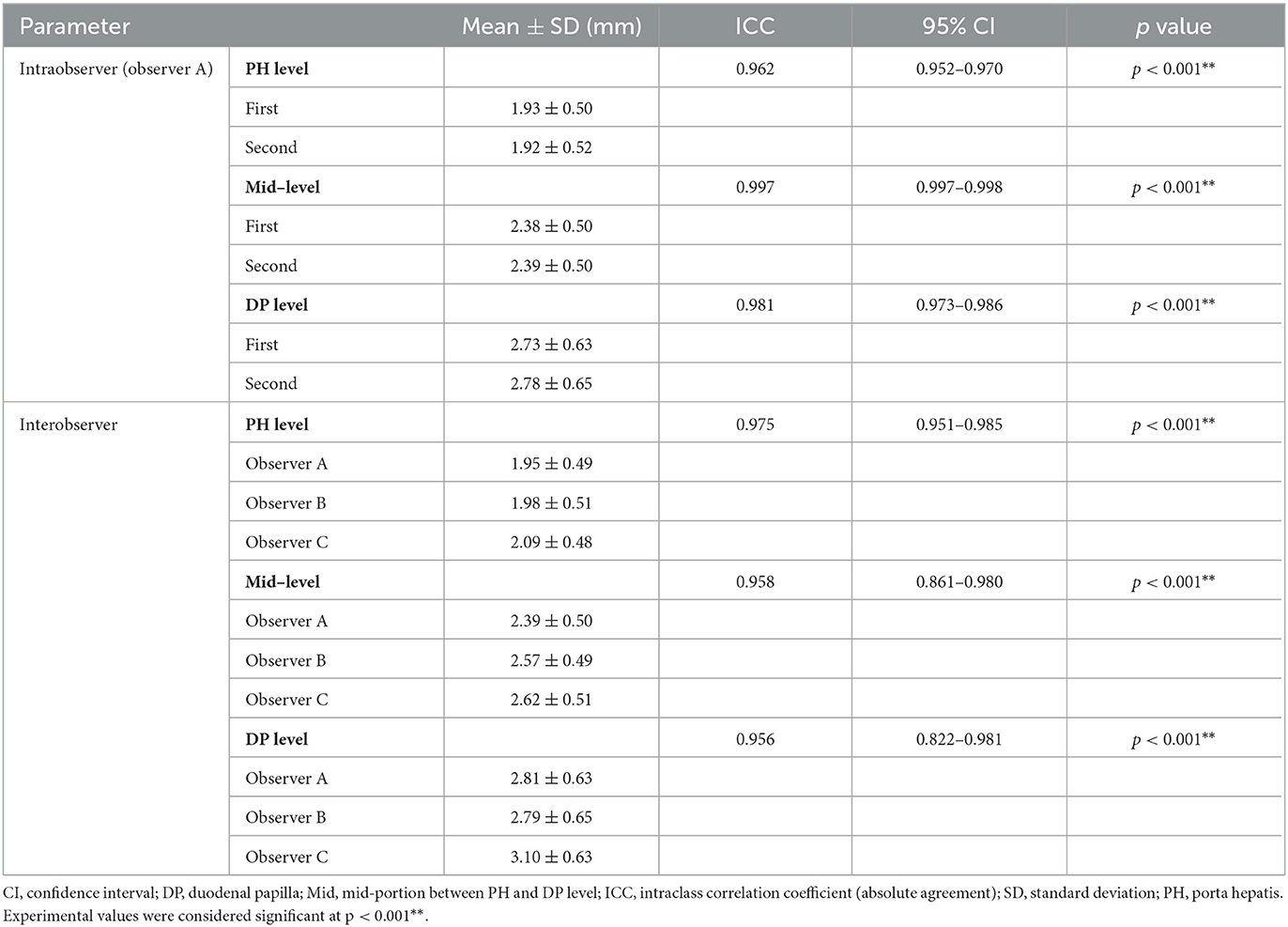
Table 3. Intraobserver and interobserver reliability for the CBD diameter at PH and DP level using ICC and their 95% CI.
4. Discussion
This study focused on the normal reference range in different BW groups and correlation between CBD diameter and BW using CT. Based on our findings, there was a significant difference in the CBD diameter in different BW groups. Moreover, positive linear correlation between CBD diameter and BW was observed. Thus, we devised a new method using Ao diameter, the CBD: Ao ratio, which is not affected by BW. To the best of the author's knowledge, this new method has not been reported in veterinary medicine.
To date, in veterinary medicine, specific reference range of CBD diameter using CT has not been established for different BW groups in normal dogs. A recent previous study evaluating the CBD diameter using CT have been reported for small breed dogs weighing <15 kg in 50 normal dogs (11). However, that study was performed only in a narrow range of BW and the number of dogs used was small. In our study, the BW range was wide (1.55–29.5 kg), and the number of dogs used was large with 283. In the previous study, the mean CBD diameter was 1.85 ± 0.63 mm at the PH level and 2.70 ± 0.51 mm at the DP level (11). Mean CBD diameters of the previous study were similar to those of Class 1 (PH; 1.69 ± 0.29, DP; 2.33 ± 0.34) and Class 2 (PH; 1.92 ± 0.35, DP; 2.90 ± 0.36), and Class 3 (PH; 2.20 ± 0.43, DP; 3.35 ± 0.49) in our study, but mean CBD diameters of Class 4 (PH; 2.79 ± 0.49, DP; 3.83 ± 0.50) were larger than those of the previous study (11). It was inferred that the reason for these differences was that the BW of Class 4 (15 kg≤BW < 30 kg) was larger compared to the BW used in previous study (11). Therefore, in a wide range of BW, it may be difficult to use the values of the previous study. Furthermore, results of our study showed significant differences in the CBD diameters for different BW groups and its values were significant higher in Class 4 (PH; 2.79 ± 0.49, Mid; 3.14 ± 0.44, DP; 3.83 ± 0.50) than Class 1 (PH; 1.69 ± 0.29, Mid; 2.06 ± 0.25, DP; 2.33 ± 0.34). Thus, we assumed that CBD diameter was affected by BW in normal dogs, and that different normal reference range of CBD diameters should be used for different BW groups.
BW is one of the most representative physiological indicators in dogs and humans. Our study showed positive linear correlations between CBD diameter and BW, which was similar to report in previous human medicine (14). However, other human studies have suggested that there was no association between CBD diameter and BW (15–17). We suppose that the reason for these results may be the differences in the variability of physiques between dogs and humans. In a human study, the participants were usually adults and their height (153.42–173.38 cm) and weight (39.15–63.65 kg) showed relatively small differences in physique (17). However, dogs vary significantly in physique between breeds, such as the Maltese and Great Pyrenees (18), causing significant differences and correlations in CBD diameter between dogs with large differences in physique. Additionally, obesity is an indicator that affects the physique index, but since the BCS of the dogs used in our study was ideal (BCS 4 or 5), it was inferred that obesity did not affect the results of our study (19). Moreover, in a previous study in veterinary medicine, no correlation was observed between BW and the CBD diameter at the PH level, although BW showed a mild correlation with the CBD diameter at the DP level (11). We inferred that the reason for these results may be because of differences such as number of samples, range of BW, and breeds.
Since the CBD diameter significantly differs according to BW, we attempted to devise a new indicator that can evaluate CBD diameter regardless of the BW. The aortic diameter has been used in ratio studies as a reliable landmark (20, 21). Due to the tortuous course of the aorta, it may appear elliptical, leading to an overestimation of the actual diameter when using the major axis of the ellipse (22). Therefore, minor axis diameter of the aorta was measured in this study. We investigated whether the CBD: Ao ratio showed a constant value in different BW groups. The results confirmed that the CBD: Ao ratio remained constant between different BW groups (p > 0.05). Therefore, this new index could be used regardless of the BW.
In previous veterinary study, CBD diameters showed significant differences at PH and DP level (11). Therefore, in our study, CBD diameters were measured at different sites; PH, Mid and DP level. There were significant differences in the CBD diameters for the three different sites, which is similar to the results of a previous veterinary medicine study. As a result, it is important to use different reference ranges for PH, Mid, and DP levels.
In this study, age showed no correlation with CBD diameters at each level. This result was similar with reports in previous human medicine (23). However, other human medicine studies have reported significant positive correlations between age and CBD diameter (14–17, 24–26). Although the reason for this discrepancy is not clear, the CBD diameter can be considered to have increased with age because the longitudinal smooth muscle band and the intervening connective tissue fragments were accompanied by loss of the reticulo-endothelial network of the ductal wall (16, 27). The reason for difference with that in humans was possibly because that the age range of humans was significantly wider (range: 18–85 years) than the relatively narrow age range in our study (range: 0.5–17 years) (17).
There was no significant difference in CBD diameter between sexes in our study. Several studies in human medicine emphasized that CBD diameters did not differ according to sex, which is same with our study (15–17).
While a previous veterinary study proposed 3 mm as the normal CBD diameter in dogs on US examination, our study showed that the normal CBD diameters in some dogs are larger than 3 mm (10). Several human studies have suggested that measurements of abdominal organ size were larger on CT compared to US (28, 29). The reason for such differences may be related to the characteristics of the imaging modality. While US can clearly distinguish specific abdominal organs from surrounding fat or other soft tissue, CT may have less clear organ contouring or delineation, which can lead to larger measurements on CT compared to US (28–30).
Intra- and interobserver reliability analyses were conducted to estimate reproducibility and reliability of measurement. We used ICC with 95% CI as described by Fleiss in which if the ICC value is 0.81–0.99, it is considered almost perfect agreement (31). In our study, intra- and interclass correlation coefficient showed almost perfect agreement. Therefore, through these results, it can be considered that the values of the reference ranges that we set are reliable.
Our study had several limitations. First, determination of the exact normal cutoff value for identifying abnormal conditions was not established because abnormal subjects were not included in this study. However, we established the upper limit of the normal CBD diameter for clinical recommendations. Future studies should aim to add and compare an abnormal group to set the normal cutoff value. Second, because of the retrospective nature of the study, CT machines used in this study were different. Some scan parameters of the images used in this study, including slice thickness, kVp, and mAs, were slightly different. However, since this study was conducted on a large scale with 283 dogs, it could be inferred that the difference due to the scan parameters would be relatively small. Lastly, the number of dogs in each BW group was unequal. Therefore, further studies should be conducted in a condition where the number of subjects in each BW group is similar.
In conclusion, based on our review of the literature, this is the first study to establish a normal reference range of the CBD diameter between different BW groups by using CT. Since the CBD diameter differs depending on BW, using a different normal reference range for different BW group is essential, while CBD: Ao ratio can be used valuable regardless of BW.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Jeonbuk National University (Approval No. JBNU 2022-037). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
Conception and design, acquisition of data, and drafting the article: YK and HY. Analysis and interpretation of data, revising article for intellectual content, and final approval of the completed article: YK, SK, DK, DI, KL, and HY. Agreement to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: YK, KL, and HY. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1A6A1A03033084).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wilkinson AR, DeMonaco SM, Panciera DL, Otoni CC, Leib MS, Larson MM. Bile duct obstruction associated with pancreatitis in 46 dogs. J Veter Internal Med. (2020) 34:1794–800. doi: 10.1111/jvim.15879
2. Mayhew PD, Savigny MR, Otto CM, Brown DC, Brooks MB, Bentley AM, et al. Evaluation of coagulation in dogs with partial or complete extrahepatic biliary tract obstruction by means of thromboelastography. J Am Vet Med Assoc. (2013) 242:778–85. doi: 10.2460/javma.242.6.778
3. Bowen DJ, Clemmons RM, Meyer DJ, Dorsey-Lee MR. Platelet functional changes secondary to hepatocholestasis and elevation of serum bile acids. Thromb Res. (1988) 52:649–54. doi: 10.1016/0049-3848(88)90137-5
4. Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. (2009) 15:1677. doi: 10.3748/wjg.15.1677
5. Kanda H, Nimura Y, Yasui A, Uematsu T, Kamiya S, Machiki Y, et al. Hepatic blood flow after acute biliary obstruction and drainage in conscious dogs. Hepatogastroenterology. (1996) 43:235–40.
6. Horrow MM. Ultrasound of the extrahepatic bile duct: issues of size. Ultrasound Q. (2010) 26:67–74. doi: 10.1097/RUQ.0b013e3181e17516
7. Parulekar SG. Transabdominal sonography of bile ducts. Ultrasound Q. (2002) 18:187–202. doi: 10.1097/00013644-200209000-00004
8. Leveille R, Biller DS, Shiroma JT. Sonographic evaluation of the common bile duct in cats. J Veter Internal Med. (1996) 10:296–9. doi: 10.1111/j.1939-1676.1996.tb02065.x
9. Smith I, Monkemuller K, Wilcox CM. Incidentally identified common bile duct dilatation. J Clin Gastroenterol. (2015) 49:810–5. doi: 10.1097/MCG.0000000000000394
10. Zeman R, Taylor K, Rosenfield A, Schwartz A, Gold J. Acute experimental biliary obstruction in the dog: sonographic findings and clinical implications. Am J Roentgenol. (1981) 136:965–7. doi: 10.2214/ajr.136.5.965
11. Park H-Y, Cho Y-G, Lee Y-W, Choi H-J. Evaluation of gallbladder and common bile duct size and appearance by computed tomography in dogs. J Vet Sci. (2018) 19:653–9. doi: 10.4142/jvs.2018.19.5.653
12. Rahmani V, Molazem M, Jamshidi S, Vali Y, Hanifeh M. Evaluation of gallbladder volume and contraction index with three-dimensional ultrasonography in healthy dogs. J Veter Med Sci. (2015) 77:1157–61. doi: 10.1292/jvms.14-0537
13. Foley WD, Wilson CR, Quiroz FA, Lawson TL. Demonstration of the normal extrahepatic biliary tract with computed tomography. J Comput Assist Tomogr. (1980) 4:48–52. doi: 10.1097/00004728-198002000-00009
14. Niederau C, Müller J, Sonnenberg A, Scholten T, Erckenbrecht J, Fritsch WP, et al. Extrahepatic bile ducts in healthy subjects, in patients with cholelithiasis, and in postcholecystectomy patients: a prospective ultrasonic study. J Clin Ultrasound. (1983) 11:23–7. doi: 10.1002/jcu.1870110106
15. Mahour GH, Wakim KG, Ferris DO. The common bile duct in man: its diameter and circumference. Ann Surg. (1967) 165:415. doi: 10.1097/00000658-196703000-00011
16. Peng R, Zhang L, Zhang X-M, Chen T-W, Yang L, Huang X-H, et al. Common bile duct diameter in an asymptomatic population: A magnetic resonance imaging study. World J Radiol. (2015) 7:501. doi: 10.4329/wjr.v7.i12.501
17. Lal N, Mehra S, Lal V. Ultrasonographic measurement of normal common bile duct diameter and its correlation with age, sex and anthropometry. J Clin Diagn Res. (2014) 8:AC01. doi: 10.7860/JCDR/2014/8738.5232
18. McGreevy PD, Georgevsky D, Carrasco J, Valenzuela M, Duffy DL, Serpell JA. Dog behavior co-varies with height, bodyweight and skull shape. PLoS ONE. (2013) 8:e80529. doi: 10.1371/journal.pone.0080529
19. Lee J, Kim SS, Kwon D, Cho Y, Lee K, Yoon H. Measurement of renal cortical thickness using ultrasound in normal dogs: a reference range study considering bodyweight and body condition score. Veter Radiol Ultrasound. (2022) 63:337–44. doi: 10.1111/vru.13053
20. D'Anjou MA, Penninck D, Cornejo L, Pibarot P. Ultrasonographic diagnosis of portosystemic shunting in dogs and cats. Veter Radiol Ultrasound. (2004) 45:424–37. doi: 10.1111/j.1740-8261.2004.04076.x
21. Mareschal A, D'anjou MA, Moreau M, Alexander K, Beauregard G. Ultrasonographic measurement of kidney-to-aorta ratio as a method of estimating renal size in dogs. Veter Radiol Ultrasound. (2007) 48:434–8. doi: 10.1111/j.1740-8261.2007.00274.x
22. Ouriel K, Green RM, Donayre C, Shortell CK, Elliott J, DeWeese JA. An evaluation of new methods of expressing aortic aneurysm size: relationship to rupture. J Vasc Surg. (1992) 15:12–8. doi: 10.1016/0741-5214(92)70008-9
23. Horrow MM, Horrow JC, Niakosari A, Kirby CL, Rosenberg HK. Is age associated with size of adult extrahepatic bile duct: sonographic study. Radiology. (2001) 221:411–4. doi: 10.1148/radiol.2212001700
24. Senturk S, Miroglu TC, Bilici A, Gumus H, Tekin RC, Ekici F, et al. Diameters of the common bile duct in adults and postcholecystectomy patients: a study with 64-slice CT. Eur J Radiol. (2012) 81:39–42. doi: 10.1016/j.ejrad.2010.11.007
25. Bowie JD. What is the upper limit of normal for the common bile duct on ultrasound: how much do you want it to be? Am J Gastroenterol. (2000) 95:897–900. doi: 10.1111/j.1572-0241.2000.01925.x
26. Wu CC, Ho YH, Chen CY. Effect of aging on common bile duct diameter: A real-time ultrasonographic study. J Clin Ultrasound. (1984) 12:473–8. doi: 10.1002/jcu.1870120804
27. Kialian G, Aznaurian A. The age-related characteristics of the muscular layer of the common bile duct in man. Morfologiia. (1995) 108:10–2.
28. Hyginus NA, Felicitas UI, Chioma LA. Correlation of ultrasound and computed tomography measurements of the pancreas in a normal adult nigerian population. J Pancreas. (2021) 22:21–7.
29. Narayana V, Roberson PL, Pu AT, Sandler H, Winfield RH, McLaughlin PW. Impact of differences in ultrasound and computed tomography volumes on treatment planning of permanent prostate implants. Int J Radiat Oncol Biol Phys. (1997) 37:1181–5. doi: 10.1016/S0360-3016(96)00618-9
30. Berthelet E, Liu M, Truong P, Czaykowski P, Kalach N, Yu C, et al. slice index and thickness: impact on organ contouring in radiation treatment planning for prostate cancer. J Appl Clin Med Phys. (2003) 4:365–73. doi: 10.1120/jacmp.v4i4.2511
Keywords: canine, computed tomography, biliary system, CBD diameter to Ao ratio, CBD size
Citation: Kim Y, Kim S-S, Kwon D, Im D, Lee K and Yoon H (2023) Computed tomographic quantitative evaluation of common bile duct size in normal dogs: A reference range study considering body weight. Front. Vet. Sci. 10:1137400. doi: 10.3389/fvets.2023.1137400
Received: 04 January 2023; Accepted: 13 March 2023;
Published: 30 March 2023.
Edited by:
Annamaria Grandis, University of Bologna, ItalyReviewed by:
Masahiro Tamura, Rakuno Gakuen University, JapanLuca Manassero, University of Turin, Italy
Copyright © 2023 Kim, Kim, Kwon, Im, Lee and Yoon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hakyoung Yoon, aHl5b29uQGpibnUuYWMua3I=
 Yein Kim1
Yein Kim1 Sung-Soo Kim
Sung-Soo Kim Danbee Kwon
Danbee Kwon Kichang Lee
Kichang Lee Hakyoung Yoon
Hakyoung Yoon