
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 20 June 2023
Sec. Animal Reproduction - Theriogenology
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1134244
This article is part of the Research TopicMolecular, Cellular and Physiological Determinants of Sperm Fertility and Freezability in MammalsView all 9 articles
Zinc has a critical physiological role in sperm function. The purpose of this study was to investigate the effect of different sources of zinc on sperm quality. For this purpose, 18 Zandi lambs with an average weight of 32 ± 1.2 kg were subjected to three treatments in a completely randomized design. Experimental treatments include (1) control treatment of basal diet without zinc supplementation, (2) basal diet with 40 mg/kg of zinc supplementation from zinc sulfate source and (3) basal diet with 40 mg/kg of zinc supplementation with organic source. At the end of feeding period, lambs were slaughtered. To determine the effect of experimental treatments on sperm quality, the testes were transferred to the laboratory. After that, epididymal spermatozoa were evaluated for sperm motility parameters, abnormal morphology, viability, membrane functionality, malondialdehyde (MDA) and antioxidant activity [glutathione peroxidase (GPx), superoxide dismutase (SOD), total antioxidant capacity (TAC)], sperm concentration and testosterone level. Zinc sulfate administration decreased MDA levels compared to other treatments and increased GPx and TAC activity compared to the control (P < 0.05), although SOD activity was not affected by any supplementation. Also, the use of zinc sulfate supplementation increased the percentage of total and progressive motility compared to the control group (P < 0.05). Membrane integrity and sperm viability were also affected by zinc sulfate supplementation (P < 0.05). Therefore, the results of this study showed that the use of zinc sulfate, can improve sperm motility and survival indices and its antioxidant capacity.
Zinc is one of the essential nutrients for animals and humans which is required for many antioxidant functions, growth, reproduction and safety (1). The second-most prevalent mineral in the body, zinc has crucial roles in development, immunological response, reproduction, gene expression, and the wool production (2). Zinc supplements are divided into mineral resources (such as sulfate and zinc oxide) and organic sources (such as zinc-methionine, zinc-protein and zinc-lysine). Zinc is essential for numerous physiological functions in animals, plants and humans (3). Moreover, zinc has a critical physiological role in the function of sperm cells, which includes the effect on their motility and maintenance of their natural morphology (4, 5). Zinc is vital in maintaining sperm characteristics (4, 5). It has been shown that zinc deficiency impairs spermatogenesis in rats and causes atrophy of the seminiferous tubule. Consumption of zinc supplements in buffalo has improved reproductive performance (6). It has been reported that supplementation of zinc improved reproductive performance, lamb production, and health (7). Zinc is one of the contributing factors in the antioxidant system which causes dismutation of superoxide anion due to aerobic metabolism and converts it to hydrogen peroxide. In addition, it acts as a cofactor for coping with oxidative stress (8).
Zinc in semen is directly related to normal morphology, viability, and directly related to sperm motility (9). Elements such as selenium and zinc are essential for sperm production and testicular growth (10). Zinc is an antioxidant element and has, protective role against free radicals (11). Adding zinc supplement to the diet eliminates many side effects of free radicals (12). The quality of the sperm and the fertility of rams are influenced by a number of variables, including breed, age, season, and nutritional management (13).
Zinc supplementation improves sperm motility in male buffaloes (14). Sperm membrane integrity is essential not only for sperm metabolism but also for favorable changes in characteristics of male and female gametes (15). Insufficient intake of Zn impairs the antioxidant defense system (16). In another study, the addition of zinc nanoparticles to bovine sperm after freezing-thawing process increased membrane integrity and mitochondrial activity of sperm compared to the control group (17). Zinc in human semen plays an essential role in the physiological function of sperm, and its deficiency results in reduced levels of low sperm quality and reduced chances of fertility (10, 18). Antioxidants protect sperm from ROS production, DNA damage, plasma and mitochondrial membrane damage, reduced motility and viability, untimely maturation, damage from the freeze-thaw process, improve quality sperm, and increase motility, viability and fertility of sperm (19–21).
Moreover, to our knowledge, there are no reports about various zinc supplements on ram sperm quality parameters. Therefore, the objective of this study was to evaluate the effect of various zinc supplements on sperm quality parameters. We evaluated the conventional sperm parameters such as motility and viability and the presence and direct markers of the oxidative status, such as malondialdehyde (MDA) levels and antioxidants GPx, SOD and TAC (total antioxidant capacity).
Chemicals were obtained from Merck (Darmstadt, Germany) and Sigma-Aldrich Company (St. Louis, MO).
During the experiment, in breeding season 18 lambs with a mean age of 120 ± 10 days and an average weight of 32 ± 1.2 kg were randomly divided into three groups. Eighteen individual cages were used to keep the lambs in the experimental period. Experimental treatments include (1) control treatment of basal diet without zinc supplementation, (2) basal diet with 40 mg/kg of zinc supplementation from zinc sulfate source and (3) basal diet with 40 mg/kg of zinc supplementation with its organic origin. All experiments were performed in the University of Tehran, Iran.
The diets were formulated in accordance with the NRC (22) recommendations and animals were fed a total mixed ration ad libitum. Every lamb was housed in a separate pen with a cement floor and access to individual feeding and watering. At 08 and 16 hours, feed was provided twice daily in quantities that permitted 10% refusal. The amount of supplements was adjusted daily based on DMI of individual lambs. The current experiment lasted for 85 days. At the end of feeding period, lambs were slaughtered following a 12 h feed removal, based on the standard slaughter protocol in experimental abattoir of the farm of college of agriculture. To determine the effect of experimental treatments on sperm quality of experimental lambs, the testes were transferred to the laboratory. The tunica vaginalis of the testicles was removed in the laboratory. Epididymides with vas deferens were detached from the testis. A 35 mm petri dish was used to hold each cauda epididymis after it had been dissected free and rinsed with 0.9% saline. With the use of forceps and a scalpel, many incisions were made in the tubuli of the cauda epididymides and immersed in of tris buffer for sperm migration for 10 min. Collected sperm were washed once by centrifugation (5 minute) with tris base extender at 400 g (23). The sperm concentration was evaluated by a hemocytometer (24).
Blood samples were taken from wing vein into EDTA anticoagulant. The tubes were centrifuged (10 min, 1500 g) and plasma were separated and stored at −20°C until assessment. Plasma testosterone level was evaluated by ELISA using commercial ELISA kit.
Assessment of sperm motility was conducted using computer assisted sperm analyzer (CASA V 12.2; Hamilton Thorne Biosciences, Beverly, MA, USA). For each sample, at least 200 sperm were examined using normal procedures (37°C, 60 frames/s). The motility parameters evaluated for each sperm contained the curvilinear velocity (VCL, μm/s), the average path velocity (VAP, μm/s), the straight-line velocity (VSL, μm/s), the straightness (STR, %; VSL/VAP), the linearity (LIN, %; VSL/VCL), the beat cross frequency (BCF, Hz) and the amplitude of lateral head displacement (ALH, μm) (25).
To assess sperm morphology, 15 μl of semen was placed in tubes containing 1 ml of Hancock solution (426 mM sodium, 21.4 mM formalin, 304.29 mM Na2HPO4 and 99.42 mM K2HPO4) (26). A minimum of 200 spermatozoa were analyzed for morphologic abnormalities (head abnormalities, detached heads, abnormal mid-pieces and tail defects) using a phase contrast microscope (Labomed LX400; Labomed Inc., Culver City, CA) ( × 400 magnification).
By staining with nigrosin-eosin, the vitality of the sperm was evaluated (27). By combining 10 μl of semen with 10 μl of stain on a heated slide and quickly spreading it with a second slide, sperm smears were created. A phase-contrast microscope (Labomed LX400; Labomed Inc., Culver City, CA) was used to examine 200 cells for viability ( × 400). Only unstained sperm heads (white color) were regarded viable, whereas spermatozoa that showed either partial or total purple staining were regarded non-viable.
For evaluating this parameter, 10 μl of semen sample were gently mixed with 100 μl of hyposmotic solution and incubated for 30 minutes in a warm water bath (37°C) (28). Afterwards, 5 μl of the sample was placed on a preheated slide and covered with a slide. The slide was then placed on the hot plate of a contrast phase microscope (Labomed LX400; Labomed Inc., Culver City, CA). Two hundred sperm were counted with a magnification of 400 ×.
The thiobarbituric acid (TBA) reaction was used to determine the MDA concentration as a marker of the lipid peroxidation in the semen samples (28). Briefly, 1 ml of trichloroacetic acid was added to each sample. Centrifugation was performed at 1200 × g for 10 min and then 1 mL thiobarbituric acid (0.375%) was added to supernatant. For 10 minutes, the tubes were incubated in boiling water. After cooling to room temperature, the samples were examined with a UV/Visible spectrophotometer (T80 UV/VIS PJ Instruments Ltd, UK) at 532 nm.
The antioxidant system was evaluated by defining the TAC, and GPx and SOD activities (29). They were determined spectrophotometrically by Olympus AU 400 automatic biochemistry analyzer (Olympus, Tokyo, Japan), converting absorbance to specific units with a calibration curve. TAC was calculated by including the kit's reactivity, measuring absorbance at 600 nm, and converting absorbance to mmol/l. In the presence of oxidized glutathione (cumene hydroperoxide) and NADPH, GPx was assessed. The oxidized glutathione was subsequently reduced by GPx while simultaneously oxidizing NADPH to NADP+, resulting in a reduction in absorbance at 340 nm. The degree of inhibition of the oxidation of 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazoliumchloride (INT) to the red formazan dye by superoxide radicals (generated by a xanthine/xanthine oxidase system) was used to determine SOD. Under the test conditions, one unit of SOD prevents a 50% drop in INT. At 505 nm, the absorbance was measured.
SAS software (version 9.1) was selected to analyze the data. For checking the normality of the data, the Shapiro-Wilk test was used. The effects of the treatments were then tested using linear mixed-effect models [PROC MIXED; (30)]. Tukey's test was selected for comparing treatments when the models were significant. The significance level was P < 0.05. Results are presented as mean ± SEM.
Motility and velocity parameters of ram treated with a different form of zinc are presented in Table 1. Total motility was higher in the zinc sulfate treatment (p < 0.05) compared to other treatments. Also, PM showed higher motility (P < 0.05) in the zinc sulfate treatment than others. Motion parameters (VAP, VSL, VCL, ALH, LIN, BCF and STR) showed numerically higher values compared to organic and control supplementation, while there was no significant difference.
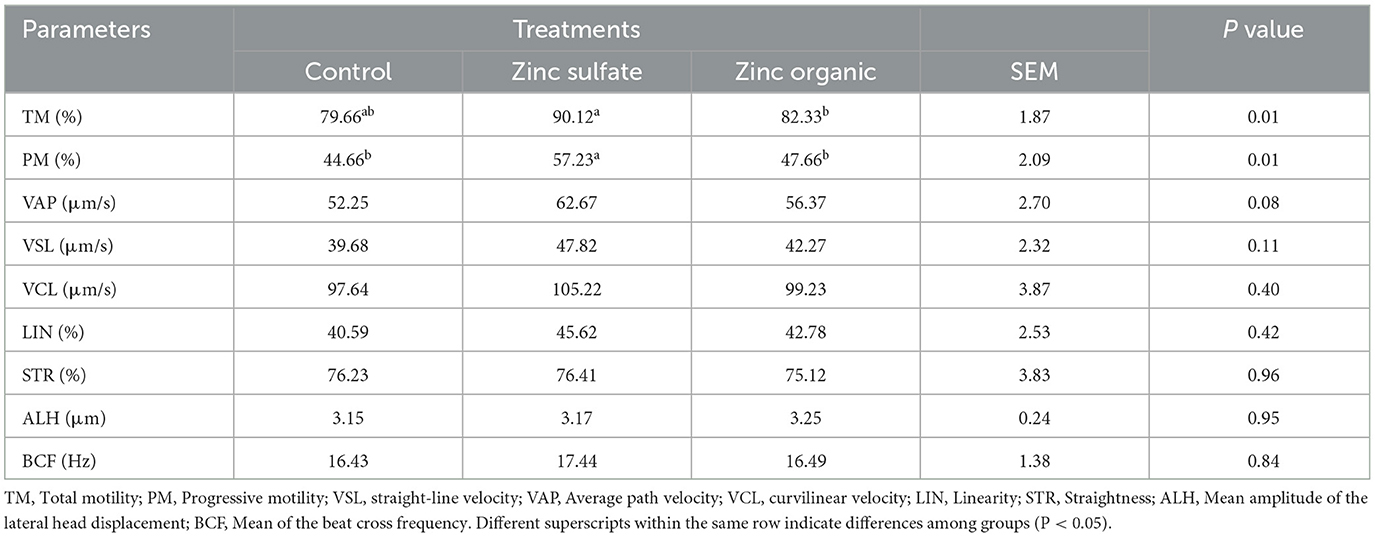
Table 1. Effect of zinc supplementation on semen motility parameters in Zandi lambs analyzed by CASA.
The use of the 40 mg/kg dry matter of zinc sulfate improved GPx, testosterone level and TAC compared to the control group. SOD activity and sperm concentration were not affected by any treatment (Table 2). The MDA level was found to be lower (P < 0.05) in the 40 mg/kg dry matter of zinc sulfate in comparison to the control group.
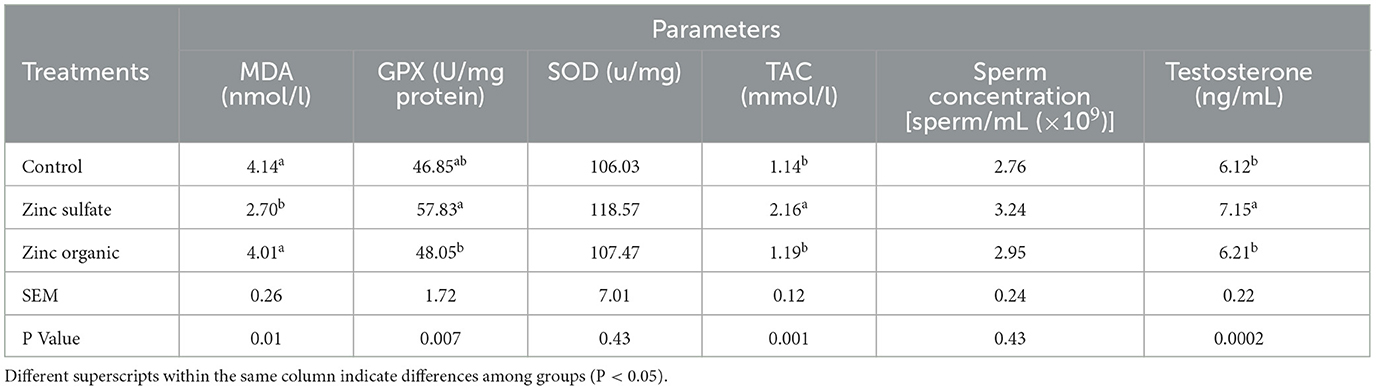
Table 2. Effect of zinc supplementation on malondialdehyde (MDA) concentration, glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity, total antioxidant capacity (TAC), sperm concentration and testosterone level of Zandi lambs.
Data on abnormal sperm structure, membrane integrity, and viability are presented in Table 3. Sperm viability and membrane integrity were higher in the group with 40 mg/kg dry matter of zinc sulfate and in the organic zinc group compared to the control group. When compared to the control group, there was no significant difference in abnormal morphology after zinc addition.
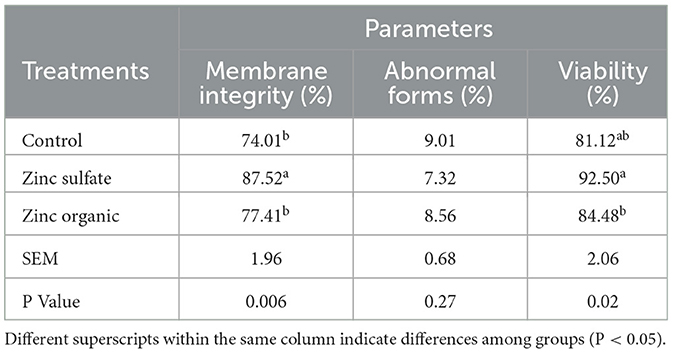
Table 3. Effect of zinc supplementation on viability (eosin/negrosin), membrane integrity, abnormal forms of Zandi lambs.
Studies have shown that zinc is very important in sperm membrane stability, viability, motility, and morphology. Optimal zinc plasma concentrations increase sperm concentration, motility, and antioxidant activity (31). On the other hand, excessive zinc consumption can lead to testicular destruction and loss of sperm motility in mice (32). The role of zinc in the production of vital enzymes has been proven to be an essential component of semen. Zinc may also indirectly affect the secretion of gonadotropic hormones through the pituitary gland (33). The lack of effect of zinc supplementation on sperm concentration in this test and other reports is due to the insufficient amount of zinc in the basal diet.
Rahman et al. (34) in their study on 16 male goats showed that the use of zinc supplementation as zinc sulfate significantly increased the amount of semen volume and sperm motility compared to the control group, but no significant relationship between sperm concentration and percentage of live sperm Zinc was observed in the receiving group and the control group.
Afifi et al. (35) evaluated the effect of zinc oxide nanoparticles on the activity of some antioxidant enzymes and sperm characteristics in diabetic mice. Their study showed that zinc oxide nanoparticles increase the activity of some antioxidant enzymes, including superoxide dismutase. They observed an increase in sperm motility and concentration compared to the control group. Similar to these results, zinc supplementation significantly increased the motility sperm (5). Similar studies have been reported in rabbits (36). Zinc sulfate supplementation with a level of 17.5 micromoles had an increasing effect on the percentage of total sperm (37). In a study on Barbari buck semen, volume, sperm motility, sperm viability and survival, and acrosome health improved (38). However, in some studies, contradictory results have been seen. For example, in a study conducted on ram sperm, the results showed that zinc sulfate has no significant effect on sperm motility (39). Jahanbin et al. (17) did not observe any significant effect on sperm motility characteristics by adding high levels of zinc in the form of zinc nanoparticles to the semen of Holstein bulls. Similar to the results of this study, zinc supplementation caused a significant increase in sperm survival (16). Kumar et al. (40) observed that zinc consumption in bulls in the form of zinc sulfate and zinc propionate increased sperm viability. Similar to the results of the present study, the use of zinc sulfate supplements has significantly increased the health of sperm membranes. Omu et al. (41) also reported that zinc supplementation improves sperm membrane health in men. In addition, KendallandTelfer (42) reported that zinc intake can improve sperm membrane health in sheep sperm. Similar to our findings, Jahanbin et al. (17) observed that membrane integrity was improved by adding zinc nanoparticles compared to control in bovine sperm. Changes in membrane integrity caused by cold shock damage, ice formation, and osmotic stress are the key factors decreasing the motility and viability of frozen sperm (43), and reactive oxygen species have been reported (types of active oxygen, which leads to oxidation) (29). Therefore, improving antioxidant indices by consuming zinc can lead to their improvements. In contrast, Orzołek et al. (44) found that turkey ejaculates enriched with Zn and kept for 48 hours had a few lower sperm parameters, including total and progressive motility and mitochondrial membrane potential. Perhaps the use of a high concentration of Zn in the study by Orzołek et al. (44) caused the reduction in sperm quality.
There is a significant relationship between testosterone and plasma zinc concentrations, so zinc supply is essential for sperm function (45). Zinc is essential for the considerable cell division required for semen production because it plays a significant role in the metabolism of nucleic acids and proteins (46). Zinc supplementation increases daily sperm production and lowers the percentage of defective spermatozoa (40, 47). In a study of the effect of organic and mineral resources on the concentration of organic hormones quantitative and qualitative traits of semen in male calves were examined for 6 months. By adding zinc to the diet, quantitative and qualitative traits of semen such as volume, motility, density and percentage of live sperm and testosterone concentration increased significantly (40). The effect of organic zinc and selenium supplementation was investigated in a study on sheep and contrary to our results, malondialdehyde concentration was not affected by experimental groups (48).
Rahman et al. (34) also reported that consumption of up to 200 parts per million zinc in the form of zinc sulfate for three months did not effect increasing sperm concentration in goats. In many studies, zinc sources as an oral supplement in the diet have an antioxidant role. It improves sperm quality indicators by stabilizing the antioxidant role in removing free radicals from the environment (49). Although in this experiment, zinc supplementation did not affect sperm concentration, but in an experiment, the use of organic and inorganic zinc supplementation in a bull diet improved the quantitative characteristics of ejaculation volume, sperm concentration and sperm count and sperm viability (40). It has been demonstrated that zinc plays a crucial function in the activity of many enzyme systems, and the role of this element in spermatogenesis and their maturation is well established (50). Zinc deficiency has severe effects on reproductive growth and yield of rams (51). Minimum dietary zinc concentrations of 30 mg/kg dry matter are recommended to prevent adverse effects on reproductive performance (51).
In the present study, supplementation of 40 mg/kg dry Zinc sulfate increased GPx and TAC activity while malondialdehyde (MDA) decreased. In addition, there was a higher percentage of total and progressive motility, membrane integrity and sperm viability.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Department of Animal and Poultry Science, College of Aburaihan, University of Tehran, Tehran, Iran. Written informed consent was obtained from the owners for the participation of their animals in this study.
MN designed and coordinated the study and collected data. AN and SM carried out the experiments and collected data. MN, AN, and SM wrote the manuscript and analyzed the data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fadl A, Abdelnaby E, El-seadawy I, Kotp M, El-Maaty A M A, El-Sherbiny H. Eco-friendly synthesized zinc oxide nanoparticles improved frozen-thawed semen quality and antioxidant capacity of Rams. J Adv Vet Res. (2022) 12:259–64.
2. Fadayifar A, Aliarabi H, Tabatabaei MM, Zamani P, Bahari A, Malecki M, et al. Improvement in lamb performance on barley based diet supplemented with zinc. Livest Sci. (2012) 144:285–9. doi: 10.1016/j.livsci.2011.12.002
3. Prasad A S. Zinc in human health: effect of zinc on immune cells. Molecular medicine. (2008) 14:353–7. doi: 10.2119/2008-00033.Prasad
4. Oliveira C, Badu C, Ferreira W, Kamwa E, Lana A. Effects of dietary zinc supplementation on spermatic characteristics of rabbit breeders. In: 8th World Rabbit Congress, Mexico. (2004)
5. Fadl AM, Abdelnaby EA, El-Sherbiny HR. Supplemental dietary zinc sulphate and folic acid combination improves testicular volume and haemodynamics, testosterone levels and semen quality in rams under heat stress conditions. Reprod Domestic Anim. (2022) 57:567–76. doi: 10.1111/rda.14096
6. Zeedan K I, El-Malky O, Komonna O. Productive and reproductive performance of buffaloes fed on rations supplemented with Biogen-Zinc at late pregnancy period. In: Proceedings of the 2nd Scientific Conference of animal wealth research in the Middle East and North Africa, Cairo International Convention Center, 24-26 October, 2009. Massive Conferences and Trade Fairs. (2009).
7. Hatfield P, Snowder G, Head Jr W, Glimp H, Stobart R, Besser T. Production by ewes rearing single or twin lambs: effects of dietary crude protein percentage and supplemental zinc methionine. J Anim Sci. (1995) 73:1227–38. doi: 10.2527/1995.7351227x
8. Najafi A, Mehdipour M, Mohammadi H, Mehdipour Z, Khorrami B, Nazari M. Effect of tempol and straw size on rooster sperm quality and fertility after post-thawing. Sci Rep. (2022) 12:12192. doi: 10.1038/s41598-022-16507-6
9. Alavi-Shoushtari S, Rezai S A, Ansari M, Khaki A. Effects of the seminal plasma zinc content and catalase activity on the semen quality of water buffalo (Bubalus bubalis) bulls. Pak J Biol Sci. (2009) 12:134–9. doi: 10.3923/pjbs.2009.134.139
10. Colagar A H, Marzony E T, Chaichi M J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. (2009) 29:82–8. doi: 10.1016/j.nutres.2008.11.007
11. Jain A, Varma M, Agrawal BK, Jadhav A. Serum zinc and malondialdehyde concentrations and their relation to total antioxidant capacity in protein energy malnutrition. J Nutr Sci Vitaminol. (2008) 54:392–5. doi: 10.3177/jnsv.54.392
12. Salama AA, Caja G, Albanell E, Such X, Casals R, Plaixats J. Effects of dietary supplements of zinc-methionine on milk production, udder health and zinc metabolism in dairy goats. J Dairy Res. (2003) 70:9–17. doi: 10.1017/S0022029902005708
13. Al-Anazi Y, Al-Mutary M, Alfuraiji M. Al-himaidi A, Al-Ghadi M, Ammari A. Seasonal variations in scrotal circumference and semen characteristics of Naimi and Najdi rams in Saudi Arabia South African. J Animal Sci. (2017) 47:454–9. doi: 10.4314/sajas.v47i4.4
14. Osman K, El-Shamaa I, Ibrahim M, Gabr S. Impact of zinc supplement on some reproductive traits in Egyptian buffalo bulls. In: Proc. 3rd All Africa Conf. Anim. Agric. and 11th Conf. Egyptian Soc. Anim. Prod. Alexandria (2000).
15. Roy B, Baghel R, Mohanty T, Mondal G. Zinc and male reproduction in domestic animals: A review. Indian J Anim Nutr. (2013) 30:339–50.
16. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. (2012) 10:1–31. doi: 10.1186/1477-7827-10-49
17. Jahanbin R, Yazdanshenas P, Rahimi M, Hajarizadeh A, Tvrda E, Nazari SA, et al. In vivo and in vitro evaluation of bull semen processed with zinc (Zn) nanoparticles. Biol Trace Elem Res. (2021) 199:126–35. doi: 10.1007/s12011-020-02153-4
18. Ma J, Bi J, Sun B, Li H, Li Y, Wang S. Zinc improves semen parameters in high-fat diet-induced male rats by regulating the expression of LncRNA in testis tissue. Biol Trace Elem Res. (2023) 1–13 doi: 10.1007/s12011-022-03550-7
19. Zigo M, Kerns K, Sen S, Essien C, Oko R, Xu D, et al. Zinc is a master-regulator of sperm function associated with binding, motility, and metabolic modulation during porcine sperm capacitation. Communications Biology. (2022) 5:1–12. doi: 10.1038/s42003-022-03485-8
20. Abedin SN, Baruah A, Baruah KK, Kadirvel G, Katiyar R, Khargharia G, et al. In Vitro and in vivo studies on the efficacy of zinc-oxide and selenium nanoparticle in cryopreserved goat (Capra hircus) spermatozoa. Biol Trace Elem Res. (2023) 1–20 doi: 10.1007/s12011-022-03551-6
21. El-Speiy M, El-Sawy M, Sadaka T, Abd-Elaal M, Habib M, Abdella M, Khattab M S. Impact of Alpinia galanga and zinc on semen quality and some reproductive hormone constituents in California rabbit bucks. Zygote. (2023) 31:1–7. doi: 10.1017/S0967199423000011
22. NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington, DC: National Academy of Science (2007).
23. Alvarez M, Tamayo-Canul J, Martinez-Rodriguez C, Lopez-Uruena E, Gomes-Alves S, Anel L, et al. Specificity of the extender used for freezing ram sperm depends of the spermatozoa source (ejaculate, electroejaculate or epididymis). Anim Reprod Sci. (2012) 132:145–54. doi: 10.1016/j.anireprosci.2012.05.006
24. Sariozkan S, Bucak M N, Tuncer PB, Tasdemir U, Kinet H, Ulutas PA. Effects of different extenders and centrifugation/washing on postthaw microscopic-oxidative stress parameters and fertilizing ability of Angora buck sperm. Theriogenology. (2010) 73:316–23. doi: 10.1016/j.theriogenology.2009.09.015
25. Mehdipour M, Daghigh Kia H, Martinez-Pastor F. Poloxamer 188 exerts a cryoprotective effect on rooster sperm and allows decreasing glycerol concentration in the freezing extender. Poult Sci. (2020) 99:6212–20. doi: 10.1016/j.psj.2020.08.041
26. Mehdipour M, Daghigh Kia H, Najafi A, Vaseghi Dodaran H, Garcia-Alvarez O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology. (2016) 73:297–303. doi: 10.1016/j.cryobiol.2016.10.008
27. Mehdipour M, Daghigh Kia H, Moghaddam G, Hamishehkar H. Effect of egg yolk plasma and soybean lecithin on rooster frozen-thawed sperm quality and fertility. Theriogenology. (2018) 116:89–94. doi: 10.1016/j.theriogenology.2018.05.013
28. Mehdipour M, Kia H D, Najafi A, Martínez-Pastor F. Type III antifreeze protein (AFP) improves the post-thaw quality and in vivo fertility of rooster spermatozoa. Poult Sci. (2021) 100:101291. doi: 10.1016/j.psj.2021.101291
29. Mehdipour M, Daghigh-Kia H, Najafi A, Mehdipour Z, Mohammadi H. Protective effect of rosiglitazone on microscopic and oxidative stress parameters of ram sperm after freeze-thawing. Sci Rep. (2022) 12:13981. doi: 10.1038/s41598-022-18298-2
30. SAS S. STAT User's Guide: Statistics. Version 9.1. Cary, NC: Statistical Analysis System Institute. Inc (2002)
31. Chia SE, Ong CN, Chua LH, Ho LM, Tay SK. Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J &rology. (2000) 21:53–7.
32. Turgut G, Abban G, Turgut S, Take G. Effect of overdose zinc on mouse testis and its relation with sperm count and motility. Biol Trace Elem Res. (2003) 96:271–9. doi: 10.1385/BTER:96:1-3:271
33. Hurley W, Doane R. Recent developments in the roles of vitamins and minerals in reproduction. J Dairy Sci. (1989) 72:784–804. doi: 10.3168/jds.S0022-0302(89)79170-0
34. Rahman H, Qureshi M, Khan R. Influence of dietary zinc on semen traits and seminal plasma antioxidant enzymes and trace minerals of b eetal bucks. Reprod Domestic Anim. (2014) 49:1004–7. doi: 10.1111/rda.12422
35. Afifi M, Almaghrabi O A, Kadasa N M. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. BioMed Res Int. (2015) 2015. doi: 10.1155/2015/153573
36. Emmanuel D, Amaka A, Okezie E, Sunday U, Ethelbert O. Epididymal sperm characteristics, testicular morphometric traits and growth parameters of rabbit bucks fed dietary saccharomyces cerevisiae and/or zinc oxide. Braz J Poult Sci. (2019) 21. doi: 10.1590/1806-9061-2018-0803
37. Daghigh Kia H, Jafari S. The effect of adding different levels of zinc sulfate on Ghezel ram sperm function during the freeze-thawing process in out of breeding season. Animal Sciences Journal. (2018) 31:59–70.
38. Kumar P, Yadav B, Yadav S. Effect of zinc and selenium supplementation on semen quality of Barbari bucks. Indian J Anim Res. (2014) 48:366–9. doi: 10.5958/0976-0555.2014.00457.9
39. Pouya M, Khodaei-Motlagh M, Khalt-Abadi Frahani AH, Mirzaei M. Effect of different levels of zinc sulfate of semen extender in Farahani ram breed sperm quality after freezing-thawing. J Cell & Tissue. (2017) 8:374–86. doi: 10.52547/JCT.8.4.374
40. Kumar N, Verma RP, Singh LP, Varshney VP, Dass RS. Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus $ \bf\times $ Bos taurus) bulls. Reprod Nutr Devt. (2006) 46:663–75. doi: 10.1051/rnd:2006041
41. Omu A E, Dashti H, Al-Othman S. Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome. Eur J Obstet Gynecol Reprod. (1998) 79:179–84. doi: 10.1016/S0301-2115(97)00262-5
42. Kendall N, Telfer S: The use of a soluble glass zinc cobalt and selenium bolus to correct zinc deficiency in sheep. In: Proceedings of the British Society of Animal Science. Cambridge: Cambridge University Press. (1998).
43. Maxwell W, Watson P. Recent progress in the preservation of ram semen. Anim Reprod Sci. (1996) 42:55–65. doi: 10.1016/0378-4320(96)01544-8
44. Orzołek A, Rafalska KT, Otowska WA, Kordan W, Korzekwa AJ, Kozłowski K. Influence of zinc and manganese nanoparticles on selected parameters of Turkey spermatozoa stored in a liquid state at 4°C. Animals. (2021) 11:3289. doi: 10.3390/ani11113289
45. Mocchegiani E, Romeo J, Malavolta M, Costarelli L, Giacconi R, Diaz L-E, et al. Zinc: dietary intake and impact of supplementation on immune function in elderly. Age. (2013) 35:839–60. doi: 10.1007/s11357-011-9377-3
47. Underwood E, Somers M. Studies of zinc nutrition in sheep. I The relation of zinc to growth, testicular development, and spermatogenesis in young rams. Aust J Agricult Res. (1969) 20:889–97. doi: 10.1071/AR9690889
48. Ghorbani A, NooriyanSoroor ME, Moeini MM. The effect of organic zinc and selenium supplementation on feed intake, digestibility, and rumen fermentation parameters in sheep. Animal Sci J. (2017) 30:17–36.
49. Mehdipour M, Daghigh Kia H, Najafi A, Mohammadi H, Alvarez-Rodriguez M. Effect of crocin and naringenin supplementation in cryopreservation medium on post-thaw rooster sperm quality and expression of apoptosis associated genes. PLoS ONE. (2020) 15:e0241105. doi: 10.1371/journal.pone.0241105
50. Biswajit R, Nagpaul P, Pankaj P, Mohanty T, Raina V, Ghosh S. Effect of Zn supplementation (ZnSO4) on sperm morphometry of Murrah buffalo bulls (Bubalus bubalis). Buffalo Bulletin. (2010) 29:21–5.
Keywords: sperm, zinc, diet, antioxidant, motility
Citation: Mousavi Esfiokhi SH, Norouzian MA and Najafi A (2023) Effect of different sources of dietary zinc on sperm quality and oxidative parameters. Front. Vet. Sci. 10:1134244. doi: 10.3389/fvets.2023.1134244
Received: 30 December 2022; Accepted: 05 June 2023;
Published: 20 June 2023.
Edited by:
Zongliang Carl Jiang, University of Florida, United StatesCopyright © 2023 Mousavi Esfiokhi, Norouzian and Najafi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abouzar Najafi, YWJvemFyLm5hamFmaUB1dC5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.