- 1College of Animal Science and Technology, Hebei North University, Zhangjiakou, Hebei, China
- 2Zhangjiakou Animal Husbandry Technology Promotion Station, Zhangjiakou, Hebei, China
- 3National Key Laboratory of Veterinary Public Health Security, Key Laboratory of Animal Epidemiology and Zoonosis of Ministry of Agriculture, Beijing, China
- 4National Animal Protozoa Laboratory and College of Veterinary Medicine, China Agricultural University, Beijing, China
Following the discovery of Eimeria kongi, we investigated the pathogenicity, immunogenicity, endogenous development and drug sensitivity of this coccidian. Coccidia-free rabbits were inoculated with 1 × 102 to 5 × 104 sporulated oocysts of E. kongi before challenge 14 days post inoculation. E. kongi was moderately pathogenic and induced good immunity against re-infection. All inoculated doses results in reduced food intake and body weight gain, and an inoculation oocyst dose of 1 × 103 or higher caused various degrees of diarrhea. Except for one death of the highest dose group, all rabbits recovered 12 days post inoculation. An inoculation dose of 1 × 103 or 1 × 104 oocysts conferred the most effective protection from re-infection, which reduced oocyst output by approximately 99% and maintained body weight gain. Four generations of schizogony were observed, and the endogenous development mainly occurred in the jejunum and ileum of rabbits. E. kongi was most sensitive to sulfachloropyrazine sodium, followed by decoquinate; it is resistant to diclazuril. Both decoquinate and sulfachloropyrazine sodium may be effective in the control of E. kongi infection.
1. Introduction
It was recognized that the monoxenous coccidia of the genus Eimeria Schneider, 1875 (Apicomplexa: Eimeriidae) is a protozoan parasite, which can cause coccidiosis in animals including livestock and is mainly characterized by severe enteritis involving diarrhea (1, 2). The infection is transmitted by the fecal-oral route of the sporulated oocysts, and its life cycle includes three different phases, schizogony (proliferation of schizozoites in schizonts), gametogony (forming gametes, zygotes, and oocysts) in the hosts, and sporogony (maturation of the oocysts) in the external environment. The parasites possess narrow host spectra and each species of Eimeria displays different pathogenicity in their specific hosts (3, 4).
Eleven species of Eimeria have been identified as pathogens of rabbit coccidiosis (5), namely E. stiedae, E. vejdovskyi, E. flavescens, E. media, E. intestinalis, E. perforans, E. piriformis, E. coecicola, E. exigue, E. magna, and E irresidua. Coccidiosis can be classified into two forms: hepatic coccidiosis that occurs because of E. stiedae and intestinal coccidiosis that occurs because of the remaining members of Eimeria. it has been reported that parasites of Eimeria species can induce significant economic losses in the farm and pet industries due to high infection and mortality rates (6). Cui et al. conducted a survey on the infection rate of coccidia in rabbits in some areas of Hebei Province. The results showed that the infection rate of coccidia in rabbits was relatively high, with the average infection rate of 96.7% in young rabbits, 75.1% in adult young rabbits and 55.7% in breeding rabbits (7). In 2017, Cui et al. discovered a new species, E. kongi, in Zhangjiakou of Hebei, China. E. kongi was identified as a new rabbit coccidia species based on morphological characteristics, prepatent period and phylogenetic analyses of the 18S rDNA gene and the first internal transcribed spacer (ITS-1) (8). The aims of this paper was to study the pathogenicity, immunogenicity, endogenous development, and drug sensitivity of E. kongi.
2. Materials and methods
2.1. Test materials
2.1.1. Coccidia strain
E. kongi was provided by the Animal Parasitology Laboratory of Hebei North University and was subcultured before our experiments (8).
2.1.2. Rabbits
Weaned New Zealand white (NZW) rabbits (35-day-old) with similar body weights were obtained from a local rabbitry. They were housed in a coccidia-free environment with free access to water and in-house feed free of anticoccidial drugs. Feed and water were heated at 80°C for 2–3 h to kill any coccidia oocysts. The rabbits were confirmed as coccidia-free if there were no coccidian oocysts after three fecal examinations once every other day. Coccidia-free rabbits were selected for the experiments until approximately 45-day-old. Experimental rabbits were subjected to a bivalent vaccine against rabbit hemorrhagic disease virus (RHDV) and Pasteralla multocida at 37 and 62 days of age.
2.1.3. Drugs
Decoquinate was purchased from Hubei Yunmei Technology Co., Ltd. (Wuhan, Hubei province, China), diclazuril from Hebei Pude Animal Pharmaceutical Co., Ltd. (Luquan, Shijiazhuang, Hebei province, China), and sulfachloropyrazine sodium from Hebei Zhongbei Jiamei Biotechnology Co., Ltd. (Zanhuang, Shijiazhuang, Hebei province, China). Xylazine Hydrochloride Injection from Dunhua Shengda Animal Pharmaceutical Co., Ltd. (Dunhua, Jilin province, China).
2.2. Experimental methods
2.2.1. Pathogenicity and immunogenicity of E. kongi
Thirty coccidia-free rabbits of 45-day-old were randomly assigned to 6 groups of 5 rabbits each. They were inoculated with 1 × 102 (group I), 1 × 103 (group II), 1 × 104 (group III), and 5 × 104 (group IV) sporulated oocysts of E. kongi, respectively, as the immunized groups. One group (Group V) was unimmunized and challenge control (UCC) group, and another group (Group VI) was unimmunized and unchallenged control (UUC) group. Fourteen days post inoculation (DPI), all rabbits of Groups I–V were orally challenged with 1 × 104 sporulated oocysts.
Water intake and feed consumption was recorded and feces was examined daily. The rabbits were weighed every 7 days within 14 DPI. Feces were collected and weighed 7–14 days post inoculation and 7 to 14 days post challenge (DPC), oocysts per gram of feces was measured by the McMaster method, and the total oocyst output of each group was calculated.
McMaster method: Two grams of feces were soaked for 2–5 min in 10 ml of water in mortar, the fecal slurry was then mixed with 50 ml of saturated sodium chloride solution and homogenized using a glass rod, and filtered through a metal sieve. Following agitation, the sample was drawn from the suspension using a pipette, and introduced into two chambers of a McMaster oocysts counting slide which was then placed on the stage of a compound light microscope. After 3 min, the number of oocysts in 1 cm2 of the chamber was counted, and the average of the two chambers was calculated and then multiplied by 200 to obtain the number of OPG.
2.2.2. Endogenous development of E. kongi
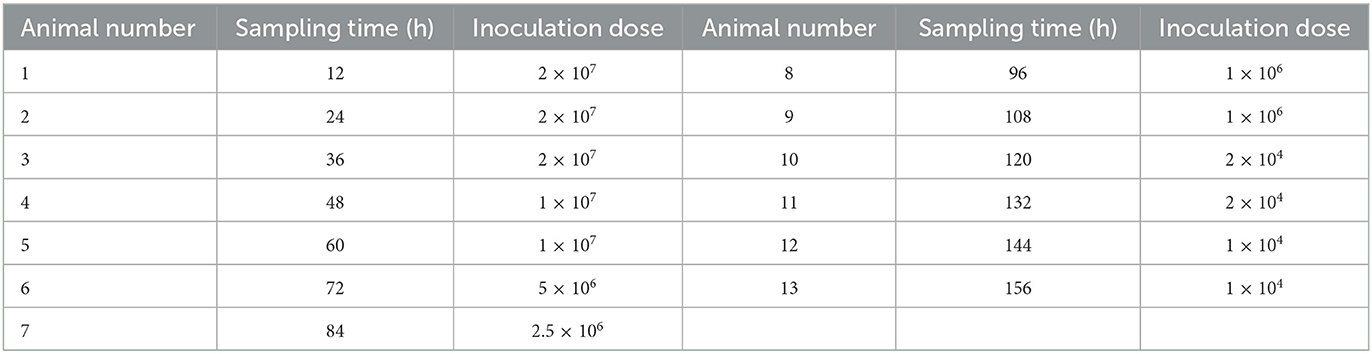
To study the development of E. kongi, 39 coccidia-free rabbits of 45-day-old were inoculated with E. kongi oocysts at doses from 1 × 104 to 2 × 107, which was selected on the basis of the pathogenicity experiment, and then sacrificed at various time points for the observation of development stages of E. kongi (Table 1).
Three rabbits at each time point after inoculation was euthanized by Xylazine Hydrochloride Injection (1 ml/kg body weight) into the ear vein. Duodenum, jejunum, ileum, cecum, colon, and rectum were collected aseptically and fixed in 10% formalin for 48 h, respectively. The collected tissues were then dehydrated, embedded in paraffin, and sectioned. The tissue sections were stained with hematoxylin and eosin (HE) and observed under a light microscope. Size of at least 30 schizonts were measured. 30 microscope fields were observed in each generation, and the number of type A schizont and type B schizont in each field was recorded, and the ratio of type A schizont to type B schizont was calculated.
2.2.3. Drug sensitivity of E. kongi
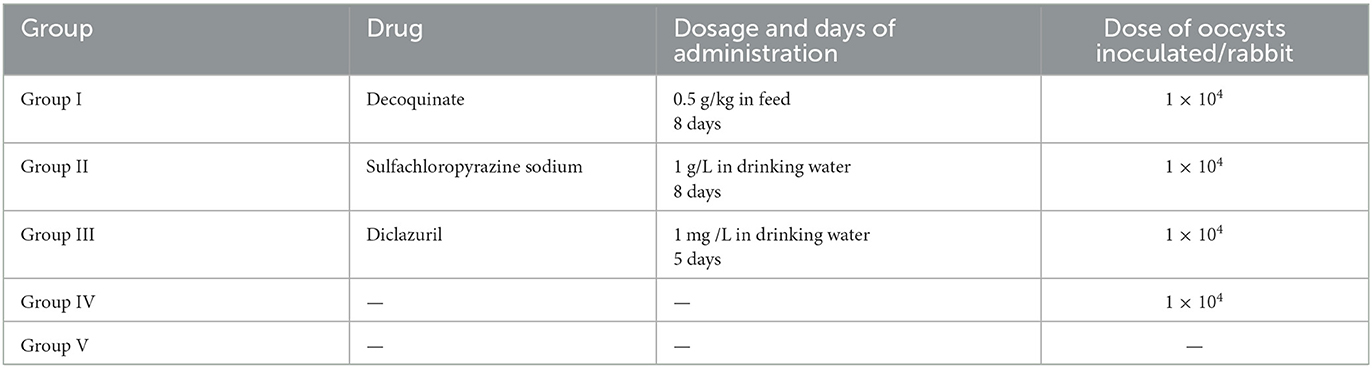
Fourty rabbits of 45-day-old coccidia-free were divided into 5 groups with 8 rabbits per group. Group I was decoquinate treatment group, group II was sulfachloropyrazine sodium treatment group, group III was diclazuril treatment group, group IV was inoculated and untreated control group, and group V was uninoculated and untreated control group. Each rabbit in groups I–IV was inoculated with 1 × 10 4 oocysts. Three drugs were given separately the day before inoculation, decoquinate at a dose of 0.5 g/kg in feed and sulfachloropyrazine sodium at a dose of 1 g/L in drinking water were given for 8 consecutive days, and diclazuril was administered at a dose of 1 mg /L in drinking water for 5 consecutive days (Table 2). The animals were observed daily for food intake, water consumption and clinical signs, and body weight was measured every 7 days within 14 days post inoculation (DPI). Feces was collected daily and oocysts in feces were enumerated using the McMaster method from 7 to 14 days post inoculation (DPI).
2.3. Data analysis
The SPSS19.0 software was used for statistical analysis by analysis of variance and student's t-test. * indicates P < 0.05, ** indicates P < 0.01, without a right superscript asterisk, indicates P > 0.05.
3. Results
3.1. Pathogenicity and immunogenicity of E. kongi
3.1.1. Clinical signs
After inoculation oocysts of E. kongi, rabbits exhibited a decrease in appetite and had soft feces. These clinical signs appeared on the 3rd day after inoculation of 5 × 104 oocysts, and on days 4–7 after the 1 × 102, 1 × 103 or 1 × 104 inoculation dose. The rabbits in the highest dose group had very little food and were lethargic, and all had different degrees of diarrhea, and one rabbit had alternating episodes of constipation and diarrhea and died on day 8. In the 1 × 104 group, 60% of the rabbits had diarrhea and in the 1 × 103 group 20% had diarrhea. No diarrhea was seen in the lowest inoculation dose group (1 × 102). On days 8–9, the rabbits in the 1 × 102 and 1 × 103 groups returned to normal, and rabbits of all groups appeared normal by day 12.
All groups except for the UUC group were challenged with 1 × 104 oocysts 14 days after the inoculation. After the challenge, the rabbits of the 1 × 102 − 1 × 104 immunized groups developed milder clinical signs. The animals in the 1 × 102 and 1 × 103 immunized groups exhibited decreased appetite, while the rabbits in the 1 × 104 immunization group did not consume any feed and were of poor condition on day 4, but none of the animals in these groups had soft feces or diarrhea. The highest immunization dose group (5 × 104) consumed no feed on day 4 and 50% of them developed soft feces, and 25% had diarrhea. Starting from day 7, conditions of all rabbits of immunized challenged groups began to improve, and they all appeared normal by day 12. Clinical signs of the UCC group were similar to those observed after the immunization dose of 1 × 104, with decreased feed intake and soft feces on day 4, and diarrhea in 60% rabbits on day 5–6. All rabbits of the UCC group appeared normal 12 days after challenge.
3.1.2. Changes in body weight gain and oocyst output
At 14 days post inoculation (DPI), the average daily weight gain of the rabbits in each immunized group was significantly different from that of the rabbits in the UUC group (P < 0.01) (Figure 1A), body weight gain with increasing inoculation doses was reduced by 21.71, 27.40, 44.84, and 65.84%, respectively.
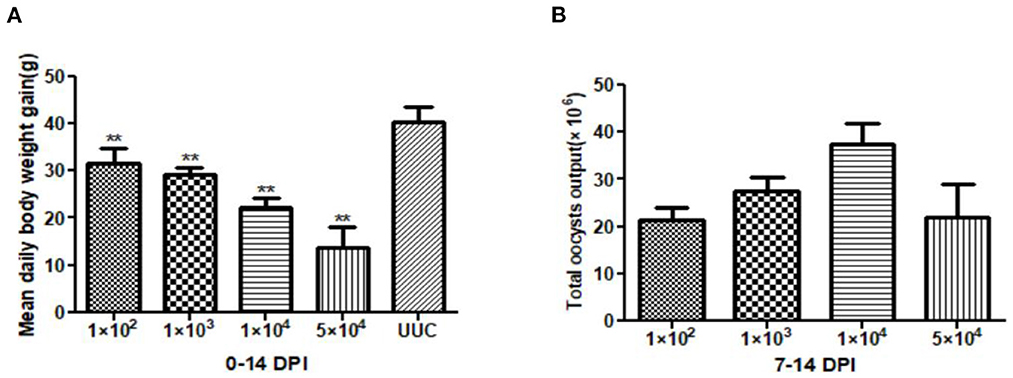
Figure 1. Average daily weight gain of rabbits and total oocyst output in pathogenicity tests. (A) Average daily weight gain of rabbits 0–14 days after initial oocyst inoculation. (B) Total oocyst output 7–14 days after inoculation with different doses of sporulated E. kongi oocysts. DPI: day post initial inoculation. **P < 0.01 (Student's t-test).
The total oocyst output after immunization ranged from 2.13 × 107 to 3.74 × 107, with the 1 × 104 dose group having the highest oocyst output and the 1 × 102 dose group the lowest oocyst output (Figure 1B). Interestingly, total oocyst output of the highest inoculation dose (5 × 104) was similar to the output of the 1 × 102 dose group.
The average daily weight gain of the rabbits in the 1 × 102, 1 × 103, and 1 × 104 immunized and challenged groups at 14 days post challenge (DPC) was not significantly different from that of rabbits in the UUC group (P > 0.05). The average daily weight gain of rabbits in the 5 × 104 immunized challenge group was significantly lower than that of the UUC group (P < 0.01) (Figure 2A).
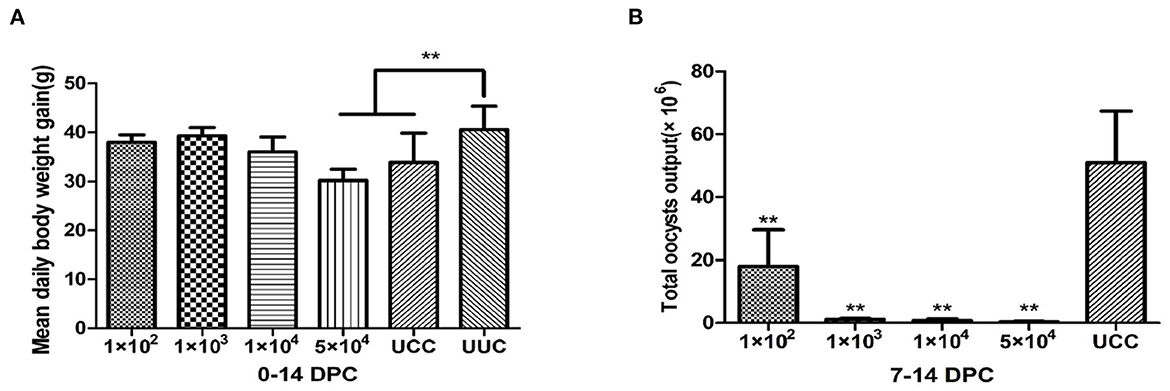
Figure 2. Average daily weight gain of rabbits and total oocyst output in immunogenicity tests. (A) Average daily weight gain of rabbits 0–14 days after challenge. (B) Total oocyst output 7–14 days after challenge with 1 × 104 sporulated oocysts. DPC, day post challenge; UCC, unimmunized, challenged control group; UUC, unimmunized, unchallenged control group. **P < 0.01 (Student's t-test).
The total oocyst output of rabbits in all immunized and challenged groups was significantly lower than that of the UCC (P < 0.01) (Figure 2B). Among the immunized groups, the rabbits in the 1 × 102 immunization dose group had the highest oocyst output (1.8 × 107 oocysts), but the output was still 64.6% lower than that of the UCC group. The oocyst output of the other 3 immunized and challenged groups decreased by 97.7, 98.5, and 99.2% in the 1 × 103, 1 × 104 and 5 × 104 groups, respectively, compared with the UCC group.
3.2. Endogenous development of E. kongi
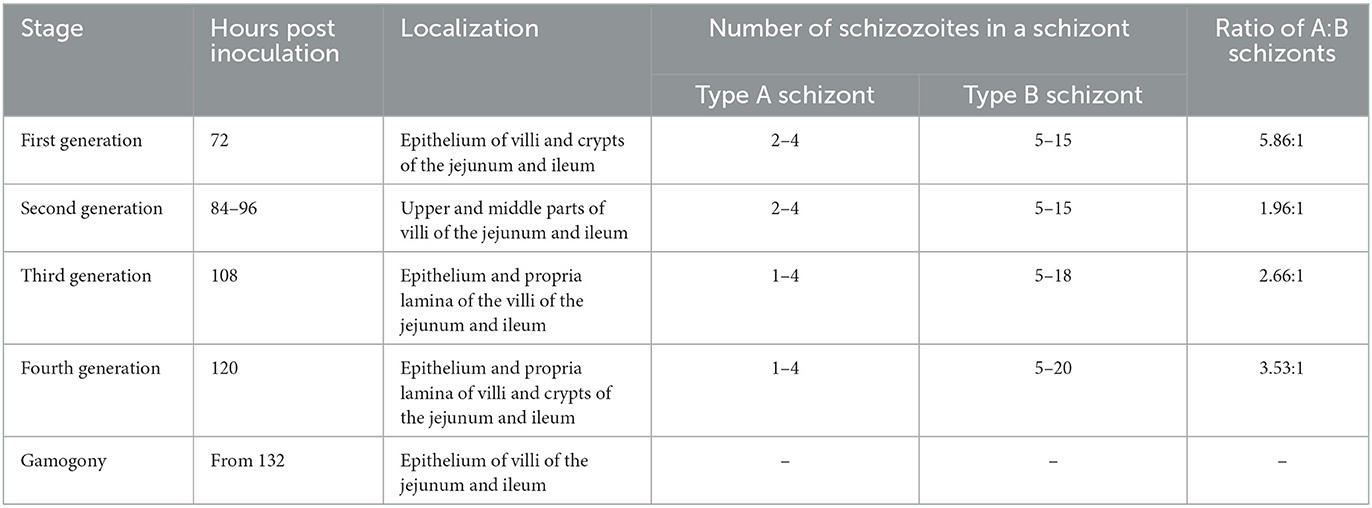
The tissue sections of intestines of rabbit infected with parasites were observated by light microscope to study endogenous development of E. kongi. Development stage was observed within a parasitophorous vacuole mainly in the epithelium of villi of jejunum and ileum. Four generations schizonts and one gametogony were observed, with two types of schizonts in each generation, namely type A schizont and type B schizont, both of which were ellipsoid. Type A schizont formed short and round, stubby schizozoites, while type B schizont gave rise to slender schizozoites.
The first generation schizonts were first seen 72 h post inoculation (p.i.) in the epithelium of villi and crypts of jejunum and ileum. The type A schizonts measured 6.42 (4.43–8.81) × 5.29 (3.41–6.99) μm, with 2–4 schizozoites (Figure 3A), whereas the type B schizonts measured on average 9.63 (7.86–10.92) × 8.25 (6.97–9.27) μm and gave rise to 5~15 slender schizozoites (Figure 3B). The ratio of the number of A to B schizonts was 5.86:1 (Table 3).
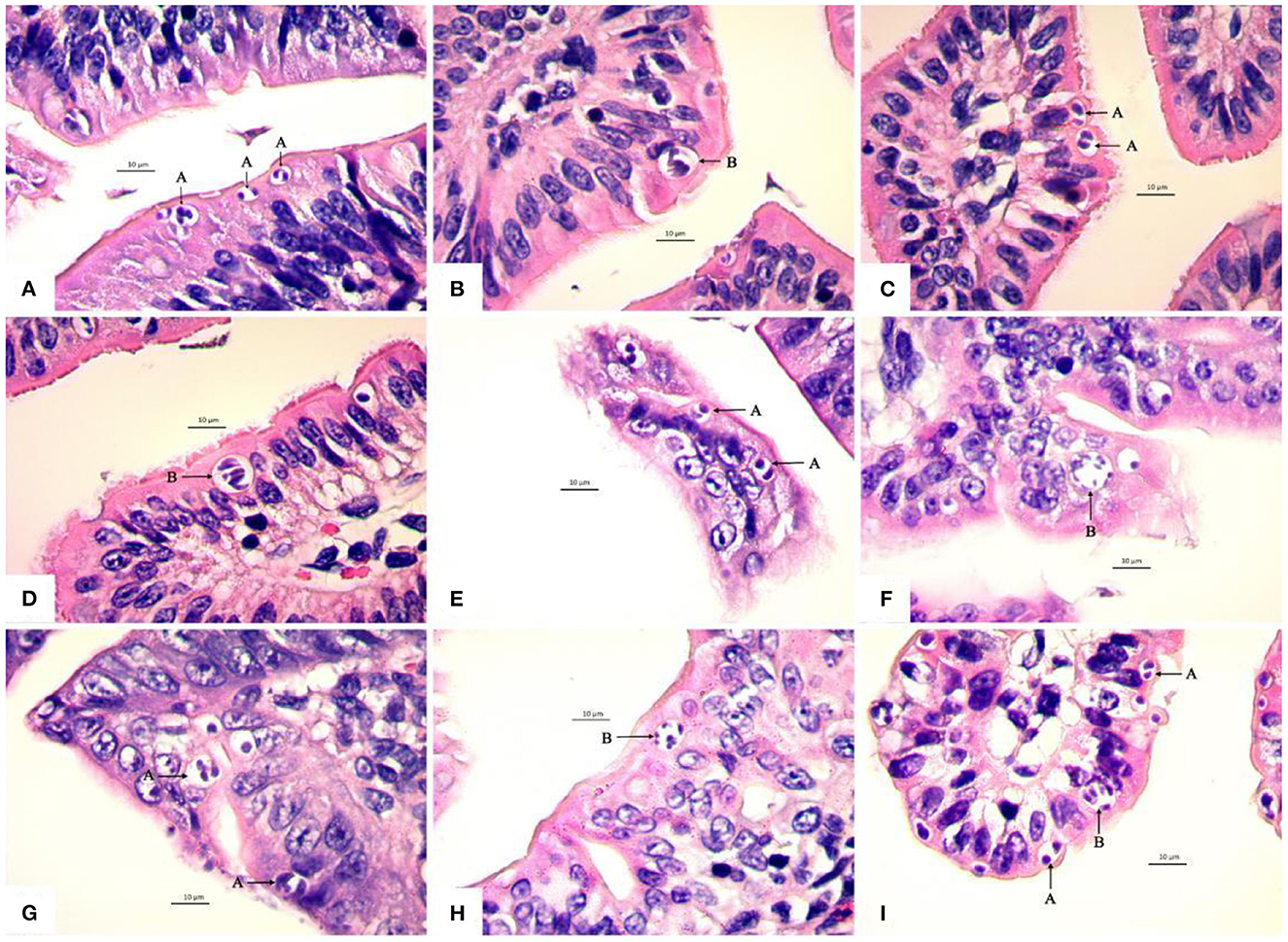
Figure 3. Light micrographs showing four schizogonies of E. kongi in the jejunum and ileum of experimentally infected rabbits (×1,000). Scale bar = 10 μm. (A) The type A schizonts (A) of the first generation at 72 h p.i. (B) The type B schizont (B) of the first generation at 72 h p.i. (C) The type A schizonts (A) of the second generation at 84 h p.i. (D) The type B schizont (B) of the second generation at 84 h p.i. (E) The type A schizonts (A) of the second generation at 96 h p.i. (F) The type B schizont (B) of the second generation at 96 h p.i. (G) The type A schizont (A) of the third generation at 108 h p.i. (H) The type B schizont (B) of the third generation at 108 h p.i. (I) The type A schizonts (A) and type B schizont (B) of the fourth generation at 120 h p.i.
The second generation schizonts occurred in the upper and middle parts of jejunum and ileum villi and were seen 84–96 h p.i. The type A schizont measured 6.77 (4.23–10.29) × 5.43 (3.44–7.12) μm and gave rise to 2–4 schizozoites (Figures 3C, E), whereas type B schizonts measured 9.91 (7.61–14.24) × 8.11 (5.94–12.15) μm and were estimated to produce 5–15 elongated schizozoites (Figures 3D, F). The ratio of A:B schizonts was 1.96:1 (Table 3).
The third generation of schizogony was observed 108 h p.i., mainly developing in epithelium and propria lamina of the villi of the jejunum and ileum. The type A schizont measured 7.11 (4.93–9.82) × 5.64 (3.37–8.39) μm and harbored 1–4 schizozoites (Figure 3G), whereas type B schizonts measured 9.72 (7.96–11.98) × 7.73 (6.27–9.71) μm and contained 5–18 schizozoites (Figure 3H). The ratio of A:B schizonts was 2.66:1 (Table 3).
The fourth generation schizonts were noted 120 h p.i. and they developed in the epithelium and propria lamina of villi and crypts of jejunum and ileum. The type A schizont measured 7.16 (3.86–11.44) × 5.63 (3.36–9.56) μm had 1–4 schizozoites (Figure 3I), whereas the type B schizont measured 9.47 (7.27–11.36) × 8.20 (6.11–10.89) μm and contained 5–20 schizozoites (Figure 3I). The ratio of A:B schizonts was 3.53:1 (Table 3).
At 132 h p.i., E. kongi entered gametogony. Additional to schizonts, macrogamonts and microgamonts were observed in epithelium of villi of the jejunum and ileum.
The average size of macrogamonts was 17.48 × 16.31 μm (Figure 4A), and microgamonts 11.56 × 10.62 μm, both of which wre spherical or subspherical (Figure 4A). The protoplasm of microgamonts was darker and more concentrated than that of macrogamonts, forming a dark purple protoplasm, whereas macrogamonts were distinguishable from schizonts and microgamonts by their large central nucleus with a prominent nucleolus and many basophilic wall forming bodies at their periphery along the membrane.
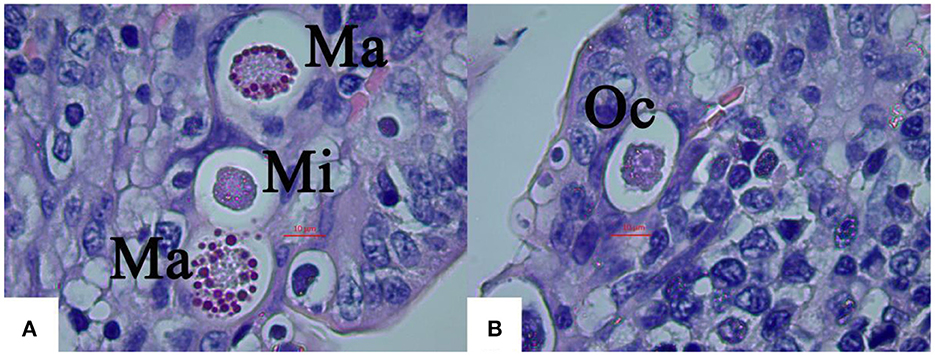
Figure 4. Light micrographs of gametogony of E. kongi experimentally infected rabbits (×1,000). Scale bar = 20 μm. (A) The microgamonts (Mi) and macrogamonts (Ma) of E. kongi in epithelial cells of ileal villi of rabbit at 132 h p.i. (B) Developing oocysts (Oc) at 144 h p.i.
Numerous oocysts at different stages of maturation could be distinguished at 144 h p.i, which was slightly ellipsoid (Figure 4B). Fully formed oocysts occurred in the feces of the infected rabbits 6 DPI.
3.3. Drug sensitivity of E. kongi
E. kongi-inoculated rabbits treated with decoquinate or sulfachloropyrazine sodium gained significantly more weight than the inoculated and untreated rabbits 0–14 DPI (P < 0.01), and the body weight gain of the two treated groups was comparable with that of the uninoculated and untreated control group (P > 0.05) (Figure 5A). The decoquinate and sulfachloropyrazine sodium-treated rabbits also excreted significantly less oocysts than the inoculated and untreated control group rabbits 7–14 DPI (P < 0.01), and the two drugs inhibited oocyst output by 80.47 and 99.97%, respectively (Figure 5B).
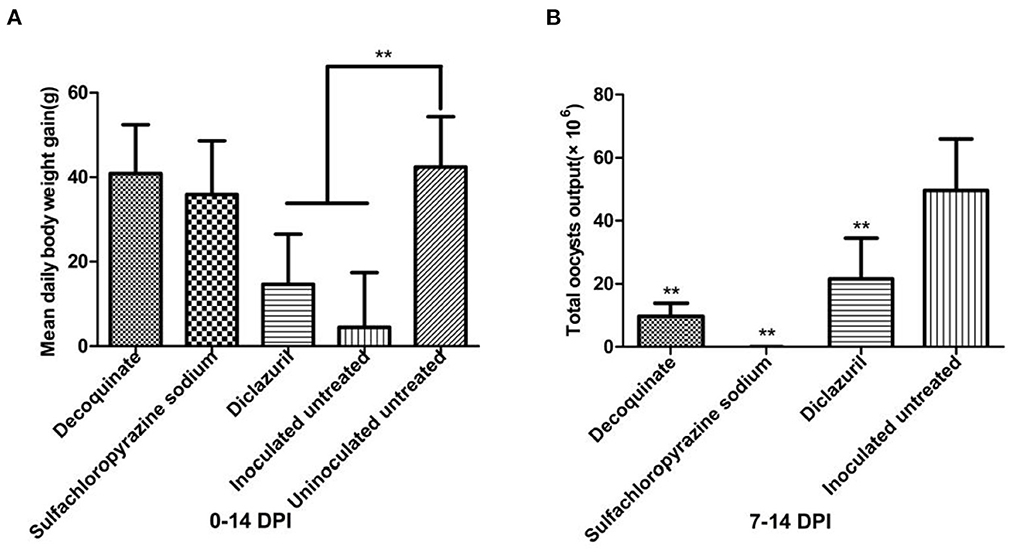
Figure 5. Comparison of mean daily body weight gain and total oocysts output in different groups. in drug sensitivity experiment of E. kongi. (A) Average daily weight gain. (B) Total oocyst output. DPI, day post inoculation. **P < 0.01 (Student's t-test).
The daily body weight gain of diclazuril-treated rabbits was significantly lower than that of the inoculated and untreated rabbits control group 0–14 DPI (P < 0.01) (Figure 5A). Diclazuril inhibited oocyst output by 56.61%, the total oocyst output of diclazuril-treated rabbits was significantly less than that of the inoculated and untreated group rabbits 7–14 DPI (P < 0.01) (Figure 5B). The diclazuril-treated rabbits and the inoculated and untreated group exhibited decreased appetite and had diarrhea, whereas the rabbits in the other experimental groups appeared normal.
4. Discussion
4.1. Pathogenicity and immunogenicity
The pathogenicity and immunogenicity of rabbit coccidia were evaluated based on clinical signs, weight gain and oocyst output (9–11). Zhangjiakou strain of E. kongi (E. kongi-ZJK) was determined to be moderately pathogenic according to the pathogenicity assessment criteria that rabbits caused by moderately pathogenic coccidia species showed symptoms of depression of growth, some cases of diarrhoea, mortality depending on the doses (12). In this study, except for rabbits in the 1 × 102 infection group, rabbits in the other infection dose groups had different degrees of diarrhea and impaired weight gain. Diarrhea and decreased body weight gain were dose-dependent, but the total oocyst output was not linearly related to the inoculation dose. The total oocyst output of rabbits in the 1 × 104 dose group was the highest, higher than that of the highest inoculation dose group (5 × 104), which was most likely due to the crowding effect of coccidia, where fecundity decreases with increasing infective dose (13, 14). Pathogenicity can vary with Eimeria species and strains (12, 15). The pathogenicity of E. kongi is similar to that of E. media, E. magna, E. irresidua, E. piriformis, i.e., they have moderate pathogenicity.
In the highest dose group (5 × 104), one rabbit died from coccidiosis 8 DPI, which was found to have lesions both the jejunum and ileum, but no lesions in other parts. The intestinal serosa was obviously hyperemia. The intestinal lumen was significantly expanded, and intestinal contents was thin, and a lot of mucus-like substance and the button-like necrosis were existed. The microscopy of scraping from the intestinal mucosa showed a large number of oocysts.
Weight gain and oocyst output after challenge infection as a criterion of immunogenicity be considered. Coudert et al. (9) showed that E. intestinalis is strongly immunogenic, In contrast, E. flavescens and E. piriformis (Coudert, pers. comm.) are weakly immunogenic. Other species, such as E. irresidua, E. media, E. magna could be considered as middle immunogenic (16). In this study, the immunization dose of 1 × 102, 1 × 103 and 1 × 104 were most effective based on body weight gain, which was comparable with the weight gain of UUC group. Body weight gain of the 5 × 104 immunized group was significantly lower than that of the UUC group, which may be related to the serious damage to intestinal tissues caused by the large immunization dose, proliferation of intestinal pathogenic bacteria, and synergistic effects of bacteria and coccidia oocysts of challenge. However, the highest immunization dose (5 × 104), as well as the 1 × 103 and 1 × 104 immunization doses, almost completely suppressed oocyst excretion in feces, whereas the lowest immunization dose (1 × 102) reduced oocyst output only by 65%. Based on body weight gain and oocyst output, E. kongi confers good immunogenicity, and an inoculation dose of 1 × 103 – 1 × 104 would provide the most effective protection from coccidia infection.
4.2. Endogenous development
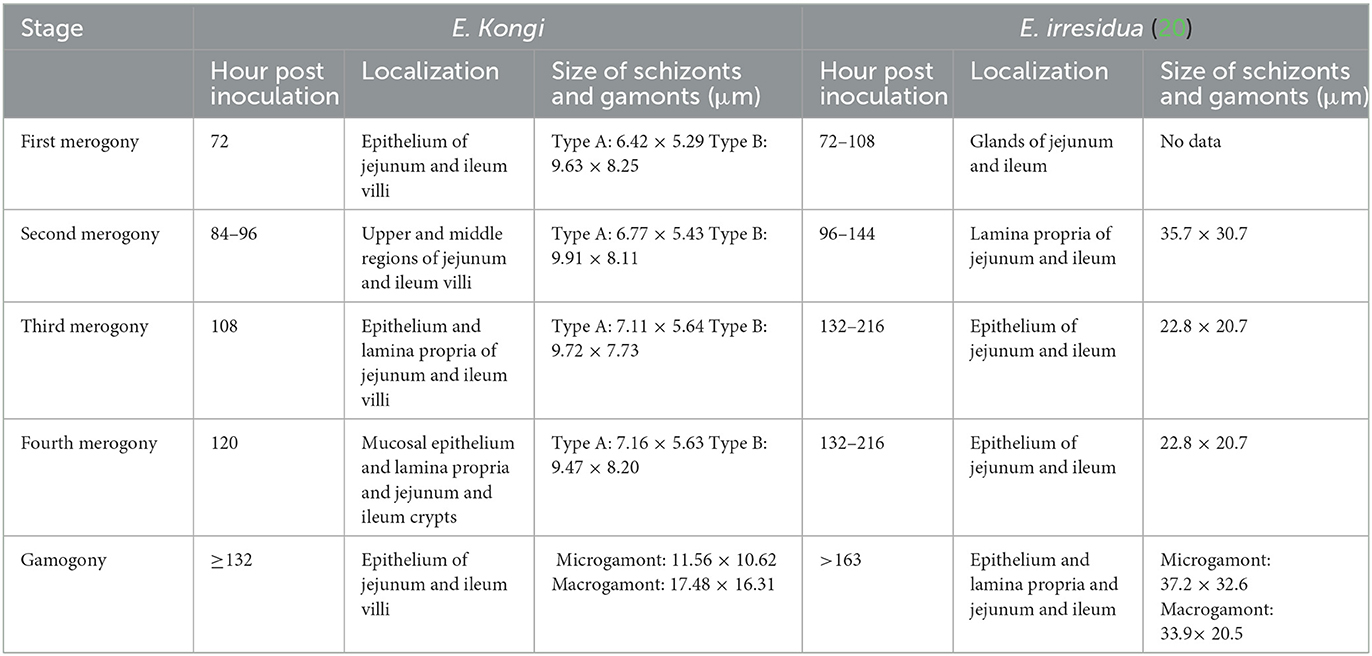
Schizogony are generally classified to generations based on the localization and distribution of coccidian schizonts, the time when a large number of mature schizonts emerge, the size and number of schizozoites, and the ratio of A: B schizonts. Pakandl et al. (17). In this study, schizogony of E. kongi could be divided into 4 generations mainly based on the localization, distribution of coccidian schizonts and the ratio of A: B schizonts (Table 3). E. intestinalis, E. magna, E. media and E. irresidua are parasitic in the jejunum and ileum (18–21). Our research suggests that the endogenous development of E. kongi proceeds in the jejunum and ileum of rabbits.
For E. kongi, there was no difference in the size of type A schizont and type B schizont between different generations, nor in the number of schizozoites in type B schizont (Tables 3, 4). The number of type A schizonts was more than that of type B schizonts; the mean ratio of type A to type B schizonts ranged from 1.86 to 5.86. As the localization of schizont appearance and the ratio of type A schizont to type B schizont were consistent at 84 h p.i. and 96 h p.i., the second asexual generation was considered at 84–96 h p.i.
Although E. kongi resembles E. irresidua in shape and structure, they differ significantly in size and shape index of oocyst and prepatent time (8). By comparison, both E. kongi and E. irresidua have four generations of schizogony, but there are significant differences in the location of asexual generation and gamogony, as well as the size of schizonts and gametophytes (Table 4).
Four generations of schizonts have also been reported for other coccidia species, such as E. flavescens by light microscopic examination of HE stained tissue sections Wang et al. (22), and E. magna by light and electron microscopic examination Pakandl et al. (19). However, 5 generations of schizogony of the same coccidia species, E. flavescens (17, 22) and E. magna were reported by Pakandl et al. (19) by electron or light microscopy. The classification of different number of schizont generations for the same species coccidia could be due to different study methods.
The endogenous development and generation division of E. kongi could be confirmed by transmission electron microscopy, which would also be able to show whether type A schizonts and type B schizonts contain polynucleate schizozoites and uninucleate schizozoites, respectively (16, 17, 19, 23, 24).
The endogenous development and division of asexual generations of E. kongi will be further determine by transmission electron microscopy, and whether type A schizonts and type B schizonts contain polynucleate and uninucleate schizozoites, respectively.
4.3. Drug sensitivity
Many factors may affect the sensitivity of parasites to drug treatment. Previous exposures to other drugs and long-term use of the same drug could induce drug resistance (25, 26). Coccidia in different regions or locations can also have different susceptibily to drug treatment (27, 28). Decoquinate has been demonstrated in in-house and university studies to be an active coccidiostat when fed daily at 0.5 mg/kg body weight during periods of oocyst exposure. In our study, treatment of E. kongi with 0.5 g/kg of decoquinate in feed was effective.
In a previous study in artificially infected rabbits (29), diclazuril was shown to be highly effective against E. intestinalis, E. magna and E. perforans in semi-field conditions (30). Diclazuril was also optimally effective at 1 ppm in the feed in the prevention and cure of intestinal and hepatic coccidiosis in rabbit (29). The superior efficacy of curative use of diclazuril and sulphachloropyrazine against rabbit coccidiosis was reported (31). In this study, we observed that E. kongi was resistant to the recommended clinical dose of diclazuril, and the rabbits experienced severe growth inhibition and oocyst discharge after treatment. Sulfachloropyrazine sodium was the most effective drug of 3 anticoccidial drugs tested in this study. Although both decoquinate and sulfachloropyrazine sodium effectively inhibited the reproduction of E. kongi, sulfachloropyrazine sodium inhibited oocyst output in feces by 99.97% compared with 80.47% inhibition by decoquinate. Infected rabbits treated with both drugs maintained normal growth performance. Both decoquinate and sulfachloropyrazine sodium were effective in the control of E. kongi infection.
5. Conclusion
E. kongi was moderately pathogenic and induced good immunity against re-infection. Four generations of schizogony were observed, and the endogenous development mainly occurred in the jejunum and ileum of rabbits. Eimeria kongi was most sensitive to sulfachloropyrazine sodium, followed by decoquinate; it is resistant to diclazuril. Both decoquinate and sulfachloropyrazine sodium were effective in the control of Eimeria kongi infection.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the specialized Ethics Committee of Hebei North University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
PC and XS conceived and designed the experiments. SF, YS, and PW performed the study of pathogenicity, immunogenicity, and endogenous development. CG and XG tested the sensitivity of Eimeria kongi to three drugs. LG collected and analyzed data. SF and YS wrote the first manuscript. XS critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by the Natural Science Foundation of Hebei Province and Key Basic Research Project (grant number C2019405052) and Key Project of Science and Technology Research in colleges and universities of Hebei Province (grant number ZD2018032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dubey P, Bauer C. A review of Eimeria infections in horses and other equids. Vet Parasitol. (2018) 256:58–70. doi: 10.1016/j.vetpar.2018.04.010
2. Dvm BB, Katherine MS. Ruminant coccidiosis. Vet Clin North Am Food Anim Pract. (2020) 36:187–203. doi: 10.1016/j.cvfa.2019.12.006
3. Joyner LP, Long PL. The specific characters of the Eimeria, with special reference to the coccidia of the fowl. Avian Pathol. (1974) 3:145–57. doi: 10.1080/03079457409353827
4. Daugschies A, Najdrowski M. Eimeriosis in cattle: current understanding. J Vet Med B Infect Dis Vet Public Health. (2005) 52:417–27. doi: 10.1111/j.1439-0450.2005.00894.x
5. Coudert P, Licois D, Drouet-Viard F. “Eimeria species and strains of rabbits,” in: J. Eckert, R. Braun, M.W. Shirley, P. Coudert, eds, Biotechnology:guidelines on techniques in coccidiosis research Luxembourg: European Commission (1995). p. 52.
6. Yan W, Wang W, Wang T, Suo X, Qian W, Wang S. et al. Simultaneous identification of three highly pathogenic Eimeria species in rabbits using a multiplex PCR diagnostic assay based on ITS1–58S rRNA-ITS2 fragments. Vet Parasitol. (2013) 193:284–8. doi: 10.1016/j.vetpar.2012.11.013
7. Cui P, Fang S, Gu X, Guo B. Investigation on rabbit coccidial species and infection rate in areas of Hebei province. Acta Agric Boreali-Occidentalis Sinica. (2010) 19:21–4. doi: 10.3724/spj.1142.2010.40491
8. Cui P, Liu H, Fang S, Gu X. Wang P, Liu C, et al. A new species of Eimeria (Apicomplexa: Eimeriidae) from Californian rabbits in Hebei Province, China. Parasitol Int. (2017) 66:677–80. doi: 10.1016/j.parint.2017.06.009
9. Coudert P, Licois D, Provot F, Drouet-Viard F. Eimeria sp. from the rabbit (Oryctolagus cuniculus): pathogenicity and immunogenicity of Eimeria intestinalis. Parasitol Res. (1993) 79:186–90. doi: 10.1007/BF00931890
10. Shi T, Bao G, Fu Y. SuoX, Hao L. A low-virulence Eimeria intestinalis isolate from rabbit (Oryctolagus cuniculus) in China: molecular identification, pathogenicity, and immunogenicity. Parasitol Res. (2014) 113:1085–90. doi: 10.1007/s00436-013-3744-1
11. Tao G, Wang Y, Li C, Gu X, Cui P, Fang S, et al. High pathogenicity and strong immunogenicity of a Chinese isolate of Eimeria magna Pérard, 1925. Parasitol Int. (2017) 66:207–9. doi: 10.1016/j.parint.2017.01.014
12. Coudert P, Licois D, Provot F, Drouet-Viard F. “Pathogenicity of different strains of rabbit coccidian,” in J. Eckert, R. Braun, M.W. Shirley, P. Coudert, eds, Biotechnology:guidelines on techniques in coccidiosis research. Luxembourg:European Commission (1995). p. 66.
13. Williams RB. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J Parasitol. (2001) 31:1056–69. doi: 10.1016/S0020-7519(01)00235-1
14. Johnston WT, Shirley MW, Smith AL, Gravenor MB. Modelling host cell availability and the crowding effect in Eimeria infections. Int J Parasitol. (2001) 31:1070–81. doi: 10.1016/S0020-7519(01)00234-X
15. Abu-Akkada SS. Awad AM. Isolation, propagation, identification and comparative pathogenicity of five Egyptian field strains of Eimeria tenella from broiler chickens in five different provinces in Egypt. Res Vet Sci. (2012) 92:92–5. doi: 10.1016/j.rvsc.2010.10.023
16. Pakandl M. Coccidia of rabbit: a review. Folia Parasitol. (2009) 56:153–66. doi: 10.14411/fp.2009.019
17. Pakandl M, Cernik F, Coudert P. The rabbit Coccidium Eimeria flavescens Marotel and Guilhon, 1941: an electron microscopic study of its life cycle. Parasitol Res. (2003) 91:304–11. doi: 10.1007/s00436-003-0946-y
18. Licois D, Coudert P, Bahagia S, Rossi GL. Endogenous development of Eimeria intestinalis in rabbits (Oryctolagus cuniculus). J Parasitol. (1992) 78:1041–8. doi: 10.2307/3283227
19. Pakandl M, Ahmed NE, Licois D, Coudert P. Eimeria magna Perard, 1925: study of the endogenous development of parental and precocious strains. Vet Parasitol. (1996) 65:213–222. doi: 10.1016/S0304-4017(96)00975-2
20. Pakandl M, Gaca K, Licois D, Coudert P. Eimeria media Kessel 1929: comparative study of endogenous development between precocious and parental strains. Vet Res. (1996) 27:465–72.
21. Norton CC, Catchpole J, Joyner LP. Redescriptions of Eimeria irresidua Kessel and Jankiewicz, 1931 and E. flavescens Marotel and Guilhon, 1941 from the Domestic Rabbit. Parasitology. (1979) 79:231–248. doi: 10.1017/S0031182000053312
22. Wang Y, Zhao J, Wang Q, Zhang L, Ning C, Lin K. Endogenous development of Eimeria flavescens in domestic rabbit. Chin J Zool. (2010) 45:91–7. doi: 10.1515/joc.2010.31.2.75
23. Pakandl M, Coudert P. Life cycle of Eimeria vejdovskyi Pakandl, 1988: electron microscopy study. Parasitol Res. (1999) 85:850–4. doi: 10.1007/s004360050644
24. Elfayoumi H, Abdel-Haleem HM. Electron microscopic study of the developmental stages of Eimeria intestinalis cheissin, 1948 in domestic rabbit (Oryctolagus Cuniculus) with reference to endodyogeny. J Egypt Soc Parasitol. (2014) 44:531–8. doi: 10.12816/0007857
25. Peeters JE, Geeroms R, Varewyck H, Bouquet Y, Lampo P, Halen P. Immunity and effect of clopidol/methyl benzoquate and robenidine before and after weaning on rabbit coccidiosis in the field. Res Vet Sci. (1983) 35:211–6. doi: 10.1016/S0034-5288(18)32181-7
26. Peeters JE, Geeroms R. Efficacy of diclazuril against robenidine resistant Eimeria magna in rabbits. Vet Rec. (1989) 124:589–90. doi: 10.1136/vr.124.22.589
27. Cam Y, Atasever A, Eraslan G, Kibar M, Atalay O, Beyaz L, et al. Eimeria stiedae: Experimental infection in rabbits and the effect of treatment with toltrazuril and ivermectin. Exp Parasitol. (2008) 119:164–72. doi: 10.1016/j.exppara.2008.01.005
28. Pakandl M. Efficacy of salinomycin, monensin and lasalocid against spontaneous Eimeria infection in rabbits. Folia Parasitol. (1986) 33:195–8. doi: 10.1016/S0065-308X(08)60345-0
29. Vanparijs O, Hermans L, Van-Der FL, Marsboom R. Efficacy of diclazuril in the prevention and cure of intestinal and hepatic coccidiosis in rabbits. Vet Parasitol. (1989) 32:109–17. doi: 10.1016/0304-4017(89)90111-8
30. Vanparijs O, Desplenter L, Marsboom R. Efficacy of diclazuril in the control of intestinal coccidiosis in rabbits. Vet Parasitol. (1989) 34:185–90. doi: 10.1016/0304-4017(89)90049-6
Keywords: Eimeria kongi, pathogenicity, immunogenicity, endogenous development, drug sensitivity
Citation: Fang S, Shi Y, Wang P, Guan C, Gu X, Guan L, Cui P and Suo X (2023) Study on the pathogenicity, immunogenicity, endogenous development and drug sensitivity of Eimeria kongi. Front. Vet. Sci. 10:1134193. doi: 10.3389/fvets.2023.1134193
Received: 30 December 2022; Accepted: 06 February 2023;
Published: 06 March 2023.
Edited by:
Charoonluk Jirapattharasate, Mahidol University, ThailandReviewed by:
Mingmin Lu, Nanjing Agricultural University, ChinaSivapong Sungpradit, Mahidol University, Thailand
Copyright © 2023 Fang, Shi, Wang, Guan, Gu, Guan, Cui and Suo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Cui, ZGt5anNjQDEyNi5jb20=; Xun Suo, c3VveHVuQGNhdS5lZHUuY24=
 Sufang Fang1
Sufang Fang1 Ping Cui
Ping Cui Xun Suo
Xun Suo