- 1Department of Small Animal Medicine and Surgery, University of Veterinary Medicine, Hannover, Germany
- 2Centre for Systems Neuroscience, University of Veterinary Medicine Hannover, Hannover, Germany
- 3Hannover Medical School, Institute for Laboratory Animal Science, Hannover, Germany
- 4Department of Clinical Science and Services, Royal Veterinary College, Hatfield, United Kingdom
Behavioral problems are highly prevalent in domestic dogs, negatively affecting the quality of life of dogs and their owners. In humans and dogs, neuropsychological or neurobehavioral disorders can be associated with deviations in various neurotransmitter systems. Previous evidence has revealed correlations between urinary neurotransmitters and various behavioral disorders; however, a causal relationship has not yet been conclusively demonstrated. Non-invasive urinary neurotransmitter analysis may identify specific biomarkers, which enable a more differentiated assessment of canine behavioral disorders in the future and contribute to more effective neuromodulatory treatment decisions and monitoring. This approach could offer new insights into underlying pathomechanisms of canine neurobehavioral disorders. This study assessed urinary neurotransmitter levels and the descriptive behavior profile of 100 dogs using established rating scales (Canine Behavioral Assessment and Research Questionnaire, Attention Deficit Hyperactivity Disorder Rating Scale, Dog Personality Questionnaire, Canine Cognitive Dysfunction Rating Scale), and explored relationships between these variables. No correlation was found between urinary neurotransmitters and the assessed behavior profiles; however, age-, sex- and neuter-related influences were identified. The lack of correlation could be explained by the many confounding factors influencing both behavior and urinary neurotransmitter excretion, including age, sex and neuter status effects, and methodological issues e.g., low discriminatory power between anxiety and aggression in the descriptive behavior evaluation. Urinary neurotransmitter testing could not be validated as a tool for canine behavior evaluation in this study. However, reliable assessment methods with low susceptibility to human biases could be valuable in the future to support behavioral-phenotype diagnoses.
1. Introduction
Behavioral problems are a common concern in domestic dogs, estimated to affect 72.5–84.5 per cent of dogs (1, 2). Behaviors that owners consider “problematic” varies widely between owners, but includes a range of presentations: aggression (e.g., to owners or other dogs), anxiety (e.g., in specific contexts such as being left alone) and hyperactivity (e.g., attention-deficit/hyperactivity disorder (ADHD)-like behavior) (1, 3, 4). Dogs are highly social animals and their lives are often interwoven with their owners' daily lives. Thus, behavioral issues negatively affect the quality of life of not only affected dogs but also of their owners (5, 6). In many cases behavioral problems threaten quality and quantity of life, by contributing to owner decisions to relinquish their dog or behavioral euthanasia at a young age (1, 7–9). Problematic behavior is routinely assessed via patient's history, ruling out or considering medical causes as a contributing factor, and a veterinarian and/or clinical behaviorist's on-site evaluation (10–13).
In research settings, validated behavioral questionnaires completed by canine caregivers, or behavioral tests are commonly used to assess canine behavior (14–17).
To investigate neuropsychological conditions in humans, in addition to traditional psychometric scales, evidence emphasizes measuring urinary neurotransmitters and their potential usage as biomarkers (18, 19). Previous studies have found increases (dopamine, glutamate, tryptophan, serotonin) and decreases (norepinephrine, γ-aminobutyric acid [GABA]) in certain neurotransmitters in the urine of autistic children compared to healthy controls (20–23). In another investigation, elevated urinary epinephrine levels after trauma in children correlated with developing symptoms of acute posttraumatic stress disorder (24). ADHD symptoms in children and adults were linked to shifts in norepinephrine and epinephrine urinary excretion, as well as diminished phenylethylamine urine levels (25–28). Depression and anxiety symptoms were associated with enhanced urinary norepinephrine and epinephrine concentrations (29–31). Moreover, urinary dopamine metabolites strongly correlated with suicidality in depression, outperforming the cerebrospinal fluid assessment (32). For clinical application, urinary neurotransmitter analysis is commercially available in human medicine. It can assist clinicians in the differentiated workup of neuropsychological diseases, as well as selecting and monitoring effective treatment strategies (25, 33–37). However, despite the correlations indicated in multiple studies, a causal relationship between urinary neurotransmitters and the diagnosis of mental health issues has not yet been conclusively demonstrated (18). So far, urinary neurotransmitter testing is only diagnostic for a single condition, pheochromocytoma, an adrenal tumor associated with increased urinary norepinephrine (38, 39).
In veterinary medicine, biomarkers for canine behavioral and neurological disorders were also investigated in former non-targeted metabolic screenings and more targeted research approaches (37, 40–44). Tryptophan and lipid metabolites in the blood were found to correlate with canine ADHD-like behavior (40). Enhanced blood levels of glutamine and γ-glutamyl glutamine, as well as differences in molecules implicated in oxidative stress, tryptophan and lipid metabolism were detected in fearful dogs (41, 42). A low serotonin serum concentration was identified in aggressive dogs (43). A recent exploratory study focusing on urinary neurotransmitter analysis, compared samples of kennel boarding dogs with pet dogs kept in their homes (44). Increased norepinephrine and dopamine levels were identified in the kennel boarding dogs, presumably caused by stress exposure (44). Another study demonstrated that urinary neurotransmitter testing could also serve as a beneficial tool in canine epilepsy (37). Significant differences in the excretion of certain urinary neurotransmitters (glycine, serotonin, norepinephrine/epinephrine ratio, GABA/glutamate ratio) were identified between dogs affected by idiopathic epilepsy and healthy controls (37). Presented evidence corroborates correlations between neurobehavioral diseases and neurotransmitters/metabolites in the blood and urine of dogs, similar to previous findings in humans.
To date, serum serotonin analysis is commercially available to support the management of canine behavioral problems associated with fear or aggression (45). Further expansion of neurotransmitter analysis could offer new insights in certain pathway disturbances and underlying pathomechanisms of canine neurobehavioral diseases. Non-invasive urinary neurotransmitter analysis may identify specific biomarkers, which enable a more differentiated assessment of canine behavioral disorders in the future and contribute to more effective neuromodulatory treatment decisions and monitoring. The objective of this study was to evaluate whether characteristic urinary neurotransmitter deviations correlate with questionnaire assessed canine behavioral profiles.
2. Materials and methods
Urine samples and behavioral data from 100 privately owned dogs were collected at the University of Veterinary Medicine Hannover, Germany (TiHo) between January and June 2020. Recruited dogs were owned by TiHo staff and students, which were contacted via email. All owners were informed and gave consent. Included dogs were at least 6 months of age, of any breed and both sexes, and without chronic diseases or drug treatment. Dogs with owner-reported behavioral problems, as well as dogs with unremarkable behavior were included; however, given the threshold for the presence or absence of “problem behavior” likely differs between owners, detailed behavioral analysis exploring individual owner-reported behaviors without asking if they were problematic to the owner and/or their household was conducted by the below listed, formerly validated questionnaires. Urine samples were collected by the owners via the non-invasive free catch method. For the urinary neurotransmitter analysis, morning urine from the first or second void of the fasting dog was used. Milk products, fruit and vegetables were avoided for 48 h and strenuous exercise was avoided for 24 h before sampling. During the collection process entire females were not in heat. Samples were preserved in prepared tubes containing 50 mg oxalic acid for 10 ml urine and were immediately transferred to the TiHo laboratory. There, aliquots were stored continuously frozen at −80°C until their shipment on dry ice for external neurotransmitter analysis.
Nine urinary neurotransmitters (serotonin, histamine, glycine, phenylethylamine, dopamine, epinephrine, norepinephrine, glutamate, GABA) were quantified utilizing high-performance liquid chromatography triple-quadrupole mass spectrometry/mass spectrometry technology (Doctor's Data, St. Charles, IL, USA). Urinary creatinine levels were assessed via enzymatic colorimetric–kinetic Jaffé method (46). They served as a reference factor to calculate urine density and determine neurotransmitter levels relative to creatinine concentration. Usually, neurotransmitter levels in human urine samples are analyzed with the applied technology. However, recently multiple studies investigating urinary neurotransmitter concentrations have been carried out in dogs also identifying biologically reasonable results (37, 47–49).
Descriptive behavioral data of the dogs as continuous variables were gained via a web-based standardized owner questionnaire, hosted on LimeSurvey (LimeSurvey GmbH, Hamburg, Germany) and based on four previously validated canine behavioral questionnaires exploring a range of behavioral presentations in dogs {Canine Behavioral Assessment and Research Questionnaire [C-BARQ] (14), Attention Deficit Hyperactivity Disorder Rating Scale [ADHD RS] (15), Dog Personality Questionnaire [DPQ] (50), and Canine Cognitive Dysfunction Rating Scale [CCDR] (51)}.
3. Results
A total of 100 dogs were included in the study, of which 42 were males (neutered: 62 percent) and 58 females (neutered: 64 percent). The mean age was 5.35 (+/–SD 3.87) years, the mean weight was 17.03 (+/-SD 9.21) kg, and the ratio of purebred to crossbred dogs was 60:40.
Comparing the urinary neurotransmitter data revealed no correlation between neurotransmitters and the behavioral profile from any of the four canine questionnaires. A multivariate analysis of variance (MANOVA) was used to compare the multiple sample means of the nine neurotransmitters as a function of sex/neuter status and age and their interaction. The analysis indicated a significant effect concerning the main effects of sex/neuter status (p ≤ 0.001), age (p ≤ 0.001), as well as their interaction (p ≤ 0.02) on urinary neurotransmitter excretion. Since all nine neurotransmitters had a significant effect, the effect on particular neurotransmitters was subsequently determined with follow-up analyses of variance (ANOVA). Here, the neurotransmitters were set as dependent variables, with sex/neuter status and age and their interaction as independent variables. The results of the ANOVAs were adjusted for multiple testing using the Bonferroni criterion. The analyses identified an effect of the sex and neuter status on the urinary concentration of histamine (p ≤ 0.05), norepinephrine (p ≤ 0.05), phenylethylamine (p ≤ 0.05) and dopamine (p ≤ 0.001). Age significantly affected the urinary levels of norepinephrine (p ≤ 0.01) and dopamine (p ≤ 0.001).
Principal component analysis (PCA) was used to reduce the complexity of multiple variables (age, sex) and put them into context with the three most significant neurotransmitters (norepinephrine, dopamine, GABA/glutamate ratio). The linear combinations of all variables were projected into two dimensions, preserving most of the variance information (60.5%) of the original data. The projections visualize age- and sex/neuter-dependent clusters of the excreted neurotransmitters as 95% confidence ellipses (Figure 1).
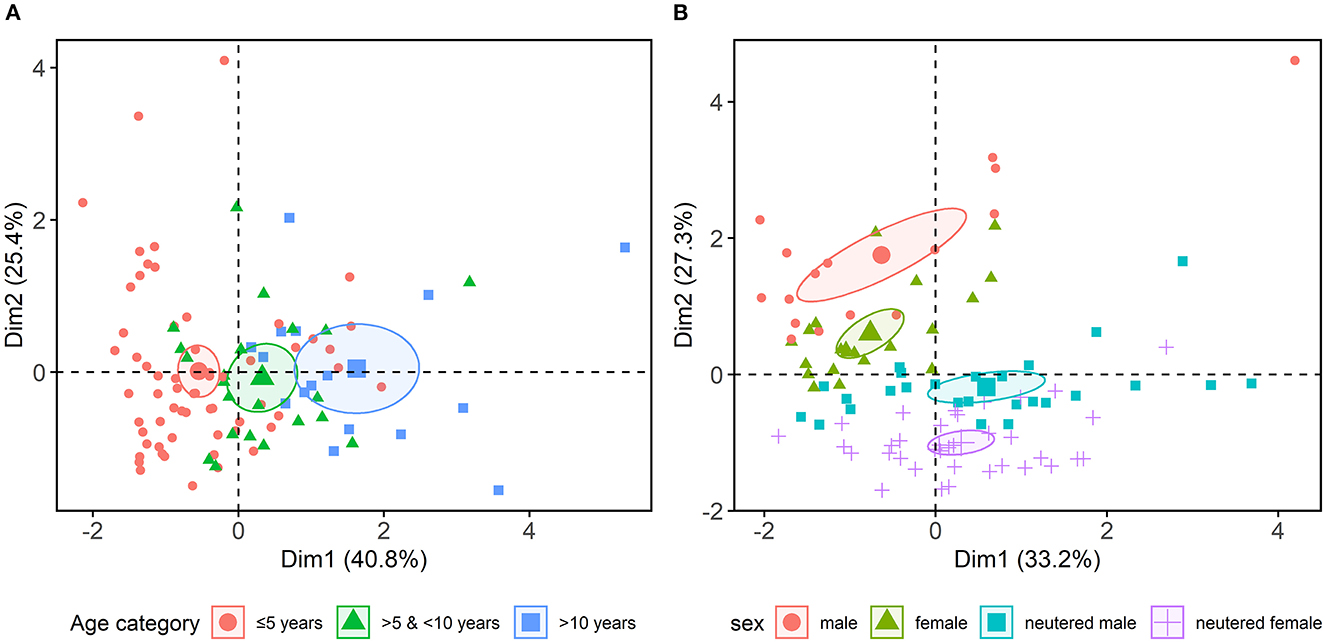
Figure 1. Combined principal component analysis, focussing on age, sex, neutering and the three most significant neurotransmitter variables (norepinephrine, dopamine, γ-aminobutyric acid/glutamate ratio) revealed (A) age- and (B) age sex/neuter-dependent clusters, indicating a hormonal influence on urinary neurotransmitters excretion.
Further, 11 behavioral C-BARQ factors were z-transformed from multiple raters and analyzed in a PCA. Due to the variance in the original data, the projections in the first two dimensions captured 34.6% of the information. A subsequent cluster analysis with the k-means algorithm revealed two formations (indicated with 95% confidence ellipses in Figure 2). When non-correlating C-BARQ factors (chasing, trainability) were excluded, the first cluster covered factors representing social fear and aggression behavior (dog-directed fear, dog-directed aggression, stranger-directed fear, stranger-directed aggression, and owner-directed aggression). The second cluster included excitability, attachment or attention-seeking, separation-related behavior, nonsocial fear, and pain sensitivity. No significant results were found in the other questionnaires (ADHD RS, DPQ, CCDR).

Figure 2. Cleaned cluster analysis showed clustering of Canine Behavioral Assessment and Research Questionnaire (C-BARQ) factors reflecting social fear and aggression (dog-directed fear [DDF], dog-directed aggression [DDA], stranger-directed fear [SDF], stranger-directed aggression [SDA], owner-directed aggression [ODA]) and another clustering of mixed factors (excitability [E], attachment or attention-seeking [AAS], separation-related behavior [SRB], nonsocial fear [NSF], pain sensitivity [PS]), when non correlating C-BARQ factors (chasing, trainability) were previously excluded, indicating a low discriminatory power of fear and aggression.
4. Discussion
The present study investigated whether urinary neurotransmitter deviations correlate with descriptive canine behavioral profiles. Evidence in human medicine has previously revealed a correlation between urinary neurotransmitters and neuropsychological disorders (18, 19). In veterinary medicine, blood screenings in dogs also showed an association between neurotransmitters/metabolites and neurobehavioral diseases (40–43). Limited pioneering studies in dogs, indicated non-invasive assessment of urinary neurotransmitters and their usage as potential biomarkers of stress and epilepsy (37, 44), which might be also a valuable approach to canine behavioral health care. In the current study no significant correlations between urinary neurotransmitter levels and canine behavioral profiles were found. However, an effect of age, sex and neuter status on canine urinary neurotransmitter excretion demonstrated in this study, indicates a hormonal influence (Figure 1). These findings are consistent with those of human studies, showing age- and sex-related variations in urinary neurotransmitter excretion of dopamine, epinephrine and norepinephrine (52, 53). Sex differences in the urinary neurotransmitter profile were assumed to be caused by associated differences in behavioral patterns of both sexes in humans, which could not be corroborated for dogs in this study (53).
Although, urinary neurotransmitter deviations have been previously associated with different neuropsychological conditions in humans, those results were not transferable to the investigated canine population, and its value for canine behavioral medicine could not be confirmed from these results. This lack of conformity is following some earlier serum serotonin level studies and may be explained by the fact that a direct correlation between the central nervous system (CNS) and the urinary neurotransmitter levels only have been shown to a limited extent in previous studies (18, 19, 54, 55). During neurotransmitter transfer through the body, transporters of the blood-brain barrier (BBB) and the kidney modulate their concentration, whereby the BBB is not permeable for every neurotransmitter (18). In addition to the CNS synthesis, neurotransmitters are also produced in the peripheral nervous system, the gut microbiome, in most body organs and can be influenced by nutrition (56–63). To minimize potential bias, solely healthy dogs without organ diseases and normal renal function were included in the current study. Furthermore, certain nutrients classified as external neurotransmitter or precursor sources were restricted before sample acquisition (62, 64, 65). A recent study was able to demonstrate a correlation of serine, glycine and norepinephrine levels between the cerebrospinal fluid, blood and urine of dogs, indicating an association of the CNS and peripheral neurotransmitters (47). Despite internal and external influences, previous investigations of canine urinary neurotransmitters found associations to stress exposure and epilepsy (37, 44). Therefore, urinary neurotransmitter analysis might be used as adjunctive screening tool for stress responses or other canine neurological diseases in the future.
The C-BARQ scores were affected by an age-related sex and neuter status effect. Intact dogs of both sexes had higher overall C-BARQ scores with advanced age, indicating a hormonal influence (Figure 2). This accords with earlier studies that found variables such as breed, sex and neuter status significantly affecting the C-BARQ scores (66–70). In addition, the exposure period to gonadal hormones in females has been negatively correlated with fear and aggression scores (67).
Cleaned cluster analysis showed clustering of C-BARQ factors reflecting social fear and aggression and another clustering of mixed behavior factors (Figure 2). These findings imply that if analyzed as an overall score, the C-BARQ primarily reflects anxiety and fearful behavior, with low discriminatory power of fear and aggression using the statistical approach applied in the investigated study population. This seems to be consistent with other studies. For example, in a Portuguese population, dog-directed fear and aggression factors were overlapping and in a study from Japan, fear and aggression items were similarly merged (71, 72). The overlap might be biologically driven e.g., fear being the emotional state underlying aggressive behavioral responses in some individual dogs, or may reflect owners' lack of ability to interpret and report dog behavior (73). Regional differences are considered another reason, and a comparison of matching groups on an international level has been recommended (66, 72).
A main limitation of the study is behavior being exclusively assessment via owner-reported questionnaires. Such data may involve owner related bias, due to the subjective interpretation of their dogs' behavior, understanding of the terminology in each questionnaire, and potentially social desirability effects. Therefore, it must be considered that the actual behavior profile of the dogs might not have been reflected in the analyses. Behavior is complex and so are behavioral disorders (74); in contrast, current behavior classification systems reduce the many facets of behavior to simpler descriptive terms e.g., aggression (74). More in-depth behavioral investigations utilizing multi-axis models may offer a valuable option for behavioral analysis in the future (74).
Behavior assessment of dogs presenting with behavioral problems remains a challenging task and is subject to the subjective biases of human interpretation (e.g., the knowledge and experience of the assessor). Urinary neurotransmitter analysis has the potential to serve as a novel, objective complement in canine behavior evaluation, especially for longitudinal studies; however, the tool could not be validated in this study population (75). Nevertheless, urinary neurotransmitter testing deserves further studies in more severely affected populations e.g., studies of specific behavioral cases diagnosed in a behavioral clinic vs. behaviorally healthy controls matched on influential factors such as age, sex and neuter status, to better understand their potential role in canine behavior assessment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Collection of urine samples was performed using the non-invasive free catch method and did not require ethical approval. Descriptive behavioral data of the dogs were gained via a web-based standardized owner questionnaire. The study was conducted following the guidelines of the University of Veterinary Medicine Hannover and approved by the Thesis Committee of the University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
TS participated in the planning of the study, carried out the main practical work, the recruitment, the sample acquisition, and drafted the manuscript. HV designed and coordinated the study. SM supported sample acquisition. SM and HV made essential contributions to the conception and acquisition of data. ST performed the statistical analysis and wrote sections of the manuscript. SM, ST, RP, and HV critically reviewed and edited the manuscript for important intellectual content. All authors contributed to the manuscript revision, read, and approved the final manuscript.
Funding
This work was financially supported by the Biotechnology and Biological Sciences Research Council (BBSRC, grant code BB/P001874/1). This Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 491094227 “Open Access Publication Funding” and the University of Veterinary Medicine Hannover, Foundation.
Acknowledgments
The authors thank the Biotechnology and Biological Sciences Research Council [BBSRC, (www.bbsrc.ac.uk), Grant code BB/P001874/1] for their financial support to HV and RP. Further they would like to thank Doctor's data for the sample analysis. Special thanks go to the participating owners and the dogs providing the canine urine samples for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADHD, attention-deficit hyperactivity disorder; GABA, γ-aminobutyric acid; TiHo, University of Veterinary Medicine Hannover; C-BARQ, Canine Behavioral Assessment & Research Questionnaire; ADHD RS, Attention Deficit Hyperactivity Disorder Rating Scale; DPQ, Dog Personality Questionnaire; CCDR, Canine Cognitive Dysfunction Rating Scale; MANOVA, multivariate analysis of variance; ANOVA, analysis of variance; PCA, principal component analysis; CNS, central nervous system; BBB, blood-brain barrier.
References
1. Salonen M, Sulkama S, Mikkola S, Puurunen J, Hakanen E, Tiira K, et al. Prevalence, comorbidity, and breed differences in canine anxiety in 13,700 Finnish pet dogs. Sci Rep. (2020) 10:2962. doi: 10.1038/s41598-020-59837-z
2. Mikkola S, Salonen M, Puurunen J, Hakanen E, Sulkama S, Araujo C, et al. Aggressive behaviour is affected by demographic, environmental and behavioural factors in purebred dogs. Sci Rep. (2021) 11:9433. doi: 10.1038/s41598-021-88793-5
3. Ramos D, Reche-Junior A, Henzel M, Mills DS. Canine behaviour problems in Brazil: a review of 180 referral cases. Vet Rec. (2020) 186:e22. doi: 10.1136/vr.105539
4. Kobelt AJ, Hemsworth PH, Barnett JL, Coleman GJ, A. Survey of dog ownership in suburban australia—conditions and behaviour problems. Appl Anim Behav Sci. (2003) 82:137–48. doi: 10.1016/S0168-1591(03)00062-5
5. Payne E, Bennett PC, McGreevy PD. Current perspectives on attachment and bonding in the dog-human dyad. Psychol Res Behav Manag. (2015) 8:71–9. doi: 10.2147/PRBM.S74972
6. Buller K, Ballantyne KC. Living with and loving a pet with behavioral problems: pet owners' experiences. J Vet Behav. (2020) 37:41–7. doi: 10.1016/j.jveb.2020.04.003
7. Salman MD, Hutchison J, Ruch-Gallie R, Kogan L, New JC, Kass PH, et al. Behavioral reasons for relinquishment of dogs and cats to 12 shelters. J Appl Anim Welf Sci. (2000) 3:93–106. doi: 10.1207/S15327604JAWS0302_2
8. O'Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. Vet J. (2013) 198:638–43. doi: 10.1016/j.tvjl.2013.09.020
9. González-Ramírez MT, Vanegas-Farfano M, Landero-Hernández R. Differences in stress and happiness between owners who perceive their dogs as well behaved or poorly behaved when they are left alone. J Vet Behav. (2018) 28:1–5. doi: 10.1016/j.jveb.2018.07.010
10. Seibert LM, Landsberg GM. Diagnosis and management of patients presenting with behavior problems. Vet Clin North Am Small Anim Pract. (2008) 38:937–50. doi: 10.1016/j.cvsm.2008.04.001
11. Horwitz DF. Diagnosis and treatment of canine separation anxiety and the use of clomipramine hydrochloride (Clomicalm). J Am Anim Hosp Assoc. (2000) 36:107–9. doi: 10.5326/15473317-36-2-107
12. Turner DC. Treating canine and feline behaviour problems and advising clients. Appl Anim Behav Sci. (1997) 52:199–204. doi: 10.1016/S0168-1591(96)01122-7
13. Mills DS, Demontigny-Bédard I, Gruen M, Klinck MP, McPeake KJ, Barcelos AM, et al. Pain and problem behavior in cats and dogs. Animals. (2020) 10:2. doi: 10.3390/ani10020318
14. Hsu Y, Serpell JA. Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. J Am Vet Med Assoc. (2003) 223:1293–300. doi: 10.2460/javma.2003.223.1293
15. Vas J, Topal J, Pech E, Miklosi A. Measuring attention deficit and activity in dogs: a new application and validation of a human adhd questionnaire. Appl Anim Behav Sci. (2006) 103:105–17. doi: 10.1016/j.applanim.2006.03.017
16. Gruen ME, Case BC, Foster ML, Lazarowski L, Fish RE, Landsberg G, et al. The use of an open field model to assess sound-induced fear and anxiety associated behaviors in Labrador Retrievers. J Vet Behav. (2015) 10:338–45. doi: 10.1016/j.jveb.2015.03.007
17. Konok V, Dóka A, Miklósi Á. The behavior of the domestic dog (canis familiaris) during separation from and reunion with the owner: a questionnaire and an experimental study. Appl Anim Behav Sci. (2011) 135:300–8. doi: 10.1016/j.applanim.2011.10.011
18. Marc DT, Ailts JW, Campeau DC, Bull MJ, Olson KL. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: validity and clinical applicability. Neurosci Biobehav Rev. (2011) 35:635–44. doi: 10.1016/j.neubiorev.2010.07.007
19. Ailts J, Ailts D, Bull M. Urinary Neurotransmitter Testing: Myths and Misconceptions. Oseola, WI: NeuroScience Inc. (2007).
20. Barthelemy C, Bruneau N, Cottet-Eymard JM, Domenech-Jouve J, Garreau B, Lelord G, et al. Urinary free and conjugated catecholamines and metabolites in autistic children. J Autism Dev Disord. (1988) 18:583–91. doi: 10.1007/BF02211876
21. Gevi F, Belardo A, Zolla L, A. Metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochimica et Biophysica Acta. (2020) 1866:165859. doi: 10.1016/j.bbadis.2020.165859
22. Liang Y, Ke X, Xiao Z, Zhang Y, Chen Y, Li Y, et al. Untargeted metabolomic profiling using uhplc-qtof/ms reveals metabolic alterations associated with autism. Biomed Res Int. (2020) 2020:6105608. doi: 10.1155/2020/6105608
23. Liang Y, Xiao Z, Ke X, Yao P, Chen Y, Lin L, et al. Urinary metabonomic profiling discriminates between children with autism and their healthy siblings. Med Sci Monit. (2020) 26:e926634. doi: 10.12659/MSM.926634
24. Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute ptsd symptoms in child trauma victims. Psychoneuroendocrinology. (2005) 30:121–8. doi: 10.1016/j.psyneuen.2004.06.004
25. Kusaga A, Yamashita Y, Koeda T, Hiratani M, Kaneko M, Yamada S, et al. Increased urine phenylethylamine after methylphenidate treatment in children with Adhd. Ann Neurol. (2002) 52:372–4. doi: 10.1002/ana.10302
26. Pliszka SR, Maas JW, Javors MA, Rogeness GA, Baker J. Urinary catecholamines in attention-deficit hyperactivity disorder with and without comorbid anxiety. J Am Acad Child Adolesc Psychiatry. (1994) 33:1165–73. doi: 10.1097/00004583-199410000-00012
27. Hanna GL, Ornitz EM, Hariharan M. Urinary catecholamine excretion and behavioral differences in adhd and normal boys. J Child Adolesc Psychopharmacol. (1996) 6:63–73. doi: 10.1089/cap.1996.6.63
28. Baker GB, Bornstein RA, Rouget AC, Ashton SE, van Muyden JC, Coutts RT. Phenylethylaminergic mechanisms in attention-deficit disorder. Biol Psychiatry. (1991) 29:15–22. doi: 10.1016/0006-3223(91)90207-3
29. Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. (2004) 57:353–8. doi: 10.1016/S0022-3999(04)00064-9
30. Roy A, Pickar D, Douillet P, Karoum F, Linnoila M. Urinary monoamines and monoamine metabolites in subtypes of unipolar depressive disorder and normal controls. Psychol Med. (1986) 16:541–6. doi: 10.1017/S0033291700010308
31. Koslow SH, Maas JW, Bowden CL, Davis JM, Hanin I, Javaid J. Csf and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis. Arch Gen Psychiatry. (1983) 40:999–1010. doi: 10.1001/archpsyc.1983.01790080081011
32. Roy A, Pollack S. Are cerebrospinal fluid or urinary monoamine metabolite measures stronger correlates of suicidal behavior in depression? Neuropsychobiology. (1994) 29:164–7. doi: 10.1159/000119081
33. Dutton J, Copeland LG, Playfer JR, Roberts NB. Measuring L-dopa in plasma and urine to monitor therapy of elderly patients with Parkinson's disease treated with L-dopa and a dopa decarboxylase inhibitor. Clin Chem. (1993) 39:629–34. doi: 10.1093/clinchem/39.4.629
34. Zametkin AJ, Karoum F, Linnoila M, Rapoport JL, Brown GL, Chuang L-W, et al. Stimulants, urinary catecholamines, and indoleamines in hyperactivity: a comparison of methylphenidate and dextroamphetamine. Arch Gen Psychiatry. (1985) 42:251–5. doi: 10.1001/archpsyc.1985.01790260045005
35. Bornstein RA, Baker GB. Neuropsychological performance and urinary phenylethylamine in tourette's syndrome. J Neuropsychiatry Clin Neurosci. (1991) 3:417–21. doi: 10.1176/jnp.3.4.417
36. Likhitweerawong N, Thonusin C, Boonchooduang N, Louthrenoo O, Nookaew I, Chattipakorn N, et al. Profiles of urine and blood metabolomics in autism spectrum disorders. Metab Brain Dis. (2021) 36:1641–71. doi: 10.1007/s11011-021-00788-3
37. Schmidt T, Meller S, Talbot SR, Berk BA, Law TH, Hobbs SL, et al. Urinary neurotransmitter patterns are altered in canine epilepsy. Front Vet Sci. (2022) 9:893013. doi: 10.3389/fvets.2022.893013
38. Westphal SA. Diagnosis of a pheochromocytoma. Am J Med Sci. (2005) 329:18–21. doi: 10.1097/00000441-200501000-00004
39. Duncan MW, Compton P, Lazarus L, Smythe GA. Measurement of norepinephrine and 3,4-dihydroxyphenylglycol in urine and plasma for the diagnosis of pheochromocytoma. N Engl J Med. (1988) 319:136–42. doi: 10.1056/NEJM198807213190303
40. Puurunen J, Sulkama S, Tiira K, Araujo C, Lehtonen M, Hanhineva K, et al. A non-targeted metabolite profiling pilot study suggests that tryptophan and lipid metabolisms are linked with adhd-like behaviours in dogs. BBF. (2016) 12:27. doi: 10.1186/s12993-016-0112-1
41. Puurunen J, Tiira K, Lehtonen M, Hanhineva K, Lohi H. Non-targeted metabolite profiling reveals changes in oxidative stress, tryptophan and lipid metabolisms in fearful dogs. BBF. (2016) 12:7. doi: 10.1186/s12993-016-0091-2
42. Puurunen J, Tiira K, Vapalahti K, Lehtonen M, Hanhineva K, Lohi H. Fearful dogs have increased plasma glutamine and gamma-glutamyl glutamine. Sci Rep. (2018) 8:15976. doi: 10.1038/s41598-018-34321-x
43. Cakiroglu D, Meral Y, Sancak AA, Cifti G. Relationship between the serum concentrations of serotonin and lipids and aggression in dogs. Vet Rec. (2007) 161:59–61. doi: 10.1136/vr.161.2.59
44. Albright JD, Ng ZY. Measurement of neurotransmitters excreted in the urine of behaviorally healthy dogs in home and boarding kennel conditions. J Vet Behav. (2022) 48:74–7. doi: 10.1016/j.jveb.2021.09.001
45. Laboklin. Klein R, Laboruntersuchungen Bei Verhaltensauffälligen Hunden Und Katzen. (2020). Available online at: https://laboklin.de/de/ laboruntersuchungen-bei-verhaltensauffaelligen-hunden-und-katzen/ (accessed December 12, 2022).
46. Delanghe JR, Speeckaert MM. Creatinine determination according to jaffe-what does it stand for? NDT Plus. (2011) 4:83–6. doi: 10.1093/ndtplus/sfq211
47. Meller S, Hildebrandt R, Gramer M, Richter Assencio F, Volk HA. Comparison of neurotransmitters concentration in canine cerebrospinal fluid, blood, and urine samples measured via highperformance liquid chromatography. In: Proceedings of the 33rd On-line Symposium ESVN-ECVN; 2021 September 17-18. (2021). p. 1–53.
48. Berk BA, Law TH, Wessmann A, Bathen-Nöthen A, Knebel A, Tipold A, et al. Neurotransmitter concentrations in urine associated with the consumption of a medium-chain triglyceride (MCT) supplement in dogs with idiopathic epilepsy. In: Proceedings of the 32nd Symposium ESVN-ECVN; 2019 September 12-14 (Wroclaw). (2020). p. 2990–3057.
49. Berk BA, Ottka C, Hong Law T, Packer RMA, Wessmann A, Bathen-Nöthen A, et al. Metabolic fingerprinting of dogs with idiopathic epilepsy receiving a ketogenic medium-chain triglyceride (Mct) oil. Front Vet Sci. (2022) 9:935430. doi: 10.3389/fvets.2022.935430
50. Wright HF, Mills DS, Pollux PMJ. Development and validation of a psychometric tool for assessing impulsivity in the domestic dog (Canis Familiaris). Int J Comp Psychol. (2011) 24:210–25. doi: 10.46867/IJCP.2011.24.02.03
51. Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ. The canine cognitive dysfunction rating scale (Ccdr): a data-driven and ecologically relevant assessment tool. Vet J. (2011) 188:331–6. doi: 10.1016/j.tvjl.2010.05.014
52. Gerlo EA, Schoors DF, Dupont AG. Age- and sex-related differences for the urinary excretion of norepinephrine, epinephrine, and dopamine in adults. Clin Chem. (1991) 37:875–8. doi: 10.1093/clinchem/37.6.875
53. Lundberg U. Sex differences in behaviour pattern and catecholamine and cortisol excretion in 3-6 year old day-care children. Biol Psychol. (1983) 16:109–17. doi: 10.1016/0301-0511(83)90057-1
54. Riggio G, Mariti C, Sergi V, Diverio S, Gazzano A. Serotonin and tryptophan serum concentrations in shelter dogs showing different behavioural responses to a potentially stressful procedure. Vet Sci. (2020) 8:1. doi: 10.3390/vetsci8010001
55. Rayment DJ, Peters RA, Marston LC, De Groef B. Relationships between serum serotonin, plasma cortisol, and behavioral factors in a mixed-breed, -sex, and -age group of pet dogs. J Vet Behav. (2020) 38:96–102. doi: 10.1016/j.jveb.2020.05.007
56. Gershon MD. 5-Hydroxytryptamine (Serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. (2013) 20:14–21. doi: 10.1097/MED.0b013e32835bc703
57. Buu NT, Duhaime J, Kuchel O. Handling of dopamine and dopamine sulfate by isolated perfused rat kidney. Am J Physiol. (1986) 250:F975–9. doi: 10.1152/ajprenal.1986.250.6.F975
58. Mazzoli R, Pessione E. The neuro-endocrinological role of microbial glutamate and gaba signaling. Front Microbiol. (2016) 7:1934. doi: 10.3389/fmicb.2016.01934
59. Franklin IK, Wollheim CB. Gaba in the endocrine pancreas: its putative role as an islet cell paracrine-signalling molecule. J General Physiol. (2004) 123:185–90. doi: 10.1085/jgp.200409016
60. Pérez-Alvarez A, Hernández-Vivanco A, Albillos A. Past, present and future of human chromaffin cells: role in physiology and therapeutics. Cell Mol Neurobiol. (2010) 30:1407–15. doi: 10.1007/s10571-010-9582-0
61. Ziegler MG, Kennedy B, Elayan H. Rat renal epinephrine synthesis. J Clin Invest. (1989) 84:1130–3. doi: 10.1172/JCI114276
62. Briguglio M, Dell'Osso B, Panzica G, Malgaroli A, Banfi G, Zanaboni Dina C, et al. Dietary neurotransmitters: a narrative review on current knowledge. Nutrients. (2018) 10:591. doi: 10.3390/nu10050591
63. Gazzano A, Casini L, Macchioni F, Mariti C, Baragli P, Preziuso G, et al. L-tryptophan supplementation increases serotonin blood levels in dogs fed a dissociated carbohydrate-based diet. Dog Behavior. (2021) 7:3. doi: 10.4454/db.v7i3.147
64. Lieberman HR, Corkin S, Spring BJ, Wurtman RJ, Growdon JH. The effects of dietary neurotransmitter precursors on human behavior. Am J Clin Nutr. (1985) 42:366–70. doi: 10.1093/ajcn/42.2.366
65. Meyers S. Use of neurotransmitter precursors for treatment of depression. Altern Med Rev. (2000) 5:64−71.
66. Tonoike A, Nagasawa M, Mogi K, Serpell JA, Ohtsuki H, Kikusui T. Comparison of owner-reported behavioral characteristics among genetically clustered breeds of dog (canis familiaris). Sci Rep. (2015) 5:17710. doi: 10.1038/srep17710
67. Starling M, Fawcett A, Wilson B, Serpell J, McGreevy P. Behavioural risks in female dogs with minimal lifetime exposure to gonadal hormones. PLoS ONE. (2019) 14:e0223709. doi: 10.1371/journal.pone.0223709
68. Serpell JA, Hsu YA. Effects of breed, sex, and neuter status on trainability in dogs. Anthrozoös. (2005) 18:196–207. doi: 10.2752/089279305785594135
69. Duffy DL, Hsu Y, Serpell JA. Breed Differences in Canine Aggression. Appl Anim Behav Sci. (2008) 114:441–60. doi: 10.1016/j.applanim.2008.04.006
70. Hsu Y, Sun L. Factors associated with aggressive responses in pet dogs. Appl Anim Behav Sci. (2010) 123:108–23. doi: 10.1016/j.applanim.2010.01.013
71. Canejo-Teixeira R, Almiro PA, Serpell JA, Baptista LV, Niza M. Evaluation of the factor structure of the canine behavioural assessment and research questionnaire (C-barq) in european portuguese. PLoS ONE. (2018) 13:e0209852. doi: 10.1371/journal.pone.0209852
72. Nagasawa M, Tsujimura A, Tateishi K, Mogi K, Ohta M, Serpell JA, et al. Assessment of the factorial structures of the C-barq in Japan. J Vet Med Sci. (2011) 73:869–75. doi: 10.1292/jvms.10-0208
73. Galac S, Knol B. Fear-motivated aggression in dogs: patient characteristics, diagnosis and therapy. Animal Welfare. (1997) 6:9–15. doi: 10.1017/S0962728600019357
74. Fatjó J, Bowen J. Making the case for multi-axis assessment of behavioural problems. Animals. (2020) 10:383. doi: 10.3390/ani10030383
75. Berk BA, Packer RMA, Law TH, Wessmann A, Bathen-Nothen A, Jokinen TS, et al. A double-blinded randomised dietary supplement crossover trial design to investigate the short-term influence of medium chain fatty acid (mct) supplement on canine idiopathic epilepsy: study protocol. BMC Vet Res. (2019) 15:181. doi: 10.1186/s12917-019-1915-8
Keywords: neurotransmitter, behavior, behavioral problems, questionnaire, C-BARQ, urinary, canine
Citation: Schmidt T, Meller S, Talbot SR, Packer RMA and Volk HA (2023) Urinary neurotransmitter analysis and canine behavior assessment. Front. Vet. Sci. 10:1124231. doi: 10.3389/fvets.2023.1124231
Received: 14 December 2022; Accepted: 17 January 2023;
Published: 06 February 2023.
Edited by:
Deborah Wells, Queen's University Belfast, United KingdomReviewed by:
Angelo Gazzano, University of Pisa, ItalyGeorge M. Strain, Louisiana State University, United States
Copyright © 2023 Schmidt, Meller, Talbot, Packer and Volk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Holger Andreas Volk,  SG9sZ2VyLlZvbGsmI3gwMDA0MDt0aWhvLWhhbm5vdmVyLmRl
SG9sZ2VyLlZvbGsmI3gwMDA0MDt0aWhvLWhhbm5vdmVyLmRl
 Teresa Schmidt
Teresa Schmidt Sebastian Meller
Sebastian Meller Steven Roger Talbot
Steven Roger Talbot Rowena Mary Anne Packer
Rowena Mary Anne Packer Holger Andreas Volk
Holger Andreas Volk