- 1Department of Veterinary and Animal Sciences, CES University, Medellin, Colombia
- 2Department of Veterinary and Animal Sciences, University of Copenhagen, Copenhagen, Denmark
- 3Instituto Colombiano de Medicina Tropical, CES University, Medellin, Colombia
- 4Department for Food Safety and Veterinary Issues, Danish Agriculture & Food Council, Copenhagen, Denmark
Control of Salmonella in pig/pork production is important to protect public health because pork is one of the main sources of human infection. Moreover, antimicrobial use in pig farms should be kept low to minimize development and transmission of antimicrobial resistance. This pilot study evaluated the productivity and Salmonella seroprevalence in pigs administered organic acids (OA) compared to pigs given growth promoters in one farm in Antioquia, Colombia. Two groups each consisting of 60 pigs of 6-weeks of age were studied for 4 months. One group was provided feed and water with OA (Selko pH® and Selacid®), whereas the other group (control) received antimicrobial growth promoters according to routine feeding practices (tylosin and zinc bacitracin). Blood samples were taken three times (T1–T3) and pigs were weighted five times to calculate daily weight gain (DWG) and feed conversion ratio (FCR). Initially when the pigs were 6 weeks old (T1), the Salmonella seroprevalence was 1.7% in both groups. When the pigs were 11 weeks old (T2), the seroprevalence was significantly lower in pigs provided OA compared to the control group (19 vs. 47%, P < 0.001), whereas when the pigs were 23 weeks old (T3), the seroprevalence did not differ between the groups (62 vs. 77%; P = 0.075). The cumulative DWG was significantly higher in the intervention group than in the control group (713 vs. 667 g/day; P < 0.001). The cumulative FCR did not differ between groups (2.80 vs. 2.77; P = 0.144). The pilot study indicates that cleaning the water pipes and administrating OA improve productivity in pigs and delay exposure to Salmonella spp. when compared with growth promoters. Thus, OA could replace antimicrobial growth promoters and reduce antimicrobial use and resistance. However, the study should be repeated before firmer conclusions can be drawn.
1. Introduction
Salmonellosis is a foodborne, zoonotic disease that is generally self-limiting (1). Worldwide, non-typhoidal Salmonella is ascribed to ~93.8 million human cases of acute gastroenteritis and 155,000 deaths annually (2, 3). In the United States, the cost of human salmonellosis is estimated to be around $2.9 billion per year (4). Denmark has carried out intensive programs to control Salmonella in the animal production chain since 1990s which has resulted in a low human incidence, i.e., 11.8 Salmonella cases per 100,000 habitants were registered in 2021 (5). In Colombia, human salmonellosis is underreported and considered an endemic disease with sporadic outbreaks. According to the Colombian National Institute of Health, 7,219 Salmonella cases were reported between 2000 and 2013 with S. Typhimurium (33.7%) being the most common serotype detected followed by S. Enteritidis (28.6%), S. Dublin (3.3%) and S. Derby (2.1%) (6, 7).
The distribution of the different Salmonella serotypes varies according to food source and geographical area (6). Infection with S. Enteritidis is often associated with consumption of eggs and poultry meat, whereas the other globally important serotype S. Typhimurium is related mainly to consumption of pork (6, 8). In 2020, 13.0% of the human cases of salmonellosis reported in the European Union (EU) were due to consumption of contaminated pork. In 2015, the Salmonella prevalence was 28.2% on pork carcasses at abattoirs in Colombia with S. Typhimurium, S. Agama and S. Agona being the main serotypes found (9). Comprehensive control of Salmonella throughout the food value chain can decrease the incidence of human salmonellosis (6, 10, 11).
Subclinical salmonellosis in pigs constitutes a source of Salmonella. After weaning, the pigs excrete Salmonella and infect other pigs in the pen. Excretion of Salmonella may increase at times of stress such as during transport to the abattoir and in the lairage area, resulting in high risks for contamination of the carcasses during slaughter unless adequate measures are taken (11–14). In Colombia, the between-farm Salmonella seroprevalence was 42.9% (n = 350) in 2020 in the seven main pig producing provinces (8, 15–17). Half of the Salmonella strains tested (n = 41) showed concurring resistance to penicillin, cefuroxime, tetracycline and trimethoprim/sulfamethoxazole. These types of antimicrobial resistances (AMR) in Salmonella may be ascribed to the routine use of antimicrobials supplemented to pig feed used in Colombia (3, 18) and is of concern for effective treatment of human salmonellosis (16). Moreover, reduced AMR levels are not just of benefit to human health but will also ensure that pigs suffering from diseases caused by bacterial pathogens can be treated.
Although the use of antimicrobial growth promoters is banned in some parts of the world including the EU, they are still allowed and commonly used in Colombia for disease control and to improve growth in livestock. However, use of such growth promoters leads to development of AMR. For this reason, alternatives have been sought to replace the antimicrobial growth promoters including preventive measures focusing on improving the health of pigs while maintaining productivity (19). Without maintenance of productivity, the farmers cannot be expected to change habits and replace antimicrobial growth promoters with alternatives.
Organic acids (OA) can be used to control Salmonella and promote growth in pigs. When administrated in water and/or feed, the OA cause a decreased pH to 3.8–4.2 at which the growth of many gastro-intestinal bacteria except lactobacilli is altered or directly inhibited. OA also modulate the intestinal fermentation patterns of feed creating a better gastro-intestinal environment with improved utilization of feed and growth (20–23). These positive effects of OA on feed conversion rate and growth performance are also described in poultry including increased egg production. The ability to decrease Salmonella colonization depends on the type of OA used (24). Although the antibacterial effect of OA is well known in theory, published results of efficacy in on-farm studies vary, with some reporting beneficial effects (25–28) while others fail to demonstrate any effect (29–31). Thus, further evidence is needed to establish at which concentrations and combinations OA could be used to control Salmonella spp. in pigs and to elucidate their effect on productivity (21, 32).
The purpose of this pilot study was to evaluate the effect of OA on the productivity and the Salmonella seroprevalence in pigs from weaning to slaughter. We undertook a clinical trial, comparing the effect of provision of OA with antimicrobial growth promoters in a pig farm in Antioquia, Colombia. The hypothesis was that OA supplements to water and feed were equally effective as the growth promoters. This would open up for a possible replacement of antimicrobial growth promoters with OA in line with the principles of prudent use of antimicrobials.
2. Materials and methods
2.1. Herd description
This study was endorsed by the Institutional Committee Cuidado y Uso de los Animales (CICUA) at the CES University, Medellin, Colombia (Code No. 206/Act No. 38). The study farm produced piglets as well as finisher pigs was selected in Antioquia, Colombia. The farm had a known positive status for Salmonella.
The farm had a total of 500 sows. The piglets were weaned at 4 weeks of age, where after they remained for 7 weeks in the weaning facilities. They were then moved to the growing facilities, where they would stay for 6 weeks. Finally, they were moved to the finishing pens where they remained for 6 weeks until slaughter. The feed was produced on the farm. The composition of the feed is shown in Supplementary Table S1. The pens were equipped with portable waterers to measure water consumption.
2.2. Baseline sampling
Prior to the start of the trial, sampling of blood, rectal swabs and fecal material was performed to determine the within-farm Salmonella seroprevalence and to confirm presence of Salmonella in the herd. In August 2020, blood samples and rectal swabs were taken from 10 lactating sows, 30 weaned piglets, 30 growing pigs and 30 finishing pigs. Subsequently in September and December 2020 as well as in April 2021, a total of 130 samples were collected, processed and analyzed in three different ways. The first 40 samples consisted of rectal swabs, which were transported in Selenite-Cystine medium (Instituto Colombiano de Medicina Tropical (ICMT), Medellin, Colombia) and processed at two different laboratories. The following 30 samples were fecal samples, each with a volume of around 25 g, and collected directly from the rectum of individual pigs to increase the sensitivity of the subsequent laboratory analysis. The fecal samples were placed in sterile plastic bags. The remaining 60 samples consisted of fecal swab samples which were transported in Aimes transport medium (ICMT, Medellin, Colombia).
2.3. Experimental design and sampling
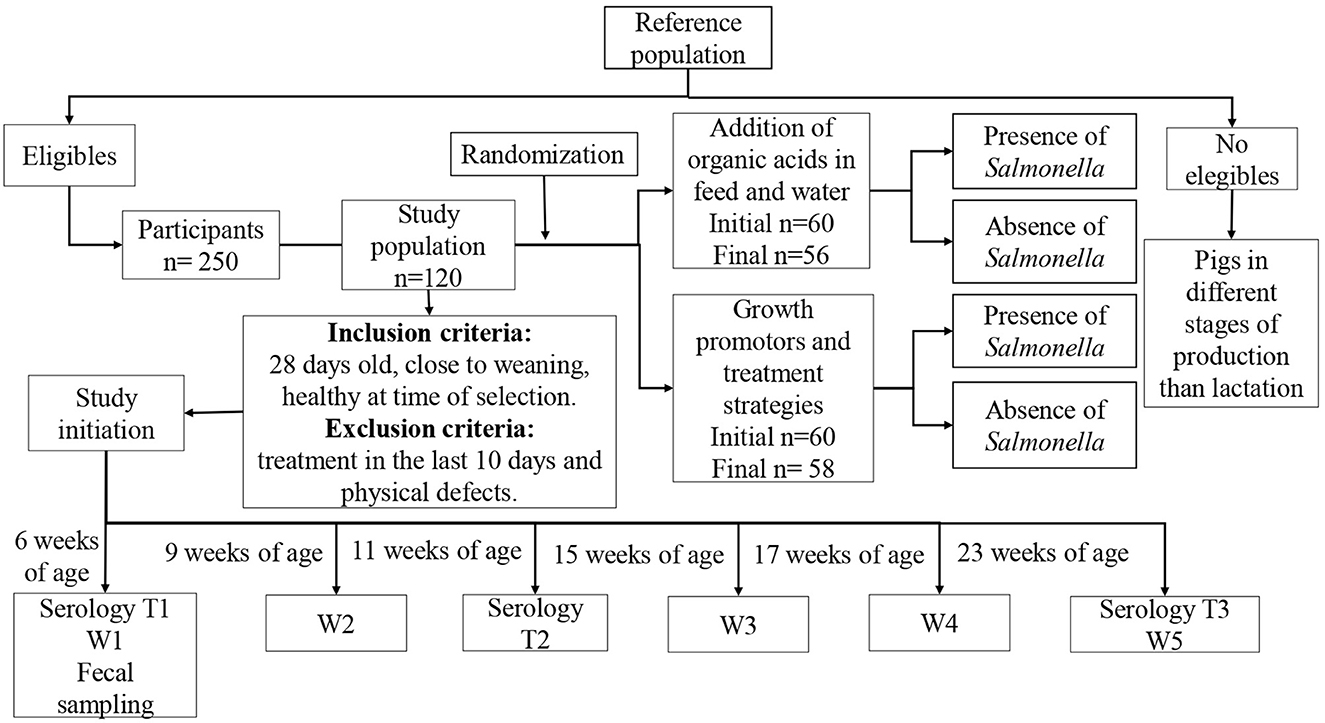
A parallel, randomized, controlled clinical trial was performed at the selected pig farm including 120 individual pigs. The sample size was based on logistical and economic considerations. The piglets were randomly divided into two groups of 60 pigs each. Each individual pig was ear tagged with an identification number to ensure proper follow-up (Figure 1).
The inclusion criteria were piglets close to weaning at ~28 days of age and healthy at the time of sampling. The exclusion criteria were piglets that presented physical defects or that had received any antimicrobial treatment up to 10 days before the selection of the animals. At the time of sample selection, 250 piglets met the inclusion criteria and the formula K = N/n (K = sample interval, N = total population units and n = sample size) was used to determine the number of animals to be included in the study.
The farm veterinarian oversaw the assignment of pigs to each group in the pens before the sampling was initiated. Each pen was completely separated from other pens preventing the pigs in one group from having physical contact with other pigs. The trial started when the pigs were 6 weeks of age with a follow-up time of 4 months.
In September 2021, water samples were taken and microbiological and physicochemical analyses were performed to determine the dosage of Selko pH® (Trouw Nutrition, Tres Cantos, Madrid) to be added to the water. These water quality analyses were done as the effect of Selko pH® depends on the characteristics of the of water including pH, hardness, concentrations of minerals and organic matter as well as bacterial concentration. The results showed a high degree of fecal contamination of the water with an E. coli count of 1,944 CFU/100 ml, fecal coliforms of 3,888 CFU/100 ml, but with no isolation of Salmonella spp. It was therefore decided by the owner of the farm to disinfect the water pipes with 0.4 ml/l of citric acid solution (GREEN DAC® ECOLAB, Bogota, Colombia) before beginning the clinical trial to ensure the effect of the OA treatment. Subsequent water samples obtained after cleaning the pipes contained 0 E. coli CFU/100 ml, 8 CFU/100 ml of fecal coliforms and absence of Salmonella spp. During the clinical trial, the pipes were cleaned every month using citric acid in the same way as described above.
The drinking water for the intervention group was supplemented with Selko pH® that contains E 236 formic acid, E 260 acetic acid, E 295 ammonium formate, E 300 L-Ascorbic acid, E 330 citric acid, E 4 copper and E 6 zinc. Based on the water characteristics it was decided to add 0.8 ml/liter of Selko pH® to the water to ensure the expected effect. This dosage was administered during the first 4 h of the day, every other day throughout the follow-up period. Likewise, Selacid® (Trouw Nutrition®, Tres Cantos, Madrid) that contains E 200 sorbic acid, E 236 formic acid, E 260 acetic acid, E270 lactic acid, E 280 propionic acid, E 295 ammonium formate and E 330 citric acid was added to the feed. Two kg of Selacid® per ton was added to weaner feed, whereas 1.5 kg per ton was used in grower and fattener feed during the entire study. The concentration of the individual compounds in the two commercial products were not declared and such information could not be obtained from the company. In the control group, tylosin phosphate 10% (1 kg per ton) was added to the weaner feed for the first 7 days of the study. Moreover, 15% zinc bacitracin (300 g per ton) was added to the grower feed for about 1 month.
Before starting the intervention with 6 weeks old piglets, initial (T1) blood samples and rectal swabs were obtained from each the 60 piglets. These samples were analyzed in pools of two yielding a total of 30 pooled samples to determine the Salmonella seroprevalence and the proportion of pigs excreting Salmonella.
Blood samples were taken again when the pigs were 11 weeks old (T2) and at the end of the observation period, when the pigs were 23 weeks of age (T3). At the beginning of the observation period, each pig was weighed (W1). Weighing was repeated when the pigs were 9, 15, 17, and 23 weeks old (W2–W5) and these measurements were used to calculate the daily weight gain (DWG) using the formula: weight in kg gained/#days between weighing. Similar for feed conversion ratio (FCR), the following formula was used: kg consumed/weight in kg gained in the period. Feed consumption was estimated from the data sheet delivered to the farm manager and workers in charge of supplying feed to the pigs, and on which they noted the number of packages of feed supplied to each pen. The amount of feed in kg consumed by pigs in each pen and each group of pigs was then calculated.
2.4. Serological and microbiological analysis
The blood samples were stored and transported in a refrigerator (4–5°C) within 24 h after sample collection. Subsequently, serum was extracted and the ELISA diagnostic kit IDEXX® Swine Salmonella Ab (IDEXX, Barcelona, Spain) was used to evaluate the seroprevalence of Salmonella spp., using a cut-off of 40% optical density.
The 30 pooled rectal swabs were duly marked and transported within 24 h at 4–5°C to the Veterinary and Zootechnical Laboratory of ICMT, which was in charge of processing and analyzing the samples. Upon arrival at the laboratory, the samples were inoculated into peptonized water at an adjusted ratio of 1:10 weight/volume and incubated at 36 ± 1°C for 18 ± 2 h after which 1 ml was incubated in selenite cystine broth and incubated at 36 ± 1°C for 24 ± 2 h. On day three, 0.1 ml of the broth was inoculated onto Xylose Lysine Deoxycholate (XDL; ICMT, Colombia) agar and Hecktoen agar (ICMT, Colombia) and incubated at 36 ± 1°C for 18 ± 2 h. Suspected Salmonella spp. colonies were selected from both agar media and re-streaked onto MacConkey agar (ICMT, Colombia) to obtain pure colonies after incubation at 36 ± 1°C for 18 ± 2 h. Subsequently, suspected isolates were tested by urea and sulfide-indole-motility tests as well as Gram staining. Finally, suspected isolates were subjected to PCR to confirm the serogroup and serotype, using the primers and conditions previously described by Cardona-Castro et al. (33).
2.5. Statistical analysis
Data from all pigs were used. The serological samples of the animals that died during the follow-up period were filled according to the mode of the results. For the statistical analyses, SPSS® version 21 CES university license, Microsoft Office Excel 2003 (Microsoft Corporation, Redmond, USA), JAMOVI version 1.8.4 of free distribution and EPIDAT 3.1 of free distribution was used.
A univariate analysis was carried out to describe the distribution of pigs included in the study according to their sex, age, weight, Salmonella seroprevalence and the line (breeder or finisher). To check for normality of the distribution of quantitative variables, Shapiro-Wilk normality test was performed. Next, bivariate analyses were undertaken investigating the association between the different variables, with a focus on the effect of treatment. Parametric tests were used for dependent quantitative variables that were normally distributed (T-student test), whereas non-parametric tests were used for the non-normally distributed variables (Mann-Whitney U test). Chi-square test was used for the count data variables, and the Fisher exact test was used when one or more of the expected cell values were <5. For all analyses, the P-value was reported using a significance value of α = 0.05 (34, 35). Due to the limited number of samples, no attempts were made to model the seroprevalence over time using repeated measurements models.
3. Results
3.1. Salmonella baseline
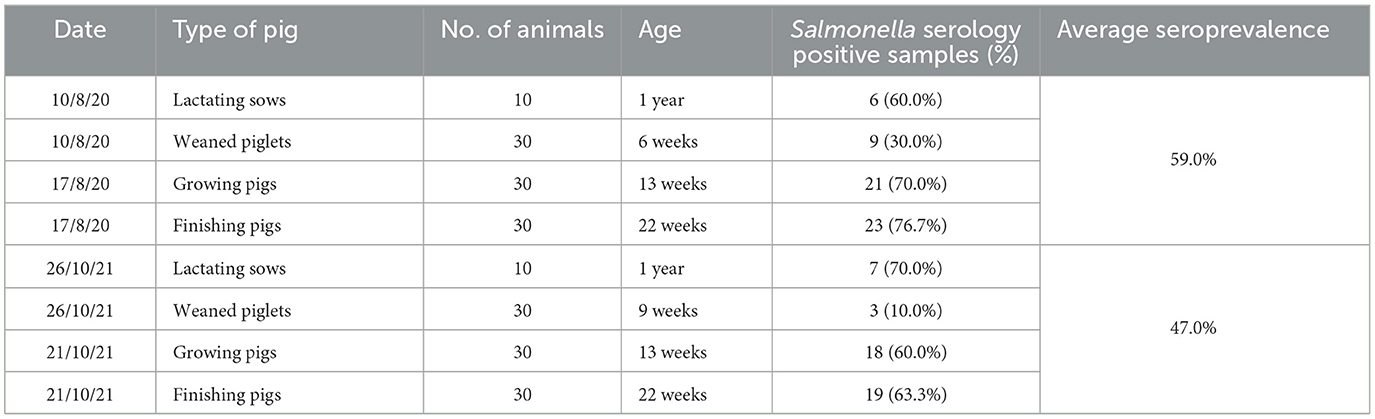
In August 2020, the baseline seroprevalence of Salmonella was 59.0% in the pig herd. Only one Salmonella-positive sample was found and confirmed by PCR among the 100 fecal samples analyzed. The 130 fecal samples obtained between September 2020 and April 2021 were all negative for Salmonella. In 2021, the results of the second baseline sampling analyzing 100 blood samples yielded a seroprevalence of 47.0% (Table 1).
3.2. Clinical trial
The distribution of the pigs according to sex, age, line, Salmonella prevalence and weight is presented in Table 2 and shows no statistical difference between the two groups. During the observation period, six animals died among including four pigs from the intervention group and two pigs in the control group (Supplementary Figure S1). Based on a necropsy examination, the pigs died due to infarction, hemorrhage, meningitis, intestinal torsion and pneumonia. Hence, the causes of death were not related to the water and feed additions and this level of mortality was normal at the farm.
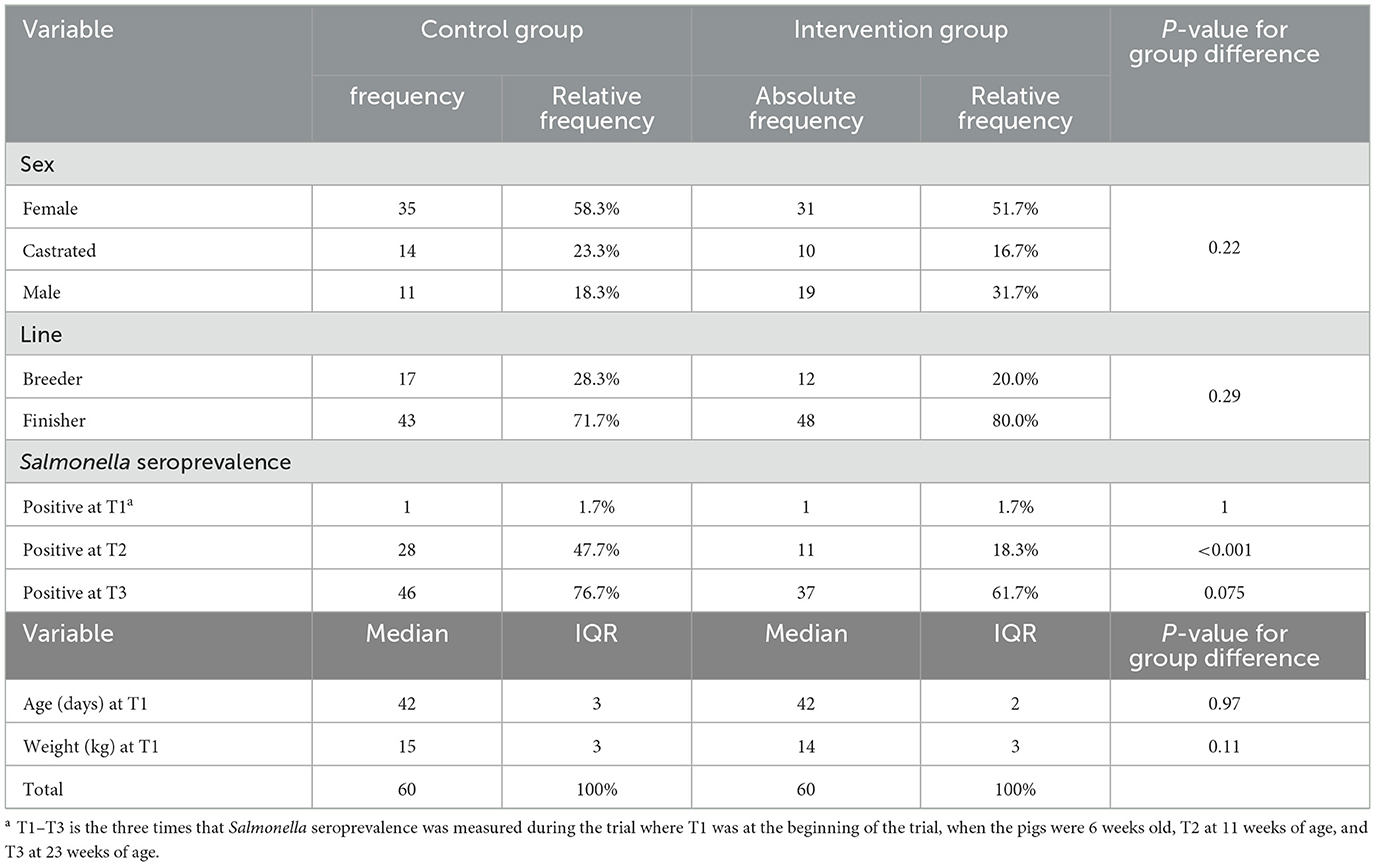
Table 2. Descriptive analysis of characteristics and Salmonella seroprevalence of 120 weaned piglets included in the clinical trial with organic acids.
At T1, when the pigs were 6 weeks of age a Salmonella seroprevalence of 1.7% was found in both groups. At T2, when the pigs were 11 weeks of age a Salmonella seroprevalence of 18.3% was observed in the intervention group vs. 47.7% in the control group, showing a statistically significant difference between groups (P < 0.001). Finally, at T3 where the pigs were 23 weeks of age a Salmonella non-significant seroprevalence of 61.7% was observed in the intervention group vs. 76.7% in the control group (P = 0.075) (Supplementary Figure S2).
The median and the interquartile range (IQR) of the weight of the pigs at the different times of measurements are shown in Figure 2. There was a statistically significant difference between the groups at W4 (P < 0.001) where the pigs were 17 weeks old with a better performance in the intervention group, where the median weight was 65.0 kg per pig (IQR = 10.0 kg) vs. 61.0 kg in the control group (IQR = 9.5 kg). Likewise, at W5 where the pigs were 23 weeks old, the growth performance was significantly higher (P = 0.024) in the intervention group, where the median weight was 101.0 kg per pig (IQR 12.5 kg) vs. 97.0 kg in the control group (IQR 11.0 kg).
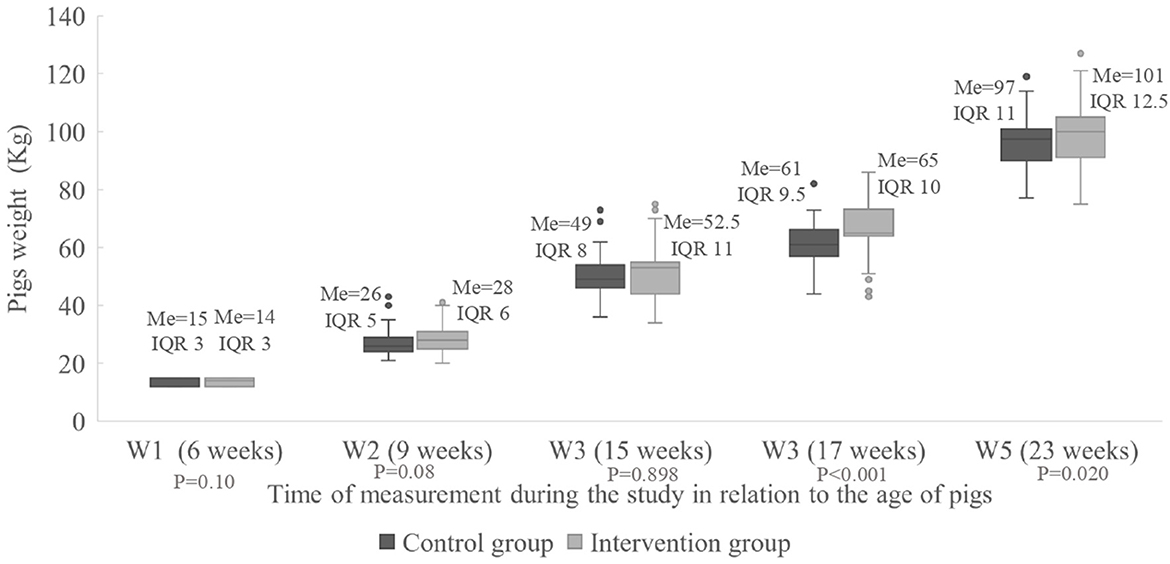
Figure 2. Weight of the individual pigs (kg) for the intervention and control groups measured five times during the study. Me, median; IQR, interquartile range; W1–5, Weight all time 1–5.
For DWG3, there was a statistically significant difference between treatment groups (P < 0.001), showing higher values in the intervention group, which had a median of 722 g/pig/day (IQR 22 g/pig/day) vs. a median of 611 g/day (IQR 78 g/pig/day) in the control group (Figure 3). There was no difference between groups for DWG1, DWG2, and DWG4. However, the median of the cumulative DWG was 743 g/pig/day (IQR 12 g/pig/day) for the intervention group vs. 666 g/pig/day (IQR 10 g/pig/day) for the control group, showing a statistically significant difference (P < 0.001).
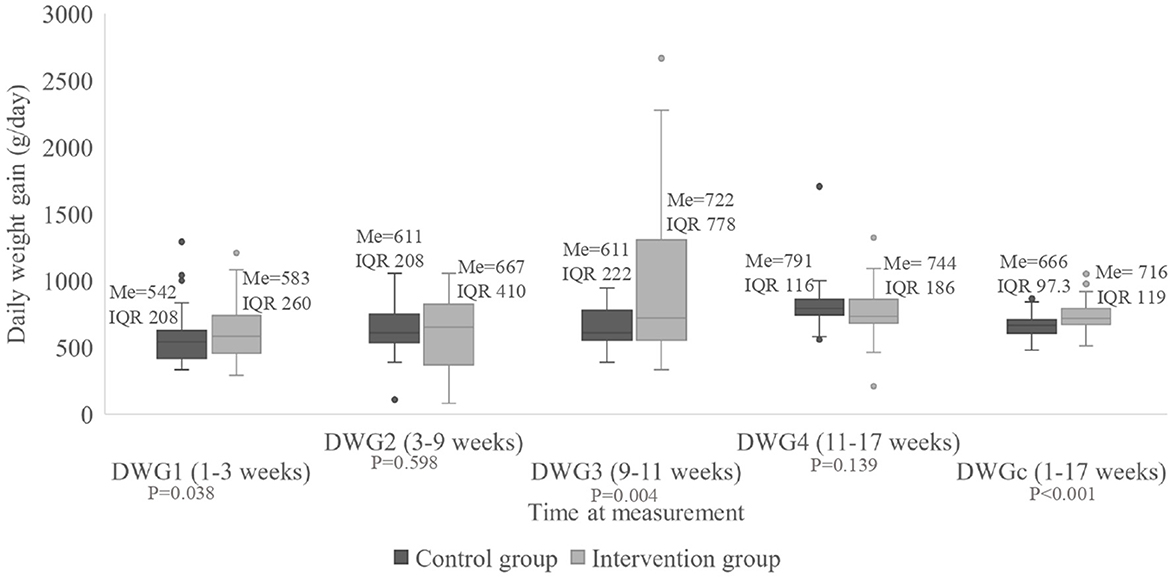
Figure 3. Daily weight gain of the pigs (g/day) divided according to group, measured at four times during the study as well as for the entire period. Me, median; IQR, interquartile range; DWGc, cumulative daily weight gain.
Regarding FCR, a statistically significant difference (P = 0.025) was observed at FCR3 where a median of 2.4 kg of feed per kg of weight gained (IQR 1.8 kg) was estimated for the intervention group vs. 2.8 kg (IQR 0.9 kg) in the control group. For FCR4, a statistically significant difference (P = 0.009) was observed where a median of 3.1 kg of feed per kg of weight gained (IQR 0.7 kg) was estimated for the intervention group vs. 2.8 kg (IQR 0.4 kg) in the control group (Figure 4). However, there was no significant difference (P = 0.14) when the median cumulative FCR was compared between groups, as the pigs in the intervention group used 2.8 kg of feed per kg weight gained (IQR 0.6 kg) vs. 2.7 kg of feed (IQR 0.4 kg) the control group.
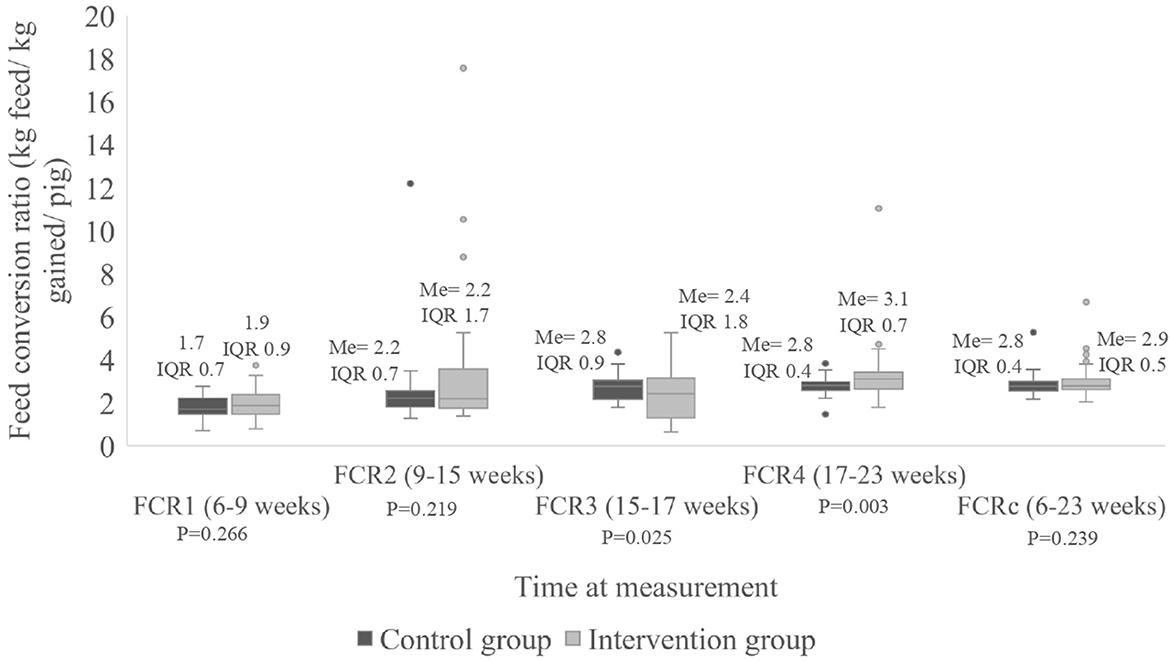
Figure 4. Feed conversion ratio (FCR) per pig (in kg/kg gained/pig) divided according to group, measured at four times during the study as well as for the entire period. Me, median; IQR, interquartile range; FCRc, cumulative feed conversion ratio.
In the intervention group, the total feed consumption was 13,120 kg vs. 12,680 kg in the control group (P = 0.61). This corresponded to an average feed consumption of 2 kg per animal per day for the intervention group and 1.9 kg for the control group (P = 0.87). Furthermore, the total water consumption for the intervention group was 78,144 L and for the control group 70,310 L. The water consumption variable was not normally distributed; the median consumption was 620 L/day (IQR 460) for the intervention group and 560 L of water/day (IQR 410) for the control group. The difference in water consumption was not statistically significant (P = 0.09).
4. Discussion
The baseline results showed a high Salmonella seroprevalence of 59.0%, which did not concord with the low proportion of pigs excreting the bacteria as shown by the culture-based detection method (1.0%) (36). To investigate this further, several methods and growth media were used to increase the sensitivity (37). However, these efforts were not successful in increasing the number of Salmonella-positive samples. This may be because of the known low sensitivity of culturing Salmonella spp. in fecal samples from pigs (8, 32, 38–40). Moreover, the regular administration of growth promoters to piglets on the farm could have reduced the Salmonella spp. excretion (40). Therefore, it was decided to measure only Salmonella seroprevalence during the study as an indication of the Salmonella prevalence.
There was a significantly lower Salmonella seroprevalence in the group of pigs provided organic acids (OA) (18.3%) compared with the control group (47.7%) at T2 (11 weeks). Contrary, at T1 (6 weeks) and T3 (15 weeks), there was no statistical difference in seroprevalence. OA favor the growth of lactobacilli, which contributes to a low pH, limit bacterial growth in the intestines and stimulates the immune system in a non-specific way; all of which decrease the probability of Salmonella colonization (3, 22, 41, 42). For this reason, the use of OA may have delayed the excretion and spread of Salmonella during the post-weaning period. However, as the observation period progressed, the majority of the pigs were eventually exposed to Salmonella spp. at some point. These findings are in agreement with the literature (3, 41, 43). Pigs develop partial immunity to Salmonella when the spread and exposure to the pathogen is reduced. Such partial immunity development is the core of a Salmonella reduction strategy as pigs at the time of slaughter will have a lower probability excreting Salmonella (36, 44). It is well-known that Salmonella cannot be eradicated without culling the farm (36, 44). There is a positive association between herd serology and the prevalence of Salmonella on the carcass as a low seroprevalence is associated with less prevalence on the carcass, less excretion and less overall contamination with Salmonella at the abattoir (45).
In Colombia, it is customary practice to use growth promoters like tylosin and zinc bacitracin. However, this is not in line with the principles of prudent use as it will lead to development of AMR and growth promoters are now banned in many countries (46, 47). Growth promoters are used as they are believed to support increased growth and reduce the severity of post-weaning diarrhea. In our study, pigs provided OA had a better cumulative DWG and weight productively than pigs administered growth promoters. The OA are feed additives that are metabolized by the animal, allowing their use without the risk of residues accumulating in the meat. The use of OA is already increasing as a response to strengthened regulations and consumer concerns on the use of antimicrobials in many countries (48). The mode of action of OA includes modulating stimulus that benefits the development of the mucosa, the length of microvilli, intestinal cell growth and therefore the absorptive capacity of the intestine is improved (3, 19).
The weight measured at time points W4 (17 weeks) and W5 (23 weeks) and the cumulative DWG during the study showed a better growth performance of pigs administered OA compared with the control group, which supports findings in other studies (49–56). van der Heijden et al. concluded that Selko pH® added to water at a concentration of 0.2% significantly reduced the seroprevalence of Salmonella and improved the productive performance of pigs (57). Likewise, formic, citric and benzoic acids can lead to improved growth when added to feed provided to weaned and growing pigs (23). A better DWG translates into less time spent by the pig in the herd, as well as a more efficient use of feed nutrients that represent one of the main costs of production (58).
Although there was a statistically significant difference (P = 0.025) at FCR3 and FCR4 (P = 0.009), favoring the intervention group and control group respectively, there was no significant difference in the cumulative FCR between the two groups. This may because the staff in charge of supplying the feed to the pigs did not fully take into account the pigs that died during the trial when calculating the feed to be administered. Therefore, the number of pigs used to calculate the feed provided was likely a little too high which may explain that no difference was found in the cumulative FCR between the groups (59).
The cleaning of the water pipes on the farm with citric acid before and during the study improved the water quality, which likely also resulted in healthier pigs (60, 61). Water is a potential source of various pig pathogens causing diseases that affect weight gain and feed conversion (62). For this reason, it is recommended to clean the water pipes regularly. The combination of cleaning of the pipes and the use of OA may be responsible for the higher overall productivity and apparently slower spread of Salmonella in the group administered OA. At the abattoir, such pigs are expected to have a lower probability of excreting Salmonella (44).
Salmonella antibodies can remain at measurable levels up to 3 months in the pig, which means that positives animals can be found even when they no longer are infected or excreting Salmonella spp. (36). Pigs included in the clinical trial may have experienced exposure to the pathogen without excreting Salmonella during sampling. Additionally, presence of antibodies in the individual animal may not be directly related to a carrier stage or probability of shedding Salmonella spp. (63). Hence, it is a limitation of our study that no other diagnostic tests were applied that could confirm whether pigs were excreting Salmonella (13). Post-harvest sampling of lymph nodes and ileocecal contents of the pigs may have increased the likelihood of detecting Salmonella if present, and thereby allowing a better assessment of how OA impacted the Salmonella levels in the pigs (37).
Selacid® was supplied at different concentrations during the study. It is known that different concentrations of OA can affect the Salmonella seroprevalence in pigs as shown by Calveyra et al. who concluded that at a concentration of 0.1%, OA had no significant effect on the Salmonella level whereas it did have a significant effect on improving daily weight gain in the pigs (64).
The dosage of Selko pH® we administered to drinking water (0.8 ml/L) was slightly lower than the dosage recommended by the technical data sheet (1–2 ml/L) from the manufacturer (65). The total estimated cost of the growth promoters added to the administered feed was 32 US$ as compared to 57 US$ for OA added to water and feed. The relative low dosage of OA may have had a reduced effect on Salmonella in the intervention group (25, 30, 66). On the other hand, the additional supplement of OA in the feed probably compensated for the lower concentration of Selko pH® used in the water. The types and concentrations of different OA products—as well as their costs—should be further investigated for their effect on Salmonella and overall productivity as the effect of the acids varies significantly depending on the components present in the feed (23, 59, 63). Moreover, attention should be given to palatability of the OA to ensure that the pigs do not consume less water or feed. Contrary to traditional organic acids, Selko pH® has the advantage that it is safe to use as it can be given to pigs in relative high concentrations without risking that the pigs stop drinking because of palatability issues.
For future research, it is recommended to include pig farms with known high prevalence of Salmonella spp., serial sampling and analyses of 25-g of fecal samples to increase the sensitivity.
5. Conclusion
This pilot study indicates that administration of OA in combination with regular cleaning of water pipes can improve productivity and delay exposure to Salmonella spp. when compared with commonly used antimicrobial growth promoters. A substitution of antimicrobial growth promoters with OA will lower antimicrobial use and resistance, while ensuring productivity. However, the study should be repeated before firmer conclusions can be drawn regarding productivity and the Salmonella spp. reduction potential of OA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was endorsed by the Institutional Committee Cuidado y Uso de los Animales (CICUA) at the CES University, Medellin, Colombia (Code No. 206/Act No. 38).
Author contributions
AD, LA, and MR-H were responsible for the conception of the study, experimental design, and manuscript writing. MR-H was responsible of collection of samples in the farm, data analysis, and interpretation. NC-C, LR-R, and LV-A were responsible for conception of the study and experimental design. All authors contributed to the article and approved the submitted version.
Funding
This work was part of the project entitled Salmonella Control in the Colombian Pig Industry that received financial support by the Ministry of Foreign Affairs (Danida) through the grant number 18-M07-KU.
Acknowledgments
The authors are grateful to ICMT veterinary laboratory (ICTM, Medellin, Colombia) and staff for help in processing and performing the serological and microbiological analyses. The Danish Agriculture & Food Council and University of Copenhagen are thanked for opening their doors for the Colombian co-authors to learn from their expertise and knowledge. Finally, we thank Janeth Perez (Department of Veterinary and Animal Sciences, CES University, Medellin, Colombia) for her help with the statistical analysis.
Conflict of interest
LA works for an organization that gives advice to livestock producers and meat producing companies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1123137/full#supplementary-material
References
1. Catering E, Díaz-Guevara P, Moreno J, Bautista A, Monta L, Realpe ME, et al. Laboratory surveillance of Salmonella enterica from human clinical cases in Colombia 2005 – 2011. Enferm Infecc Microbiol Clin. (2017) 35:417–25. doi: 10.1016/j.eimc.2016.02.023
2. Mogollón Vergara D, Rodríguez Gutiérrez V, Verjan García N. Prevalence and molecular identification of Salmonella spp. isolated from commercialized eggs at Ibague, Colombia. Revista de Salúd Animal. (2016) 38:164–72.
3. Food and Agriculture Organization, World Health Organization. Interventions for the Control of Non-typhoidal Salmonella spp. in Beef and Pork: Meeting Report and Systematic Review. (2016). Available online at: https://apps.who.int/iris/handle/10665/249529 (accessed December 12, 2022).
4. Donado-Godoy P, Clavijo V, León M, Arevalo A, Castellanos R, Bernal J, et al. Counts, serovars, and antimicrobial resistance phenotypes of Salmonella on raw chicken meat at retail in Colombia. J Food Prot. (2014) 77:227. doi: 10.4315/0362-028X.JFP-13-276
5. National Food Institute, Technical University of Denmark. Annual Report on Zoonoses in Denmark 2021. National Food Institute (2022). Available online at: https://www.food.dtu.dk/ (accessed December 12, 2022).
6. Carvajal-Restrepo H, Sánchez-Jiménez MM, Díaz-Rodríguez S, Cardona-Castro N. Detection of Salmonella human carriers in Colombian outbreak areas. J Infect Dev Ctries. (2017) 11:228. doi: 10.3855/jidc.8023
7. Instituto Nacional de Salud Dirección Redes en Salud, Pública. Características de los Aislamientos de Salmonella spp. Colombia Resultados de la Vigilancia 2000-2013. (2014). Available online at: https://www.ins.gov.co/BibliotecaDigital/informe-vigilancia-por-laboratorio-de-salmonella-spp-2000-2013.pdf (accessed December 12, 2022).
8. Rondón-Barragán IS, Arcos EC, Mora-Cardona L, Fandiño C. Characterization of Salmonella species from pork meat in Tolima, Colombia. Revista Colombiana de Ciencias Pecuarias. (2015) 28:74–82.
9. Ayala-Romero C, Ballen-Parada C, Rico-Gaitan M, Chamorro-Tobar I, Zambrano-Moreno D, Poutou-Piñales R. Prevalencia de Salmonella spp. en ganglios mesentéricos de porcinos en plantas de beneficio colombianas. RevMVZ Córdoba. (2018) 23:6474. doi: 10.21897/rmvz.1242
10. European Food Safety Authority. The European Union one health 2020 zoonoses report. EFSA J. (2021) 19:6971. doi: 10.2903/j.efsa.2021.6406
11. Alban L, Stärk KDC. Where should the effort be put to reduce the Salmonella prevalence in the slaughtered swine carcass effectively? Prevent Vet Med. (2005) 68:63. doi: 10.1016/j.prevetmed.2005.01.001
12. Betancur Vélez E. Control de Salmonella en cerdos con uso de aditivos y sin el uso de antibióticos en el alimento, en cinco granjas porcícolas del municipio de Santa Rosa de Osos (Antioquia) (Master's thesis). Corporación Universitaria Lasallista, Caldas, Antioquia (2018).
13. Ruggeri J, Foresti F, Pavesi R, Terrini A, Giudici F, Padoan D, et al. The synergistic effect of organic acids, phytochemicals and a permeabilizing complex reduces Salmonella Typhimurium 1,4,[5],12:i-shedding in pigs. Vet Res Commun. (2018) 42:209. doi: 10.1007/s11259-018-9723-3
14. Bonardi S. Salmonella in the pork production chain and its impact on human health in the European Union. Epidemiol Infect. (2017) 145:1513. doi: 10.1017/S095026881700036X
15. Arguello H, Álvarez-Ordoñez A, Carvajal A, Rubio P, Prieto M. Role of slaughtering in Salmonella spreading and control in pork production. J Food Prot. (2013) 76:899. doi: 10.4315/0362-028X.JFP-12-404
16. Giraldo-Cardona JP, Gualdrón-Ramírez D, Chamorro-Tobar I, Pulido-Villamarín A, Santamaría-Durán N, Castañeda-Salazar R, et al. Salmonella spp. prevalence, antimicrobial resistance and risk factor determination in Colombian swine farms. Pesquisa Veterinaria Brasileira. (2019) 39:816. doi: 10.1590/1678-5150-pvb-6156
17. Pulido-Villamarín AP, Santamaría-Durán AN, Castañeda-Salazar R, Chamorro-Tobar I, Carrascal-Camacho AK, Aranda-Silva M, et al. Evaluación de anticuerpos frente a tres bacterias zoonóticas y factores de riesgo asociados en explotaciones porcinas de Colombia. Rev Sci Tech. (2020) 39:923. doi: 10.20506/rst.39.3.3188
18. Miller GY, Xuanli L, McNamara P, Barber DA. Influence of Salmonella in pigs preharvest and during pork processing on human health costs and risks from pork. J Food Protect. (2005) 68:1788–98. doi: 10.4315/0362-028X-68.9.1788
19. Mroz Z. Organic acids as potential alternatives to antibiotic growth promoters for pigs. Adv Pork Prod. (2005) 16:169–82.
20. van der Wolf PJ, van Schie FW, Elbers ARW, Engel B, Van der Heijden HMJF, Huneman WA, et al. Epidemiology: administration of acidified drinking water to finishing pigs in order to prevent Salmonella infections. Vet Q. (2011) 2176:20–5. doi: 10.1080/01652176.2001.9695097
21. Gómez-García M, Sol C, Nova PJG de, Puyalto M, Mesas L, Puente H, et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porcine Health Manag. (2019) 4:1. doi: 10.1186/s40813-019-0139-4
22. Michiels J, Missotten J, Rasschaert G, Dierick N, Heyndrickx M, Smet SDE. Effect of organic acids on Salmonella colonization and shedding in weaned piglets in a seeder model. J Food Protect. (2012) 75:1974. doi: 10.4315/0362-028X.JFP-12-210
23. Liu Y, Espinosa CD, Abelilla JJ, Casas GA, Lagos LV, Lee SA, et al. Non-antibiotic feed additives in diets for pigs: a review. Anim Nutr. (2018) 4:113. doi: 10.1016/j.aninu.2018.01.007
24. van Immerseel F, Russell JB, Flythe MD, Gantois I, Timbermont L, Pasmans F, et al. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. (2006) 35:182. doi: 10.1080/03079450600711045
25. Creus E, Pérez JF, Peralta B, Baucells F, Mateu E. Effect of acidified feed on the prevalence of Salmonella in market-age pigs. Zoonoses Public Health. (2007) 54:314. doi: 10.1111/j.1863-2378.2007.01069.x
26. Rasschaert G, Michiels J, Tagliabue M, Missotten J, de Smet S, Heyndrickx M. Effect of organic acids on Salmonella shedding and colonization in pigs on a farm with high Salmonella prevalence. J Food Prot. (2016) 79:51. doi: 10.4315/0362-028X.JFP-15-183
27. Taube VA, Neu ME, Hassan Y, Verspohl J, Beyerbach M, Kamphues J. Effects of dietary additives (potassium diformate/organic acids) as well as influences of grinding intensity (coarse/fine) of diets for weaned piglets experimentally infected with Salmonella Derby or Escherichia coli. J Anim Physiol Anim Nutr. (2009) 93:350. doi: 10.1111/j.1439-0396.2008.00894.x
28. Walia K, Argüello H, Lynch H, Leonard FC, Grant J, Yearsley D, et al. Effect of feeding sodium butyrate in the late finishing period on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev Vet Med. (2016) 131:79. doi: 10.1016/j.prevetmed.2016.07.009
29. de Busser EV, Dewulf J, Nollet N, Houf K, Schwarzer K, de Sadeleer L. Effect of organic acids in drinking water during the last 2 weeks prior to slaughter on Salmonella shedding by slaughter pigs and contamination of carcasses. Zoonoses Public Health. (2009) 32:129. doi: 10.1111/j.1863-2378.2008.01172.x
30. Letellier A, Messier S, Lessard L, Quessy S. Assessment of various treatments to reduce carriage of Salmonella in swine. Can J Vet Res. (2000) 3:27–31. doi: 10.31274/safepork-180809-1037
31. Walsh M, Sholly D, Kelly D, Cobb M, Trapp S. The effects of supplementing weanling pig diets with organic and inorganic acids on growth performance and microbial shedding. Swine Research Report. (2003). p. 89–98.
32. Lynch H, Leonard FC, Walia K, Lawlor PG, Duffy G, Fanning S, et al. Investigation of in-feed organic acids as a low-cost strategy to combat Salmonella in grower pigs. Prev Vet Med. (2017) 139:50. doi: 10.1016/j.prevetmed.2017.02.008
33. Cardona-Castro N, Sánchez-Jiménez M, Lavalett L, Múñoz N, Moreno J. Development and evaluation of a multiplex polymerase chain reaction assay to identify Salmonella serogroups and serotypes. Diagn Microbiol Infect Dis. (2009) 65:327. doi: 10.1016/j.diagmicrobio.2009.07.003
34. Dani Daniel WW. Bioestadística: Base Para el Análisis de las Ciencias de la Salud. México: Limusa Wiley (2010). 736 p.
35. Martínez-González MA, Sánchez-Villegas A, Faulín Fajardo FJ. Bioestadística Amigable. Madrid: Díaz de Santos (2009). 919 p.
36. Kranker S, Alban L, Boes J, Dahl J. Longitudinal study of Salmonella enterica serotype typhimurium infection in three Danish farrow-to-finish swine herds. J Clin Microbiol. (2003) 4:2282. doi: 10.1128/JCM.41.6.2282-2288.2003
37. World Health Organization, Food and Agriculture Administration. Codex Alimentarius. Anteproyecto de directrices para el control de Salmonella spp. no tifoidea en la carne de bovino y cerdo. (2016). Available online at: http://www.who.int/foodsafety/consumer/5keys/en/ (accessed December 12, 2022).
38. Organización Mundial de Sanidad Animal. Código Sanitario para los Animales Terrestres. Salmonelosis (2022). Available online at: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahm/3.10.07_SALMONELLOSIS.pdf (accessed December 12, 2022).
39. Mejía Silva WJ. Epidemiología de la salmonelosis porcina en granjas de Cataluña y determinación de los factores de riesgo de la infección (Master's thesis). Universidad Autónoma de Barcelona, Barcelona, España (2003).
40. Wells JE, Kalchayanand N, Berry ED, Oliver W. Effects of antimicrobials fed as dietary growth promoters on faecal shedding of Campylobacter, Salmonella and shiga-toxin producing Escherichia coli in swine. J Appl Microbiol. (2013) 114:318. doi: 10.1111/jam.12065
41. Dibner JJ, Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poultry Res. (2002) 11:453. doi: 10.1093/japr/11.4.453
42. Boyen F, Haesebrouck F, Vanparys A, Volf J, Mahu M. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet Microbiol. (2008) 132:319. doi: 10.1016/j.vetmic.2008.05.008
43. Boyen F, Haesebrouck F, Maes D, van Immerseel F, Ducatelle R, Pasmans F. Non-typhoidal Salmonella infections in pigs: a closer look at epidemiology, pathogenesis and control. Vet Microbiol. (2008) 130:1. doi: 10.1016/j.vetmic.2007.12.017
44. Alban L, Baptista FM, Møgelmose V, Sørensen LL, Christensen H, Aabo S, et al. Salmonella surveillance and control for finisher pigs and pork in Denmark - a case study. Food Res Int. (2012) 45:656. doi: 10.1016/j.foodres.2011.02.050
45. Sørensen LL, Alban L, Nielsen B, Dahl J. The correlation between Salmonella serology and isolation of Salmonella in Danish pigs at slaughter. Vet Microbiol. (2004) 101:131. doi: 10.1016/j.vetmic.2004.02.016
46. Porkcolombia. Normas Colombianas. Revista Porkcolombia (2019). Available online at: https://porkcolombia.co/wp-content/uploads/2019/10/ED-249-PORKCOLOMBIA-DIGITAL.pdf (accessed January 25, 2023).
47. Dirección de Medicamentos y Tecnologías en Salud. Plan Nacional de Respuesta a la Resistencia a los Antimicrobianos Plan Estratégico. Bogotá: Dirección de Medicamentos y Tecnologías en Salud (2018).
48. Pacheco Nuñez GL, Palma Berrios JM. Efecto del uso de acidificantes (CitroZimTM y CitroZim-NaTM) en dietas de lechones en etapa de inicio (Master's thesis). Universidad de Zamorano, Zamorano (2015).
49. Luise D, Motta V, Salvarani C, Chiappelli M, Fusco L, Bertocchi M, et al. Long-term administration of formic acid to weaners: influence on intestinal microbiota, immunity parameters and growth performance. Anim Feed Sci Technol. (2017) 232:160. doi: 10.1016/j.anifeedsci.2017.06.015
50. Guggenbuhl P, Séon A, Quintana AP, Nunes CS. Effects of dietary supplementation with benzoic acid (VevoVitall®) on the zootechnical performance, the gastrointestinal microflora and the ileal digestibility of the young pig. Livest Sci. (2007) 108:218. doi: 10.1016/j.livsci.2007.01.068
51. Papatsiros V, Tassis P, Tzika E, Papaioannou D, Petridou E, Alexopoulos C, et al. Effect of benzoic acid and combination of benzoic acid with a probiotic containing Bacillus Cereus var. toyoi in weaned pig nutrition. Pol J Vet Sci. (2011) 14:117. doi: 10.2478/v10181-011-0017-8
52. Halas D, Hansen CF, Hampson DJ, Mullan BP, Kim JC, Wilson RH, et al. Dietary supplementation with benzoic acid improves apparent ileal digestibility of total nitrogen and increases villous height and caecal microbial diversity in weaner pigs. Anim Feed Sci Technol. (2010) 160:137. doi: 10.1016/j.anifeedsci.2010.07.001
53. Diao H, Gao Z, Yu B, Zheng P, He J, Yu J, et al. Effects of benzoic acid (VevoVitall®) on the performance and jejunal digestive physiology in young pigs. J Anim Sci Biotechnol. (2016) 7:32. doi: 10.1186/s40104-016-0091-y
54. Upadhaya SD, Lee KY, Kim IH. Protected organic acid blends as an alternative to antibiotics in finishing pigs. Asian Australas J Anim Sci. (2014) 27:1600. doi: 10.5713/ajas.2014.14356
55. Kuang Y, Wang Y, Zhang Y, Song Y, Zhang X, Lin Y, et al. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Anim Feed Sci Technol. (2015) 208:145. doi: 10.1016/j.anifeedsci.2015.07.010
56. Long SF, Xu YT, Pan L, Wang QQ, Wang CL, Wu JY, et al. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim Feed Sci Technol. (2018) 235:23. doi: 10.1016/j.anifeedsci.2017.08.018
57. van der Heijden M, van Dam H, Niewerth D, Frankena K. Effectiveness of Salmonella control strategies in fattening pigs. In: Safe Pork. (2005).
58. Pfuderer S, Bennett RM, Brown A, Collins LM. A flexible tool for the assessment of the economic cost of pig disease in growers and finishers at farm level. Prev Vet Med. (2022) 208:105757. doi: 10.1016/j.prevetmed.2022.105757
59. Garzón Rivero D, Espitia Forero ÉT. Crisis de los contenedores una mirada desde el contexto global y sus implicaciones en Colombia (Master's thesis). Institución Universitaria Colegios de Colombia, Bogotá (2022).
60. de Sousa C, Colmenares MC, Correia A. Contaminación bacteriológica en los sistemas de distribución de agua potable: revisión de las estrategias de control. Bol Mal Salud Amb. (2008) 48:17.
61. Quintero Barrera LD, Pulido-Villamarín A del P, Chamorro-Tobar I. Determinación de la presencia de Salmonella spp. en el agua de granjas porcícolas de diferentes regiones de Colombia (Master's thesis). Universidad Pontificia Javeriana, Bogotá] (2020).
62. Rivas-Nichorzon M, Alfaro-Escalona M, Silva-Acuña R, Gomez-Piñeres E. Calidad bacteriológica y pH del agua en una unidad de producción porcina ubicada en el Rincón de Monagas, estado Monagas, Venezuela. Zootec Trop. (2016) 34:127–33.
63. Parker S, Waddell L, Wilhelm B, Raji A, Sanchez J, Fazil A, et al. Assessment of the efficacy and quality of evidence for five on-farm interventions for Salmonella reduction in grow-finish swine: a systematic review and meta-analysis. Prev Vet Med. (2012) 107:1. doi: 10.1016/j.prevetmed.2012.07.011
64. Calveyra JC, Nogueira MG, Kich JD, Biesus LL, Vizzotto R, Berno L, et al. Effect of organic acids and mannanoligosaccharide on excretion of Salmonella typhimurium in experimentally infected growing pigs. Res Vet Sci. (2012) 93:46–7. doi: 10.1016/j.rvsc.2011.08.018
65. Trouw Nutrition. Selko ®-pH (2018). Available online at: www.selko.com (accessed December 12, 2022).
Keywords: Salmonella, organic acids, pigs, seroprevalence, growth promoters, growth performance
Citation: Roldan-Henao M, Dalsgaard A, Cardona-Castro N, Restrepo-Rivera L, Veloza-Angulo LC and Alban L (2023) Pilot study of the productivity and Salmonella seroprevalence in pigs administered organic acids. Front. Vet. Sci. 10:1123137. doi: 10.3389/fvets.2023.1123137
Received: 14 December 2022; Accepted: 13 February 2023;
Published: 03 March 2023.
Edited by:
Kohei Makita, Rakuno Gakuen University, JapanReviewed by:
Ilias Giannenas, Aristotle University of Thessaloniki, GreeceAshenafi Feyisa Beyi, Iowa State University, United States
Akira Fukuda, Rakuno Gakuen University, Japan
Copyright © 2023 Roldan-Henao, Dalsgaard, Cardona-Castro, Restrepo-Rivera, Veloza-Angulo and Alban. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuela Roldan-Henao, bWFudWVsYXJvbGRhbkBvdXRsb29rLmNvbQ==
 Manuela Roldan-Henao
Manuela Roldan-Henao Anders Dalsgaard
Anders Dalsgaard Nora Cardona-Castro
Nora Cardona-Castro Lina Restrepo-Rivera
Lina Restrepo-Rivera Luis Carlos Veloza-Angulo1
Luis Carlos Veloza-Angulo1 Lis Alban
Lis Alban