- 1College of Animal Science and Veterinary Medicine, Henan Institute of Science and Technology, Xinxiang, Henan, China
- 2Life Science College, Luoyang Normal University, Luoyang, Henan, China
Chinese yam polysaccharide (CYP) has received attention in recent years owing to its positive nutritional and medicinal characteristics. Copper is an essential trace metal in animals, which plays an important role in iron absorption and hemoglobin synthesis. However, no published study has evaluated Chinese yam polysaccharide copper complex (CYP-Cu) as a dietary additive in broilers. This study was conducted to investigate the effects of dietary CYP-Cu on growth performance, immunity, and oxidative resistance in broilers. A total of 360 1-day-old 817 broiler chickens were randomly divided into 4 groups, with 3 replicates of 30 birds each and were fed a basal diet with the addition of 0 (control group), 0.02, 0.10, and 0.50 g/kg CYP-Cu. The feeding trial lasted 48 days. On day 28 and day 48, 6 broilers in each group were slaughtered, respectively. Then the parameters of growth and carcass, serum biochemistry, immunity, and antioxidation, and the expression level of hepatic antioxidative genes were investigated. The results showed that compared with the control group, the supplementation of dietary CYP-Cu could improve the indexes of the growth, carcass, serum biochemistry, immunity and oxidation resistance in broilers, such as average daily gain (ADG), the slaughter percentage (SP), semi-evisceration weight percentage (SEWP), eviscerated carcass weight percentage (EWP), breast muscle percentage (BMP), leg muscle percentage (LMP), serum albumin (ALB), high density lipoprotein (HDL), insulin-like growth factor I (IGF-I), triiodothyronine (T3), thyroxine (T4), growth hormone (GH), insulin (INS), immunoglobulin M (IgM), immunoglobulin G (IgG), immunoglobulin A (IgA), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 6 (IL-6), complement 3 (C3), complement 4 (C4), total superoxide dismutase (T-SOD), total antioxidative capacity (T-AOC), glutathione peroxidase (GSH-Px), and glutathione s-transferase (GSH-ST); these parameters in the 0.10 g/kg CYP-Cu treated group were significantly increased (P < 0.05) in the total trial period, with the exceptions that feed conversion ratio (FCR) and serum low density lipoprotein (LDL), malondialdehyde (MDA) were decreased in the total trial period. In addition, the antioxidative gene mRNA expression of Nuclear factor E2-related factor 2 (Nrf2), Superoxide dismutase 1 (SOD1), Superoxide dismutase 2 (SOD2), and Catalase (CAT) were upregulated in the liver (P < 0.05). These results indicated that the supplementation of dietary CYP-Cu improved the growth, immunity, and oxidation resistance of broilers, and the addition of 0.10 g/kg CYP-Cu in broiler diets is recommended, which suggests that CYP-Cu may be a promising green feed additive in the poultry industry.
Introduction
Chinese yam polysaccharide (CYP) extracted from the Chinese yam, a very popular tuber crop for food and medical purposes in China (1), has received attention in recent years, owing to its positive nutritional and medicinal characteristics (2–5). Previous studies have shown that the pharmacological effects of CYP include promoting growth performance, lowering blood glucose and blood lipid, and enhancing anti-oxidation and immunity in animal (6–9). It is known that CYP has good antioxidant activity, and removed hydroxyl radical capacity (4). CYP could enhance the cellular and humoral immune activities in chickens induced by Newcastle disease virus vaccine, and promote proliferation of peripheral blood lymphocytes induced by concanavalin A (9). Another study has shown that mice in a CYP group had heavier body weights than controls, and that CYP could regulate inflammatory responses and oxidative stress in mice (9). Our previous study showed that the supplementation of dietary CYP (0.50 g/kg) promoted thymus index, serum immunoglobulin A (IgA), complement 3 (C3), complement 4 (C4), insulin-like growth factor I (IGF-I), triiodothyronine (T3), thyroxine (T4), insulin (INS), growth hormone (GH), interleukin 2 (IL-2), interleukin 4 (IL-4), and interleukin 6 (IL-6) levels in broilers (10).
An important issue is the application of trace metals in the diets of livestock and poultry. Copper is an essential trace metal in the body, it plays an important role in iron absorption and hemoglobin synthesis (11), and also has strong antimicrobial activity (12). However, inorganic copper is not well absorbed, and can lead to poisoning and environmental pollution, while organic copper is more easily absorbed and used, and reduces environmental pollution and toxic effects (12).
Recent research has shown that the addition of polysaccharides and metal complexes can be effective substances against bacteria (13–15). To date, no published study has evaluated Chinese yam polysaccharide copper complex (CYP-Cu) as a dietary additive in animals. In this paper, we prepared CYP-Cu, investigated the effects of its dietary inclusion upon the growth performance, serum immunity levels, and antioxidant capacity in broilers, and discussed the immune function of CYP-Cu in birds, as a strategy to replace antibiotic use and improve growth performance and immunity in poultry.
Materials and methods
Preparation of CYP-Cu
The Chinese yam polysaccharide for the test was purchased from Shaanxi Hana Biotechnology Co., Ltd (Xi'an city, China), with particle size passing through an 80 mesh sieve (≥95.00%), dry weight loss < 5%, and polysaccharide content ≥30% (total carbohydrate ≥90.40%). The trial copper sulfate was purchased from Tianjin Fenghua Chemical Reagent Factory (analytically pure, Tianjin city, China); molecular formula: CuSO4-5H2O; molecular weight: 249.68, content: 99.00%. To prepare the CYP-Cu, a water bath was adjusted to 60–70°C; 4 g of CYP and 1 g of sodium citrate were placed into a beaker, and 120 mL of water was added and mixed well; stir until its temperature up to 60–70°C. Sodium hydroxide was added in a dropwise fashion to increase alkalinity; then the saturated copper sulfate solution was added dropwise (to maintain the alkaline environment) until flocculent material appeared; at this point, no more was added. The water bath was stirred for 1 h; the contents were centrifuged while hot, the supernatant was separated and 95% ethanol was added. The solution was left overnight at 4°C, and then centrifuged to produce the precipitate, which is the CYP-Cu. The content of copper in CYP-Cu was determined to be 1591.59 ± 6.07 mg/kg (16).
Experimental design
In this experiment, 360 1-day-old healthy 817 broilers (39.54 ± 0.51 g, sex balance), a commercial Chinese crossbred broiler produced by crossing a fast-growing broiler cock and layer hen (17), were selected from the same batch at Henan Fengyuan Poultry Co (Xinxiang city, China). The broilers were randomly placed into 4 groups with 3 replicates, with 30 broilers per replicate. The control group was fed only a basal diet (without CYP-Cu); the 3 treated groups were supplemented with 0.02, 0.10, and 0.50 g/kg of CYP-Cu in the basal diet, respectively. The experimental feeding lasted until the chickens were 48 days old, and was divided into two stages, 1–28 and 29–48 days.
Diets and management
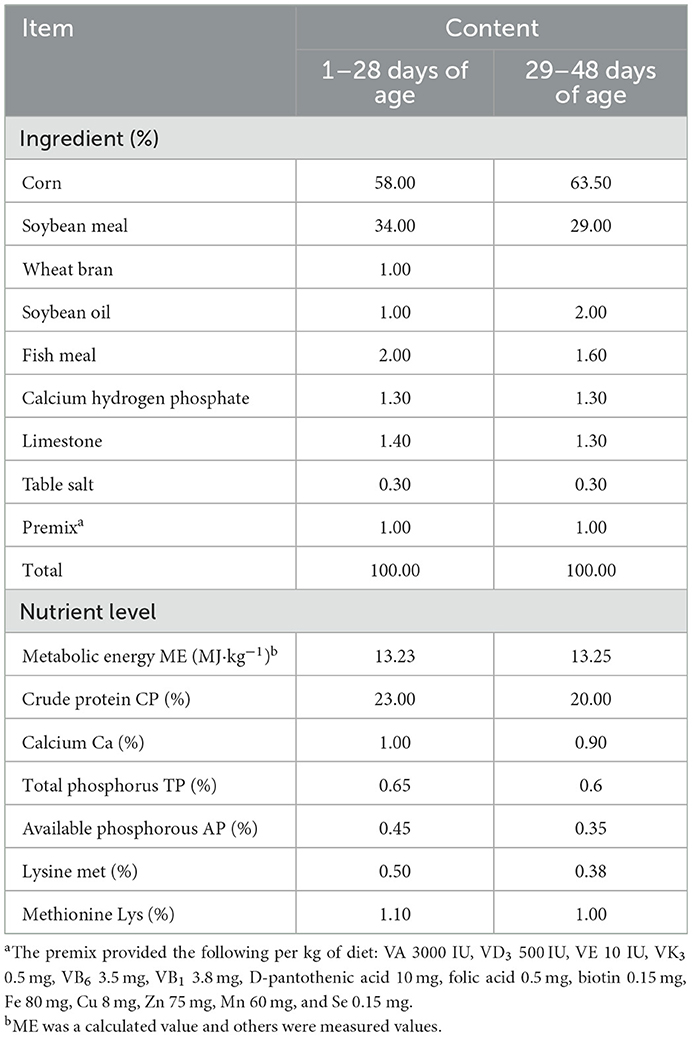
The broilers were fed commercial diets formulated with reference to the NRC (1994) nutritional requirement standards for broilers. The composition and nutritional levels of the basic diet for broilers are shown in Table 1.
All birds were kept under continuous lighting for 24h, and birdhouse temperature was kept at 32°C for the first 3 days, cooled 2–3°C every week, and dropped to 21°C on the 35th until end of the trial. During the experiment, broilers were caged, and had ad-libitum access to diet and water; in addition, routine management followed the company's procedures.
Growth and carcass parameters measured
During the feeding trial period, the amount of intake and leftovers were recorded daily, and body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) of broilers were calculated at the end of the trial, respectively. On day 28 and day 48, after collecting blood samples, 6 broilers (3 males and 3 females) in each group were slaughtered, respectively. The data of carcass weight, semi-evisceration weight, evisceration weight, breast muscle, and leg muscle were measured. Then the slaughter percentage (SP), semi-evisceration weight percentage (SEWP), eviscerated carcass weight percentage (EWP), breast muscle percentage (BMP), and leg muscle percentage (LMP) of broilers were determined. The samples of liver tissue were taken from the same position and kept at −80°C until analysis.
Blood collection
On day 28 and day 48, 6 broilers (3 males and 3 females) were randomly selected from each group for the collection of blood samples. After weighing, the blood was drawn from the wing veins of birds, and put into centrifuge tubes for overnight coagulation at 4°C. Following centrifugation (3,000 g, 10 min, at 4°C), the serum was collected into sterile tubes and kept at −80°C until analysis.
Serum biochemical, immune, and antioxidative parameters measured
Serum biochemistry parameters, such as serum albumin (ALB), low density lipoprotein (LDL), and high density lipoprotein (HDL), were obtained by using a 7,180 automatic analyzer (Hitachi High-Technologies Co., Ltd., Tokyo, Japan). The assays of serum biochemical, immune, and antioxidative profiles included insulin-like growth factor I (IGF-I), triiodothyronine (T3), thyroxine (T4), growth hormone (GH), insulin (INS), immunoglobulin M (IgM), immunoglobulin G (IgG), immunoglobulin A (IgA), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 6 (IL-6), complement 3 (C3), complement 4 (C4), total superoxide dismutase (T-SOD), total antioxidative capacity (T-AOC), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and glutathione s-transferase (GSH-ST); concentrations were analyzed by ELISA according to the manufacturer's instruction (Nanjing Jiancheng Bioengineering Institute, Nanjing city, China).
Assay of hepatic antioxidative gene expressions
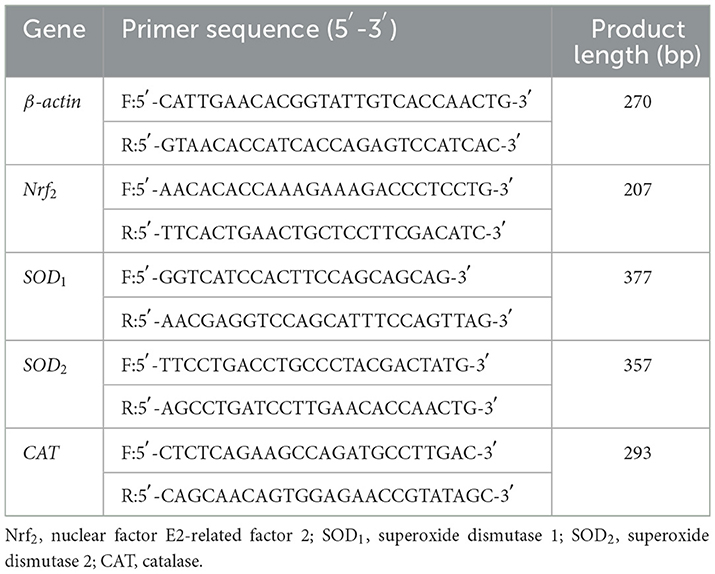
Total RNAs were isolated from hepatic tissues using Trizol reagent (Invitrogen, Thermo Fisher Scientific, USA) following the manufacturer's instructions. The quality and quantity of RNA was detected with a NanoPhotometer® spectrophotometer (Implen, Westlake Village, CA, USA), and RNA integrity wasdetected with 1.0% agarose gelelectrophoresis. Total RNAs were transcribed into cDNA using M-MLV reverse transcriptase (Promega, Madison, WI, USA). The genes of Nuclear factor E2-related factor 2 (Nrf2), Superoxide dismutase 1 (SOD1), Superoxide dismutase 2 (SOD2), and Catalase (CAT) were selected for the detection of hepatic antioxidative gene expression profiles. The primer sequences for quantitative real-time PCR (qRT-PCR) assay are shown in Table 2. The qRT-PCR was carried out in a QuantStudio 6 Flex Real-Time PCR System (ABI, Carlsbad, CA, USA) with the miScript SYBR Green PCR kit (Qiagen, GmbH, Hilden, Germany) following the manufacturer's instructions. In the present study, β-actin as a housekeeping gene, the relative expression of the target genes was calculated by 2−ΔΔCt method.
Statistical analysis
All data obtained from this experiment were analyzed using One-Way ANOVA in SPSS 26.0 for Windows (IBM Corp., Chicago, IL, USA), and presented as mean ± standard error of the means (SEM), and significant differences among all groups were determined at P < 0.05 by Duncan's multiple range tests.
Results
Growth performances
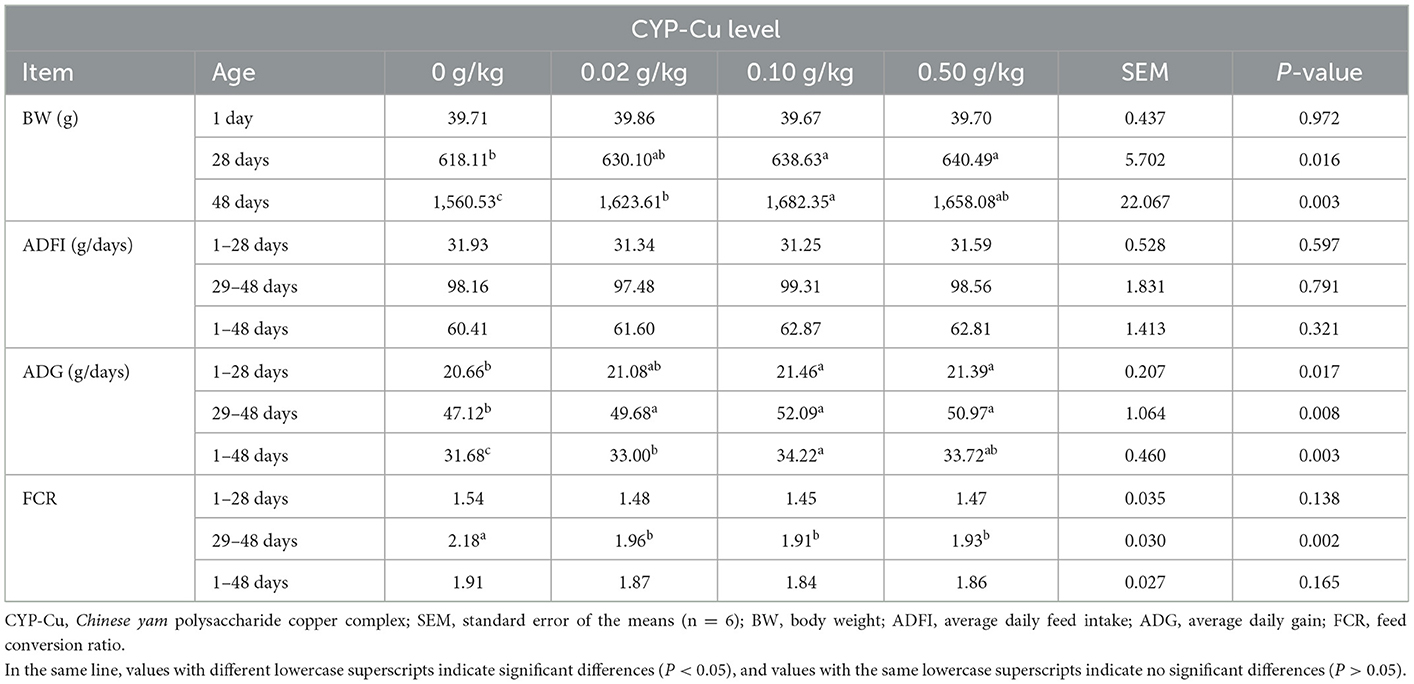
The effect of dietary CYP-Cu on growth performances evaluated in this study is shown in Table 3. The BW of broilers on day 28 and day 48 was affected by the addition of CYP-Cu, the BW of the 0.10 and 0.50 g/kg CYP-Cu group was significantly higher than that of the control group on day 28 (P < 0.05), while the BW of the 0.10 g/kg group was significantly higher than that of control group on day 48 (P < 0.05). Compared with the control, the ADG of the treatments increased with the addition of CYP-Cu, and the 0.10 g/kg group had the highest ADG in all trial periods (P < 0.05). However, no difference of ADFI between the treatments and the control were observed in all trial periods (P > 0.05). By contrast, the FCR of the treatments in 29–48 days periods was significantly lower than that of control group (P < 0.05), while no difference of FCR between the treatments and the control were observed in 1–28 and 1–48 days periods (P > 0.05). However, the FCR of the treatments had a downward trend with the addition of CYP-Cu, and the 0.10 g/kg group had the lowest FCR in all trial periods.
Carcass performances
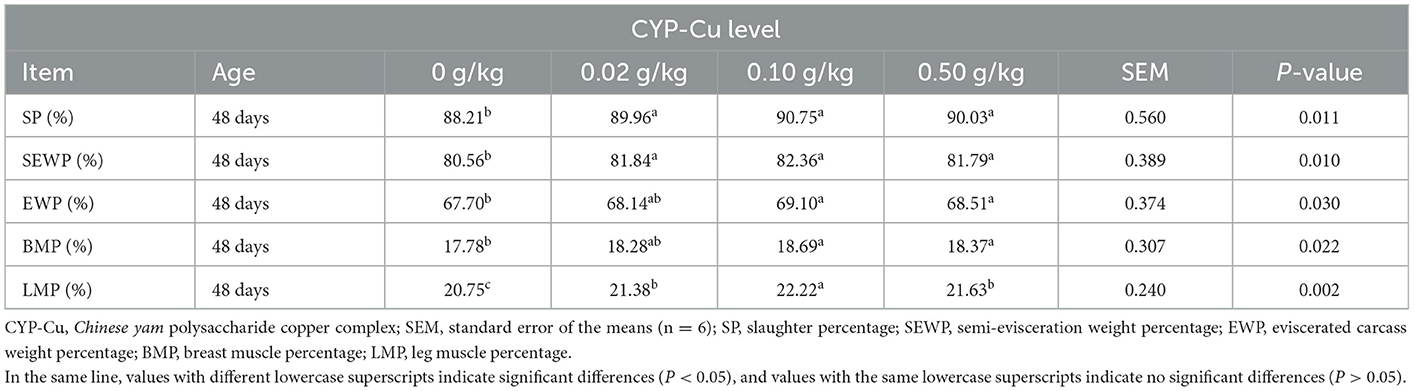
The supplementation of dietary CYP-Cu had a positive effect on the carcass performances of broilers on day 48 (Table 4). Compared to that of the control, SP, SEWP, EWP, BMP, and LMP of 48-day broilers showed a gradual increase with the addition of CYP-Cu. Among them, the carcass performances of the 0.10 and 0.50 g/kg CYP-Cu group showed a significantly greater increase than those of the control in 48-day broilers (P < 0.05).
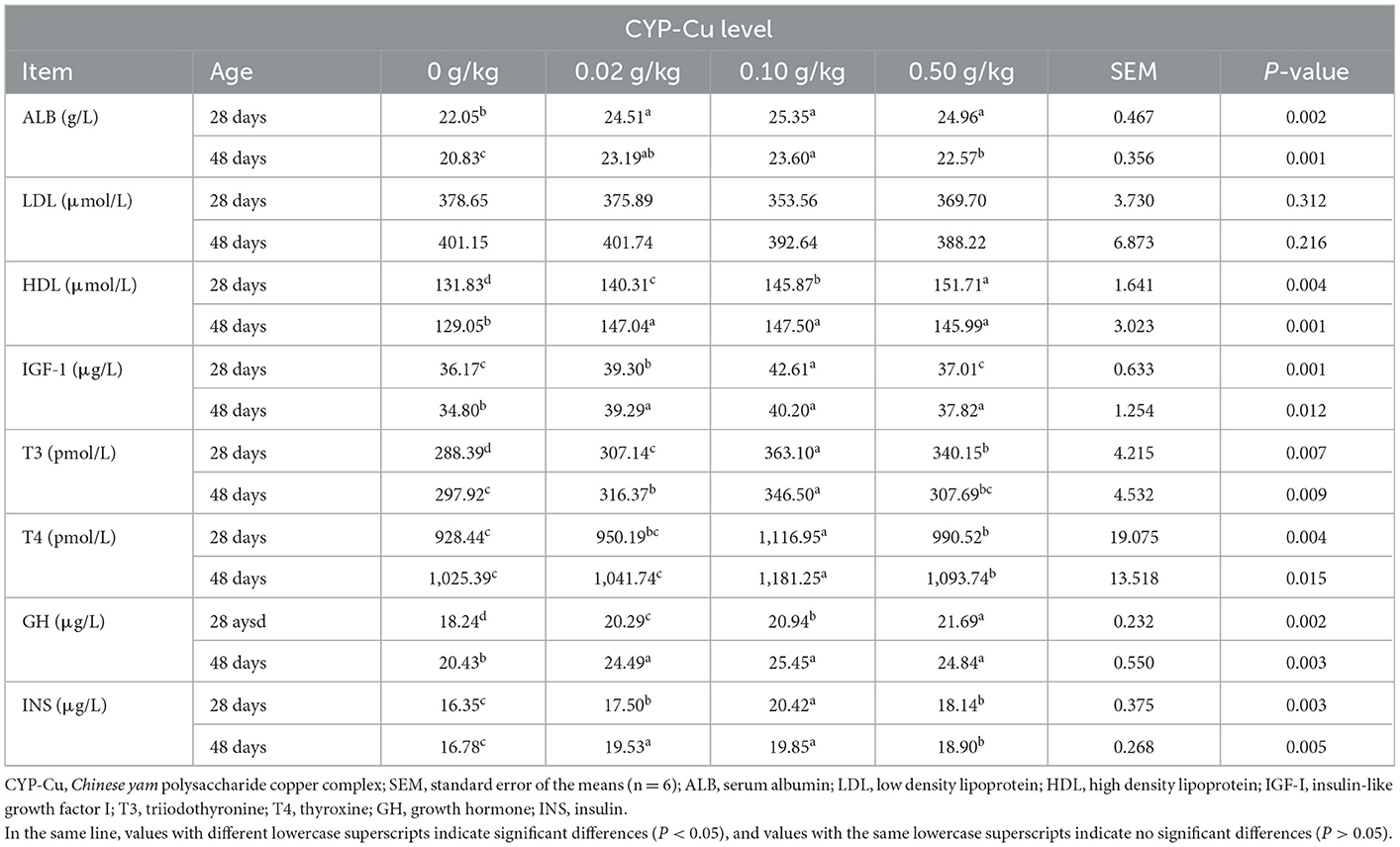
Serum growth factors and biochemical parameters
As presented in Table 5, the concentrations of serum growth factors, IGF-I, T3, T4, GH, and INS, in the 3 treated groups expressed a higher tendency than those in the control group of broilers on day 28 and day 48. The results of the 0.10 g/kg CYP-Cu group were the most significant (P < 0.05), with the exception of serum GH concentration in the 0.50 g/kg group, which had the highest level on day 28 (P < 0.05).
According to Table 5, the densities of ALB and HDL in the 3 treated groups were significantly higher than those in the control group on day 28 and day 48 (P < 0.05). ALB levels in the 0.10 g/kg CYP-Cu group were highest in broilers on day 28 and day 48 (P < 0.05), while HDL level was highest in the 0.50 g/kg CYP-Cu group on day 28 and in the 0.10 g/kg CYP-Cu group on day 48 (P < 0.05). Interestingly, the densities of LDL in the 3 treated groups had a decreasing trend with increasing dietary CYP-Cu supplementation, but there were no significant differences in LDL levels between the treated and the control groups (P > 0.05).
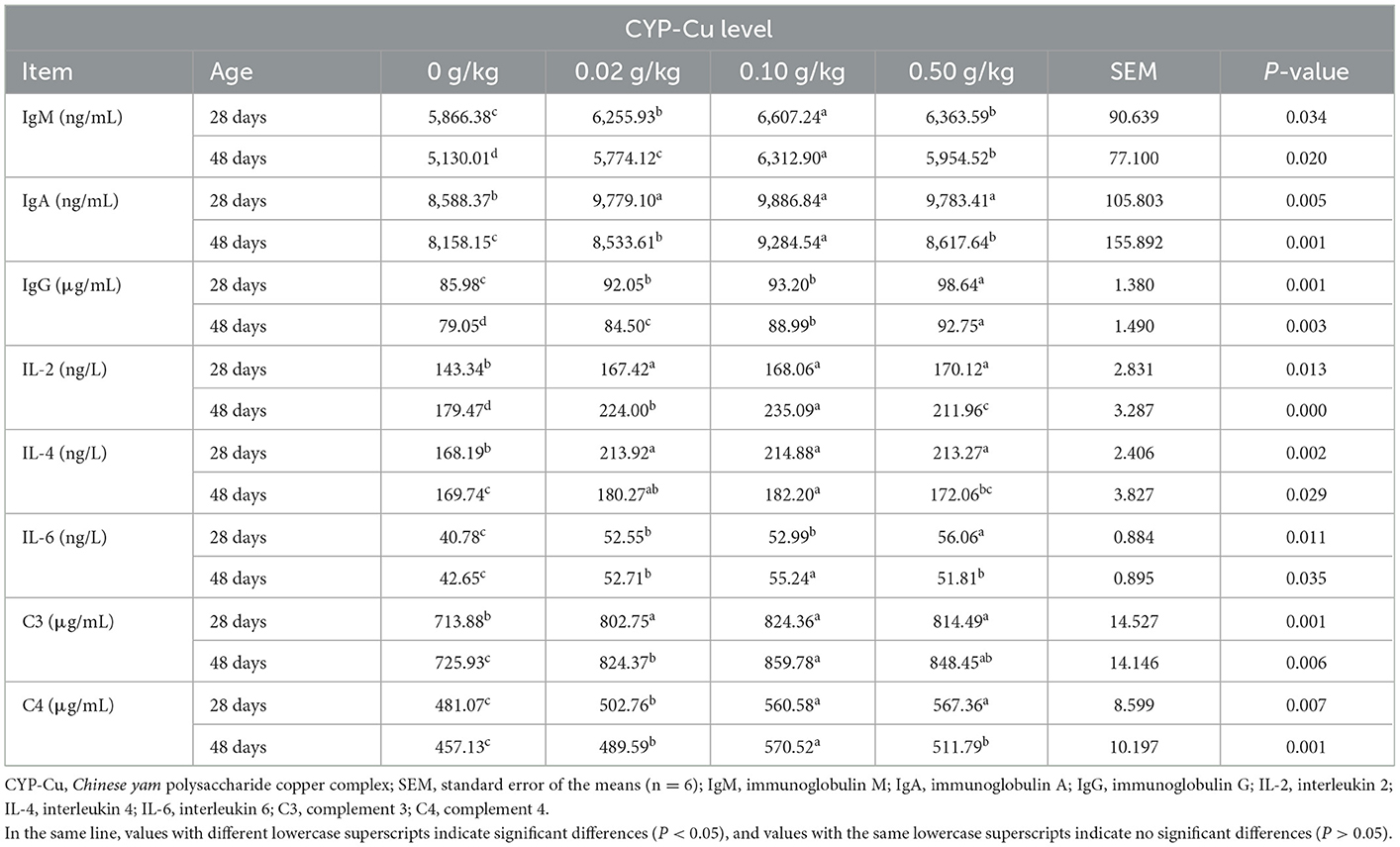
Serum immune parameters
The serum immune parameters in broilers on day 28 and day 48 are shown in Table 6. Compared with the control group, the serum levels of IgM, IgA, IgG, IL-2, IL-4, IL-6, C3, and C4 in the 3 treated groups were significantly higher on day 28 (P < 0.05). The dietary supplementations of 0.10 and 0.50 g/kg CYP-Cu were more effective. On day 48, the serum concentrations of IgM, IgA, IgG, IL-2, IL-6, C3, and C4 in the 3 treated groups were significantly higher than those in the control group (P < 0.05), meanwhile, the serum levels of IL-2 in the 0.02 and 0.10 g/kg CYP-Cu groups were significantly higher than those in the control group (P < 0.05). The additions of 0.10 g/kg CYP-Cu in the diet of broilers on day 48 seemed to have a greater effect on serum immune parameters.
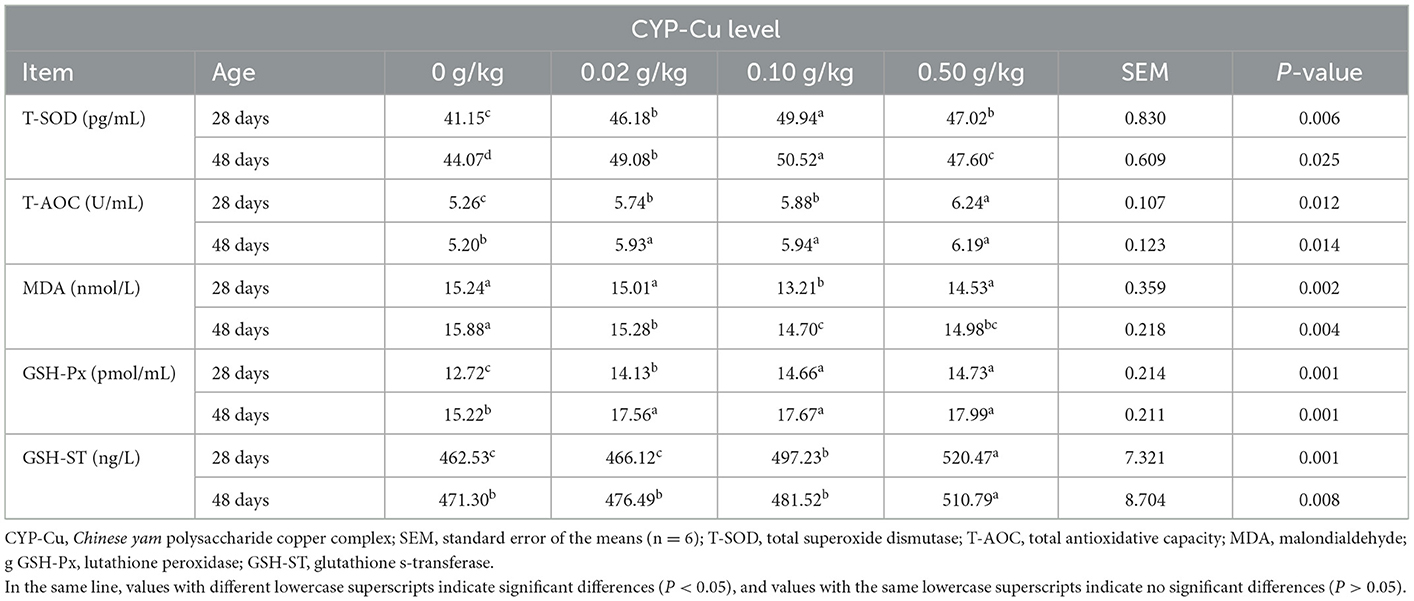
Serum antioxidative parameters
According to Table 7, the dietary supplementation of CYP-Cu played a positive role in serum antioxidative parameters in broilers on day 28 and day 48. Compared with that of the control, the serum concentrations of T-SOD, T-AOC, GSH-ST, and GSH-Px in the treated groups were higher on day 28 and day 48. Therein, the results of the 0.10 and 0.50 g/kg CYP-Cu groups were the most significant on day 28 (P < 0.05), while the results of the 0.50 g/kg CYP-Cu group seemed to be more effective on day 48 (P < 0.05). Interestingly, the serum concentration of MDA in the treated groups was downregulated in contrast to the control on day 28 and day 48. The serum concentration of MDA of the 0.10 and 0.50 g/kg CYP-Cu groups showed a greater decline (P < 0.05). These results indicated that the supplementation of CYP-Cu in the diet of broilers could raise oxidation resistance.
Hepatic antioxidative gene expression profiles
In this experiment, the antioxidant genes, CAT, Nrf2, SOD1, SOD2, and mRNA expression in the livers of broilers on day 48 were investigated (Figure 1, Supplementary Table S1). According to Figure 1, the antioxidative gene mRNA expression of Nrf2, SOD1, SOD2 and CAT were significantly upregulated in the liver in the treated groups compared to the control group (P < 0.05), while among all the treated groups, the antioxidative genes had the highest mRNA expression level in the 0.10 g/kg group. The results show that the dietary supplementation of 0.10 g/kg CYP-Cu could improve mRNA expression of the antioxidant genes CAT, Nrf2, SOD1, and SOD2 in the livers of broilers on day 48, and enhance their antioxidant capacity.
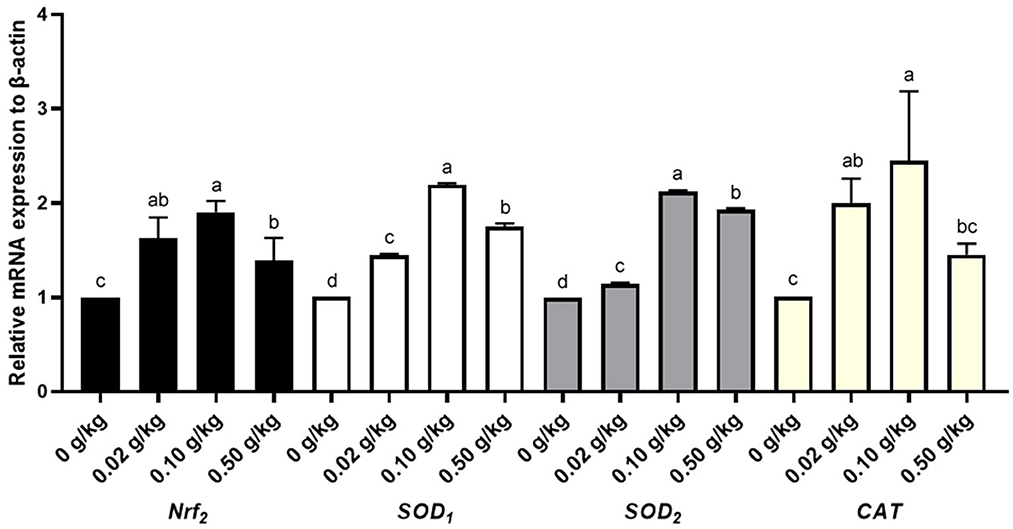
Figure 1. Hepatic antioxidative gene expression profiles in 48-day-old broilers. Nrf2, nuclear factor E2-related factor 2; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; CAT, catalase. In the figure, antioxidative gene expression profiles of Nrf2, SOD1, SOD2, and CAT, in the livers of 48-day-old broilers were detected by qRT-PCR. The values with different lowercase superscripts indicate significant differences (P < 0.05), while values with the same lowercase superscripts indicate no significant differences (P > 0.05).
Discussion
In recent years, increasing numbers of studies have shown that polysaccharide metal complexes display diverse biological activities, such as improving growth, antioxidation, immune regulation, and antibacterial activity (14, 18–21). The objective of this paper was to determine the effect of supplementation of CYP-Cu in broiler diets on growth performance, immune function, and antioxidant capacity. The treated results showed that such supplementation improved the growth and carcass performance of broiler chickens, and a dietary supplementation of 0.10 g/kg CYP-Cu was the optimal level. Cui et al. (22) reported that the supplementation of Enteromorpha prolifera polysaccharide-iron (III) complex (2 mg Fe/kg body weight) by intragastric administration has a considerable effect on the growth performance and blood indexes of rats with iron deficiency anemia, indicating that Enteromorpha prolifera polysaccharide-iron (III) complex could be used as a new iron fortifier to replace traditional iron supplements for gastrointestinal irritation and poor absorption. Another study reported the effects of pectin oligosaccharide and zinc chelate (80 mg/kg) on growth performance, antioxidant ability, and gut function in broilers, and found that dietary supplementation with pectin oligosaccharide and zinc chelate was more effective than feeding pectin oligosaccharide and zinc sulfate alone (23). Gao et al. (20) showed that soybean polysaccharide iron complex (5.00–20.00 mg/mL) had different impacts on foodborne bacteria, promoting the growth of the beneficial bacteria, Bacillus licheniformis, and inhibiting the proliferation of the pathogenic bacteria, Staphylococcus aureus, thus improving the body's growth and immunity. These studies indicate that, compared with polysaccharide and metal alone, polysaccharide and metal complexes improve animal growth and immunity because of the synergistic interaction between polysaccharides and metals, and the enhancement of intestinal health and nutrient absorption by promoting the proliferation of beneficial bacteria to the competitive exclusion of pathogenic bacteria. Consistent with previous studies, our results showed that CYP-Cu had a positive effect on growth performance of broilers, implying a good synergy.
In the present study, the addition of CYP-Cu in diet can enhance the immunity of broilers by improving the concentrations of IgM, IgA, IgG, IL-2, IL-4, IL-6, C3, and C4 in serum at 28 days and in serum IgM, IgA, IgG, IL-2, IL-6, C3, and C4 in group 48 days, and we recommend the supplementation of 0.10 g/kg CYP-Cu in broiler diets. However, little is known about the underlying principle by which dietary CYP-Cu improves the immune performance of broilers. Gao et al. (19) demonstrated that Ulva polysaccharide iron complex (200 and 400 mg/mL) could promote the proliferation of lymphocytes, improve the activities of murine macrophages, and restore serum levels of IFN-γ, and IL-10 in immune-deficient mice to normal; furthermore, the Ulva polysaccharide iron complex exhibits excellent hematopoietic capacity. Dey et al. (24) found that chitosan conjugated green copper oxide nanoparticles (50 μg/mL) inhibited the proliferation of breast cancer and cervical cancer cells in vivo in a Balb/C mouse model, and increased pro-inflammatory cytokines and CD4+ populations; the author showed that the potential mechanism of chitosan conjugated green copper oxide nanoparticles is not only through inducing cellular immunity by activating immune cells, but they also lead to a humoral immune response through an IgG reaction. Another study reports that copper-loaded chitosan nanoparticles (100 mg/kg) in broiler diets improves the growth of poultry, and raises the serum levels of IgA, IgM, C3, and C4, suggesting that it can be used as a substitute for antibiotics (25). Although previous studies have exhibited the immune activities of polysaccharide metal complexes in animals, further research is needed to explain the underlying mechanisms by which dietary CYP-Cu improves immune performance in animals.
Free radicals, in vivo, are products that are inevitably generated through metabolism in a broad range of biochemical reactions; when accumulation is excessive they can lead to oxidative stress and subsequent tissue damage (26–28). Antioxidants are the substances that protect organisms against damage caused by oxidation, and they are classified into two groups, exogenous and endogenous antioxidants, according to origin. To date, increasing numbers of studies indicate that natural botanical polysaccharides are potential antioxidants (29, 30). In this paper, an evaluation about the effects of the supplementation of dietary CYP-Cu on serum antioxidative parameters of broilers demonstrated that dietary CYP-Cu significantly increased the serum concentrations of T-SOD, T-AOC, GSH-ST, GSH-Px, and decreased the serum concentrations of MDA of broilers (0.10 g/kg CYP-Cu group), which revealed that CYP-Cu had strong antioxidant activity. A previous study shown that lotus root polysaccharide iron complex could significantly increase the antioxidant capacity of mice by increasing the serum levels of CAT, SOD, and GSH-Px (31), and showed a trend of first increasing from 2 to 8 mg/mL and then decreasing. Similarly, Dong et al. (32) found that Flammulina velutipes polysacchrides and polysacchride-iron (III) complex inhibited the MDA production of health mice liver and improve its antioxidant capacity. Li et al. (33) found that novel biochanin a-chromium (III) complex enhanced the oxidative capacity of the db/db mice and improved the oxidative stress injury caused by hyperglycemia through decreasing the content of MDA, and increasing the content of CAT, SOD, and GSH-Px in liver of mice. Our study also demonstrated that dietary CYP-Cu could improve the antioxidant capacity of broilers.
On the other hand, the current study showed that the supplementation of dietary CYP-Cu in broilers had the ability to upregulate the mRNA expression of hepatic antioxidant genes, CAT, Nrf2, SOD1, and SOD2, and the dietary supplementation of 0.10 g/kg CYP-Cu in broilers was more effective in promoting antioxidant gene expressions. Gene expressions of antioxidant enzymes such as SOD, glutathione peroxidase, and heme oxygenase-1 are the key mechanism by which the body responds to various reactive oxygen species during inflammation, trauma, or other stressful conditions (34). SODs are a family of metalloproteins that catalyze the change of radical superoxide to oxygen and hydrogen peroxide, and protect organisms against oxidative stress. SOD1 and SOD2, such as Cu–Zn SOD and Mn SOD, are located in the cytoplasm and mitochondria (35, 36). Chinese wolfberry and Astragalus extract (1%) notably improved the activities of SOD1, T-AOC, and CAT, and decreased MDA in Tibetan pig livers, and promoted the expression levels of hepatic antioxidant genes (e.g., CAT and SOD1); this indicates that the extracts mechanism of regulating antioxidation is the signaling pathway of peroxisome antioxidant-oxidant stress in Tibetan pig livers (37). Another study investigated the effect of a plateau condition on oxidation in Tibetan pigs and Duroc × Landrace × Yorkshire (DLY) pigs, and found that Tibetan pigs exhibited higher SOD, GSH-Px, and T-AOC levels, but lower MDA levels in the liver and heart. Furthermore, the mRNA levels of SOD, GSH-Px, and Nrf2 in the liver and heart of Tibetan pigs were higher than those in DLY pigs, and the authors suggest that Tibetan pigs held a stronger antioxidant activity through the AMPK/p38 MAPK/Nrf2-ARE signaling pathways (38). Wang and Li (18) found that the Zinc-HSP (Hohenbuehelia serotina polysaccharides) complex (20 mg/mL) not only had superoxide anion radical scavenging ability, but also did not affect the biological activity of Hohenbuehelia serotina polysaccharides when combined with zinc. The dietary supplementation of Enteromorpha prolifera polysaccharide-zinc (EP-Zn) complex (400 mg EP-Zn/kg) in chickens upregulated the mRNA expression levels of the antioxidant genes Nrf2, CAT1, and SOD1 in breast muscle and lipid metabolism genes ACC, FADS1, PPAR-α, and CPT1 in liver, thereby improving the growth performance and meat quality of chickens (39). In this paper, dietary supplementation of CYP-Cu has been shown to have effective free radical scavenging and antioxidant activities in broilers, which indicates that CYP-Cu has great potential for the application in poultry.
Conclusion
The current results showed that dietary CYP-Cu improved growth performance, serum immune function, and antioxidant capacity in broilers, and upregulated the hepatic antioxidant gene CAT, Nrf2, SOD1, and SOD2 mRNA expression of broilers, and the supplementation of 0.10 g/kg CYP-Cu in broiler diet is recommended.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Animal Protection and Utilization Committee of Henan Institute of Science and Technology (No. 2021HIST018, Xinxiang, P. R. China).
Author contributions
JZ: design, investigation, writing—original manuscript, and editing. YJ, MC, JD, and YC: carried out the experiments and analyzed the data. MS: conceptualization, methodology, and supervision. ZM: project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (Henan Province, China; 22IRTSTHN026), and the Science and Technology Program of Henan Province (Henan Province, China; 212102110169), and the Key Scientific Research Projects of Colleges and Universities in Henan Province (Henan Province, China; 23B230003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1123002/full#supplementary-material
References
1. Epping J, Laibach N. An underutilized orphan tuber crop-Chinese yam: a review. Planta. (2020) 252:58. doi: 10.1007/s00425-020-03458-3
2. Li Q, Li W, Gao Q, Zou Y. Hypoglycemic effect of Chinese yam (Dioscorea opposita rhizoma) polysaccharide in different structure and molecular weight. J Food Sci. (2017) 82:2487–94. doi: 10.1111/1750-3841.13919
3. Huang R, Shen M, Yu Y, Liu X, Xie J. Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. Int J Biol Macromol. (2020) 165(Pt A):635–44. doi: 10.1016/j.ijbiomac.2020.09.213
4. Zhou S, Huang G, Chen G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. (2021) 361:130089. doi: 10.1016/j.foodchem.2021.130089
5. Li Z, Xiao W, Xie J, Chen Y, Yu Q, Zhang W, et al. Isolation, characterization and antioxidant activity of yam polysaccharides. Foods. (2022) 11:800. doi: 10.3390/foods11060800
6. Qiu Y, Hu YL, Cui BA, Zhang HY, Kong XF, Wang DY, et al. Immunopotentiating effects of four Chinese herbal polysaccharides administered at vaccination in chickens. Poult Sci. (2007) 86:2530–5. doi: 10.3382/ps.2007-00076
7. Ju Y, Xue Y, Huang J, Zhai Q. Wang XH. Antioxidant Chinese yam polysaccharides and its pro-proliferative effect on endometrial epithelial cells. Int J Biol Macromol. (2014) 66:81–5. doi: 10.1016/j.ijbiomac.2014.01.070
8. Fan Y, He Q, Luo A, Wang M, Luo A. Characterization and antihyperglycemic activity of a polysaccharide from Dioscorea opposita Thunb roots. Int J Mol Sci. (2015) 16:6391–401. doi: 10.3390/ijms16036391
9. Wang Y, Liu Y, Zhang Y, Huo Z, Wang G, He Y, et al. Effects of the polysaccharides extracted from Chinese yam (Dioscorea opposite Thunb) on cancer-related fatigue in mice. Food Funct. (2021) 12:10602–14. doi: 10.1039/D1FO00375E
10. Deng J, Zhang J, Chang Y, Wang S, Shi M, Miao Z. Effects of Chinese yam polysaccharides on the immune function and serum biochemical indexes of broilers. Front Vet Sci. (2022) 9:1013888. doi: 10.3389/fvets.2022.1013888
11. Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, et al. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr. (2006) 136:1236–41. doi: 10.1093/jn/136.5.1236
12. Mitra D, Kang ET, Neoh KG. Antimicrobial copper-based materials and coatings: potential multifaceted biomedical applications. ACS Appl Mater Interfaces. (2020) 12:21159–82. doi: 10.1021/acsami.9b17815
13. Abdel-Daim MM, Eissa IAM, Abdeen A, Abdel-Latif HMR, Ismail M, Dawood MAO, et al. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol. (2019) 69:44–50. doi: 10.1016/j.etap.2019.03.016
14. Ghonam HE, Abu Yousef MA, Gohar YM, Almeer R, Barakat KM. A new antidiabetic foot bacteria formula from marine chitosan nanosilver-metal complex. Environ Sci Pollut Res Int. (2021) 28:60833–41. doi: 10.1007/s11356-021-14958-4
15. Liang J, Sun D, Yang Y, Li M, Li H, Chen L. Discovery of metal-based complexes as promising antimicrobial agents. Eur J Med Chem. (2021) 224:113696. doi: 10.1016/j.ejmech.2021.113696
16. Fan M, Qian Y, Yue W, Yang Y, Zhang X, Ma S, et al. Preparation and characterization of metal-tea polysaccharide complexes and their inhibition on α-glucosidase. J Food Biochem. (2021) 45:e13689. doi: 10.1111/jfbc.13689
17. Chen Y, Qiao Y, Xiao Y, Chen H, Zhao L, Huang M, et al. Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian Aust J Anim Sci. (2016) 29:855–64. doi: 10.5713/ajas.15.0840
18. Wang L, Li X. Preparation, physicochemical property and in vitro antioxidant activity of zinc-Hohenbuehelia serotina polysaccharides complex. Int J Biol Macromol. (2019) 121:862–9. doi: 10.1016/j.ijbiomac.2018.10.118
19. Gao X, Qu H, Gao Z, Zeng D, Wang J, Baranenko D, et al. Protective effects of Ulva pertusa polysaccharide and polysaccharide-iron (III) complex on cyclophosphamide induced immunosuppression in mice. Int J Biol Macromol. (2019) 133:911–9. doi: 10.1016/j.ijbiomac.2019.04.101
20. Gao W, Jiang L, Wan Z, Zeng XA. Antibacterial and probiotic promotion potential of a new soluble soybean polysaccharide-iron (III) complex. Int J Biol Macromol. (2020) 163:2306–13. doi: 10.1016/j.ijbiomac.2020.09.063
21. Li X, Jiang F, Liu M, Qu Y, Lan Z, Dai X, et al. Synthesis, characterization, and bioactivities of polysaccharide metal complexes: a review. J Agric Food Chem. (2022) 70:6922–42. doi: 10.1021/acs.jafc.2c01349
22. Cui J, Li Y, Yu P, Zhan Q, Wang J, Chi Y, et al. A novel low molecular weight Enteromorpha polysaccharide-iron (III) complex and its effect on rats with iron deficiency anemia (IDA). Int J Biol Macromol. (2018) 108:412–8. doi: 10.1016/j.ijbiomac.2017.12.033
23. Wang Z, Yu H, Xie J, Cui H, Gao X. Effect of pectin oligosaccharides and zinc chelate on growth performance, zinc status, antioxidant ability, intestinal morphology and short-chain fatty acids in broilers. J Anim Physiol Anim Nutr. (2019) 103:935–46. doi: 10.1111/jpn.13076
24. Dey A, Manna S, Kumar S, Chattopadhyay S, Saha B, Roy S. Immunostimulatory effect of chitosan conjugated green copper oxide nanoparticles in tumor immunotherapy. Cytokine. (2020) 127:154958. doi: 10.1016/j.cyto.2019.154958
25. Wang C, Wang MQ, Ye SS, Tao WJ, Du YJ. Effects of copper-loaded chitosan nanoparticles on growth and immunity in broilers. Poult Sci. (2011) 90:2223–8. doi: 10.3382/ps.2011-01511
26. Veskoukis AS, Tsatsakis AM, Kouretas D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones. (2012) 17:11–21. doi: 10.1007/s12192-011-0293-3
27. Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, et al. Antioxidants and human diseases. Clin Chim Acta. (2014) 436:332–47. doi: 10.1016/j.cca.2014.06.004
28. Neha K, Haider MR, Pathak A, Yar MS. Medicinal prospects of antioxidants: a review. Eur J Med Chem. (2019) 178:687–704. doi: 10.1016/j.ejmech.2019.06.010
29. Long LN, Kang BJ, Jiang Q, Chen JS. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult Sci. (2020) 99:744–51. doi: 10.1016/j.psj.2019.10.043
30. Long LN, Zhang HH, Wang F, Yin YX, Yang LY, Chen JS. Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult Sci. (2021) 100:101028. doi: 10.1016/j.psj.2021.101028
31. Yuan S, Dong PY, Ma HH, Liang SL Li L, Zhang XF. Antioxidant and biological activities of the Lotus root polysaccharide-iron (III) complex. Molecules. (2022) 27:7106. doi: 10.3390/molecules27207106
32. Dong YR, Cheng SJ Qi GH, Yang ZP, Yin SY, Chen GT. Antimicrobial and antioxidant activities of Flammulina velutipes polysaccharides and polysaccharide-iron (III) complex. Carbohydr Polym. (2017) 161:26–32. doi: 10.1016/j.carbpol.2016.12.069
33. Li P, Li M, Lou X, Zhao B, Ma Q, Bian Y, et al. Evaluation of hypoglycemic activity and sub-acute toxicity of the novel biochanin a-chromium (III) complex. Molecules. (2022) 27:5786. doi: 10.3390/molecules27185786
34. Li S, Li H, Xu X, Saw PE, Zhang L. Nanocarrier-mediated antioxidant delivery for liver diseases. Theranostics. (2020) 10:1262–80. doi: 10.7150/thno.38834
35. Eleutherio ECA, Silva Magalhães RS, de Araújo Brasil A, Monteiro Neto JR, de Holanda Paranhos L. SOD1, more than just an antioxidant. Arch Biochem Biophys. (2021) 697:108701. doi: 10.1016/j.abb.2020.108701
36. Kim YS, Gupta Vallur P, Phaëton R, Mythreye K, Hempel N. Insights into the dichotomous regulation of SOD2 in Cancer. Antioxidants. (2017) 6:86. doi: 10.3390/antiox6040086
37. Hao Z, Li Z, Huo J, Li J, Liu F, Yin P. Effects of Chinese wolfberry and Astragalus extract on the antioxidant capacity of Tibetan pig liver. PLoS ONE. (2021) 16:e0245749. doi: 10.1371/journal.pone.0245749
38. Hu H, Li Y, Yang Y, Xu K, Yang L, Qiao S, et al. Effect of a plateau environment on the oxidation state of the heart and liver through AMPK/p38 MAPK/Nrf2-ARE signaling pathways in Tibetan and DLY Pigs. Animals. (2022) 12:1219. doi: 10.3390/ani12091219
Keywords: Chinese yam polysaccharide copper complex, growth performance, immunity, oxidation resistance, broilers
Citation: Zhang J, Jin Y, Cao M, Deng J, Chang Y, Shi M and Miao Z (2023) Effects of dietary Chinese yam polysaccharide copper complex on growth performance, immunity, and antioxidant capacity of broilers. Front. Vet. Sci. 10:1123002. doi: 10.3389/fvets.2023.1123002
Received: 13 December 2022; Accepted: 30 January 2023;
Published: 16 February 2023.
Edited by:
Abdelrazeq M. Shehata, Al-Azhar University, EgyptReviewed by:
Hisham Shoukry, Al-Azhar University, EgyptDaniel Hernandez-Patlan, Faculty of Higher Studies Cuautitlán, National Autonomous University of Mexico, Mexico
Sharif Hasan Siddiqui, University of Rochester, United States
Copyright © 2023 Zhang, Jin, Cao, Deng, Chang, Shi and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Miao,  bWlhb3poaWd1bzE5OThAMTI2LmNvbQ==
bWlhb3poaWd1bzE5OThAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jinzhou Zhang
Jinzhou Zhang Yan Jin1†
Yan Jin1† Jiahua Deng
Jiahua Deng Zhiguo Miao
Zhiguo Miao